UHPLC-MS/MS Assay for Quantification of Legubicin, a Novel Doxorubicin-Based Legumain-Activated Prodrug, and Its Application to Pharmacokinetic and Tissue Distribution Studies
Abstract
1. Introduction
2. Methodologies, Validation, Results, and Discussion
2.1. Method Development
2.1.1. Sample Preparation Method for Detecting Free Leu-DOX and DOX
2.1.2. Sample Preparation Method for Detecting Albumin Conjugate of Legubicin
2.2. Method Validation
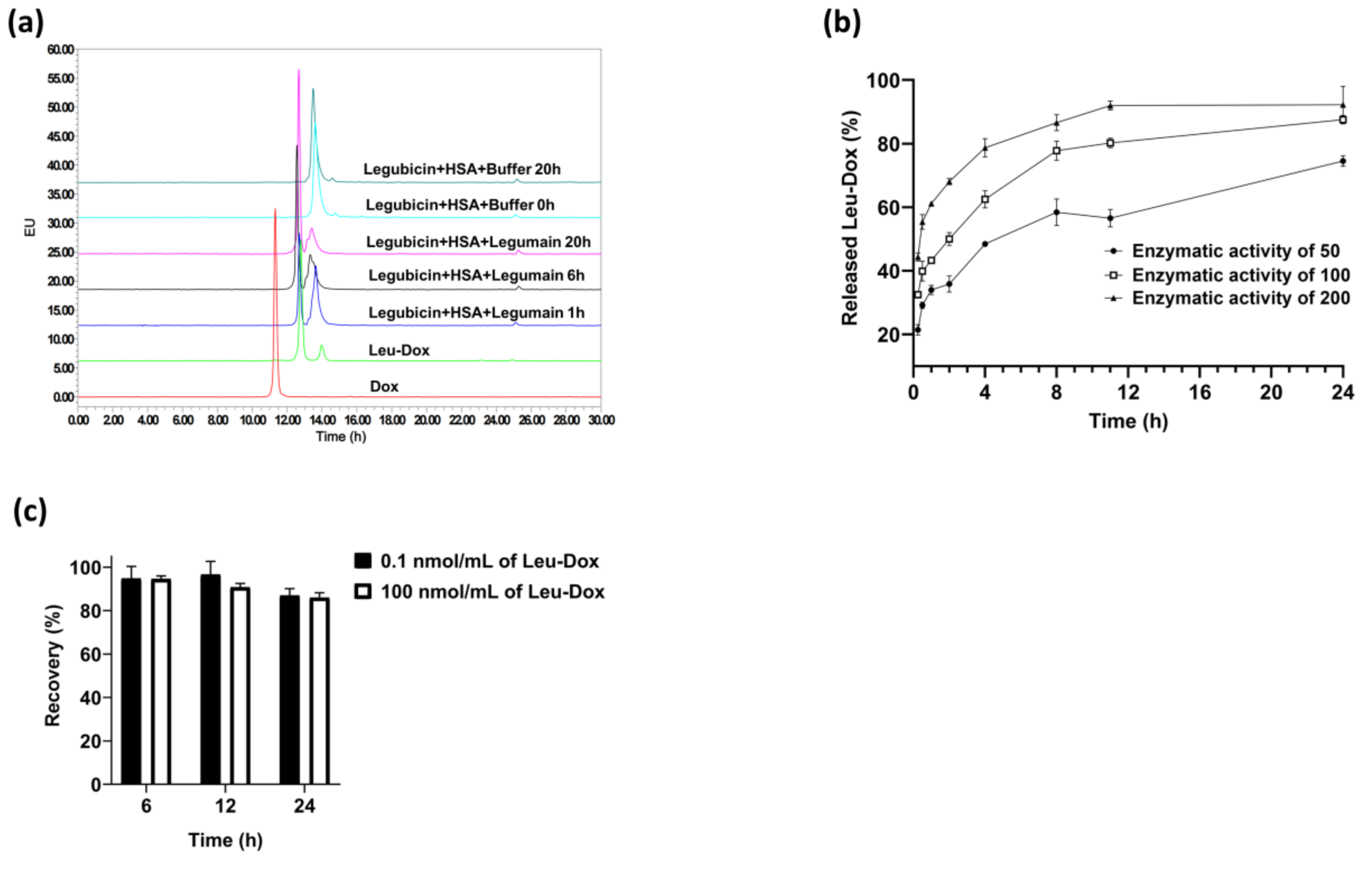
2.2.1. Selectivity, Specificity, and Carry-Over
2.2.2. Linearity
2.2.3. Accuracy and Precision
2.2.4. Extraction Recovery and Matrix Effect
2.2.5. Dilution Integrity
2.2.6. Stability
2.3. Pharmacokinetic Study in SD Rats
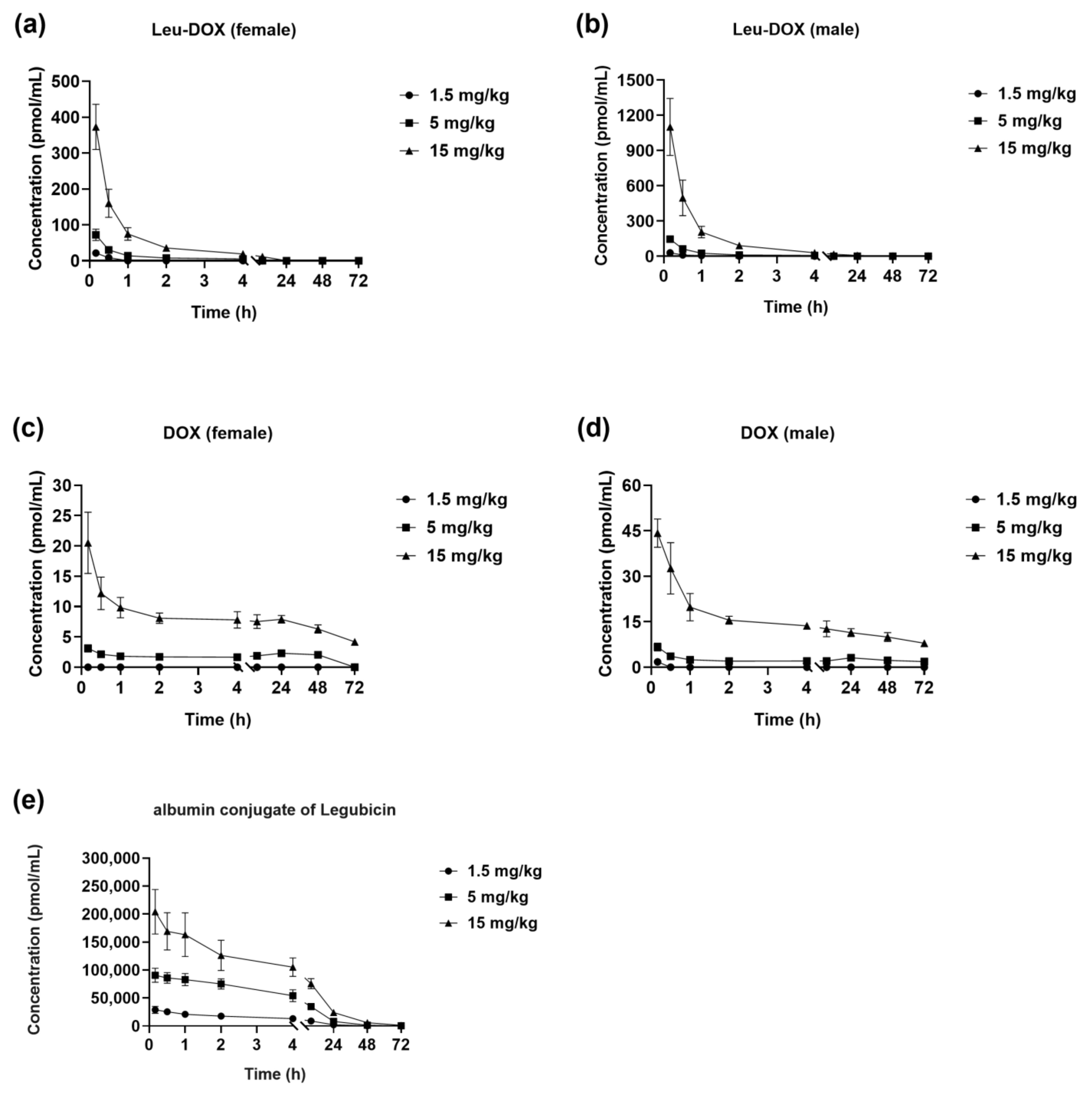
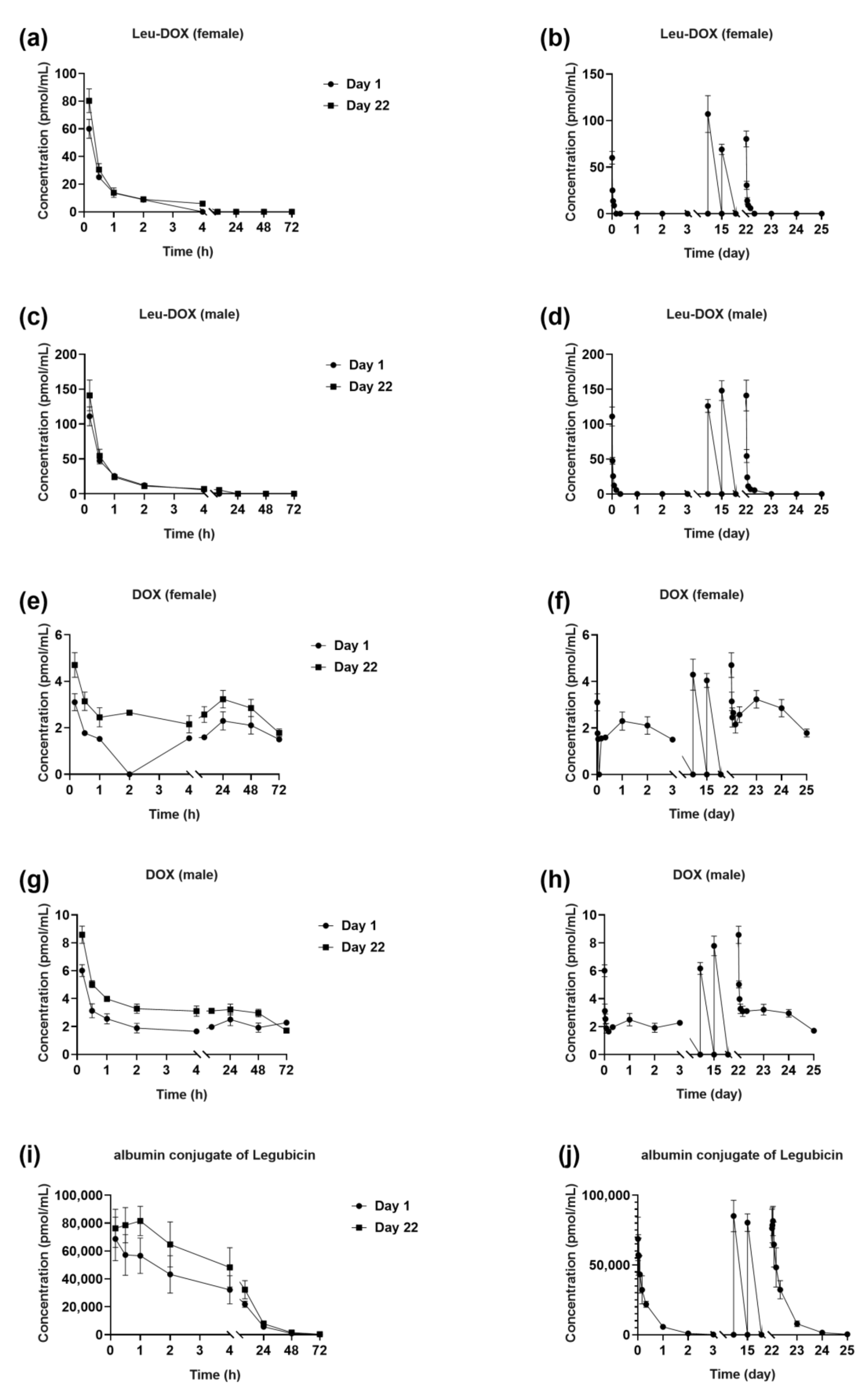
2.3.1. Single-Dose Studies
2.3.2. Multiple-Dose Studies
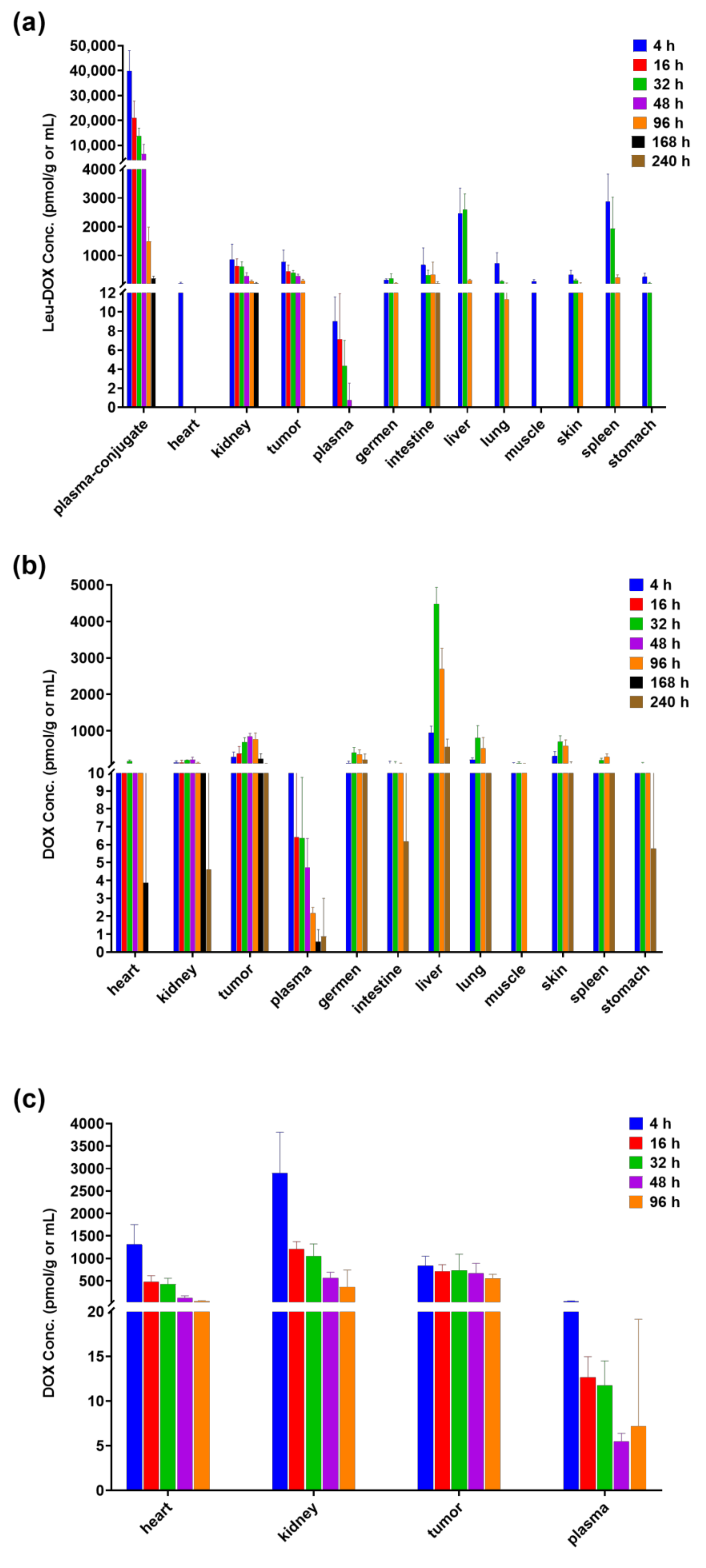
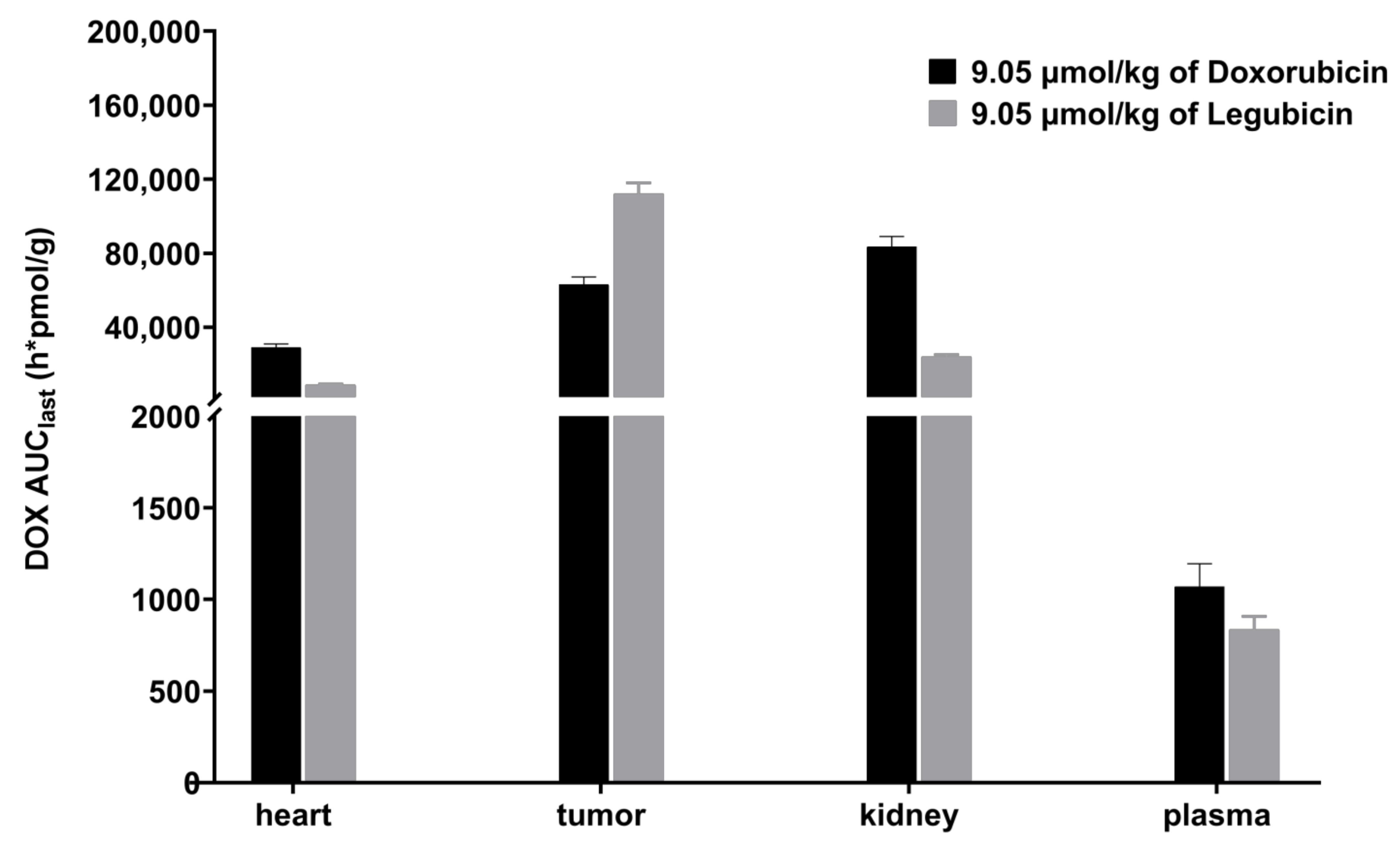
2.4. Tissue-Distribution Study in Tumor-Bearing Mice
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Animals
3.3. Instrumentation and UHPLC-MS/MS Parameters
3.4. Stock and Working Solutions Preparation
3.5. Calibration Standards and Quality Control Samples Preparation
3.6. Preparation of Administration Formulation
3.7. Sample Pretreatment
3.7.1. Plasma Samples
3.7.2. Tumor and Tissue Samples
3.8. Method Validation
3.8.1. Selectivity, Specificity and Carry-Over
3.8.2. Linearity, LOD and LOQ
3.8.3. Accuracy and Precision
3.8.4. Extraction Recovery and Matrix Effect
3.8.5. Dilution Integrity
3.8.6. Stability Studies
3.9. Animal Experiments
3.10. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Cancer Institute. Cancer Drug Information: Doxorubicin Hydrochloride. 2023. Available online: https://www.cancer.gov/about-cancer/treatment/drugs/doxorubicinhydrochloride (accessed on 1 January 2024).
- Al-Malky, H.S.; Al Harthi, S.E.; Osman, A.M.M. Major obstacles to doxorubicin therapy: Cardiotoxicity and drug resistance. J. Oncol. Pharm. Pract. 2019, 26, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.M.; Dando, P.M.; Rawlings, N.D.; Brown, M.A.; Young, N.E.; Stevens, R.A.; Hewitt, E.; Watts, C.; Barrett, A.J. Cloning, Isolation, and Characterization of Mammalian Legumain, an Asparaginyl Endopeptidase. J. Biol. Chem. 1997, 272, 8090–8098. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.M.; Fortunato, M.; Barrett, A.J. Activation of human prolegumain by cleavage at a C-terminal asparagine residue. Biochem. J. 2000, 352, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Breistøl, K.; Hendriks, H.R.; Fodstad, Ø. Superior Therapeutic Efficacy of N-L-leucyl-doxorubicin Versus Doxorubicin in Human Melanoma Xenografts Correlates with Higher Tumour Concentrations of Free Drug. Eur. J. Cancer 1999, 35, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Jiang, Z.; Chen, S.; Wu, Z.; Chen, K.; Wu, Y. Legumain correlates with neuroblastoma differentiation and can be used in prodrug design. Chem. Biol. Drug Des. 2017, 91, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Luo, Y.; Sun, C.; Liu, Y.; Kuo, P.; Varga, J.; Xiang, R.; Reisfeld, R.; Janda, K.D.; Edgington, T.S.; et al. Targeting Cell-Impermeable Prodrug Activation to Tumor Microenvironment Eradicates Multiple Drug-Resistant Neoplasms. Cancer Res. 2006, 66, 970–980. [Google Scholar] [CrossRef] [PubMed]
- Kratz, F. Albumin as a drug carrier: Design of prodrugs, drug conjugates and nanoparticles. J. Control. Release 2008, 132, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Maliszewska, O.; Plenis, A.; Oledzka, I.; Kowalski, P.; Miekus, N.; Bien, E.; Krawczyk, M.A.; Adamkiewicz-Drozynska, E.; Baczek, T. Optimization of LC method for the quantification of doxorubicin in plasma and urine samples in view of pharmacokinetic, biomedical and drug monitoring therapy studies. J. Pharm. Biomed. Anal. 2018, 158, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Harahap, Y.; Ardiningsih, P.; Corintias Winarti, A.; Purwanto, D.J. Analysis of the Doxorubicin and Doxorubicinol in the Plasma of Breast Cancer Patients for Monitoring the Toxicity of Doxorubicin. Drug Des. Dev. Ther. 2020, 14, 3469–3475. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Y.; Ma, W.; Wang, X.; Tu, P. Validated LC-MS/MS method for simultaneous determination of doxorubicin and curcumin in polymeric micelles in subcellular compartments of MCF-7/Adr cells by protein precipitation-ultrasonic breaking method. Biomed. Chromatogr. 2017, 31, e3892. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Wang, J.; Guo, Q.; Tu, P. Simultaneous determination of doxorubicin and curcumin in rat plasma by LC-MS/MS and its application to pharmacokinetic study. J. Pharm. Biomed. Anal. 2015, 111, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Wang, X.; Wang, Y.; Xu, G.; Zhang, H.; Dai, W.; He, B.; Zhang, Q.; Ji, J.; Wang, X. A sensitive liquid chromatography/electrospray tandem mass spectroscopy method for simultaneous quantification of a disulfide bond doxorubicin conjugation prodrug and activated doxorubicin: Application to cellular pharmacokinetic and catabolism studies. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1065–1066, 96–103. [Google Scholar] [CrossRef] [PubMed]
- De Jong, J.; Klein, I.; Bast, A.; van der Vijgh, W.J. Analysis and pharmacokinetics of N-/-leucyldoxorubicin and metabolites in tissues of tumor-bearing BALB/c mice. Cancer Chemother. Pharmacol. 1992, 31, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Qi, P.; Li, P.; Qiao, L.; Xue, H.; Ma, Y.; Wei, S.; Yang, X.; Zhang, H.; Zhang, Y.; Wang, Y.; et al. Simultaneous quantification of pirarubicin, doxorubicin, cyclophosphamide, and vincristine in human plasma of patients with non-Hodgkin’s lymphoma by LC-MS/MS method. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2023, 1224, 123754. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.G.; Kim, D.K.; Shin, Y.; Park, R.; Cho, Y.Y.; Lee, J.Y.; Kang, H.C.; Lee, H.S. Liquid Chromatography-Tandem Mass Spectrometry for the Simultaneous Determination of Doxorubicin and its Metabolites Doxorubicinol, Doxorubicinone, Doxorubicinolone, and 7-Deoxydoxorubicinone in Mouse Plasma. Molecules 2020, 25, 1254. [Google Scholar] [CrossRef] [PubMed]
- Mazuel, C.; Grove, J.; Gerin, G.; Keenan, K.P. HPLC-MS/MS determination of a peptide conjugate prodrug of doxorubicin, and its active metabolites, leucine-doxorubicin and doxorubicin, in dog and rat plasma. J. Pharm. Biomed. Anal. 2003, 33, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Shao, N.; Jin, Y.; Zhang, L.; Jiang, H.; Xiong, N.; Su, F.; Xu, H. Determination of non-liposomal and liposomal doxorubicin in plasma by LC-MS/MS coupled with an effective solid phase extraction: In comparison with ultrafiltration technique and application to a pharmacokinetic study. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1072, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Sottani, C.; Tranfo, G.; Bettinelli, M.; Faranda, P.; Spagnoli, M.; Minoia, C. Trace determination of anthracyclines in urine: A new high-performance liquid chromatography/tandem mass spectrometry method for assessing exposure of hospital personnel. Rapid Commun. Mass. Spectrom. 2004, 18, 2426–2436. [Google Scholar] [CrossRef] [PubMed]
- Bavlovic Piskackova, H.; Oiestad, E.L.; Vanova, N.; Lengvarska, J.; Sterbova-Kovarikova, P.; Pedersen-Bjergaard, S. Electromembrane extraction of anthracyclines from plasma: Comparison with conventional extraction techniques. Talanta 2021, 223 Pt 2, 121748. [Google Scholar] [CrossRef]
- Kratz, F. DOXO-EMCH (INNO-206): The first albumin-binding prodrug of doxorubicin to enter clinical trials. Expert. Opin. Investig. Drugs 2007, 16, 855–866. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Guidance for Industry: Bioanalytical Method Validation Guidance for Industry. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/bioanalytical-method-validation-guidance-industry (accessed on 1 January 2024).
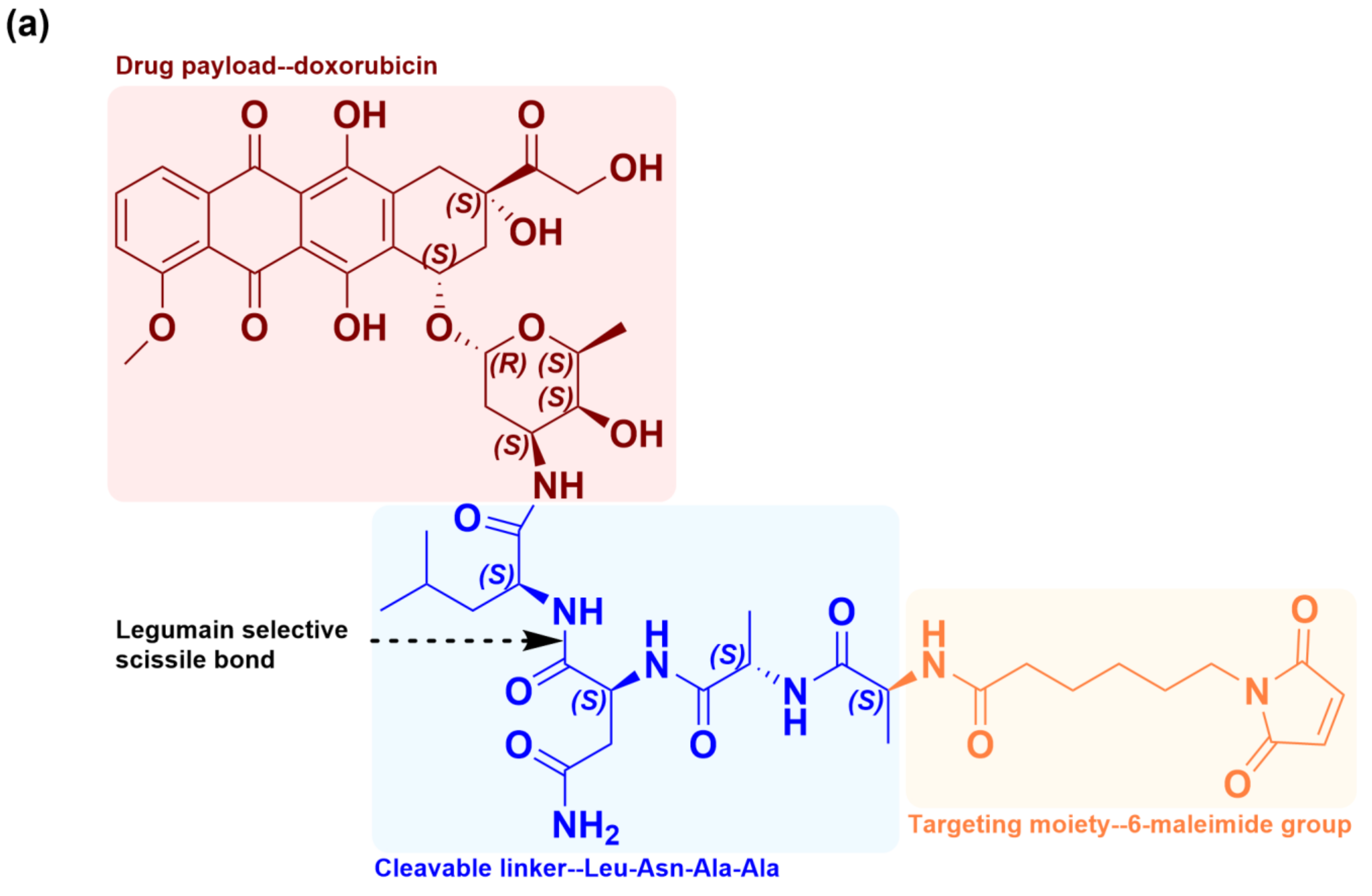
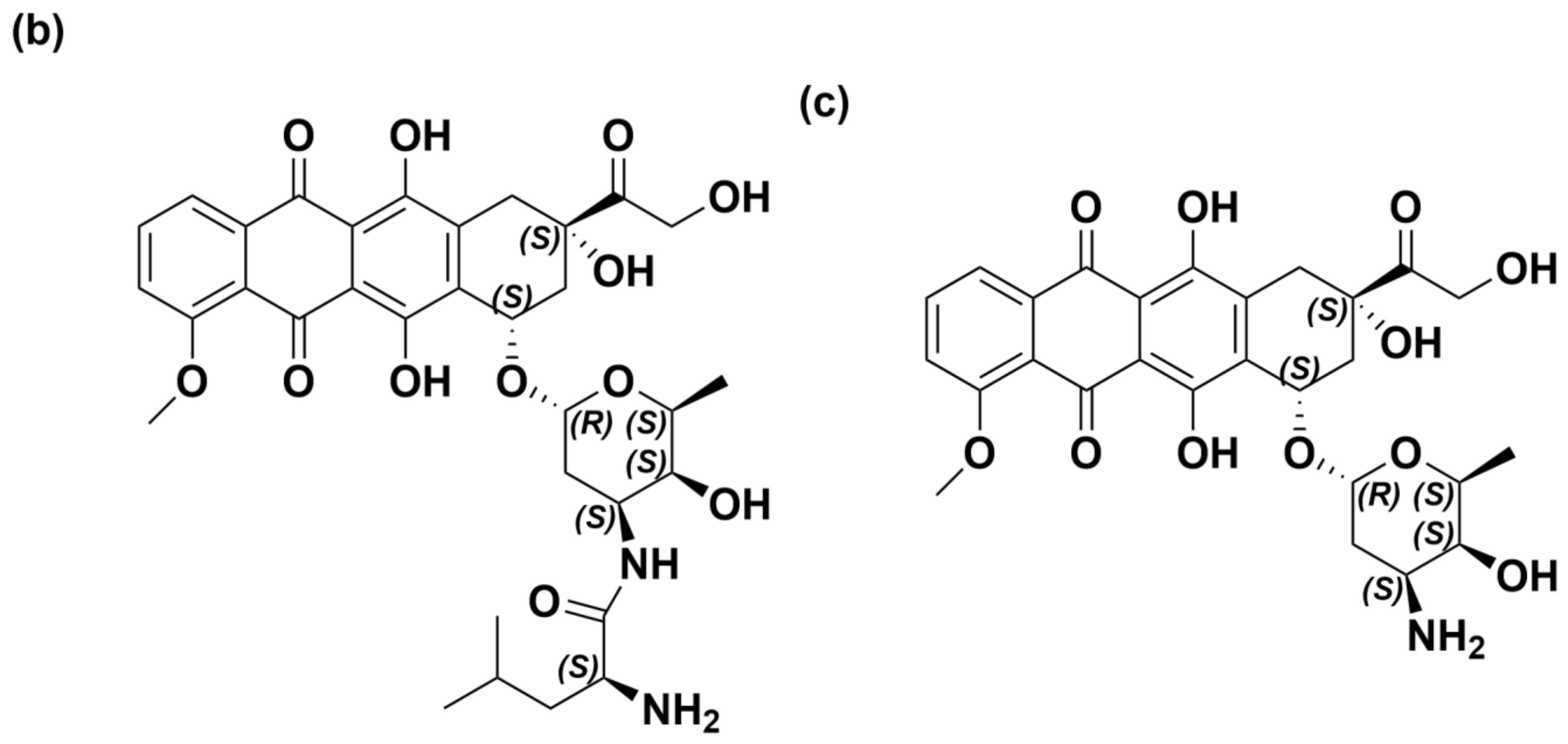

| Matrix | Analyte | Calibration Range | Calibration Curve | r | LOD | LOQ |
|---|---|---|---|---|---|---|
| Rat plasma | Leu-DOX | 5–5000 pmol/mL | y = 0.00432x + 0.00255 | 0.9978 | 0.261 pmol/mL | 0.870 pmol/mL |
| DOX | 1.5–1500 pmol/mL | y = 0.01672x + 0.00190 | 0.9985 | 0.179 pmol/mL | 0.595 pmol/mL | |
| Albumin conjugate of legubicin | 0.1–100 nmol/mL | y = −0.00044x2 + 0.23550x + 0.00173 | 0.9979 | 0.006 nmol/mL | 0.021 nmol/mL | |
| Mouse plasma | Leu-DOX | 3–3000 pmol/mL | y = 0.00007x − 0.00001 | 0.9975 | 0.667 pmol/mL | 2.222 pmol/mL |
| DOX | 1–1000 pmol/mL | y = 0.00018x + 0.00003 | 0.9988 | 0.144 pmol/mL | 0.481 pmol/mL | |
| Albumin conjugate of legubicin | 0.1–100 nmol/mL | y = 0.00002x − 0.00004 | 0.9966 | 0.005 nmol/mL | 0.018 nmol/mL | |
| Mouse tumor | Leu-DOX | 9–9000 pmol/mL | y = 0.00397x + 0.00561 | 0.9979 | 1.019 pmol/mL | 3.396 pmol/mL |
| DOX | 3–3000 pmol/mL | y = 0.00313x + 0.00486 | 0.9971 | 0.709 pmol/mL | 2.362 pmol/mL | |
| Mouse tissue | Leu-DOX | 9–9000 pmol/mL | y = 0.00348x + 0.00157 | 0.9928 | 2.000 pmol/mL | 6.667 pmol/mL |
| DOX | 3–3000 pmol/mL | y = 0.00708x + 0.00016 | 0.9961 | 0.750 pmol/mL | 2.500 pmol/mL |
| Analytes | Nominal Concentration a | Intra-Day | Inter-Day | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st Day | 2nd Day | 3rd Day | |||||||||||
| Measured Concentration (Mean ± SD) a | Accuracy (% Bias) | Precision (% RSD) | Measured Concentration (Mean ± SD) a | Accuracy (% Bias) | Precision (% RSD) | Measured Concentration (Mean ± SD) a | Accuracy (% bias) | Precision (% RSD) | Measured Concentration a | Accuracy (% bias) | Precision (% RSD) | ||
| Leu-DOX | 5 | 5.61 ± 0.30 | 12.2 | 5.4 | 5.55 ± 0.35 | 11.0 | 6.4 | 5.03 ± 0.31 | 0.5 | 6.2 | 5.40 ± 0.41 | 7.9 | 7.5 |
| 10 | 9.52 ± 0.53 | −4.8 | 5.6 | 9.86 ± 0.66 | −1.4 | 6.7 | 10.26 ± 0.59 | 2.6 | 5.8 | 9.88 ± 0.64 | −1.2 | 6.5 | |
| 200 | 183.00 ± 13.97 | −8.5 | 7.6 | 193.17 ± 8.45 | −3.4 | 4.4 | 189.50 ± 8.89 | −5.3 | 4.7 | 188.56 ± 10.97 | −5.7 | 5.8 | |
| 4000 | 3688.33 ± 210.09 | −7.8 | 5.7 | 3876.67 ± 122.26 | −3.1 | 3.2 | 3930.00 ± 309.13 | −1.8 | 7.9 | 3831.67 ± 238.46 | −4.2 | 6.2 | |
| DOX | 1.5 | 1.49 ± 0.07 | −0.5 | 4.5 | 1.41 ± 0.05 | −6.3 | 3.7 | 1.44 ± 0.10 | −4.2 | 6.8 | 1.44 ± 0.08 | −3.7 | 5.5 |
| 3 | 2.91 ± 0.10 | −3.0 | 3.4 | 2.90 ± 0.13 | −3.3 | 4.6 | 2.84 ± 0.12 | −5.5 | 4.2 | 2.88 ± 0.12 | −3.9 | 4.0 | |
| 60 | 54.73 ± 4.06 | −8.8 | 7.4 | 55.03 ± 4.30 | −8.3 | 7.8 | 58.65 ± 1.12 | −2.3 | 1.9 | 56.14 ± 3.74 | −6.4 | 6.7 | |
| 1200 | 1106.67 ± 58.54 | −7.8 | 5.3 | 1143.33 ± 75.28 | −4.7 | 6.6 | 1138.33 ± 31.89 | −5.1 | 2.8 | 1129.44 ± 57.03 | −5.9 | 5.0 | |
| Albumin conjugate of legubicin | 0.1 | 0.11 ± 0.01 | 8.0 | 5.6 | 0.10 ± 0.01 | 1.0 | 7.9 | 0.11 ± 0.00 | 5.8 | 6.3 | 0.11 ± 0.01 | 5.8 | 6.3 |
| 0.2 | 0.21 ± 0.01 | 6.5 | 4.2 | 0.20 ± 0.01 | 1.5 | 4.9 | 0.21 ± 0.01 | 4.2 | 4.8 | 0.21 ± 0.01 | 4.2 | 4.8 | |
| 4 | 4.39 ± 0.39 | 9.7 | 9.0 | 4.11 ± 0.26 | 2.7 | 6.4 | 4.25 ± 0.36 | 6.3 | 8.0 | 4.25 ± 0.34 | 6.3 | 8.0 | |
| 80 | 91.07 ± 6.55 | 13.8 | 7.2 | 91.77 ± 3.79 | 14.7 | 4.1 | 80.73 ± 5.29 | 9.8 | 8.2 | 87.86 ± 7.21 | 9.8 | 8.2 | |
| Analytes | Nominal Concentration a | Extraction Recovery (%, Mean ± SD) | RSD (%) | Matrix Factor (%, Mean ± SD) | RSD (%) |
|---|---|---|---|---|---|
| Leu-DOX | 10 | 102.1 ± 6.5 | 6.4 | 99.4 ± 10.8 | 10.9 |
| 200 | 98.2 ± 3.2 | 3.3 | 98.7 ± 7.0 | 7.0 | |
| 4000 | 96.6 ± 5.4 | 5.6 | 96.5 ± 5.5 | 5.7 | |
| DOX | 3 | 93.7 ± 8.4 | 8.9 | 108.8 ± 4.5 | 4.1 |
| 60 | 97.8 ± 3.2 | 3.3 | 98.6 ± 2.7 | 2.8 | |
| 1200 | 96.9 ± 7.9 | 8.2 | 103.6 ± 9.6 | 9.3 | |
| Albumin conjugate of legubicin | 0.2 | 94.1 ± 4.5 | 4.8 | 84.3 ± 3.2 | 3.8 |
| 4 | 90.0 ± 6.1 | 6.8 | 99.4 ± 7.6 | 7.7 | |
| 80 | 89.7 ± 8.8 | 9.9 | 107.4 ± 5.9 | 5.5 |
| Analytes | Nominal Concentration a | Short-Term Stability at Room Temperature (4 h) | Freeze and Thaw Stability at −80 °C/Room Temperature (3 Cycles) | Auto-Sampler Stability at 2–8 °C (72 h) | Long-Term Stability at −80 °C (70 days) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measured Concentration (Mean ± SD) a | Bias (%) | RSD (%) | Measured Concentration (Mean ± SD) a | Bias (%) | RSD (%) | Measured Concentration (Mean ± SD) a | Bias (%) | RSD (%) | Measured Concentration (Mean ± SD) a | Bias (%) | RSD (%) | ||
| Leu-DOX | 10 | 9.76 ± 0.41 | −2.4 | 4.2 | 10.20 ± 0.70 | 2.0 | 6.8 | 9.96 ± 0.48 | −0.4 | 4.8 | 10.07 ± 1.50 | 0.7 | 15.0 |
| 4000 | 3871.67 ± 390.25 | −3.2 | 10.1 | 3775.00 ± 329.89 | −5.6 | 8.7 | 3758.33 ± 422.58 | −6.0 | 11.2 | 4300.00 ± 240.83 | 7.5 | 5.6 | |
| DOX | 3 | 3.04 ± 0.18 | 1.2 | 5.9 | 3.01 ± 0.15 | 0.4 | 5.1 | 3.13 ± 0.25 | 4.4 | 7.9 | 2.98 ± 0.29 | −0.6 | 9.7 |
| 1200 | 1238.33 ± 100.48 | 3.2 | 8.1 | 1221.67 ± 97.45 | 1.8 | 8.0 | 1211.67 ± 104.58 | 1.0 | 8.6 | 1190.00 ± 40.50 | −0.8 | 3.4 | |
| Albumin conjugate of legubicin | 0.2 | 0.19 ± 0.01 | −7.1 | 5.8 | 0.18 ± 0.01 | −10.6 | 5.3 | 0.18 ± 0.01 | −8.7 | 4.2 | 0.17 ± 0.00 | −13.7 | 2.2 |
| 80 | 87.30 ± 8.98 | 9.1 | 10.3 | 81.23 ± 2.80 | 1.5 | 3.5 | 80.17 ± 2.65 | 0.2 | 3.3 | 84.62 ± 9.77 | 5.8 | 11.5 | |
| Albumin conjugate of legubicin PK parameters | 1.5 mg/kg | 5 mg/kg | 15 mg/kg | |||
| AUCINF_obs (h*pmol/mL) | 222,000 ± 47,600 | 873,000 ± 155,000 | 2,020,000 ± 331,000 | |||
| AUClast (h*pmol/mL) | 218,000 ± 47,400 | 869,000 ± 152,000 | 2,000,000 ± 324,000 | |||
| C0 (pmol/mL) | 30,900 ± 7290 | 96,300 ± 19,300 | 224,000 ± 48,100 | |||
| Cl_obs (mL/h/kg) | 6.4 ± 1.53 | 5.33 ± 1.05 | 6.87 ± 1.16 | |||
| Cmax (pmol/mL) | 28,900 ± 6310 | 97,300 ± 8270 | 204,000 ± 39,800 | |||
| t1/2z (h) | 8.43 ± 2.6 | 9.72 ± 1.01 | 10.7 ± 2.31 | |||
| Tmax $ (h) | 0.167 [0.167, 0.167] | 0.334 [0.167, 1] | 0.167 [0.167, 0.167] | |||
| Vss_obs (mL/kg) | 66.7 ± 9.85 | 59.2 ± 5.7 | 98.6 ± 19.3 | |||
| Vz_obs (mL/kg) | 73.5 ± 10.4 | 73.8 ± 9.09 | 105 ± 26.2 | |||
| Leu-DOX PK parameters | 1.5 mg/kg, Female | 1.5 mg/kg, Male | 5 mg/kg, Female | 5 mg/kg, Male | 15 mg/kg, Female | 15 mg/kg, Male |
| AUClast (h*pmol/mL) | 6.44 ± 2.02 | 11.2 ± 2.1 | 52.0 ± 16.2 | 94.3 ± 10.5 | 336 ± 62.5 | 1030 ± 244 |
| Cmax (pmol/mL) | 21.5 ± 7.78 | 29.5 ± 2.74 | 72.1 ± 16.0 | 145 ± 23.9 | 373 ± 63.2 | 1100 ± 243 |
| Tmax $ (h) | 0.167 [0.167, 0.167] | 0.167 [0.167, 0.167] | 0.167 [0.167, 0.167] | 0.167 [0.167, 0.167] | 0.167 [0.167, 0.167] | 0.167 [0.167, 0.167] |
| DOX PK parameters | 5 mg/kg, Female | 5 mg/kg, Male | 15 mg/kg, Female | 15 mg/kg, Male | ||
| AUClast (h*pmol/mL) | 100 ± 5.38 | 173 ± 24.3 | 486 ± 46.4 | 789 ± 99.7 | ||
| Cmax (pmol/mL) | 3.11 ± 0.615 | 6.65 ± 1.30 | 20.5 ± 5.06 | 44.2 ± 4.65 | ||
| Tmax $ (h) | 0.167 [0.167, 0.167] | 0.167 [0.167, 0.167] | 0.167 [0.167, 0.167] | 0.167 [0.167, 0.167] |
| Albumin conjugate of legubicin PK parameters | Single dose | Multiple dose | ||
| AUCINF_obs (h*pmol/mL) | 563,000 ± 91,200 | 814,000 ± 156,000 | ||
| AUClast (h*pmol/mL) | 560,000 ± 90,900 | 808,000 ± 156,000 | ||
| C0 (pmol/mL) | 75,600 ± 17,600 | 80,400 ± 20,500 | ||
| Cl_obs (mL/h/kg) | 8.21 ± 1.35 | 5.75 ± 1.23 | ||
| Cmax (pmol/mL) | 68,700 ± 15,600 | 88,600 ± 12,200 | ||
| t1/2z (h) | 9.32 ± 0.994 | 10.0 ± 1.63 | ||
| Tmax $ (h) | 0.167 [0.167, 0.167] | 0.5 [0.167, 1] | ||
| Vss_obs (mL/kg) | 97.7 ± 23.2 | 67.6 ± 9.15 | ||
| Vz_obs (mL/kg) | 110 ± 18.3 | 81.5 ± 12.8 | ||
| Leu-DOX PK parameters | Single dose, female | Single dose, male | Multiple dose, female | Multiple dose, male |
| AUClast (h*pmol/mL) | 38.7 ± 1.04 | 81.0 ± 13.3 | 60.4 ± 6.43 | 112 ± 14.0 |
| Cmax (pmol/mL) | 60.0 ± 6.89 | 111 ± 13.5 | 80.3 ± 8.57 | 141 ± 22.0 |
| Tmax $ (h) | 0.167 [0.167, 0.167] | 0.167 [0.167, 0.167] | 0.167 [0.167, 0.167] | 0.167 [0.167, 0.167] |
| DOX PK parameters | Single dose, female | Single dose, male | Multiple dose, female | Multiple dose, male |
| AUClast (h*pmol/mL) | 94.5 ± 44.7 | 123 ± 44.1 | 174 ± 36.7 | 207 ± 11.2 |
| Cmax (pmol/mL) | 3.10 ± 0.367 | 6.01 ± 0.430 | 4.70 ± 0.534 | 8.58 ± 0.616 |
| Tmax $ (h) | 0.167 [0.167, 0.167] | 0.167 [0.167, 0.167] | 0.167 [0.167, 0.167] | 0.167 [0.167, 0.167] |
| Legubicin Administration | Doxorubicin Administration | |||
|---|---|---|---|---|
| Plasma/Tissue | AUClast (h*nmol/g) | T/P Ratio | AUClast (h*nmol/g) | T/P Ratio |
| Plasma | 0.835 | 1.0 | 1.07 | 1.0 |
| Heart | 8.89 | 10.7 | 29.0 | 27.1 |
| Tumor | 112 | 134.1 | 63.1 | 59.0 |
| Kidney | 24.1 | 28.9 | 83.5 | 78.0 |
| Biological Matrix | Analyte | Q1 a (m/z) | Q3 b (m/z) | DP c (V) | CE d (V) | CXP e (V) | EP f (V) | Dwell Time (ms) |
|---|---|---|---|---|---|---|---|---|
| Rat plasma | Leu-DOX | 657.5 | 243.1 | 70 | 18 | 13 | 10 | 100 |
| DOX | 544.0 | 397.0 | 98 | 18 | 13 | 10 | 100 | |
| Phenacetin | 180.0 | 110.1 | 120 | 27 | 13 | 10 | 100 | |
| Mouse plasma/tumor/tissue | Leu-DOX | 657.4 | 243.2 | 70 | 25 | 13 | 10 | 50 |
| DOX | 544.2 | 397.2 | 100 | 16 | 13 | 10 | 50 | |
| Phenacetin | 180.1 | 110 | 90 | 27 | 13 | 10 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, L.; Yu, Q.; Zhuang, M.; Yang, C.; Liu, Y.; Li, Y.; Liu, C.; Shen, X.; Chang, Y. UHPLC-MS/MS Assay for Quantification of Legubicin, a Novel Doxorubicin-Based Legumain-Activated Prodrug, and Its Application to Pharmacokinetic and Tissue Distribution Studies. Molecules 2024, 29, 775. https://doi.org/10.3390/molecules29040775
Ma L, Yu Q, Zhuang M, Yang C, Liu Y, Li Y, Liu C, Shen X, Chang Y. UHPLC-MS/MS Assay for Quantification of Legubicin, a Novel Doxorubicin-Based Legumain-Activated Prodrug, and Its Application to Pharmacokinetic and Tissue Distribution Studies. Molecules. 2024; 29(4):775. https://doi.org/10.3390/molecules29040775
Chicago/Turabian StyleMa, Liyuan, Qiaoling Yu, Meng Zhuang, Chen Yang, Yuan Liu, Yuling Li, Cheng Liu, Xiaoyan Shen, and Yan Chang. 2024. "UHPLC-MS/MS Assay for Quantification of Legubicin, a Novel Doxorubicin-Based Legumain-Activated Prodrug, and Its Application to Pharmacokinetic and Tissue Distribution Studies" Molecules 29, no. 4: 775. https://doi.org/10.3390/molecules29040775
APA StyleMa, L., Yu, Q., Zhuang, M., Yang, C., Liu, Y., Li, Y., Liu, C., Shen, X., & Chang, Y. (2024). UHPLC-MS/MS Assay for Quantification of Legubicin, a Novel Doxorubicin-Based Legumain-Activated Prodrug, and Its Application to Pharmacokinetic and Tissue Distribution Studies. Molecules, 29(4), 775. https://doi.org/10.3390/molecules29040775







