Recent Developments in Functional Polymers via the Kabachnik–Fields Reaction: The State of the Art
Abstract
1. Introduction
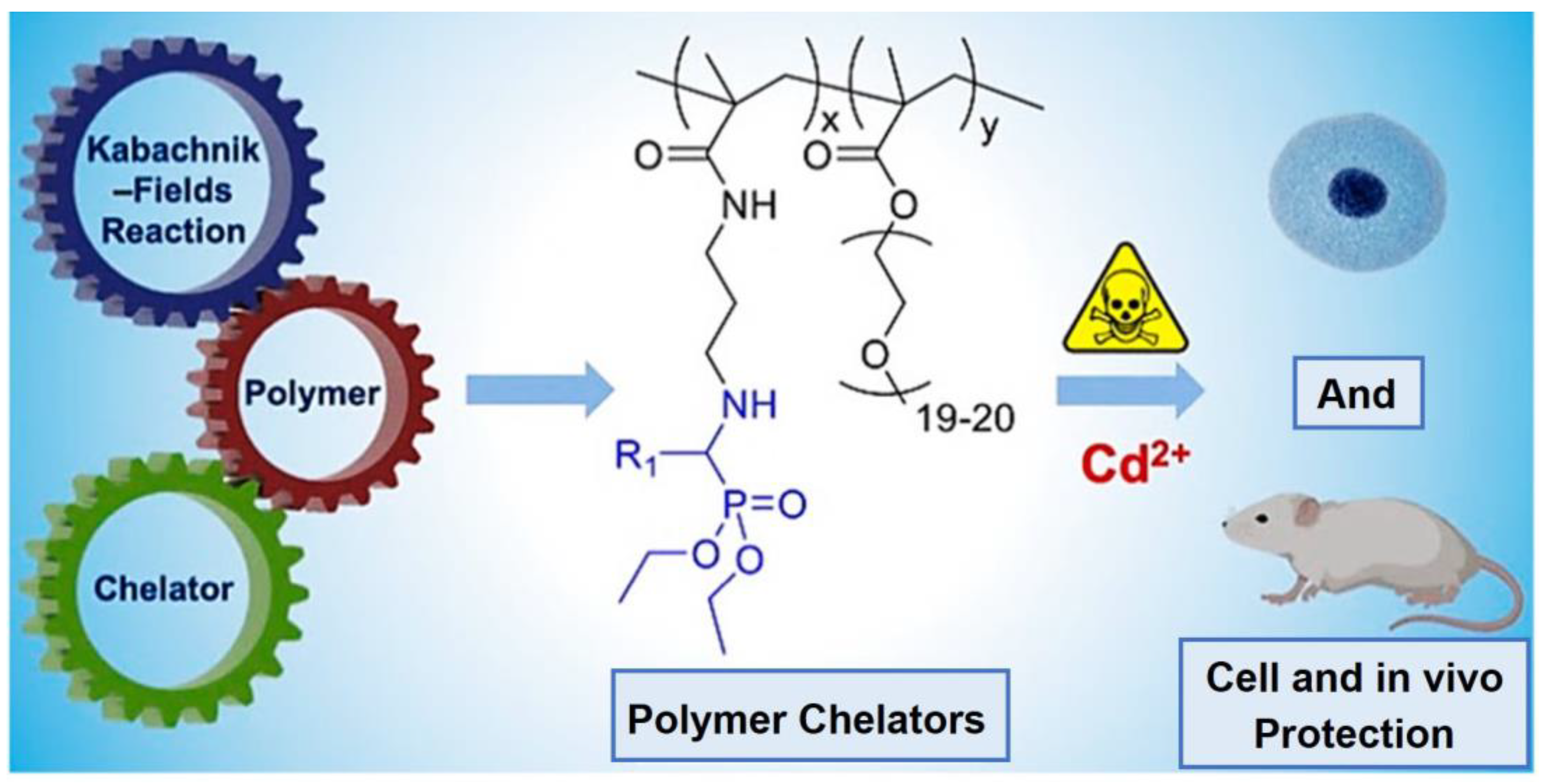
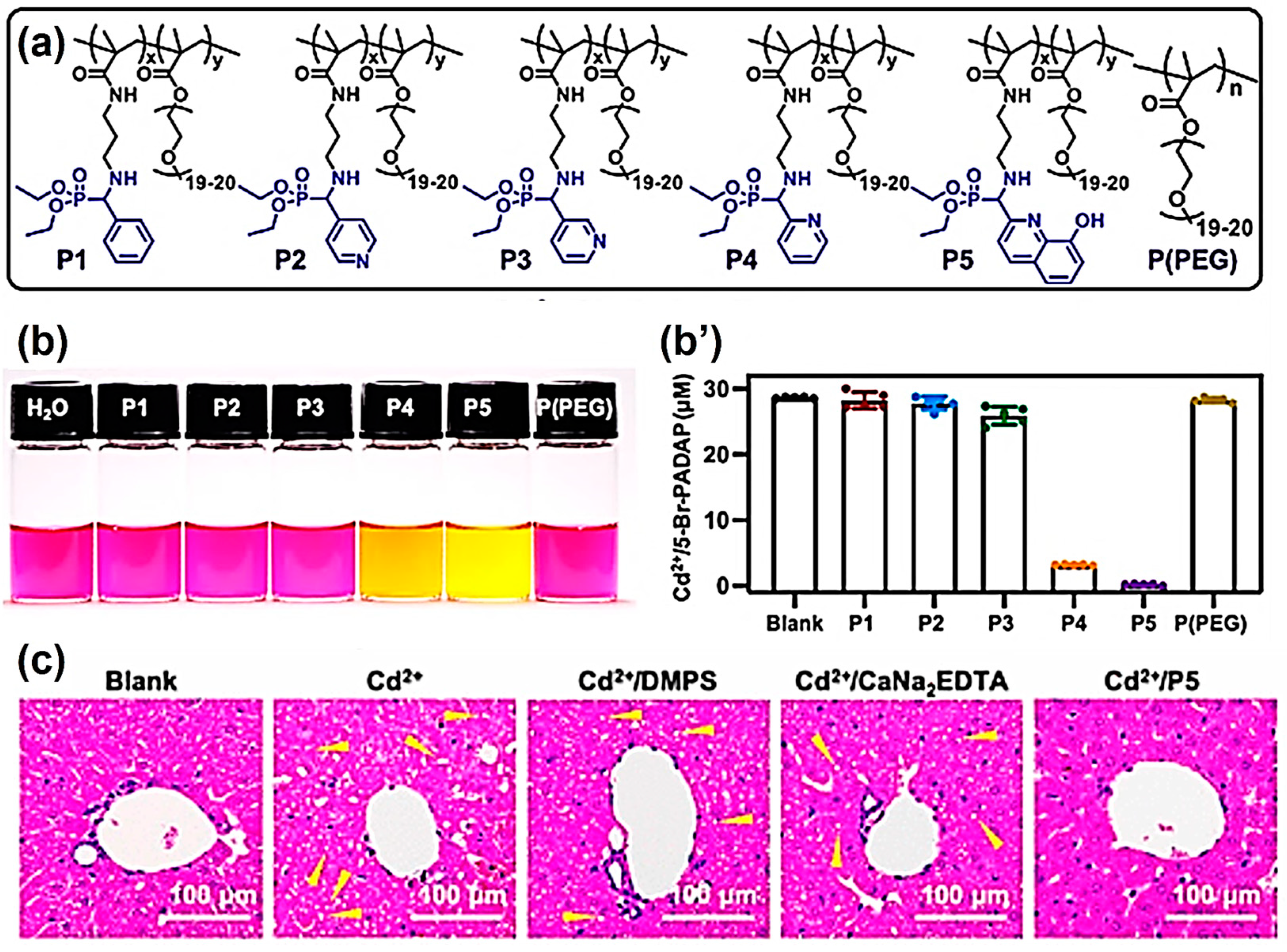
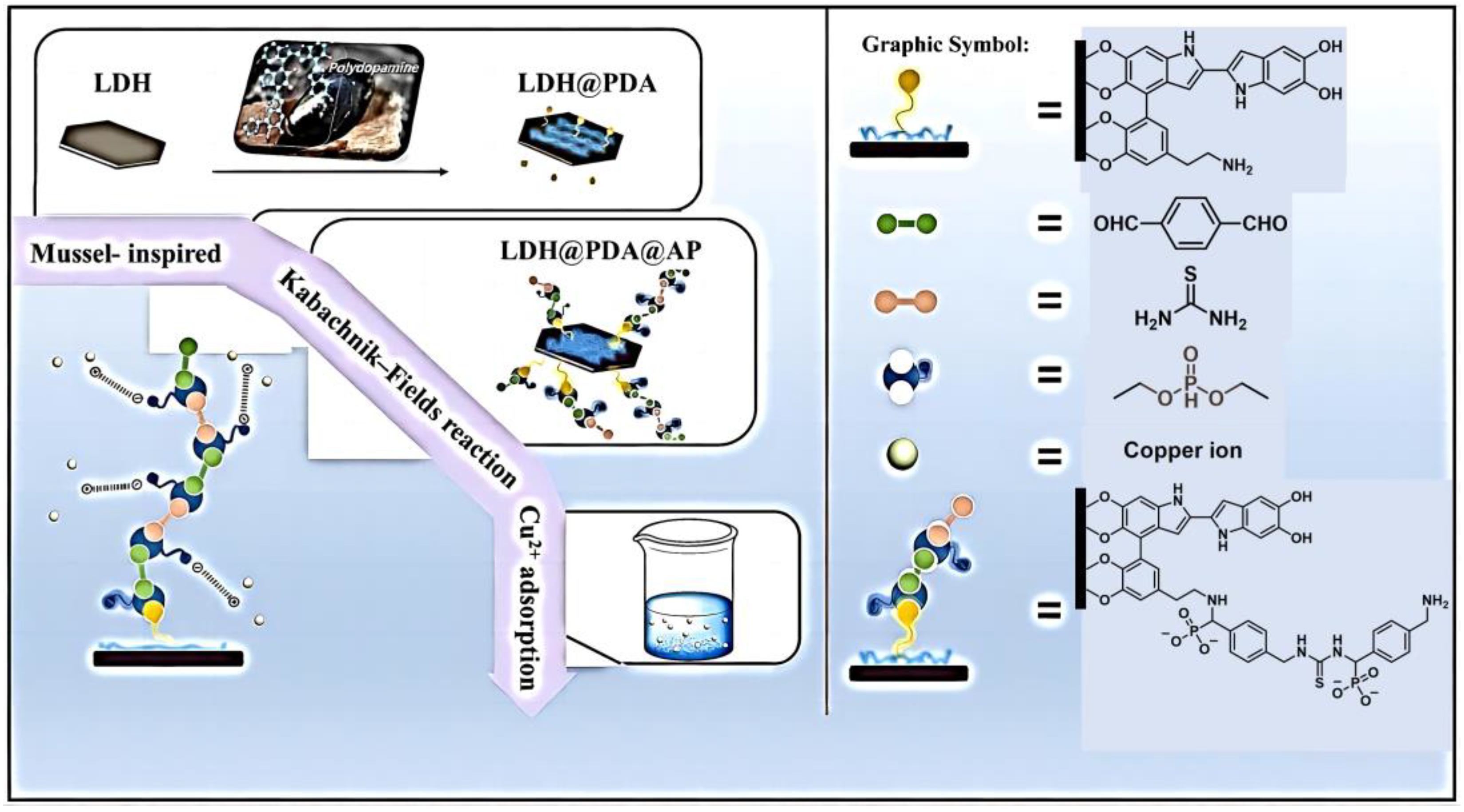
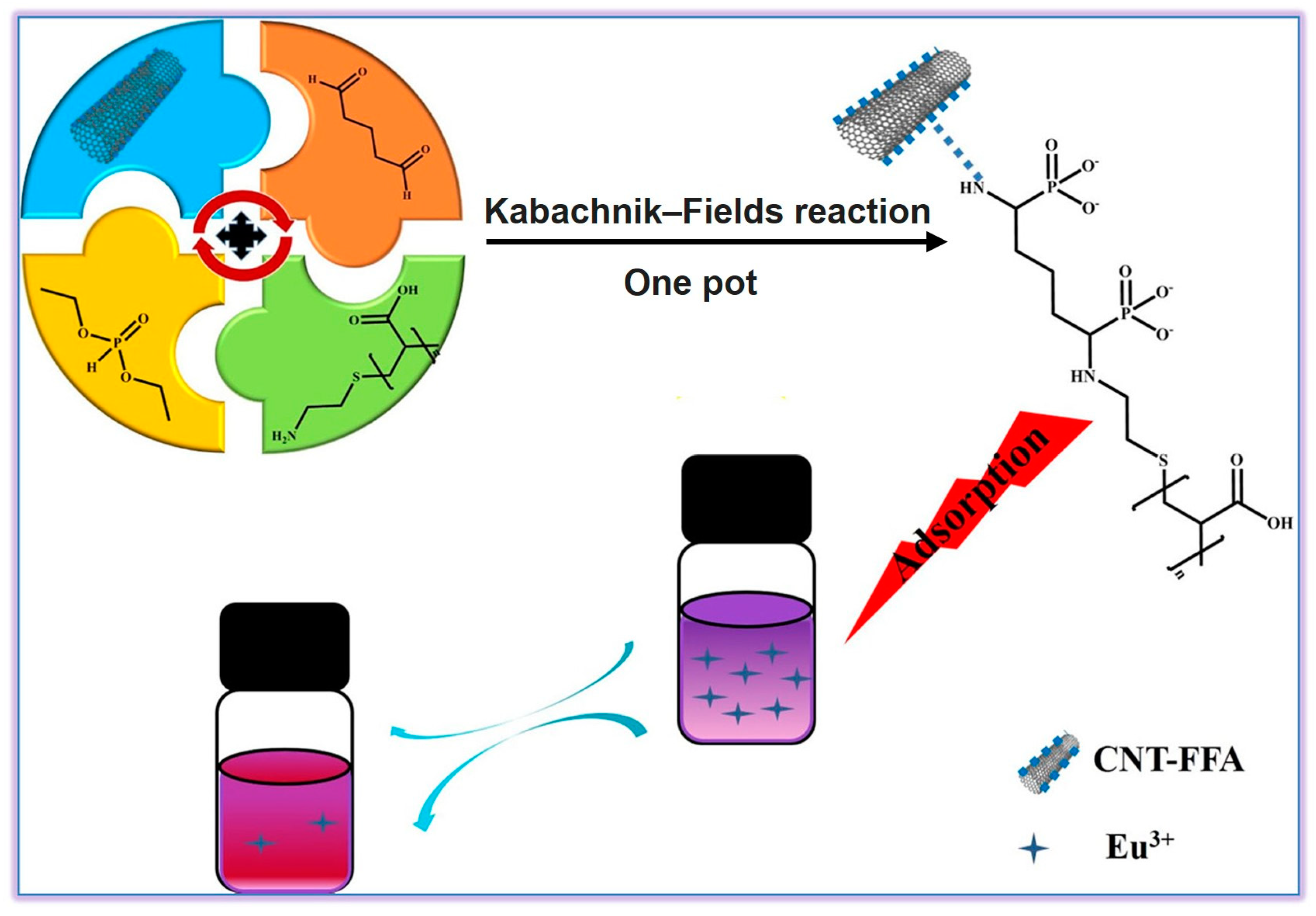
2. Heavy Metal Adsorbents
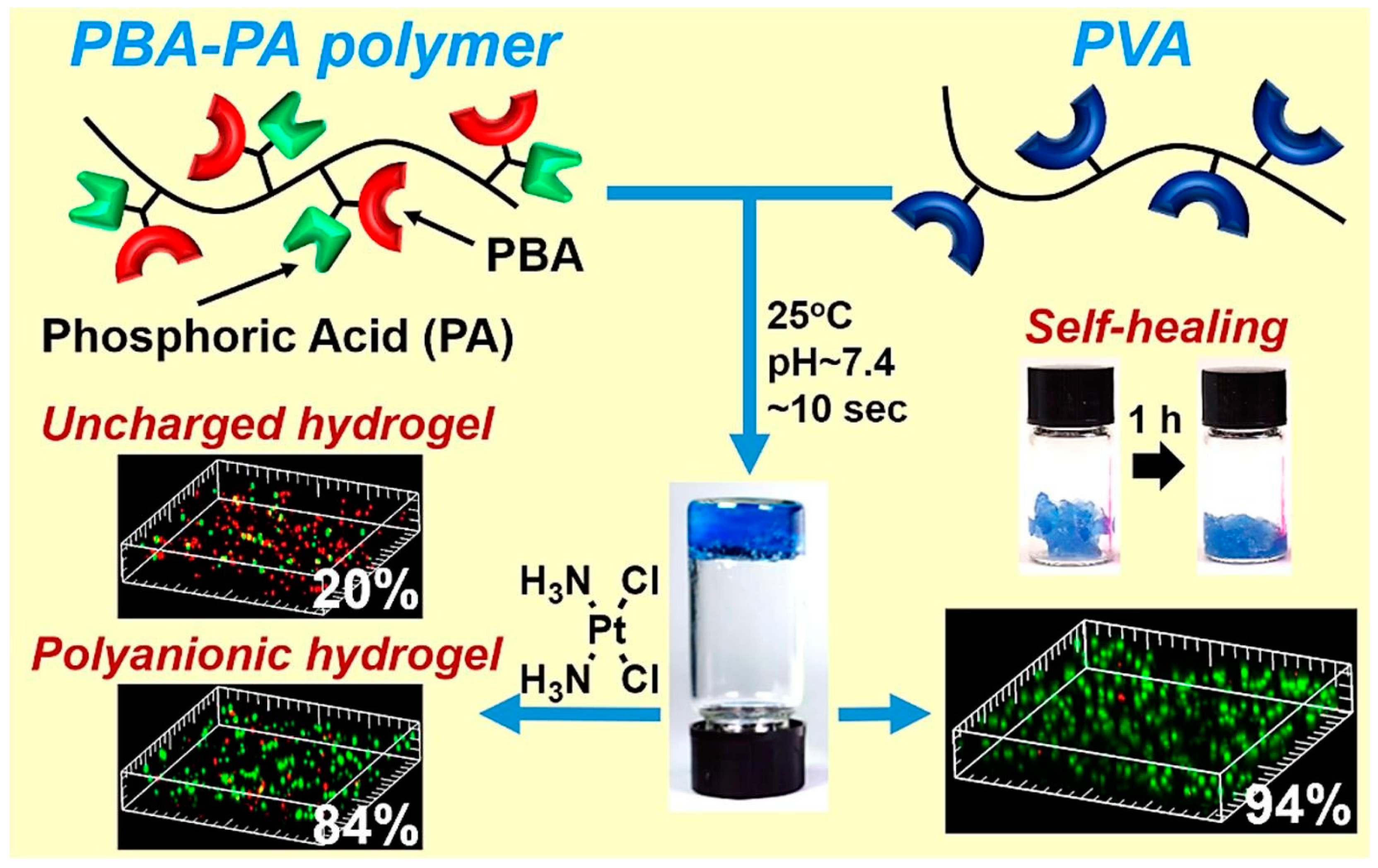
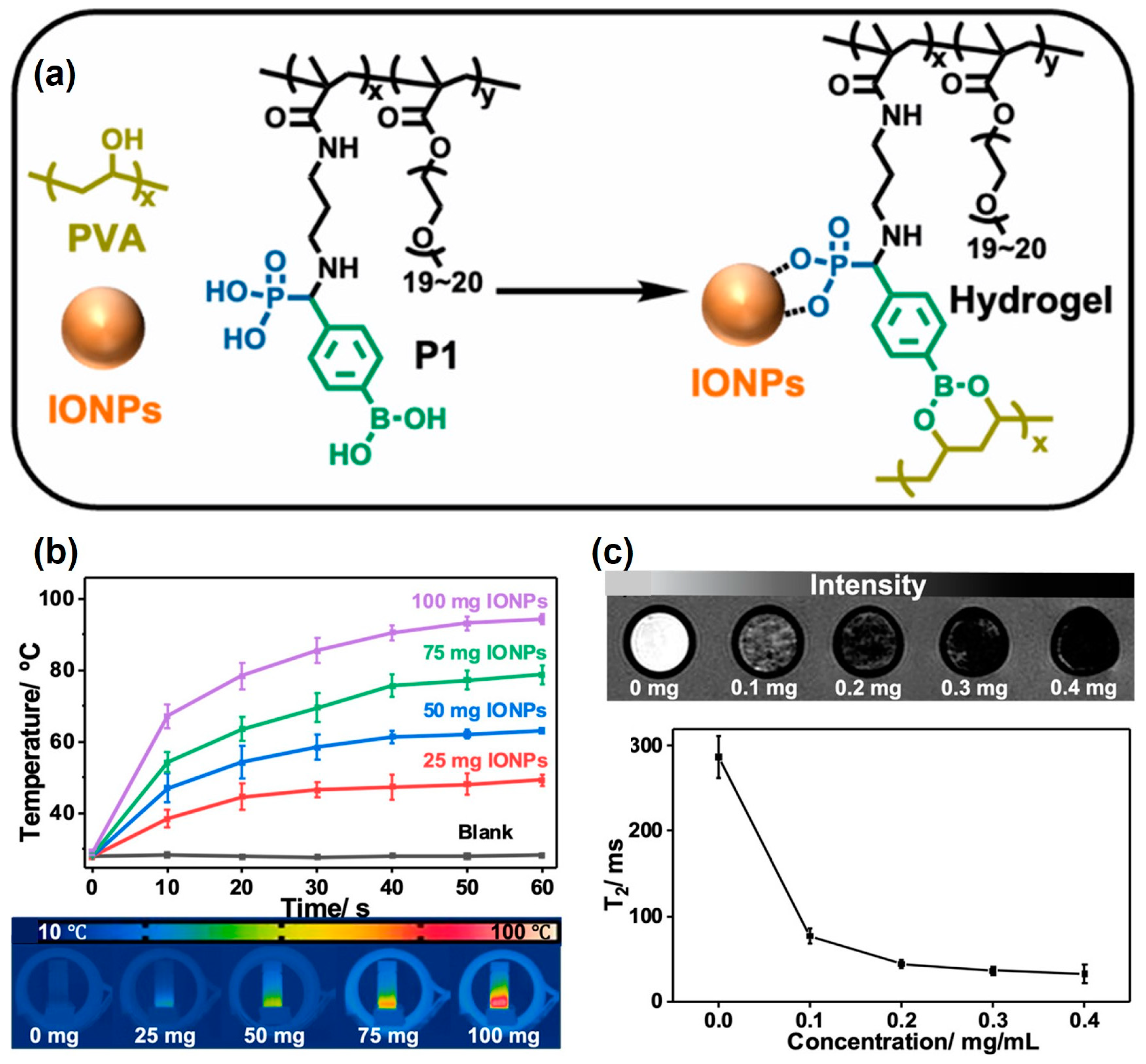
3. Multifunctional Hydrogels
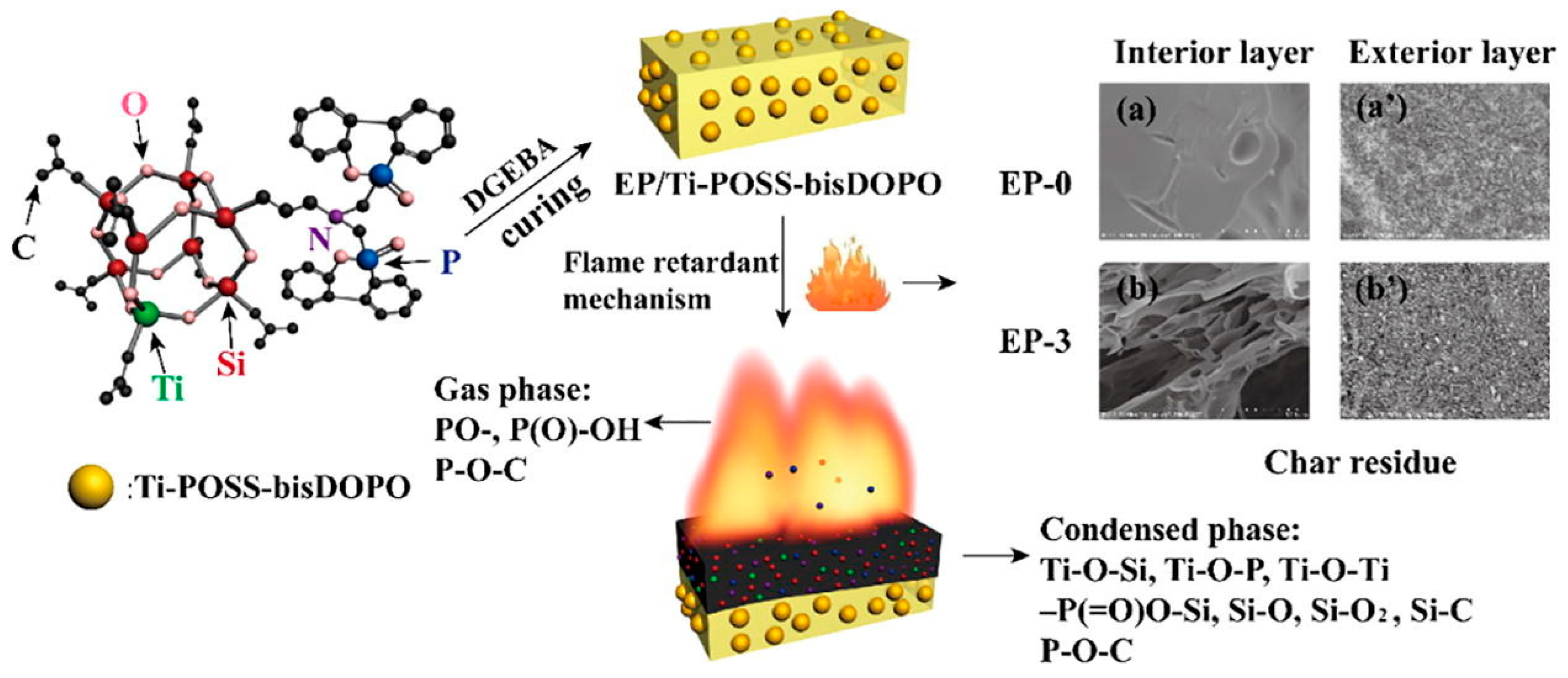
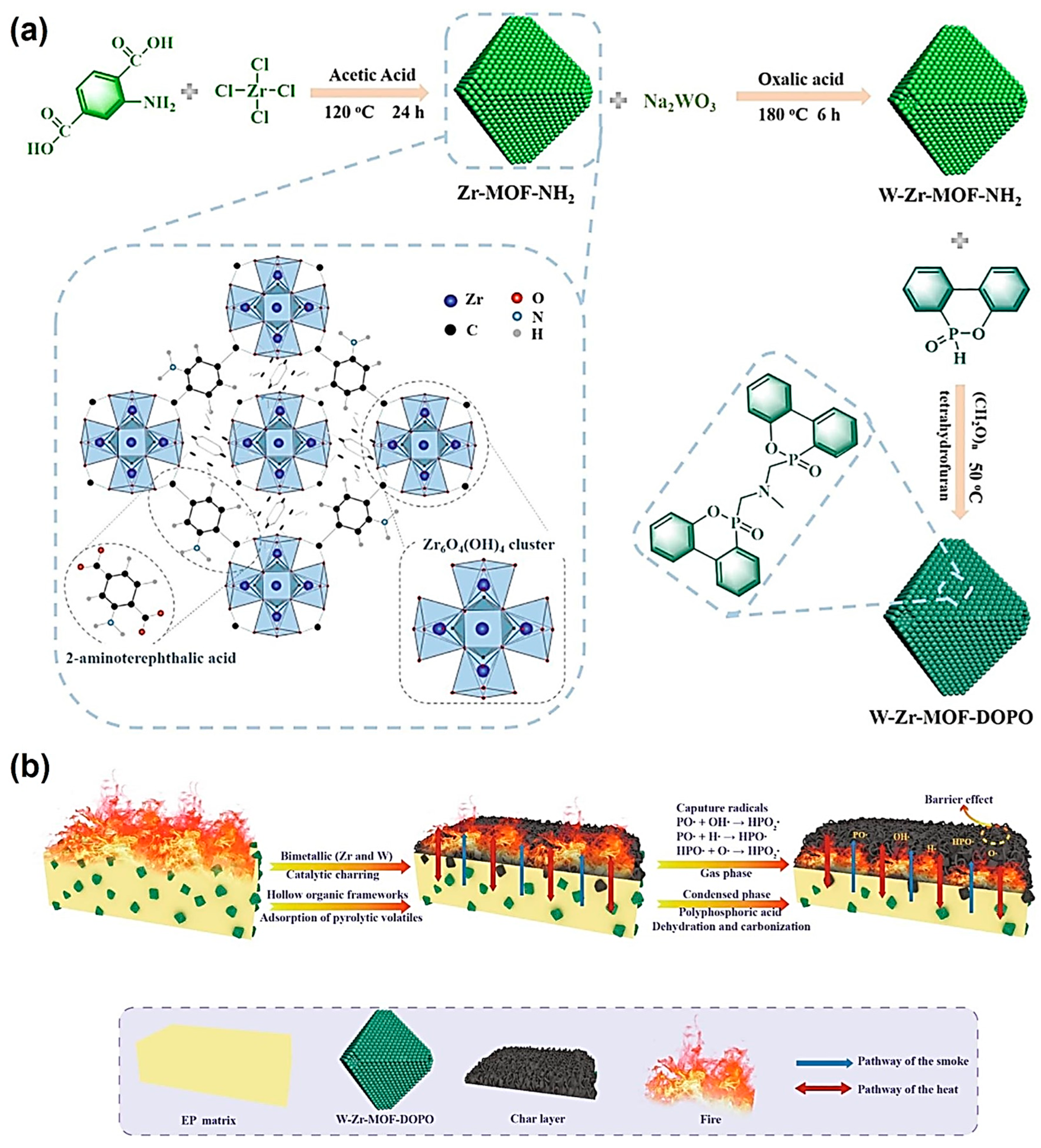
4. Flame Retardants
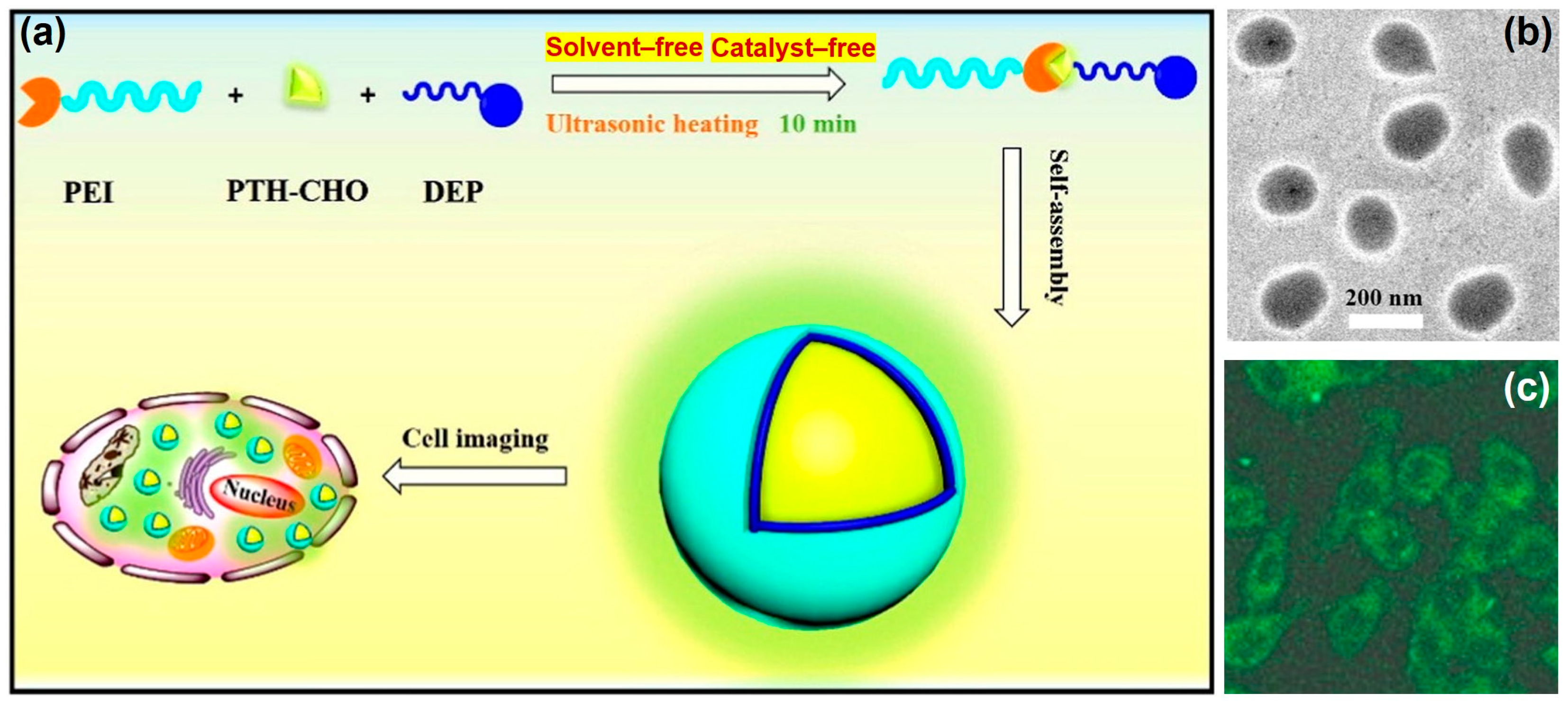
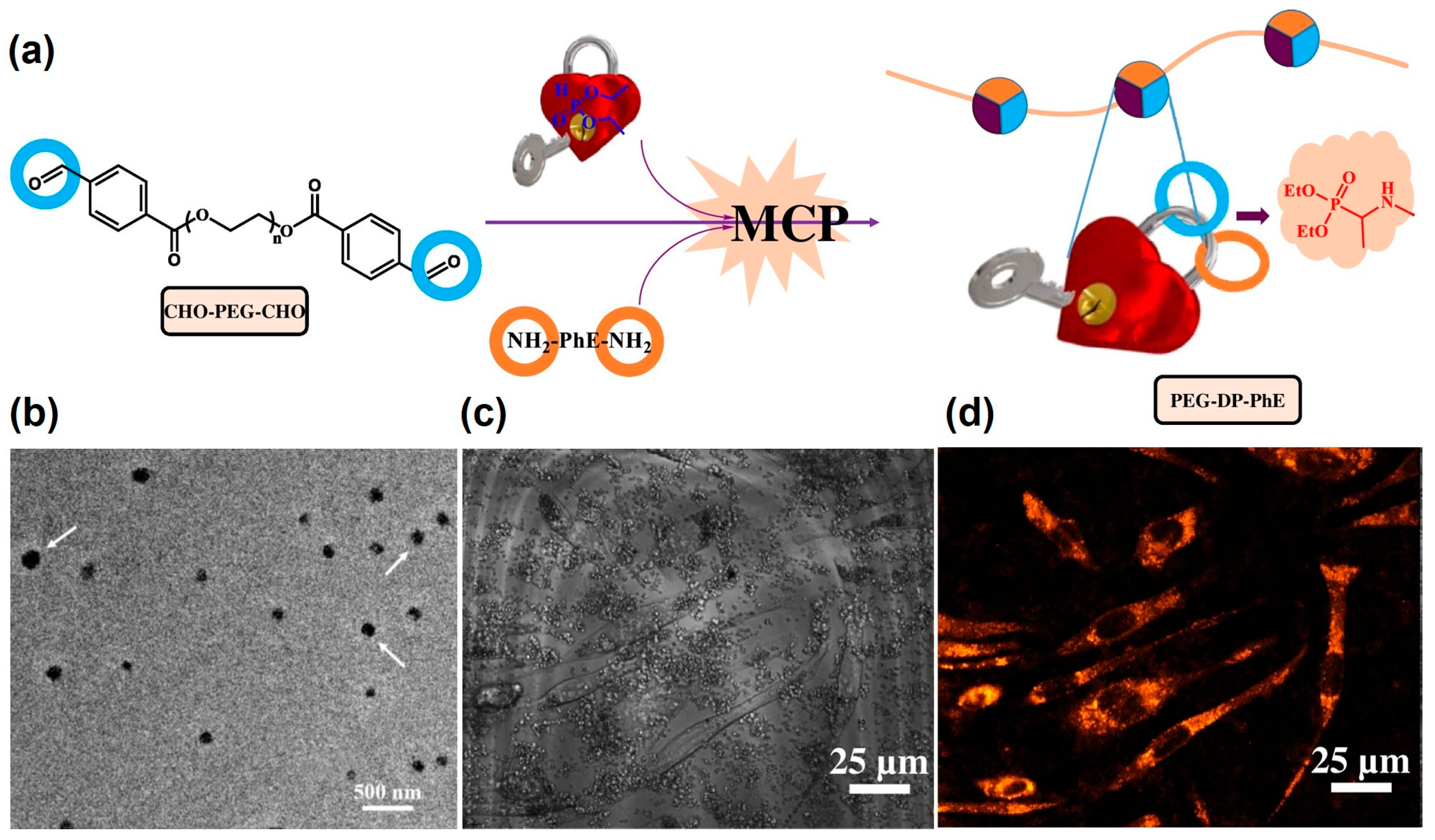
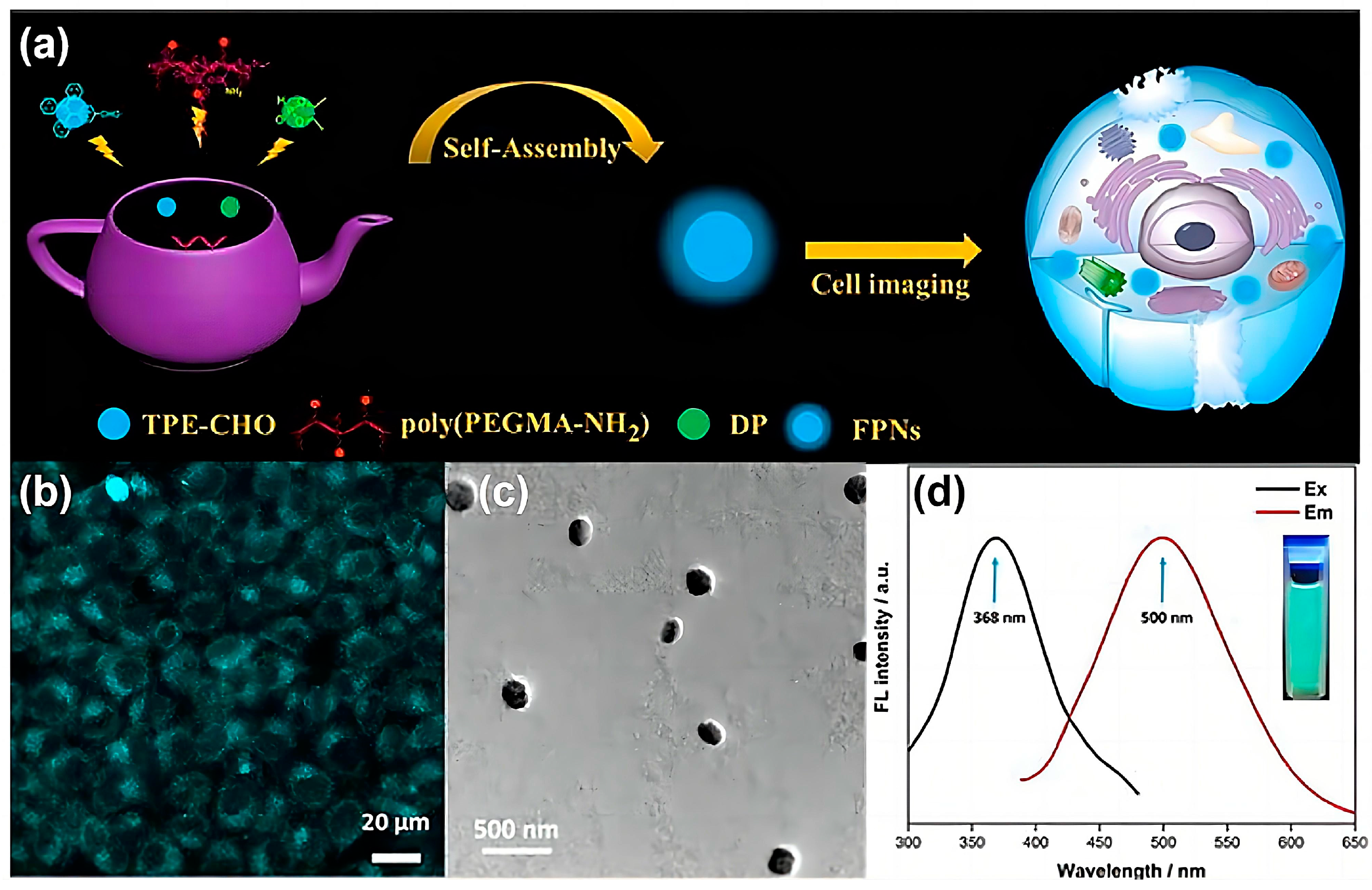
5. Bioimaging Probes
6. Other Applications
7. Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, J.; Luo, Z.; Yu, L.; Zhang, P.; Wei, H.; Yu, Y. A new soft-matter material with old chemistry: Passerini multicomponent polymerization-induced assembly of AIE-active double-helical polymers with rapid visible-light degradability. Chem. Sci. 2020, 11, 8224–8230. [Google Scholar] [CrossRef]
- Tuten, B.T.; De Keer, L.; Wiedbrauk, S.; Van Steenberge, P.H.M.; D’hooge, D.R.; Barner-Kowollik, C. Visible-light-induced Passerini multicomponent polymerization. Angew. Chem. Int. Ed. 2019, 58, 5672–5676. [Google Scholar] [CrossRef]
- Xie, J.; Niu, N.; Fu, X.; Su, X.; Wang, D.; Qin, A.; Han, T.; Tang, B.Z. Catalyst-Free Synthesis of Diverse Fluorescent Polyoxadiazoles for the Facile Formation and Morphology Visualization of Microporous Films and Cell Imaging. Chem. Sci. 2023, 14, 903–915. [Google Scholar] [CrossRef]
- Kakuchi, R. Multicomponent reactions in polymer synthesis. Angew. Chem. Int. Ed. 2014, 53, 46–48. [Google Scholar] [CrossRef]
- Mao, T.; Liu, G.; Wu, H.; Wei, Y.; Gou, Y.; Wang, J.; Tao, L. High throughput preparation of UV-protective polymers from essential oil extracts via the Biginelli reaction. J. Am. Chem. Soc. 2018, 140, 6865–6872. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.V.; Chavan, J.U.; Dalal, D.S.; Shinde, V.S.; Beldar, A.G. Biginelli reaction: Polymer supported catalytic approaches. ACS Comb. Sci. 2019, 21, 105–148. [Google Scholar] [CrossRef] [PubMed]
- Boukis, A.C.; Llevot, A.; Meier, M.A.R. High glass transition temperature renewable polymers via biginelli multicomponent polymerization. Macromol. Rapid Commun. 2016, 37, 643–649. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Yang, L.; Liu, G.; Wei, Y.; Zeng, Y.; Tao, L. Polymer chelator prepared via the Kabachnik–Fields reaction for the in vivo prevention of heavy-metal damage. Chem. Mater. 2022, 34, 9558–9568. [Google Scholar] [CrossRef]
- He, X.; Zeng, Y.; Liu, G.; Tian, Y.; Wei, Y.; Zhao, L.; Yang, L.; Tao, L. Magnetic self-healing hydrogel from difunctional polymers prepared via the Kabachnik–Fields reaction. ACS Macro Lett. 2022, 11, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zeng, Y.; Lv, T.; Mao, T.; Wei, Y.; Jia, S.; Gou, Y.; Tao, L. High-throughput preparation of radioprotective polymers via Hantzsch’s reaction for in vivo X-ray damage determination. Nat. Commun. 2020, 11, 6214. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Chen, Y.; Liu, H.; Chen, P.; Peng, F.; Qi, H. Color-tunable, excitation-dependent, and water stimulus-responsive room-temperature phosphorescence cellulose for versatile applications. Adv. Mater. 2023, 35, 2304032. [Google Scholar] [CrossRef]
- Chalitangkoon, J.; Monvisade, P. Dual ph/thermal-dependent coloring polymeric dye through mannich reaction of chitosan: Synthesis and characterization. Carbohydr. Polym. 2019, 223, 115049. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Wu, W.; Wei, Z.; Zhu, D.; Tu, H.; Zhang, A. Synthesis of functional catechols as monomers of mussel-inspired biomimetic polymers. Green Chem. 2018, 20, 912–920. [Google Scholar] [CrossRef]
- Resetco, C.; Frank, D.; Dikić, T.; Claessens, S.; Verbrugge, T.; Du Prez, F.E. Thiolactone-based polymers for formaldehyde scavenging coatings. Eur. Polym. J. 2016, 82, 166–174. [Google Scholar] [CrossRef]
- Satoh, K.; Ozawa, S.; Mizutani, M.; Nagai, K.; Kamigaito, M. Sequence-regulated vinyl copolymers by metal-catalysed step-growth radical polymerization. Nat. Commun. 2010, 1, 6. [Google Scholar] [CrossRef]
- Zeng, Y.; He, X.; Ma, Z.; Gou, Y.; Wei, Y.; Pan, S.; Tao, L. Coral-friendly and non-transdermal polymeric UV filter via the Biginelli reaction for in vivo UV protection. Cell Rep. Phys. Sci. 2023, 4, 101308. [Google Scholar] [CrossRef]
- Peng, F.; Liu, H.; Hu, S.; Yue, F.; Xiao, D.; Guo, L.; Qi, H. High throughput preparation of antioxidant polysaccharide-based polymers with UV-resistant and antibacterial performance. Food Hydrocoll. 2022, 133, 107936. [Google Scholar] [CrossRef]
- Dong, J.; Liu, M.; Jiang, R.; Huang, H.; Wan, Q.; Wen, Y.; Tian, J.; Dai, Y.; Zhang, X.; Wei, Y. Synthesis and biological imaging of cross-linked fluorescent polymeric nanoparticles with aggregation-induced emission characteristics based on the combination of raft polymerization and the Biginelli reaction. J. Colloid Interface Sci. 2018, 528, 192–199. [Google Scholar] [CrossRef]
- Meier, M.A.R.; Hu, R.; Tang, B.Z. Multicomponent reactions in polymer science. Macromol. Rapid Commun. 2021, 42, 2100104. [Google Scholar] [CrossRef]
- Rivera, D.G.; Ricardo, M.G.; Vasco, A.V.; Wessjohann, L.A.; Van der Eycken, E.V. On-resin multicomponent protocols for biopolymer assembly and derivatization. Nat. Protoc. 2021, 16, 561–578. [Google Scholar] [CrossRef]
- Fields, E.K. The synthesis of esters of substituted amino phosphonic acids1a. J. Am. Chem. Soc. 1952, 74, 1528–1531. [Google Scholar] [CrossRef]
- Cherkasov, R.A.; Galkin, V.I. The kabachnik–fields reaction: Synthetic potential and the problem of the mechanism. Russ. Chem. Rev. 1998, 67, 857. [Google Scholar] [CrossRef]
- Mucha, A.; Kafarski, P.; Berlicki, Ł. Remarkable potential of the α-aminophosphonate/phosphinate structural motif in medicinal chemistry. J. Med. Chem. 2011, 54, 5955–5980. [Google Scholar] [CrossRef]
- Fiore, C.; Sovic, I.; Lukin, S.; Halasz, I.; Martina, K.; Delogu, F.; Ricci, P.C.; Porcheddu, A.; Shemchuk, O.; Braga, D.; et al. Kabachnik-Fields reaction by mechanochemistry: New horizons from old methods. ACS Sustain. Chem. Eng. 2020, 8, 18889–18902. [Google Scholar] [CrossRef]
- Kostelnik, T.I.; Scheiber, H.; Cappai, R.; Choudhary, N.; Lindheimer, F.; de Guadalupe Jaraquemada-Peláez, M.; Orvig, C. Phosphonate chelators for medicinal metal ions. Inorg. Chem. 2021, 60, 5343–5361. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Rao, W.; Zhao, P.; Wang, L.; Liu, Y.; Yu, C. An efficient organic/inorganic phosphorus–nitrogen–silicon flame retardant towards low-flammability epoxy resin. Polym. Degrad. Stabil. 2020, 178, 109195. [Google Scholar] [CrossRef]
- Wang, X.; Wu, T.; Hong, J.; Dai, J.; Lu, Z.; Yang, C.; Yuan, C.; Dai, L. Organophosphorus modified hollow bimetallic organic frameworks: Effective adsorption and catalytic charring of pyrolytic volatiles. Chem. Eng. J. 2021, 421, 129697. [Google Scholar] [CrossRef]
- Moldenhauer, F.; Kakuchi, R.; Theato, P. Synthesis of polymers via Kabachnik-Fields polycondensation. ACS Macro Lett. 2016, 5, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, Y.; Yang, B.; Zhu, C.; Wei, Y.; Tao, L. ‘One pot’ synthesis of well-defined poly(aminophosphonate)s: Time for the Kabachnik–Fields reaction on the stage of polymer chemistry. Polym. Chem. 2014, 5, 1857–1862. [Google Scholar] [CrossRef]
- Kakuchi, R.; Theato, P. Efficient multicomponent postpolymerization modification based on Kabachnik-Fields reaction. ACS Macro Lett. 2014, 3, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Bachler, P.R.; Schulz, M.D.; Sparks, C.A.; Wagener, K.B.; Sumerlin, B.S. Aminobisphosphonate polymers via raft and a multicomponent Kabachnik–Fields reaction. Macromol. Rapid Commun. 2015, 36, 828–833. [Google Scholar] [CrossRef]
- Lee, S.-M.; Laldawngliana, C.; Tiwari, D. Iron oxide nano-particles-immobilized-sand material in the treatment of Cu(II), Cd(II) and Pb(II) contaminated waste waters. Chem. Eng. J. 2012, 195–196, 103–111. [Google Scholar] [CrossRef]
- Reshmy, R.; Philip, E.; Madhavan, A.; Pugazhendhi, A.; Sindhu, R.; Sirohi, R.; Awasthi, M.K.; Pandey, A.; Binod, P. Nanocellulose as green material for remediation of hazardous heavy metal contaminants. J. Hazard. Mater. 2022, 424, 127516. [Google Scholar] [CrossRef]
- Wang, R.; Liang, R.; Dai, T.; Chen, J.; Shuai, X.; Liu, C. Pectin-based adsorbents for heavy metal ions: A review. Trends Food Sci. Technol. 2019, 91, 319–329. [Google Scholar] [CrossRef]
- Bilal, M.; Shah, J.A.; Ashfaq, T.; Gardazi, S.M.H.; Tahir, A.A.; Pervez, A.; Haroon, H.; Mahmood, Q. Waste biomass adsorbents for copper removal from industrial wastewater—A review. J. Hazard. Mater. 2013, 263, 322–333. [Google Scholar] [CrossRef]
- Bhattacharjee, Y. A sluggish response to humanity’s biggest mass poisoning. Science 2007, 315, 1659–1661. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Guo, C.; Hao, J.; Zhao, Z.; Long, H.; Li, M. Adsorption of heavy metal ions by sodium alginate based adsorbent-a review and new perspectives. Int. J. Biol. Macromol. 2020, 164, 4423–4434. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Zheng, Y.-M.; Zhang, B.-G.; Dai, Y.-R. A critical review on the electrospun nanofibrous membranes for the adsorption of heavy metals in water treatment. J. Hazard. Mater. 2021, 401, 123608. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Li, Z. Application of lignin and its derivatives in adsorption of heavy metal ions in water: A review. ACS Sustain. Chem. Eng. 2018, 6, 7181–7192. [Google Scholar] [CrossRef]
- Tusek–Bozic, L. Aminophosphonate metal complexes of biomedical potential. Curr. Med. Chem. 2013, 20, 2096–2117. [Google Scholar] [CrossRef] [PubMed]
- Soyekwo, F.; Liu, C.; Zhao, L.; Wen, H.; Huang, W.; Cai, C.; Kanagaraj, P.; Hu, Y. Nanofiltration membranes with metal cation-immobilized aminophosphonate networks for efficient heavy metal ion removal and organic dye degradation. ACS Appl. Mater. Interfaces 2019, 11, 30317–30331. [Google Scholar] [CrossRef]
- Dou, J.; Chen, J.; Huang, Q.; Huang, H.; Mao, L.; Deng, F.; Wen, Y.; Zhu, X.; Zhang, X.; Wei, Y. Preparation of polymer functionalized layered double hydroxide through mussel-inspired chemistry and Kabachnik–Fields reaction for highly efficient adsorption. J. Environ. Chem. Eng. 2020, 8, 103634. [Google Scholar] [CrossRef]
- Ghobadi, M.; Gharabaghi, M.; Abdollahi, H.; Boroumand, Z.; Moradian, M. MnFe2O4-graphene oxide magnetic nanoparticles as a high-performance adsorbent for rare earth elements: Synthesis, isotherms, kinetics, thermodynamics and desorption. J. Hazard. Mater. 2018, 351, 308–316. [Google Scholar] [CrossRef]
- Wilfong, W.C.; Kail, B.W.; Bank, T.L.; Howard, B.H.; Gray, M.L. Recovering rare earth elements from aqueous solution with porous amine–epoxy networks. ACS Appl. Mater. Interfaces 2017, 9, 18283–18294. [Google Scholar] [CrossRef] [PubMed]
- Jacinto, J.; Henriques, B.; Duarte, A.C.; Vale, C.; Pereira, E. Removal and recovery of critical rare elements from contaminated waters by living gracilaria gracilis. J. Hazard. Mater. 2018, 344, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Liu, Y.; Dou, J.; Huang, Q.; Lei, Y.; Chen, J.; Wen, Y.; Li, Y.; Zhang, X.; Wei, Y. Highly efficient removal of Eu3+ ions using carbon nanotubes-based polymer composites synthesized from the combination of diels-alder and multicomponent reactions. J. Mol. Liq. 2020, 308, 112964. [Google Scholar] [CrossRef]
- Tian, Y.; Zeng, Y.; Li, Y.; He, X.; Wu, H.; Wei, Y.; Wu, Y.; Wang, X.; Tao, L. Polyanionic self-healing hydrogels for the controlled release of cisplatin. Eur. Polym. J. 2020, 133, 109773. [Google Scholar] [CrossRef]
- Zhou, Y.; Chu, F.; Qiu, S.; Guo, W.; Zhang, S.; Xu, Z.; Hu, W.; Hu, Y. Construction of graphite oxide modified black phosphorus through covalent linkage: An efficient strategy for smoke toxicity and fire hazard suppression of epoxy resin. J. Hazard. Mater. 2020, 399, 123015. [Google Scholar] [CrossRef]
- Ai, Y.-F.; Xia, L.; Pang, F.-Q.; Xu, Y.-L.; Zhao, H.-B.; Jian, R.-K. Mechanically strong and flame-retardant epoxy resins with anti-corrosion performance. Compos. Part B Eng. 2020, 193, 108019. [Google Scholar] [CrossRef]
- Huo, S.; Song, P.; Yu, B.; Ran, S.; Chevali, V.S.; Liu, L.; Fang, Z.; Wang, H. Phosphorus-containing flame retardant epoxy thermosets: Recent advances and future perspectives. Prog. Polym. Sci. 2021, 114, 101366. [Google Scholar] [CrossRef]
- Wu, H.; Zeng, B.; Chen, J.; Wu, T.; Li, Y.; Liu, Y.; Dai, L. An intramolecular hybrid of metal polyhedral oligomeric silsesquioxanes with special titanium-embedded cage structure and flame retardant functionality. Chem. Eng. J. 2019, 374, 1304–1316. [Google Scholar] [CrossRef]
- Long, Z.; Liu, M.; Jiang, R.; Zeng, G.; Wan, Q.; Huang, H.; Deng, F.; Wan, Y.; Zhang, X.; Wei, Y. Ultrasonic-assisted Kabachnik-Fields reaction for rapid fabrication of AIE-active fluorescent organic nanoparticles. Ultrason. Sonochem. 2017, 35, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Huang, L.; Liu, M.; Deng, F.; Huang, H.; Tian, J.; Wen, Y.; Cao, Q.; Zhang, X.; Wei, Y. Ultrafast microwave-assisted multicomponent tandem polymerization for rapid fabrication of AIE-active fluorescent polymeric nanoparticles and their potential utilization for biological imaging. Mat. Sci. Eng. C-Mater. 2018, 83, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Jiang, R.; Liu, M.; Wan, Q.; Xu, D.; Tian, J.; Huang, H.; Wen, Y.; Zhang, X.; Wei, Y. Microwave-assisted multicomponent reactions for rapid synthesis of AIE-active fluorescent polymeric nanoparticles by post-polymerization method. Mat. Sci. Eng. C-Mater. 2017, 80, 578–583. [Google Scholar] [CrossRef]
- Zhang, L.; Deng, H.; Jing, Y.; Tao, L.; Fu, Q. An effective compatibilizer for tin fluorophosphate glass/polymer composites obtained from “one pot” KF-RAFT polymerization. Compos. Sci. Technol. 2018, 168, 336–345. [Google Scholar] [CrossRef]
- Mady, M.F.; Abdel-Azeim, S.; Kelland, M.A. Investigation of the antiscaling performance of phosphonated chitosan for upstream petroleum industry application. ACS Sustain. Chem. Eng. 2021, 9, 16494–16505. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, R.; He, X.; Zhu, C.; Tao, L. Recent Developments in Functional Polymers via the Kabachnik–Fields Reaction: The State of the Art. Molecules 2024, 29, 727. https://doi.org/10.3390/molecules29030727
Yuan R, He X, Zhu C, Tao L. Recent Developments in Functional Polymers via the Kabachnik–Fields Reaction: The State of the Art. Molecules. 2024; 29(3):727. https://doi.org/10.3390/molecules29030727
Chicago/Turabian StyleYuan, Rui, Xianzhe He, Chongyu Zhu, and Lei Tao. 2024. "Recent Developments in Functional Polymers via the Kabachnik–Fields Reaction: The State of the Art" Molecules 29, no. 3: 727. https://doi.org/10.3390/molecules29030727
APA StyleYuan, R., He, X., Zhu, C., & Tao, L. (2024). Recent Developments in Functional Polymers via the Kabachnik–Fields Reaction: The State of the Art. Molecules, 29(3), 727. https://doi.org/10.3390/molecules29030727







