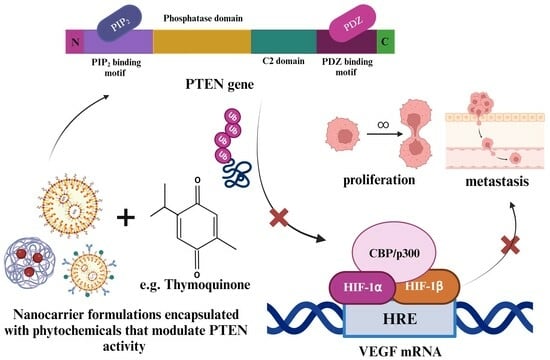
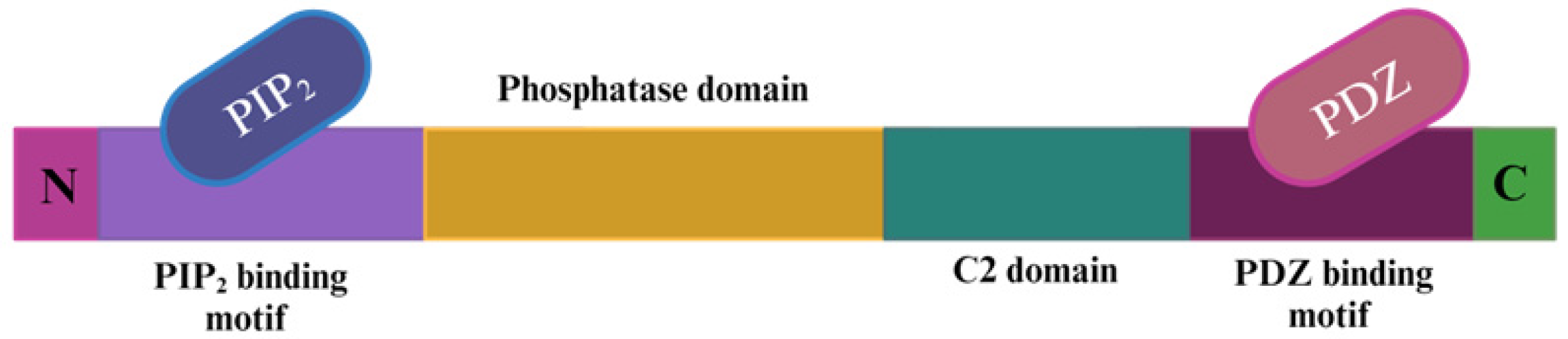
The Role of the PTEN Tumor Suppressor Gene and Its Anti-Angiogenic Activity in Melanoma and Other Cancers
Abstract
1. Introduction
2. The Tumor Suppressor Phosphatase Tensin Homolog (PTEN)
3. Transcriptional, Epigenetic, and Post-Translational Modifications of PTEN
4. What Is Angiogenesis?
5. FDA-Approved Drugs for the Treatment of Angiogenesis
6. The Link between PTEN and Angiogenesis
7. Phytochemicals That Display Anti-Angiogenic Activity through the Upregulation of PTEN Activity
8. Enhancing the Efficacy of Phytochemicals Used to Upregulate PTEN Activity Using Nanocarriers
9. Discussion
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Barsouk, A. Epidemiology of Melanoma. Med. Sci. 2021, 9, 63. [Google Scholar] [CrossRef]
- Rabbie, R.; Ferguson, P.; Molina-Aguilar, C.; Adams, D.J.; Robles-Espinoza, C.D. Melanoma subtypes: Genomic profiles, prognostic molecular markers and therapeutic possibilities. J. Pathol. 2019, 247, 539–551. [Google Scholar] [CrossRef]
- Long, G.V.; Swetter, S.M.; Menzies, A.M.; Gershenwald, J.E.; Scolyer, R.A. Cutaneous melanoma. Lancet 2023, 402, 485–502. [Google Scholar] [CrossRef]
- El Sharouni, M.A.; van Diest, P.J.; Witkamp, A.J.; Sigurdsson, V.; van Gils, C.H. Subtyping Cutaneous Melanoma Matters. JNCI Cancer Spectr. 2020, 4, pkaa097. [Google Scholar] [CrossRef]
- Paluncic, J.; Kovacevic, Z.; Jansson, P.J.; Kalinowski, D.; Merlot, A.M.; Huang, M.L.H.; Lok, H.C.; Sahni, S.; Lane, D.J.R.; Richardson, D.R. Roads to melanoma: Key pathways and emerging players in melanoma progression and oncogenic signaling. Biochim. Et Biophys. Acta (BBA)—Mol. Cell Res. 2016, 1863, 770–784. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Chen, J.; He, L.; Stiles, B.L. PTEN: Tumor Suppressor and Metabolic Regulator. Front. Endocrinol. 2018, 9, 338. [Google Scholar] [CrossRef]
- Hannan, E.J.; O’Leary, D.P.; MacNally, S.P.; Kay, E.W.; Farrell, M.A.; Morris, P.G.; Power, C.P.; Hill, A.D.K. The significance of BRAF V600E mutation status discordance between primary cutaneous melanoma and brain metastases: The implications for BRAF inhibitor therapy. Medicine 2017, 96, e8404. [Google Scholar] [CrossRef] [PubMed]
- Waite, K.A.; Eng, C. Protean PTEN: Form and Function. Am. J. Hum. Genet. 2002, 70, 829–844. [Google Scholar] [CrossRef] [PubMed]
- Debela, D.T.; Muzazu, S.G.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New approaches and procedures for cancer treatment: Current perspectives. SAGE Open Med. 2021, 9, 20503121211034366. [Google Scholar] [CrossRef] [PubMed]
- Mousa, S.A.; Davis, P.J. Chapter 1—Angiogenesis and Anti-Angiogenesis Strategies in Cancer. In Anti-Angiogenesis Strategies in Cancer Therapeutics; Mousa, S.A., Davis, P.J., Eds.; Academic Press: Boston, MA, USA, 2017; pp. 1–19. [Google Scholar] [CrossRef]
- Abdalla, A.M.E.; Xiao, L.; Ullah, M.W.; Yu, M.; Ouyang, C.; Yang, G. Current Challenges of Cancer Anti-angiogenic Therapy and the Promise of Nanotherapeutics. Theranostics 2018, 8, 533–548. [Google Scholar] [CrossRef]
- Dillon, L.M.; Miller, T.W. Therapeutic targeting of cancers with loss of PTEN function. Curr. Drug Targets 2014, 15, 65–79. [Google Scholar] [CrossRef]
- Morris, L.G.; Chan, T.A. Therapeutic targeting of tumor suppressor genes. Cancer 2015, 121, 1357–1368. [Google Scholar] [CrossRef]
- Wigerup, C.; Påhlman, S.; Bexell, D. Therapeutic targeting of hypoxia and hypoxia-inducible factors in cancer. Pharmacol. Ther. 2016, 164, 152–169. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, S.; Huynh-Do, U. The Role of PTEN in Tumor Angiogenesis. J. Oncol. 2012, 2012, 141236. [Google Scholar] [CrossRef] [PubMed]
- Murthy, S.K. Nanoparticles in modern medicine: State of the art and future challenges. Int. J. Nanomed. 2007, 2, 129–141. [Google Scholar]
- Foulkes, R.; Man, E.; Thind, J.; Yeung, S.; Joy, A.; Hoskins, C. The regulation of nanomaterials and nanomedicines for clinical application: Current and future perspectives. Biomater. Sci. 2020, 8, 4653–4664. [Google Scholar] [CrossRef]
- Molinari, F.; Frattini, M. Functions and Regulation of the PTEN Gene in Colorectal Cancer. Front. Oncol. 2013, 3, 326. [Google Scholar] [CrossRef]
- Chang, F.; Lee, J.T.; Navolanic, P.M.; Steelman, L.S.; Shelton, J.G.; Blalock, W.L.; Franklin, R.A.; McCubrey, J.A. Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: A target for cancer chemotherapy. Leukemia 2003, 17, 590–603. [Google Scholar] [CrossRef]
- Manning, B.D.; Cantley, L.C. AKT/PKB signaling: Navigating downstream. Cell 2007, 129, 1261–1274. [Google Scholar] [CrossRef] [PubMed]
- Hobert, J.A.; Eng, C. PTEN hamartoma tumor syndrome: An overview. Genet. Med. 2009, 11, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Aquila, S.; Santoro, M.; Caputo, A.; Panno, M.L.; Pezzi, V.; De Amicis, F. The Tumor Suppressor PTEN as Molecular Switch Node Regulating Cell Metabolism and Autophagy: Implications in Immune System and Tumor Microenvironment. Cells 2020, 9, 1725. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Goel, V.; Haluska, F.G. PTEN signaling pathways in melanoma. Oncogene 2003, 22, 3113–3122. [Google Scholar] [CrossRef]
- Lee, Y.R.; Chen, M.; Pandolfi, P.P. The functions and regulation of the PTEN tumour suppressor: New modes and prospects. Nat. Rev. Mol. Cell Biol. 2018, 19, 547–562. [Google Scholar] [CrossRef]
- Carlos-Reyes, Á.; López-González, J.S.; Meneses-Flores, M.; Gallardo-Rincón, D.; Ruíz-García, E.; Marchat, L.A.; Astudillo-de la Vega, H.; Hernández de la Cruz, O.N.; López-Camarillo, C. Dietary Compounds as Epigenetic Modulating Agents in Cancer. Front. Genet. 2019, 10, 79. [Google Scholar] [CrossRef]
- Mirmohammadsadegh, A.; Marini, A.; Nambiar, S.; Hassan, M.; Tannapfel, A.; Ruzicka, T.; Hengge, U.R. Epigenetic silencing of the PTEN gene in melanoma. Cancer Res. 2006, 66, 6546–6552. [Google Scholar] [CrossRef]
- Aronchik, I.; Kundu, A.; Quirit, J.G.; Firestone, G.L. The antiproliferative response of indole-3-carbinol in human melanoma cells is triggered by an interaction with NEDD4-1 and disruption of wild-type PTEN degradation. Mol. Cancer Res. 2014, 12, 1621–1634. [Google Scholar] [CrossRef] [PubMed]
- Kotelevets, L.; Trifault, B.; Chastre, E.; Scott, M.G.H. Posttranslational Regulation and Conformational Plasticity of PTEN. Cold Spring Harb. Perspect. Med. 2020, 10, a036095. [Google Scholar] [CrossRef]
- Nguyen Huu, T.; Park, J.; Zhang, Y.; Park, I.; Yoon, H.J.; Woo, H.A.; Lee, S.R. Redox Regulation of PTEN by Peroxiredoxins. Antioxidants 2021, 10, 302. [Google Scholar] [CrossRef]
- Numajiri, N.; Takasawa, K.; Nishiya, T.; Tanaka, H.; Ohno, K.; Hayakawa, W.; Asada, M.; Matsuda, H.; Azumi, K.; Kamata, H.; et al. On–off system for PI3-kinase–Akt signaling through S-nitrosylation of phosphatase with sequence homology to tensin (PTEN). Proc. Natl. Acad. Sci. USA 2011, 108, 10349–10354. [Google Scholar] [CrossRef]
- González-Santamaría, J.; Campagna, M.; Ortega-Molina, A.; Marcos-Villar, L.; de la Cruz-Herrera, C.F.; González, D.; Gallego, P.; Lopitz-Otsoa, F.; Esteban, M.; Rodríguez, M.S.; et al. Regulation of the tumor suppressor PTEN by SUMO. Cell Death Dis. 2012, 3, e393. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Paluch, B.E.; Wang, X.; Jiang, X. PTEN at a glance. J. Cell Sci. 2012, 125, 4687–4692. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.Y.; Kwon, S.M. Angiogenesis and its therapeutic opportunities. Mediat. Inflamm. 2013, 2013, 127170. [Google Scholar] [CrossRef] [PubMed]
- North, S.; Moenner, M.; Bikfalvi, A. Recent developments in the regulation of the angiogenic switch by cellular stress factors in tumors. Cancer Lett. 2005, 218, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Dratkiewicz, E.; Simiczyjew, A.; Mazurkiewicz, J.; Ziętek, M.; Matkowski, R.; Nowak, D. Hypoxia and Extracellular Acidification as Drivers of Melanoma Progression and Drug Resistance. Cells 2021, 10, 862. [Google Scholar] [CrossRef]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef]
- Hillen, F.; Griffioen, A.W. Tumour vascularization: Sprouting angiogenesis and beyond. Cancer Metastasis Rev. 2007, 26, 489–502. [Google Scholar] [CrossRef]
- Escudier, B.; Gore, M. Axitinib for the Management of Metastatic Renal Cell Carcinoma. Drugs R D 2011, 11, 113–126. [Google Scholar] [CrossRef]
- Mittal, K.; Wood, L.S.; Rini, B.I. Axitinib in Metastatic Renal Cell Carcinoma. Biol. Ther. 2012, 2, 5. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Axitinib. 2020. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=202324 (accessed on 26 April 2021).
- Kazazi-Hyseni, F.; Beijnen, J.H.; Schellens, J.H.M. Bevacizumab. Oncologist 2010, 15, 819–825. [Google Scholar] [CrossRef]
- Arriaga, Y.; Becerra, C.R. Adverse Effects of Bevacizumab and Their Management in Solid Tumors. Support. Cancer Ther. 2006, 3, 247–250. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. Bevacizumab. 2020. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=125085 (accessed on 26 April 2021).
- Hoy, S.M. Cabozantinib: A review of its use in patients with medullary thyroid cancer. Drugs 2014, 74, 1435–1444. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. COMETRIQ. 2020. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=203756 (accessed on 26 April 2021).
- Coppin, C. Everolimus: The first approved product for patients with advanced renal cell cancer after sunitinib and/or sorafenib. Biologics 2010, 4, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Porta, C.; Osanto, S.; Ravaud, A.; Climent, M.-A.; Vaishampayan, U.; White, D.A.; Creel, P.; Dickow, B.; Fischer, P.; Gornell, S.S.; et al. Management of adverse events associated with the use of everolimus in patients with advanced renal cell carcinoma. Eur. J. Cancer 2011, 47, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. EVEROLIMUS. 2020. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=022334 (accessed on 26 April 2021).
- Galustian, C.; Dalgleish, A. Lenalidomide: A novel anticancer drug with multiple modalities. Expert. Opin. Pharmacother. 2009, 10, 125–133. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Lenalidomide. 2020. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=021880 (accessed on 26 April 2021).
- Rodríguez, A.P.G. Management of the adverse effects of lenalidomide in multiple myeloma. Adv. Ther. 2011, 28, 1. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Lenvatinib Mesylate. 2020. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2015/206947Orig1s000ltr.pdf (accessed on 26 April 2021).
- Lorusso, L.; Newbold, K. Lenvatinib: A new option for the treatment of advanced iodine refractory differentiated thyroid cancer? Future Oncol. 2015, 11, 1719–1727. [Google Scholar] [CrossRef]
- Keisner, S.V.; Shah, S.R. Pazopanib. Drugs 2011, 71, 443–454. [Google Scholar] [CrossRef]
- US Food and drug administration. Pazopanib 2020. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=215837 (accessed on 26 April 2021).
- Fuchs, C.S.; Tomasek, J.; Yong, C.J.; Dumitru, F.; Passalacqua, R.; Goswami, C.; Safran, H.; dos Santos, L.V.; Aprile, G.; Ferry, D.R.; et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): An international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014, 383, 31–39. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Ramucirumab. 2020. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2014/125477Orig1s000ltr.pdf (accessed on 26 April 2021).
- Strumberg, D.; Schultheis, B. Regorafenib for cancer. Expert. Opin. Investig. Drugs 2012, 21, 879–889. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Regorafenib 2020. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=203085 (accessed on 26 April 2021).
- US Food and Drug Administration. Sorafenib 2020. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=021923 (accessed on 26 April 2021).
- Kane, R.C.; Farrell, A.T.; Saber, H.; Tang, S.; Williams, G.; Jee, J.M.; Liang, C.; Booth, B.; Chidambaram, N.; Morse, D.; et al. Sorafenib for the Treatment of Advanced Renal Cell Carcinoma. Clin. Cancer Res. 2006, 12, 7271. [Google Scholar] [CrossRef]
- Izzedine, H.; Buhaescu, I.; Rixe, O.; Deray, G. Sunitinib malate. Cancer Chemother. Pharmacol. 2007, 60, 357–364. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Sunitinib 2020. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=021938 (accessed on 26 April 2021).
- US Food and Drug Administration. Vandetanib 2020. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2011/022405s000ltr.pdf (accessed on 26 April 2021).
- Commander, H.; Whiteside, G.; Perry, C. Vandetanib. Drugs 2011, 71, 1355–1365. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. Ziv-Aflibercept 2020. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2012/125418Orig1s000ltr.pdf (accessed on 26 April 2021).
- Patel, A.; Sun, W. Ziv-aflibercept in metastatic colorectal cancer. Biologics 2014, 8, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Albadari, N.; Deng, S.; Li, W. The transcriptional factors HIF-1 and HIF-2 and their novel inhibitors in cancer therapy. Expert. Opin. Drug Discov. 2019, 14, 667–682. [Google Scholar] [CrossRef] [PubMed]
- Hicklin, D.J.; Ellis, L.M. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J. Clin. Oncol. 2005, 23, 1011–1027. [Google Scholar] [CrossRef] [PubMed]
- Ellis, L.M.; Hicklin, D.J. VEGF-targeted therapy: Mechanisms of anti-tumour activity. Nat. Rev. Cancer 2008, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, J.; Li, G.; Wei, J.; Chen, H.; Zhang, C.; Zhao, J.; Wang, Y.; Dang, S.; Li, X.; et al. Fresh red raspberry phytochemicals suppress the growth of hepatocellular carcinoma cells by PTEN/AKT pathway. Int. J. Biochem. Cell Biol. 2018, 104, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Stefanska, B.; Rudnicka, K.; Bednarek, A.; Fabianowska-Majewska, K. Hypomethylation and induction of retinoic acid receptor beta 2 by concurrent action of adenosine analogues and natural compounds in breast cancer cells. Eur. J. Pharmacol. 2010, 638, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Yu, Y.; Padhye, S.B.; Sarkar, F.H.; Majumdar, A.P. Difluorinated-curcumin (CDF) restores PTEN expression in colon cancer cells by down-regulating miR-21. PLoS ONE 2013, 8, e68543. [Google Scholar] [CrossRef]
- Arafa, E.S.A.; Zhu, Q.; Shah, Z.I.; Wani, G.; Barakat, B.M.; Racoma, I.; El-Mahdy, M.A.; Wani, A.A. Thymoquinone up-regulates PTEN expression and induces apoptosis in doxorubicin-resistant human breast cancer cells. Mutat. Res. 2011, 706, 28–35. [Google Scholar] [CrossRef]
- Kim, D.-H.; Suh, J.; Surh, Y.-J.; Na, H.-K. Regulation of the tumor suppressor PTEN by natural anticancer compounds. Ann. N. Y. Acad. Sci. 2017, 1401, 136–149. [Google Scholar] [CrossRef]
- Lubecka-Pietruszewska, K.; Kaufman-Szymczyk, A.; Stefanska, B.; Cebula-Obrzut, B.; Smolewski, P.; Fabianowska-Majewska, K. Sulforaphane Alone and in Combination with Clofarabine Epigenetically Regulates the Expression of DNA Methylation-Silenced Tumour Suppressor Genes in Human Breast Cancer Cells. J. Nutr. Nutr. 2015, 8, 91–101. [Google Scholar] [CrossRef]
- Garbiec, E.; Cielecka-Piontek, J.; Kowalówka, M.; Hołubiec, M.; Zalewski, P. Genistein—Opportunities Related to an Interesting Molecule of Natural Origin. Molecules 2022, 27, 815. [Google Scholar]
- Sarao, L.; Kaur, S.; Malik, T.; Singh, A. Chapter 19—Genistein and daidzein. In Nutraceuticals and Health Care; Kour, J., Nayik, G.A., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 331–341. [Google Scholar] [CrossRef]
- Tuli, H.S.; Tuorkey, M.J.; Thakral, F.; Sak, K.; Kumar, M.; Sharma, A.K.; Sharma, U.; Jain, A.; Aggarwal, V.; Bishayee, A. Molecular Mechanisms of Action of Genistein in Cancer: Recent Advances. Front. Pharmacol. 2019, 10, 1336. [Google Scholar] [CrossRef]
- Kamal, M.M.; Akter, S.; Lin, C.N.; Nazzal, S. Sulforaphane as an anticancer molecule: Mechanisms of action, synergistic effects, enhancement of drug safety, and delivery systems. Arch. Pharm. Res. 2020, 43, 371–384. [Google Scholar] [CrossRef]
- Santos, A.C.; Pereira, I.; Magalhães, M.; Pereira-Silva, M.; Caldas, M.; Ferreira, L.; Figueiras, A.; Ribeiro, A.J.; Veiga, F. Targeting Cancer Via Resveratrol-Loaded Nanoparticles Administration: Focusing on In Vivo Evidence. AAPS J. 2019, 21, 57. [Google Scholar] [CrossRef]
- Spagnuolo, C.; Russo, G.L.; Orhan, I.E.; Habtemariam, S.; Daglia, M.; Sureda, A.; Nabavi, S.F.; Devi, K.P.; Loizzo, M.R.; Tundis, R.; et al. Genistein and cancer: Current status, challenges, and future directions. Adv. Nutr. 2015, 6, 408–419. [Google Scholar] [CrossRef]
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X. A Review of Curcumin and Its Derivatives as Anticancer Agents. Int. J. Mol. Sci. 2019, 20, 1033. [Google Scholar] [CrossRef]
- Aqil, F.; Munagala, R.; Jeyabalan, J.; Vadhanam, M.V. Bioavailability of phytochemicals and its enhancement by drug delivery systems. Cancer Lett. 2013, 334, 133–141. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Kampan, N.C.; Madondo, M.T.; McNally, O.M.; Quinn, M.; Plebanski, M. Paclitaxel and Its Evolving Role in the Management of Ovarian Cancer. Biomed. Res. Int. 2015, 2015, 413076. [Google Scholar] [CrossRef]
- Ma, P.; Mumper, R.J. Paclitaxel Nano-Delivery Systems: A Comprehensive Review. J. Nanomed. Nanotechnol. 2013, 4, 1000164. [Google Scholar] [CrossRef]
- Pal, R.R.; Rajpal, V.; Singh, P.; Saraf, S.A. Recent Findings on Thymoquinone and Its Applications as a Nanocarrier for the Treatment of Cancer and Rheumatoid Arthritis. Pharmaceutics 2021, 13, 775. [Google Scholar] [CrossRef]
- Odeh, F.; Ismail, S.I.; Abu-Dahab, R.; Mahmoud, I.S.; Al Bawab, A. Thymoquinone in liposomes: A study of loading efficiency and biological activity towards breast cancer. Drug Deliv. 2012, 19, 371–377. [Google Scholar] [CrossRef]
- Danhier, F.; Feron, O.; Préat, V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control. Release 2010, 148, 135–146. [Google Scholar] [CrossRef]
- Zhao, M.; Lei, C.; Yang, Y.; Bu, X.; Ma, H.; Gong, H.; Liu, J.; Fang, X.; Hu, Z.; Fang, Q. Abraxane, the Nanoparticle Formulation of Paclitaxel Can Induce Drug Resistance by Up-Regulation of P-gp. PLoS ONE 2015, 10, e0131429. [Google Scholar] [CrossRef]
- Gradishar, W.J. Albumin-bound paclitaxel: A next-generation taxane. Expert. Opin. Pharmacother. 2006, 7, 1041–1053. [Google Scholar] [CrossRef]
- Barenholz, Y. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Rafiyath, S.M.; Rasul, M.; Lee, B.; Wei, G.; Lamba, G.; Liu, D. Comparison of safety and toxicity of liposomal doxorubicin vs. conventional anthracyclines: A meta-analysis. Exp. Hematol. Oncol. 2012, 1, 10. [Google Scholar] [CrossRef]
- Kanwal, U.; Irfan Bukhari, N.; Ovais, M.; Abass, N.; Hussain, K.; Raza, A. Advances in nano-delivery systems for doxorubicin: An updated insight. J. Drug Target. 2018, 26, 296–310. [Google Scholar] [CrossRef]
- Silverman, J.A.; Deitcher, S.R. Marqibo® (vincristine sulfate liposome injection) improves the pharmacokinetics and pharmacodynamics of vincristine. Cancer Chemother. Pharmacol. 2013, 71, 555–564. [Google Scholar] [CrossRef]
- Yang, F.; Jiang, M.; Lu, M.; Hu, P.; Wang, H.; Jiang, J. Pharmacokinetic Behavior of Vincristine and Safety Following Intravenous Administration of Vincristine Sulfate Liposome Injection in Chinese Patients with Malignant Lymphoma. Front. Pharmacol. 2018, 9, 991. [Google Scholar] [CrossRef]
- Doval, D.; Kumar Sharma, S.; Kumar, M.; Khandelwal, V.; Choudhary, D. Cytarabine ears—A side effect of cytarabine therapy. J. Oncol. Pharm. Pract. 2019, 26, 471–473. [Google Scholar] [CrossRef]
- Nirmala, M.J.; Kizhuveetil, U.; Johnson, A.; Balaji, G.; Nagarajan, R.; Muthuvijayan, V. Cancer nanomedicine: A review of nano-therapeutics and challenges ahead. RSC Adv. 2023, 13, 8606–8629. [Google Scholar] [CrossRef]
- Passero, F.C.; Grapsa, D.; Syrigos, K.N.; Saif, M.W. The safety and efficacy of Onivyde (irinotecan liposome injection) for the treatment of metastatic pancreatic cancer following gemcitabine-based therapy. Expert. Rev. Anticancer. Ther. 2016, 16, 697–703. [Google Scholar] [CrossRef]
- Dinndorf, P.A.; Gootenberg, J.; Cohen, M.H.; Keegan, P.; Pazdur, R. FDA drug approval summary: Pegaspargase (oncaspar) for the first-line treatment of children with acute lymphoblastic leukemia (ALL). Oncologist 2007, 12, 991–998. [Google Scholar] [CrossRef]
- Walker, P.L.; Dang, N.H. Denileukin diftitox as novel targeted therapy in non-Hodgkin’s lymphoma. Clin. J. Oncol. Nurs. 2004, 8, 169–174. [Google Scholar] [CrossRef]
- Berges, R. Eligard®: Pharmacokinetics, Effect on Testosterone and PSA Levels and Tolerability. Eur. Urol. Suppl. 2005, 4, 20–25. [Google Scholar] [CrossRef]
- Chang, J.I.C.; Bucci, J. Unusual side effect from a luteinizing hormone-releasing hormone agonist, leuprorelin, in the treatment of prostate cancer: A case report. J. Med. Case Rep. 2016, 10, 323. [Google Scholar] [CrossRef]
- Hong, M.; Shi, H.; Wang, N.; Tan, H.Y.; Wang, Q.; Feng, Y. Dual Effects of Chinese Herbal Medicines on Angiogenesis in Cancer and Ischemic Stroke Treatments: Role of HIF-1 Network. Front. Pharmacol. 2019, 10, 696. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, N.; Han, S.; Wang, D.; Mo, S.; Yu, L.; Huang, H.; Tsui, K.; Shen, J.; Chen, J. Dietary Compound Isoliquiritigenin Inhibits Breast Cancer Neoangiogenesis via VEGF/VEGFR-2 Signaling Pathway. PLoS ONE 2013, 8, e68566. [Google Scholar] [CrossRef]
- Peng, F.; Tang, H.; Liu, P.; Shen, J.; Guan, X.; Xie, X.; Gao, J.; Xiong, L.; Jia, L.; Chen, J.; et al. Isoliquiritigenin modulates miR-374a/PTEN/Akt axis to suppress breast cancer tumorigenesis and metastasis. Sci. Rep. 2017, 7, 9022. [Google Scholar] [CrossRef]
- Li, X.; Lu, Q.; Xie, W.; Wang, Y.; Wang, G. Anti-tumor effects of triptolide on angiogenesis and cell apoptosis in osteosarcoma cells by inducing autophagy via repressing Wnt/β-Catenin signaling. Biochem. Biophys. Res. Commun. 2018, 496, 443–449. [Google Scholar] [CrossRef]
- Li, X.; Zang, A.; Jia, Y.; Zhang, J.; Fan, W.; Feng, J.; Duan, M.; Zhang, L.; Huo, R.; Jiao, J.; et al. Triptolide reduces proliferation and enhances apoptosis of human non-small cell lung cancer cells through PTEN by targeting miR-21. Mol. Med. Rep. 2016, 13, 2763–2768. [Google Scholar] [CrossRef]
- Wang, F.-R.; Jiang, Y.-S. Effect of treatment with baicalein on the intracerebral tumor growth and survival of orthotopic glioma models. J. Neuro-Oncol. 2015, 124, 5–11. [Google Scholar] [CrossRef]
- Lu, C.; Wang, H.; Chen, S.; Yang, R.; Li, H.; Zhang, G. Baicalein inhibits cell growth and increases cisplatin sensitivity of A549 and H460 cells via miR-424-3p and targeting PTEN/PI3K/Akt pathway. J. Cell. Mol. Med. 2018, 22, 2478–2487. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, Z.; Zhang, S.; Ren, J.; Zhang, R.; Zeng, H.; Li, Q.; Wu, G. Inhibitory action of Celastrol on hypoxia-mediated angiogenesis and metastasis via the HIF-1α pathway. Int. J. Mol. Med. 2011, 27, 407–415. [Google Scholar] [CrossRef]
- Zhu, B.; Wei, Y. Antitumor activity of celastrol by inhibition of proliferation, invasion, and migration in cholangiocarcinoma via PTEN/PI3K/Akt pathway. Cancer Med. 2020, 9, 783–796. [Google Scholar] [CrossRef]

| Pro-Angiogenic Factors | Function | Reference |
| Chemokines (CXCL)-1, -2, -3, -5, -6, -7, -8 | Chemokines are structurally related cytokines that play a crucial role in inflammation, immunity, and angiogenesis. CC and CXCL chemokines both play an integral role in tumor angiogenesis, which is required for sustained tumor proliferation. The CXCL chemokines, identified by the presence of the glutamic-leucine-arginine (ELR) motif at the N-terminal, can be divided into ELR+ chemokines that promote angiogenesis and ELR− chemokines that suppress angiogenesis or have angiostatic effects. | [31] |
| Vascular endothelial growth factor (VEGF)-A, -B, -C, -D, E and placenta growth factor (PlGF)-1 and -2 | The VEGF gene secretes six glycoproteins, namely: VEGF-A, -B, -C, -D, -E, and placenta growth factor (PlGF)-1 and -2. The predominant glycoprotein associated with tumor angiogenesis is VEGF-A, which can give rise to five isoforms through mRNA alternative splicing, namely VEGF111, VEGF121, VEGF165, VEGF189, and VEGF206. The predominant isoform, VEGF165, is overexpressed by several malignant tumors, such as malignant melanoma, and the expression of isoforms is tissue-specific; thus, these isoforms play pertinent roles in vasculogenesis and tumor angiogenesis. The binding of the glycoprotein VEGF-A to its receptor, VEGFR1, triggers the activation of several signaling pathways that ensure sustained endothelial cell survival, mitogenesis, migration, differentiation, vascular permeability, and recruitment of endothelial progenitor cells from the bone marrow to the tumor vasculature. | [31] |
| Basic fibroblast growth factor (bFGF) | Stimulates angiogenesis in melanoma through the upregulation of VEGF and matrix metalloproteinase (MMP) expression and promotes endothelial cell proliferation and migration. | [31,32] |
| HIF-1, -2, -3 | The predominant expression of hypoxia-inducible factors (HIFs) occurs due to the stabilization of hypoxia when tumors outgrow their vascular network. Furthermore, stabilization of hypoxia leads to the transcription of genes that promote enhanced angiogenesis, energy metabolism, cell survival, radiation resistance, invasion, and metastasis. | [31,32] |
| Platelet-derived growth factor (PDGF)-A, -B, -C, -D | Plays a role in the autocrine stimulation of cancer as it directly stimulates the growth of cancer cells. Additionally, it plays a role in the paracrine stimulation of cancer as it indirectly stimulates carcinogenesis through the stimulation of angiogenesis. | [31] |
| Transforming growth factor beta (TGF-β)-1, -2, -3 | Transforming growth factor beta (TGF-β) is a cytokine involved in the promotion of carcinogenesis through various hallmarks of cancer, such as the evasion of apoptosis and stimulation of angiogenesis. The TGF-β family comprises three isoforms: TGF-β-1, -2 and -3, where TGF-β-1 is the predominant isoform linked to the stimulation of VEGF, which in turn directly stimulates angiogenesis in melanoma. | [31] |
| Interleukin-8 (IL-8) | Tumor-derived IL-8 promotes angiogenesis, tumor proliferation, and migration in melanoma. Similarly, IL-8 derived from endothelial cells promotes the migration of tumor cells. | [31,32] |
| Matrix metalloproteinases (MMP-2, -3, -7, -9 and -14) | Proteases are involved in bone resorption, wound healing, and angiogenesis. The MMPs produced by tumor cells facilitate angiogenesis, tumor growth, and metastasis. MMP-2 and MMP-9 are the main drivers of angiogenesis in melanoma through the degradation of the extracellular matrix (ECM) and the activation of VEGF and TGF-β. | [31,32] |
| Antiangiogenic factors | Function | Reference |
| Angiostatin | Angiostatin formation occurs through the proteolytic digestion of plasminogen. Angiostatin selectively inhibits endothelial cell proliferation after administration of serum/urine from tumor-bearing mice. Furthermore, in human prostate carcinoma cells (PC-3), the release of urokinase (uPA) and free sulfhydryl donors (FSDs) generates angiostatin from plasminogen, and non-cell-derived angiostatin suppresses angiogenesis in vitro and in vivo, highlighting the potential use of recombinant angiostatin as an anti-angiogenic therapeutic/drug. | [31,32,33] |
| Endostatin | The cleavage of collagen XVIII, an important component of the basement membrane in the extracellular matrix, results in the formation of a 20 kda C—terminal fragment, which possesses anti-angiogenic activity. The anti-angiogenic activity is elicited by inhibiting proliferation, migration, and enhancing apoptosis in endothelial cells. Endostatin also suppresses angiogenesis through the competitive inhibition of VEGFR1 and VEGFR2, thereby enhancing the production of thrombospondin-1, which in turn suppresses angiogenesis. | [31,32] |
| Thrombospondin (TSP)-1 | Thrombospondins are a family of extracellular matrix (ECM) proteins, predominantly found in embryonic and adult tissues, consisting of five members: TSP1, TSP2, TSP3, TSP4, and TSP5. Thrombospondin-1 has been identified as an endogenous inhibitor of angiogenesis through the inhibition of endothelial cell migration, proliferation, and induction of apoptosis in endothelial cells. Thrombospondin-1 also elicits anti-angiogenic activity through the inhibition of VEGFR2 through the ligation of its receptor (CD47). | [31,32] |
| Therapy | Mechanism | Side-Effects | Cancer | References |
|---|---|---|---|---|
| Axitinib (Inlyta) | Selectively inhibits in vitro VEGFR-1, -2 and -3 at sub-nanomolar concentrations. In in vivo pre-clinical models, it demonstrated anti-angiogenic activity through the modulation of VEGFR-1, -2 and -3. | Hypertension, diarrhoea, nausea, hand-foot syndrome, fatigue, and hypothyroidism | Advanced Renal cell carcinoma | [39,40,41] |
| Bevacizumab (Avastin®) | Monoclonal antibody that neutralizes circulating VEGF such that VEGF cannot bind the tyrosine kinase receptors (VEGFR-1, -2 and -3). This results in the lowering of interstitial pressure, thereby increasing in vascular permeability and enhancing the delivery of chemotherapeutic agents. | Hypertension, proteinuria, epistaxis, thrombosis, and gastrointestinal bleeding | Metastatic colorectal cancer | [42,43,44] |
| Cabozantinib (Cometriq®) | A small molecule inhibitor that inhibits multiple tyrosine kinase receptors implicated in the pathogenesis of medullary thyroid cancer i.e., RET, MET and VEGFR-2 | Gastrointestinal perforation, hemorrhage, hypertension, and venous thrombosis | Progressive, unresectable locally advanced or metastatic medullary thyroid cancer | [45,46] |
| Everolimus (Afinitor®) | Inhibits mTORC2 linked to the PI3K and VEGF pathway. It is used concurrently with drugs such as sorafenib and sunitinib that inhibit angiogenesis | Stomatitis, Asthenia, fatigue, rash, diarrhea, nausea, mucosal inflammation, oedema peripheral, infections, dyspnea, pneumonitis, anemia, lymphopenia, thrombocytopenia, hypercholesterolemia, hypertriglyceridemia, hyperglycemia, and elevated creatinine | Clear cell metastatic renal cell cancer | [47,48,49] |
| Lenalidomide (Revlimid®) | Inhibits angiogenesis through the inhibition of pertinent angiogenic factors i.e., VEGF, bFGF and HIF. Lenalidomide also inhibits endothelial cell migration, adhesion, capillary tube formation and endothelial cell apoptosis in 3D collagen cultures. | Neutropenia, thrombocytopenia, anemia, infections, and thrombosis | Multiple myeloma | [16,50,51,52] |
| Lenvatinib mesylate (Lenvima®) | Inhibits angiogenesis through the inhibition of multiple tyrosine kinase receptors, namely VEGFR1-3, FGFR1-4, RET, c-KIT, and PDGFRβ. | Hypertension, diarrhea, fatigue or asthenia, appetite loss, weight loss and nausea | Progressiveradioiodine-refractory differentiated thyroid cancer | [53,54] |
| Pazopanib (Votrient®) | Anti-angiogenic activity through the inhibition of VEGFR1-3 and PDGFR-α28 and PDGFR-β on endothelial cells. Inhibition of tyrosine kinase receptors results in the inhibition of pathways that promote cell proliferation, cell survival, vascular permeability, and cell migration. | Diarrhea, hypertension, and elevation of liver enzymes | Metastatic renal cell carcinoma | [55,56] |
| Ramucirumab (Cyramza®) | Anti-angiogenic activity through the inhibition of VEGFR-2 on endothelial cells. The inhibition of VEGFR-2 enables the inhibition of signaling pathways in endothelial cells that promote cell proliferation, cell survival, increased vascular permeability and differentiation. | Fatigue, abdominal pain, appetite loss, vomiting, constipation, anemia, dysphagia, hypertension, hemorrhage, arterial thromboembolism, venous thromboembolism, proteinuria, gastrointestinal perforation, fistula formation, infusion-related reaction, and cardiac failure | Advanced gastric cancer or gastro-esophageal junction adenocarcinoma | [57,58] |
| Regorafenib (Stivarga®) | Orally active, diphenylurea multikinase inhibitor of VEGFR1-3, c-KIT, TIE-2, PDGFR-β, FGFR-1, RET, RAF-1, BRAF and p38 MAP kinase. The inhibition of several tyrosine kinase receptors leads to the inhibition of angiogenesis and oncogenesis | Hand-foot skin reaction, rash, desquamation, alopecia, fatigue, hypertension, mucotitis, diarrhea, and thyroid dysfunction | Metastatic colorectal cancer | [59,60] |
| Sorafenib (Nexavar®) | Inhibits angiogenesis and oncogenesis through the inhibition of the following tyrosine kinase receptors, i.e., RAF kinase, PDGF, VEGFR 2-3, and c-KIT | Reversible skin rashes, hand-foot skin reaction, diarrhea, hypertension, and sensory neuropathic changes | Advanced renal cell carcinoma | [61,62] |
| Sunitinib (Sutent®) | Exhibits anti-tumor and anti-angiogenic effects through the inhibition of several kinases namely, PDGFR-α, PDGFR-β, VEGFR1-3, KIT, FLT3, CSF-1R, and RET | Left ventricle dysfunction, hemorrhagic events, hypertension, fatigue, diarrhea, mucositis/stomatitis, vomiting, abdominal pain, constipation, nausea, anorexia, altered taste, headache, dyspnea, cough, skin discoloration, rash, hand-foot syndrome, arthralgia, back-pain, and myalgia | Gastrointestinal stromal tumor and metastatic renal cell carcinoma | [63,64] |
| Vandetanib (Caprelsa®) | Antagonist of VEGFR-2, EGFR, and RET kinase resulting in antiangiogenic and antineoplastic activity | Diarrhea/colitis, rash, dermatitis acneiform/acne, nausea, hypertension, hypertensive crisis, accelerated hypertension, headache, fatigue, appetite loss, abdominal pain, dry skin, vomiting, asthenia, ECG QT prolonged, photosensitivity radiation, insomnia, nasopharyngitis, dyspepsia, hypocalcemia, cough, pruritus, weight loss, proteinuria, and depression | Medullary thyroid cancer | [65,66] |
| Ziv-aflibercept (Zaltrap®) | A recombinant protein that comprises of the extracellular domains from both VEGFR-1 and VEGFR-2 fused to the fc (a) region of human IgG1. Ziv-aflibercept is a pseudo receptor that binds VEGFA, VEGFB, and PlGF resulting in the inhibition of angiogenesis | Urinary tract infection, leukopenia, neutropenia, thrombocytopenia, appetite loss, dehydration, headache, hypertension, epistaxis, dysphonia, dyspnea, oropharyngeal pain, rhinorrhea, diarrhea, stomatitis, abdominal pain, hemorrhoids, rectal hemorrhage, proctalgia, palmer-plantar erythrodysesthesia syndrome, skin hyperpigmentation, proteinuria, serum creatinine increased, fatigue, asthenia, AST increased, ALT increased, and weight loss | Metastatic colorectal cancer | [67,68] |
| Phytochemical | Source | Mechanism | References |
|---|---|---|---|
| Red raspberry extract | Rubus idaeus L. (red raspberry) | Red raspberry extract enhanced PTEN activity through demethylating the PTEN promoter and inhibiting DNA methyltransferase-1 (DNMT-1) expression, thus, decreasing Akt activation in hepatocellular carcinoma (HepG2) | [72] |
| Resveratrol | Grapes, apples, blueberries, plums and peanuts | Resveratrol in combination with Vitamin D3 demethylated the PTEN promoter and downregulated DNMT in breast cancer cells (MCF-7) | [73] |
| Curcumin | Curcuma longa L. (turmeric) | An analogue of curcumin (difluorinated curcumin) displayed antiproliferative activity against 5-fluorouracil (5-FU) + oxaliplatin resistant colon cancer cells, downregulated miR21 in chemo-resistant colon cancer (HCT116 and HT-29) cells, and restored PTEN function | [74] |
| Sulforaphane | Cruciferous vegetables such as kale (Brassica oleracea L. var. acephala), cauliflower (B. oleracea var Botrytis L.), cabbage (B. oleracea L. var capitata), and broccoli (B. oleracea var. italica) | Demethylated the PTEN promoter, thereby inhibiting the PI3K/Akt pathway and angiogenesis | [76,77] |
| Genistein | Soybeans, legumes, broccoli, cauliflower and sunflowers | At 1 µM, genistein upregulated PTEN expression by 1.2-fold in the metastastic breast cancer cell (Hs578t) | [78,79,80] |
| Indole-3-carbinol | Cruciferous vegetables such as Kale (Brassica oleracea L. var. acephala), cauliflower (B. oleracea var botrytis L.), cabbage (B. oleracea L. var capitata), and broccoli (B. oleracea var. italica) | At 200 µM, indole-3-carbinol displayed a 2-fold and 10-fold increase in PTEN protein levels of G361 and SKMEL30 melanoma cells, respectively | [28] |
| Thymoquinone | Nigella sativa L. (black cumin) | At 50 µM, thymoquinone upregulated PTEN mRNA levels in doxorubicin resistant breast cancer (MCF-7/DOX) by 7.9-fold after 24 h | [75] |
| Phytochemical | Source | Limitation/s | Nanocarrier Formulation | Mechanism | Reference |
|---|---|---|---|---|---|
| Paclitaxel | Taxus brevifolia Nutt (Pacific Yew) and Taxus baccata L. (English Yew) | Low water solubility | Lipid-based nanocarrier | The encapsulation of paclitaxel in lipid-based nanocarriers enhanced solubility and efficacy of paclitaxel against various cancers such as ovarian, metastatic breast, and non-small cell lung cancer | [88] |
| Thymoquinone | Nigella sativa L. (Black cumin) | Poor biological stability, short half-life, hydrophobicity, and low bioavailability | Liposomes | The encapsulation of thymoquinone in liposomes enhanced biological stability, increased the half-life, decreased hydrophobicity and enhanced bioavailability. Thymoquinone liposomes (TQ-LP) displayed an ED50 of 350 µM on periodontal ligament fibroblasts (normal cells) whereas free thymoquinone displayed an ED50 of 85 µM, thus, TQ-LP displayed a lower antiproliferative effect on normal cells, which is favorable | [90] |
| Nanocarrier | Drugs | Name | Indications | Side Effects Compared to Free Drug | FDA Approval Date | References |
|---|---|---|---|---|---|---|
| Nanoparticle | Albumin-Paclitaxel (nab-paclitaxel) | Abraxane | Metastatic breast cancer | The absence of cremophor in the paclitaxel formulation results in decreased neutropenia and rapid improvement of peripheral neutropathy with albumin-paclitaxel. | 2005 | [91,92,93] |
| Pegylated liposome | Doxorubicin | Doxil | Ovarian, metastatic breast cancer, Kaposi sarcoma | Doxil also displayed increased cardiac safety, less nausea, vomiting and neutropenia but the main dose limiting side effect of Doxil is hand-foot syndrome, which leads to tenderness and peeling of the skin. This side effect limits the dose that can be given compared with doxorubicin. | 1995 | [91,94,95] |
| Liposome | Doxorubicin | Myocet | Breast cancer | Myocet displayed improved cardiac safety, less nausea, vomiting and neutropenia. Furthermore, Myocet does not cause hand-foot syndrome and may be used at the same dosing as doxorubicin in treatment regimens, enhancing efficacy. | 2000 | [91,95,96] |
| Liposome | Vincristine | Marqibo | Philadelphia chromosome-negative lymphoblastic leukemia | The main adverse effect of vincristine is neurotoxicity, which could be detected at lower doses of administration of vincristine resulting in the capping of the dose for vincristine to 1.4–2 mg/m2. The dose for Marqibo was not capped and more vincristine could be delivered with a similar toxicity profile to vincristine at low doses. | 2012 | [91,97,98] |
| Liposome | Cytarabine | Depocyt | Lymphomatous malignant meningitis | Arachnoiditis, neurotoxicity, cardiotoxicity, fever, cerebellar toxicity, corneal toxicity, hepato-renal insufficiency, necrotizing enterocolitis, pancreatitis, acute respiratory distress, and dermatological side effects. | 1999 | [99,100] |
| Liposome | Irinotecan | Onivyde | Pancreatic cancer | Diarrhea, nausea, vomiting, neutropenia, and febrile neutropenia | 2015 | [100,101] |
| Pegylated conjugate | L-Asparaginase | Oncaspar | Acute lymphoblastic leukemia | Venous thromboembolism, pancreatitis, and hyperglycemia | 2006 | [100,102] |
| Recombinant DNA derived cytotoxic protein | Denileukin diftitox | Ontak | Cutaneous T cell lymphoma | Acute hypersensitivity reactions, asthenia. Nausea, vomiting and dehydration | 1999 | [100,103] |
| Polymeric nanoparticles | Leuprolide acetate | Eligard | Advanced prostate cancer | Hot flushes, fatigue testicular atrophy, dizziness, gynecomastia, and nausea | 2002 | [100,104,105] |
| Trastuzumab covalently linked to DM1 via the stable thioether linker MCC | DM1 | Kadcyla | HER2+ breast cancer | Nausea, fatigue, thrombocytopenia, headache, constipation, diarrhea, epistaxis | 2013 | [100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maphutha, J.; Twilley, D.; Lall, N. The Role of the PTEN Tumor Suppressor Gene and Its Anti-Angiogenic Activity in Melanoma and Other Cancers. Molecules 2024, 29, 721. https://doi.org/10.3390/molecules29030721
Maphutha J, Twilley D, Lall N. The Role of the PTEN Tumor Suppressor Gene and Its Anti-Angiogenic Activity in Melanoma and Other Cancers. Molecules. 2024; 29(3):721. https://doi.org/10.3390/molecules29030721
Chicago/Turabian StyleMaphutha, Jacqueline, Danielle Twilley, and Namrita Lall. 2024. "The Role of the PTEN Tumor Suppressor Gene and Its Anti-Angiogenic Activity in Melanoma and Other Cancers" Molecules 29, no. 3: 721. https://doi.org/10.3390/molecules29030721
APA StyleMaphutha, J., Twilley, D., & Lall, N. (2024). The Role of the PTEN Tumor Suppressor Gene and Its Anti-Angiogenic Activity in Melanoma and Other Cancers. Molecules, 29(3), 721. https://doi.org/10.3390/molecules29030721