Silicas with Covalently Anchored Fluorosolvatochromic Dyes Suitable for Naked-Eye Detection of Colourless Compounds
Abstract
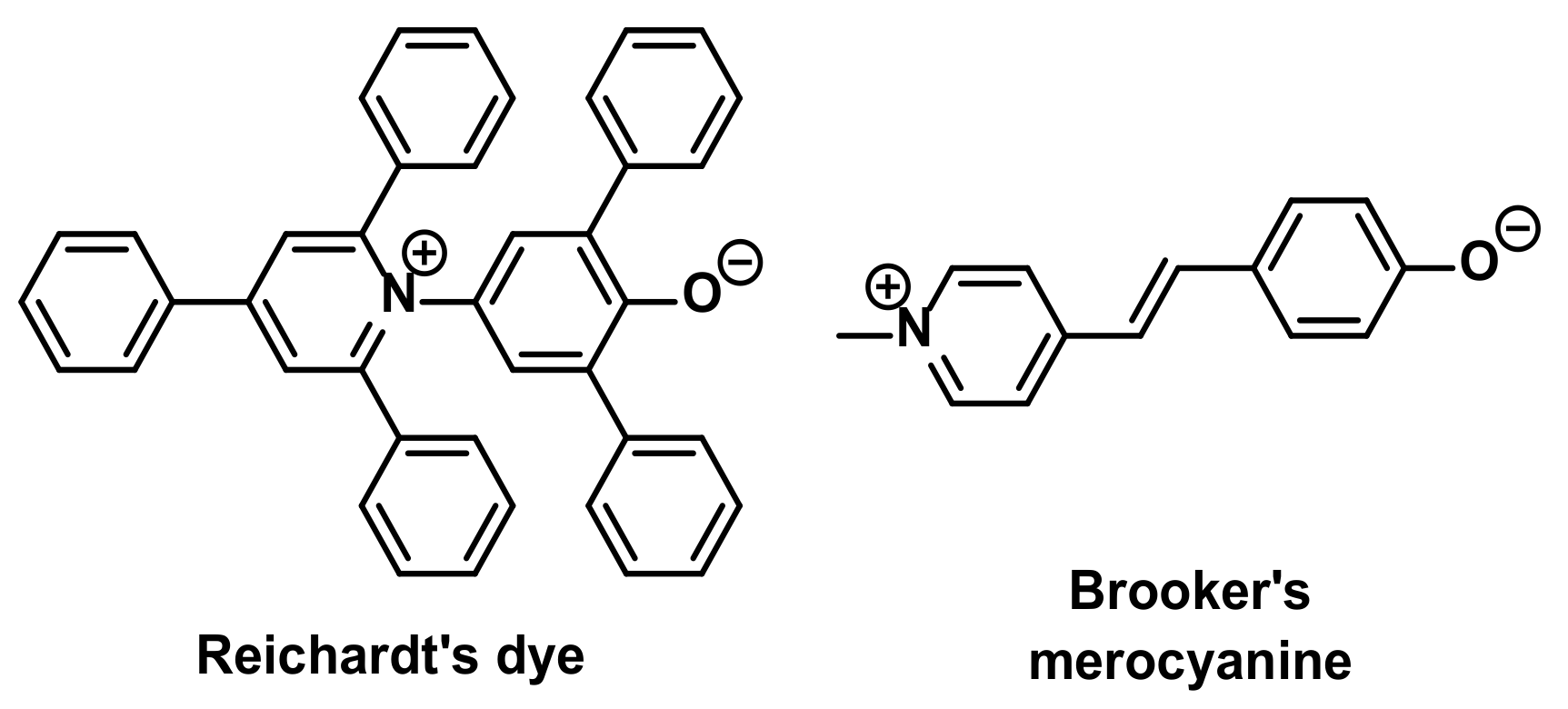
1. Introduction
2. Results and Discussion
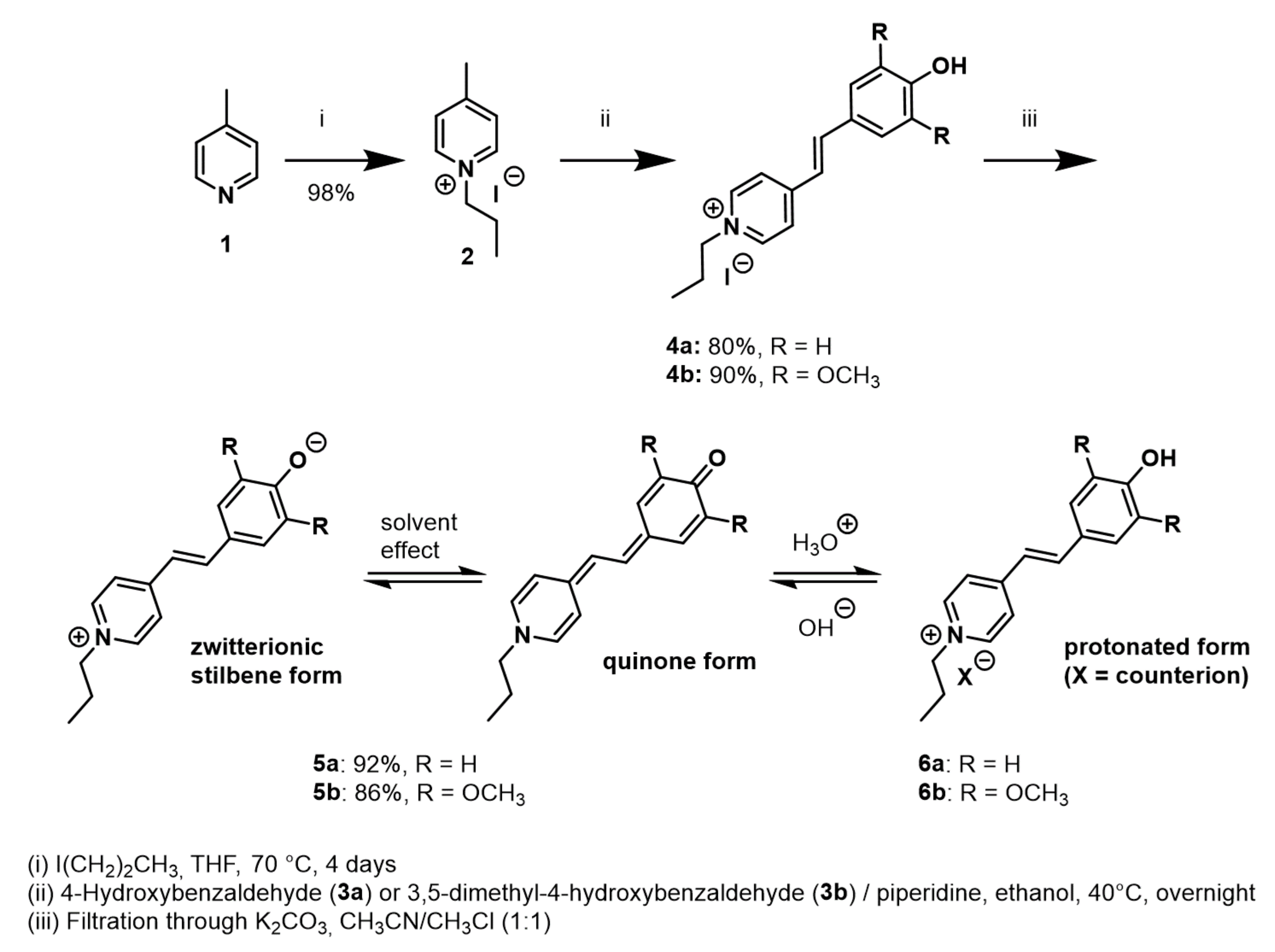
2.1. Preparation of Model Stilbazolium Dyes 5
2.2. Fluorosolvatochromic Behaviour of Stilbazolium Dyes 5 in Solution
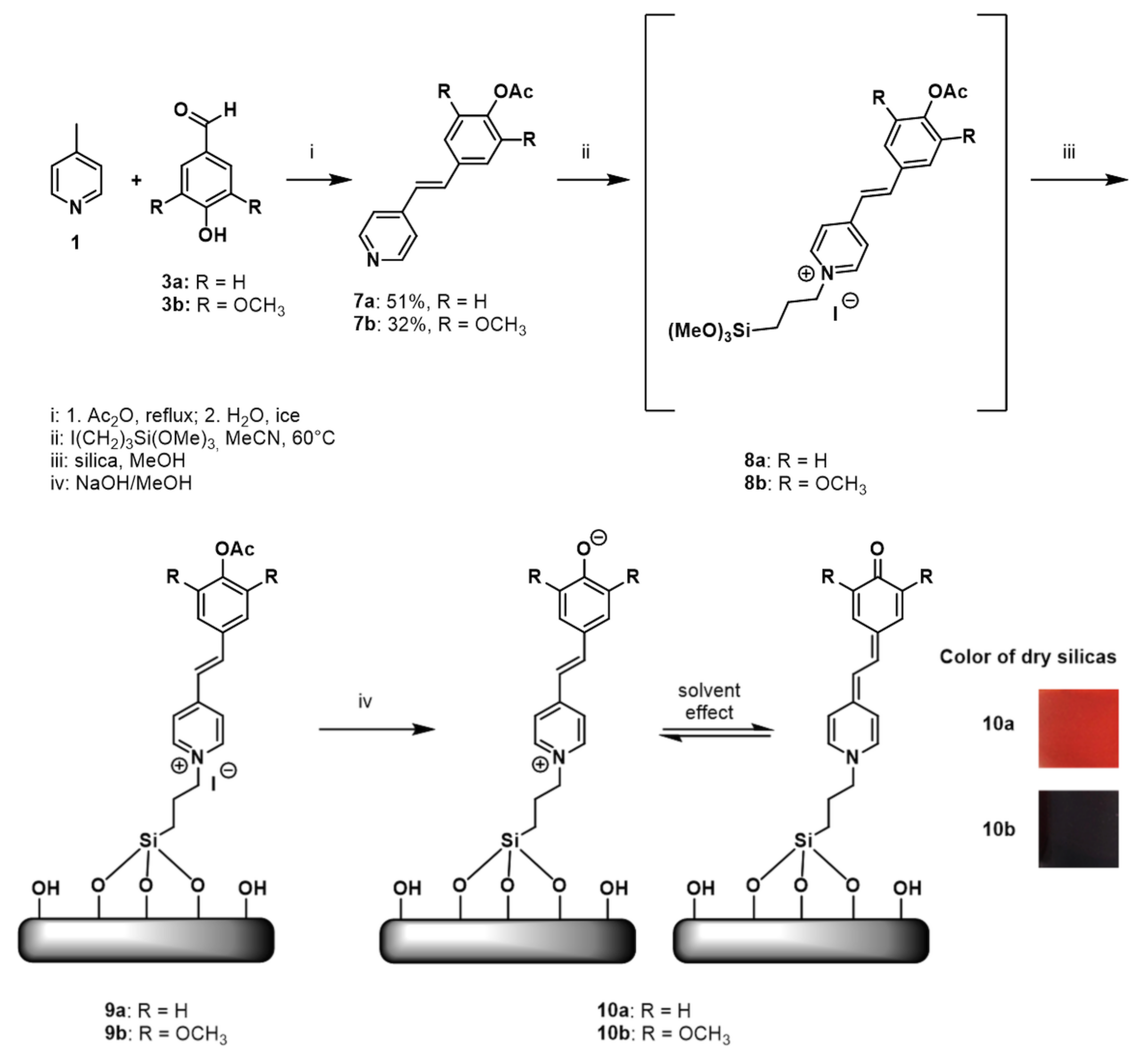
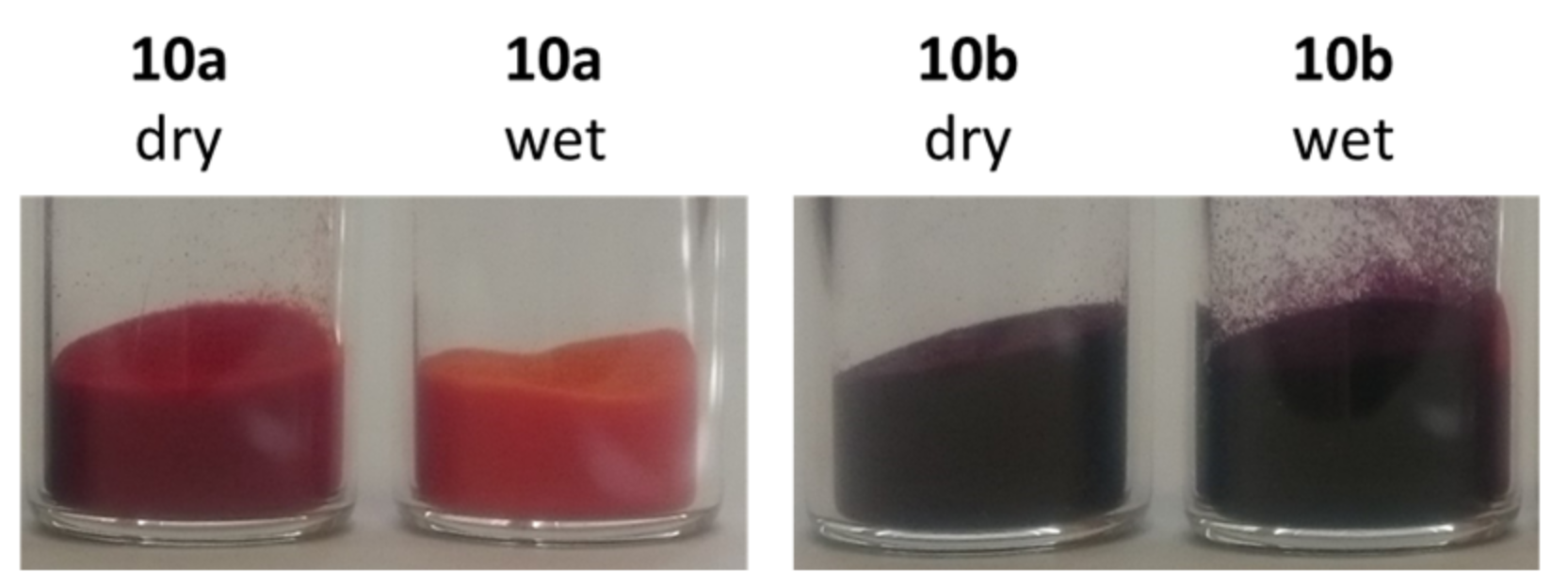
2.3. Preparation of Fluorosolvatochromic Silicas 10
2.4. Fluorosolvatochromic Properties of the Prepared Silicas
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Nuclear Magnetic Resonance
3.3. High-Resolution Mass Spectrometry
3.4. Elemental Analysis
3.5. UV–Vis Spectroscopy
3.6. Fluorescence Spectroscopy
3.7. Optical Images
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marini, A.; Muñoz-Losa, A.; Biancardi, A.; Mennucci, B. What is Solvatochromism? J. Phys. Chem. B 2010, 114, 17128–17135. [Google Scholar] [CrossRef]
- Reichardt, C. Solvatochromic Dyes as Solvent Polarity Indicators. Chem. Rev. 1994, 94, 2319–2358. [Google Scholar] [CrossRef]
- Budag, R.; Giusti, L.A.; Machado, V.G.; Machado, C. Quality analysis of automotive fuel using solvatochromic probes. Fuel 2006, 85, 1494–1497. [Google Scholar] [CrossRef]
- Gotor, R.; Bell, J.; Rurack, K. Tailored fluorescent solvatochromic test strips for quantitative on-site detection of gasoline fuel adulteration. J. Mater. Chem. C 2019, 7, 2250–2256. [Google Scholar] [CrossRef]
- Schäfer, K.; Ihmels, H. Ratiometric Detection of Water in Acetonitrile with 9-Hydroxybenzo[b]Quinolizinium as Fluorosolvatochromic Probe. J. Fluoresc. 2017, 27, 1221–1224. [Google Scholar] [CrossRef] [PubMed]
- Gaines, G.L. Solvatochromic compound as an acid indicator in nonaqueous media. Anal. Chem. 1976, 48, 450–451. [Google Scholar] [CrossRef]
- Mitewa, M.; Mateeva, N.; Antonov, L.; Deligeorgiev, T. Spectrophotometric Investigation of the Complex Formation of Aza-15-Crown-5 Containing Styryl Dyes with Ba2+ and Ca2+ Cations. Dyes Pigm. 1995, 27, 219–225. [Google Scholar] [CrossRef]
- Malins, C.; Doyle, A.; MacCraith, B.D.; Kvasnik, F.; Landl, M.; Simon, P.; Kalvoda, L.; Lukas, R.; Pufler, K.; Babusik, I. Personal ammonia sensor for industrial environments. J. Environ. Monit. 1999, 1, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Citterio, D.; Omagari, M.; Kawada, T.; Sasaki, S.; Suzuki, Y.; Suzuki, K. Chromogenic betaine lariates for highly selective calcium ion sensing in aqueous environment. Anal. Chim. Acta 2004, 504, 227–234. [Google Scholar] [CrossRef]
- Stock, R.I.; Dreyer, J.P.; Nunes, G.E.; Bechtold, I.H.; Machado, V.G. Optical Chemosensors and Chemodosimeters for Anion Detection Based on Merrifield Resin Functionalized with Brooker’s Merocyanine Derivatives. ACS Appl. Polym. Mater. 2019, 1, 1757–1768. [Google Scholar] [CrossRef]
- Hubert, C.; Fichou, D.; Valat, P. A solvatochromic dye doped polymer for detection of polar additives in hydrocarbon blends. Polymer 1995, 36, 2663–2666. [Google Scholar] [CrossRef]
- Koopmans, C.; Ritter, H. Color Change of N-Isopropylacrylamide Copolymer Bearing Reichardts Dye as Optical Sensor for Lower Critical Solution Temperature and for Host–Guest Interaction with β-Cyclodextrin. J. Am. Chem. Soc. 2007, 129, 3502–3503. [Google Scholar] [CrossRef]
- Lee, J.; Chang, H.T.; An, H.; Ahn, S.; Shim, J.; Kim, J.-M. A protective layer approach to solvatochromic sensor. Nat. Commun. 2013, 4, 2461. [Google Scholar] [CrossRef]
- Nandi, L.G.; Nicoleti, C.R.; Bellettini, I.C.; Machado, V.G. Optical Chemosensor for the Detection of Cyanide in Water Based On Ethyl(hydroxyethyl)cellulose Functionalized with Brooker’s Merocyanine. Anal. Chem. 2014, 86, 4653–4656. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Pyo, M.; Lee, S.-H.; Kim, J.; Ra, M.; Kim, W.-Y.; Park, B.J.; Lee, C.W.; Kim, J.-M. Hydrochromic conjugated polymers for human sweat pore mapping. Nat. Commun. 2014, 5, 3736. [Google Scholar] [CrossRef]
- Nordhaus, M.A.; Krongauz, V.V.; Hai, T.T. Synthesis of solvatochromic merocyanine dyes and their immobilization to polymers. J. Appl. Polym. Sci. 2017, 134, 1–14. [Google Scholar] [CrossRef]
- Pasch, P.; Papadopoulos, J.; Goralczyk, A.; Hofer, M.L.; Tabatabai, M.; Müller, T.J.J.; Hartmann, L. Highly Fluorescent Merocyanine and Cyanine PMMA Copolymers. Macromol. Rapid Commun. 2018, 39, 1800277. [Google Scholar] [CrossRef]
- Crowther, D.; Liu, X.M. Covalent lmmobilisation of Solvatochromic Dyes. J. Chem. Soc. Chem. Commun. 1995, 23, 2445. [Google Scholar] [CrossRef]
- Fiorilli, S.; Onida, B.; Barolo, C.; Viscardi, G.; Brunel, D.; Garrone, E. Tethering of Modified Reichardt’s Dye on SBA-15 Mesoporous Silica: The Effect of the Linker Flexibility. Langmuir 2007, 23, 2261–2268. [Google Scholar] [CrossRef]
- Descalzo, A.B.; Marcos, M.D.; Monte, C.; Martínez-Máñez, R.; Rurack, K. Mesoporous silica materials with covalently anchored phenoxazinone dyes as fluorescent hybrid materials for vapour sensing. J. Mater. Chem. 2007, 17, 4716–4723. [Google Scholar] [CrossRef]
- Wintzheimer, S.; Oppmann, M.; Dold, M.; Pannek, C.; Bauersfeld, M.-L.; Henfling, M.; Trupp, S.; Schug, B.; Mandel, K. Indicator Supraparticles for Smart Gasochromic Sensor Surfaces Reacting Ultrafast and Highly Sensitive. Part. Part. Syst. Charact. 2019, 36, 1900254. [Google Scholar] [CrossRef]
- Gvishi, R.; Narang, U.; Bright, F.V.; Prasad, P.N. Probing the Microenvironment of Polymer-Impregnated Composite Glass Using Solvatochromic Dye. Chem. Mater. 1995, 7, 1703–1708. [Google Scholar] [CrossRef]
- Imai, Y.; Chujo, Y. Solvatochromic Characterization of Organic–Inorganic Polymer Hybrids with Pyridinium N-Phenolate Betaine Dyes. Macromolecules 2000, 33, 3059–3064. [Google Scholar] [CrossRef]
- Lu, Z.-Z.; Zhang, R.; Li, Y.-Z.; Guo, Z.-J.; Zheng, H.-G. Solvatochromic behavior of a nanotubular metal organic framework for sensing small molecules. J. Am. Chem. Soc. 2011, 133, 4172–4174. [Google Scholar] [CrossRef] [PubMed]
- Ascherl, L.; Evans, E.W.; Hennemann, M.; Nuzzo, D.D.; Hufnagel, A.G.; Beetz, M.; Friend, R.H.; Clark, T.; Bein, T.; Auras, F. Solvatochromic covalent organic frameworks. Nat. Commun. 2018, 9, 3802. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Z.; Zhao, Y.; Zhou, Y.; Xue, B.; Han, Y.; Wang, Y.; Mu, X.; Zang, S.; Zhou, X.; et al. A water-soluble two-dimensional supramolecular organic framework with aggregation-induced emission for DNA affinity and live-cell imaging. J. Mater. Chem. B 2019, 7, 1435–1441. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Yin, X.; Ding, B.; Sun, G.; Ke, T.; Chen, J.; Yu, J. Colorimetric strips for visual lead ion recognition utilizing polydiacetylene embedded nanofibers. J. Mater. Chem. A 2014, 2, 18304–18312. [Google Scholar] [CrossRef]
- Nakabayashi, K.; Takahashi, T.; Sugawara, R.; Lo, C.-T.; Mori, H. Benzothiadiazole-based donor–acceptor nanoparticles with solvatochromic and thermoresponsive properties. React. Funct. Polym. 2018, 131, 350–360. [Google Scholar] [CrossRef]
- Arshad, F.; Pal, A.; Rahman, M.A.; Ali, M.; Khan, J.A.; Sk, M.P. Insights on the solvatochromic effects in N-doped yellow orange emissive carbon dots. New J. Chem. 2018, 42, 19837–19843. [Google Scholar] [CrossRef]
- Pramanik, A.; Biswas, S.; Kumbhakar, P. Solvatochromism in highly luminescent environmental friendly carbon quantum dots for sensing applications: Conversion of bio-waste into bio-asset. Spectrochim. Acta Part A 2018, 191, 498–512. [Google Scholar] [CrossRef]
- Baranowska-Korczyc, A.; Stelmach, E.; Paterczyk, B.; Maksymiuk, K.; Michalska, A. Ultrasmall self-assembly poly(N-isopropylacrylamide-butyl acrylate) (polyNIPAM-BA) thermoresponsive nanoparticles. J. Colloid Interface Sci. 2019, 542, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Nayl, A.A.; Abd-Elhamid, A.I.; Aly, A.A.; Bräse, S. Recent progress in the applications of silica-based nanoparticles. RSC Adv. 2022, 12, 13706–13726. [Google Scholar] [CrossRef] [PubMed]
- Marcus, Y. Preferential solvation. Part 3.—Binary solvent mixtures. J. Chem. Soc. Faraday Trans. 1989, 85, 381–388. [Google Scholar] [CrossRef]
- Li, X.C.; King, T.A.; Pallikari-Viras, F. Characteristics of composites based on PMMA modified gel silica glasses. J. Non-Cryst. Solids 1994, 170, 243–249. [Google Scholar] [CrossRef]
- Onida, B.; Fiorilli, S.; Borello, L.; Viscardi, G.; Macquarrie, D.; Garrone, E. Mechanism of the Optical Response of Mesoporous Silica Impregnated with Reichardt’s Dye to NH3 and Other Gases. J. Phys. Chem. B 2004, 108, 16617–16620. [Google Scholar] [CrossRef]
- Szkop, M.; Kliszcz, B.; Kasprzak, A.A. A simple and reproducible protocol of glass surface silanization for TIRF microscopy imaging. Anal. Biochem. 2018, 549, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, C. Über Pyridinium-N-phenol-betaine und ihre Verwendung zur Charakterisierung der Polarität von Lösungsmitteln, VI1) Erweiterung der Lösungsmittelpolaritätsskala durch Bestimmung neuer molarer Übergangsenergien (ET-Werte). Justus Liebigs Ann. Chem. 1971, 752, 64–67. [Google Scholar] [CrossRef]
- Morley, J.O.; Morley, R.M.; Docherty, R.; Charlton, M.H. Fundamental Studies on Brooker’s Merocyanine. J. Am. Chem. Soc. 1997, 119, 10192–10202. [Google Scholar] [CrossRef]
- Brooker-1951 Brooker, L.G.S.; Keyes, G.H.; Heseltine, D.W. Color and Constitution. XI.1 Anhydronium Bases of p-Hydroxystyryl Dyes as Solvent Polarity Indicators. J. Am. Chem. Soc. 1951, 73, 5350–5356. [Google Scholar] [CrossRef]
- Garcia-Guzman, C.; Fernandez, A.; Avlonitis, N.; Bradley, M.; Vendrell, M. Red-Fluorescent Activatable Probes for the Detection of Hydrogen Peroxide in Living Cells. Comb. Chem. High Throughput Screen. 2016, 19, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Dutkiewicz, M. Classification of Organic Solvents based on Correlation between Dielectric β Parameter and Empirical Solvent Polarity Parameter . J. Chem. Soc. Faraday Trans. 1990, 86, 2237–2241. [Google Scholar] [CrossRef]
- Reichardt, C.; Harbusch-Görnert, E. Über Pyridinium-N-phenolat-Betaine und ihre Verwendung zur Charakterisierung der Polarität von Lösungsmitteln, X. Erweiterung, Korrektur und Neudefinition der ET Lösungsmittelpolaritätsskala mit Hilfe eines lipophilen penta-tert-butyl-substituierten Pyridinium N-phenolat-Betainfarbstoffes. Liebigs Ann. Chem. 1983, 5, 721–896. [Google Scholar] [CrossRef]
- Lippert, E.; Lüder, W.; Moll, F.; Nägele, W.; Boos, H.; Prigge, H.; Seibold-Blankenstein, I. Umwandlung von Elektronenanregungsenergie. Angew. Chem. 1961, 73, 695–706. [Google Scholar] [CrossRef]
- Dennis, K.J.; Luong, T.; Reshwan, M.L.; Minch, M.J. Isomerizable N-alkylmerocyanine dyes as probes of micellar solubilization sites. J. Phys. Chem. 1993, 97, 8328–8335. [Google Scholar] [CrossRef]
- Kumar, U.; Kato, T.; Frechet, J.M.J. Use of intermolecular hydrogen bonding for the induction of liquid crystallinity in the side chain of polysiloxanes. J. Am. Chem. Soc. 1992, 114, 6630–6639. [Google Scholar] [CrossRef]

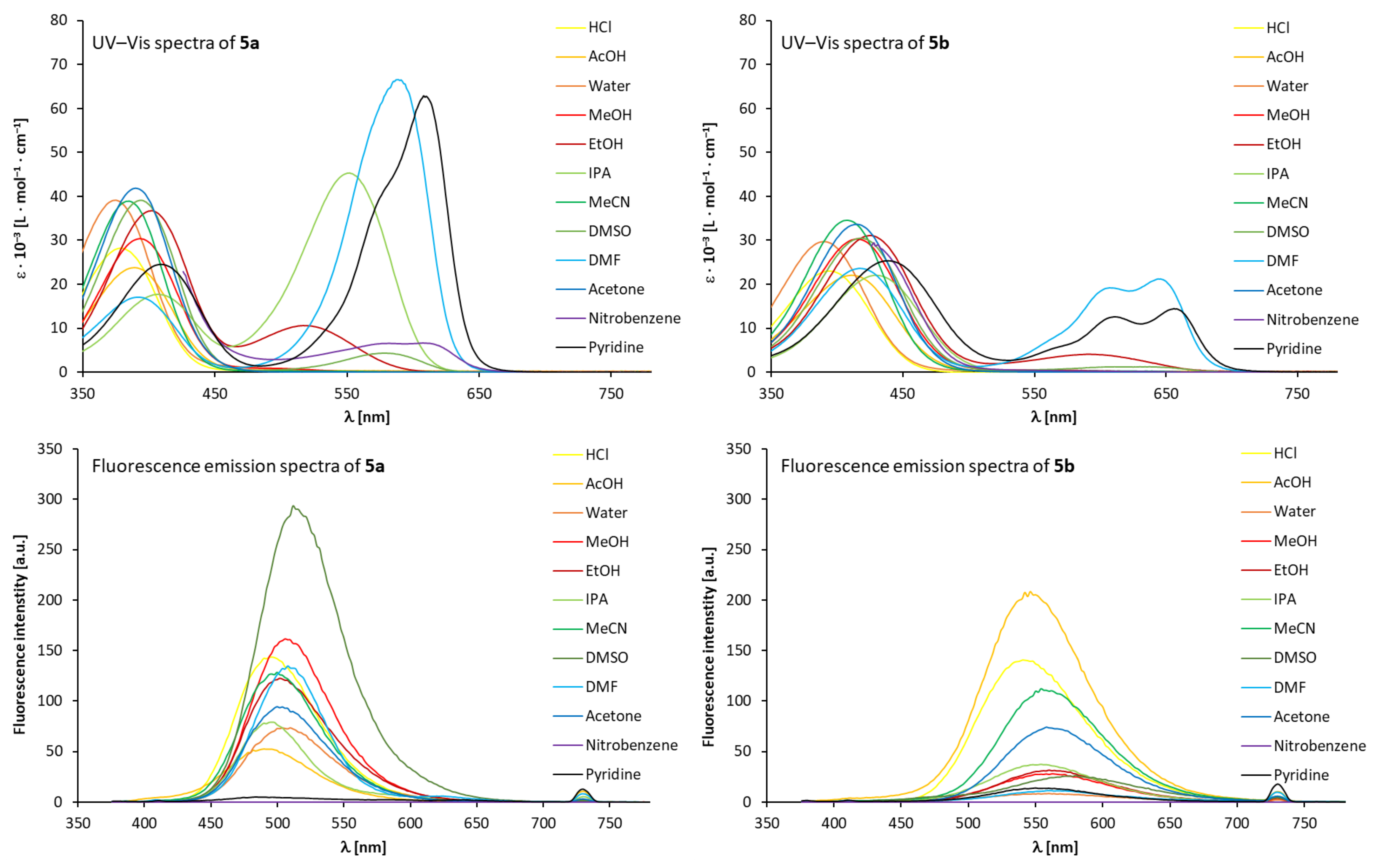


| Stilbazolium Dye 5a | Stilbazolium Dye 5b | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Solvent a | λmax,1 [nm] | λmax,2 [nm] | λmax,3 [nm] | λem [nm] | Stokes shift [nm] | λmax,1 [nm] | λmax,2 [nm] | λmax,3 [nm] | λem [nm] | Stokes Shift [nm] |
| Hydrochloric acid | 378 | - | - | 496 | 130 | 391 | - | - | 540 | 174 |
| Acetic acid | 387 | - | - | 490 | 124 | 410 | - | - | 546 | 180 |
| Water | 375 | - | - | 510 | 144 | 390 | - | - | 560 | 194 |
| Methanol | 393 | - | - | 506 | 140 | 417 | - | - | 562 | 196 |
| Ethanol | 402 | 516 | - | 502 | 136 | 426 | 592 | - | 560 | 194 |
| Isopropyl alcohol | 406 | 552 | - | 496 | 130 | 430 | - | - | 554 | 188 |
| Acetonitrile | 385 | - | - | 500 | 134 | 408 | - | - | 554 | 188 |
| Dimethyl sulfoxide | 395 | - | 581 | 512 | 146 | 416 | - | - | 580 | 214 |
| N,N-Dimethylformamide | 391 | - | 591 | 508 | 142 | 419 | 607 | 644 | 562 | 196 |
| Acetone | 390 | - | - | 500 | 134 | 415 | - | - | 558 | 192 |
| Nitrobenzene | - b | - | 607 | - | - | - b | - | - | - | - |
| Pyridine | 499 | 577 | 607 | - | - | 439 | 610 | 656 | 550 | 184 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navrátilová, T.; Havlík, M.; Tatar, A.; Hricková, K.; Dolenský, B. Silicas with Covalently Anchored Fluorosolvatochromic Dyes Suitable for Naked-Eye Detection of Colourless Compounds. Molecules 2024, 29, 673. https://doi.org/10.3390/molecules29030673
Navrátilová T, Havlík M, Tatar A, Hricková K, Dolenský B. Silicas with Covalently Anchored Fluorosolvatochromic Dyes Suitable for Naked-Eye Detection of Colourless Compounds. Molecules. 2024; 29(3):673. https://doi.org/10.3390/molecules29030673
Chicago/Turabian StyleNavrátilová, Tereza, Martin Havlík, Ameneh Tatar, Karolína Hricková, and Bohumil Dolenský. 2024. "Silicas with Covalently Anchored Fluorosolvatochromic Dyes Suitable for Naked-Eye Detection of Colourless Compounds" Molecules 29, no. 3: 673. https://doi.org/10.3390/molecules29030673
APA StyleNavrátilová, T., Havlík, M., Tatar, A., Hricková, K., & Dolenský, B. (2024). Silicas with Covalently Anchored Fluorosolvatochromic Dyes Suitable for Naked-Eye Detection of Colourless Compounds. Molecules, 29(3), 673. https://doi.org/10.3390/molecules29030673






