Highly Promiscuous Flavonoid Di-O-glycosyltransferases from Carthamus tinctorius L.
Abstract
1. Introduction
2. Results
2.1. Screening of Safflower Flavonoid O-Glycosyltransferases
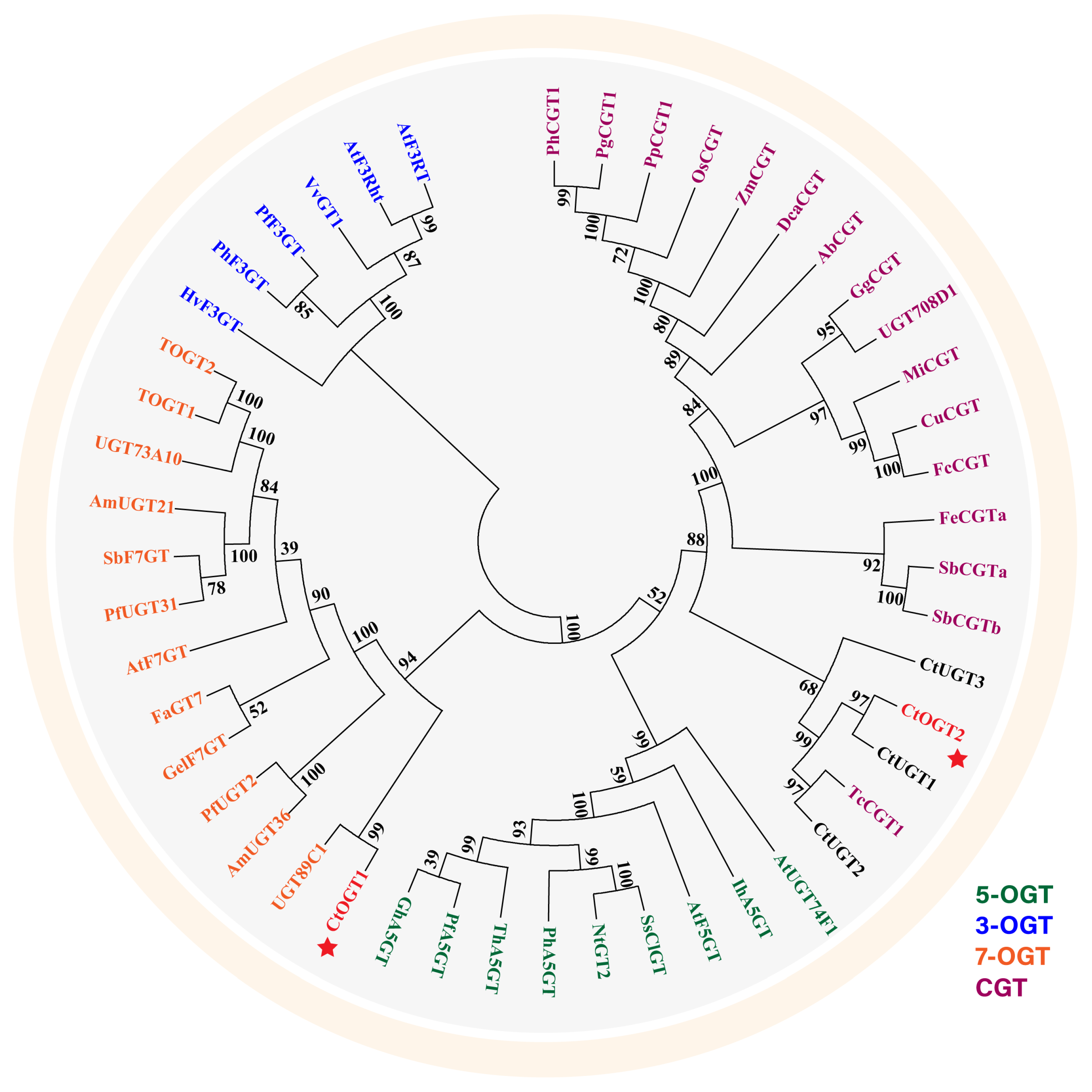
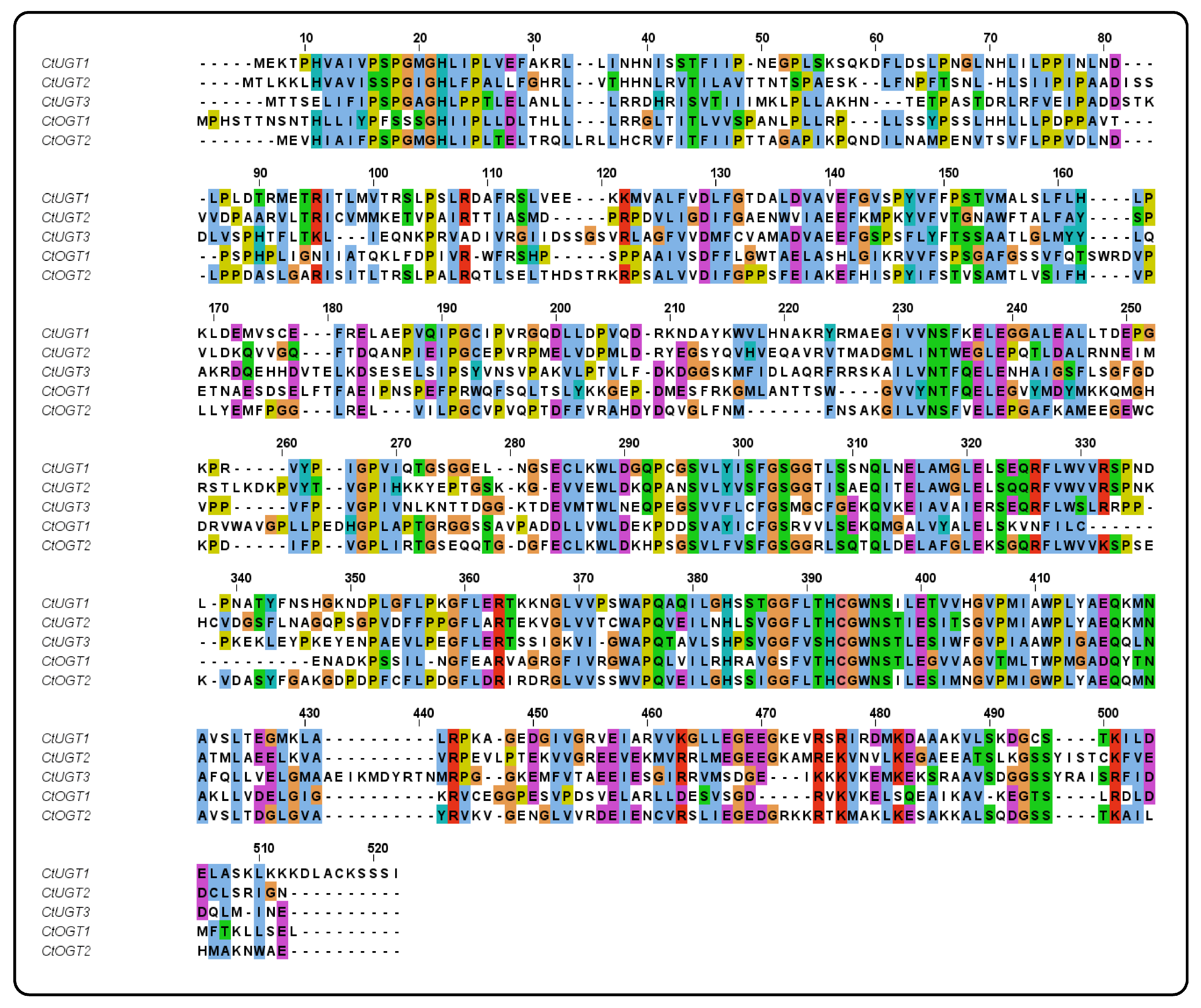
2.2. Phylogenetic and Multiple Sequence Alignment Analysis of CtOGTs
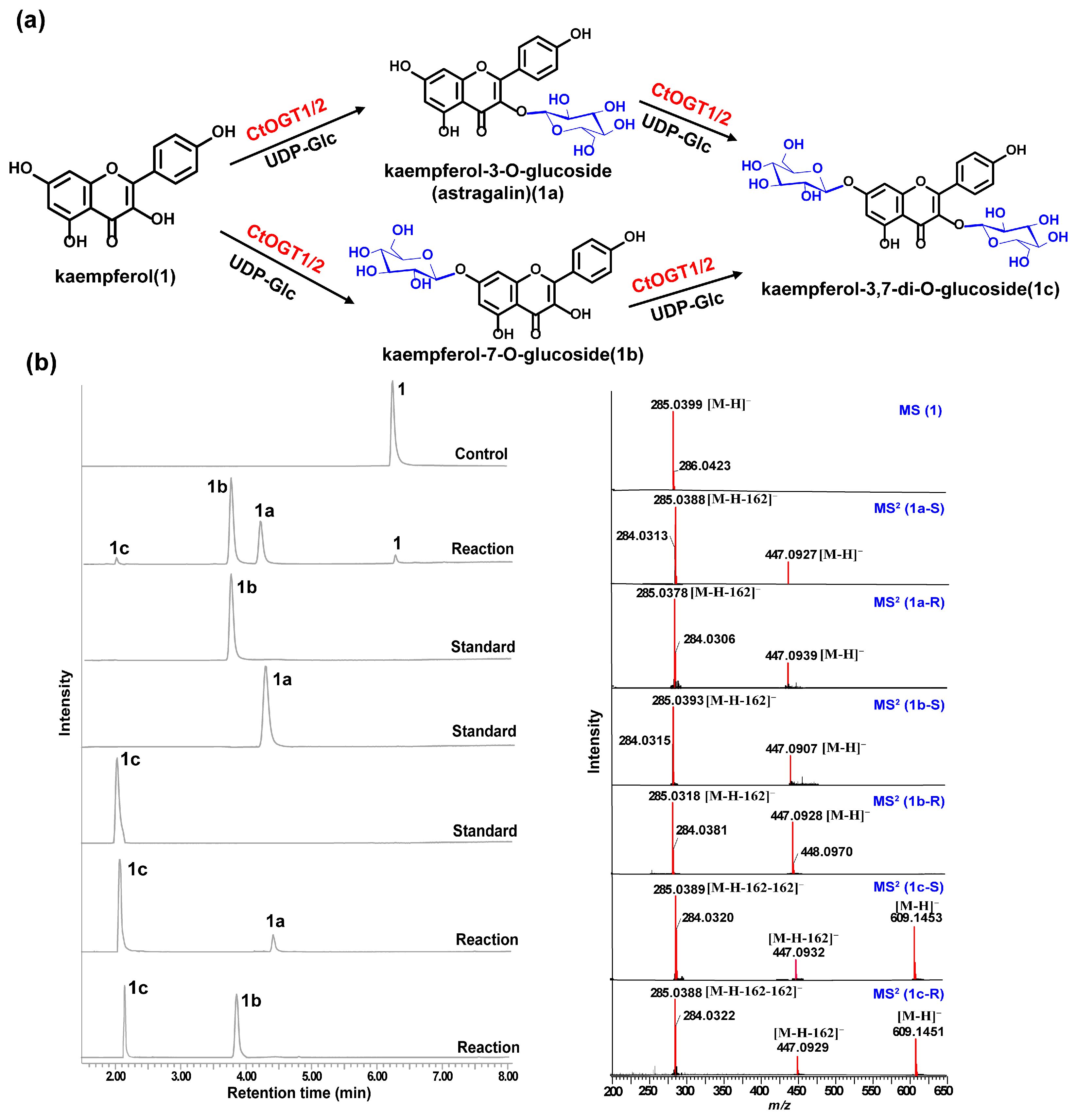
2.3. Cloning and Functional Characterization of CtOGTs
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Phylogenetic Analysis and Sequence Alignment
4.3. Molecular Cloning
4.4. Expression and Purification of CtOGT1 and CtOGT2
4.5. Enzyme Activity Assay
4.6. General Procedures
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, J.S.; Ko, H.C.; Hur, O.S.; Sang, G.K.; Kyoung, Y.R. Morphological and oil compositions in safflower (Carthamus tinctorius L.) germplasm of different geographical groups. Korean J. Agric. Sci. 2016, 28, 84–91. [Google Scholar] [CrossRef]
- Delshad, E.; Yousefi, M.; Sasannezhad, P.; Rakhshandeh, H.; Ayati, Z. Medical uses of Carthamus tinctorius L. (Safflower): A comprehensive review from Traditional Medicine to Modern Medicine. Electron. Physician 2018, 4, 6672–6681. [Google Scholar] [CrossRef] [PubMed]
- Ao, H.; Feng, W.; Peng, C. Hydroxysafflor Yellow A: A promising therapeutic agent for a broad spectrum of diseases. Evid.-Based Complement. Altern. Med. 2018, 2018, 8259280. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Xue, Y.; Guo, D.; Sun, L.; Guo, M. Carthami Flos: A review of its ethnopharmacology, pharmacology and clinical applications. Rev. Bras. 2015, 25, 553–566. [Google Scholar] [CrossRef]
- Wong, G.; He, S.; Siragam, V.; Bi, Y.; Mbikay, M.; Chretien, M.; Qiu, X. Antiviral activity of quercetin-3-β-O-D-glucoside against Zika virus infection. Virol. Sin. 2017, 32, 545–547. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, J.; Kim, S.J.; Kim, J.H. Quercetin-3-O-glucoside suppresses pancreatic cancer cell migration induced by tumor-deteriorated growth factors in vitro. Oncol. Rep. 2016, 35, 2473–2479. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, A. Mechanisms of antidiabetic effects of flavonoid rutin. Biomed. Pharmacother. 2017, 96, 305–312. [Google Scholar] [CrossRef]
- Elizabeth, T.D.; Diego, M.E.; Oswaldo, O.M.; Liliana, S.C. Rutin: Family farming products’ extraction sources, industrial applications and current trends in biological activity protection. Molecules 2023, 28, 5864. [Google Scholar]
- Caparica, R.; Júlio, A.; Araújo, M.E.M.; Baby, A.R.; Fonte, P.; Costa, J.G.; Santos, D.A.T. Anticancer activity of rutin and its combination with ionic liquids on renal cells. Biomolecules 2020, 10, 233. [Google Scholar] [CrossRef]
- Nishina, A.; Sato, D.; Yamamoto, J.; Kobayashi-Hattori, K.; Hirai, Y.; Kimura, H. Antidiabetic-like effects of naringenin-7-O-glucoside from edible chrysanthemum ‘Kotobuki’ and naringenin by activation of the PI3K/Akt pathway and PPARγ. Chem. Biodivers. 2019, 16, e1800434. [Google Scholar] [CrossRef]
- Penso, J.; Cordeiro, K.C.; da Cunha, C.R.; da Silva Castro, P.F.; Martins, D.R.; Lião, L.M.; Rocha, M.L.; de Oliveira, V. Vasorelaxant activity of 7-β-O-glycosides biosynthesized from flavonoids. Eur. J. Pharmacol. 2014, 733, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Han, X.Z.; Gao, S.; Cheng, Y.N.; Sun, Y.Z.; Liu, W.; Tang, L.L.; Ren, D.M. Protective effect of naringenin-7-O-glucoside against oxidative stress induced by doxorubicin in H9c2 cardiomyocytes. Biosci. Trends 2012, 6, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.S.; Seo, Y. Antiadipogenic activity of isohamnetin 3-O-β-D-glucopyranoside from Salicornia herbacea. Immunopharmacol. Immunotoxicol. 2012, 34, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Devi, V.G.; Rooban, B.N.; Sasikala, V.; Sahasranamam, V.; Abraham, A. Isorhamnetin-3-glucoside alleviates oxidative stress and opacification in selenite cataract in vitro. Toxicol. In Vitro 2010, 24, 1662–1669. [Google Scholar] [CrossRef] [PubMed]
- Zaki, A.A.; Xu, X.; Wang, Y.; Shie, P.H.; Qiu, L. A new anti-inflammatory flavonoid glycoside from tetraena aegyptia. Nat. Prod. Res. 2021, 35, 1985–1990. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Islam, M.S.; Uddin, M.N.; Hasan, M.M.I.; Akanda, M.R. The potential health benefits of the isoflavone glycoside genistin. Arch. Pharm. Res. 2020, 43, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Yue, S.; Tang, Y.; Li, S.; Duan, J.A. Chemical and biological properties of quinochalcone C-glycosides from the florets of Carthamus tinctorius. Molecules 2013, 18, 15220–15254. [Google Scholar] [CrossRef]
- Xian, B.; Wang, R.; Jiang, H.; Zhou, Y.; Yan, J.; Huang, X.; Chen, J.; Wu, Q.; Chen, C.; Xi, Z.; et al. Comprehensive review of two groups of flavonoids in Carthamus tinctorius L. Biomed. Pharmacother. 2022, 153, 113462. [Google Scholar] [CrossRef]
- Peng, M.; Shahzad, R.; Gul, A.; Subthain, H.; Shen, S.; Lei, L.; Zheng, Z.; Zhou, J.; Lu, D.; Wang, S.; et al. Differentially evolved glucosyltransferases determine natural variation of rice flavone accumulation and UV-tolerance. Nat. Commun. 2017, 8, 1975. [Google Scholar] [CrossRef]
- Cornell, H.V.; Hawkins, B.A. Herbivore responses to plant secondary compounds: A test of phytochemical coevolution theory. Am. Nat. 2003, 161, 507–522. [Google Scholar] [CrossRef]
- Jones, P.; Vogt, T. Glycosyltransferases in secondary plant metabolism: Tranquilizers and stimulant controllers. Planta 2001, 213, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Yao, M.; Wang, Y.; Ding, M.; Zha, J.; Xiao, W.; Yuan, Y. Advances in engineering UDP-sugar supply for recombinant biosynthesis of glycosides in microbes. Biotechnol. Adv. 2020, 41, 107538. [Google Scholar] [CrossRef] [PubMed]
- Hoffmeister, D.; Dräger, G.; Ichinose, K.; Rohr, J.; Bechthold, A. The C-Glycosyltransferase UrdGT2 is unselective toward d- and l-configured nucleotide-bound rhodinoses. J. Am. Chem. Soc. 2003, 125, 4678–4679. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Xi, Z.; Xian, B.; Chen, C.; Huang, X.; Jiang, H.; Chen, J.; Peng, C.; Pei, J. Identification and characterization of CtUGT3 as the key player of astragalin biosynthesis in Carthamus tinctorius L. J. Agric. Food Chem. 2023, 71, 16221–16232. [Google Scholar] [CrossRef]
- Qi, S.; He, B.; Wang, H.; Duan, Y.; Wang, L.; Gao, Y.; Guo, M. A muti-substrate flavonol O-glucosyltransferases from safflower. Molecules 2023, 28, 7613. [Google Scholar] [CrossRef]
- Hiromoto, T.; Honjo, E.; Noda, N.; Tamada, T.; Kazuma, K.; Suzuki, M.; Blaber, M.; Kuroki, R. Structural basis for acceptor-substrate recognition of UDP-glucose: Anthocyanidin 3-O-glucosyltransferase from Clitoria ternatea. Protein Sci. 2015, 24, 395–407. [Google Scholar] [CrossRef]
- Zong, G.; Li, J.; Gao, Y.; Fei, S.; Liu, X.; Wang, X.; Shen, Y. Overexpression, purification, biochemical and structural characterization of rhamnosyltransferase UGT89C1 from Arabidopsis thaliana. Protein Expr. Purif. 2019, 156, 44–49. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 12, 2725–2729. [Google Scholar] [CrossRef]
- He, J.B.; Zhao, P.; Hu, Z.M.; Liu, S.; Kuang, Y.; Zhang, M.; Li, B.; Yun, C.H.; Qiao, X.; Ye, M. Molecular and structural characterization of a promiscuous C-glycosyltransferase from Trollius chinensis. Angew. Chem. Int. Ed. Engl. 2019, 58, 11513–11520. [Google Scholar] [CrossRef]
- Zhang, L.L.; Tian, K.; Tang, Z.H.; Chen, X.J.; Bian, Z.X.; Wang, Y.T.; Lu, J.J. Phytochemistry and pharmacology of Carthamus tinctorius L. Am. J. Chin. Med. 2016, 44, 197–226. [Google Scholar] [CrossRef]
- Xie, K.; Chen, R.; Li, J.; Wang, R.; Chen, D.; Dou, X.; Dai, J. Exploring the catalytic promiscuity of a new glycosyltransferase from Carthamus tinctorius. Org. Lett. 2014, 16, 4874–4877. [Google Scholar] [CrossRef] [PubMed]
- Kovinich, N.; Saleem, A.; Arnason, J.T.; Miki, B. Functional characterization of a UDP-glucose: Flavonoid 3-O-glucosyltransferase from the seed coat of black soybean (Glycine max (L.) Merr.). Phytochemistry 2010, 71, 1253–1263. [Google Scholar] [CrossRef]
- Sun, W.; Liang, L.; Meng, X.; Li, Y.; Gao, F.; Liu, X.; Wang, S.; Gao, X.; Wang, L. Biochemical and molecular characterization of a flavonoid 3-O-glycosyltransferase responsible for anthocyanins and flavonols biosynthesis in Freesia hybrida. Front. Plant Sci. 2016, 7, 410. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.L.; Wang, S.; Xu, Z.; Li, M.; Chen, K.; Zhang, Y.; Hu, Z.; Zhang, M.; Zhang, Z.; Qiao, X.; et al. Highly promiscuous flavonoid 3-O-glycosyltransferase from Scutellaria baicalensis. Org. Lett. 2019, 21, 2241–2245. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Shi, X.; Yang, C.; Zhao, X.; Zhuang, J.; Liu, Y.; Gao, L.; Xia, T. Two UDP-glycosyltransferases catalyze the biosynthesis of bitter flavonoid 7-O-neohesperidoside through sequential glycosylation in tea plants. J. Agric. Food Chem. 2022, 70, 2354–2365. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; He, J.; Chen, K.; Wang, Z.L.; Liu, J.; Qiao, X.; Ye, M. Molecular cloning and biochemical characterization of a new flavonoid glycosyltransferase from the aquatic plant lotus. Biochem. Biophys. Res. Commun. 2019, 510, 315–321. [Google Scholar] [CrossRef]
- Huang, J.; Li, J.; Yue, J.; Huang, Z.; Zhang, L.; Yao, W.; Guan, R.; Wu, J.; Liang, J.; Duan, L.; et al. Functional characterization of a novel glycosyltransferase (UGT73CD1) from Iris tectorum Maxim. for the substrate promiscuity. Mol. Biotechnol. 2021, 63, 1030–1039. [Google Scholar] [CrossRef]
- Gao, J.; Ma, B.; Lu, Y.; Zhang, Y.; Tong, Y.; Guo, S.; Gao, W.; Huang, L. Investigating the catalytic activity of glycosyltransferase on quercetin from Tripterygium wilfordii. ACS Omega 2020, 5, 1414–1421. [Google Scholar] [CrossRef]
- Jung, J.; Liu, H.; Borg, A.J.E.; Nidetzky, B. Solvent engineering for nonpolar substrate glycosylation catalyzed by the UDP-glucose-dependent glycosyltransferase UGT71E5: Intensification of the synthesis of 15-hydroxy cinmethylin β-d-glucoside. J. Agric. Food Chem. 2023, 71, 13419–13429. [Google Scholar] [CrossRef]
- Dorjjugder, N.; Taguchi, G. Production of flavonoid 7-O-glucosides by bioconversion using Escherichia coli expressing a 7-O-glucosyltransferase from Tobacco (Nicotiana tabacum). Appl. Biochem. Biotechnol. 2022, 194, 3320–3329. [Google Scholar] [CrossRef]
- Liu, X.; Li, L.; Zhao, G.R. Systems metabolic engineering of Escherichia coli coculture for de novo production of genistein. ACS Synth. Biol. 2022, 11, 1746–1757. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tian, C.; Xia, Y.; Mutanda, I.; Wang, K.; Wang, Y. Production of plant-specific flavones baicalein and scutellarein in an engineered E. coli from available phenylalanine and tyrosine. Metab. Eng. 2019, 52, 124–133. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, J.; Zhang, G.; Ding, W.; Duan, L.; Yang, J.; Kui, L.; Cheng, X.; Ruan, J.; Fan, W.; et al. Engineering yeast for the production of breviscapine by genomic analysis and synthetic biology approaches. Nat. Commun. 2018, 9, 448. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Liu, Y.; Li, G.; Savolainen, O.; Chen, Y.; Nielsen, J. De novo biosynthesis of bioactive isoflavonoids by engineered yeast cell factories. Nat. Commun. 2021, 12, 6085. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Mu, Y.; Chang, X.; Li, G.; Dong, Z.; Sun, J.; Jin, S.; Wang, X.; Zhang, L.; Jin, S. Functional verification and screening of protein interacting with the slPHB3. Plant Signal Behav. 2022, 1, 2025678. [Google Scholar] [CrossRef]


| No. | Compounds | Molecular Formula | Type |
|---|---|---|---|
| 1 | Acacetin | C16H12O5 | Flavonoid |
| 2 | Acacetin-7-O-β-d-glucuronide (Tilianin) | C22H22O10 | Flavonoid |
| 3 | Acacetin-7-O-alpha-l- rhamnopyranoside | C22H22O9 | Flavonoid |
| 4 | Acacetin-7-O-β-d-apiofuranosyl (1→6)-O-β-d-glucoside | C27H30O14 | Flavonoid |
| 5 | Apigenin | C15H10O5 | Flavonoid |
| 6 | Apigenin-6,8-di-C-β-d-glucopyranoside | C27H30O15 | Flavonoid |
| 7 | 6-Hydroxyapigenin (Scutellarein) | C15H10O6 | Flavonoid |
| 8 | Luteolin | C15H10O6 | Flavonoid |
| 9 | Cynaroside | C21H20O11 | Flavonoid |
| 10 | Luteolin-7-O-(6″-O-acetyl)-β- glucopyranoside | C23H22O12 | Flavonoid |
| 11 | Naringenin | C15H12O5 | Flavonoid |
| 12 | Naringin | C27H32O14 | Flavonoid |
| 13 | Scutellarin | C21H18O12 | Flavonoid |
| 14 | Kaempferol | C15H10O6 | Flavonol |
| 15 | Kaempferide | C16H12O6 | Flavonol |
| 16 | Kaempferol-3-O-β-d-glucoside (Astragalin) | C21H20O11 | Flavonol |
| 17 | Kaempferol-3-O-β-d-rutinoside | C27H30O15 | Flavonol |
| 18 | kaempferol-3-O-β-d-glucopyranosyl- 7-O-β-d-glucopyranoside | C27H30O16 | Flavonol |
| 19 | Kaempferol-3-O-β-sophoroside (Sophoraflavonoloside) | C27H30O16 | Flavonol |
| 20 | 6-Hydroxykaempferol | C15H10O7 | Flavonol |
| 21 | 6-Hydroxykaempferol-3-O-β-glucoside | C21H20O12 | Flavonol |
| 22 | 6-Hydroxykaempferol-7-O-β-glucoside | C21H20O12 | Flavonol |
| 23 | 6-Hydroxykaempferol-3,6-di-O-β- glucoside | C27H30O17 | Flavonol |
| 24 | 6-Hydroxykaempferol-3,7-di-O-β- glucoside | C27H30O17 | Flavonol |
| 25 | 6-Hydroxykaempferol-6,7-di-O-β- glucoside | C27H30O17 | Flavonol |
| 26 | 6-Hydroxykaempferol-3,6,7-tri-O-β- glucoside | C33H40O22 | Flavonol |
| 27 | 6-Hydroxykaempferol-3,6-di-O-β-glucoside-7-O-β-glucuronide | C33H38O23 | Flavonol |
| 28 | 6-Hydroxykaempferol-3-O-β- rutinoside-6-O-β-7-glucoside | C33H40O21 | Flavonol |
| 29 | 6-Hydroxykaempferol-3-O-β-rutinoside | C27H30O16 | Flavonol |
| 30 | Quercetin | C15H14O9 | Flavonol |
| 31 | Quercetin-3-O-β-d-glucoside (Isoquercetin) | C21H20O12 | Flavonol |
| 32 | Quercetin-3-O-β-d-galactosid (Hyperoside) | C21H20O12 | Flavonol |
| 33 | Quercetin-7-O-β-glucoside | C21H20O12 | Flavonol |
| 34 | Quercetin-3,7-di-O-β-glucoside | C27H30O17 | Flavonol |
| 35 | Quercetin-3-O-α-l-rhamnoside-7-O-β- glucuronide | C27H30O16 | Flavonol |
| 36 | Rutin | C27H30O16 | Flavonol |
| 37 | Myricetin | C15H10O8 | Flavonol |
| 38 | Eriodictyol | C15H12O6 | Flavanone |
| 39 | (2S)-4′,5,6,7-tetrahydroxy flavanone 6-O-β-d-glucoside | C21H27O10 | Favanone |
| 40 | (2R)-5,6,7,4′-tetrahydroxyflavanone- 6,7-diglucoside | C27H32O16 | Flavanone |
| 41 | (2S)-5,6,7,4′-tetrahydroxyflavanone- 6,7-diglucoside | C27H32O16 | Flavanone |
| 42 | Saffloflavonesides A | C21H18O9 | Flavanone |
| 43 | Saffloflavonesides B | C21H18O9 | Flavanone |
| 44 | Hydroxysafflor yellow A (Safflomin A) | C27H32O16 | Quinochalcones |
| 45 | Hydroxysafflor yellow B (Safflomin B) | C27H32O16 | Quinochalcones |
| 46 | Hydroxysafflor yellow A-4′-O-β-d- glucopyranosid | C33H42O21 | Quinochalcones |
| 47 | 3′-hydroxyhydroxysafflor yellow A | C27H32O17 | Quinochalcones |
| 48 | Safflomin C | C30H30O14 | Quinochalcones |
| 49 | Isosafflomin C | C27H29O15 | Quinochalcones |
| 50 | Methylsafflomin C | C28H31O15 | Quinochalcones |
| 51 | Methylisosafflomin C | C28H31O15 | Quinochalcones |
| 52 | Anhydrosafflor yellow B | C48H52O26 | Quinochalcones |
| 53 | Safflor yellow A | C27H30O15 | Quinochalcones |
| 54 | Safflor yellow B | C48H54O27 | Quinochalcones |
| 55 | Cartormin | C27H29O13N | Quinochalcones |
| 56 | Isocartormin | C27H29O13N | Quinochalcones |
| 57 | Tinctormine | C27H31O14N | Quinochalcones |
| 58 | Saffloquinoside A | C27H29O15 | Quinochalcones |
| 59 | Saffloquinoside B | C34H38O17 | Quinochalcones |
| 60 | Saffloquinoside C | C27H30O15 | Quinochalcones |
| 61 | Saffloquinoside D | C27H31O16 | Quinochalcones |
| 62 | Saffloquinoside E | C30H29O14 | Quinochalcones |
| 63 | Carthamine | C43H42O22 | Quinochalcones |
| 64 | Hydroxyethylcarthamin | C45H46O23 | Quinochalcones |
| 65 | Precarthamin | C44H43O23 | Quinochalcones |
| 66 | Neocarthamin | C21H22O11 | Quinochalcones |
| 67 | Carthamone | C21H20O11 | Quinochalcones |
| Gene | Primer | Sequence (5′-3′) |
|---|---|---|
| CtOGT1 | CtOGT1-F | ATGCCTCACTCAACCACCAAC |
| CtOGT1-R | TTAGAGCTCCGATAGAAGCTT | |
| CtOGT1-BamHI-F | TTAGAGCTCCGATAGAAGCTT ATGCCTCACTCAACCACCAAC | |
| CtOGT1-BamHI-R | ACGGAGCTCGAATTCGGATCC TTAGAGCTCCGATAGAAGCTT | |
| CtOGT2 | CtOGT2-F | ATGGAAGTTCACATAGCCATT |
| CtOGT2-R | TTACTCAGCCCACTTTTTAGC | |
| CtOGT2-BamHI-F | ATGGAAGTTCACATAGCCATT TTAGAGCTCCGATAGAAGCTT | |
| CtOGT2-BamHI-R | ACGGAGCTCGAATTCGGATCC TTACTCAGCCCACTTTTTAGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Xia, M.; Han, Y.; Tan, H.; Chen, Y.; Song, X.; Yuan, S.; Zhang, Y.; Su, P.; Huang, L. Highly Promiscuous Flavonoid Di-O-glycosyltransferases from Carthamus tinctorius L. Molecules 2024, 29, 604. https://doi.org/10.3390/molecules29030604
Xu X, Xia M, Han Y, Tan H, Chen Y, Song X, Yuan S, Zhang Y, Su P, Huang L. Highly Promiscuous Flavonoid Di-O-glycosyltransferases from Carthamus tinctorius L. Molecules. 2024; 29(3):604. https://doi.org/10.3390/molecules29030604
Chicago/Turabian StyleXu, Xiaoyu, Meng Xia, Yang Han, Honghu Tan, Yanying Chen, Xinqi Song, Shijun Yuan, Yifeng Zhang, Ping Su, and Luqi Huang. 2024. "Highly Promiscuous Flavonoid Di-O-glycosyltransferases from Carthamus tinctorius L." Molecules 29, no. 3: 604. https://doi.org/10.3390/molecules29030604
APA StyleXu, X., Xia, M., Han, Y., Tan, H., Chen, Y., Song, X., Yuan, S., Zhang, Y., Su, P., & Huang, L. (2024). Highly Promiscuous Flavonoid Di-O-glycosyltransferases from Carthamus tinctorius L. Molecules, 29(3), 604. https://doi.org/10.3390/molecules29030604






