Abstract
Consumers in developed and Western European countries are becoming more aware of the impact of food on their health, and they demand clear, transparent, and reliable information from the food industry about the products they consume. They recognise that food safety risks are often due to the unexpected presence of contaminants throughout the food supply chain. Among these, mycotoxins produced by food-infecting fungi, endogenous toxins from certain plants and organisms, pesticides, and other drugs used excessively during farming and food production, which lead to their contamination and accumulation in foodstuffs, are the main causes of concern. In this context, the goals of this review are to provide a comprehensive overview of the presence of toxic molecules reported in foodstuffs since 2020 through the Rapid Alert System for Food and Feed (RASFF) portal and use chromatography to address this challenge. Overall, natural toxins, environmental pollutants, and food-processing contaminants are the most frequently reported toxic molecules, and liquid chromatography and gas chromatography are the most reliable approaches for their control. However, faster, simpler, and more powerful analytical procedures are necessary to cope with the growing pressures on the food chain supply.
1. Introduction
Currently, consumers, particularly those in developed and Western European countries, are more aware of the direct impact of food on their health. Consequently, they demand a food industry with a greater degree of information about the products they acquire, which requires this information to be clear, transparent, and reliable [1,2]. Consumers’ perception of food safety risks is recognised in Europe as one of the main pillars directly associated with the subjective perception of food quality. Accordingly, ensuring food safety is an essential aspect of the European food industry to protect the health of consumers, as they have no choice but to trust both manufacturers and farmers when it comes to food production, as well as authorities regarding the enforcement of regulations [2,3]. Hence, in Europe, food safety has become an issue of public concern, implying that the food industry must carry out routine controls throughout the different steps of the food chain to ensure food safety and avoid risks for consumers.
Food safety risks are primarily associated with the unexpected occurrence of contaminants throughout the food supply chain. These contaminants originate from biotic and abiotic sources [4]. Biotic contaminants refer to the occurrence of pathogenic microorganisms, such as bacteria, viruses, fungi, and parasites, whereas abiotic contaminants are those derived from the presence of chemical substances and their derived/transformed products.
Focusing on abiotic contaminants, these chemical substances can be unintentionally present in foods because of the different steps of their production, processing, and transportation. Similarly, they can also be caused by environmental pollution [5]. Many of these chemical substances play an important role in the production and distribution of food, as is the case with pesticides and veterinary drugs, which help improve crop yields and livestock production, as well as reduce production costs and food prices. However, their use can lead to the presence of chemical residues in food, which constitutes a potential risk to the health of consumers. Among the most relevant abiotic contaminants present in food, the following groups can be distinguished:
- I.
- Natural toxins: Toxic chemical substances produced by different organisms. The following categories are included in this group: mycotoxins, plant toxins, and marine toxins.
- II.
- Environmental pollutants: Chemical substances released into the air, water, or soil as a result of industrial or agricultural practices. Pesticides, xenohormones, and veterinary drug residues are also included in this group.
- III.
- Food-processing contaminants: Chemical substances naturally formed in food during industrial processes or cooking.
In the European Union (EU), the authority responsible for evaluating and ensuring food safety is the European Food Safety Authority (EFSA), which evaluates and ensures food safety. This body issues scientific opinions on the different identified food risks. Subsequently, based on the evaluations and reports prepared by the EFSA, the European Commission and EU member states establish European regulations for the correct management of food risks [6]. Thus, within European legislation, there are specific regulations that address the establishment of maximum residue and contaminant limits related to the presence of these chemical substances in foods [7,8,9].
To identify and manage different food risks so that it is possible to protect the health of consumers, the EU has a coordinated food alert system called the Rapid Alert System for Food and Feed (RASFF) [10]. This system allows the rapid exchange of information on food risks between member countries so that immediate measures can be taken, such as removing contaminated products from the market. This tool includes an interactive database called the RASFF window of public access that provides summary information about food alerts notified in the EU (currently limited to 2020 and later) [10]. Thanks to this database tool, consumers, business operators, and authorities around the world can have access to information on recent food recalls and public health warnings in countries linked to this system.
Chromatography is a leading analytical approach for the fast and reliable analysis of molecules in diverse and complex matrices, including foodstuffs. Accordingly, liquid chromatography and gas chromatography cover a wide range of molecules, and their coupling with mass spectrometry makes chromatography a powerful and reliable technique for assessing food composition and safety, enabling the determination of toxic compounds in foods at levels far below those that could threaten human health and well-being. This review aims to provide an overview of food alerts notified in the EU for different categories of abiotic contaminants in recent years (2020–2023) using the information provided by the RASFF window database and highlight the most relevant chemical contaminants responsible for the notification issued. Hence, information on contamination pathways, toxic or adverse effects, and the occurrence of these contaminants in food is included. Examples of the most relevant approaches for determining the presence of these contaminants in food samples using chromatographic techniques are also described. Finally, recent developments and future perspectives for the control and assessment of food safety are discussed.
2. Methodology to Review the Food Alerts and Literature
The RASFF window database has been employed to provide a comprehensive overview of food alerts notified in the EU in recent years [10]. The search was limited to the period from January 2020 to June 2023, and it focused only on alerts related to abiotic organic chemical contaminants in food. For this purpose, in the hazard category of the database intended for “risk”, the following filters were selected: “Biocontaminants” OR “Biotoxins” OR “Chemical contamination” OR “Environmental pollutants” OR “Industrial contaminants” OR “Mycotoxins” OR “Natural toxins” OR “Pesticide residues” OR “Residues of veterinary medicinal products”. The data collected were processed and evaluated to determine the main compounds responsible for the alerts in each category. The distribution of the alerts was determined using Microsoft Excel. In addition, to provide a comprehensive overview of the most relevant approaches for determining the presence of these organic chemical compounds in food samples using chromatographic techniques, both Google Scholar and PubMed databases were employed. Accordingly, the following keywords were used: “Food contaminants” OR “Chromatographic analysis”. The results obtained from this search were limited to articles written in English and published in peer-reviewed journals over the last five years. Finally, the abstracts of the retrieved studies were read, and relevant information was used to obtain the data presented in this review.
3. Major Classes of Toxic Molecules Reported on Foodstuffs
As shown in Figure 1, the highest number of food alerts registered in the EU in recent years was due to the occurrence of pesticide residues in food (almost 60% of the total food alerts issued), followed by the presence of mycotoxins (29% of the total food alerts issued). In contrast, the number of food alerts in the other contaminant categories was significantly lower, with marine toxins having the lowest incidence (0.4% of the food alerts). Each category of chemical contaminants is described in more detail below, highlighting the main compounds responsible for food alerts issued in these categories.
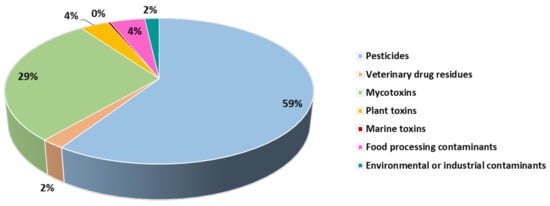
Figure 1.
Distribution of food alerts reported between January 2020 and June 2023 in different food contaminant categories (data collected from the Rapid Alert System Feed and Food (RASFF) window, 2023 [10]).
3.1. Pesticides
Pesticides are very useful for protecting plants from harmful organisms, including weeds, and improving agricultural production. However, the use of pesticides on crops may lead to the presence of residues of these compounds, including their metabolites, as well as products resulting from their degradation or reactions [11].
Pesticides include herbicides, fungicides, acaricides, plant growth regulators, and repellents. They can be classified according to different aspects such as their use, toxicity, chemical structure, or media lifetime. Based on their chemical structures, it is possible to differentiate organophosphorus pesticides, carbamates, pyrethroids, organochlorines, triazines, benzimidazoles, and nitro compounds [12,13]. Among the recent food alerts reported for the occurrence of pesticides in foods, a huge number have been related to the occurrence of organophosphorus pesticides, such as chlorpyrifos and dimethoate (Figure 2a). It is also worth highlighting the alerts referred to the use of ethylene oxide as a pesticide (Figure 2a), which has been used to sterilise some spices, certain dried herbs, and dried vegetables to control food-borne pathogens such as Salmonella and Escherichia coli. However, within these food alerts, the occurrence of other pesticides also stands out, such as acetamiprid, prochloraz, tricyclazole, carbendazim, and dimethoate (Figure 2a), which are frequently detected in food products at concerning levels.
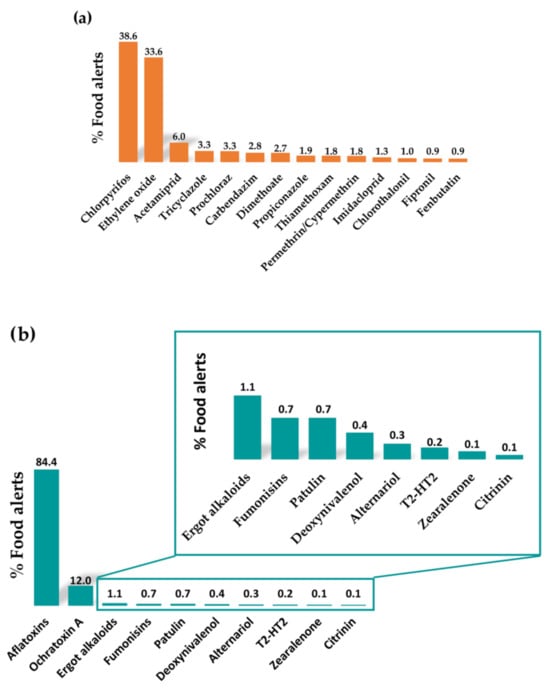
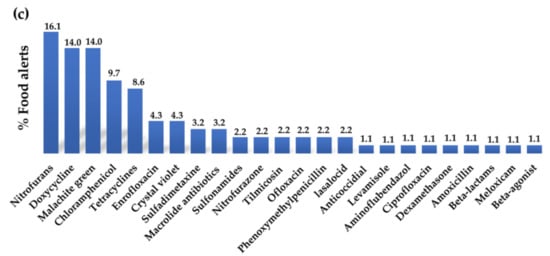
Figure 2.
Percentage of food alerts corresponding to the main contaminants detected in the categories of (a) pesticides, (b) mycotoxins, and (c) veterinary drug residues between January 2020 and June 2023 in the Rapid Alert System Feed and Food (RASFF) portal (data collected from RASFF window, 2023 [10]).
Owing to the adverse toxic effects of pesticide residues on human health (Table 1), pesticides cannot be marketed or used without prior authorisation. Accordingly, pesticides are regulated by Regulation (EC) No. 1107/2009 [14], and maximum residue limits (MRLs) for authorised pesticides in foods and feed are included in Regulation (EC) No. 396/2005 [7]. This regulation also contains information on official controls of pesticide residues in foods of plant and animal origin.
3.2. Mycotoxins
Mycotoxins are toxic secondary metabolites produced naturally by several species of mould that can grow on food under certain temperature and humidity conditions. Their production is maximal between 24 and 28 °C, whereas their occurrence is lower at refrigeration temperatures [15]. A wide variety of mycotoxins can affect the health of humans and animals, depending on the type of mould that produces them. The most important of these are those produced by mould of the genera Aspergillus, Penicillium, and Fusarium. According to food alerts reported in recent years, the most common are aflatoxins (B1, B2, G1, G2, M1, and M2, produced by Aspergillus flavus and Aspergillus parasiticus), followed by ochratoxin A (produced by Aspergillus ochraceus and Penicillium verrucosum), patulin (produced by Penicillium, Aspergillus, and Byssochlamys), fusarium toxins (such as fumonisins, deoxynivalenol, T-2 toxin, HT-2 toxin, and zearalenone, produced by the genus Fusarium), and others such as alternariol, citrinin, and ergot alkaloids (Figure 2b).
These mycotoxins can appear at any stage of the food chain in crops contaminated with processed foods. Unprocessed or raw foods, which are more likely to be contaminated with mycotoxins, include cereals, oilseeds, fruits, vegetables, nuts, dried fruits, coffee beans, cocoa beans, and spices. Likewise, these compounds present great technological and thermal stability, so they can resist processes such as grinding and drying as well as subsequent cooking. Consequently, mycotoxins can also be present in processed products, such as cereal-based products (e.g., bread, pasta, and breakfast cereals), beverages (e.g., wine, coffee, cocoa, beer, and juices), baby foods, and even in foods of animal origin (e.g., milk and cheese) [16].
The most common pathway of food contamination with mycotoxins is in the fields during cultivation; however, they can also appear during harvest and storage, or even at several of these stages at the same time. For this reason, owing to their high chemical stability, the most suitable risk management measure to reduce the occurrence of mycotoxins in food, as well as human risk exposure, is by applying the Codes of Hygienic Practice. Currently, there are several Codes of Practice for the prevention and reduction of mycotoxins in some foods, such as patulin and ochratoxin A [17,18,19,20].
The intake of mycotoxins can lead to both acute and long-term adverse effects depending on the type and amount ingested. The acute effects of mycotoxins are primarily gastrointestinal disorders. In contrast, the main disorders derived from long-term exposure to these compounds can be nephrotoxic, hepatotoxic, carcinogenic, genotoxic, and mutagenic, among others. Likewise, some mycotoxins are immunosuppressive, reducing resistance to infectious diseases such as patulin. Table 1 describes the effects of each mycotoxin.
Because of the possible health risks associated with exposure to these compounds by consumers, the EU has a regulation that allows monitoring the occurrence of mycotoxins in food by establishing maximum concentration limits of these contaminants in certain products. Thus, these limits are established in the Commission Regulation (EU) 2023/915 of 25 April 2023 on maximum levels for certain contaminants in food and repealing Regulation (EC) No 1881/2006 [9]. It is also worth noting that the criteria for sampling and analysis of mycotoxins in food have been harmonised in the Commission Regulation (EC) No 401/2006 of 23 February 2006 due to the wide heterogeneous distribution of mycotoxins in foods [21].
3.3. Veterinary Drug Residues
Veterinary drug residues can be defined as all pharmacologically active substances, whether active ingredients, excipients, or degradation products and their metabolites, that remain in foods obtained from the animals to which the veterinary drug product has been administered [22]. The health and well-being of food-producing animals are essential to ensure food safety. For this reason, in animal production, a wide variety of drugs are used for therapeutic or zootechnical purposes, because by preventing diseases in animals, public health is also protected.
However, the use of these drugs must be carried out in a responsible and controlled manner to avoid fraudulent practices and abusive use. Otherwise, incorrect administration of these drugs can lead to poor metabolism by the animal, resulting in the occurrence of drug residues remaining in the muscle after slaughter or in the milk after milking [22]. These situations would lead to the entry of these substances into the food chain, posing a serious risk to the health of consumers.
It has been reported that prolonged exposure to these drug residues, even at low doses, can cause different side effects, such as allergic reactions, toxicity, and microbiological, teratogenic, or even carcinogenic effects [23,24,25]. Therefore, controlling veterinary drug residues in food is of utmost importance in the field of food safety. Accordingly, in the EU, substances that are authorised for therapeutic use in animals, as well as those forbidden, are regulated, including MRLs for those allowed (Regulation (EC) No 470/2009 and Commission Regulation (EU) No 37/2010) [8,14]. At the same time, these regulations also establish waiting times that must be respected after administration of the drug before products of animal origin are destined for human consumption.
Despite this, there are a high number of food alerts regarding these compounds (Figure 1). These alerts are notified when quantities of drug residues greater than the MRLs established in legislation are detected or when substances not authorised for veterinary use in animals are used and detected in animal-derived products. The veterinary drug residues most frequently detected in food alerts are antibiotics and antimicrobial substances, such as nitrofurans (e.g., nitrofurazone), sulphonamides (e.g., sulfadimethoxine), and dyes (e.g., malachite green and crystal violet) (Figure 2c). Among the antibiotics, the most common are tetracyclines, particularly doxycycline, followed by chloramphenicol, fluoroquinolones (particularly enrofloxacin), and macrolide antibiotics. The toxic effects of these compounds are summarised in Table 1.
3.4. Plant Toxins
Many plants synthesise natural toxins resulting from their secondary metabolism as a defence mechanism; therefore, they are usually highly toxic. Currently, there is increasing awareness about the potential danger that their intake through contaminated food can pose to human health, since until recent years, their occurrence in different foodstuffs has been underestimated [26]. Within this group of contaminants, the food alerts reported in recent years are related to the occurrence of alkaloids, particularly pyrrolizidine and tropane alkaloids (Figure 3a). Generally, these alkaloids are secondary metabolites of plants that grow as weeds in crop fields, leading to contamination of plant-derived products during their production [27]. There are five main families of flowering plants able to produce pyrrolizidine alkaloids: Asteraceae (tribes Senecioneae and Eupatorieae), Boraginaceae, Fabaceae (genus Crotalaria), Apocynaceae, and Orchidaceae [28]. On the other hand, tropane alkaloids are naturally produced by the following plant families: Brassicaceae, Solanaceae, Erythroxylaceae, and Convolvulaceae [29]. The intake of these alkaloids can cause mild disorders (digestive disorders, headache, etc.) to serious situations (neurotoxic, nephrotoxic, hepatotoxic, carcinogenic, mutagenic, and teratogenic effects) or can even be lethal (Table 1). Pyrrolizidine alkaloids can also lead to chronic diseases after long-term exposure, whereas the chronic toxicity of tropane alkaloids has not been demonstrated to date [26]. Both alkaloids have been detected in plant-based products at high concentrations. Pyrrolizidine alkaloids are commonly found in teas, herbal teas, spices, aromatic herbs, honey, and in food supplements. In contrast, tropane alkaloids are mainly found in cereals, pseudocereals, legumes, grains, and derived bakery products. Nonetheless, these alkaloids have also been detected in other products to a lesser extent [26,27,28,29]. To keep consumers’ exposure to these alkaloids as low as possible throughout their diet, the EU has set maximum concentration limits for these toxins in food products. These limits are included in the Commission Regulation (EU) 2023/915 of 25 April 2023 on maximum levels for certain contaminants in food and repealing Regulation (EC) No 1881/2006 [9].
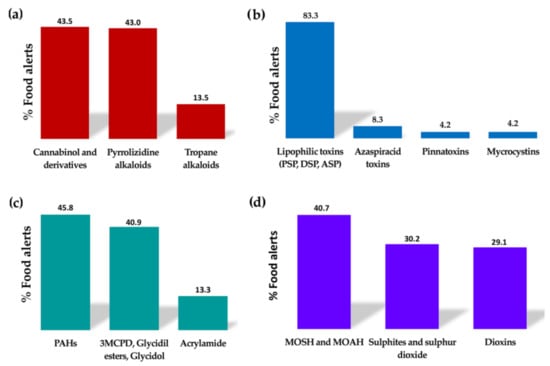
Figure 3.
Percentage of food alerts corresponding to the main contaminants detected in the categories of (a) plant toxins, (b) marine toxins, (c) food-processing contaminants, and (d) environmental or industrial contaminants within the period from January 2020 to June 2023 in the Rapid Alert System Feed and Food (RASFF) portal (data collected from the RASFF window, 2023 [10]).
Another toxic compound naturally produced by plants that has caused many food alerts in recent years is the tetrahydrocannabinol (THC) and its derivatives (Figure 3a), which are psychoactive compounds synthesised by the hemp plant (Cannabis sativa). In fact, what is present in high concentrations in the crop and in the harvested plant is the precursor of the active molecule, which is then transformed after the application of heat [30]. In the EU, hemp varieties grown and used for food purposes must have a maximum THC content of 0.2% (w/w) in accordance with Regulation (EU) No. 1307/2013 [31]. Consequently, foods derived from hemp that are authorised to be marketed in the EU are those obtained from the seeds of these hemp varieties, such as oil, hemp protein, and hemp flour. Consequently, it has been estimated that these substances may occur mainly as contaminants in bread, pasta, breakfast cereals, cereal bars, bakery products, teas, beers, and dietary supplements when hemp-derived products are used as ingredients [30]. Nonetheless, small amounts of data are currently available on the actual exposure of the population to these compounds, as well as on the presence of THC in foods of animal origin and on the transfer rate of these compounds from feed to foods of animal origin. The intake of these psychoactive substances mainly affects the central nervous system and is capable of increasing the heart rate even at low doses [30].
3.5. Marine Toxins
In general, marine toxins are naturally produced by microalgal species that occur in freshwater and oceans. Consequently, these toxins can contaminate drinking water or accumulate in shellfish and fish when they feed on algae or on other fish that have previously been fed on algae. Nonetheless, some fish species can naturally synthesise toxins (e.g., tetrodotoxins produced by pufferfish) [32].
Regarding the food alerts reported in recent years for marine toxins (Figure 3b), three types of lipophilic toxins stand out: amnesic shellfish poisoning (ASP), mainly related to the presence of domoic acid; diarrhetic shellfish poisoning (DSP), mainly related to the presence of okadaic acid and its derivatives; and paralytic shellfish poisoning (PSP), mainly related to the presence of saxitoxins. The details and effects of the toxins are listed in Table 1.
Intake of these toxins can be a potential hazard to consumers because they can cause several adverse effects (Table 1). However, the main problem with the use of these toxins is that they cannot be reliably eliminated by cooking or freezing because of their high stability [32]. For this reason, the main method to minimise the risk of exposure to these compounds is to limit and control the harvesting area of molluscan shellfish, as well as to remove and discard the viscera in fish.
3.6. Food-Processing Contaminants
Industrial or food-processing contaminants are a group of compounds that are naturally generated during food processing and cooking, which have potentially harmful effects on the health of consumers. These contaminants are generated from precursors present in foods (e.g., carbohydrates and amino acids) that undergo chemical changes during processing. These processing procedures include fermentation, drying, smoking, refining, frying, baking, roasting, and grilling [33].
Many foods must be cooked to make them edible and digestible. The application of heat treatment ensures suitable hygiene and microbiological stability of the products. Moreover, thermal processing is crucial for the development of aromas, flavours, and colours in the product. However, food-processing contaminants are also generated through the same chemical reactions that occur during the cooking and preservation procedures of food [33].
Among these contaminants, polycyclic aromatic hydrocarbons (PAHs) are the most relevant. Most of the food alerts collected in this category were caused by the presence of these compounds (Figure 3c). PAHs are chemical compounds composed of carbon and hydrogen atoms that contain two or more aromatic groups. They are mainly formed during incomplete combustion or pyrolysis of organic matter (T > 400 °C). Their occurrence in food can be the result of environmental pollution (industrial activities, heating, forest fires, etc.) or due to food-processing procedures, including smoking, drying, or heating (roasting, barbecues, etc.) [34]. Within PAHs, benzopyrenes have been frequently detected at high levels in food alerts reported in recent years [10]. Cereals, cereal-based products, and fish and fishery products (especially smoked) are the main foods contributing to the total dietary exposure of the population to PAHs. High-fat and high-protein foods prepared on grills or barbecues also contribute to this exposure [34].
Nonetheless, in recent years, many food alerts have emerged for other compounds within the category of processing contaminants, such as acrylamide, 3-monochloropropane diol (3-MCPD), 3-MCPD esters, and glycidyl esters (Figure 3c). These substances have been classified as compounds with possible neoplastic actions, among other effects (Table 1). Acrylamide is formed by the reaction of asparagine (an amino acid) with reducing sugars (mainly glucose and fructose) in the Maillard reaction [34]. Therefore, acrylamide likely appears when cooking or processing starchy foods such as potatoes or cereals at high temperatures (>120 °C) and low moisture levels (i.e., frying, toasting, or roasting). The main dietary sources of acrylamide exposure are coffee, fried potatoes, cookies, crackers, toasts, sliced bread, and certain baby foods.
In contrast, 3-MCPD and its esters, glycidol, and glycidyl esters are generated when high temperatures (>200 °C) are applied to foods high in fat, as in the case of oil refining. These substances were first detected in soy sauce and palm oil. However, it is currently known that the main contributors to total dietary exposure to these contaminants are margarines and derivatives, as well as fats and oils of vegetable origin, followed by bread, pastries, and smoked preserved meat [35].
A common feature of all these food-processing contaminants is their wide distribution in foods, which is an important issue for their monitoring. Thus, consumers’ exposure to these contaminants is transversal and does not generally affect a single specific food or technological process. This means that, to manage the risk of these contaminants, it is necessary to adjust industrial processes and achieve consumer awareness to reduce risk. Currently, the establishment of maximum concentration limits in legislation is the most effective risk management measure to protect consumers from these contaminants. Thus, the Commission Regulation (EU) 2023/915 of 25 April 2023 on maximum levels for certain contaminants in food and repealing Regulation (EC) No 1881/2006 sets limits for PAHs, 3-MCPD and its esters, and glycidil esters [9]. On the other hand, acrylamide has its own regulation (Commission Regulation (EU) 2017/2158), which includes mitigation measures to reduce the presence of acrylamide in foods, reference levels, and a series of codes of good practices that seek to reduce consumer exposure to acrylamide, such as temperature control, cooking time, and the correct selection of raw material measures [36]. Similarly, there is also a Code of Good Practice to prevent and reduce contamination of PAHs in foods produced by smoking and drying procedures [37].
3.7. Environmental or Industrial Contaminants
This category includes chemical compounds related to environmental pollution, as they can be released into air, water, or soil, often because of industrial or agricultural activities. Consequently, these substances can enter the food chain at different stages of production, processing, and transport of food products. Many compounds can be included in this group, such as polychlorinated biphenyls (PCBs), dioxins, persistent chlorinated pesticides, brominated flame retardants, and metals (e.g., arsenic, cadmium, lead, and mercury). However, most food alerts reported in recent years in this category have been mainly related to the occurrence of mineral oils (MOH), dioxins, sulphites, and sulphur dioxide (Figure 3d).
MOH is a chemical compound derived from petroleum distillation and refining processes. It can be divided into two main types based on chemical structures: mineral oil saturated hydrocarbons (MOSHs) and mineral oil aromatic hydrocarbons (MOAHs). These compounds can enter the food chain through environmental pollution, the use of lubricants for machinery, release agents, food or feed additives, processing aids, and migration from food contact materials [38]. Accordingly, a large variety of foods may be contaminated with MOH, including oils of vegetable origin, dairy products, cereals and cereal-based products, baby foods, potato chips, legumes, nuts, canned fish, chocolate and chocolate-based products, spices, salads, and ready-to-eat dishes. The potential human health risks derived from MOH intake of MOH varies widely. Currently, MOAHs can act as a genotoxic carcinogen, whereas MOSHs can accumulate in the lymphoid system and damage the liver [38]. However, awareness of the occurrence of these contaminants in food began a few years ago, so it is a relatively recent topic. For this reason, there is currently no regulation regarding assessments and risk management, as well as their monitoring in food products. Nonetheless, data on the presence of these compounds are being collected with the participation of food business operators, manufacturers, processors, and distributors of food contact materials to establish possible regulations in the future.
Dioxins are referred to as polychlorinated dibenzo-p-dioxins (PCDDs) and dibenzofurans (PCDFs). These compounds have no technological or other uses but are generated in many thermal and industrial processes as unwanted and often unavoidable by-products. Thus, they can be generated during the bleaching of paper pulp with chlorine, electrochemical production of chlorine with graphite electrodes, in the textile industry, automobile combustion engines, heating systems, waste incineration, and volcanic eruptions. [39]. Owing to their lipophilic nature, these contaminants tend to accumulate in the fatty tissues of animals. Therefore, the main source of human exposure to these compounds is through diet, mainly through the intake of foods of animal origin with a high fat content. Long-term exposure to contaminants can negatively affect the nervous, endocrine, immune, and reproductive systems. Similarly, these substances may also cause cancer [39]. Nonetheless, these contaminants are usually found in low levels in many foods. In fact, their presence in the environment in Europe has decreased since the 1970s thanks to the efforts of public authorities and industry. In 2001, the EU adopted a strategy to significantly reduce the levels of these compounds in the environment, feed, and foodstuffs to ensure a high level of public health protection [40]. Moreover, the maximum concentration levels for these compounds are currently regulated by Commission Regulation (EU) 2023/915 on 25 April 2023 [9].
Regarding sulphites and sulphur dioxide, these chemical compounds are authorised as food additives in several foodstuffs as preservatives to avoid the growth of bacteria, yeast, and fungi, or as antioxidants to prevent browning processes in fruit and vegetables. Sometimes, they are also used to halt ongoing fermentation during winemaking. Similarly, sulphites are naturally present in our bodies and in some foods, such as onions, rice, apples, cabbages, and wine. However, in some individuals, these substances can cause adverse reactions to the immune system. In 2022, the EFSA carried out a renewed risk assessment for all food additives approved in the EU, concluding that there are gaps in the toxicity data that do not allow confirmation of the extent of certain adverse health effects associated with sulphites and sulphur dioxide [41]. Among these adverse effects, negative effects on the central nervous system were observed, such as a delayed response of nerve cells to stimuli and early signs of nervous system dysfunction. Likewise, it was observed that the estimated intake of these compounds by the population potentially exceeded the safe dose by up to 12.5% in children (3–10 years old) and up to 60% in adults [41]. Therefore, it is important to monitor the levels of these compounds in food products. Accordingly, the food alerts reported in the last few years refer to products that did not comply with the current regulations for these compounds (Regulation (EU) No 1169/2011) [42] or that the presence of these additives was not correctly stated on the food label.

Table 1.
Origins, contamination pathways, and most relevant toxic effects of the main abiotic organic contaminants.
Table 1.
Origins, contamination pathways, and most relevant toxic effects of the main abiotic organic contaminants.
| Compound | Origin and Food Contamination | Toxic Effects | Refs. |
|---|---|---|---|
| Pesticides | |||
| Benzimidazoles |
|
| [43,44] |
| Carbamates |
|
| [45,46] |
| Nitro compounds or dinitroaniline pesticides |
|
| [47,48] |
| Organochlorine pesticides |
|
| [49] |
| Organophosphorous pesticides |
|
| [50,51] |
| Pyrethroids |
|
| [52] |
| Triazines |
|
| [53,54] |
| Veterinary drug residues | |||
| Beta-lactams |
|
| [55,56] |
| Dyes (malachite green and crystal violet) |
|
| [57] |
| Fluoroquinolones |
|
| [58] |
| Macrolide antibiotics |
|
| [59] |
| Nitrofuranzone |
|
| [60] |
| Sulphonamides |
|
| [61] |
| Tetracyclines |
|
| [62] |
| Plant toxins | |||
| Cannabinoids and derivatives |
|
| [63,64] |
| Pyrrolizidine alkaloids |
|
| [28,65,66] |
| Tropane alkaloids |
|
| [29,67] |
| Marine toxins | |||
| Amnesic shellfish poisoning (ASP) |
|
| [34] |
| Diarrhetic shellfish poisoning (DSP) |
|
| [34] |
| Paralytic shellfish poisoning (PSP) |
|
| [34] |
| Mycotoxins | |||
| Aflatoxins |
|
| [68,69] |
| Alternariol |
|
| [70,71,72] |
| Citrinin |
|
| [73,74,75,76] |
| Deoxynivalenol |
|
| [77] |
| Ergot alkaloids |
|
| [78] |
| Fumonisins |
|
| [79] |
| Ochratoxin A |
|
| [77,80] |
| Patulin |
|
| [81,82] |
| T-2 and HT-2 toxins |
|
| [83] |
| Zearalenone |
|
| [84] |
| Food-processing contaminants | |||
| Acrylamide |
|
| [85,86] |
| Glycidol and glycidyl esters |
|
| [87,88] |
| Polycyclic aromatic hydrocarbons (PAHs) |
|
| [89,90] |
| 3-MCPD and its esters |
|
| [87,91] |
| Environmental or industrial contaminants | |||
| Dioxins and furans |
|
| [92,93] |
| Mineral oil hydrocarbons (MOHs) |
|
| [94] |
| Sulphites and sulphur dioxide |
|
| [95,96,97] |
4. Overview of the Main Chromatographic Approaches Used to Assess Food Safety
Regardless of the target contaminants and food matrices analysed, LC is consistently the preferred chromatographic approach to be used and reported in the last five years (2018–2023, as reviewed in [98]). This is often preceded by sample preparation using commercial SPE, QuEChERS, among other procedures [99]. Sample preparation and extraction is an important step that will dictate the success of the chromatographic analysis, and for this reason, specific information about the extraction procedures followed in each of the selected reports is also presented in Table 2. There have been some improvements to these standard protocols for the extraction of the mycotoxin citrinin in cereals, food supplements, and red yeast rice using molecularly imprinted polymers as sorbents in the SPE procedure [75] or the extraction of the toxin okadaic acid in clams using magnetic SPE [100]. Regarding QuEChERS, successful downscale ability to extract tropane alkaloids in leafy vegetables is noteworthy [67]. Deep eutectic solvents (DES) have also been employed in the liquid–liquid microextraction of organophosphorus and pyrethroid pesticides from fruit juices and teas [50,52]. Another interesting report on the analysis of PAHs in nutritional supplements containing omega-3 and fish oil involved fabric sorbent-phase extraction (FPSE) [89]. The application of covalent organic frameworks (COFs) and metal organic frameworks (MOFs) to obtain sorbents with augmented retention capabilities has been successfully explored in recent years, particularly for the extraction of antibiotics from different foodstuffs (reviewed in [101,102]). Overall, from the reports compiled in Table 2, it is clear that, despite the improvements and innovations introduced in the extraction procedure, MS detection is essential to obtain a better analytical performance. However, as observed in the determination of citrinin in red yeast rice, the use of improved extraction protocols (MISPE) partially compensates for the lack of MS detection systems, clearly improving the analytical performance of the methodologies reported using the HPLC-FLD architecture [73,75]. GC-MS is used less frequently than LC for the analysis of food contaminants, because its range of applications is limited to volatile and semi-volatiles compounds. A derivatisation procedure to obtain volatile molecules is sometimes possible; however, this can make the procedure longer and more prone to errors, resulting in poorer analytical performance. Nevertheless, it is worthwhile to refer to the use of GC-MS/MS to detect dioxins and furans in different meats, salmon, and fish oils [92], or more recently, to detect genotoxic carcinogens of vegetable origin in infant formulas and elderly milk powders [87].

Table 2.
Toxic molecules reported in foodstuffs and the methodology used to assess their safety found in the literature in the last five years.
Table 2.
Toxic molecules reported in foodstuffs and the methodology used to assess their safety found in the literature in the last five years.
| Compound | Sample | Extraction Method | Analysis | Results (LODs/Recoveries) | Ref. |
|---|---|---|---|---|---|
| Pesticides | |||||
| Imidacloprid, acetamiprid, clothianidin, and atrazine | Fruits and vegetables | QuEChERS: 5 g sample, 5 mL ACN; 0.6 g MgSO4, and 0.2 g PSA | LC-MS/MS | 0.08–141 μg/kg/70–110% | [103] |
| Organophosphorus pesticides | Juices, water, tomato, cucumber, and honey samples | 75 mL sample; nanocomposite comprising metal-organic framework MIL-101(Cr), and graphene nanopowder | GC-MS | 0.005–15.0 µg/kg/84–110% | [51] |
| Organophosphorus pesticides | Vegetables | 30 min sonication of 4 g homogenised samples mixed with 8 mL ACN; collect the filtrate; repeat three times; combine and evaporate (50 °C N2 stream); redissolve (1 mL acetone); MSPE: add 25 mg Fe3O4@COF@Zr4+ to the sample solution; 30 min vortex; discard supernatant; elute (1 mL acetone; 8 min US); 0.22 μm filtration | GC-FPD | 0.7–3.0 μg/kg/87–121% | [104] |
| Organophosphorus pesticides (phosalone and chlorpyrifos) | Red grape juice and sour cherry juice | 10 mL sample; DES-UALLME: choline chloride/4-chlorophenol (408 μL) | HPLC-UV | 0.070–0.096 ng/mL/87.3–116.7% | [105] |
| 14 organophosphorous pesticides | Fruits and vegetables | 2 g sample; SPME: N-doped C-(C3N4@MOF) fibre coating | GC–MS | 0.23–7.5 ng/g/82.6–118% | [50] |
| Pyrethroids (transfluthrin, fenpropathrin, fenralerate, ethofenprox, and bifenthrin) | Tea beverages and fruit juices | 5 mL sample; DES-DLLME: Hexafluoro-isopropanol-based hydrophobic DES (0.15 g) | HPLC-DAD | 0.06–0.17 ng/mL | [52] |
| Neonicotinoids | Water | 2 mg MOFs + 1 mL NEOs standards; 5 min incubation; centrifugation (14,000 rpm, 2 min); 500 μL MeOH ultrasonic elution; vacuum evaporator, 100 μL mobile phase solubilisation | LC-MS | 0.02–0.1 ng/mL | [106] |
| Veterinary drug residues | |||||
| 52 veterinary drug residues | Mutton or leg meat | 5 g sample; QuEChERS: modified with reduced graphene oxide-melamine sponge (r-GO@MeS) | UPLC–MS/MS | LOD: 0.02–2.0 μg/kg LOQ: 0.05–5.0 μg/kg/63.7–109.5% | [107] |
| 103 veterinary drug residues | Milk and dairy products | 5 g liquid milk or 1 g milk powder; QuEChERS with dispersive solid phase: 100 mg C18 and 300 mg anhydrous sodium sulphate | UPLC-MS/MS | LOQ: 0.1–5 μg/kg (milk) and 0.5–25 μg/kg (milk powder)/>60% | [108] |
| Beta-lactams, quinolones, sulphonamides, and tetracyclines | Fish, poultry, and red meat | 1 g sample; SPE: 5 mL ACN | LC-MS/MS | LOD: 0.3–15 µg/kg, LOQ: 0.8–45.3 µg/kg/82–119% | [109] |
| Sulphonamides | Pork, milk, and water | 100 mL sample loaded through the TPB-DMTP-COF column; washing (3 mL water); drying; elution (8 mL MA); drying (N2 flow); eluent re-dissolved (1.0 mL ultrapure water) | LC–MS/MS | 0.5–1.0 ng/L | [110] |
| Malachite green and crystal violet | Hairtail fish | 5 g sample; dSPE: NiO/ZnO-coated carbon microspheres, 3 mL 3:7 MeOH–H2O, 4 mL 9:1 MeOH | UPLC-UV | 0.50 μg/L (malachite green) and 0.35 μg/L (crystal violet) | [57] |
| 8 nitrofurans | Muscle, milk, eggs, honey, and casings | 2 g sample; hydrolysis and derivatisation, followed by ethyl acetate extraction | UHPLC-MS/MS | 93.5–127.5% recovery | [60] |
| Doxycycline | Chicken claws | 2 g sample; extraction with 5 mL 5% TCA | UHPLC−MS/MS | 5 μg/kg/80–110% | [111] |
| Estrogens | Milk and cosmetics | 5 mL milk + perchloric acid (100 μL, 10% v/v); homogenisation and centrifugation (3 min 10,000 rpm); supernatant pH adjusted to 4 (NaOH, 1 M); 0.45 μm filtration; lotion centrifugation (10 min 10,000 rpm); supernatant pH adjusted to 4 (HCl 1 M); 0.45 μm filtration; add 40 mg MILs + 0.275 g NaCl; 5 min shaken 1500 rpm; recover MILs; 500 µL ACN elution | HPLC-UV | 5–15 ng/mL/98.5–109.3% | [112] |
| Biotoxins | |||||
| Ergot alkaloids and their epimers | Oat-based foods and food supplements (bran, flakes, flour, grass, hydroalcoholic extracts, juices, and tablets) | QuEChERS: 1 g sample; 4 mL ACN and 5 mM ammonium carbonate (85:15, v/v); dSPE: 150 mg C18:Z-Sep+ (1:1); residue reconstituted with 750 µL MeOH 50% (v/v), 0.22 µm nylon membrane filter | UHPLC–MS/MS | LOQ: 3.2 μg/kg/89.7–109% | [78] |
| Lipophilic marine toxins (yessotoxins, dinophysistoxins, okadaic acid, azazspiracids, and spirolides) | Fresh and processed shellfish | 100 g sample; QuEChERS: 2 mL MeOH/ethanol/isopropanol; dSPE: 50 mg graphene oxide/ 100 mg MgSO4 | UPLC-MS/MS | LOD: 0.10–1.47 μg/kg LOQ: 0.32–4.92 μg/kg/85–117.4% | [113] |
| Staphylococcal enterotoxin type A (SEA) | Cow’s milk | 25 g sample, clean up: pH control (pH 3.5 ± 0.5 + 5 M HCl; pH 7.5 ± 0.1 + 5 M NaOH) and TCA precipitation (20% TCA solution); protein denaturisation (5 mL 100 mM Tris-HCl, pH 8.5, 7 M guanidium hydrochloride + 10 mM EDTA); enzymatic digestion and desalting: trypsin digestion (1:100 (w/w)), 1% formic acid acidification, desalting with a GL–Tip styrene-divinylbenzene | LC–MS/MS | LOQ: 10 µg/kg/70–120% | [114] |
| Okadaic acid | Clams | MSPE: 2 g samples + 9 mL MeOH, mix; clean-up: 3 mg Fe3O4@TaTp dispersed in 200 μL MeOH, extraction (5 mL blank seawater containing okadaic acid) and derivatives incubated with Fe3O4@TaTp; rinse with 200 μL ultrapure H2O, 90% MeOH desorption (50 μL); extraction: 5 mg Fe3O4@TaTp dispersed in 200 μL MeOH, extraction with 1 mL reconstituted solution of shellfish samples spiked with okadaic acid and derivatives incubated with Fe3O4@TaTp; rinse with 200 μL ultrapure H2O, 200 μL ACN desorption; 0.22 μm nylon filtration | LC-MS/MS | 0.5 pg/mL (seawater) and 0.04 µg/kg (shellfish) | [100] |
| Pinnatoxin-G | Mussels | 2 g mussel tissue; 9 mL methanol; 2.5 mL methanolic extract hydrolysed with 313 µL 2.5 M NaOH; neutralised with 313 µL 2.5 M HCl; 0.22 µm filtration | LC–MS/MS | LOD: 0.1 µg/kg LOQ: 0.4 µg/kg/62–110% | [115] |
| Biocontaminants | |||||
| Tropane alkaloids | Leafy vegetables | 0.1 g sample; µQuEChERS: 150 mg MgSO4, and 25 mg PSA | HPLC-MS/MS | LOQ: 2.2–2.3 ng/g/82–110% | [67] |
| Histamine | Cheese and cured meat products | 10 g sample; 100 mL HNO3 (0.1 mol/L); ultrasonication (15 min, 35 kHz, 40 °C) | IC-PCD | 0.15 mg/kg/91.3–116.9% | [116] |
| Mackerel canned fish | 5 g sample; 20 mL perchloric acid 0.2 M; SPE: 0.5 g cationic exchange resin; column derivatisation: ortho-phthalaldehyde (0.1 mL), and 2-mercaptoethanol | HPLC-UV | LOD: 1.8 mg/kg LOQ: 5 mg/kg/98–99% | [117] | |
| 7 cannabinoids | Hemp products: seeds, cannabis-infused beer, energy drink, chocolates, roasted coffee and tea | Beer and energy drink (30 mL): SPE (1 mL hydrochloric acid 0.1 M/ 2 mL MeOH); chocolates, hemp seeds, and hemp tea (0.02 g): UAE (10 mL MeOH) | LC-MS | LOD: 2.19 ng/mL LOQ: 6.59 ng/mL/70.0–110% | [63] |
| 21 pyrrolizidine alkaloids | Oregano samples | 0.2 g sample; QuEChERS: 150 mg MgSO4 and 25 mg PSA | UHPLC-MS/MS | LOD: 0.1–7.5 µg/kg, LOQ: 0.5–25 µg/kg/77–96% | [65] |
| 14 pyrrolizidine alkaloids and pyrrolizidine alkaloid N-oxides | Teas and weeds | 1 g sample; 0.1 M sulphuric acid; SPE: 1% formic acid, and 5 mL MeOH/4 mL MeOH + 0.5% ammonium hydroxide | UHPLC-MS/MS | LOD: 0.001–0.4 μg/kg LOQ: 1–5 μg/kg/ 68.6–110.2% | [66] |
| Mycotoxins | |||||
| Citrinin | Red yeast rice | LLE: 30 mg sample, 2 mL H2O–acetone 2:3 (V/V) | HPLC-FLD | 4 mg/kg/109.9% | [73] |
| Nutraceutical green tea | SPE: 1 g sample, sorbent zirconia-coated silica and PSA | UHPLC-HRMS | LOQ: 0.2 μg/kg/97% | [74] | |
| Cereals, food supplements and red yeast rice | MISPE: 0.5 g sample, molecularly imprinted polymer | HPLC-FLD | 550–1105 μg/kg/75.6–90.7% | [75] | |
| Alternariol, alternariol monamethyl ether, tenuazonic acid, tentoxin, deoxynivalenol, and patulin | Cherry tomato, lettuce, and pakchoi | SPE: 1 g sample, HLB SPE cartridges (hydrophilic N-vinyl pyrrolidone and lipophilic diethyl benzene) | UHPLC-MS/MS | LOD: 0.05–3.0 μg/kg LOQ: 0.2–10.0 μg/kg 81.1–116% | [70] |
| 19 mycotoxins | Lotus seeds | QuEChERS: 1 g sample, 5 mL ACN 80% (v/v), 150 mg C18, and 150 mg MgSO4 anhydrous | UHPLC-MS/MS | 0.1–15.0 μg/kg/84.6–96.4% | [76] |
| 17 mycotoxins | Edible nuts | QuEChERS: 5 g sample, 10 mL ACN-formic acid (99.9/0.1 (v/v)); dSPE-EMR-lipid: 0.4 g NaCl, and 1.6 g anhydrous MgSO4 | LC-MS | 0.05–5 μg/kg/ 75–98% | [118] |
| Chemical and industrial contaminants | |||||
| PAHs | Nutritional supplements containing omega-3 and fish oil | FPSE: sol–gel phenyl/polydimethylsiloxane (PDMS)-coated FPSE membranes back-extracted with ACN | HPLC-UV | LOD: 2.16–2.50 ng/mL LOQ: 6.50–7.50 ng/mL/63.2–102.3% | [89] |
| Sulphites | Herbal teas | dSPE: ACN and 0.1% acetic acid + 10 mM ammonium acetate | UPLC-MS/MS | 0.51–12.1 μg/kg/83.8–102.7% | [95] |
| Sulphur dioxide | Stir-fried foods, dried fruits, preserved fruits, ginger, and shredded squid | 1 g sample; 25 mL NaOH 0.4 mM; derivatisation: 2 mL sample disodium hydrogen phosphate and potassium dihydrogen phosphate buffer (pH 5.5)/2.50 mL phthalaldehyde and 1.5 mL ammonium acetate | HPLC-FLD | LOD: 0.2 mg/kg LOQ: 0.7 mg/kg/82.32–105.08% | [97] |
| Acrylamide | French fries, bakery biscuits, and branded biscuits | 1 g defatted sample; 10 mL H2O; 0.5 mL Carrez I and Carrez II solutions; filtration (0.45 μm cellulose acetate syringe filter paper) | HPLC-DAD | LOD: 3.733 ng/μL LOQ: 11.045 ng/μL/98–110% | [85] |
| Coffee and coffee products | QuEChERS: 0.5 g roasted coffee or 2.5 g ready-to-drink (brewed) + 5 mL dichloromethane; SPE Carb/SCX/PSA cartridge; acrylamide residue transformed to 2,3-dibromoacrylamide (acrylamide-Br2) by KBr derivatisation (1 mL 15% (m/v)) and potassium bromate (100 μL 1.7% (m/v)) at acidic conditions (70 μL 10% (v/v) sulphuric acid); 0.22 μm PTFE filtration | UPLC-MS/MS | Roasted and instant coffees: LOD: 1.2 μg/kg LOQ: 4 μg/kg; Ready-to-drink coffees: LOD: 0.24 μg/kg LOQ: 0.8 μg/kg/ 99.3–102.2% | [86] | |
| Polychlorinated dibenzo-p-dioxins and furans | Boiled eggs, crab meat, beef, sheep liver, herring, cod liver, salmon, and fish oil | Dichloromethane/n-hexane (1:1, v/v); acidic silica gel (44% sulphuric acid) to remove lipids and polar interfering substances | GC-MS/MS | LOQ: 0.005–0.101 ng/mL (GC-APCI-MS/MS) and 0.006–0.201 ng/mL (GC-EI-MS/MS) | [92] |
| Glycidyl esters | Infant formulas and elderly milk powders | Transesterification by automation: 0.5 g sample, 2 g anhydrous sodium sulphate, and 2 mL distilled H2O; 10 mL hexane: ethanol (2:1, v/v); residue re-dissolved with 400 μL isooctane | GC-MS/MS | LOD: 0.8 μg/kg/91.7–111.3% | [87] |
| Sodium iron chlorophyllin and sodium copper chlorophyllin | Candies | 0.1 N hydrochloric acid (5 mL), ultrasonication (50 °C, 10 min), dilution to 20 mL (MeOH); vortex mixing, centrifugation (10,000 rpm, 10 min), filter upper layer (0.2 μm)/HPLC-MS | UHPLC-MS | LOD/LOQ: 1.2; 4.1 mg/kg (SIC); 1.4; 4.8 mg/kg (SCC), | [119] |
Legend: ACN—acetonitrile; C-(C3N4@MOF)—metal organic framework-based porous carbon; COF—covalent organic framework; DAD—diode-array detection; DES—deep eutectic solvent; DLLME—dispersive liquid–liquid microextraction; dSPE: dispersive solid-phase extraction; EMR—enhanced matrix removal; GC-APCI-MS/MS – gas chromatography-atmospheric pressure chemical ionization-tandem mass spectrometry; GC-EI-MS/MS – gas chromatography coupled with electron impact–tandem mass spectrometry; FPSE—fabric phase sorptive extraction; GC-FPD—gas chromatography equipped with flame photometric detector; GC-MS—gas chromatography–mass spectrometry; GC-MS/MS – gas chromatography tandem mass spectrometry; HCl—hydrochloric acid; HNO3—nitric acid; HPLC—high-performance liquid chromatography; HPLC-DAD—high-performance liquid chromatography with diode-array detection; HPLC-FLD – high performance liquid chromatography with fluorescence detector; HPLC-UV—high-performance liquid chromatography with ultraviolet detection; IC-PCD—ion chromatography post column derivatisation; LC—liquid chromatography; LC-MS—liquid chromatography–mass spectrometry; LLE—liquid–liquid extraction; LOD—limit of detection; LOQ—limit of quantitation; MeOH—methanol; MgSO4—magnesium sulphate; MILs—magnetic ionic liquids; MISPE: molecular imprinting solid-phase extraction MOF—metal organic framework; MSPE: magnetic solid-phase extraction; MS/MS—tandem mass spectrometry; NaCl—sodium chloride; NaOH—sodium hydroxide; PSA: primary secondary amine; PAHs—polycyclic aromatic hydrocarbons; QuEChERS—quick, easy, cheap, effective, rugged, safe; SPE: solid-phase extraction; TCA—trichloroacetic acid; TPB-DMTP-COF—triphenylbenzene-dimethoxyterephthaldehyde-COFs; UAE—ultrasound-assisted extraction; UALLME—ultrasound-assisted liquid–liquid microextraction; UHPLC—ultra-high-performance liquid chromatography; UHPLC-HRMS — ultra-high-performance liquid chromatography-high resolution mass spectrometry; μ—miniaturised.
4.1. Recent Developments and Future Perspectives in the Control of Food Safety Using Chromatographic Approaches
In recent decades, there has been growing recognition of the adverse effects of human activity on the environment, which has prompted an increase in the search for more environmentally friendly analytical methodologies, including chromatography. Large-scale multiresidue methods that enable the simultaneous analysis of a large number of compounds can reduce the number of necessary analyses. Rizzo et al. [120], for instance, proposed an analytical platform using salting-out-assisted liquid–liquid extraction of aqueous extracts combined with ultra-high-performance liquid chromatography–high-resolution tandem mass spectrometry for the screening of 88 pyrrolizidine alkaloids in food matrices with a high risk of contamination. In turn, Steiner, et al. [121] developed an LC-MS/MS-based multiclass approach for the accurate quantification of >1200 biotoxins, pesticides, and veterinary drugs in complex feeds. This approach was challenged with more than 130 real compound feed samples, providing the first insight into the co-exposure of animal feed to agricultural contaminants. A reliable and efficient method for analysing 302 targeted contaminants in catfish muscle was also developed and validated. This method was designed to detect pesticides and their metabolites at US regulatory levels as well as other lipophilic pesticides and environmental contaminants, including PAHs, PCBs, PBDEs, and other flame retardants. The sample preparation was based on the QuEChERS extraction technique. The extracted sample was divided and analysed using UHPLC-MS/MS for 128 analytes after filtration and low-pressure (LP) GC-MS/MS for 219 analytes after an automated robotic micro-SPE clean-up [122]. Another remarkable example was reported by Fialkov et al. [123], who designed an LP GC-MS system capable of achieving good separation with full analysis cycle times of less than one minute. This was accomplished by combining low-pressure GC-MS with low thermal mass resistive-heating for rapid temperature ramping and cooling of the capillary column. This method was successfully applied to replicate the EPA Method 8270 using a complex mixture of 76 semivolatile compounds, which are typically quantified using conventional GC-MS. This approach has great potential for the rapid analysis of PAHs in food samples [123]. Another methodology using GC-MS/MS has been devised to analyse 209 pesticides and persistent organic pollutants (POPs) in non-target wildlife animal liver tissues. This technique requires only 100 mg of liver tissue and allows for the detection of multiple residues in each sample [124].
Micellar liquid chromatography is a green chromatographic approach that is notable for its minimal requirement for organic modifiers, such as acetonitrile and methanol, and ease of recycling the mobile phase. This results in a reduction in excess solvents. Micellar liquid chromatography has a wide range of applications, including, but not limited to, the analysis of antibacterial substances, melamine, biogenic amines, plant protection products, flavonoids, and peptides in various biological matrices, such as milk, eggs, tissues, honey, and feed [125]. The assessment of the more environmentally friendly profile of micellar liquid chromatography was further investigated by Mohamed and Fouad [126], who proposed three alternative HPLC methods for determining the levels of sulfadiazine and trimethoprim in bovine meat and chicken muscles. After thorough evaluation using the GAPI, NEMI, and analytical eco-scale, it was concluded that micellar liquid chromatography demonstrated superior environmental performance.
4.1.1. Multidimensional Chromatography
Methods involving two consecutive chromatographic separations, hereby considered multidimensional (MD) chromatography, have great potential by combining the resolution power of the chromatographic approaches taken individually. However, these formats require sophisticated and expensive instrument configurations and expertise that can be challenging to achieve [127]. Nevertheless, advancements in LC, such as increased orthogonality, separation power, sensitivity, and the ability to hyphenate with more powerful MS detectors, are boosting foodomic investigations, leading to an increase in the number of applications, including food contaminant analyses [128,129]. In this respect, MD-LC has gained significant popularity over the past few years for separating non-volatile analytes from complex matrices. Conventional one-dimensional LC cannot resolve potential co-elutions or minimise matrix effects, which can hinder accurate quantitative analysis. However, coupling MD-LC with MS results in a notable enhancement of the separation power or peak capacity, owing to increased selectivity and sensitivity, making it a valuable tool for many applications, such as the quantification of mycotoxins [127]. Mycotoxins are major contaminants in agricultural products, and several other MD-LC approaches have been developed for their analysis, such as a multi LC-LC coupled with the ESI–MS/MS method for the determination of seven mycotoxins in beer [130], or a 2D-LC HRMS method for the simultaneous monitoring of 70 regulated and emerging mycotoxins in Pu-erh tea [131]. Other notable examples of this approach include enhanced analytical capacities for the analysis of aromatic biogenic amines using 2D heart-cutting sequential injection chromatography [132] and the determination of dangerous compounds in milk and colostrum by coupling MD-LC with HR-MS [128].
Online LC-GC streamlines the sample preparation process, thereby saving time and improving the sensitivity and reliability of analysis. This MD system integrates sample preparation in the first dimension (LC) and analysis in the second dimension (GC). The LC dimension has a high sample capacity, whereas the GC dimension offers a high separation efficiency and the ability to utilise various detectors, including MS. A recent automatised interface, named TOTAD, has been proposed to eliminate manipulation errors and offer different operation modes that enhance analytical performance (e.g., the ability to inject or transfer large volume fractions regardless of the eluent used) [127]. Another promising development in this field is a compact 2D GC system that incorporates microfabricated columns and a nanoelectromechanical system resonator as the detector. This system is eco-friendly, portable, and capable of ultra-fast chromatographic separation, making it suitable for a range of applications where size, weight, power, and speed are critical, including real-time and on-site food safety assays [133].
4.1.2. Miniaturisation of Chromatographic Architectures
The scaling down of traditional macroscale systems, including conventional chromatographic architectures, offers several advantages, including a substantial reduction in the consumption of reagents, samples, and energy, as well as faster and more cost-effective analytical processes, resulting in shorter analysis times. Furthermore, such systems are more prone to efficient automation, resulting in higher throughput and multiplexing [134]. The miniaturisation of GC methodologies involves less energy consumption, whereas the same strategy applied to LC results in a reduction in solvent consumption [135]. One example of these approaches is the bubble-in-drop (BID) microextraction of carbamate pesticides followed by GC-MS analysis. This method utilises only 1.00 μL of the extraction solvent and an air bubble volume of 0.40 μL to determine carbamates in water with good recovery rates, low limits of detection, and high enrichment factors [136].
Another path involves the development of new architectures, such as that proposed by Liao et al. [137], employing a cellular design that simultaneously performs the partial separation of analytes during the sampling process. The authors assayed this promising progressive cellular architecture in microscale GC using a range of polar and nonpolar analytes with wide molecular weights and vapour pressure variations, including alkanes, alcohols, aromatics, and phosphonate esters. Under these conditions, separations within 12 min at a column temperature of 63–68 °C and resolutions greater than two for any two homologues that differ by one methyl group were achieved [137].
Regarding LC developments in this field, nano-LC offers several advantages that align with green chemistry principles, such as reduced flow rate and solvent consumption, resulting in a lower environmental impact and cost of analysis. Common HPLC stationary phases, including C18 sorbents with particle sizes of 3–5 µm or smaller, can be used in nano-LC methods. Additionally, nano-LC methods have been found to have several advantages when applied to pesticide analysis compared to other types of LC, including requiring fewer sample preparation steps and achieving greater sensitivity. Given the increasing regulatory requirements for detecting contaminants, there is a strong demand for more capable analytical methods, and nano-LC has the potential to provide better analytical performance than other chromatographic methods [138]. Moreno-González et al. [139] reported a remarkable example of the nano-LC potential for determining pesticide residues in specific parts of bee specimens. The method developed allows for the extraction of useful information from specific bee parts of individual specimens and provides pseudo spatially resolved chemical information about pesticide contamination [139]. The presence of pyrrolizidine alkaloids in honey, tea, herbal tinctures, and milk was also determined with increased sensitivity and reduced solvent consumption using nano-LC-MS with high-resolution Orbitrap mass spectrometry [140].
4.1.3. Portable Chromatography Solutions and Chromatography-on-Chip
Polycyclic aromatic hydrocarbons (PAHs) are classified as priority hazardous substances because of their carcinogenic properties and potential threats to public health. There are strict regulations in place to prevent their release into the environment, but these regulations are not consistently enforced due to the lack of a reliable field-testing procedure. To address this challenge, Chatzimichail et al. [141] developed a hand-portable system capable of separating, identifying, and quantifying PAHs. The developed system incorporates an HPLC and a spectrally wide absorption detector, which can identify all 24 PAHs on the priority pollutant list of the United States Environmental Protection Agency [141]. In addition, an alternative chipHLPC device using fluorescence and electrospray mass spectrometry (ESI-MS) was used to obtain a rapid and on-site separation of four PAHs [142]. Another microfluidic chromatography detection system was used to measure the concentrations of saccharin sodium (SAC) and acesulfame potassium (Ace-K) in 16 commercial food samples, providing rapid detection of artificial sweeteners in food [143].
Unconventional print and media technologies have also been applied in the field of chromatography, resulting in the creation of a compact, all-in-one LabToGo system. This emerging field, referred to as office chromatography (OC), employs additive manufacturing for the 3D printing of functional components, as well as open-source hardware and software. For example, the analysis of steviol glycosides in Stevia leaves yielded results comparable to those obtained through traditional methods, while the bioanalytical screening of water samples enabled the evaluation of potential health and environmental risks [144].
5. Conclusions
This paper offers a comprehensive overview of the presence of toxic molecules in the food chain and the crucial role of chromatography to ensure food safety. It highlights the importance of stringent regulations and continuous monitoring to safeguard consumer health. The application of various chromatographic techniques, such as liquid chromatography, gas chromatography, and mass spectrometry, in the assessment of food safety has demonstrated their essential roles in the detection and quantification of contaminants and toxins in food and environmental samples. Emergent improvements in the field of chromatography are essential to obtaining faster and more robust chromatographic analysis. Overall, the review underscores the critical importance of chromatographic methods in addressing the challenges of toxic compounds in the food chain and their indispensable role in ensuring food safety and consumer well-being.
Author Contributions
Conceptualisation, J.A.M.P. and N.C.; investigation, J.A.M.P., N.C. and C.V.B.; writing—original draft preparation, J.A.M.P., N.C., C.V.B. and J.S.C.; writing—review and editing, J.A.M.P. and N.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Fundação para a Ciência e a Tecnologia (FCT) with Portuguese Government funds through the CQM Base Fund, UIDB/00674/2020 (DOI: 10.54499/UIDB/00674/2020) and the Programmatic Fund, UIDP/00674/2020 (DOI 10.54499/UIDP/00674/2020), the ARDITI-Agência Regional para o Desenvolvimento da Investigação Tecnologia e Inovação through funds from Região Autónoma da Madeira-Governo Regional, and the Interreg MAC 2014–2020 Cooperacion Territorial through AD4MAC project (MAC2/1.1 b/350), for the invited researcher contract granted to J.A.M.P. The research was also funded by the call Proyectos de Impulso a la Investigación para Jóvenes Doctores de la Universidad Rey Juan Carlos, project PROCESALK ref. M2984.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The Graphical Abstract of this review was obtained by prompt design using AI-assisted Google Bard® and Microsoft Bing®.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Macready, A.L.; Hieke, S.; Klimczuk-Kochanska, M.; Szumial, S.; Vranken, L.; Grunert, K.G. Consumer trust in the food value chain and its impact on consumer confidence: A model for assessing consumer trust and evidence from a 5-country study in Europe. Food Policy 2020, 92, 101880. [Google Scholar] [CrossRef]
- Truong, V.; Conroy, D.M.; Lang, B. The trust paradox in food labelling: An exploration of consumers’ perceptions of certified vegetables. Food Qual. Prefer. 2021, 93, 104280. [Google Scholar] [CrossRef]
- Djekic, I.; Nikolic, A.; Mujcinovic, A.; Blazic, M.; Herljevic, D.; Goel, G.; Trafiałek, J.; Czarniecka-Skubina, E.; Guiné, R.; Gonçalves, J.C.; et al. How do consumers perceive food safety risks?—Results from a multi-country survey. Food Control 2022, 142, 109216. [Google Scholar] [CrossRef]
- Kantiani, L.; Llorca, M.; Sanchis, J.; Farre, M.; Barcelo, D. Emerging food contaminants: A review. Anal. Bioanal. Chem. 2010, 398, 2413–2427. [Google Scholar] [CrossRef] [PubMed]
- EFSA—European Food Safety Authority. Chemical Contaminants in Food and Feed. Available online: https://www.efsa.europa.eu/en/topics/topic/chemical-contaminants-food-feed (accessed on 27 October 2023).
- EFSA—European Food Safety Authority. About Us. Available online: https://www.efsa.europa.eu/en/about/about-efsa (accessed on 27 October 2023).
- EC—European Commission. Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on Maximum Residue Levels of Pesticides in or on Food and Feed of Plant and Animal Origin and Amending Council Directive 91/414/EEC Text with EEA Relevance; European Commission: Brussels, Belgium, 2005. [Google Scholar]
- EC—European Commission. Commission Regulation (EU) No 37/2010 of 22 December 2009 on Pharmacologically Active Substances and Their Classification Regarding Maximum Residue Limits in Foodstuffs of Animal Origin (Text with EEA Relevance); European Commission: Brussels, Belgium, 2010. [Google Scholar]
- EC—European Commission. Commission Regulation (EU) 2023/915 of 25 April 2023 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No 1881/2006 (Text with EEA Relevance); European Commission: Brussels, Belgium, 2023. [Google Scholar]
- EC—European Commission. Rapid Alert System for Food and Feed (RASFF). Available online: https://food.ec.europa.eu/safety/rasff_en (accessed on 27 October 2023).
- EFSA—European Food Safety Authority; Aagaard, A.; Berny, P.; Chaton, P.-F.; Antia, A.L.; McVey, E.; Arena, M.; Fait, G.; Ippolito, A.; Linguadoca, A.; et al. Risk assessment for Birds and Mammals. EFSA J. 2023, 21, e07790. [Google Scholar] [CrossRef]
- Jimenez-Jimenez, S.; Casado, N.; Garcia, M.A.; Marina, M.L. Enantiomeric analysis of pyrethroids and organophosphorus insecticides. J. Chromatogr. A 2019, 1605, 360345. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, N.S.; Rovina, K.; Joseph, V.M. Classification, extraction and current analytical approaches for detection of pesticides in various food products. J. Consum. Prot. Food Saf. 2019, 14, 209–221. [Google Scholar] [CrossRef]
- EC—European Commission. Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 Concerning the Placing of Plant Protection Products on the Market and Repealing Council Directives 79/117/EEC and 91/414/EEC; European Commission: Brussels, Belgium, 2009. [Google Scholar]
- Casado, N.; Gañán, J.; Morante-Zarcero, S.; Sierra, I. New Advanced Materials and Sorbent-Based Microextraction Techniques as Strategies in Sample Preparation to Improve the Determination of Natural Toxins in Food Samples. Molecules 2020, 25, 702. [Google Scholar] [CrossRef]
- EFSA—European Food Safety Authority. Mycotoxins. Available online: https://www.efsa.europa.eu/en/topics/topic/mycotoxins (accessed on 27 October 2023).
- Codex Alimentarius Commission. Code of Practice for the Prevention and Reduction of Ochratoxin a Contamination in Coffee; CAC/RCP 69-2009; Codex Alimentarius Commission: Rome, Italy, 2009. [Google Scholar]
- Codex Alimentarius International Food Standards. Code of Practice for the Prevention and Reduction of Ochratoxin a Contamination; Codex Alimentarius Commission: Rome, Italy, 2013. [Google Scholar]
- Codex Alimentarius Commission. Code of Practice for the Prevention and Reduction of Ochratoxin a Contamination in Wine; CAC/RCP 63-2007; Codex Alimentarius Commission: Rome, Italy, 2007. [Google Scholar]
- Codex Alimentarius Commission. Code of Practice for the Prevention and Reduction of Patulin Contamination in Apple Juice and Apple Juice Ingredients in Other Beverages; CAC/RCP 50-2003; Codex Alimentarius Commission: Rome, Italy, 2003. [Google Scholar]
- EC—European Commission. Commission Regulation (EC) No 401/2006 of 23 February 2006 Laying Down the Methods of Sampling and Analysis for the Official Control of the Levels of Mycotoxins in Foodstuffs (Text with EEA Relevance); European Commission: Brussels, Belgium, 2006. [Google Scholar]
- Daeseleire, E.; Van Pamel, E.; Van Poucke, C.; Croubels, S. Chapter 6—Veterinary Drug Residues in Foods. In Chemical Contaminants and Residues in Food, 2nd ed.; Schrenk, D., Cartus, A., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 117–153. [Google Scholar] [CrossRef]
- Jedziniak, P.; Szprengier-Juszkiewicz, T.; Olejnik, M.; Zmudzki, J. Determination of non-steroidal anti-inflammatory drugs residues in animal muscles by liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta 2010, 672, 85–92. [Google Scholar] [CrossRef]
- Sai, F.; Hong, M.; Yunfeng, Z.; Huijing, C.; Yongning, W. Simultaneous Detection of Residues of 25 β2-Agonists and 23 β-Blockers in Animal Foods by High-Performance Liquid Chromatography Coupled with Linear Ion Trap Mass Spectrometry. J. Agric. Food. Chem. 2012, 60, 1898–1905. [Google Scholar] [CrossRef]
- Robert, C.; Gillard, N.; Brasseur, P.Y.; Pierret, G.; Ralet, N.; Dubois, M.; Delahaut, P. Rapid multi-residue and multi-class qualitative screening for veterinary drugs in foods of animal origin by UHPLC-MS/MS. Food Addit. Contam. Part A 2013, 30, 443–457. [Google Scholar] [CrossRef] [PubMed]
- Casado, N.; Gañán, J.; Morante-Zarcero, S.; Sierra, I. Occurrence of Pyrrolizidines and Other Alkaloids of Plant Origin in Foods. In Encyclopedia of Food Safety, 2nd ed.; Smithers, G.W., Ed.; Academic Press: Oxford, UK, 2024; pp. 518–528. [Google Scholar] [CrossRef]
- Casado, N.; Casado-Hidalgo, G.; González-Gómez, L.; Morante-Zarcero, S.; Sierra, I. Insight into the Impact of Food Processing and Culinary Preparations on the Stability and Content of Plant Alkaloids Considered as Natural Food Contaminants. Appl. Sci. 2023, 13, 1704. [Google Scholar] [CrossRef]
- Casado, N.; Morante-Zarcero, S.; Sierra, I. The concerning food safety issue of pyrrolizidine alkaloids: An overview. Trends Food Sci. Technol. 2022, 120, 123–139. [Google Scholar] [CrossRef]
- González-Gómez, L.; Morante-Zarcero, S.; Pérez-Quintanilla, D.; Sierra, I. Occurrence and Chemistry of Tropane Alkaloids in Foods, with a Focus on Sample Analysis Methods: A Review on Recent Trends and Technological Advances. Foods 2022, 11, 407. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Contaminants in the Food Chain. Scientific opinion on the risks for human health related to the presence of tetrahydrocannabinol (THC) in milk and other food of animal origin. EFSA J. 2015, 13, 4141. [Google Scholar]
- EC—European Commission. Regulation (EU) No 1307/2013 of the European Parliament and of the Council of 17 December 2013 Establishing Rules for Direct Payments to Farmers under Support Schemes within the Framework of the Common Agricultural Policy and Repealing Council Regulation (EC) No 637/2008 and Council Regulation (EC) No 73/2009; European Commission: Brussels, Belgium, 2013. [Google Scholar]
- Gerssen, A.; Pol-Hofstad, I.E.; Poelman, M.; Mulder, P.P.J.; Van den Top, H.J.; De Boer, J. Marine Toxins: Chemistry, Toxicity, Occurrence and Detection, with Special Reference to the Dutch Situation. Toxins 2010, 2, 878–904. [Google Scholar] [CrossRef]
- EFSA—European Food Safety Authority. Process Contaminants. Available online: https://www.efsa.europa.eu/en/topics/topic/process-contaminants (accessed on 27 October 2023).
- Sampaio, G.R.; Guizellini, G.M.; da Silva, S.A.; de Almeida, A.P.; Pinaffi-Langley, A.C.C.; Rogero, M.M.; de Camargo, A.C.; Torres, E. Polycyclic Aromatic Hydrocarbons in Foods: Biological Effects, Legislation, Occurrence, Analytical Methods, and Strategies to Reduce Their Formation. Int. J. Mol. Sci. 2021, 22, 6010. [Google Scholar] [CrossRef]
- Hoogenboom, L.A.P. Scientific opinion: Risks for human health related to the presence of 3-and 2-monochloropropanediol (MCPD), and their fatty acid esters, and glycidyl fatty acid esters in food. EFSA J. 2016, 14, e04426. [Google Scholar]
- EC—European Commission. Commission Regulation (EU) 2017/2158 of 20 November 2017 Establishing Mitigation Measures and Benchmark Levels for the Reduction of the Presence of Acrylamide in Food (Text with EEA Relevance); European Commission: Brussels, Belgium, 2017. [Google Scholar]
- Codex Alimentarius Commission. Code of Practice for the Reduction of Contamination of Food with Polycyclic Aromatic Hydrocarbons (PAH) from Smoking and Direct Drying Processes; CAC/RCP 68-2009; Codex Alimentarius Commission: Rome, Italy, 2009. [Google Scholar]
- EFSA—European Food Safety Authority. Mineral Oil Hydrocarbons in Food. Available online: https://www.efsa.europa.eu/en/infographics/mineral-oil-hydrocarbons-food (accessed on 27 October 2023).
- EFSA Panel on Contaminants in the Food Chain; Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L. Risk for animal and human health related to the presence of dioxins and dioxin-like PCBs in feed and food. EFSA J. 2018, 16, e05333. [Google Scholar]
- EFSA—European Food Safety Authority. Dioxins and PCBs. Available online: https://www.efsa.europa.eu/en/topics/topic/dioxins-and-pcbs (accessed on 27 October 2023).
- EFSA Panel on Food Additives and Flavourings; Younes, M.; Aquilina, G.; Castle, L.; Engel, K.H.; Fowler, P.J.; Frutos Fernandez, M.J.; Fürst, P.; Gundert-Remy, U.; Gürtler, R. Follow-up of the re-evaluation of sulfur dioxide (E 220), sodium sulfite (E 221), sodium bisulfite (E 222), sodium metabisulfite (E 223), potassium metabisulfite (E 224), calcium sulfite (E 226), calcium bisulfite (E 227) and potassium bisulfite (E 228). EFSA J. 2022, 20, e07594. [Google Scholar]
- EC—European Commission. Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the Provision of Food Information to Consumers, Amending Regulations (EC) No 1924/2006 and (EC) No 1925/2006 of the European Parliament and of the Council, and Repealing Commission Directive 87/250/EEC, Council Directive 90/496/EEC, Commission Directive 1999/10/EC, Directive 2000/13/EC of the European Parliament and of the Council, Commission Directives 2002/67/EC and 2008/5/EC and Commission Regulation (EC) No 608/2004 Text with EEA Relevance; European Commission: Brussels, Belgium, 2011. [Google Scholar]
- Bustamante-Rangel, M.; Delgado-Zamarreno, M.M.; Rodriguez-Gonzalo, E. Simple method for the determination of anthelmintic drugs in milk intended for human consumption using liquid chromatography-tandem mass spectrometry. J. Sci. Food Agric. 2022, 102, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.M.; Xu, Y.; He, P.; Zareef, M.; Li, H.; Chen, Q. Simultaneous determination of benzimidazole fungicides in food using signal optimized label-free HAu/Ag NS-SERS sensor. Food Chem. 2022, 397, 133755. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Wang, S.; Ma, W.; Li, G.; Tu, M.; Ma, Z.; Zhang, Q.; Li, H.; Li, X. Simultaneous Determination of Neonicotinoid and Carbamate Pesticides in Freeze-Dried Cabbage by Modified QuEChERS and Ultra-Performance Liquid Chromatography–Tandem Mass Spectrometry. Foods 2023, 12, 699. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Feng, T.; Wang, J.; Hao, L.; Wang, C.; Wu, Q.; Wang, Z. Preparation of magnetic porous covalent triazine-based organic polymer for the extraction of carbamates prior to high performance liquid chromatography-mass spectrometric detection. J. Chromatogr. A 2019, 1602, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Giglio, A.; Vommaro, M.L. Dinitroaniline herbicides: A comprehensive review of toxicity and side effects on animal non-target organisms. Environ. Sci. Pollut. Res. 2022, 29, 76687–76711. [Google Scholar] [CrossRef] [PubMed]
- Kovida; Sharma, V.; Koner, A.L. Rapid on-site and naked-eye detection of common nitro pesticides with ionic liquids. Analyst 2020, 145, 4335–4340. [Google Scholar] [CrossRef] [PubMed]
- Mardani, A.; Torbati, M.; Farajzadeh, M.A.; Mohebbi, A.; Mogaddam, M.R.A. Combination of homogeneous liquid–liquid extraction and dispersive liquid–liquid microextraction for extraction of some organochlorine pesticides from cocoa. Int. J. Environ. Anal. Chem. 2022, 102, 5092–5105. [Google Scholar] [CrossRef]
- Pang, Y.; Zang, X.; Li, H.; Liu, J.; Chang, Q.; Zhang, S.; Wang, C.; Wang, Z. Solid-phase microextraction of organophosphorous pesticides from food samples with a nitrogen-doped porous carbon derived from g-C(3)N(4) templated MOF as the fiber coating. J. Hazard. Mater. 2020, 384, 121430. [Google Scholar] [CrossRef]
- Moinfar, S.; Khodayari, A.; Abdulrahman, S.S.; Aghaei, A.; Sohrabnezhad, S.; Jamil, L.A. Development of a SPE/GC–MS method for the determination of organophosphorus pesticides in food samples using syringe filters packed by GNP/MIL-101(Cr) nanocomposite. Food Chem. 2022, 371, 130997. [Google Scholar] [CrossRef]
- Deng, W.; Yu, L.; Li, X.; Chen, J.; Wang, X.; Deng, Z.; Xiao, Y. Hexafluoroisopropanol-based hydrophobic deep eutectic solvents for dispersive liquid-liquid microextraction of pyrethroids in tea beverages and fruit juices. Food Chem. 2019, 274, 891–899. [Google Scholar] [CrossRef]
- Manousi, N.; Kabir, A.; Furton, K.G.; Zachariadis, G.A.; Rosenberg, E. Expanding the applicability of magnet integrated fabric phase sorptive extraction in food analysis: Extraction of triazine herbicides from herbal infusion samples. Microchem. J. 2022, 179, 107524. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, T.; Zhang, Y.; Ma, J.; Tuo, Y. Preparation and evaluation of a chitosan modified biochar as an efficient adsorbent for pipette tip-solid phase extraction of triazine herbicides from rice. Food Chem. 2022, 396, 133716. [Google Scholar] [CrossRef] [PubMed]
- Ballah, F.M.; Islam, M.S.; Rana, M.L.; Ullah, M.A.; Ferdous, F.B.; Neloy, F.H.; Ievy, S.; Sobur, M.A.; Rahman, A.T.; Khatun, M.M.; et al. Virulence Determinants and Methicillin Resistance in Biofilm-Forming Staphylococcus aureus from Various Food Sources in Bangladesh. Antibiotics 2022, 11, 1666. [Google Scholar] [CrossRef] [PubMed]
- Guironnet, A.; Wiest, L.; Vulliet, E. Improvement of the QuEChERS extraction step by matrix-dispersion effect and application on beta-lactams analysis in wastewater sludge by LC-MS/MS. Talanta 2022, 237, 122923. [Google Scholar] [CrossRef] [PubMed]
- Fei, Z.; Chen, B.; Wei, X.; Lian, L.; Wang, X.; Lou, D. NiO/ZnO-Coated Carbon Microspheres for Dispersive Solid-Phase Extraction (DSPE) of Malachite Green and Crystal Violet in Aquatic Food Products with Determination by Ultra-High-Performance Liquid Chromatography (UPLC). Anal. Lett. 2022, 56, 1192–1205. [Google Scholar] [CrossRef]
- Lu, H.-z.; Chen, Z.-r.; Xu, S.-f. Surface Molecularly Imprinted Polymers on Multi-Function MnO2-Decorated Magnetic Carbon Nanotubes for Dispersive Solid-Phase Extraction of Three Fluoroquinolones from Milk Samples. ACS Food Sci. Technol. 2022, 2, 1612–1621. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Xie, D.; Lai, H.; Qiu, Q.; Ma, X. Optimized synthesis of molecularly imprinted polymers coated magnetic UIO-66 MOFs for simultaneous specific removal and determination of multi types of macrolide antibiotics in water. J. Environ. Chem. Eng. 2022, 10, 108094. [Google Scholar] [CrossRef]
- Varga, I.; Luburić, Đ.B.; Varenina, I.; Kolanović, B.S.; Čalopek, B.; Sedak, M.; Vratarić, D.; Bilandžić, N.; Đokić, M. Residue analysis of nitrofuran metabolites in five food commodities from Croatia using ultra-high performance liquid chromatography–tandem mass spectrometry. J. Food Compos. Anal. 2023, 123, 105614. [Google Scholar] [CrossRef]
- Ning, Y.; Ye, Y.; Liao, W.; Xu, Y.; Wang, W.; Wang, A.-j. Triazine-based porous organic polymer as pipette tip solid-phase extraction adsorbent coupled with HPLC for the determination of sulfonamide residues in food samples. Food Chem. 2022, 397, 133831. [Google Scholar] [CrossRef]
- Tang, Y.; Huang, X.; Wang, X.; Wang, C.; Tao, H.; Wu, Y. G-quadruplex DNAzyme as peroxidase mimetic in a colorimetric biosensor for ultrasensitive and selective detection of trace tetracyclines in foods. Food Chem. 2022, 366, 130560. [Google Scholar] [CrossRef]
- Christodoulou, M.C.; Christou, A.; Stavrou, I.J.; Kapnissi-Christodoulou, C.P. Evaluation of different extraction procedures for the quantification of seven cannabinoids in cannabis-based edibles by the use of LC-MS. J. Food Compos. Anal. 2023, 115, 104915. [Google Scholar] [CrossRef]
- Nyland, C.R.; Moyer, D.C. Regulating for Safety: Cannabidiol Dose in Food: A Review. J. Food Prot. 2022, 85, 1355–1369. [Google Scholar] [CrossRef] [PubMed]
- Izcara, S.; Casado, N.; Morante-Zarcero, S.; Sierra, I. A Miniaturized QuEChERS Method Combined with Ultrahigh Liquid Chromatography Coupled to Tandem Mass Spectrometry for the Analysis of Pyrrolizidine Alkaloids in Oregano Samples. Foods 2020, 9, 1319. [Google Scholar] [CrossRef]
- Han, H.; Jiang, C.; Wang, C.; Wang, Z.; Chai, Y.; Zhang, X.; Liu, X.; Lu, C.; Chen, H. Development, optimization, validation and application of ultra high performance liquid chromatography tandem mass spectrometry for the analysis of pyrrolizidine alkaloids and pyrrolizidine alkaloid N-oxides in teas and weeds. Food Control 2022, 132, 108518. [Google Scholar] [CrossRef]
- González-Gómez, L.; Morante-Zarcero, S.; Pereira, J.A.M.; Câmara, J.S.; Sierra, I. Improved Analytical Approach for Determination of Tropane Alkaloids in Leafy Vegetables Based on µ-QuEChERS Combined with HPLC-MS/MS. Toxins 2022, 14, 650. [Google Scholar] [CrossRef] [PubMed]
- Caviglia, J.M.; Schwabe, R.F. Experimental Hepatocarcinogenesis. In Pathobiology of Human Disease; McManus, L.M., Mitchell, R.N., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 1866–1880. [Google Scholar] [CrossRef]
- Wang, X.; Wang, T.; Nepovimova, E.; Long, M.; Wu, W.; Kuca, K. Progress on the detoxification of aflatoxin B1 using natural anti-oxidants. Food Chem. Toxicol. 2022, 169, 113417. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Xian, Y.; Xiao, K.; Wu, Y.; Zhu, L.; He, J. Development and comparison of single-step solid phase extraction and QuEChERS clean-up for the analysis of 7 mycotoxins in fruits and vegetables during storage by UHPLC-MS/MS. Food Chem. 2019, 274, 471–479. [Google Scholar] [CrossRef]
- Islam, M.T.; Martorell, M.; Gonzalez-Contreras, C.; Villagran, M.; Mardones, L.; Tynybekov, B.; Docea, A.O.; Abdull Razis, A.F.; Modu, B.; Calina, D.; et al. An updated overview of anticancer effects of alternariol and its derivatives: Underlying molecular mechanisms. Front. Pharmacol. 2023, 14, 1099380. [Google Scholar] [CrossRef]
- Puvača, N.; Avantaggiato, G.; Merkuri, J.; Vuković, G.; Bursić, V.; Cara, M. Occurrence and Determination of Alternaria Mycotoxins Alternariol, Alternariol Monomethyl Ether, and Tentoxin in Wheat Grains by QuEChERS Method. Toxins 2022, 14, 791. [Google Scholar] [CrossRef]
- Klingelhofer, I.; Morlock, G.E. Lovastatin in lactone and hydroxy acid forms and citrinin in red yeast rice powders analyzed by HPTLC-UV/FLD. Anal. Bioanal. Chem. 2019, 411, 6655–6665. [Google Scholar] [CrossRef]
- Jia, W.; Shi, L.; Zhang, F.; Fan, C.; Chang, J.; Chu, X. Multiplexing data independent untargeted workflows for mycotoxins screening on a quadrupole-Orbitrap high resolution mass spectrometry platform. Food Chem. 2019, 278, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Lhotská, I.; Kholová, A.; Machyňáková, A.; Hroboňová, K.; Solich, P.; Švec, F.; Šatínský, D. Preparation of citrinin-selective molecularly imprinted polymer and its use for on-line solid-phase extraction coupled to liquid chromatography. Anal. Bioanal. Chem. 2019, 411, 2395–2404. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Liu, X.; Liao, X.; Shi, L.; Zhang, S.; Lu, J.; Zhou, L.; Kong, W. Simultaneous determination of 19 mycotoxins in lotus seed using a multimycotoxin UFLC-MS/MS method. J. Pharm. Pharmacol. 2019, 71, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, A.R.; Mohan, K.; Karthick Rajan, D.; Pillay, A.A.; Palanisami, T.; Sathishkumar, P.; Conterno, L. Distribution, toxicity, interactive effects, and detection of ochratoxin and deoxynivalenol in food: A review. Food Chem. 2022, 378, 131978. [Google Scholar] [CrossRef] [PubMed]
- Carbonell-Rozas, L.; Gámiz-Gracia, L.; Lara, F.J.; García-Campaña, A.M. Determination of the Main Ergot Alkaloids and Their Epimers in Oat-Based Functional Foods by Ultra-High Performance Liquid Chromatography Tandem Mass Spectrometry. Molecules 2021, 26, 3717. [Google Scholar] [CrossRef] [PubMed]
- Sohrabi, H.; Arbabzadeh, O.; Khaaki, P.; Majidi, M.R.; Khataee, A.; Woo Joo, S. Emerging electrochemical sensing and biosensing approaches for detection of Fumonisins in food samples. Crit. Rev. Food Sci. Nutr. 2022, 62, 8761–8776. [Google Scholar] [CrossRef]
- Wang, L.; Hua, X.; Shi, J.; Jing, N.; Ji, T.; Lv, B.; Liu, L.; Chen, Y. Ochratoxin A: Occurrence and recent advances in detoxification. Toxicon 2022, 210, 11–18. [Google Scholar] [CrossRef]
- Bacha, S.A.S.; Li, Y.; Nie, J.; Xu, G.; Han, L.; Farooq, S. Comprehensive review on patulin and Alternaria toxins in fruit and derived products. Front. Plant Sci. 2023, 14, 1139757. [Google Scholar] [CrossRef]
- Kharayat, B.S.; Singh, Y. Chapter 13—Mycotoxins in Foods: Mycotoxicoses, Detection, and Management. In Microbial Contamination and Food Degradation; Holban, A.M., Grumezescu, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 395–421. [Google Scholar] [CrossRef]
- Meneely, J.; Greer, B.; Kolawole, O.; Elliott, C. T-2 and HT-2 Toxins: Toxicity, Occurrence and Analysis: A Review. Toxins 2023, 15, 481. [Google Scholar] [CrossRef]
- Kang, M.; Yao, Y.; Yuan, B.; Zhang, S.; Oderinde, O.; Zhang, Z. A sensitive bimetallic copper/bismuth metal-organic frameworks-based aptasensors for zearalenone detection in foodstuffs. Food Chem. 2024, 437, 137827. [Google Scholar] [CrossRef]
- Verma, V.; Yadav, N. Acrylamide content in starch based commercial foods by using high performance liquid chromatography and its association with browning index. Curr. Res. Food Sci. 2022, 5, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cai, Z.; Zhang, N.; Hu, Z.; Zhang, J.; Ying, Y.; Zhao, Y.; Feng, L.; Zhang, J.; Wu, P. A novel single step solid-phase extraction combined with bromine derivatization method for rapid determination of acrylamide in coffee and its products by stable isotope dilution ultra-performance liquid chromatography tandem triple quadrupole electrospray ionization mass spectrometry. Food Chem. 2022, 388, 132977. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Olajide, T.M.; Cao, W. Automated methods for the determination of 2- and 3-monochloropropanediol (MCPD) esters and glycidyl esters in milk powder products by gas chromatography-tandem mass spectrometry (GC-MS/MS) and risk assessment. J. Food Compos. Anal. 2023, 116, 105084. [Google Scholar] [CrossRef]
- Goh, K.M.; Wong, Y.H.; Tan, C.P.; Nyam, K.L. A summary of 2-, 3-MCPD esters and glycidyl ester occurrence during frying and baking processes. Curr. Res. Food Sci. 2021, 4, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Gazioglu, I.; Zengin, O.S.; Tartaglia, A.; Locatelli, M.; Furton, K.G.; Kabir, A. Determination of Polycyclic Aromatic Hydrocarbons in Nutritional Supplements by Fabric Phase Sorptive Extraction (FPSE) with High-Performance Liquid Chromatography (HPLC) with Fluorescence Detection. Anal. Lett. 2020, 54, 1683–1696. [Google Scholar] [CrossRef]
- Zhu, Z.; Xu, Y.; Huang, T.; Yu, Y.; Bassey, A.P.; Huang, M. The contamination, formation, determination and control of polycyclic aromatic hydrocarbons in meat products. Food Control 2022, 141, 109194. [Google Scholar] [CrossRef]
- Ramli, N.A.S.; Roslan, N.A.; Abdullah, F.; Bilal, B.; Ghazali, R.; Abd Razak, R.A.; Ahmad Tarmizi, A.H. Determination of process contaminants 2- and 3-MCPD esters and glycidyl esters in palm-based glycerol by indirect analysis using GC-MS. Food Addit. Contam. Part A 2023, 40, 1307–1321. [Google Scholar] [CrossRef] [PubMed]
- Lyu, B.; Zhang, X.; Li, J.; Zhang, L.; Zhong, Y.; Wu, Y. Determination of polychlorinated dibenzo-p-dioxins and furans in food samples by gas chromatography-triple quadrupole mass spectrometry (GC-MS/MS) and comparison with gas chromatography-high resolution mass spectrometry (GC-HRMS). J. Food Compos. Anal. 2023, 115, 104947. [Google Scholar] [CrossRef]
- Fernandes, A.R.; Falandysz, J. Polybrominated dibenzo-p-dioxins and furans (PBDD/Fs): Contamination in food, humans and dietary exposure. Sci. Total Environ. 2021, 761, 143191. [Google Scholar] [CrossRef]
- Bauwens, G.; Conchione, C.; Sdrigotti, N.; Moret, S.; Purcaro, G. Quantification and characterization of mineral oil in fish feed by liquid chromatography-gas chromatography-flame ionization detector and liquid chromatography-comprehensive multidimensional gas chromatography-time-of-flight mass spectrometer/flame ionization detector. J. Chromatogr. A 2022, 1677, 463208. [Google Scholar] [CrossRef]
- Yang, L.; Hu, G.; Huang, Y.; Wang, C.; Liu, X.; Lu, C.; Chen, H.; Zhang, J.; Ma, G. Simple and sensitive determination of sulfites in Chinese herbal teas by ultrahigh-performance liquid chromatography tandem mass spectrometry. Anal. Methods 2022, 14, 2849–2856. [Google Scholar] [CrossRef] [PubMed]
- Bijad, M.; Hojjati-Najafabadi, A.; Asari-Bami, H.; Habibzadeh, S.; Amini, I.; Fazeli, F. An overview of modified sensors with focus on electrochemical sensing of sulfite in food samples. Eurasian Chem. Commun. 2021, 3, 116–138. [Google Scholar] [CrossRef]
- Mu, L.; Li, G.; Kang, Q.; Xu, Y.; Fu, Y.; Ye, L. Determination of sulfur dioxide in food by liquid chromatography with pre-column derivatization. Food Control 2022, 132, 108500. [Google Scholar] [CrossRef]
- Wu, Y.; Han, L.; Wu, X.; Jiang, W.; Liao, H.; Xu, Z.; Pan, C. Trends and perspectives on general Pesticide analytical chemistry. Adv. Agrochem 2022, 1, 113–124. [Google Scholar] [CrossRef]
- Yang, B.; Ma, W.; Wang, S.; Shi, L.; Li, X.; Ma, Z.; Zhang, Q.; Li, H. Determination of eight neonicotinoid insecticides in Chinese cabbage using a modified QuEChERS method combined with ultra performance liquid chromatography-tandem mass spectrometry. Food Chem. 2022, 387, 132935. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Li, J.; Feng, J.; Xiang, Y.; Zhu, J.; Li, Y.; Yu, Y.; Li, Y. Core-shell structured magnetic covalent-organic frameworks for rapid extraction and preconcentration of okadaic acid in seawater and shellfish followed with LC-MS/MS quantification. Food Chem. 2022, 374, 131778. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, A.R.; Aramesh, N.; Liu, Z.; Chen, C.; Shen, W.; Tang, S. Recent Advances in the Application of Covalent Organic Frameworks in Extraction: A Review. Crit. Rev. Anal. Chem. 2022, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Li, X.; Bai, Y.; Liu, H. Applications of metal-organic frameworks as advanced sorbents in biomacromolecules sample preparation. TrAC Trends Anal. Chem. 2018, 109, 154–162. [Google Scholar] [CrossRef]
- Montiel-León, J.M.; Duy, S.V.; Munoz, G.; Verner, M.A.; Hendawi, M.Y.; Moya, H.; Amyot, M.; Sauvé, S. Occurrence of pesticides in fruits and vegetables from organic and conventional agriculture by QuEChERS extraction liquid chromatography tandem mass spectrometry. Food Control 2019, 104, 74–82. [Google Scholar] [CrossRef]
- Li, G.; Wen, A.; Liu, J.; Wu, D.; Wu, Y. Facile extraction and determination of organophosphorus pesticides in vegetables via magnetic functionalized covalent organic framework nanocomposites. Food Chem. 2021, 337, 127974. [Google Scholar] [CrossRef]
- Heidari, H.; Ghanbari-Rad, S.; Habibi, E. Optimization deep eutectic solvent-based ultrasound-assisted liquid-liquid microextraction by using the desirability function approach for extraction and preconcentration of organophosphorus pesticides from fruit juice samples. J. Food Compos. Anal. 2020, 87, 103389. [Google Scholar] [CrossRef]
- Ma, W.; Yang, B.; Li, J.; Li, X. Amino-functional metal–organic framework as a general applicable adsorbent for simultaneous enrichment of nine neonicotinoids. Chem. Eng. J. 2022, 434, 134629. [Google Scholar] [CrossRef]
- Ji, B.; Yang, L.; Ren, C.; Xu, X.; Zhao, W.; Yang, Y.; Xu, G.; Zhao, D.; Bai, Y. A modified QuEChERS method based on a reduced graphene oxide-coated melamine sponge for multiresidue analysis of veterinary drugs in mutton by UPLC-MS/MS. Food Chem. 2023, 433, 137376. [Google Scholar] [CrossRef]
- Guo, X.; Tian, H.; Yang, F.; Fan, S.; Zhang, J.; Ma, J.; Ai, L.; Zhang, Y. Rapid determination of 103 common veterinary drug residues in milk and dairy products by ultra performance liquid chromatography tandem mass spectrometry. Front. Nutr. 2022, 9, 879518. [Google Scholar] [CrossRef]
- Al Tamim, A.; Alzahrani, S.; Al-Subaie, S.; Almutairi, M.A.; Al Jaber, A.; Alowaifeer, A.M. Fast simultaneous determination of 23 veterinary drug residues in fish, poultry, and red meat by liquid chromatography/tandem mass spectrometry. Arab. J. Chem. 2022, 15, 104116. [Google Scholar] [CrossRef]
- Wen, L.; Liu, L.; Wang, X.; Wang, M.L.; Lin, J.M.; Zhao, R.S. Spherical mesoporous covalent organic framework as a solid-phase extraction adsorbent for the ultrasensitive determination of sulfonamides in food and water samples by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2020, 1625, 461275. [Google Scholar] [CrossRef] [PubMed]
- Gajda, A.; Szymanek-Bany, I.; Nowacka-Kozak, E.; Gbylik-Sikorska, M. Risk to Public Health Regarding Doxycycline Residues in Chicken Claws. J. Agric. Food. Chem. 2022, 70, 2495–2500. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Xu, X.; Liu, Z.; Xue, S.; Zhang, L. Novel functionalized magnetic ionic liquid green separation technology coupled with high performance liquid chromatography: A rapid approach for determination of estrogens in milk and cosmetics. Talanta 2020, 209, 120542. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shi, X.; Zhao, Q.; Sun, A.; Li, D.; Zhao, J. Determination of lipophilic marine toxins in fresh and processed shellfish using modified QuEChERS and ultra-high-performance liquid chromatography-tandem mass spectrometry. Food Chem. 2019, 272, 427–433. [Google Scholar] [CrossRef]
- Koike, H.; Kanda, M.; Hayashi, H.; Matsushima, Y.; Ohba, Y.; Nakagawa, Y.; Nagano, C.; Sekimura, K.; Hirai, A.; Shindo, T.; et al. Quantification of staphylococcal enterotoxin type A in cow milk by using a stable isotope-labelled peptide via liquid chromatography-tandem mass spectrometry. Food Addit. Contam. Part A 2019, 36, 1098–1108. [Google Scholar] [CrossRef] [PubMed]
- Otero, P.; Miguéns, N.; Rodríguez, I.; Botana, L.M. LC–MS/MS Analysis of the Emerging Toxin Pinnatoxin-G and High Levels of Esterified OA Group Toxins in Galician Commercial Mussels. Toxins 2019, 11, 394. [Google Scholar] [CrossRef] [PubMed]
- Kouti, E.; Tsiasioti, A.; Zacharis, C.K.; Tzanavaras, P.D. Specific determination of histamine in cheese and cured meat products by ion chromatography coupled to fluorimetric detection. Microchem. J. 2021, 168, 106513. [Google Scholar] [CrossRef]
- Kounnoun, A.; El Maadoud, M.; Cacciola, F.; Mondello, L.; Bougtaib, H.; Alahlah, N.; Amajoud, N.; El Baaboua, A.; Louajri, A. Development and Validation of a High-Performance Liquid Chromatography Method for the Determination of Histamine in Fish Samples Using Fluorescence Detection with Pre-column Derivatization. Chromatographia 2020, 83, 893–901. [Google Scholar] [CrossRef]
- Alcantara-Duran, J.; Moreno-Gonzalez, D.; Garcia-Reyes, J.F.; Molina-Diaz, A. Use of a modified QuEChERS method for the determination of mycotoxin residues in edible nuts by nano flow liquid chromatography high resolution mass spectrometry. Food Chem. 2019, 279, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Chong, H.S.; Sim, S.; Yamaguchi, T.; Park, J.; Lee, C.; Kim, M.; Lee, G.; Yun, S.S.; Lim, H.S.; Suh, H.J. Simultaneous determination of sodium iron chlorophyllin and sodium copper chlorophyllin in food using high-performance liquid chromatography and ultra-performance liquid chromatography-mass spectrometry. Food Chem. 2019, 276, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, S.; Celano, R.; Piccinelli, A.L.; Serio, S.; Russo, M.; Rastrelli, L. An analytical platform for the screening and identification of pyrrolizidine alkaloids in food matrices with high risk of contamination. Food Chem. 2023, 406, 135058. [Google Scholar] [CrossRef]
- Steiner, D.; Sulyok, M.; Malachová, A.; Mueller, A.; Krska, R. Realizing the simultaneous liquid chromatography-tandem mass spectrometry based quantification of >1200 biotoxins, pesticides and veterinary drugs in complex feed. J. Chromatogr. A 2020, 1629, 461502. [Google Scholar] [CrossRef]
- Han, L.; Sapozhnikova, Y. Semi-automated high-throughput method for residual analysis of 302 pesticides and environmental contaminants in catfish by fast low-pressure GC-MS/MS and UHPLC-MS/MS. Food Chem. 2020, 319, 126592. [Google Scholar] [CrossRef]
- Fialkov, A.B.; Lehotay, S.J.; Amirav, A. Less than one minute low-pressure gas chromatography—Mass spectrometry. J. Chromatogr. A 2020, 1612, 460691. [Google Scholar] [CrossRef]
- Schanzer, S.; Kroner, E.; Wibbelt, G.; Koch, M.; Kiefer, A.; Bracher, F.; Muller, C. Miniaturized multiresidue method for the analysis of pesticides and persistent organic pollutants in non-target wildlife animal liver tissues using GC-MS/MS. Chemosphere 2021, 279, 130434. [Google Scholar] [CrossRef]
- Patyra, E.; Kwiatek, K. Analytical capabilities of micellar liquid chromatography and application to residue and contaminant analysis: A review. J. Sep. Sci. 2021, 44, 2206–2220. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, D.; Fouad, M.M. Application of NEMI, Analytical Eco-Scale and GAPI tools for greenness assessment of three developed chromatographic methods for quantification of sulfadiazine and trimethoprim in bovine meat and chicken muscles: Comparison to greenness profile of reported HPLC methods. Microchem. J. 2020, 157, 104873. [Google Scholar] [CrossRef]
- Espinosa, F.J.; Toledano, R.M.; Villén, J.; Cortés, J.M.; Vázquez, A.M. TOTAD interface: A review of its application for LVI and LC-GC. Rev. Anal. Chem. 2021, 40, 253–271. [Google Scholar] [CrossRef]
- Arena, K.; Mandolfino, F.; Cacciola, F.; Dugo, P.; Mondello, L. Multidimensional liquid chromatography approaches for analysis of food contaminants. J. Sep. Sci. 2021, 44, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Stilo, F.; Bicchi, C.; Reichenbach, S.E.; Cordero, C. Comprehensive two-dimensional gas chromatography as a boosting technology in food-omic investigations. J. Sep. Sci. 2021, 44, 1592–1611. [Google Scholar] [CrossRef] [PubMed]
- Campone, L.; Rizzo, S.; Piccinelli, A.L.; Celano, R.; Pagano, I.; Russo, M.; Labra, M.; Rastrelli, L. Determination of mycotoxins in beer by multi heart-cutting two-dimensional liquid chromatography tandem mass spectrometry method. Food Chem. 2020, 318, 126496. [Google Scholar] [CrossRef]
- Bogdanova, E.; Pugajeva, I.; Reinholds, I.; Bartkevics, V. Two-dimensional liquid chromatography—High resolution mass spectrometry method for simultaneous monitoring of 70 regulated and emerging mycotoxins in Pu-erh tea. J. Chromatogr. A 2020, 1622, 461145. [Google Scholar] [CrossRef]
- Acevedo, M.S.M.; Gama, M.R.; Batista, A.D.; Rocha, F.R. Two-dimensional separation by sequential injection chromatography. J. Chromatogr. A 2020, 1626, 461365. [Google Scholar] [CrossRef]
- Whiting, J.J.; Myers, E.; Manginell, R.P.; Moorman, M.W.; Anderson, J.; Fix, C.S.; Washburn, C.; Staton, A.; Porter, D.; Graf, D. A high-speed, high-performance, microfabricated comprehensive two-dimensional gas chromatograph. Lab Chip 2019, 19, 1633–1643. [Google Scholar] [CrossRef]
- Keçili, R.; Hussain, C.M. Green micro total analysis systems (GμTAS) for environmental samples. Trends Environ. Anal. Chem. 2021, 31, e00128. [Google Scholar] [CrossRef]
- Goyal, S.; Sharma, R.; Singh, J.; Asadnia, M. Green Chromatography Techniques. In Green Chemical Analysis and Sample Preparations: Procedures, Instrumentation, Data Metrics, and Sustainability; El-Maghrabey, M.H., Sivasankar, V., El-Shaheny, R.N., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 379–432. [Google Scholar] [CrossRef]
- Chullasat, K.; Huang, Z.Z.; Bunkoed, O.; Kanatharana, P.; Lee, H.K. Bubble-in-drop microextraction of carbamate pesticides followed by gas chromatography-mass spectrometric analysis. Microchem. J. 2020, 155, 104666. [Google Scholar] [CrossRef]
- Liao, W.; Zhao, X.; Lu, H.-T.; Byambadorj, T.; Qin, Y.; Gianchandani, Y.B. Progressive Cellular Architecture in Microscale Gas Chromatography for Broad Chemical Analyses. Sensors 2021, 21, 3089. [Google Scholar] [CrossRef]
- Fedorenko, D.; Bartkevics, V. Recent Applications of Nano-Liquid Chromatography in Food Safety and Environmental Monitoring: A Review. Crit. Rev. Anal. Chem. 2023, 53, 98–122. [Google Scholar] [CrossRef] [PubMed]
- Moreno-González, D.; Cutillas, V.; Hernando, M.D.; Alcántara-Durán, J.; García-Reyes, J.F.; Molina-Díaz, A. Quantitative determination of pesticide residues in specific parts of bee specimens by nanoflow liquid chromatography high resolution mass spectrometry. Sci. Total Environ. 2020, 715, 137005. [Google Scholar] [CrossRef] [PubMed]
- Jansons, M.; Fedorenko, D.; Pavlenko, R.; Berzina, Z.; Bartkevics, V. Nanoflow liquid chromatography mass spectrometry method for quantitative analysis and target ion screening of pyrrolizidine alkaloids in honey, tea, herbal tinctures, and milk. J. Chromatogr. A 2022, 1676, 463269. [Google Scholar] [CrossRef] [PubMed]
- Chatzimichail, S.; Rahimi, F.; Saifuddin, A.; Surman, A.J.; Taylor-Robinson, S.D.; Salehi-Reyhani, A. Hand-portable HPLC with broadband spectral detection enables analysis of complex polycyclic aromatic hydrocarbon mixtures. Commun. Chem. 2021, 4, 17. [Google Scholar] [CrossRef]
- Svensson, K.; Weise, C.; Westphal, H.; Södergren, S.; Belder, D.; Hjort, K. Coupling microchip pressure regulators with chipHPLC as a step toward fully portable analysis system. Sens. Actuators B Chem. 2023, 385, 133732. [Google Scholar] [CrossRef]
- Liu, C.C.; Ko, C.H.; Wang, Y.N.; Fu, L.M.; Lee, S.Z. Rapid detection of artificial sweeteners in food using microfluidic chromatography detection system. Chem. Eng. J. 2021, 425, 131528. [Google Scholar] [CrossRef]
- Schade, F.; Schwack, W.; Demirbas, Y.; Morlock, G.E. Open-source all-in-one LabToGo Office Chromatography. Anal. Chim. Acta 2021, 1174, 338702. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).