Abstract
Ester is one of the most significant functional groups in organic chemistry and is enclosed in several valued molecules. Usually, esters are prepared through the acid-catalyzed esterification reaction of carboxylic acids with alcohols, transesterification of esters with alcohols, or via activation of carboxylic acids followed by the addition of alcohols. However, these procedures typically imply the excess use of reactants and harsh reaction conditions. Visible light-mediated photoreactions have been disclosed to display a safe, sustainable, and accessible alternative to traditional methods, and to lead new reactivity modes in organic procedures. In this context, we propose a transition metal-based and organic-based photocatalyst-free synthesis of esters from alcohols induced by visible light. The methodology can be carried out using sunlight or artificial visible light as a solar simulator or a blue LED source.
1. Introduction
Esters are a very significant functional group in organic chemistry, and they are contained in several natural products, polymers, pharmaceuticals, and synthetic intermediates [1].
The most significant examples of molecules containing esters are biofuels such as biodiesel [2,3], solvents such as ethyl acetate and methyl acetate [4], pharmaceuticals [5], plastics and coatings [6,7], and herbicides and pesticides [8]. Moreover, esters are often used as flavoring components [9] and preservatives in food products [10], as additives in perfume [11], and as fragrances in the soap and cosmetic industry [12].
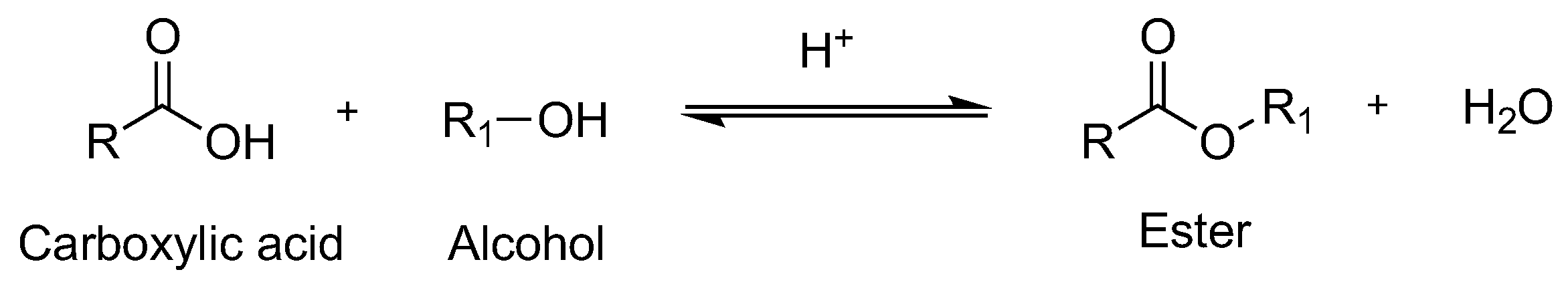
Generally, esters are prepared via Fisher esterification, an acid-catalyzed esterification reaction between carboxylic acids and alcohols, which requires either of two methods to shift the equilibrium in favor of the products [13,14,15] (Scheme 1). One is the removal of water generated as the reactions proceed, via azeotropic distillation or using dehydrating agents, and the other is the use of large excess amounts of one of the reactants, usually alcohols. The reaction fails to achieve the completion, prejudicing the product yield; it is restricted by a slow rate of reaction and a low overall conversion because of the establishment of thermodynamic equilibrium. Moreover, conventional Fisher esterification has other complications associated with the generation of side reactions (such as oxidation and etherification), corrosion of the equipment, tedious purification procedures, and the discharge of large volumes of acidic wastes. Despite being industrially significant, the esterification process has yet to overcome these drawbacks in a cost-effective and environmentally friendly way [16].

Scheme 1.
Fischer esterification.
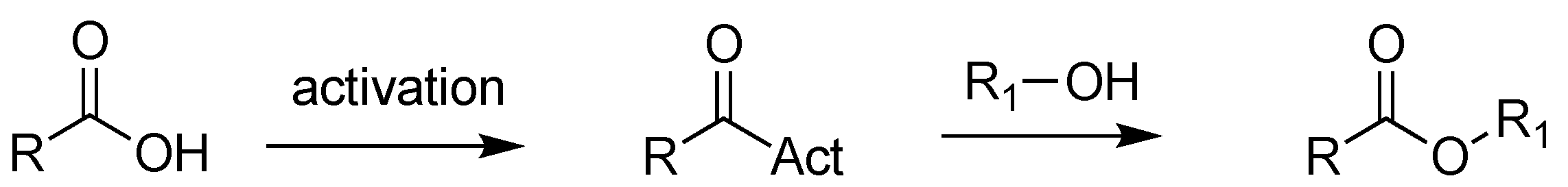
Further, esters can be achieved through the succeeding activation of carboxylic acids (e.g., via acyl chlorides, anhydrides, and activated esters) [17], a process that converts the -OH of the acid into a good leaving group, followed by the addition of alcohols, under harsh reaction conditions [18] (Scheme 2). This procedure is commonly used for the industrial synthesis of pharmaceuticals containing esters, but it presents many limits, such as additional steps, the production of a stoichiometric amount of waste products, and the usage of hazardous reagents [19].
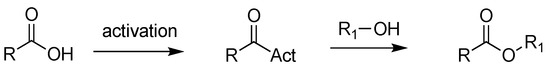
Scheme 2.
Synthesis of esters via activation of carboxylic acids.
An appealing alternative approach is the oxidative esterification of aldehydes with alcohols, but aldehydes are usually gained by selective oxidation of alcohols [10,11,12,13,14,15,16,17,18,19,20,21,22].
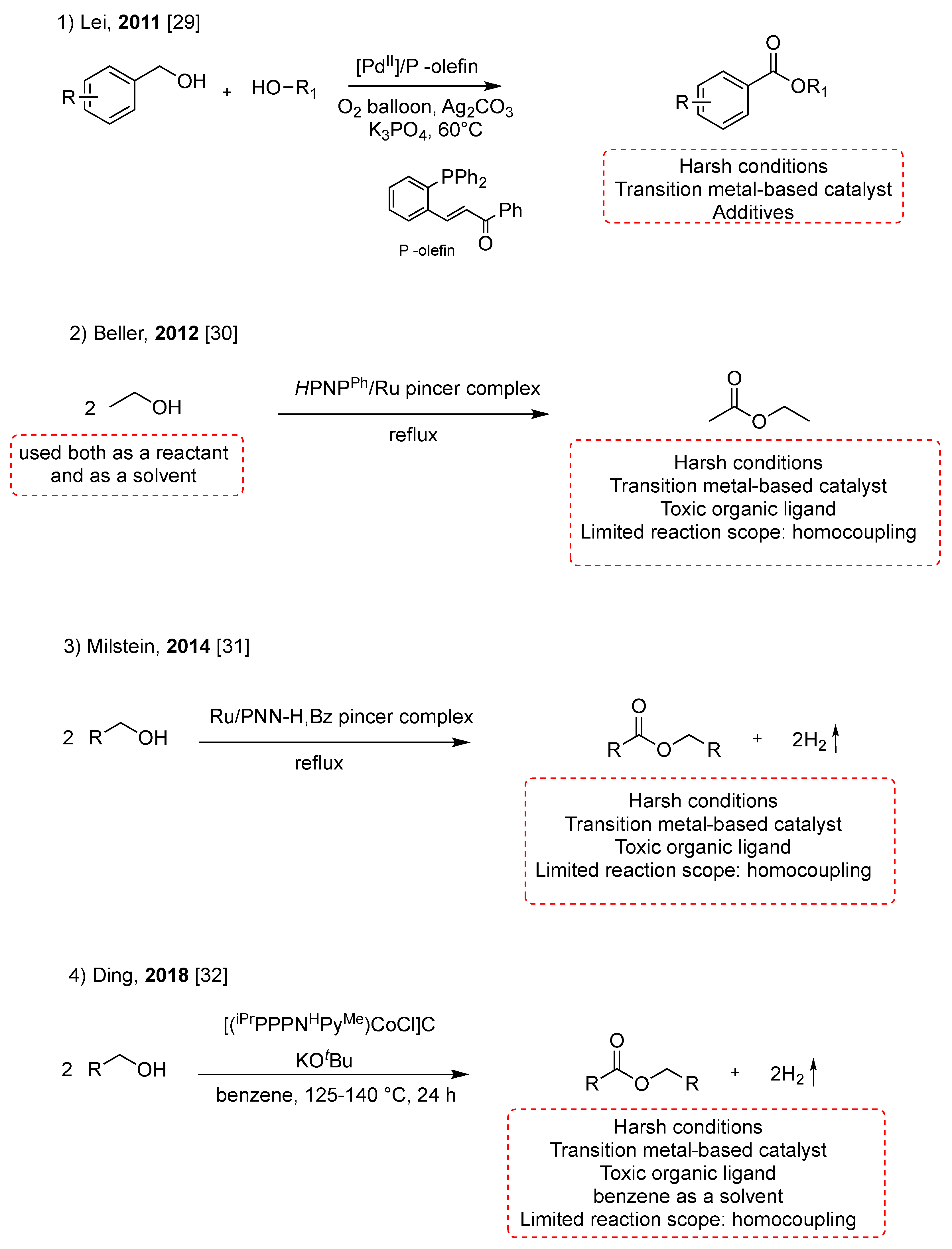
Alcohols are easily accessible, stable, and abundant compounds and are present in many naturally occurring molecules. In light of these reasons, the direct conversion of alcohols to esters is a significant focus of green and sustainable chemistry and offers an economic approach to ester’s synthesis [23,24,25,26,27,28]. In recent years, the direct transformation of alcohols into esters has been achieved using catalysts based on metals such as Pd, Ru, and Co (Scheme 3). In 2011, Lei reported a palladium-catalyzed aerobic oxidative esterification of alcohols using a P-olefin ligand [29]. The reaction was achieved between benzylic alcohols with electron-donating substituents, such as Ph and OMe, and aliphatic alcohols with average yields of 50%, at 60 °C for 12 h (Scheme 3, pathway 1). The milestone of transition metal-catalyzed oxidative esterification of alcohols was Beller’s catalytic acceptorless dehydrogenation of ethanol to ethyl acetate using a Ru-PNP complex with TONs over 15,000 (Scheme 3, pathway 2) [30]. In 2014, Milstein reported a new class of phosphinopyridyl ruthenium pincer complexes, bearing sec-amine coordination, which catalyze homocoupling of alcohols to esters under mild conditions (Scheme 3, pathway 3).
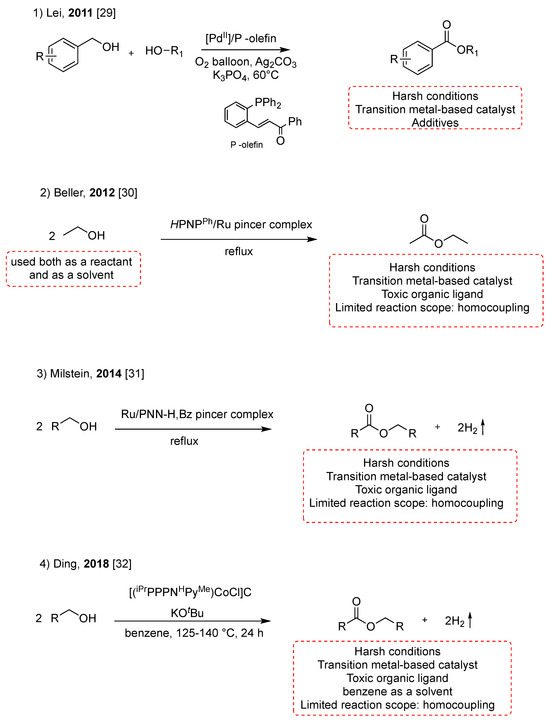
Scheme 3.
Synthesis of esters from alcohols [29,30,31,32].
The above methods require catalysts prepared with expensive precious toxic transition metals and toxic ligands, with consequent increases in the cost and challenges for the subsequent separation and purification of products. The ruthenium complexes were obtained by a two-step synthesis carried out at, respectively, 100 °C for 12 h and 65 °C for 4 h [31].
Later, Ding’s group developed a catalytic system with a tripodal cobalt complex supported by a tetradentate tripodal ligand to achieve the homocoupling of primary alcohols to esters in benzene as a solvent for 24 h at 120 °C (Scheme 3, pathway 4) [32]. Although catalysts based on non-noble metals have been proposed and studied, these catalytic systems still need many organic ligands or high pressure.
Recently, single-side organically decorated Anderson-type chrome-based catalyst [N(C4H9)4]3[CrMo6O18(OH)3{(OCH2)3CCH2OH}] to efficiently convert alcohols to esters has been reported [33]. The catalyst has been prepared by reacting (NH4)6Mo7O24·2H2O with Cr(NO3)3 to obtain (NH4)3[Cr(OH)6Mo6O18]. The compound (NH4)3[Cr(OH)6Mo6O18] was treated with pentaerythritol under reflux at 100 °C for 12 h, and the desired Anderson-type chrome-based catalyst was obtained by adding tetrabutyl bromide to the solution in batches. This pentaerythritol-decorated Anderson-type polyoxometalate has been employed to catalyze the conversion of alcohols into esters in the presence of 3 mmol H2O2 used as an oxidant at 65 °C for 36 h.
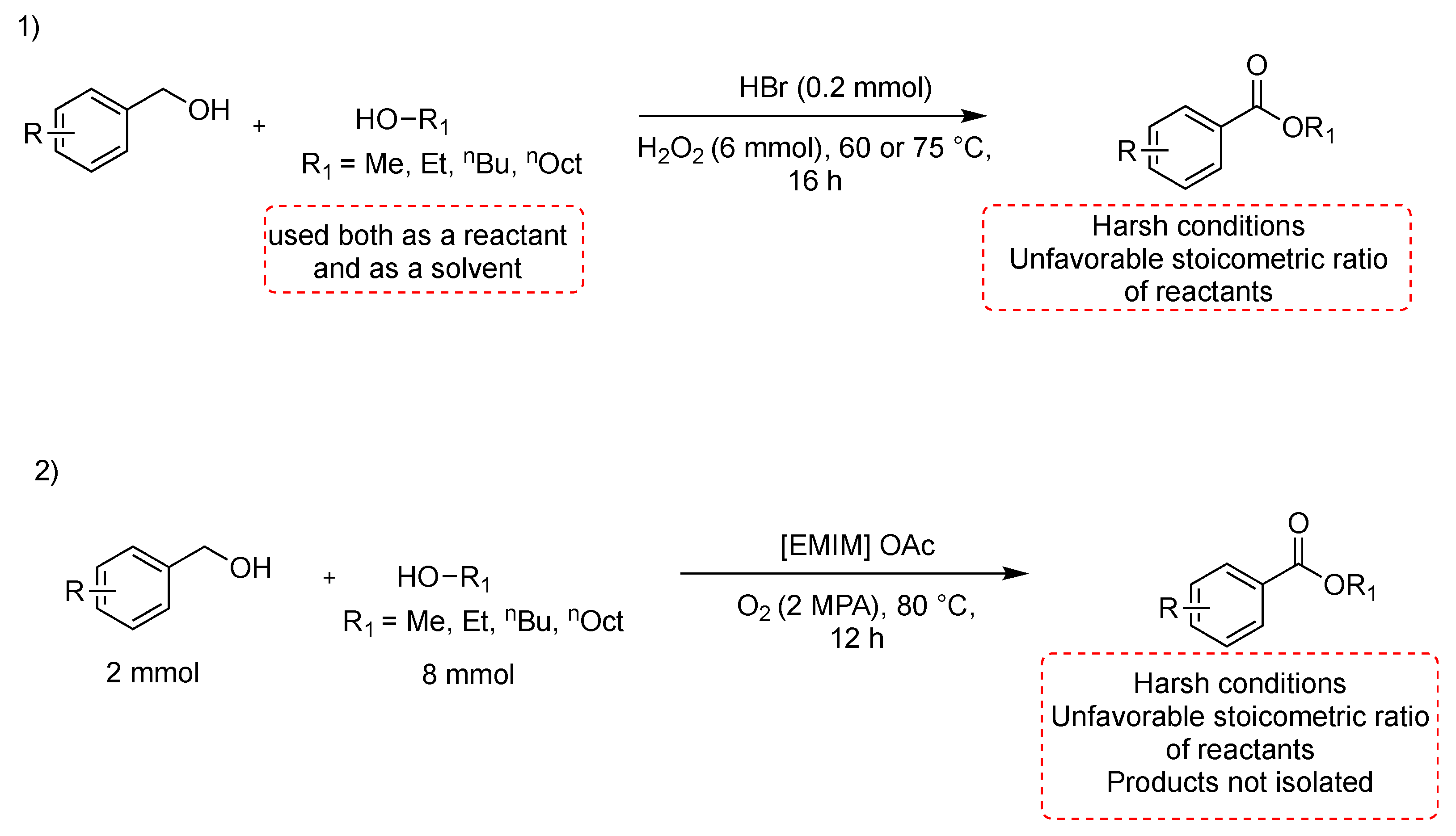
Metal-free oxidative esterification of alcohols is infrequently reported in the literature, even if it is of particular importance to the separation of a metal catalyst from products. Furthermore, transition metal-catalyzed reactions also produce hazardous waste, which is environmentally problematic and, hence, should be avoided wherever possible. Furthermore, it is also highly desirable to develop environmentally benign oxidative esterification processes without the use of any metal-based catalyst. A transition metal-free oxidative esterification of benzylic alcohols with catalytic amount of HBr in aqueous medium at 60–75 °C for 16 h has been reported [34]. The reaction proceeds in the presence of a large excess of the oxidant, and the alcohol is used as a solvent. The methodology is suitable for substituted benzylic alcohols, which have been esterified with methanol, ethanol, n-butanol, and n-octanol; it is also suitable for selective mono-esterification of ethylene glycol and glycerol (Scheme 4, path 1). Another interesting route to esters from alcohols consists of a cross-esterification between benzyl alcohols and aliphatic alcohols in the basic ionic liquid 1-ethyl-3-methylimidazolium acetate ([EMIM] OAc), used both as a solvent and as a catalyst (Scheme 4, path 2) [35]. The benzyl alcohol (2 mmol) is reacted with aliphatic alcohol (8 mmol) in [EMIM]OAc, at 80 °C for 12 h. ILS need to be dried under vacuum at 80 °C for 24 h to remove water and organic impurities, which otherwise might interfere with the catalytic activity. The ILS can be reused five times by being dried under a vacuum at 50 °C for 24 h after the extraction process. Unfortunately, when two different benzylic alcohols were employed as substrates, a mixture including their self- and cross-esterification products was generated.
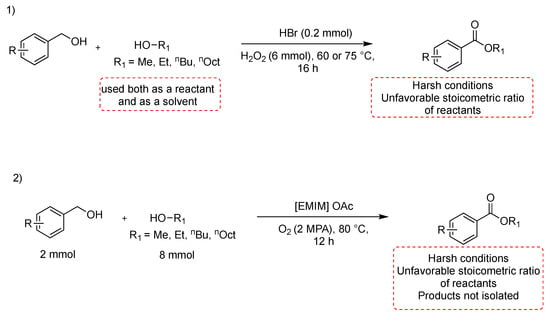
Scheme 4.
Metal-free esterification of alcohols.
These methodologies are oriented to the preparation of methyl benzoate and its analogs. Methyl benzoate is a volatile organic compound that is present naturally as a floral aroma in many plants and is capable of killing insects at different life stages. Compared with commercial pesticides, methyl benzoate is 1.3 to 3.4 times more toxic to gypsy moth larvae and brown marmorated stinkbug nymphs. It is believed to be more desirable as an insect pest toxin than a conventional synthetic insecticide due to its rapid environmental biodegradable property and potentially lower toxicity against natural enemies, humans, and other mammals. Methyl benzoate and benzoate esters are an appealing green alternative to commercially available arthropod repellents based on N, N-diethyl-3-methylbenzamide (DEET) [36]. Moreover, methyl benzoate and benzoate esters are widely used in the perfume industry [37]. They are also an important class of chemical intermediates, commonly used in plasticizers, pharmaceuticals, dyeing polyester fibers, solvents, and disinfectants [38,39,40].
Although the synthesis of benzoate esters is an important target, unfortunately, these procedures suffer from unfavorable stoichiometric ratios of the reagent and hard reaction conditions. Moreover, the use of transition metal-based catalysts, which need to be synthesized, limits the accessibility of these methodologies and their improvement in view of industrial-scale applications. It is, thus, desirable to explore the prospect of finding a new, more sustainable, easily and directly accessible, and cheaper route. Therefore, the development of a new methodology, driven by a green energy source, for effective conversion of alcohols to esters would be highly desirable. One of the most relevant research topics in organic chemistry is the development of new methodologies induced by visible light [41,42]. Photosynthesis can be defined as the transformation of light into chemical energy, efficiently used to promote chemical synthesis, allowing for the development of sustainable and efficient synthetic methodologies. Visible light can be considered a clean reagent: it activates the substrates leaving no residues in the subsequent mixture, with considerable exemplification of the work-up and purification operations. Moreover, the use of visible light, instead of thermal energy, for performing chemical processes, permits great energy savings. For these reasons, visible light-mediated organic synthesis has reached strong urgency because of the development of sustainable chemistry methods [43]. In addition, the majority of photochemical reactions require catalysts based on heavy or rare metals, such as ruthenium and iridium, or organic photocatalysts. So, a photoreaction, which arises without any catalyst, represents an interesting aim. Due to our interest in the oxidative transformations of alcohols [44], we studied the possibility of directly converting primary benzylic alcohols into esters using trichloroisocyanuric acid (TCCA) under visible light irradiation. TCCA is an inexpensive, commercially available, nontoxic, versatile, and efficient oxidizing reagent and chlorinating [45] reagent, frequently used for the disinfection of swimming pools, dishware, houses, hotels, and public places, as well as in fruit and vegetable preservation [46]. The selected reagents have several advantages: alcohols are stable, readily accessible, inexpensive, and commercially available compounds and trichloroisocyanuric acid is a safe, easy-to-handle, and shelf-stable solid. Moreover, the use of visible light allows for a display of a safe, sustainable, and accessible alternative to traditional methods, and the introduction of new reactivity modes in organic synthesis.
2. Results and Discussion
2.1. Study of the Methodology and Optimization Conditions
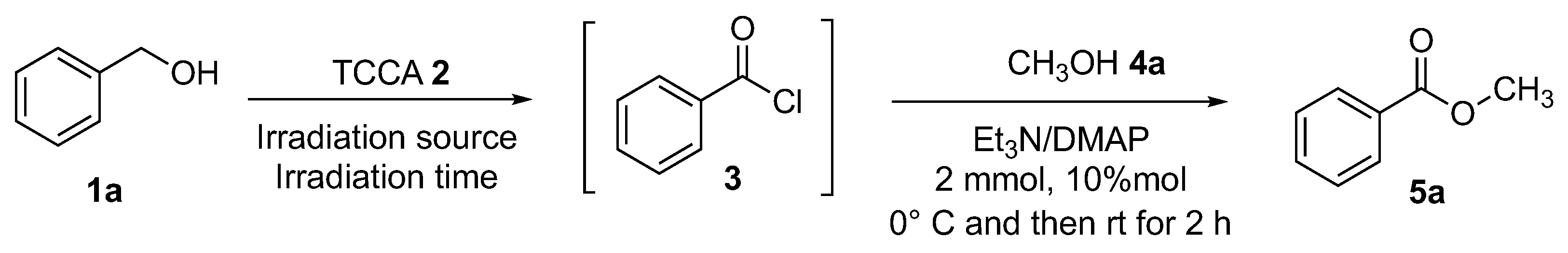
We started our investigation by reacting benzyl alcohol 1a (1.1 mmol) and TCCA 2 (1.5 mmol) in dichloromethane (2 mL) under solar simulator irradiation (Scheme 5) for 1 h (until the complete transformation of the starting alcohol 1a). The resultant benzoyl chloride 3 was quantitatively formed. The resulting mixture, containing the acyl chloride generated in situ, was added with NEt3 (2 mmol) in the presence of a catalytic amount of DMAP (10 mol%), and after 2 h at room temperature, the desired ester was formed in 67% yield (Table 1, 5a, entry 1). Several process parameters have been investigated and the whole optimization process is summarized in Table 1. For the purpose of optimizing the product yield, the reduction of the amount of TCCA 2 was investigated. The same reaction was carried out employing 1.2 mmol (Table 1, entry 2) and 1.3 mmol (Table 1, entry 3) of TCCA 2, and the ester 5a was achieved in 77% and 80%, respectively. Usually, photochemical-mediated methodologies are not clean and they are carried out at a low concentration (>1 M) in order to reduce the secondary photoreactions and, in general, to minimize the product distribution [47]. So, the amount of the solvent was doubled (Table 1, entry 4) and the product yield reached 87%. Then the irradiation time was increased to 1.5 h (Table 1, entry 5), affording an increase in yield of up to 99%. In order to reduce the overall energy expenditure requirement, natural sunlight was employed as an alternative green energy source. The use of solar energy is a favorable tool because it is a reliable, renewable, and advantageous source of energy [42]. Then the synthesis of esters was performed under sunlight irradiation, giving comparable results as reported in Table 1, entry 6. In order to extend the applicability, reliability, and reproducibility of the methodology, the use of blue LED was explored, and methyl benzoate 5a was found in 99% yield (Table 1, entry 7). A screening of solvents was carried out: cyclopentyl methyl ether (CPME) (Table 1, entry 9), tetrahydrofuran (THF) (Table 1, entry 10), acetonitrile (Table 1, entry 11), and ethyl acetate (Table 1, entry 12) were tested, but no formation of methyl benzoate 5a was detected. Using dichloroethane (Table 1, entry 13) as a solvent, ester 5a was obtained, but only in trace amounts. The same transformation was carried out in the dark and in the absence of TCCA, but no product formation was observed (Table 1, entry 14).
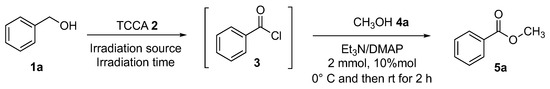
Scheme 5.
General scheme of the transformation of alcohols to esters.

Table 1.
Screening of conditions for the esterification of 1a to 5a.
2.2. Investigation of Reaction Applicability
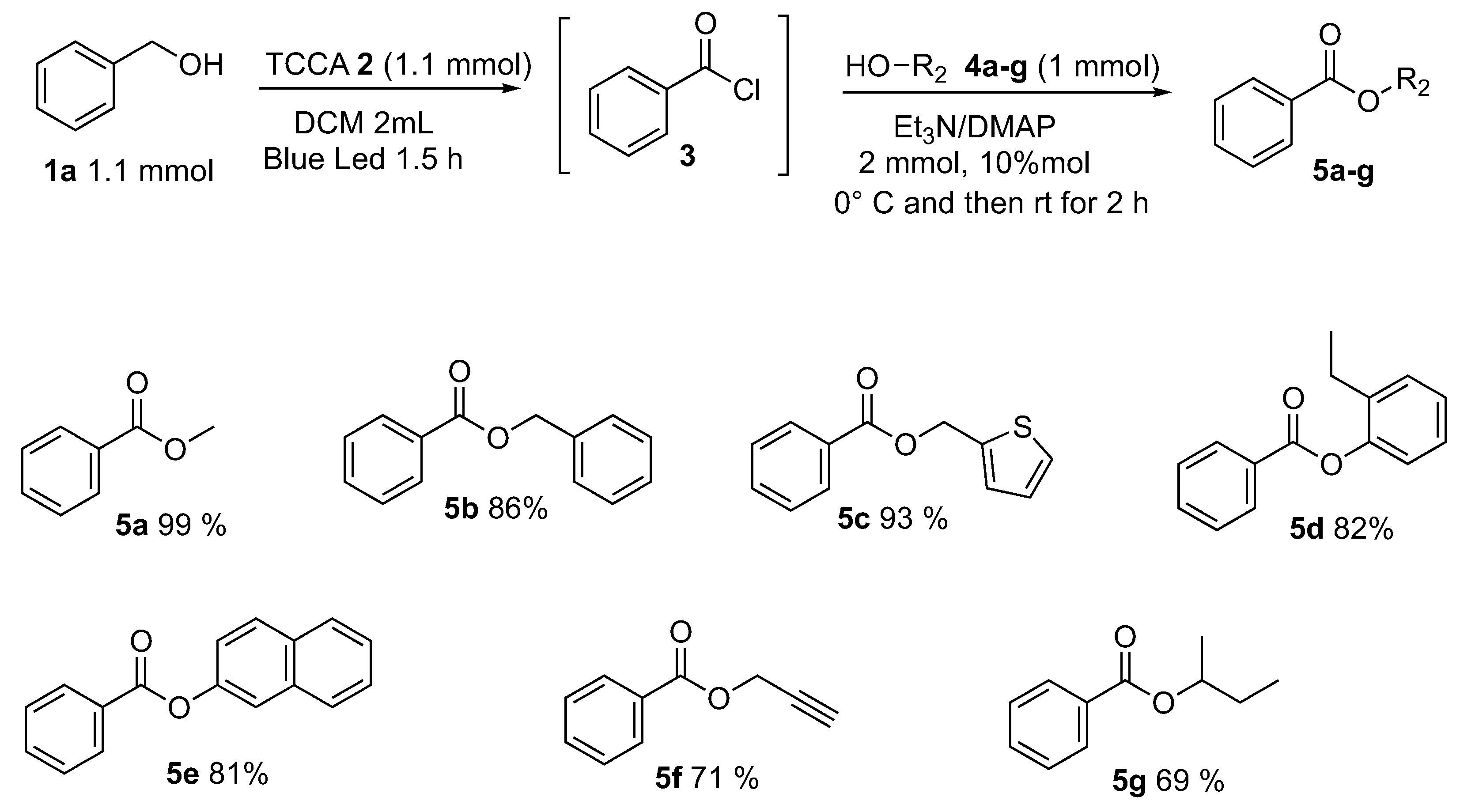
After the optimized conditions were fixed (Table 1, entry 8), the applicability and functional group tolerance were explored. An array of alcohols R2OH, keeping the reactant 1a fixed (Scheme 6), were employed and tested. Benzyl alcohol and thiophen-2-methanol furnished the corresponding esters 5b and 5c in very good yields—respectively, 86% and 93%. In order to test the applicability of the methodology, less nucleophilic phenolic alcohols, 2-ethylphenol, and naphthalen-1-ol were reacted. We were delighted to observe that phenols, which are weak nucleophiles, reacted successfully delivering the 2-ethylphenyl benzoate 5d and 2-ethylphenyl benzoate 5e in a satisfying way (82% and 81% yields). Even if the TCCA is a chlorinating reagent, the esterification showed excellent compatibility toward triple bond and prop-2-yn-1-ol furnished the corresponding prop-2-yn-1-yl benzoate 5f in 71% yield. A sterically hindered secondary alcohol, butan-2-ol, was reacted following optimized conditions affording sec-butyl benzoate 5g in 69% yield.
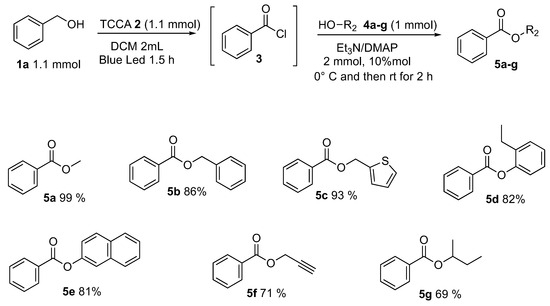
Scheme 6.
Alcohol’s scope.
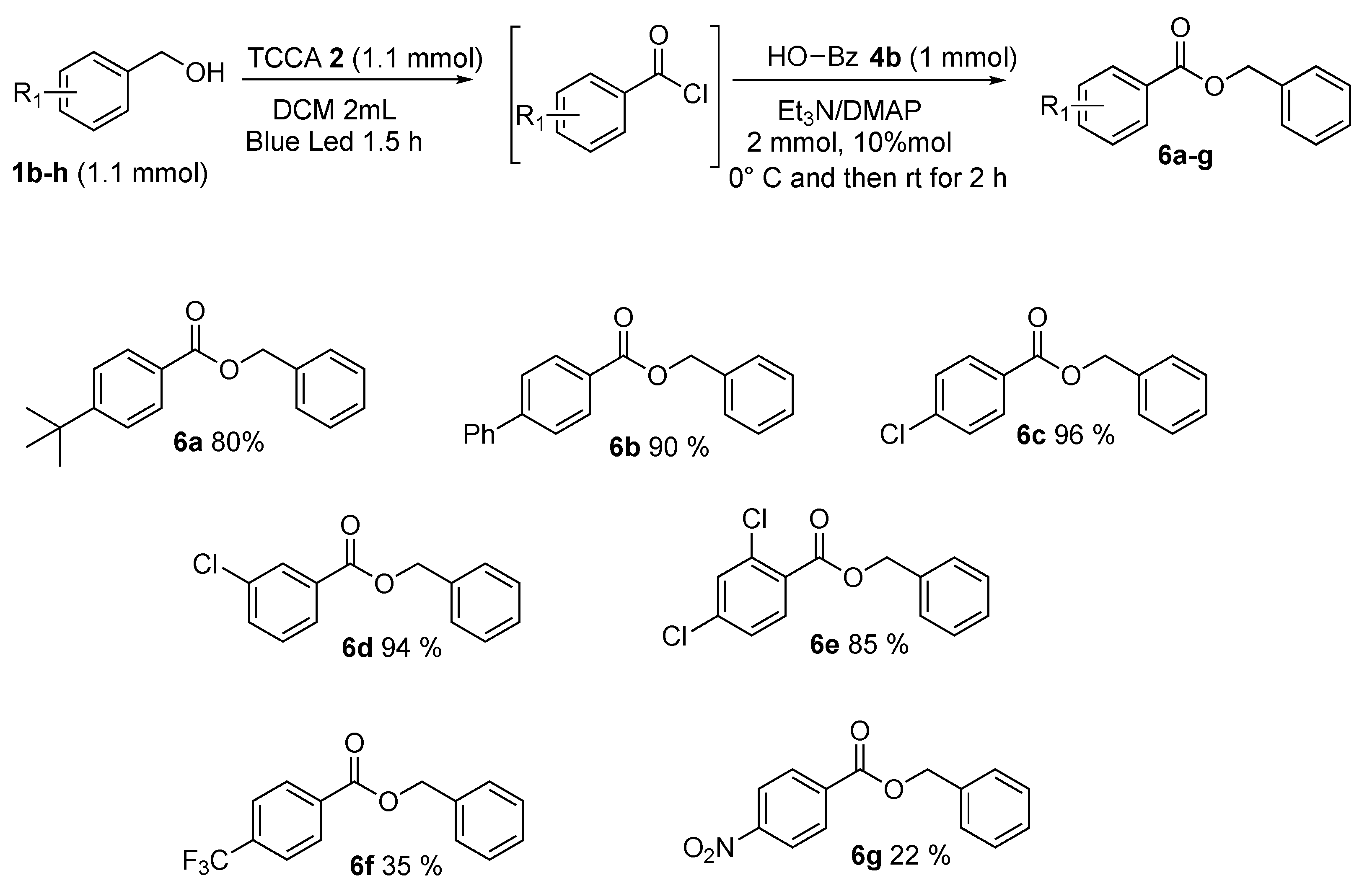
Then, the benzyl alcohols 1b–h were investigated (Scheme 7). In general, the corresponding esters were gained in good yields (Scheme 7, 6a–g). Various functional groups on aromatic rings, both electron-donating and electron-withdrawing, were studied. The steric effects of substituents on the ring of substituted benzyl alcohols were found to have no effect on the yields. Instead, the electronic properties of substituents affected the results. Electron-donating groups in para position as t-butyl and phenyl showed very good results and benzyl 4-(tert-butyl)benzoate 6a and benzyl [1,1′-biphenyl]-4-carboxylate 6b were found in, respectively, 80% and 90% yields. Benzyl alcohol with halide substituents, such as chlorine in para, meta, and orto, para positions was subjected to this procedure producing the resultant esters 6c, 6d, and 6e in very satisfactory yields, which could be further transformed by traditional cross-couplings. Strong electron-withdrawing groups, such as CF3 and NO2, provided benzyl 4-(trifluoromethyl)benzoate 6f and benzyl 4-nitrobenzoate 6g, in poor yields—respectively, 35% and 22%.
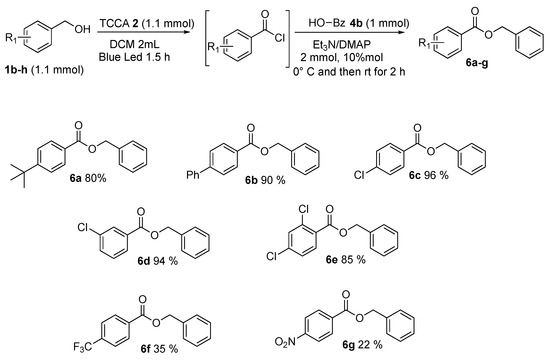
Scheme 7.
Benzyl alcohol’s scope.
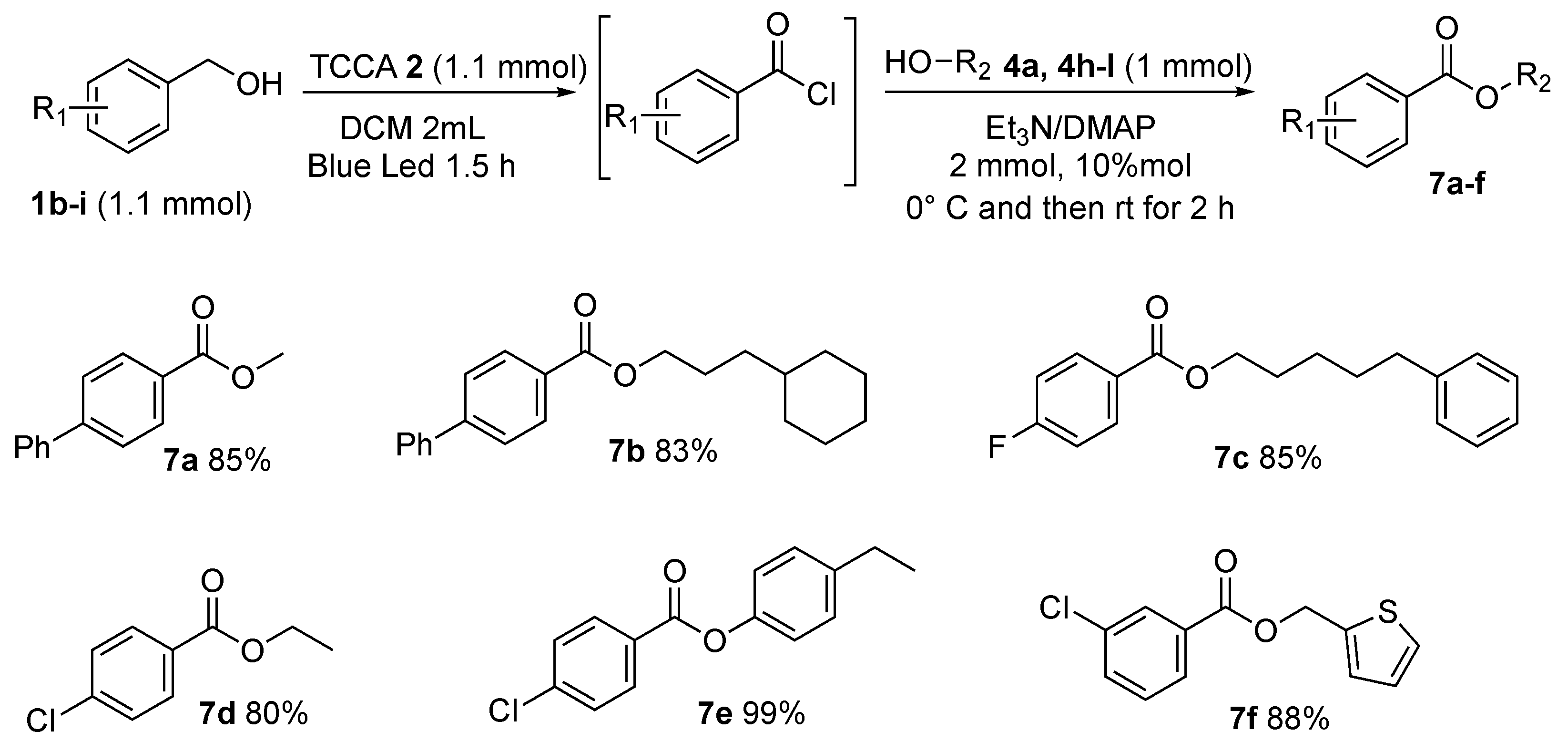
Finally, for the purpose of exploring the general applicability of the procedure, variously substituted benzyl alcohols were reacted with an array of aliphatic alcohols, benzylic alcohols, and phenols (Scheme 8, 7a–f). Of particular interest are the esters composed of alkyloxy chains of medium length, such as 3-cyclohexylpropyl [1,1′-biphenyl]-4-carboxylate 7b, and 5-phenylpentyl 4-fluorobenzoate 7c, found in, respectively, 83% and 85% yields. To prove the synthetic utility of the procedure, thiophen-2-ylmethanol was subjected to optimized conditions, producing the desired heteroaryl ester thiophen-2-ylmethyl 3-chlorobenzoate 7f in 88% yield.
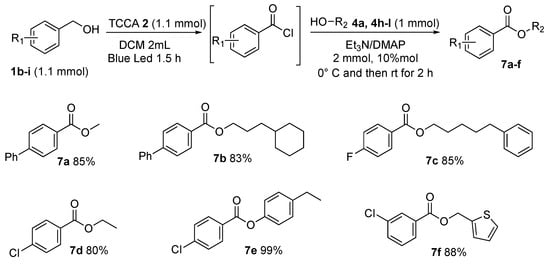
Scheme 8.
General scope.
2.3. Proposed Mechanism
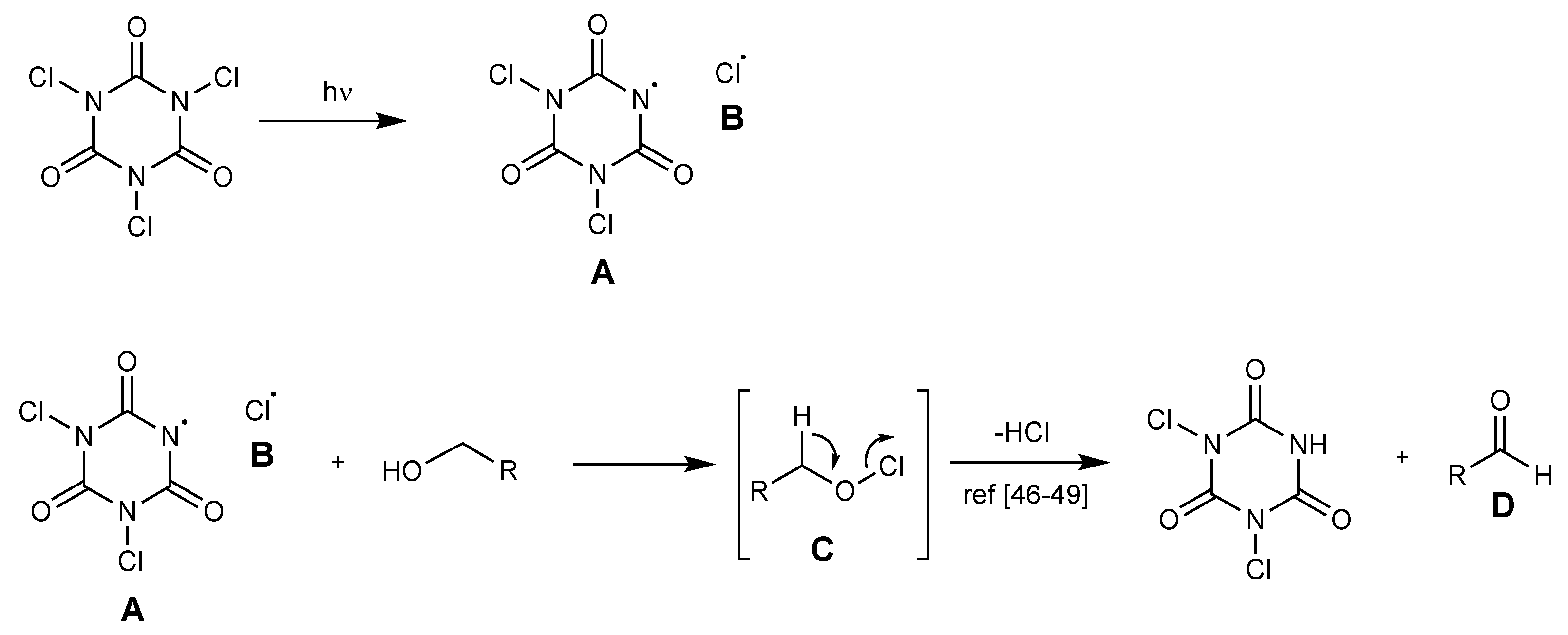
A plausible reaction mechanism has been fixed. The process is supposed to be a radical mechanism. It initiates from a visible light-assisted homolytic cleavage of the N–Cl bond in TCCA to form amidyl radical [48,49,50] A and radical chlorine atom B. Alcohol is converted into a hypochlorite compound C, which readily loses hydrogen chloride to form aldehyde D [51,52,53] (Scheme 9).
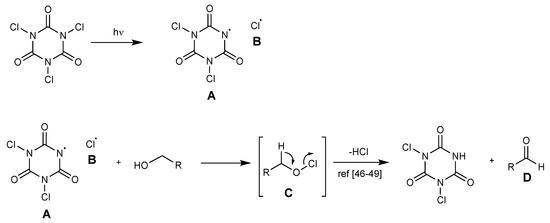
Scheme 9.
Proposed mechanism for aldehyde formation.
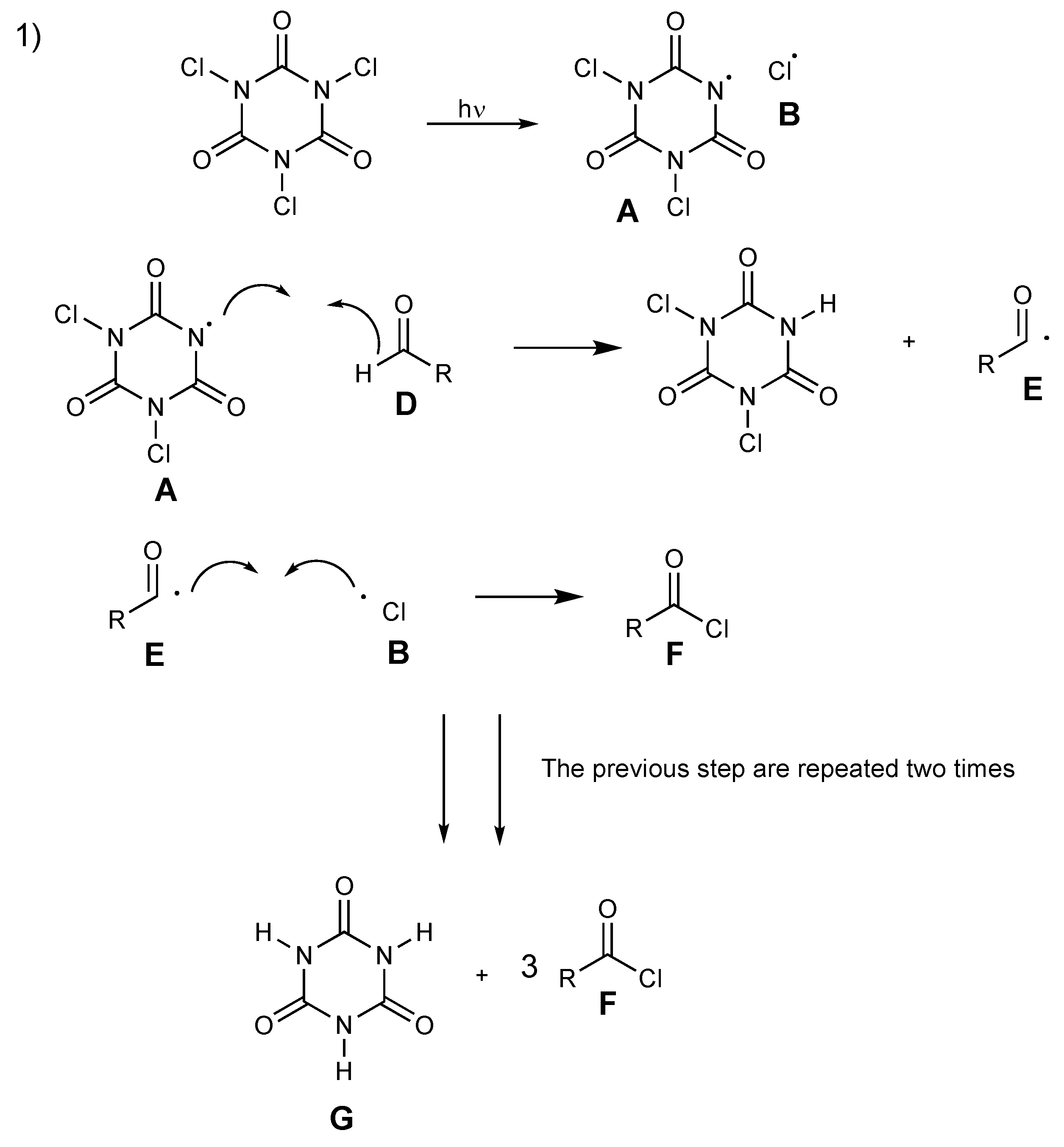
Two plausible pathways of the mechanism for the formation of acyl chlorides from aldehydes with TCCA are shown in Scheme 10 and Scheme 11. In the first possible pathway (Scheme 10), N-centered radical A abstracts a hydrogen atom at the aldehyde D to form acyl radical E. The intermediary acyl radical E and chlorine radical B react together to form acyl chloride F. These key steps are repeated until the generation of cyanuric acid G allows for the complete reaction of all chlorine atoms.
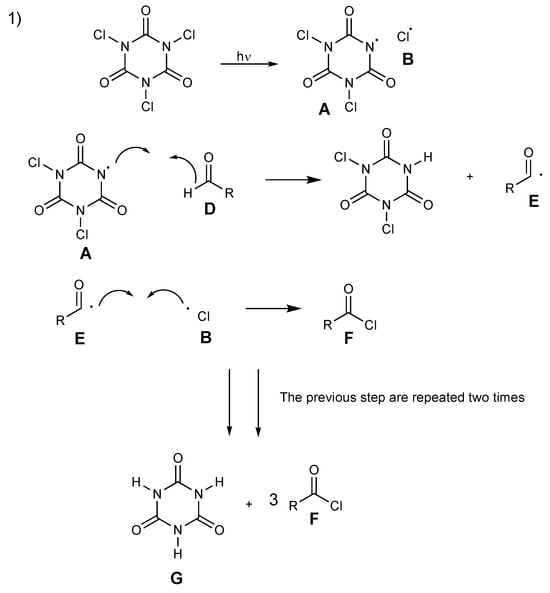
Scheme 10.
Proposed mechanism for acyl chloride formation, pathway 1.
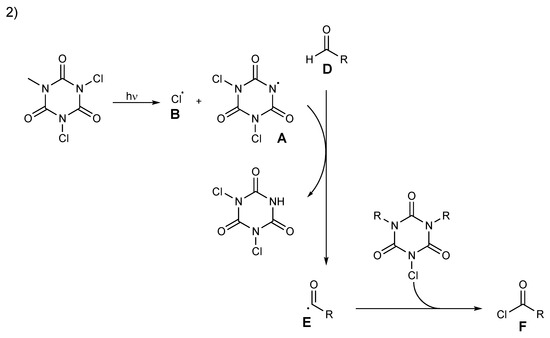
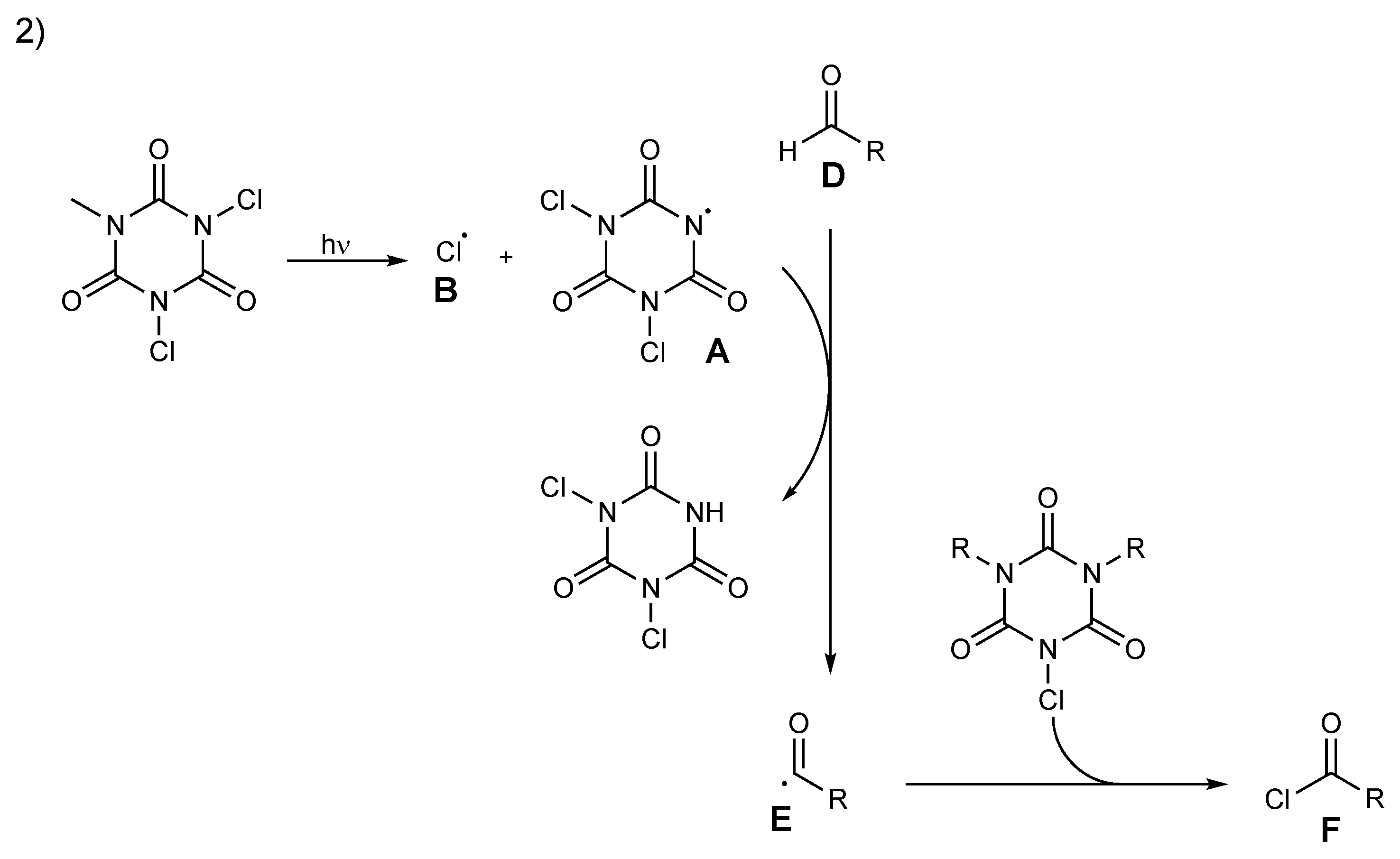
Scheme 11.
Proposed mechanism for acyl chloride formation, pathway 2.
An alternative plausible pathway, pathway 2, is proposed in Scheme 11. The reaction starts with a homolytic cleavage of the N–Cl bond of TCCA to generate radical chlorine atom B and nitrogen-centered radical A and then propagates via abstraction of aldehydes’ hydrogen by A to generate intermediate acyl radical E. Quenching of the acyl radical E by chloride transfer produces acyl chloride F and, subsequently, proceeds until the halogen source has been consumed (Scheme 11).
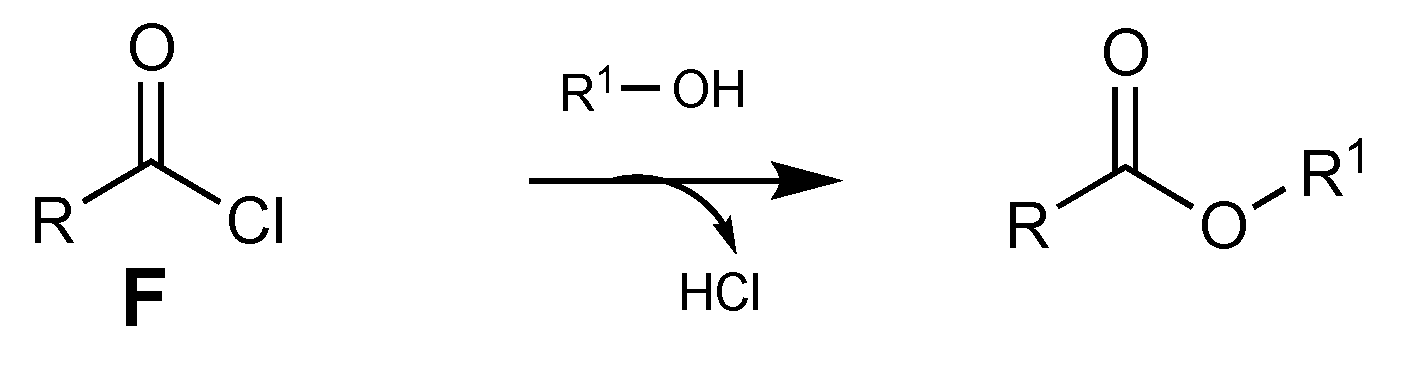
Finally, the acyl chloride F reacts with alcohol to yield the desired ester (Scheme 12) [54].
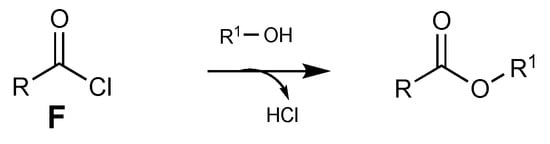
Scheme 12.
Addition of alcohol to acyl chloride.
3. Materials and Methods
3.1. Materials
All solvents and reagents were employed as acquired by commercial providers. All the reactions were carried out in an Argon atmosphere using standard procedures. All solvents were dried by common methods and distilled in an Argon atmosphere. Column chromatography was generally executed on silica gel (pore size 60 Å, 32–63 nm particle size) and reactions were monitored by thin-layer chromatography (TLC); analysis was accomplished with Merck Kieselgel 60 F254 plates and visualized using UV light at 254 nm, KMnO4, 2,4-DNP, and cerium ammonium molybdate staining. The reactions were carried out with the Abet tech sun 2000 simulator (under 100 mW/cm2 simulated AM 1.5G irradiance). Irradiation with blue light was performed with OSRAM Oslon SSL 80 LDCQ7P-1U3U (blue, λ max = 455 nm, I max = 1000 mA, 1.12 W). 1H NMR and 13C NMR spectra were recorded by a Bruker Avance III 400 spectrometer (400 MHz or 100 MHz, respectively) using CDCl3 solutions and TMS as an internal standard. Chemical shifts are indicated as parts per million (ppm, d) relative to the internal tetramethylsilane standard (TMS, d 0.00). The peak patterns are reported as follows: s, singlet; d, doublet; t, triplet; m, multiplet; q, quartet; dd, doublet of doublets; br, broad. The coupling constants, J, are indicated in Hertz (Hz). High-resolution mass spectra HRMS (HESI-FT-ORBITRAP) were performed on a Q-Exactive Thermo Scientific mass spectrometer (Waltham, MA, USA). Melting points were recorded in open capillary tubes and were uncorrected.
3.2. General Procedure for the Preparation of Methyl Benzoate 5a via Solar Simulator Irradiation
In a round bottom flask of 10 mL, furnished by a condenser, TCCA (1.3 mmol) was added to benzyl alcohol (1.1 mmol) solved in 2 mL dichloromethane, in an Ar atmosphere and at room temperature. The resultant suspension was stirred and irradiated by the solar simulator for 1.5 h in an Ar atmosphere (the progress of the synthesis was monitored by TLC until the disappearance of starting benzyl alcohol, 1a). Afterward, the obtained mixture was cooled to 0 °C and stirred under an inert atmosphere of dry argon. First, methanol (1.0 mmol) was added dropwise via syringe, then NEt3 (2.0 mmol) was added dropwise, and finally DMAP (10% mol) at once. After completion of the addition, the resulting mixture was stirred at room temperature until the disappearance of the methanol (monitored by TLC, the reaction is usually complete in about 1 h). Afterward, the solvent was removed in a vacuum and the residue was purified by flash chromatography. Pale yellow oil was obtained (0.135 g, 99% yield).
3.3. General Procedure for the Preparation of Methyl Benzoate 5a via Sunlight Irradiation
In a round bottom flask of 10 mL, furnished by a condenser, TCCA (1.3 mmol) was added to benzyl alcohol (1.1 mmol) solved in 2 mL dichloromethane, in an Ar atmosphere and at room temperature. The resultant suspension was stirred and irradiated by sunlight for 1.5 h, under an Ar atmosphere (the progress was monitored by TLC until the disappearance of the starting benzyl alcohol, 1a). Afterward, the resulting mixture was cooled to 0 °C and stirred under an inert atmosphere of dry argon. First, methanol (1.0 mmol) was added dropwise via syringe, then NEt3 (2.0 mmol) was added dropwise, and finally DMAP (10% mol) at once. After completion of the addition, the obtained mixture was stirred at room temperature until the disappearance of the methanol (monitored by TLC, the reaction is usually complete in about 1 h). Then the solvent was removed under reduced pressure, and the residue was purified by flash chromatography. Pale yellow oil was obtained (0.134 g, 98% yield).
3.4. General Procedure for the Synthesis of Esters 5a–g, 6a–g and 7a–f via Blue LED Irradiation
In a round bottom flask of 10 mL, furnished by a condenser, TCCA (1.1 mmol) was added to benzyl alcohol 1a (1.1 mmol) solved in 1 mL dichloromethane, under an Ar atmosphere, at room temperature. The resultant suspension was stirred and irradiated with blue LED (455 nm) for 1.5 h at 25 °C, in an Ar atmosphere (the progress of the reaction was monitored by TLC until the disappearance of benzyl alcohol, 1a). Afterward, the resultant mixture was cooled to 0 °C and stirred under an inert atmosphere of dry argon. First, alcohol (1.0 mmol) was added dropwise via syringe, then NEt3 (2.0 mmol) was added dropwise, and finally DMAP (10% mol) at once. After completion of the additions, the resulting mixture was stirred at room temperature until the disappearance of the starting alcohol (monitored by TLC, the reaction is generally complete in about 1 h). Then, the solvent was removed under the reduced pressure, and the residue was purified by flash chromatography.
3.5. Detection and Isolation of Benzoyl Chloride 3
In a round bottom flask of 10 mL, furnished by a condenser, TCCA (2.2 mmol) was put in a solution of benzyl alcohol, 1a (2.2 mmol), and 2 mL dichloromethane under an Ar atmosphere, at room temperature. The resultant suspension was stirred under blue LED irradiation (455 nm) for 1.5 h in an Ar atmosphere (the progress of the synthesis was monitored by TLC until the disappearance of the starting reactant). The mixture of the reaction was filtered on Celite, then the solvent was removed under the reduced pressure, and the residue was distilled.
4. Conclusions
In conclusion, a visible light-promoted cross-coupling of alcohols to esters was reported. The starting materials have several advantages: alcohols are stable, readily accessible, inexpensive, and commercially available compounds; trichloroisocyanuric acid is a safe, easy-to-handle, shelf-stable solid; and the use of visible light as a source of energy is very appealing from an ecological point of view. The conditions are mild, and the stoichiometric ratio of the reagents is ideal. The methodology has shown good versatility and applicability, and various functional groups are well tolerated, providing an alternative approach to visible light-mediated ester synthesis, which avoids the use of expensive and toxic transition metal-based catalysts and toxic organic ligands. The reaction can be carried out using different visible light sources, such as blue LED, solar simulator, and sunlight. The methodology is operationally simple and the reactants as well as the visible light sources are economical and readily accessible. The proposed procedure appears to be very suitable to prepare benzoate esters, which are an industrially relevant class of compounds.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29030570/s1, General information; General procedure for the synthesis of methyl benzoate 5a under solar simulator irradiation; General procedure for the synthesis of methyl benzoate 5a under sunlight irradiation; General procedure to esters under blue LED irradiation; Compound characterizations; Detection and characterization of benzoyl chloride 3; References [25,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73]; NMR spectra of esters; NMR spectra of benzoyl chloride (3); UV-Vis spectra.
Author Contributions
Conceptualization, writing—original draft preparation, writing—review and editing, L.D.L.; validation, formal analysis, investigation, data curation, A.D., E.C., A.P., M.C., L.P. and S.G.; supervision, review and editing, A.P. and S.G.; funding acquisition, L.D.L. All authors have read and agreed to the published version of the manuscript.
Funding
Università degli Studi di Sassari within “Finanziamento straordinario una tantum per la ricerca 2020”, (project FAR2020DELUCA).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the Supplementary Materials.
Acknowledgments
We acknowledge the Dipartimento di Scienze Chimiche, Fisiche, Matematiche e Naturali, and the Università degli Studi di Sassari for the facilities.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ishihara, K. Dehydrative condensation catalyses. Tetrahedron 2009, 65, 1085–1109. [Google Scholar] [CrossRef]
- Moser, B.R. Biodiesel Production, Properties, and Feedstocks. In Biofuels; Springer: New York, NY, USA, 2011; pp. 285–347. [Google Scholar]
- Ambat, I.; Srivastava, V.; Sillanpaa, M. Recent advancement in biodiesel production methodologies using various feedstock: A review. Renew. Sustain. Energy Rev. 2018, 90, 356–369. [Google Scholar] [CrossRef]
- Petibon, R.; Aiken, C.P.; Ma, L.; Xiong, D.; Dahn, J.R. The use of ethyl acetate as a sole solvent in highly concentrated electrolyte for Li-ion batteries. Electrochim. Acta 2015, 154, 287–293. [Google Scholar] [CrossRef]
- De Nazare de Oliveira, A.; de Oliveira, D.T.; Angèlica, R.S.; de Aguiar Andrade, E.H.; do Rosàrio da Silva, J.K.; da Rocha Filo, G.N.; Coral, N.; de Oliveira Pires, L.H.; Luque, R.; Santos do Nascimento, L.A. Efficient esterification of eugenol using a microwave-activated waste kaolin. React. Kinet. Mech. Catal. 2020, 130, 633–653. [Google Scholar] [CrossRef]
- Pereira, C.S.; Silva, V.M.; Rodrigues, A.E. Ethyl lactate as a solvent: Properties, applications and production processes—A review. Green Chem. 2011, 13, 2658–2671. [Google Scholar] [CrossRef]
- Tang, X.; Chen, E.Y.-X. Toward Infinitely Recyclable Plastics Derived from Renewable Cyclic Esters. Chem 2019, 5, 284–312. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, W. Antioxidant and antibacterial chitosan film with tea polyphenols-mediated green synthesis silver nanoparticle via a novel one-pot method. Int. J. Biol. Macromol. 2020, 155, 1252–1261. [Google Scholar] [CrossRef]
- Zare, M.; Golmakani, M.-T.; Sardarian, A. Green synthesis of banana flavor using different catalysts: A comparative study of different methods. Green Chem. Lett. Rev. 2020, 13, 83–92. [Google Scholar] [CrossRef]
- De Barros, D.P.C.; Azevedo, A.M.; Cabral, J.M.S.; Fonseca, L.P. Optimization of flavor esters synthesis by Fusarium solani pisi cutinase. J. Food Biochem. 2012, 36, 275–284. [Google Scholar] [CrossRef]
- Almeida, S.A.A.G.; de Meneses, A.C.; de Araùjo, P.H.H.; de Oliveira, D. A review on enzymatic synthesis of aromatic esters used as flavor ingredients for food, cosmetics and pharmaceuticals industries. Trends Food Sci. Technol. 2017, 69, 95–105. [Google Scholar]
- Steele, J.H.; Bozor, M.X.; Boyce, G.R. Transumtation of scent: An evaluation of the synthesis of methyl cinnamate, a commercial fragrance, via a Fischer esterification for the second-year organic laboratory. J. Chem. Educ. 2020, 97, 4127–4132. [Google Scholar] [CrossRef]
- Otera, J.; Dan-oh, N.; Nozaki, H. Novel template effects of distannoxane catalysts in highly efficient transesterification and esterification. J. Org. Chem. 1991, 56, 5307–5311. [Google Scholar] [CrossRef]
- Ishihara, K.; Ohara, S.; Yamamoto, H. 3,4,5-Trifluorobenzeneboronic Acid as an Extremely Active Amidation Catalyst. J. Org. Chem. 1996, 61, 4196–4197. [Google Scholar] [CrossRef] [PubMed]
- Storck, S.; Maier, W.F.; Salvado, I.M.M.; Ferreira, J.M.F.; Guhl, D.; Souverijins, W.; Martens, J.A. Amorphous Sn/Si Mixed Oxides, Mild Solid Lewis Acid Catalysts for Esterification and Etherification Reactions. J. Catal. 1997, 172, 414–426. [Google Scholar] [CrossRef]
- Mustafa, A.; Faisal, S.; Ahmed, I.A.; Munir, M.; Pereira Cipolatti, E.; Manoel, E.A.; Pastore, C.; di Bitonto, L.; Hanelt, D.; Nitbani, F.O.; et al. Has the time finally come for green oleochemicals and biodiesel production using large-scale enzyme technologies? Current status and new developments. Biotechnol. Adv. 2023, 69, 108275–108293. [Google Scholar] [CrossRef]
- Otera, J.; Nishikido, J. Esterification: Methods, Reactions, and Applications, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2010. [Google Scholar]
- Wipf, P. Handbook of Reagents for Organic Synthesis; Wiley: New York, NY, USA, 2005. [Google Scholar]
- Valeur, E.; Bradley, M. Amide bond formation: Beyond the myth of coupling reagents. Chem. Soc. Rev. 2009, 38, 606–631. [Google Scholar] [CrossRef] [PubMed]
- Enache, D.I.; Edwards, J.K.; Landon, P.; Solsona-Espriu, B.; Carley, A.F.; Herzing, A.A.; Watanabe, M.; Kiely, C.J.; Knight, D.W.; Hutchings, G.J. Solvent-free oxidation of primary alcohols to aldehydes using Au-Pd/TiO2 catalysts. Science 2006, 311, 362–365. [Google Scholar] [CrossRef]
- Mallat, T.; Baiker, A. Oxidation of alcohols with molecular oxygen on solid catalysts. Chem. Rev. 2004, 104, 3037–3058. [Google Scholar] [CrossRef]
- Liu, C.; Tang, S.; Zheng, L.; Liu, D.; Zhang, H.; Lei, A. Covalently Bound Benzyl Ligand Promotes Selective Palladium-Catalyzed Oxidative Esterification of Aldehydes with Alcohols. Angew. Chem. Int. Ed. 2012, 51, 5662–5666. [Google Scholar] [CrossRef]
- Tang, S.; Yuan, J.; Liu, C.; Lei, A. Direct oxidative esterification of alcohols. Dalton Trans. 2014, 43, 13460–13470. [Google Scholar] [CrossRef]
- Ekoue-Kovi, K.; Wolf, C. One-pot oxidative esterification and amidation of aldehydes. Chem. Eur. J. 2008, 14, 6302–6315. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Liang, S.; Chen, S.-Y.; Zhang, J.; Fu, S.-S.; Yu, X.-Q. A Metal-Free Oxidative Esterification of the Benzyl C–H Bond. Adv. Synth. Catal. 2012, 354, 1287–1292. [Google Scholar] [CrossRef]
- Liu, C.; Tang, S.; Lei, A. Oxidant controlled Pd-catalysed selective oxidation of primary alcohols. Chem. Commun. 2013, 49, 1324–1326. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, C.; Yuan, J.; Lei, A. Transition-metal-free aerobic oxidation of primary alcohols to carboxylic acids. New J. Chem. 2013, 37, 1700–1703. [Google Scholar] [CrossRef]
- Xia, J.; Shao, A.; Tang, S.; Gao, X.; Gao, M.; Lei, A. Palladium-catalysed oxidative cross-esterification between two alcohols. Org. Biomol. Chem. 2015, 13, 6154–6157. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, J.; Meng, L.; Deng, Y.; Li, Y.; Lei, A. Palladium-Catalyzed Aerobic Oxidative Direct Esterification of Alcohols. Angew. Chem. Int. Ed. 2011, 50, 5144–5148. [Google Scholar] [CrossRef]
- Nielsen, M.; Junge, H.; Kammer, A.; Beller, M. Towards a Green Process for Bulk-Scale Synthesis of Ethyl Acetate: Efficient Acceptorless Dehydrogenation of Ethanol. Angew. Chem. Int. Ed. 2012, 51, 5711–5713. [Google Scholar] [CrossRef]
- Fogler, E.; Garg, J.A.; Hu, P.; Leitus, G.; Shimon, L.J.W.; Milstein, D. System with Potential Dual Modes of Metal–Ligand Cooperation: Highly Catalytically Active Pyridine-Based PNNH–Ru Pincer Complexes. Chem. Eur. J. 2014, 20, 15727–15731. [Google Scholar] [CrossRef]
- Paudel, K.; Pandey, B.; Xu, S.; Taylor, D.K.; Tyer, D.L.; Lopez Torres, C.; Gallagher, S.; Kong, L.; Ding, K. Cobalt-Catalyzed Acceptorless Dehydrogenative Coupling of Primary Alcohols to Esters. Org. Lett. 2018, 20, 4478–4481. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, F.; Tao, C.; Yu, H.; Ruhlmann, L.; Wei, Y. Oxidative esterification of alcohols by a single-side organically decorated Anderson-type chrome-based catalyst. Green Chem. 2021, 23, 2652–2657. [Google Scholar] [CrossRef]
- Samanta, S.; Pappula, V.; Dinda, M.; Adimurthy, A. Transition metal-free oxidative esterification of benzylic alcohols in aqueous medium. Org. Biomol. Chem. 2014, 12, 9453–9456. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, Z.; Liu, H.; Xie, Z.; Mei, Q.; Han, B. Transformation of alcohols to esters promoted by hydrogen bonds using oxygen as the oxidant under metal-free conditions. Sci. Adv. 2018, 4, eaas9319. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Chen, J.; Zhang, A. Commercially available natural benzyl esters and their synthetic analogs exhibit different toxicities against insect pests. Sci. Rep. 2018, 8, 7902. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, P.; Dollimore, D.; Alexander, K. The use of thermogravimetry to follow the rate of evaporation of an ingredient used in perfumes. J. Ther. Anal. Calorim. 1997, 49, 595–599. [Google Scholar] [CrossRef]
- Wardzińska, E.; Penczek, P. Influence of the glycol component in dibenzoate plasticizers on the properties of plasticized PVC films. J. Appl. Polym. Sci. 2005, 97, 822–824. [Google Scholar] [CrossRef]
- Tekin, E.; de Gans, B.J.; Schubert, U.S. Ink-jet printing of polymers–from single dots to thin film libraries. J. Mater. Chem. 2004, 14, 2627–2632. [Google Scholar] [CrossRef]
- Patel, J.P.; Deshmukh, S.; Zhao, C.; Wamuo, O.; Hsu, S.L.; Schoch, A.B.; Carleen, S.A.; Matsumoto, D. An analysis of the role of nonreactive plasticizers in the crosslinking reactions of a rigid resin. J. Polym. Sci. B Polym. Phys. 2017, 55, 206–213. [Google Scholar] [CrossRef]
- Stephenson, C.R.J.; Yoon, T.P.; MacMillan, D.W.C. Visible Light Photocatalysis in Organic Chemistry; Wiley-VCH: Weinheim, Germany, 2017. [Google Scholar]
- Konig, B. Photocatalysis in Organic Synthesis—Past, Present, and Future. Eur. J. Org. Chem. 2017, 15, 1979–1981. [Google Scholar] [CrossRef]
- Prier, C.K.; Rankic, D.A.; MacMillan, D.W. Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem. Rev. 2013, 113, 5322–5363. [Google Scholar] [CrossRef]
- Dettori, G.; Gaspa, S.; Porcheddu, A.; De Luca, L. A two-step tandem reaction to prepare hydroxamic acids directly from alcohols. Org. Biomol. Chem. 2014, 12, 4582–4585. [Google Scholar] [CrossRef]
- Gaspa, S.; Carraro, M.; Pisano, L.; Porcheddu, A.; De Luca, L. Trichloroisocianuric acid: A versatile and efficient chlorinationg and oxidizing reagent. Eur. J. Org. Chem. 2019, 2019, 3544–3552. [Google Scholar] [CrossRef]
- Tilstam, U.; Weinmann, H. Trichloroisocyanuric acid: A safe and efficient oxidant. Org. Process Res. Dev. 2002, 6, 384–393. [Google Scholar] [CrossRef]
- Protti, S.; Ravelli, M.; Fagnoni, M.; Albini, A. Solar light-driven photocatalyzed alkylations. Chemistry on the window ledge. Chem. Commun. 2009, 47, 7351–7353. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Muniz, K. Selective piperidine synthesis exploiting iodine-catalyzed Csp3–H amination under visible light. ACS Catal. 2017, 7, 4122–4125. [Google Scholar] [CrossRef]
- Saika, A.J.; Boran, P.; Phukan, P. Use of bromine and bromo-organic compounds in organic synthesis. Chem. Rev. 2016, 116, 6837–7042. [Google Scholar] [CrossRef] [PubMed]
- Beckwith, A.L.J.; Goodrich, J.E. Free-radical rearrangement of N-chlor-anides: A synthesis of lactones. Aust. J. Chem. 1965, 18, 747–757. [Google Scholar] [CrossRef]
- Veisi, H. Direct oxidative conversion of alcohols, amines, aldehydes and benzyl halides into the corresponding nitriles with tricholroisocianuric acid in aqueous ammonia. Synthesis 2010, 2010, 2631–2635. [Google Scholar] [CrossRef]
- Srilakshmi Krishanaveni, N.; Suredra, K.; Rama Rao, K. A Simple and Highly Selective Biomimetic Oxidation of Alcohols and Epoxides with N-Bromosuccinimide in the Presence of β-Cyclodextrin in Water. Adv. Synth. Catal. 2004, 346, 346–350. [Google Scholar] [CrossRef]
- Filler, R. Oxidations and Dehydrogenations with N-Bromosuccinimide and Related N-Haloimides. Chem. Rev. 1963, 63, 21–43. [Google Scholar] [CrossRef]
- Wilson, S.R.; Tofigh, S.; Misra, R.N. A novel, nonoxidative method for the conversion of aldehydes to esters. J. Org. Chem. 1982, 47, 1360–1361. [Google Scholar] [CrossRef]
- Zhao, J.; Sun, H.; Lu, Y.; Li, J.; Yu, Z.; Zhu, H.; Ma, C.; Meng, Q.; Peg, X. A divergent photocatalysis strategy for selective aerobic oxidation of C(sp3)–H bonds promoted by disulfides. Green Chem. 2022, 24, 8503–8511. [Google Scholar] [CrossRef]
- Chen, P.-S.; Chou, C.-H. Pyrolytic Chemistry of Thenyl Benzoates. Tetrahedron 1996, 52, 13615–13622. [Google Scholar] [CrossRef]
- Ruso, J.S.; Rajendiran, N.; Kumaran, R.S. Metal-free synthesis of aryl esters by coupling aryl carboxylic acids and aryl boroni cacids. Tetrahedron Lett. 2014, 55, 2345–2347. [Google Scholar] [CrossRef]
- Gondo, K.; Oyamada, J.; Kitamura, T. Palladium-Catalyzed Desilylative Acyloxylation of Silicon–CarbonBonds on (Trimethylsilyl)arenes: Synthesis of Phenol Derivativesfrom Trimethylsilylarenes. Org. Lett. 2015, 17, 4778–4781. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Yuan, L.; Wang, T.; Wang, C.; Ke, J.; Zhao, J. Palladium-catalyzed oxidative carbonylation of aryl hydrazines with CO and O2 at atmospheric pressure. J. Org. Chem. 2017, 82, 4970–4976. [Google Scholar] [CrossRef] [PubMed]
- Ramanjaneyulu, B.T.; Reddy, V.; Arde, P.; Mahesh, S.; Anand, R.V. Combining Oxidative N-Heterocyclic Carbene Catalysis with Click Chemistry: A Facile One-Pot Approach to 1, 2, 3-Triazole Derivatives. Chem. Asian J. 2013, 8, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Ullah, E.; McNulty, J.; Sliwinski, M.; Robertson, A. One-step synthesis of reusable, polymer-supported tri-alkyl phosphine ligands. Application in Suzuki–Miyaura and alkoxycarbonylation reactions. Tetrahedron Lett. 2012, 53, 3990–3993. [Google Scholar] [CrossRef]
- Dai, M.-S.; Zheng, Z.-M.; Zhang, S.L. High-valent Cu (III)–CF 3 compound-mediated esterification reaction. Org. Biomol. Chem. 2023, 21, 935–939. [Google Scholar] [CrossRef]
- Rout, S.K.; Guin, S.; Ghara, K.K.; Banerjee, A.; Patel, B.K. Copper catalyzed oxidative esterification of aldehydes with alkylbenzenes via cross dehydrogenative coupling. Org. Lett. 2012, 14, 3982–3985. [Google Scholar] [CrossRef]
- Liu, H.; Dong, C.; Zhang, Z.; Wu, P.; Jiang, X. Transition-Metal-Free Aerobic Oxidative Cleavage of C–C Bonds in α-Hydroxy Ketones and Mechanistic Insight to the Reaction Pathway. Angew. Chem. Int. Ed. 2012, 51, 12570–12574. [Google Scholar] [CrossRef]
- Liu, H.; Shi, G.; Pan, S.; Jiang, Y.; Zhang, Y. Palladium-catalyzed benzylation of carboxylic acids with toluene via benzylic C–H activation. Org. Lett. 2013, 15, 4098–4101. [Google Scholar] [CrossRef] [PubMed]
- Finney, E.E.; Ogawa, K.A.; Boydston, A.J. Organocatalyzed anodic oxidation of aldehydes. J. Am. Chem. Soc. 2012, 134, 12374–12377. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, T.; Hirose, D.; Ishibashi, H. Esterification via iron-catalyzed activation of triphenylphosphine with air. ACS Catal. 2011, 1, 1469–1474. [Google Scholar] [CrossRef]
- But, T.Y.S.; Lu, J.; Toy, P.H. Organocatalytic Mitsunobu reactions with 3, 5-dinitrobenzoic acid. Synlett 2010, 2010, 1115–1117. [Google Scholar]
- Ackermann, L.; Gschrei, C.J.; Althammer, A.; Riederer, M. Cross-coupling reactions of aryl and vinyl chlorides catalyzed by a palladium complex derived from an air-stable H-phosphonate. Chem. Comm. 2006, 2006, 1419–1421. [Google Scholar] [CrossRef] [PubMed]
- Inamoto, K.; Kuroda, J.I.; Hiroya, K.; Noda, Y.; Watanabe, M.; Sakamoto, T. Synthesis and catalytic activity of a pincer-type bis (imidazolin-2-ylidene) nickel (II) complex. Organometallics 2006, 25, 3095–3098. [Google Scholar] [CrossRef]
- Tian, Y.; Ling, A.; Fang, R.; Tan, R.X.; Liu, Z.Q. Free-radical anti-Markovnikov hydroalkylation of unactivated alkenes with simple alkanes. Green Chem. 2018, 20, 3432–3435. [Google Scholar] [CrossRef]
- Chen, X.; Hu, S.; Chen, R.; Wang, J.; Wu, M.; Guo, H.; Sun, S. Fe-catalyzed esterification of amides via C–N bond activation. RSC Adv. 2018, 8, 4571–4576. [Google Scholar] [CrossRef]
- Hoffmann, H.M.R.; Haase, K. The synthesis of acyl iodides. Synthesis 1981, 1981, 715–719. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).