Abstract
Monitoring the level of biothiols in organisms would be beneficial for health inspections. Recently, 3-(2′-nitro vinyl)-4-phenylselenyl coumarin as a fluorescent probe for distinguishing the detection of the small-molecule biothiols cysteine/homocysteine (Cys/Hcy) and glutathione (GSH) was developed. By introducing 4-phenyselenium as the active site, the probe CouSeNO2/CouSNO2 was capable of detecting Cys/Hcy and GSH in dual fluorescence channels. Theoretical insights into the fluorescence sensing mechanism of the probe were provided in this work. The details of the electron excitation process in the probe and sensing products under optical excitation and the fluorescent character were analyzed using the quantum mechanical method. All these theoretical results would provide insight and pave the way for the molecular design of fluorescent probes for the detection of biothiols.
1. Introduction
Biothiols are involved in many processes of transfer and detoxification, including cell growth, redox, and so on. Small molecular biothiols, including cysteine, homocysteine, and glutathione (Cys, Hcy, and GSH, respectively), are important sulfur compounds that could protect parts of the body due to their reducibility [1,2,3]. Biothiols with structural differences would lead to different functions; meanwhile, the biothiols are related to each other. Cys is involved in the process of enzyme catalysis, detoxification, and protein synthesis. Hcy is a regulatory intermediate in the Met cycle and the precursors of Cys and methionine. GSH has a role in maintaining redox homeostasis in biological systems.
The concentration of biological biothiols will deviate from normal values under the influence of adverse factors and directly affect their functions. In this situation, diseases such as growth retardation, cardiovascular disease, liver damage, and rheumatism, etc., could be caused. Therefore, monitoring the level of biothiols in organisms would be beneficial for health inspections. Nowadays, the methods of detecting biothiols are diversified and gradually improved. Yet, different detection methods have their own advantages and drawbacks [2,4,5,6,7,8,9,10].
At present, the main methods for the detection and analysis of active sulfur species (RSS) include the high-performance liquid chromatography (HPLC) analytical method, the colorimetric method, mass spectrometry, electrochemical analysis, capillary electrophoresis, and fluorescence analysis [11,12,13,14,15,16].
According to the comparative analysis, the detection results of high-performance liquid chromatography and mass spectrometry are relatively stable and sensitive but necessitate complicated sample operations and expensive equipment. The capillary electrophoresis detection method is economical and rapid, but has slightly inferior sensitivity. Colorimetry is easy to use but usually produces a relatively big error. Although the electrochemical analysis method has the advantages of convenience and high sensitivity, it is relatively weak in terms of selectivity. In contrast, fluorescent probes have been successfully applied to many detection fields due to their advantages of high sensitivity, low background interference, high selectivity, and good biocompatibility. Combined with focusing microscope instruments, fluorescent probes are applied to real-time and in situ imaging of biological cells and tissues without causing any damage, which provides a powerful analytical technique for disease diagnosis and is becoming a popular detection method in biological and medical fields.
In recent years, remarkable progress has been made in the construction of biothiol fluorescent probes [4,17,18,19]. Many reported fluorescent probes can be responded to biothiols in cells, tissues, and a variety of amino acids. However, due to the similar structure and reactivity of biothiols, most of the fluorescent probes reported so far cannot distinguish between the biothiols of Cys, Hcy, and GSH, which hinders the research of their roles in corresponding physiological and pathological processes [20,21].
Recently, Chen et al. developed 3-(2′-nitro vinyl)-4-phenylselenyl coumarin as a fluorescent probe for distinguishing between the detection of Cys/Hcy and GSH. By introducing 4-phenyselenium as the active site, the probe CouSeNO2/CouSNO2 was capable of detecting Cys/Hcy and GSH in dual fluorescence channels [22]. For the biothiols, the first-step sensing reaction was experimentally proven to be the nucleophilic substitution of 4-phenylselenium with the thiol group. Furthermore, through two-channel fluorescent imaging, the probe CouSeNO2/CouSNO2 had been successfully applied to sense the exogenous and endogenous biothiols in living cells. Except for the Michael addition as a usual sensing reaction in reported nitroolefin fluorescent probes, the nucleophilic substitution of 4-phenylselenium in the probe CouSeNO2/CouSNO2 with the thiol group of a biothiol as the first-step sensing reaction not only accelerated the reaction to biothiols but also realized the distinction between Cys/Hcy and GSH in dual fluorescence channels. Compared with the experimental results, the theoretical research on the electronic structure, reaction sites, sensing mechanism, and fluorescent properties of the probe CouSeNO2/CouSNO2 in this work could provide insights and pave the way for the molecular design of fluorescent probes for the detection of biothiols.
2. Results and Discussion
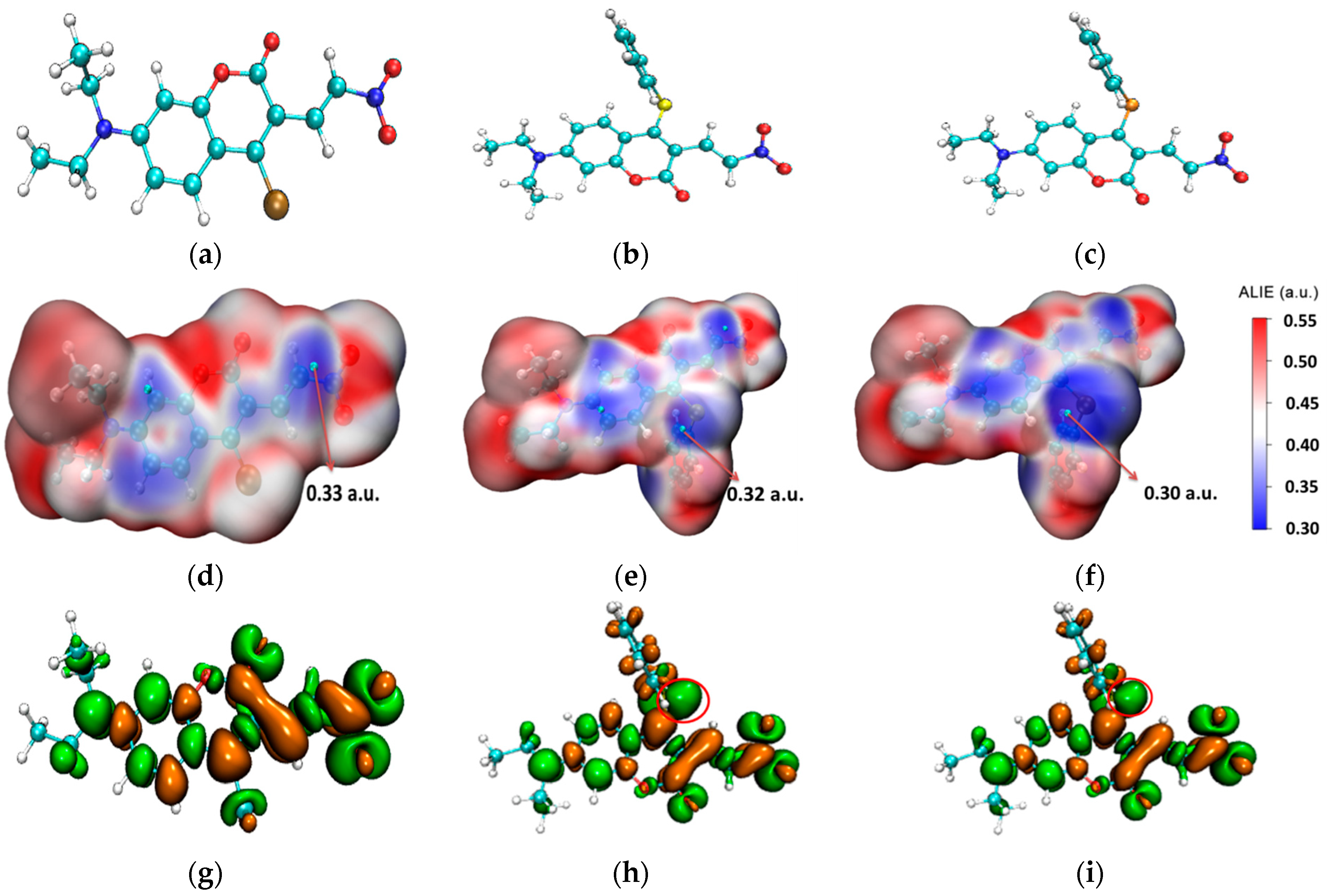
The stable molecular structures of the probes CouClNO2, CouSNO2, and CouSeNO2 are shown in Figure 1a–c. Due to no apparent spectral response with biothiols, the probe CouClNO2 was only presented for structural comparison here but not for consideration in the following theoretical research.
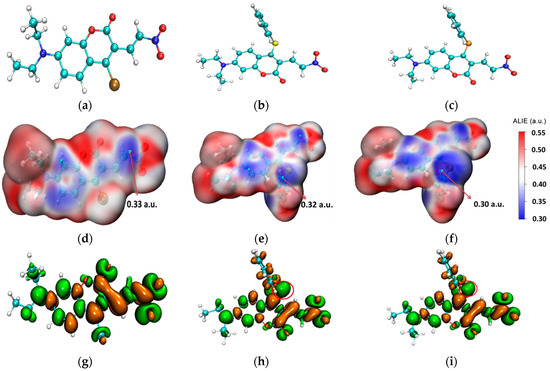
Figure 1.
(a–c) The stable molecular structures of the CouClNO2, CouSNO2, and CouSeNO2 probes; (d–f) the surface map of ALIE on the CouClNO2, CouSNO2, and CouSeNO2 probes; and (g–i) the dual descriptors of the CouClNO2, CouSNO2, and CouSeNO2 probes. (the red circle indicate S and Se atom in the CouSNO2 and CouSeNO2 probes respectively).
From the surface map of average local ionization energy (ALIE) [23] on three probes in Figure 1d–f, it could be deduced that the C=C bond in CouClNO2 is the potential electrophilic reaction site (with an ALIE value of 0.33 a.u.); otherwise, the S(Se) atom and C=C bond in the CouSNO2 and CouSeNO2 probes are the potential electrophilic reaction sites (with the ALIE values of 0.32 a.u. and 0.30 a.u. for the S and Se atoms in the CouSNO2 and CouSeNO2 probes, respectively).
Fukui function and dual descriptor, known as the important concepts in density functional reactivity theory, which was initially developed by Parr, are very popular methods for predicting reaction sites defined under the conceptual density functional theory framework [24,25,26]. The dual descriptors of the CouClNO2, CouSNO2, and CouSeNO2 probes were obtained through Multiwfn 3.8(dev) analysis based on the ORCA output results and are illustrated in Figure 1g–i. The S and Se atoms in the CouSNO2 and CouSeNO2 probes (indicated by the red circle in Figure 1h,i) were indicated to be the potential electrophilic reaction sites with biothiols, which were in agreement with the corresponding experimental results [22]. The lower ALIE value of the Se atom compared to the S atom indicated the higher sensitivity of the CouSeNO2 probe to biothiols than the CouSNO2 probe, which was also testified within the experiment work.
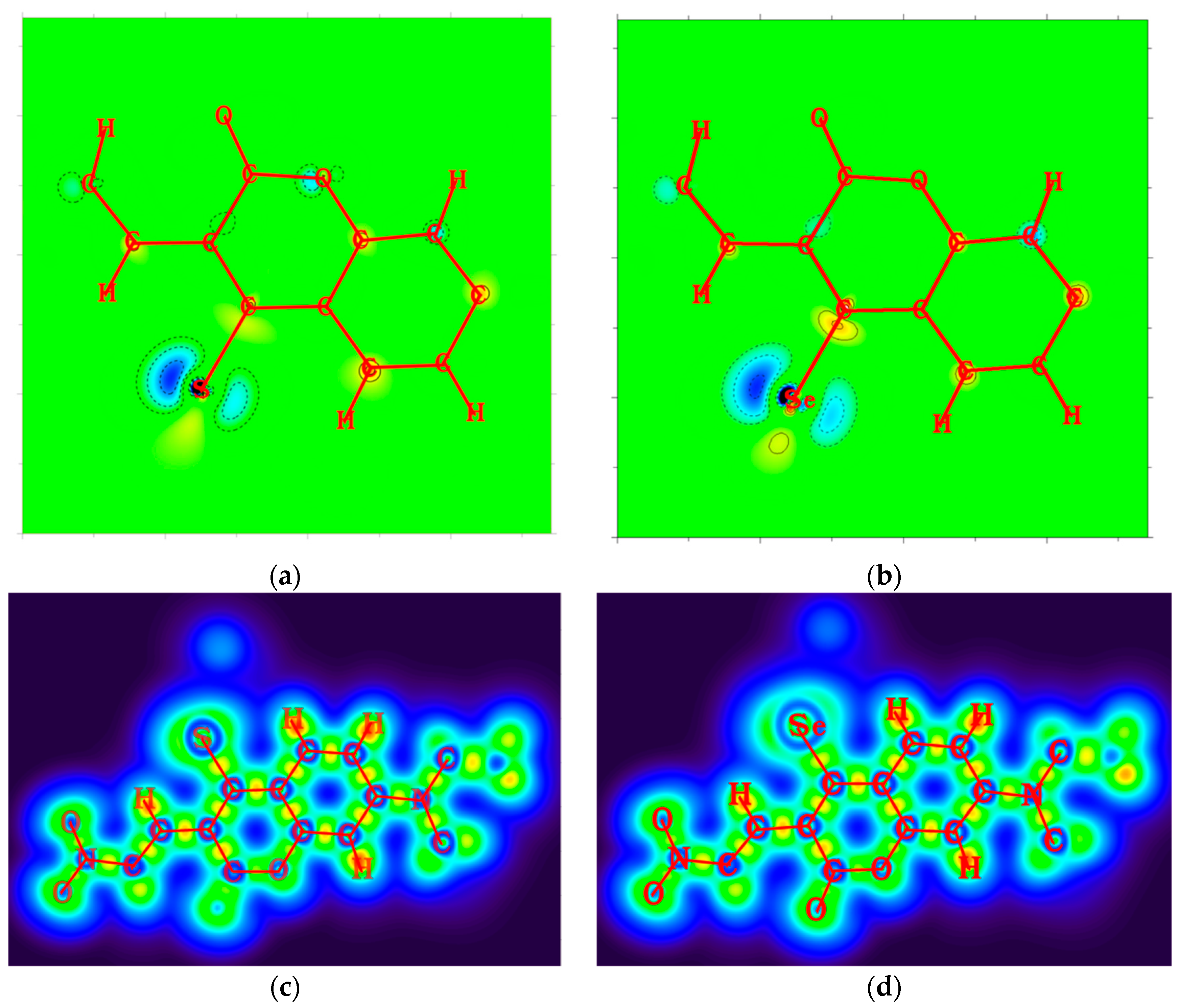
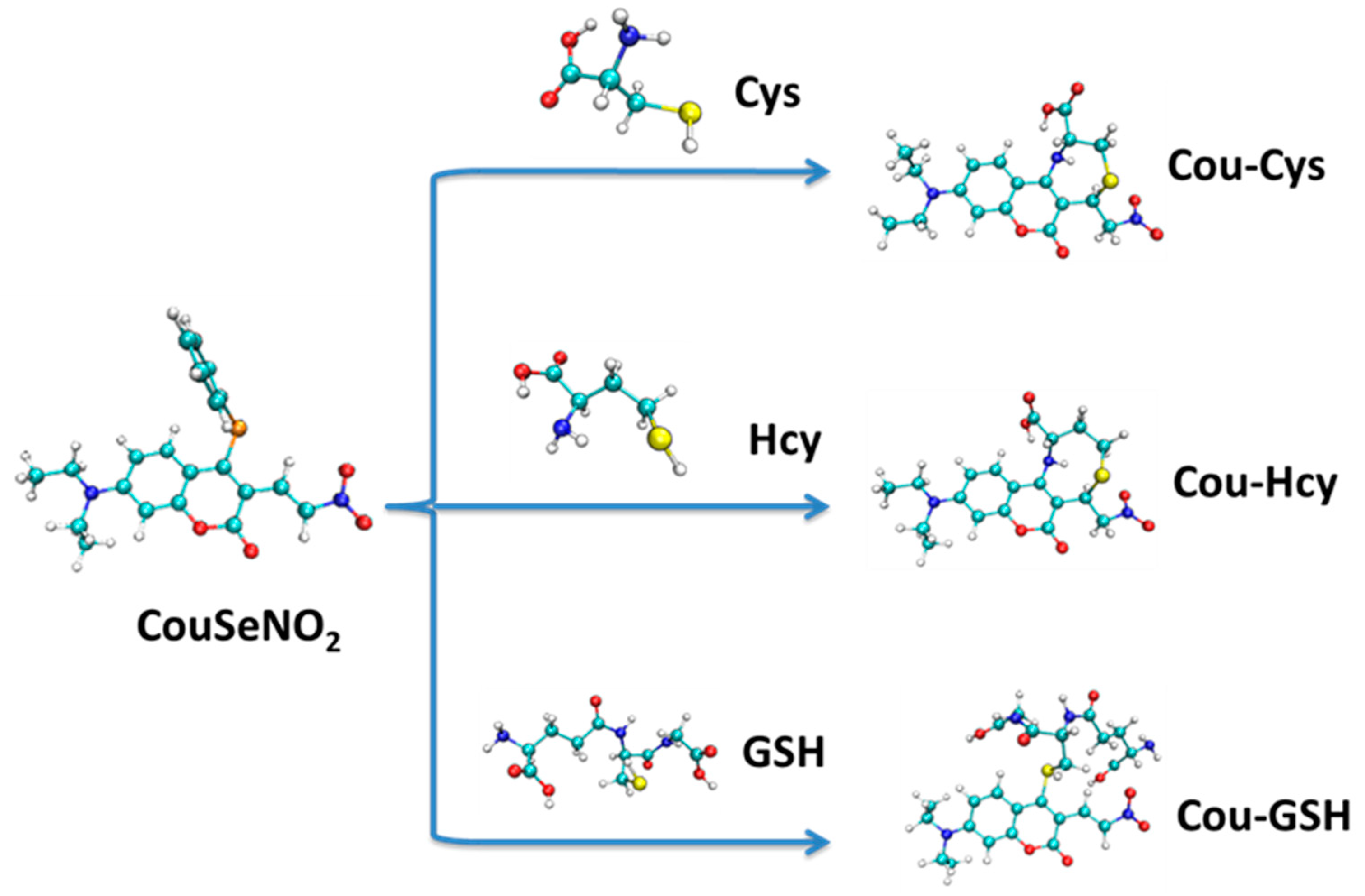
From the 2D plots of dual descriptors on the main molecular planes of the CouSNO2 and CouSeNO2 probes, as shown in Figure 2a,b, the dual descriptor absolute values of the S and Se atoms are obviously larger than the values at other places within the probe molecule. This result indicated that a substitution reaction would likely occur within the S and Se atoms when the CouSNO2 and CouSNO2 probes encountered the biothiols. The 2D localized orbital locator (lol) on the molecular planes of the CouSNO2 and CouSeNO2 probes, as shown in Figure 2c,d, also indicated that the S and Se atoms in the probe molecules were potential reaction sites. The sensing mechanism of CouSeNO2 towards biothiols is shown in Scheme 1.
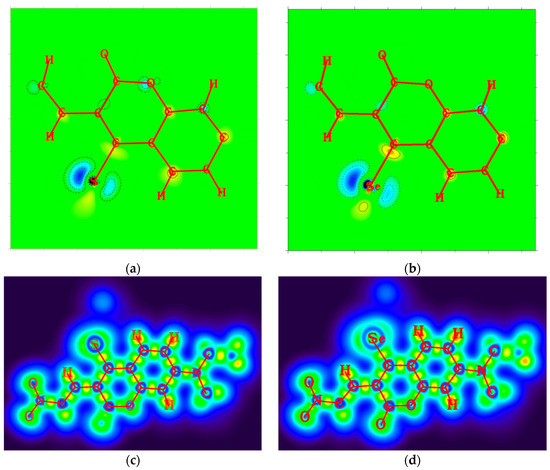
Figure 2.
(a,b) Two-dimensional plots of dual descriptors on the molecular planes of the CouSNO2 and CouSeNO2 probes; (c,d) 2D lol on the molecular planes of the CouSNO2 and CouSeNO2 probes.
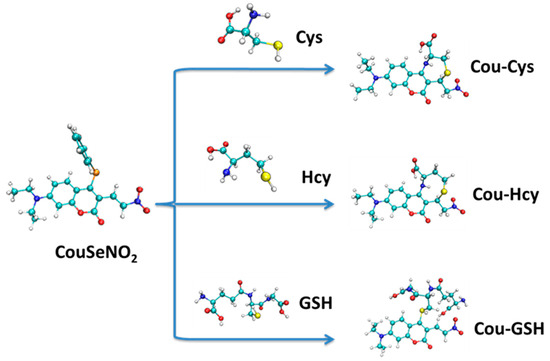
Scheme 1.
The sensing mechanism of CouSeNO2 towards biothiols.
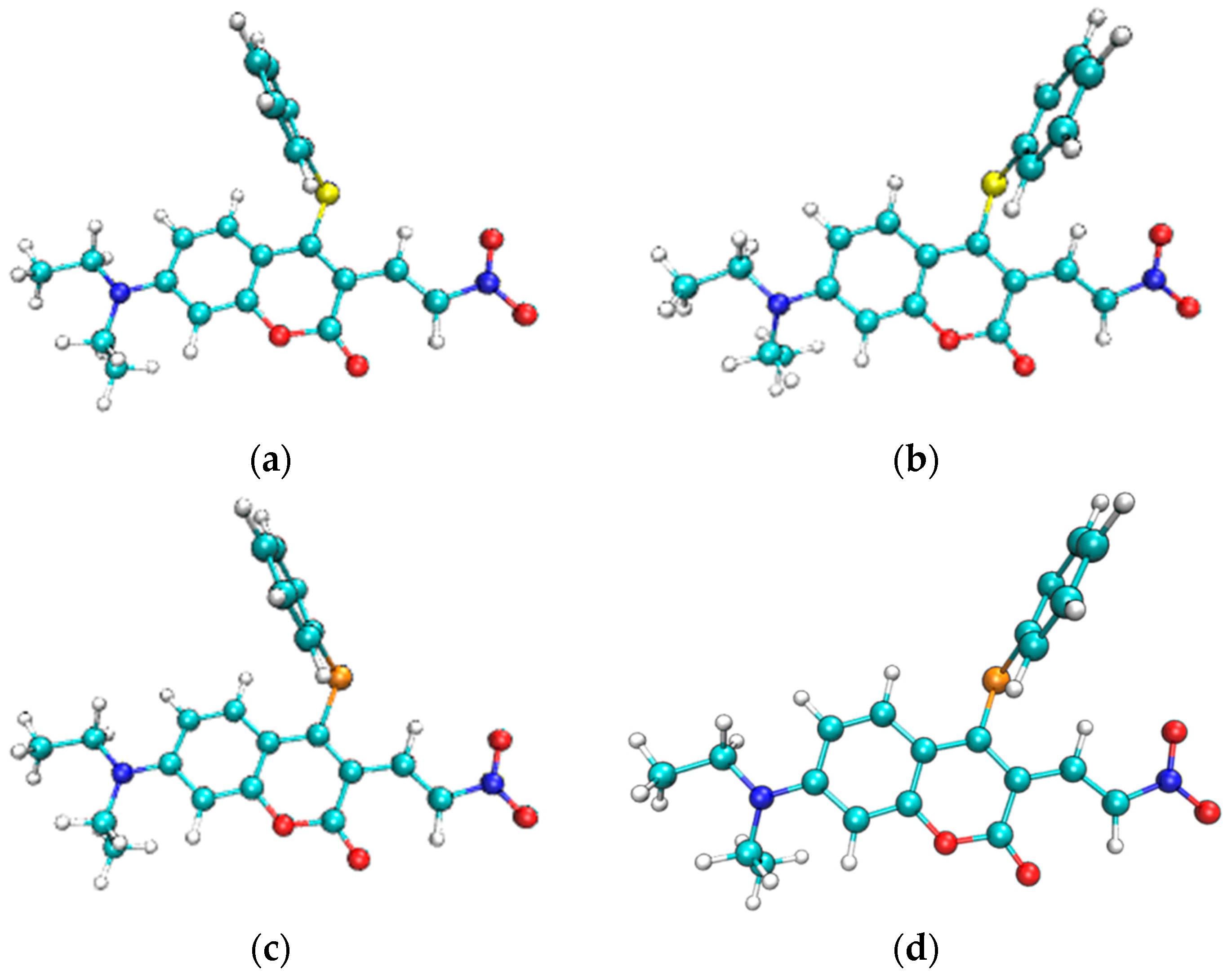
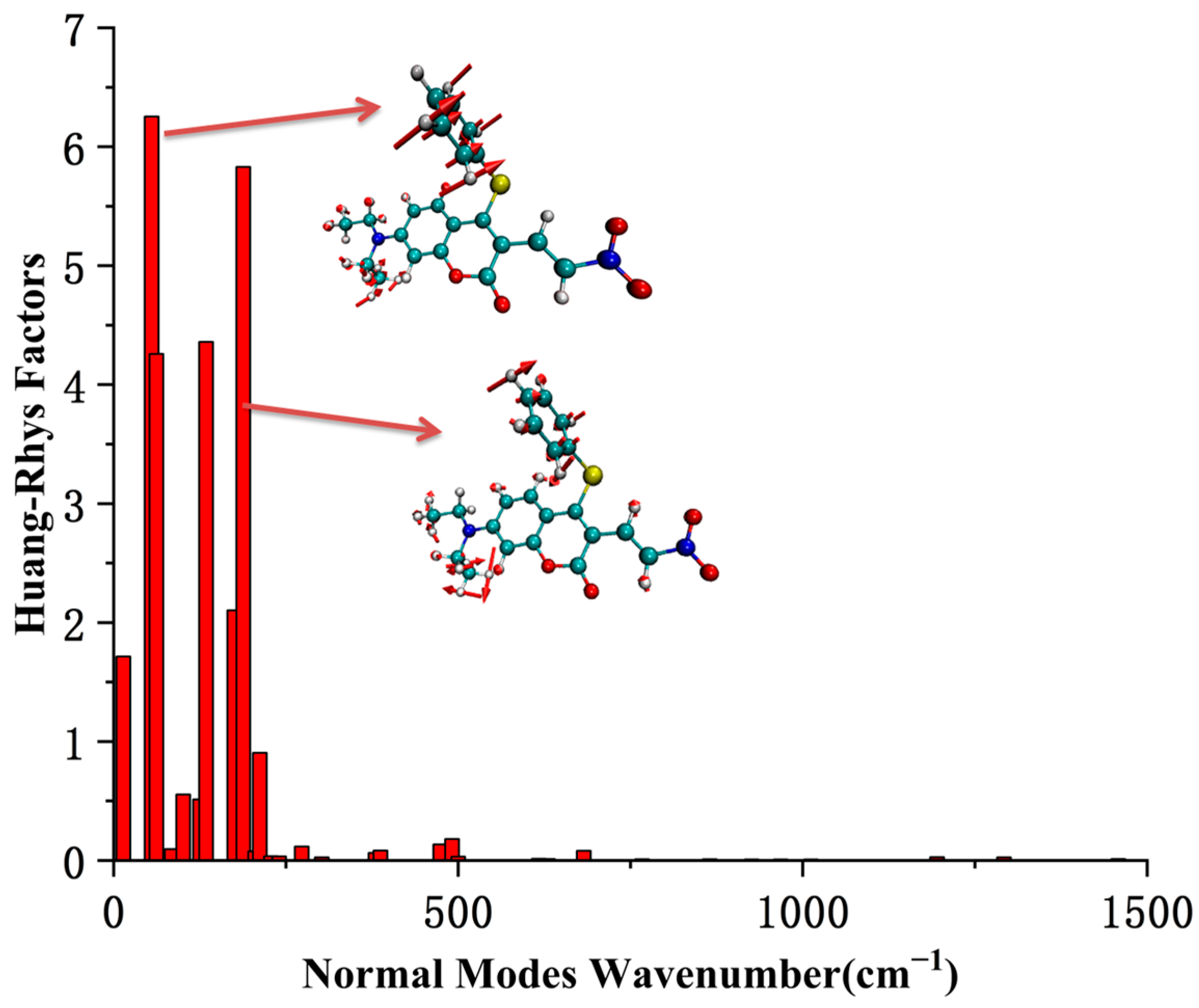
The most stable geometric structures of the ground state S0 and first excited state S1 of the CouSNO2 and CouSeNO2 probes are shown in Figure 3. It indicated a similar difference between the S0 and S1 structures of the CouSNO2 and CouSeNO2 probes, in which the benzene ring showed an obvious flip from the ground state to the first excited state. The dihedral angle, α, between the benzene ring and the main molecular plane of the CouSNO2 probe variated from 59° to 108° when the molecule was excited from S0 to S1; this change in α was from 56° to 108° in the CouSeNO2 probe. This large structural difference between S0 and S1 within the CouSNO2 and CouSeNO2 probes would lead to large reorganization energy and Huang–Rhys factors [27,28] for some normal vibration modes, as shown in Figure 4 (CouSNO2 was only shown for clarity consideration). It could be seen that the vibration mode with large Huang–Rhys factors were just corresponding with the swing of the benzene ring in the probe molecule. The reorganization energy and Huang–Rhys factors between S0 and S1 of the CouSNO2 and CouSeNO2 probes were calculated through the Dushin program [29].
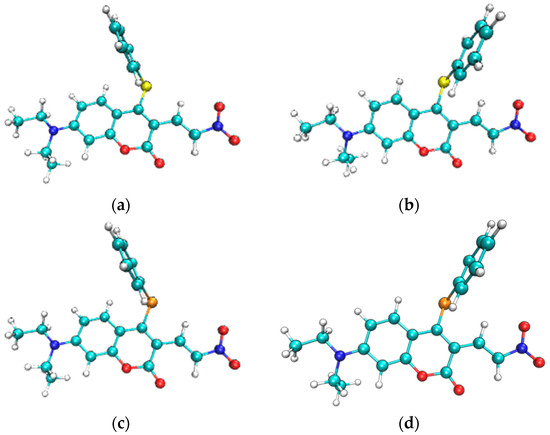
Figure 3.
(a,b) Geometric structures of the S0 and S1 of the CouSNO2 probe; and (c,d) geometric structures of the S0 and S1 of the CouSeNO2 probe.
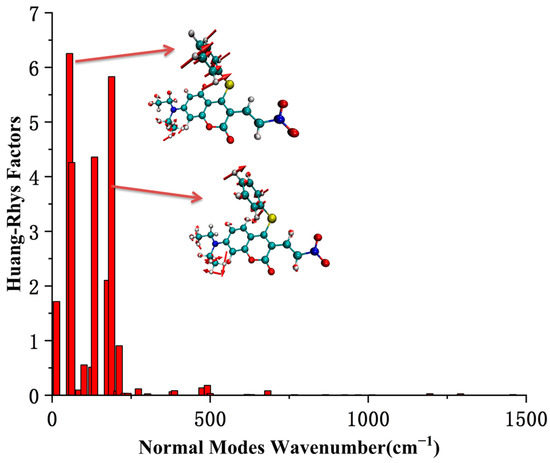
Figure 4.
The Huang–Rhys factors of the CouSNO2 probe.
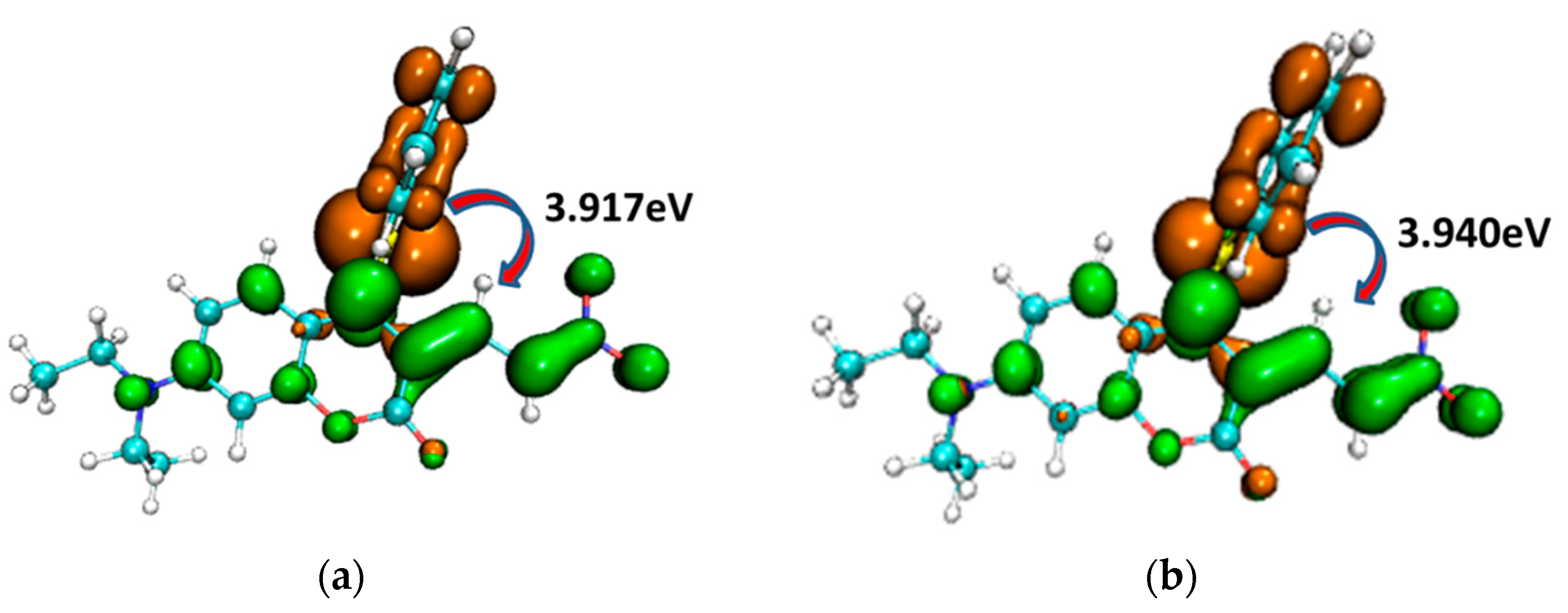
To illustrate the electron excitation process from S0 to S1 within the CouSNO2 and CouSeNO2 probes, the hole–electron (brown and green colors, respectively, in Figure 5) analyses were performed based on the TDDFT results. It could be informed that the electron was mainly excited from the benzene ring part to the main planar part of the probes. The excitation energy from S0 to S1 in CouSeNO2 (3.940 eV) was a little larger than that in CouSNO2 (3.917 eV).
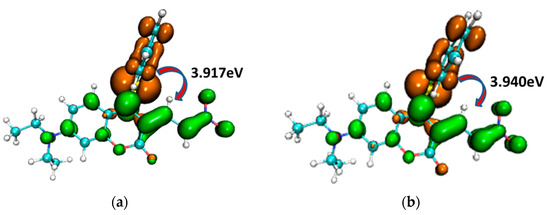
Figure 5.
Hole–electron (brown and green colors, respectively) analysis for the electron excitation process from S0 to S1 within the (a) CouSNO2 and (b) CouSeNO2 probes.
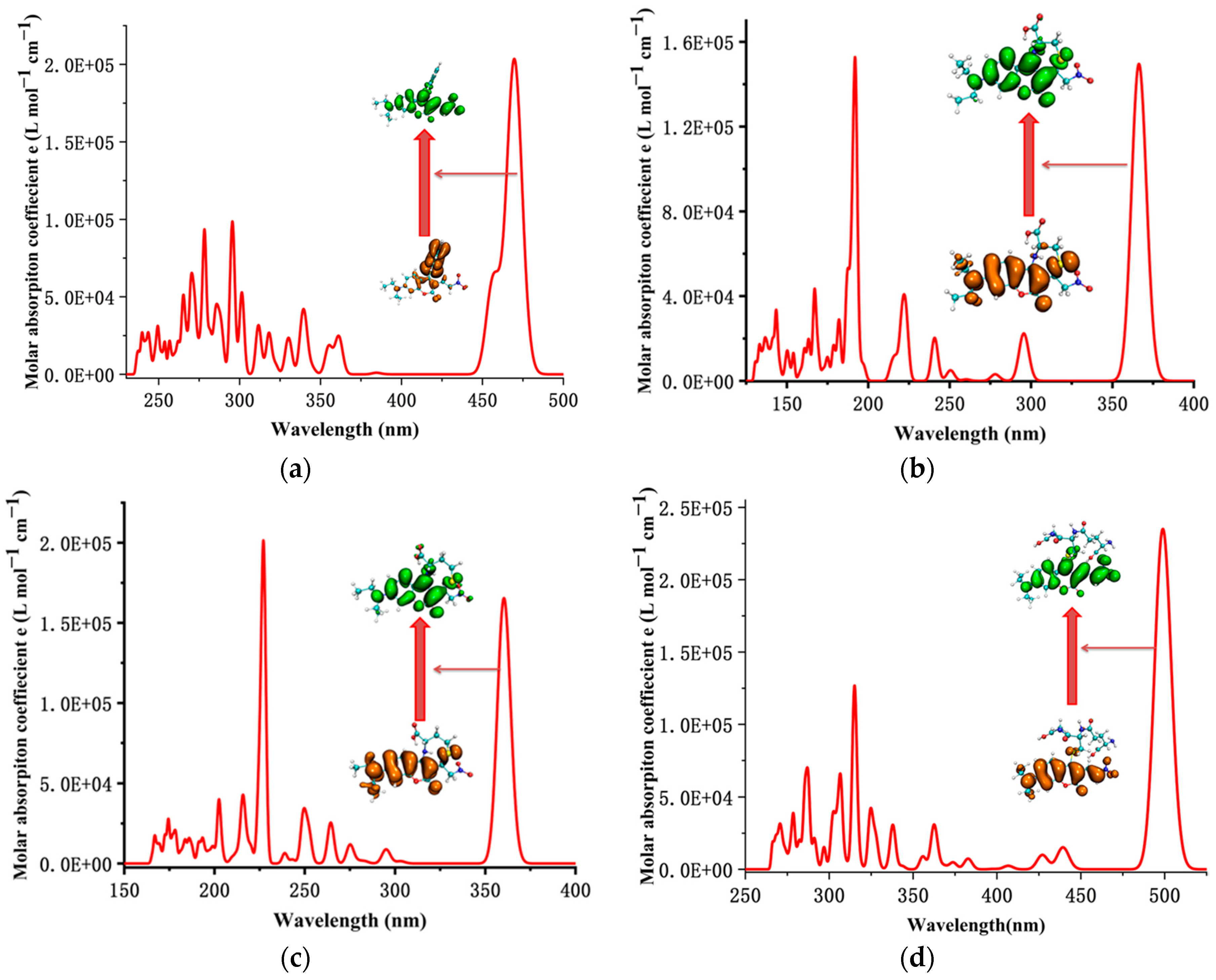
The simulated UV–Vis absorption spectrum of the CouSeNO2 probe, as shown in Figure 6a, indicated that the absorption wavelength from S0 to S1 was about 473 nm, which was near the experimental value of 480 nm and testified to the reasonable choice of the functional and basis set for electron excitation calculation on this kind of organic molecular probe. After the reaction with Cys and Hcy, the absorption wavelengths from S0 to S1 of the sensing products Cou-Cys and Cou-Hcy were changed by a blue shift to be about 360 nm and 357 nm, respectively, which were consistent with the experimental results.
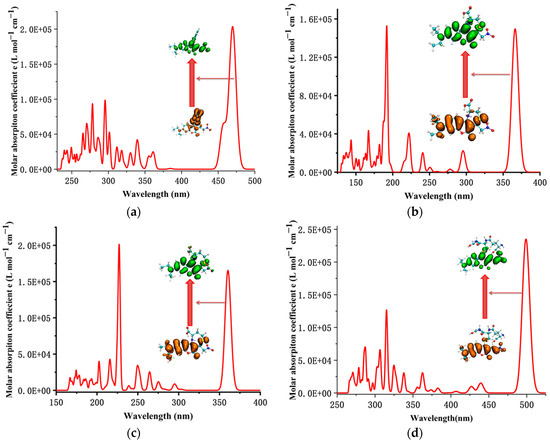
Figure 6.
The simulated UV–Vis absorption spectrum of the probe and sensing products. (a) CouSeNO2. (b) Cou-Cys. (c) Cou-Hcy. (d) Cou-GSH.
Unlike the charge transfer characteristic of electron excitation in the process from S0 to S1 within the original CouSeNO2 probe, it was shown the local excitation character for the electron excitation process from S0 to S1 within the sensing products Cou-Cys and Cou-Hcy, and this local excitation character led to a significant increase in the fluorescent intensity at about 460 nm and 451 nm, respectively, which were testified within both the theoretical and experimental results. A similar reaction between the CouSeNO2 probe and GSH occurred, which also led to the variation in the UV–Vis absorption spectrum and fluorescent intensity of the sensing product Cou-GSH. Without the seven- or eight-membered ring like in sensing products Cou-Cys and Cou-Hcy, due to the Michael addition reaction of the thiol group to the unsaturated C=C double bond, there was a red shift within the UV–Vis absorption and fluorescent spectrum of sensing product Cou-GSH compared with the original probe CouSeNO2. The theoretical absorption and emission wavelength between S0 and S1 was about 500 nm and 550 nm, respectively, which were well agreed with the experimental values of 515 nm and 562 nm, respectively. The theoretical and experimental fluorescent-related absorption and emission wavelengths are summarized in Table 1 and Table 2.

Table 1.
The main electron excitation processes in the probe and sensing product molecule.

Table 2.
The main electron emission processes in the probe and sensing product molecule.
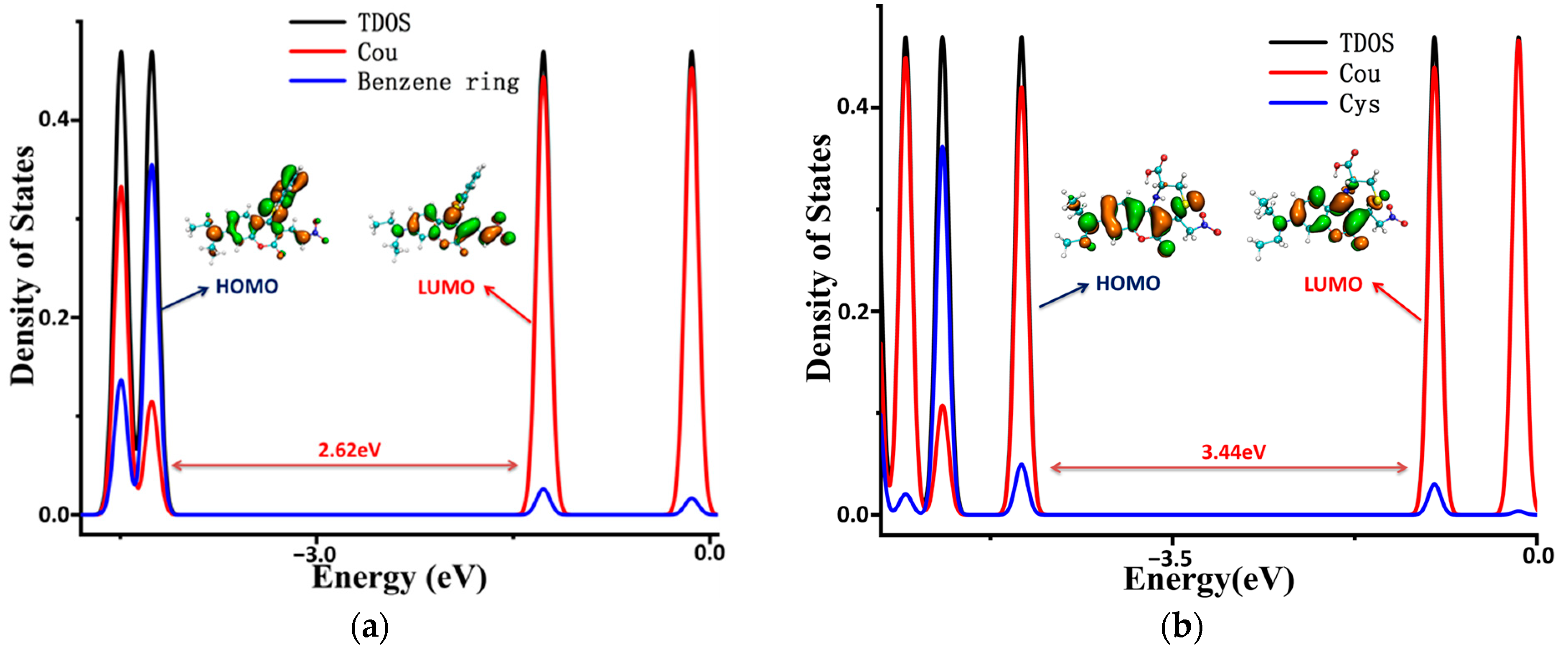
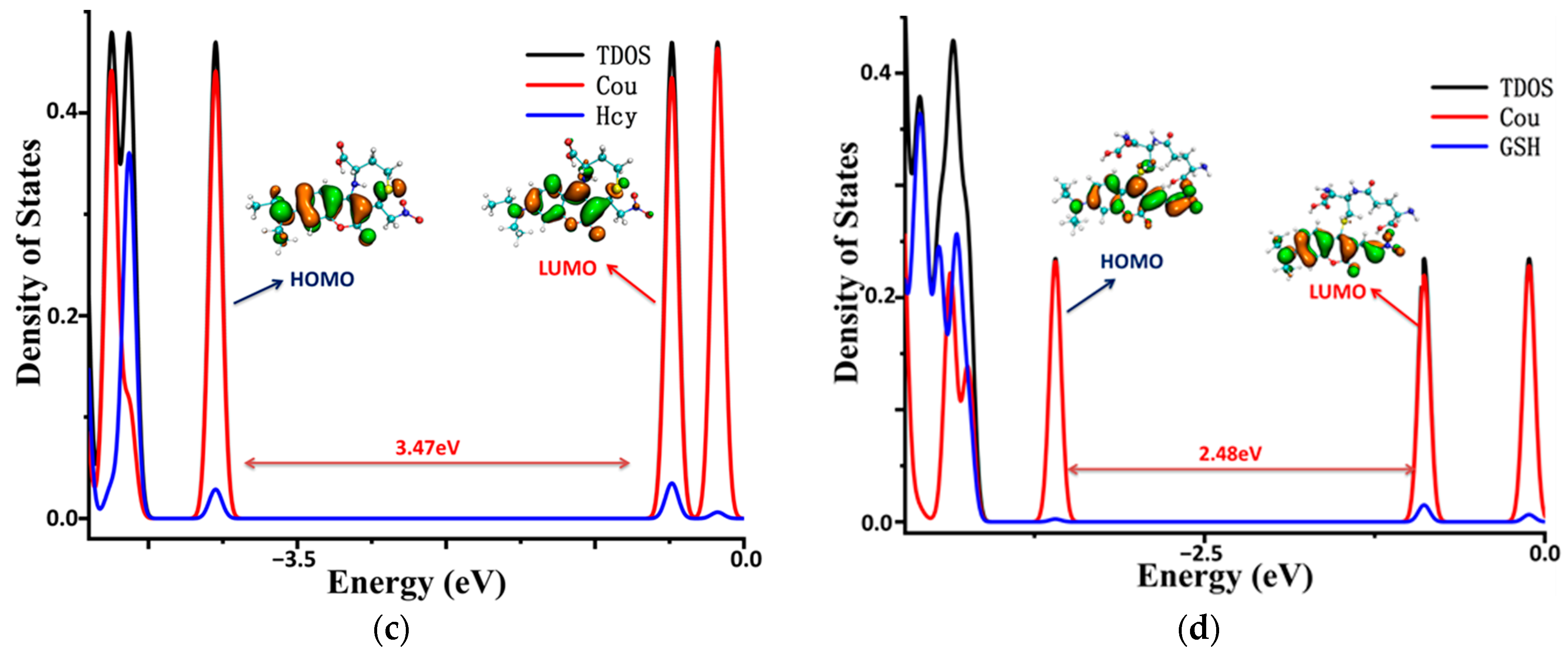
To illustrate the electronic structures of the CouSeNO2 probe and its sensing product with biothoils in more depth, the density of electronic states (DOSs) were calculated and are illustrated in Figure 7. The main orbital transition contribution to the electron excitation between S0 and S1 in the probes and sensing products was the highest occupied molecular orbit (HOMO) and the lowest unoccupied molecular orbit (LUMO), as shown in Table 1 and Table 2. The fluorescence of the probes and sensing products were decided through the electron radiation process from S1 to S0.
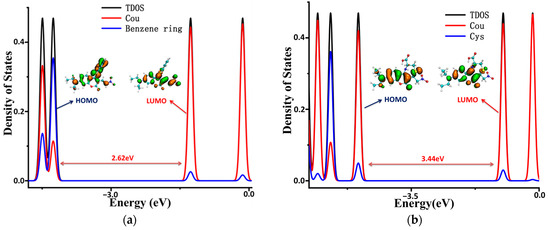
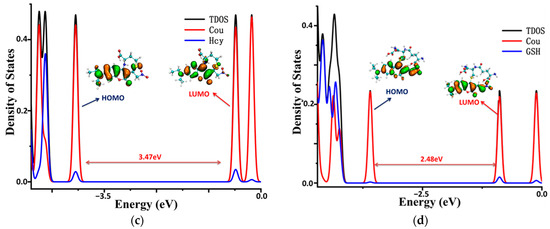
Figure 7.
The density of the electronic states of the probe and sensing products. (a) CouSeNO2. (b) Cou-Cys. (c) Cou-Hcy. (d) Cou-GSH.
The total DOS (TDOS) of the probe and sensing product molecules and the partial DOS of two individual parts in the molecules (coumarin part and benzene ring in the probe and biothoils in the sensing product) are all depicted within Figure 7. It could be seen that the obvious charge transfer characteristic in the electron excitation process between S0 and S1 in the CouSeNO2 probe, in which the HOMO was mainly contributed by the benzene ring part and the LUMO was mainly contributed by the coumarin part. This charge transfer character indicated that the ICT process led to the small oscillation strength between the S0 and S1 states and a weak fluorescent intensity in the original CouSeNO2 probe. Otherwise, the local excitation characteristic was shown in the electron excitation process between S0 and S1 in the sensing products through the probe reaction with the biothiols, which led to the corresponding significant oscillation strength and fluorescent intensity. Due to the different molecular structures of the biothoils, the sensing product Cou-GSH without its 7–8-membered rings showed a wavelength red shift in maximum absorption peak and fluorescence compared with the original CouSeNO2 probe. Contrarily, the Michael addition reaction between the thiol groups (Cys and Hcy) and the unsaturated C=C double bond in the CouSeNO2 probe led to the formation of the 7–8-membered rings in the sensing products Cou-Cys and Cou-Hcy, which made the different electronic structure variation compared with the original probe CouSeNO2. Both the wavelength of the maximum absorption peak and fluorescence took a blue shift relative to the CouSeNO2 probe. The blue and red absorption shifts of CouSeNO2 with Cys, Hcy, and GSH were clearly related to the HOMO/LUMO energy gaps of the corresponding sensing products. So, the different wavelengths and colors of the fluorescence from the sensing product with the biothiols (Cys, Hcy, and GSH) allowed for the CouSeNO2 probe to be successfully applied in distinguishing the detection of the small-molecule biothiols.
3. Theoretical Methods
The theoretical methods of the research for the fluorescent probe CouSeNO2/CouSNO2 sensing biothiols were as follows:
- The functional and basis set combination CAM-B3LYP/def2-TZVPD was used in structure optimization, corresponding vibrational frequency analysis on the probe, and sensing product conformations with ORCA program 5.1 [30,31,32,33]. Non-imaginary frequency was found in the vibrational analysis on the stable geometric structure, which confirmed the stability of the structure optimization results. The wB2GP-PLYP/def2-TZVPD combination was used in single-point energy to obtain free energy with high precision, according to benchmark research [34]. Similar calculated results were obtained in the gas phase and in several solvents with different polarities, which indicated that this fluorescent probe was insensitive to the solvent effect.
- The electronic structure and fluorescent properties of the probe and its sensing products were obtained through the Multiwfn 3.8(dev) code [35] based on the DFT and TDDFT results through the ORCA program.
- The reorganization energy and Huang–Rhys factors between the S0 and S1 states of the probe and sensing products were obtained through the Dushin program.
- Most of the figures in this work were rendered by means of VMD 1.9.3 software [36].
4. Conclusions
The electron structure and fluorescent theoretical analysis indicated a local excitation character for the electron excitation process from S0 to S1 within the sensing product of the CouSeNO2 probe’s reaction with small-molecule biothiols, including Cys/Hcy and GSH. Due to the different molecular structures of the biothoils, the sensing product Cou-GSH without its 7–8-membered rings showed a wavelength red shift in its maximum absorption peak and fluorescence compared with the original CouSeNO2 probe. Contrarily, the Michael addition reaction between the thiol groups (Cys and Hcy) and the unsaturated C=C double bond in the CouSeNO2 probe led to both the wavelengths of the maximum absorption peak and fluorescence taking a blue shift relative to the CouSeNO2 probe. So, the different wavelengths and colors of the fluorescence from the sensing product with the biothiols (Cys, Hcy, and GSH) allowed for the CouSeNO2 probe to be successfully applied in distinguishing the detection of the exogenous and endogenous biothiols in living cells. The theoretical investigation of the mechanism of fluorescent probe molecular design would provide insights into building highly efficient fluorescent probes for biothiol detection in the future.
Funding
This research was funded by Natural Science Foundation of Liaoning Province (2022-MS-389, 20180550512, JYTQN201923).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Han, H.; Wang, F.; Chen, J.; Li, X.; Fu, G.; Zhou, J.; Zhou, D.; Wu, W.; Chen, H. Changes in Biothiol Levels Are Closely Associated with Alzheimer’s Disease. J. Alzheimers Dis. 2021, 82, 527–540. [Google Scholar] [PubMed]
- Campodonico, P.R.; Alarcon-Esposito, J.; Olivares, B. Kinetics and Reaction Mechanism of Biothiols Involved in SNAr Reactions: An Experimental Study. Front. Chem. 2022, 10, 142214. [Google Scholar]
- Hu, Y.; Zeng, F. A theranostic prodrug based on FRET for real-time drug release monitoring in response to biothiols. Mater. Sci. Eng. C-Mater. Biol. Appl. 2017, 72, 77–85. [Google Scholar] [PubMed]
- Si, L.; Fu, Q.; Shi, Z.; Zhang, T.; Hou, Q.; Xu, Z.; Ai, S. The fluorescent detection of biothiols and antimicrobial study based on copper(I) iodide coordination polymer. Dye. Pigment. 2023, 215, 2134–2146. [Google Scholar]
- Nagendraraj, T.; Priya, S.V.; Annaraj, J.; Sagadevan, S. Targeted cysteine and glutathione detection in extra/intracellular systems by copper-based fluorescent imaging probes. Coord. Chem. Rev. 2023, 495, 1089–1101. [Google Scholar]
- Ma, C.; Yan, D.; Hou, P.; Liu, X.; Wang, H.; Xia, C.; Li, G.; Chen, S. Bioimaging and Sensing Thiols In Vivo and in Tumor Tissues Based on a Near-Infrared Fluorescent Probe with Large Stokes Shift. Molecules 2023, 28, 5702. [Google Scholar] [PubMed]
- Feng, Q.; Song, Y.; Ma, Y.; Deng, Y.; Xu, P.; Sheng, K.; Zhang, Y.; Li, J.; Wu, S. Molecular engineering of benzenesulfonyl analogs for visual hydrogen polysulfide fluorescent probes based on Nile red skeleton. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2023, 296, 135466. [Google Scholar]
- Sun, Y.; Wang, Y.; Lu, Y.; Kong, X.; Wei, H.; Chen, Q.; Yan, M.; Dong, B. Mitochondria-targeted and FRET-based fluorescent probe for the imaging of endogenous SO2 in living cells. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2022, 265, 110233. [Google Scholar] [CrossRef]
- Bhasin, A.K.K.; Chauhan, P.; Chaudhary, S.; Umar, A.; Baskoutas, S. Highly Sensitive and Selective Sulfite Ion Sensor Based on Biochemically Stable Iodo-Functionalized Coumarin Fluorophores. Chemistryselect 2022, 7, 224361. [Google Scholar] [CrossRef]
- Zhu, Y.; Pan, H.; Song, Y.; Jing, C.; Gan, J.-A.; Zhang, J. Mitochondria-targeted fluorescent probe for rapid detection of thiols and its application in bioimaging. Dye. Pigment. 2021, 191, 3647–3658. [Google Scholar]
- Li, H.; An, Y.; Gao, J.; Yang, M.; Luo, J.; Li, X.; Lv, J.; Li, X.; Yuan, Z.; Ma, H. Recent Advances of Fluorescence Probes for Imaging of Ferroptosis Process. Chemosensors 2022, 10, 233. [Google Scholar] [CrossRef]
- Khan, Z.G.; Patil, M.R.; Nangare, S.N.; Patil, A.G.; Boddu, S.H.S.; Tade, R.S.; Patil, P.O. Surface nanoarchitectured metal-organic frameworks-based sensor for reduced glutathione sensing: A review. J. Nanostruct. Chem. 2022, 12, 1053–1074. [Google Scholar]
- Yang, Q.; Lan, T.; He, W. Recent progress in reaction-based fluorescent probes for active sulfur small molecules. Dye. Pigment. 2021, 186, 3145–3156. [Google Scholar] [CrossRef]
- Su, S.; Chen, Q.; Wang, C.; Jing, J.; Zhang, X. A Sensitive Fluorescent Probe for Homocysteine/Cysteine in Pure Aqueous Media and Mitochondria. Chemistryselect 2021, 6, 8391–8396. [Google Scholar] [CrossRef]
- Raut, J.; Sahoo, P. Detection of Biothiols Using Some Novel Chemosensors: An Overview. Mini Rev. Org. Chem. 2021, 18, 867–884. [Google Scholar] [CrossRef]
- Liu, W.; Chen, J.; Xu, Z. Fluorescent probes for biothiols based on metal complex. Coord. Chem. Rev. 2021, 429, 241756. [Google Scholar]
- Xue, L.; Yu, D.; Sun, J.; Guan, L.; Xie, C.; Wang, L.; Jia, Y.; Tian, J.; Fan, H.; Sun, H. Rapid GSH detection and versatile peptide/protein labelling to track cell penetration using coumarin-based probes. Analyst 2023, 148, 532–538. [Google Scholar] [CrossRef]
- Jiang, H.; Yin, G.; Gan, Y.; Yu, T.; Zhang, Y.; Li, H.; Yin, P. A multisite-binding fluorescent probe for simultaneous monitoring of mitochondrial homocysteine, cysteine and glutathione in live cells and zebrafish. Chin. Chem. Lett. 2022, 33, 1609–1612. [Google Scholar] [CrossRef]
- Hao, Y.; Zhang, Y.; Luo, L.; Zhu, D.; Xu, M.; Zeng, R.; Chen, S. Deep-Red Emissive Fluorescent Probe for Sensitive Detection of Cysteine in Milk and Living Cells. Food Anal. Methods 2022, 15, 2145–2154. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, C.; Sun, H.; Ren, M.; Kong, F. A Cys-regulated fluorescent probe targeting cancer cells and their application in inflammation detection. J. Photochem. Photobiol. A-Chem. 2023, 444, 7241–7253. [Google Scholar] [CrossRef]
- Wang, Z.; Li, X.; Sun, X.; Zhang, X.; He, C.; Li, Y.; Lu, F.; Lu, X.; Fan, Q. Highly selective imaging of intratumoral hydrogen sulfide by NIR-II emissive fluorescent probes. Sens. Actuators B-Chem. 2023, 384, 6325–6337. [Google Scholar] [CrossRef]
- Chen, X.G.; Mei, Y.; Song, Q.H. A 3-(2′-nitro vinyl)-4-phenylselenyl coumarin as a fluorescent probe for distinguishing detection of Cys/Hcy and GSH. Dye. Pigment. 2022, 203, 11032. [Google Scholar] [CrossRef]
- Kohut, S.V.; Cuevas-Saavedra, R.; Staroverov, V.N. Generalized average local ionization energy and its representations in terms of Dyson and energy orbitals. J. Chem. Phys. 2016, 145, 2568–2574. [Google Scholar] [CrossRef] [PubMed]
- Zamora, P.P.; Bieger, K.; Cuchillo, A.; Tello, A.; Muena, J.P. Theoretical determination of a reaction intermediate: Fukui function analysis, dual reactivity descriptor and activation energy. J. Mol. Struct. 2021, 1227, 129369. [Google Scholar] [CrossRef]
- Sanchez-Marquez, J. New advances in conceptual-DFT: An alternative way to calculate the Fukui function and dual descriptor. J. Mol. Model. 2019, 25, 5214–5226. [Google Scholar] [CrossRef]
- Martinez-Araya, J.I. Why is the dual descriptor a more accurate local reactivity descriptor than Fukui functions? J. Math. Chem. 2015, 53, 451–465. [Google Scholar] [CrossRef]
- Wei, Y.-C.; Hsu, L.-Y. Polaritonic Huang-Rhys Factor: Basic Concepts and Quantifying Light-Matter Interactions in Media. J. Phys. Chem. Lett. 2023, 14, 2395–2401. [Google Scholar] [CrossRef]
- Filipowska, K.; Pawlikowski, M.T.; Andrzejak, M. Optical Spectra of Oligofurans: A Theoretical Approach to the Transition Energies, Reorganization Energies, and the Vibronic Activity. Molecules 2021, 26, 7458. [Google Scholar] [CrossRef]
- Reimers, J.R. A practical method for the use of curvilinear coordinates in calculations of normal-mode-projected displacements and Duschinsky rotation matrices for large molecules. J. Chem. Phys. 2001, 115, 9103–9109. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system-Version 5.0. Wiley Interdiscip. Rev.-Comput. Mol. Sci. 2022, 12, 3251–3264. [Google Scholar] [CrossRef]
- Liu, P.; Liu, Y.L.; Huang, H.; Bai, G.; Peng, Y.J. Theoretical investigation on FRET strategy of ratio metric fluorescent probe sensing hydrogen sulfide. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2023, 289, 122223. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-L.; Huang, H.; Peng, Y.-J. Fluorescent probe for simultaneous detection of human serum albumin and sulfite: A theoretical analysis. J. Mol. Struct. 2022, 1255, 132441. [Google Scholar] [CrossRef]
- Fu, L.; Huang, H.; Zuo, Z.; Peng, Y. A Single Organic Fluorescent Probe for the Discrimination of Dual Spontaneous ROS in Living Organisms: Theoretical Approach. Molecules 2023, 28, 6983. [Google Scholar] [CrossRef] [PubMed]
- Casanova-Páez, M.; Dardis, M.B.; Goerigk, L. ωB2PLYP and ωB2GPPLYP: The First Two Double-Hybrid Density Functionals with Long-Range Correction Optimized for Excitation Energies. J. Chem. Theory Comput. 2019, 15, 4735–4744. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K.K. VMD—Visual molecular dynamics. J. Mol. Graph. 1995, 14, 33–38. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).