Bis-Iridoid Glycosides and Triterpenoids from Kolkwitzia amabilis and Their Potential as Inhibitors of ACC1 and ACL
Abstract
1. Introduction
2. Results and Discussion
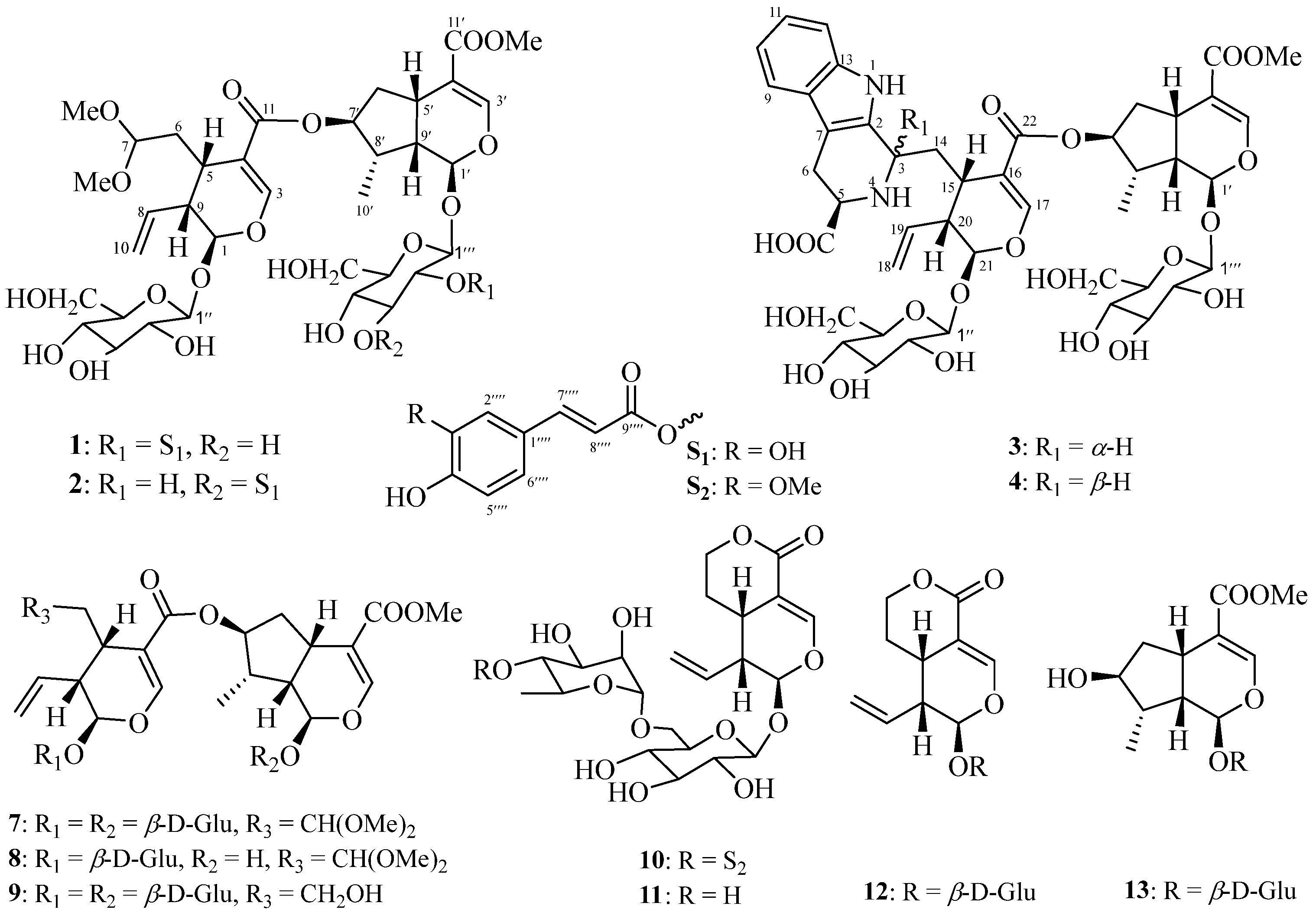
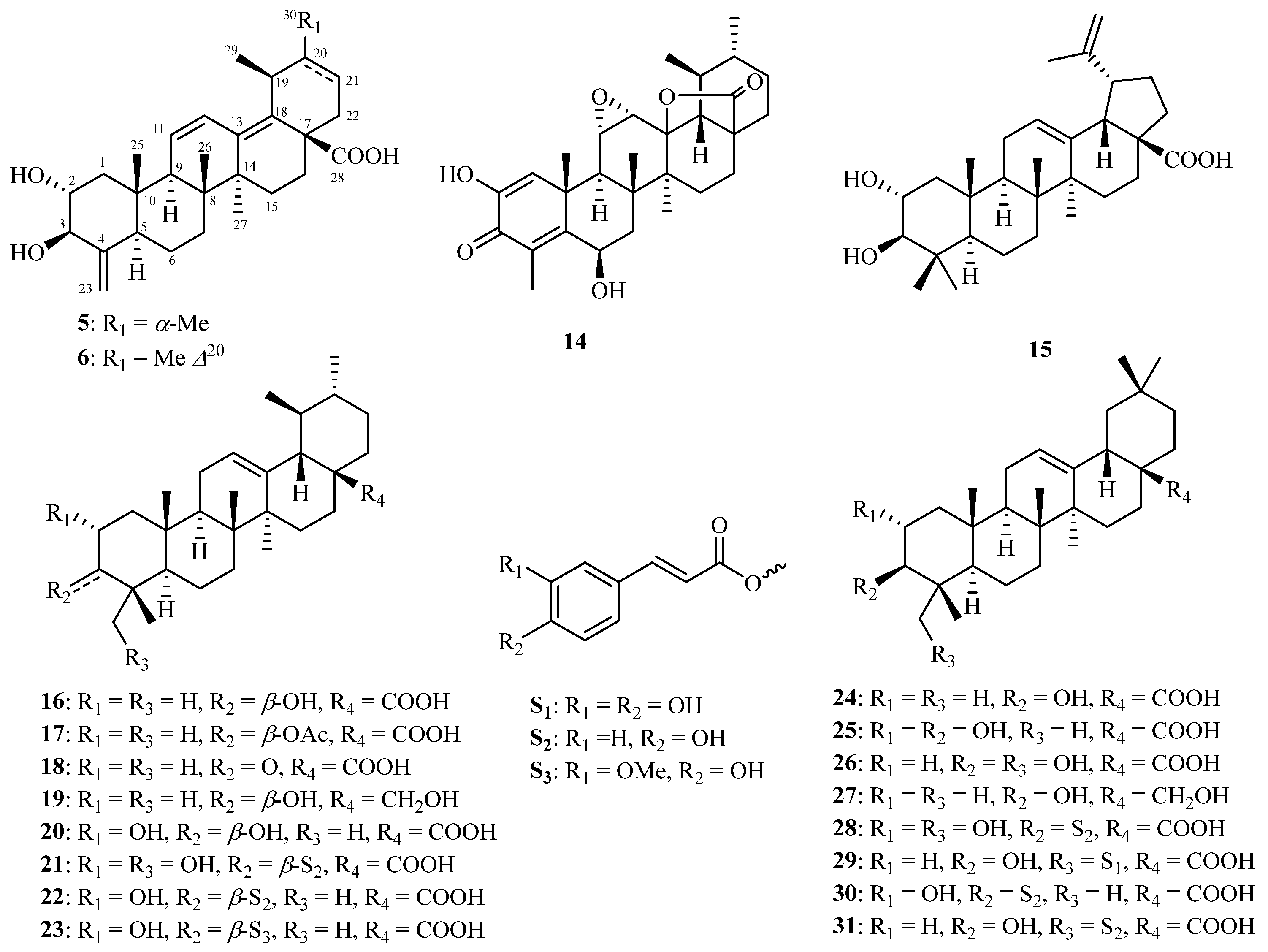
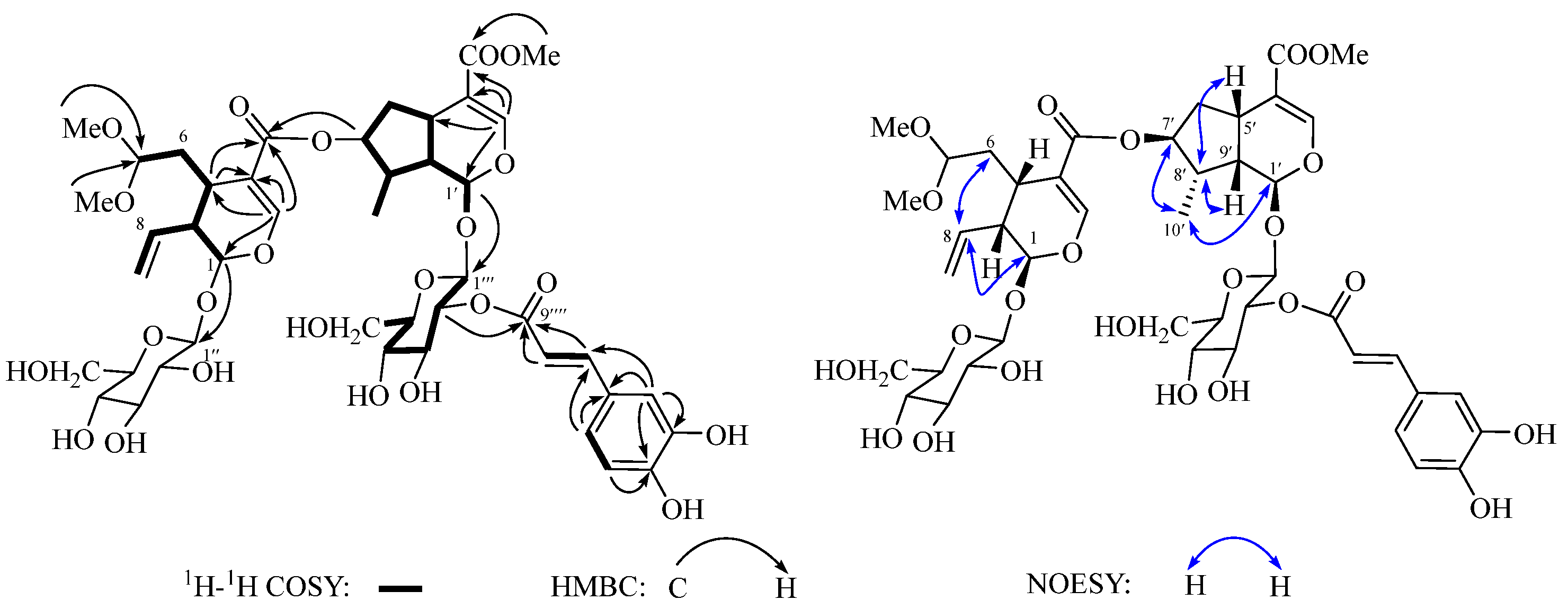
2.1. Structure Identification of Compounds 1–6
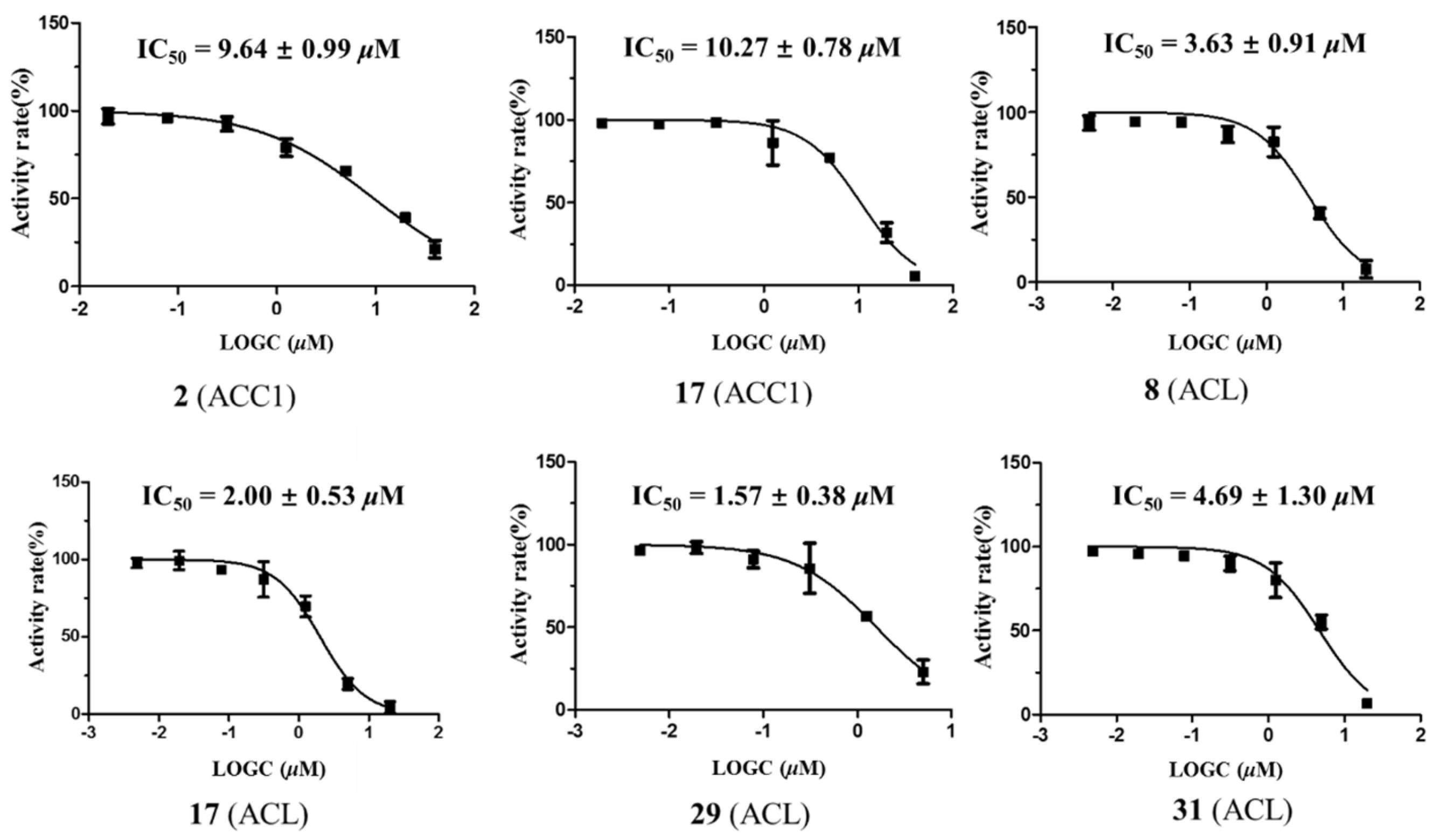
2.2. Anti-ACC1 and Anti-acl Bioactivities of the Isolated Compounds
2.3. Molecular Docking Simulation of Compounds 2, 17, and 29
2.4. Chemotaxonomic Significance
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Materials
3.3. Extraction and Isolation
3.4. Acid Hydrolysis of Saungmaygaoside C (7)
3.5. ACC1 and ACL Inhibitory Assay
3.6. Molecular Docking Simulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, H.-X.; Liu, H.; Moore, M.J.; Landrein, S.; Liu, B.; Zhu, Z.-X.; Wang, H.-F. Plastid phylogenomic insights into the evolution of the Caprifoliaceae s.l. (Dipsacales). Mol. Phylogenet. Evol. 2020, 142, 106641. [Google Scholar] [CrossRef] [PubMed]
- Li, W.J.; Zhang, L.Q.; He, P.; Li, H.Y.; Pan, X.; Zhang, W.L.; Xiao, M.F.; He, F.Y. Traditional uses, botany, phytochemistry, and pharmacology of Lonicerae japonicae flos and Lonicerae flos: A systematic comparative review. J. Ethnopharmcol. 2024, 322, 117278. [Google Scholar] [CrossRef] [PubMed]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China Part I; Chinese Medical Science and Technology Press: Beijing, China, 2020. [Google Scholar]
- Skala, E.; Szopa, A. Dipsacus and Scabiosa species-The source of specialized metabolites with high biological relevance: A review. Molecules 2023, 28, 3754. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.Y.; Li, J.; Yang, R.; Fang, L.; Zhang, Y.Q. A review: The triterpenoid saponins and biological activities of Lonicera Linn. Molecules 2020, 25, 3773. [Google Scholar] [CrossRef]
- Wei, L.L.; Luo, H.; Jin, Y.; Shu, Y.; Wen, C.L.; Qin, T.; Yang, X.R.; Ma, L.Q.; Liu, Y.; You, Y.; et al. Asperosaponin VI protects alcohol-induced hepatic steatosis and injury via regulating metabolism and ER stress. Phytomedicine 2023, 121, 155080. [Google Scholar] [CrossRef]
- Liu, Z.H.; Meng, L.J.; Wang, M.K.; Wang, L.; Liu, Y.H.; Hou, G.X.; Li, S.M.; Kang, W.Y. New iridoids from Patrinia scabiosaefolia and their hypoglycemic effects by activating PI3k/Akt signaling pathway. Fitoterapia 2023, 165, 105423. [Google Scholar] [CrossRef]
- Zhao, Z.-Y.; Wan, J.; Chen, H.-W.; Sun, Z.-S.; Tao, Y.-T.; Tong, Y.P.; Zang, Y.; Choo, Y.-M.; Wang, P.; Li, Y.-L.; et al. Major specialized natural products from the endangered plant Heptacodium miconioides, potential medicinal uses and insights into its longstanding unresolved systematic classification. Phytochemistry 2024, 228, 114259. [Google Scholar] [CrossRef]
- Wan, J.; Jiang, C.-X.; Tang, Y.; Ma, G.-L.; Tong, Y.-P.; Jin, Z.-X.; Zang, Y.; Osman, E.E.A.; Li, J.; Xiong, J.; et al. Structurally diverse glycosides of secoiridoid, bisiridoid, and triterpene-bisiridoid conjugates from the flower buds of two Caprifoliaceae plants and their ATP-citrate lyase inhibitory activities. Bioorg. Chem. 2022, 120, 105630. [Google Scholar] [CrossRef]
- Bai, G.-Q.; Zhou, T.; Zhao, J.-X.; Li, W.-M.; Han, G.-J.; Li, S.-F. The complete chloroplast genome of Kolkwitzia amabilis (Caprifoliaceae), an endangered horticultural plant in China. Mitochondrial DNA A 2017, 28, 296–297. [Google Scholar] [CrossRef]
- Park, S.H.; Burchi, G.; Roh, M.S.; Joung, Y.H. Characterization of Kolkwitzia amabilis accessions based on flowering and molecular markers. Sci. Hortic. 2014, 165, 190–195. [Google Scholar] [CrossRef]
- Naranjo, L.L.; Robacker, C.D. Kolkwitzia × abelia: A new Abelia hybrid with ornamental potential? HortScience 2022, 57, 774–776. [Google Scholar] [CrossRef]
- Fu, L.K.; Jin, J.M. China Plant Red Data Book. Rare and Endangered Plants I; Science Press: Beijing, China; New York, NY, USA, 1992; pp. 200–201. [Google Scholar]
- Wu, J.; Long, J.Y.; Liu, H.X.; Sun, G.P.; Li, J.; Xu, L.J.; Xu, C.Y. Biogenic volatile organic compounds from 14 landscape woody species: Tree species selection in the construction of urban greenspace with forest healthcare effects. J. Environ. Manag. 2021, 300, 113761. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Zang, Y.; Xiao, D.-A.; Li, N.; Li, J.M.; Jin, Z.-X.; Chen, D.-L.; Xiong, J.; Li, J.; Hu, J.-F. Stewartiacids A–N, C-23 carboxylated triterpenoids from Chinese stewartia and their inhibitory effects against ATP-citrate lyase and NF-κB. RSC Adv. 2020, 10, 3343–3356. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.-J.; Wu, X.-Y.; Zhao, Z.-Y.; Zang, Y.; Sun, Z.-S.; Li, Y.-L.; Li, N.; Xiong, J.; Choo, Y.-M.; Jin, Z.-X.; et al. Benzofurans and dibenzofurans from galls on twigs of the endangered Chinese endemic tree Parrotia subaequalis and their inhibitory properties against Staphylococcus aureus and ATP-citrate lyase. Phytochemistry 2025, 229, 114309. [Google Scholar] [CrossRef]
- Zhao, Z.-Y.; Zang, Y.; Li, J.; Choo, Y.-M.; Xiong, J.; Hu, J.-F. Fortunefuroic acid J from Keteleeria hainanensis and its dual inhibitory effects on ATP-citrate lyase and acetyl-CoA carboxylase. Chem. Biodivers. 2024, 21, e202401520. [Google Scholar] [CrossRef]
- Zhou, P.-J.; Zhao, Z.-Y.; Zang, Y.; Xiong, J.; Choo, Y.-M.; Li, J.; Hu, J.-F. Structurally diverse terpenoids from Pseudotsuga brevifolia and their inhibitory effects against ACL and ACC1 enzymes. Chin. J. Nat. Med. 2024, 22, 1–13. Available online: http://www.cjnmcpu.com/article/doi/10.1016/S1875-5364(24)60709-0 (accessed on 10 September 2024).
- He, Y.-H.; Li, K.-X.; Wu, Y.-F.; Jin, Z.-X.; Hu, J.-F.; Mao, Y.-C.; Xiong, J. Lirispirolodes A–L, a new class of sesquiterpene-monoterpene heterodimers with anti-neuroinflammatory activity from the rare medicinal plant Liriodendron chinense. Chin. J. Nat. Med. 2024, 22, 1–17. Available online: http://www.cjnmcpu.com/article/doi/10.1016/S1875-5364(24)60702-8 (accessed on 21 August 2024).
- Win, N.N.; Kodama, T.; Lae, K.Z.W.; Win, Y.Y.; Ngwe, H.; Abe, I.; Morita, H. Bis-iridoid and iridoid glycosides: Viral protein R inhibitors from Picrorhiza kurroa collected in Myanmar. Fitoterapia 2019, 134, 101–107. [Google Scholar] [CrossRef]
- Aimi, N.; Seki, H.; Sakai, S.-I.; Haginiwa, J. Kinginoside, a new acyl group carrying iridoid bioside from Lonicera morrowii. Chem. Pharm. Bull. 1993, 41, 1882–1884. [Google Scholar] [CrossRef][Green Version]
- Cambie, R.C.; Lal, A.R.; Rickard, C.E.F.; Tanaka, N. Chemistry of Fijian plants V.1) Constituents of Fagraea gracilipes A. Gray. Chem. Pharm. Bull. 1990, 38, 1857–1861. [Google Scholar] [CrossRef]
- Bianco, A.; Passacantilli, P. 8-Epiloganin, an iridoid glucoside from Odontites verna. Phytochemistry 1981, 20, 1873–1876. [Google Scholar] [CrossRef]
- Saito, Y.; Takashima, Y.; Okamoto, Y.; Komiyama, T.; Ohsaki, A.; Gong, X.; Tori, M. Two new norursane-type triterpenoids from Dipsacus chinensis collected in China. Chem. Lett. 2012, 41, 372–373. [Google Scholar] [CrossRef]
- Hemmateenejad, B.; Javidnia, K.; Nematollahi, M.; Elyasi, M. QSAR studies on the antiviral compounds of natural origin. J. Iran. Chem. Soc. 2009, 6, 420–435. [Google Scholar] [CrossRef]
- Seebacher, W.; Simic, N.; Weis, R.; Saf, R.; Kunert, O. Complete assignment of 1H and 13C NMR resonances of oleanolic acid, 18α-oleanolic acid, ursolic acid and their 11-oxo derivatives. Magn. Reson. Chem. 2003, 41, 636–638. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Min, K.; Huang, G.-L.; Chen, Y.-G. Compounds from the acid hydrolysate of glycosides of Boschniakia himalaica. Chem. Nat. Compd. 2019, 55, 105–106. [Google Scholar] [CrossRef]
- Min, B.S.; Kim, Y.H.; Lee, S.M.; Jung, H.J.; Lee, J.S.; Na, M.K.; Lee, C.O.; Lee, J.P.; Bae, K.H. Cytotoxic triterpenes from Crataegus pinnatifida. Arch. Pharm. Res. 2000, 23, 155–158. [Google Scholar] [CrossRef]
- Acebey-Castellon, I.L.; Voutquenne-Nazabadioko, L.; Mai, H.D.T.; Roseau, N.; Bouthagane, N.; Muhammad, D.; Debar, E.L.M.; Gangloff, S.C.; Litaudon, M.; Sevenet, T.; et al. Triterpenoid saponins from Symplocos lancifolia. J. Nat. Prod. 2011, 74, 163–168. [Google Scholar] [CrossRef]
- Sashida, Y.; Ogawa, K.; Mori, N.; Yamanouchi, T. Triterpenoids from the fruit galls of Actinidia polygama. Phytochemistry 1992, 31, 2801–2804. [Google Scholar] [CrossRef]
- Numata, A.; Yang, P.M.; Takahashi, C.; Fujiki, R.; Nabae, M.; Fujita, E. Cytotoxic triterpenes from a Chinese medicine, Goreishi. Chem. Pharm. Bull. 1989, 37, 648–651. [Google Scholar] [CrossRef]
- Häberlein, H.; Tschiersch, K.-P. Triterpenoids and flavonoids from Leptospermum scoparium. Phytochemistry 1994, 35, 765–768. [Google Scholar] [CrossRef]
- Tchivounda, H.P.; Koudogbo, B.; Besace, Y.; Casadevall, E. Triterpene saponins from Cylicodiscus gabunensis. Phytochemistry 1991, 30, 2711–2716. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.A.; Ko, H.J.; Lee, H.; Haque, M.A.; Park, I.-S.; Lee, D.-S.; Woo, E.-R. Oleanane triterpenoids from Akebiae caulis exhibitory effects on Aβ42 induced fibrillogenesis. Arch. Pharm. Res. 2017, 40, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Nam, I.Y.; Kim, J.W.; Shin, T.Y.; Lim, J.P. Pentacyclic triterpenoids from Ilex macropoda. Arch. Pharm. Res. 2002, 25, 617–620. [Google Scholar] [CrossRef] [PubMed]
- Tapondjou, A.L.; Ngounou, N.F.; Lontsi, D.; Sondengam, B.L.; Martin, M.-T.; Bodo, B. Pentacyclic triterpenes from Myrianthus liberecus. Phytochemistry 1995, 40, 1761–1764. [Google Scholar] [CrossRef]
- Pan, H.F.; Lundgren, L.N.; Andersson, R. Triterpene caffeates from bark of Betula pubescens. Phytochemistry 1994, 37, 795–799. [Google Scholar] [CrossRef]
- Rudiyansyah; Garson, M.J. Secondary metabolites from the wood bark of Durio zibethinus and Durio kutejensis. J. Nat. Prod. 2006, 69, 1218–1221. [Google Scholar] [CrossRef]
- Chang, C.-I.; Kuo, C.-C.; Chang, J.-Y.; Kuo, Y.-H. Three new oleanane-type triterpenes from Ludwigia octovalvis with cytotoxic activity against two human cancer cell lines. J. Nat. Prod. 2004, 67, 91–93. [Google Scholar] [CrossRef]
- Barakat, H.H.; Nawwar, M.A.M.; Buddrus, J.; Linscheid, M. Niloticol, a phenolic glyceride and two phenolic aldehydes from the roots of Tamarix nilotica. Phytochemistry 1987, 26, 1837–1838. [Google Scholar] [CrossRef]
- Xuan, W.-D.; Chen, H.-S.; Yuan, Z.-X.; Zhu, P. Chemical constituents of Nauclea officinalis. Chin. J. Nat. Prod. 2005, 3, 181–183. [Google Scholar]
- Zou, J.; Xiao, L.; Zhang, J.J.; Wang, L.; Zhao, C.L.; Ye, J.H.; Zhang, Q.L.; He, K. Chemical constituents of Pteris ensiformis from Guizhou. Biochem. Syst. Ecol. 2023, 110, 104718. [Google Scholar] [CrossRef]
- Pel, P.; Kim, Y.-M.; Chin, Y.-W. Chemical constituents with anti-allergic activity from the barks of Cinnamomum cambodianum collected in Cambodia. Bull. Korean Chem. Soc. 2015, 36, 384–387. [Google Scholar] [CrossRef]
- Vermes, B.; Seligmann, O.; Wagner, H. Synthesis of biologically active tetrahydro-furofuranlignan-(syringing, pinoresinol)-mono- and bis-glucosides. Phytochemistry 1991, 30, 3087–3089. [Google Scholar] [CrossRef]
- Zhu, H.C.; Wu, J.T.; Xu, S.Q.; Zhao, W.; Naseem, A.; Pan, J.; Guan, W.; Kuang, H.X.; Liu, Y.; Yang, B.Y. A new neolignan glycoside from the roots of Eleutherococcus senticosus (Rupr. Et Maxim) Maxim and its chemotaxonomic significance. Biochem. Syst. Ecol. 2024, 113, 104788. [Google Scholar] [CrossRef]
- Della Greca, M.; Ferrara, M.; Fiorentino, A.; Monaco, P.; Previtera, L. Antialgal compounds from Zanteddeschia aethiopica. Phytochemistry 1998, 49, 1299–1304. [Google Scholar] [CrossRef]
- Kuang, H.-X.; Xia, Y.-G.; Yang, B.-Y.; Wang, Q.-H.; Lü, S.-W. Lignan constituents from Chloranthus japonicus Sieb. Arch. Pharm. Res. 2009, 32, 329–334. [Google Scholar] [CrossRef]
- Yu, Z.-P.; Wang, Y.-Y.; Yu, S.-J.; Bao, J.; Yu, J.-H.; Zhang, H. Absolute structure assignment of an iridoid-monoterpenoid indole alkaloid hybrid from Dipsacus asper. Fitoterapia 2019, 135, 99–106. [Google Scholar] [CrossRef]
- Aimi, N.; Seki, H.; Sakai, S.-I. Synthesis of lyaloside, a prototypal β-carboline gluco indole alkaloid in Rubiaceous plants. Chem. Pharm. Bull. 1992, 40, 2588–2590. [Google Scholar] [CrossRef]
- Saiga, Y.; Iijima, I.; Ishida, A.; Miyagishima, T.; Takamura, N.; Oh-Ishi, T.; Matsumoto, M.; Matsuoka, Y. Synthesis of 1,2,3,4-tetrahydro-β-carboline derivatives as hepatoprotective agents. IV. Positional isomers of 1,2,3,4-tetrahydro-2-methylthiothiocarbonyl-β-carboline-3-carboxylic acid and its 1-alkylated derivatives. Chem. Pharm. Bull. 1987, 35, 3705–3712. [Google Scholar] [CrossRef]
- Yu, J.-H.; Yu, Z.-P.; Wang, Y.-Y.; Bao, J.; Zhu, K.-K.; Yuan, T.; Zhang, H. Triterpenoids and triterpenoid saponins from Dipsacus asper and their cytotoxic and antibacterial activities. Phytochemistry 2019, 162, 241–249. [Google Scholar] [CrossRef]
- Zhang, J.-S.; Qian, Y.; Xin, Z.-Q.; Cao, X.-X.; Yang, Z.; Zhang, H. Bioactive pentacyclic triterpenoids from the whole plants of Perocephalus hookeri. Phytochemistry 2022, 195, 113040. [Google Scholar] [CrossRef]
- Pham, T.; Walden, E.; Huard, S.; Pezacki, J.; Fullerton, M.D.; Baetz, K. Fine-tuning acetyl-CoA carboxylase 1 activity through localization: Functional genomics reveals a role for the lysine acetyltransferase NuA4 and sphingolipid metabolism in regulating ACC1 activity and localization. Genetics 2022, 221, iyac086. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.J.; Zhang, L.; Xu, S.W.; Shen, A.-Z. ATP-citrate lyase (ACLY) in lipid metabolism and atherosclerosis: An updated review. Prog. Lipid Res. 2020, 77, 101006. [Google Scholar] [CrossRef] [PubMed]
- Bates, J.; Vijayakumar, A.; Ghoshal, S.; Marchand, B.; Yi, S.L.; Kornyeyev, D.; Zagorska, A.; Hollenback, D.; Walker, K.; Liu, K.; et al. Acetyl-CoA carboxylase inhibition disrupts metabolic reprogramming during hepatic stellate cell activation. J. Hepatol. 2020, 73, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, J.M.; Kiazand, M. Bempedoic acid reduces major adverse cardiovascular events for statin-intolerant patients. J. Gen. Intern. Med. 2024, 39, 1536–1538. [Google Scholar] [CrossRef]
- Harriman, G.; Greenwood, J.; Bhat, S.; Huang, X.Y.; Wang, R.Y.; Paul, D.; Tong, L.; Saha, A.K.; Westlin, W.F.; Kapeller, R.; et al. Acetyl-CoA carboxylase inhibition by ND-630 reduces hepatic steatosis, improves insulin sensitivity, and modulates dyslipidemia in rats. Proc. Natl. Acad. USA 2016, 113, E1796–E1805. [Google Scholar] [CrossRef]
- Koerner, S.K.; Hanai, J.-I.; Bai, S.; Jernigan, F.E.; Oki, M.; Komaba, C.; Shuto, E.; Sukhatme, V.P.; Sun, L.J. Design and synthesis of emodin derivatives as novel inhibitors of ATP-citrate lyase. Eur. J. Med. Chem. 2017, 126, 920–928. [Google Scholar] [CrossRef]
- Quattrini, A.M.; Wu, T.; Soong, K.; Jeng, M.-S.; Benayahu, Y.; McFadden, C.S. A next generation approach to species delimitation reveals the role of hybridization in cryptic species complex of corals. BMC Evol. Biol. 2019, 19, 116. [Google Scholar] [CrossRef]
- Eich, E. Solanaceae and Convolvulaceae: Secondary Metabolites: Biosynthesis, Chemotaxonomy, Biological and Economic Significance; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Kim, H.K.; Saifullah; Khan, S.; Wilson, E.G.; Kricun, S.D.P.; Meissner, A.; Goraler, S.; Deelder, A.M.; Choi, Y.H.; Verpoorte, R. Metabolic classification of South American Ilex species by NMR-based metabolomics. Phytochemistry 2010, 71, 773–784. [Google Scholar] [CrossRef]
- Kim, H.K.; Choi, Y.H.; Verpoorte, R. NMR-based plant metabolomics: Where do we stand, where do we go? Trends Biotechnol. 2011, 29, 267–275. [Google Scholar] [CrossRef]
- Vajda, V.; Pucetaite, M.; McLoughlin, S.; Engdahl, A.; Heimdal, J.; Uvdal, P. Molecular signatures of fossil leaves provide unexpected new evidence for extinct plant relationships. Nat. Ecol. Evol. 2017, 1, 1093–1099. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Göker, M. TYGS is an automated high-throughput platform for state-of-the-art genome-based taxonomy. Nat. Commun. 2019, 10, 2182. [Google Scholar] [CrossRef] [PubMed]
- Koltai, H.; Namdar, D. Cannabis phytomolecule ‘Entourage’: From domestication to medical use. Trends Plant Sci. 2020, 25, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Dayrat, B. Towards integrative taxonomy. Biol. J. Linn. Soc. 2005, 85, 407–415. [Google Scholar] [CrossRef]
- Will, K.W.; Mishler, B.D.; Wheeler, Q.D. The perils of DNA barcoding and the need for integrative taxonomy. Syst. Biol. 2005, 54, 844–851. [Google Scholar] [CrossRef]
- Schlick-Steiner, B.C.; Steiner, F.M.; Seifert, B.; Stauffer, C.; Christian, E.; Crozier, R.H. Integrative taxonomy: A multisource approach to exploring biodiversity. Annu. Rev. Entomol. 2010, 55, 421–438. [Google Scholar] [CrossRef]
- Meng, S.Y.; Wang, Z.M.; Ye, L. Integrative taxonomy supports two new species of Rhodiola (Crassulaceae) in Xizang. China Divers. 2022, 14, 289. [Google Scholar] [CrossRef]
- Bendamene, S.; Boutaghane, N.; Sayagh, C.; Magid, A.A.; Kabouche, Z.; Bensouici, C.; Voutquenne-Nazabadioko, L. Bis-iridoids and other constituents from Scabiosa semipapposa. Phytochem. Lett. 2022, 49, 202–210. [Google Scholar] [CrossRef]
- Duan, X.-Y.; Ai, L.-Q.; Qian, C.-Z.; Zhang, M.-D.; Mei, R.-Q. The polymer iridoid glucosides isolated from Dipsacus asper. Phytochem. Lett. 2019, 33, 17–21. [Google Scholar] [CrossRef]
- Tomassini, L.; Foddai, S.; Serafini, M.; Cometa, M.F. Bis-iridoid glucosides from Abelia chinensis. J. Nat. Prod. 2000, 63, 998–999. [Google Scholar] [CrossRef]
- Podányi, B.; Reid, R.S.; Kocsis, A.; Szabó, L. Laciniatoside V: A new bis-iridoid glucoside, isolation and structure elucidation by 2D NMR spectroscopy. J. Nat. Prod. 1989, 52, 135–142. [Google Scholar] [CrossRef]
- Jensen, S.R.; Lyse-Petersen, S.E.; Nielsen, B.J. Novel bis-iridoid glycosides from Dipsacus sylvestris. Phytochemistry 1979, 18, 273–277. [Google Scholar] [CrossRef]
- Xiong, J.; Wu, X.-Y.; Wang, P.-P.; Lau, C.; Fan, H.; Ma, G.-L.; Tang, Y.; Li, J.; Hu, J.-F. Acylated iridoid diglycosides from the cultivated endangered ornamental tree Gmelina hainanensis. Phytochem. Lett. 2018, 25, 17–21. [Google Scholar] [CrossRef]
- Xiong, J.; Zhou, P.-J.; Jiang, H.-W.; Huang, T.; He, Y.-H.; Zhao, Z.-Y.; Zang, Y.; Choo, Y.-M.; Wang, X.; Chittiboyina, A.G.; et al. Forrestiacids A and B, pentaterpene inhibitors of ACL and lipogenesis: Extending the limits of computational NMR methods in the structure assignments of complex natural products. Angew. Chem. Int. Ed. 2021, 60, 22270–22275. [Google Scholar] [CrossRef] [PubMed]


| No. | 1 | 2 | ||
|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC | |
| 1 | 5.52, d (5.5) | 97.8 | 5.52, d (5.5) | 97.8 |
| 3 | 7.43, br s | 153.3 | 7.45, br s | 153.3 |
| 4 | 112.0 | 112.0 | ||
| 5 | 2.89, m | 29.5 | 2.91, m | 29.4 |
| 6 | 2.06, m; 1.62, m | 33.2 | 2.06, m; 1.62, m | 33.2 |
| 7 | 4.50, dd (6.9, 4.4) | 104.1 | 4.50, dd (7.3, 4.7) | 104.1 |
| 8 | 5.74, m | 135.9 | 5.74, m | 135.9 |
| 9 | 2.67, m | 45.4 | 2.70, m | 45.4 |
| 10 | 5.26, br d (17.0) | 119.8 | 5.31, br d (17.1) | 119.8 |
| 5.20, br d (10.6) | 5.27, br d (10.2) | |||
| 11 | 168.3 | 168.3 | ||
| 1′ | 5.47, d (1.8) | 95.5 | 5.34, d (2.0) | 97.4 |
| 3′ | 7.23, br s | 150.7 | 7.43, br s | 152.5 |
| 4′ | 114.5 | 113.4 | ||
| 5′ | 2.93, m | 30.9 | 3.11, m | 32.4 |
| 6′ | 2.17, m; 1.77, m | 39.6 | 2.31, m; 1.75, m | 40.3 |
| 7′ | 5.10, m | 78.6 | 5.20, m | 78.3 |
| 8′ | 1.92, m | 40.0 | 2.12, m | 41.0 |
| 9′ | 2.22, m | 47.2 | 2.12, m | 47.1 |
| 10′ | 1.03, d (7.0) | 12.8 | 1.08, d (5.6) | 13.7 |
| 11′ | 168.7 | 169.3 | ||
| 1″ | 4.69, d (7.9) | 100.0 | 4.68, d (8.0) | 100.1 |
| 2″ | 3.19, m | 74.6 | 3.19, m | 74.6 |
| 3″ | 3.38, m | 78.0 | 3.38, m | 78.0 |
| 4″ | 3.28, m | 71.5 | 3.28, m | 71.5 |
| 5″ | 3.30, m | 78.4 | 3.30, m | 78.4 |
| 6″ | 3.93, dd (12.1, 1.5) | 62.7 | 3.89, dd (12.0, 2.0) | 62.7 |
| 3.69, dd (12.1, 5.8) | 3.70, dd (12.0, 5.9) | |||
| 1‴ | 4.86, d (8.1) | 97.6 | 4.79, d (8.0) | 100.1 |
| 2‴ | 4.79, dd (9.0, 8.1) | 74.5 | 3.39, m | 73.2 |
| 3‴ | 3.62, m | 75.8 | 5.06, dd (9.3, 9.2) | 78.8 |
| 4‴ | 3.38, m | 71.7 | 3.52, dd (9.4, 9.3) | 69.8 |
| 5‴ | 3.38, m | 78.5 | 3.27, m | 78.2 |
| 6‴ | 3.92, br d (12.4) | 62.8 | 3.92, dd (12.0, 2.0) | 62.5 |
| 3.68, dd (overlapped) | 3.66, dd (12.0, 6.3) | |||
| 1″″ | 127.6 | 127.7 | ||
| 2″″ | 7.03, s | 115.3 | 7.04, s | 115.1 |
| 3″″/4″″ | 149.8/147.0 | 149.8/147.0 | ||
| 5″″ | 6.78, d (8.0) | 116.5 | 6.76, d (8.0) | 116.5 |
| 6″″ | 6.94, d (8.0) | 123.2 | 6.94, d (8.0) | 123.0 |
| 7″″ | 7.47, d (15.9) | 147.0 | 7.59, d (15.9) | 146.9 |
| 8″″ | 6.16, d (15.9) | 114.7 | 6.32, d (15.9) | 115.3 |
| 9″″ | 168.0 | 169.0 | ||
| 7-OMe | 3.28, s (6H) | 53.5 or 52.8 | 3.29, s (6H) | 53.6 or 52.7 |
| 11′-OMe | 3.15, s | 51.7 | 3.68, s | 51.7 |
| No. | 3 | 4 | ||
|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC | |
| 2 | 130.2 | 130.0 | ||
| 3 | 4.51, br d (11.3) | 53.2 | 4.84, dd (3.7, 3.7) | 52.7 |
| 5 | 3.89, dd, (11.7, 4.0) | 59.6 | 4.12, dd (9.1, 5.7) | 55.0 |
| 6 | 3.44, m; 3.01, m | 24.0 | 3.39, m; 3.13, m | 24.1 |
| 7/8 | 108.4/138.5 | 107.3/138.4 | ||
| 9 | 7.48, d (7.4) | 119.8 | 7.47, d (7.8) | 119.2 |
| 10 | 7.04, dd (7.4, 7.0) | 120.7 | 7.04, dd (8.0, 7.2) | 120.5 |
| 11 | 7.13, dd (8.0, 7.0) | 123.5 | 7.14, dd (7.8, 7.2) | 123.5 |
| 12 | 7.31, d (8.0) | 112.2 | 7.36, d (8.0) | 112.4 |
| 13 | 127.5 | 127.4 | ||
| 14 | 2.45, m; 2.25, m | 34.6 | 2.36, m; 2.17, m | 35.3 |
| 15 | 3.09, m | 32.7 | 3.16, m | 31.5 |
| 16 | 108.9 | 110.8 | ||
| 17 | 7.87, br s | 157.3 | 7.64, br s | 154.9 |
| 18 | 5.40, br d (17.8); 5.26, br d (10.4) | 119.2 | 5.34, br d (17.2); 5.30, br d (10.3) | 120.8 |
| 19 | 5.85, m | 135.2 | 5.93, m | 135.0 |
| 20 | 2.81, m | 45.3 | 2.18, m | 45.6 |
| 21 | 5.92, d (8.3) | 97.6 | 5.63, d (7.1) | 97.8 |
| 22 | 171.2 | 169.3 | ||
| 23 | 173.5 | 174.0 | ||
| 1′ | 5.27, d (5.5) | 97.5 | 5.28, d (4.9) | 97.6 |
| 3′ | 7.41, br s | 152.7 | 7.44, br s | 152.7 |
| 4′ | 113.0 | 113.1 | ||
| 5′ | 3.12, m | 32.8 | 3.11, m | 32.8 |
| 6′ | 2.35, m; 1.75, m | 40.5 | 2.34, m; 1.76, m | 40.4 |
| 7′ | 5.29, m | 79.8 | 5.28, m | 79.1 |
| 8′ | 2.16, m | 41.3 | 2.14, m | 41.1 |
| 9′ | 2.08, m | 47.0 | 2.07, m | 47.1 |
| 10′ | 1.09, d (6.7) | 14.1 | 1.04, d (6.7) | 13.9 |
| 11′ | 169.3 | 169.4 | ||
| 1″ | 4.84, d (7.7) | 100.5 | 4.75, d (7.8) | 100.4 |
| 2″ | 3.24, m | 74.7 | 3.21, m | 74.7 |
| 3″ | 3.40, m | 78.0 | 3.38, m | 78.0 |
| 4″ | 3.28, m | 71.7 | 3.25, m | 71.7 |
| 5″ | 3.41, m | 78.8 | 3.25, m | 78.6 |
| 6″ | 4.02, br d (11.7); 3.66, m | 63.1 | 3.97, dd (12.0, 2.0); 3.65, m | 62.8 |
| 1‴ | 4.65, d (7.7) | 100.2 | 4.65, d (7.7) | 100.2 |
| 2‴ | 3.17, m | 74.6 | 3.17, m | 74.7 |
| 3‴ | 3.35, m | 77.9 | 3.38, m | 78.0 |
| 4‴ | 3.23, m | 71.6 | 3.25, m | 71.6 |
| 5‴ | 3.29, m | 78.4 | 3.34, m | 78.4 |
| 6‴ | 3.88, br d (11.5); 3.61, m | 62.8 | 3.89, dd (12.0, 2.0); 3.62, m | 62.8 |
| OMe | 3.68, s | 51.8 | 3.69, s | 51.8 |
| No. | 5 | 6 | ||
|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC | |
| 1α | 1.13, dd (overlapped) | 47.4 | 1.13, dd (overlapped) | 47.3 |
| 1β | 2.27, dd (12.4, 5.3) | 2.27, dd (12.7, 5.3) | ||
| 2 | 3.54, m | 74.2 | 3.54, m | 74.1 |
| 3 | 3.76, d (9.1) | 79.6 | 3.77, d (9.0) | 79.6 |
| 4 | 151.5 | 151.5 | ||
| 5 | 1.75, br d (overlapped) | 51.1 | 1.77, br d (12.6) | 51.1 |
| 6 | 1.53, m | 22.2 | 1.55, m | 22.2 |
| 7 | 1.42, m | 32.0 | 1.43, m | 32.0 |
| 8 | 43.5 | 43.2 | ||
| 9 | 2.15, d (2.9) | 53.4 | 2.17, d (2.8) | 53.4 |
| 10 | 39.2 | 39.3 | ||
| 11 | 6.56, dd (11.8, 2.9) | 126.5 | 6.50, dd (10.6, 2.8) | 126.7 |
| 12 | 5.72, d (11.8) | 127.6 | 5.77, d (10.6) | 127.6 |
| 13 | 138.9 | 136.3 | ||
| 14 | 42.0 | 42.5 | ||
| 15 | 1.97, m; 1.07, m | 26.1 | 1.07, m | 26.3 |
| 16 | 2.23, m; 1.35, m | 34.2 | 1.90, m; 1.68, m | 33.4 |
| 17 | 47.3 | 47.7 | ||
| 18 | 136.8 | 134.9 | ||
| 19 | 2.88, m | 38.5 | 3.22, m | 37.5 |
| 20 | 1.70, m | 36.2 | 137.6 | |
| 21 | 1.96, m; 1.19, m | 26.1 | 5.43, br d (5.0) | 121.0 |
| 22 | 1.73, m | 35.3 | 2.77, dd (16.0, 6.2) | 38.9 |
| 1.73, m | 1.74, br d (16.0) | |||
| 23 | 5.16, 4.73, br s | 105.0 | 5.17, 4.74, br s | 105.5 |
| 25 | 0.75, s | 16.6 | 0.76, s | 16.6 |
| 26 | 0.90, s | 17.1 | 0.93, s | 17.3 |
| 27 | 1.00, s | 20.9 | 0.99, s | 20.2 |
| 28 | 180.3 | 182.4 | ||
| 29 | 1.05, d (7.2) | 21.9 | 1.18, d (7.4) | 21.4 |
| 30 | 0.92, d (7.1) | 20.2 | 1.66, s | 21.8 |
| Compound | IC50 a | |
|---|---|---|
| ACC1 | ACL | |
| 2 | 9.6 ± 1.0 μM | >20 |
| 8 | >20 | 3.6 ± 0.9 μM |
| 17 | 10.3 ± 0.8 μM | 2.0 ± 0.5 μM |
| 29 | >20 | 1.6 ± 0.4 μM |
| 31 | >20 | 4.7 ± 1.3 μM |
| ND 630 b | 2.0 ± 0.1 nM | NT d |
| BMS 303141 c | NT d | 0.3 ± 0.1 μM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, J.; Zhao, Z.-Y.; Wang, C.; Jiang, C.-X.; Tong, Y.-P.; Zang, Y.; Choo, Y.-M.; Li, J.; Hu, J.-F. Bis-Iridoid Glycosides and Triterpenoids from Kolkwitzia amabilis and Their Potential as Inhibitors of ACC1 and ACL. Molecules 2024, 29, 5980. https://doi.org/10.3390/molecules29245980
Wan J, Zhao Z-Y, Wang C, Jiang C-X, Tong Y-P, Zang Y, Choo Y-M, Li J, Hu J-F. Bis-Iridoid Glycosides and Triterpenoids from Kolkwitzia amabilis and Their Potential as Inhibitors of ACC1 and ACL. Molecules. 2024; 29(24):5980. https://doi.org/10.3390/molecules29245980
Chicago/Turabian StyleWan, Jiang, Ze-Yu Zhao, Can Wang, Chun-Xiao Jiang, Ying-Peng Tong, Yi Zang, Yeun-Mun Choo, Jia Li, and Jin-Feng Hu. 2024. "Bis-Iridoid Glycosides and Triterpenoids from Kolkwitzia amabilis and Their Potential as Inhibitors of ACC1 and ACL" Molecules 29, no. 24: 5980. https://doi.org/10.3390/molecules29245980
APA StyleWan, J., Zhao, Z.-Y., Wang, C., Jiang, C.-X., Tong, Y.-P., Zang, Y., Choo, Y.-M., Li, J., & Hu, J.-F. (2024). Bis-Iridoid Glycosides and Triterpenoids from Kolkwitzia amabilis and Their Potential as Inhibitors of ACC1 and ACL. Molecules, 29(24), 5980. https://doi.org/10.3390/molecules29245980








