Analysis of Polyphenols During Alcoholic Fermentation of Red Grape Aglianico (Vitis vinifera L.): Potential Winemaking Optimization and Pomace Valorization
Abstract
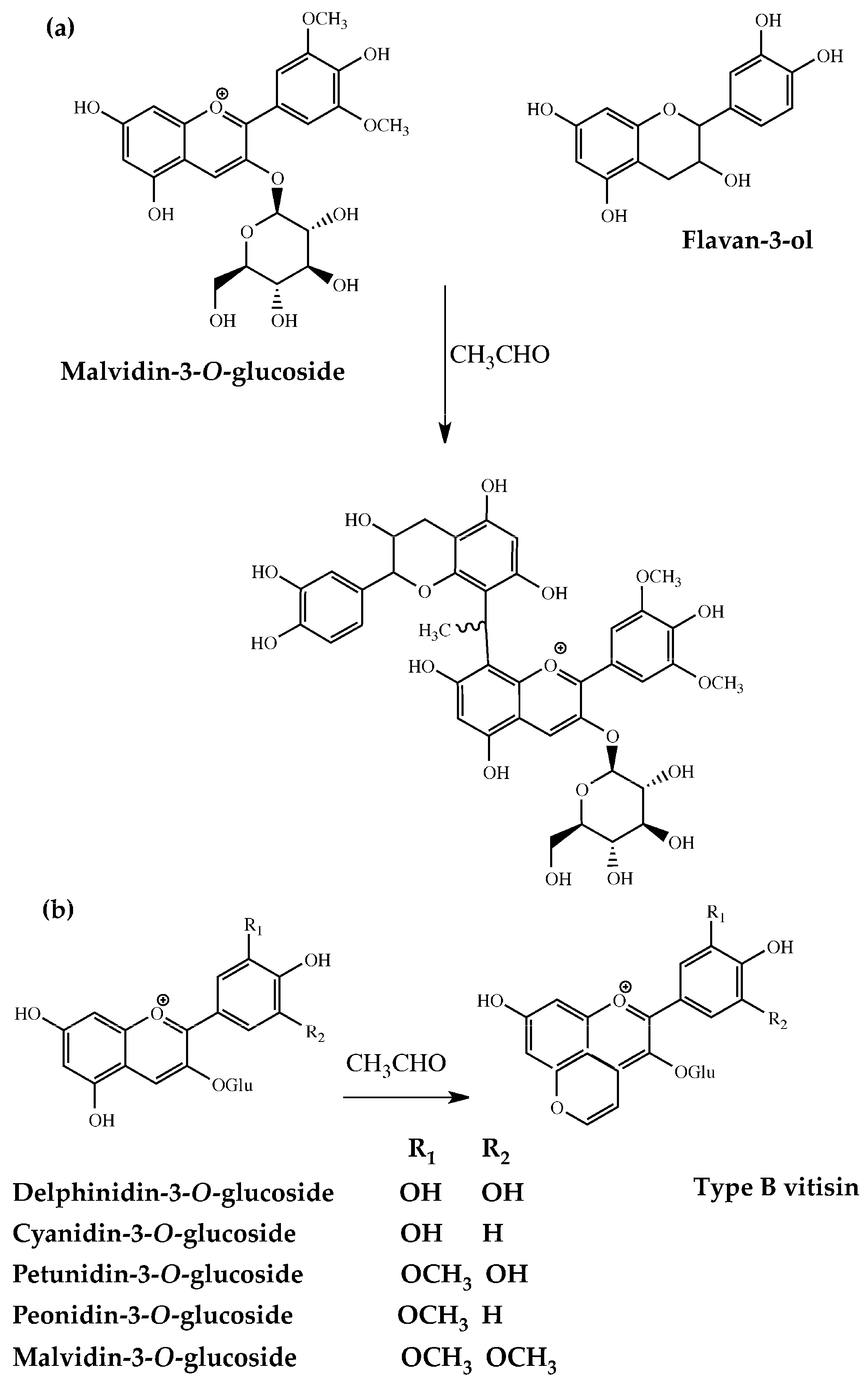
1. Introduction
2. Results and Discussions
2.1. Analytical Strategy
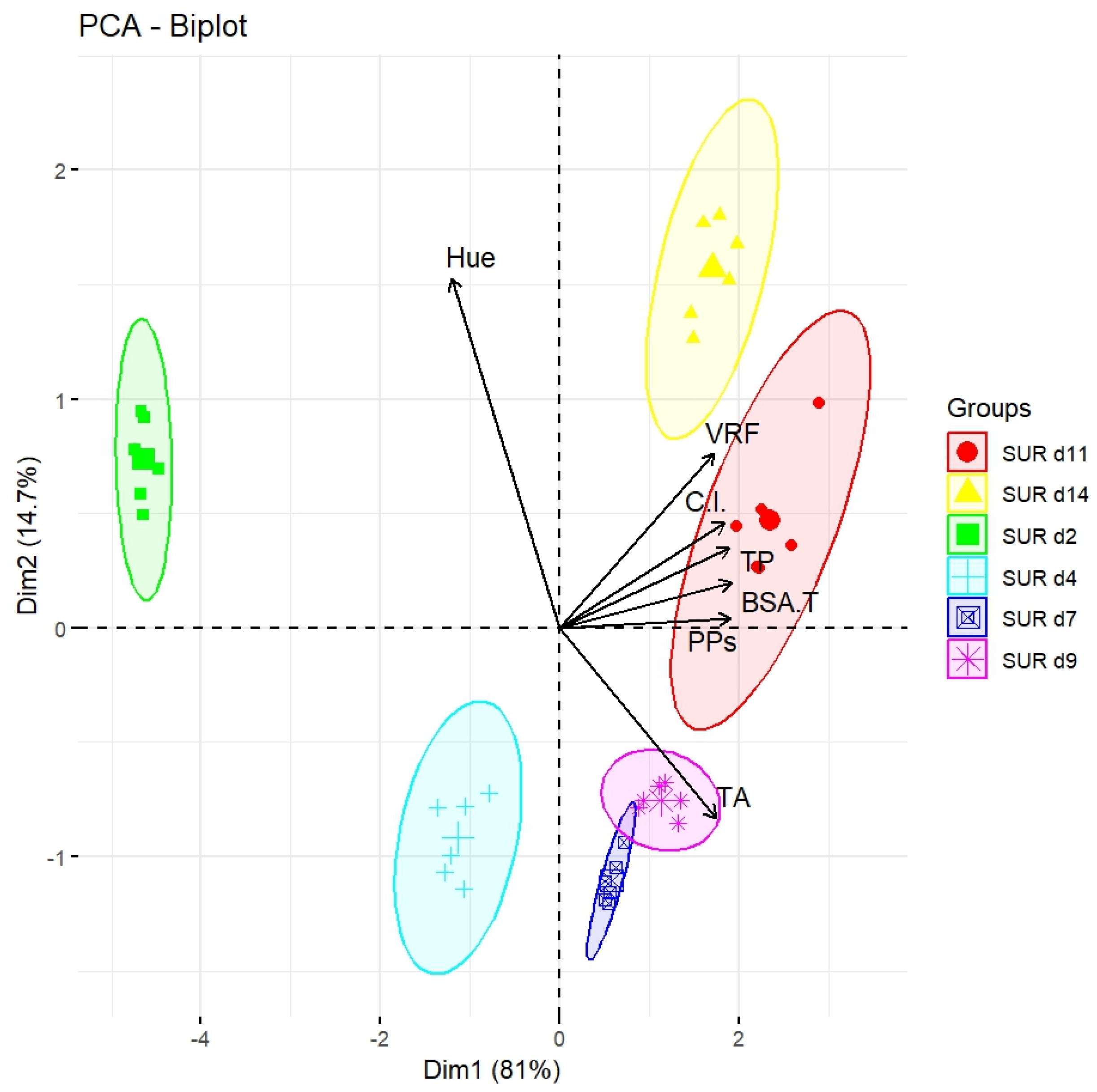
2.2. Polyphenol Analysis in the Liquid Must (SUR)
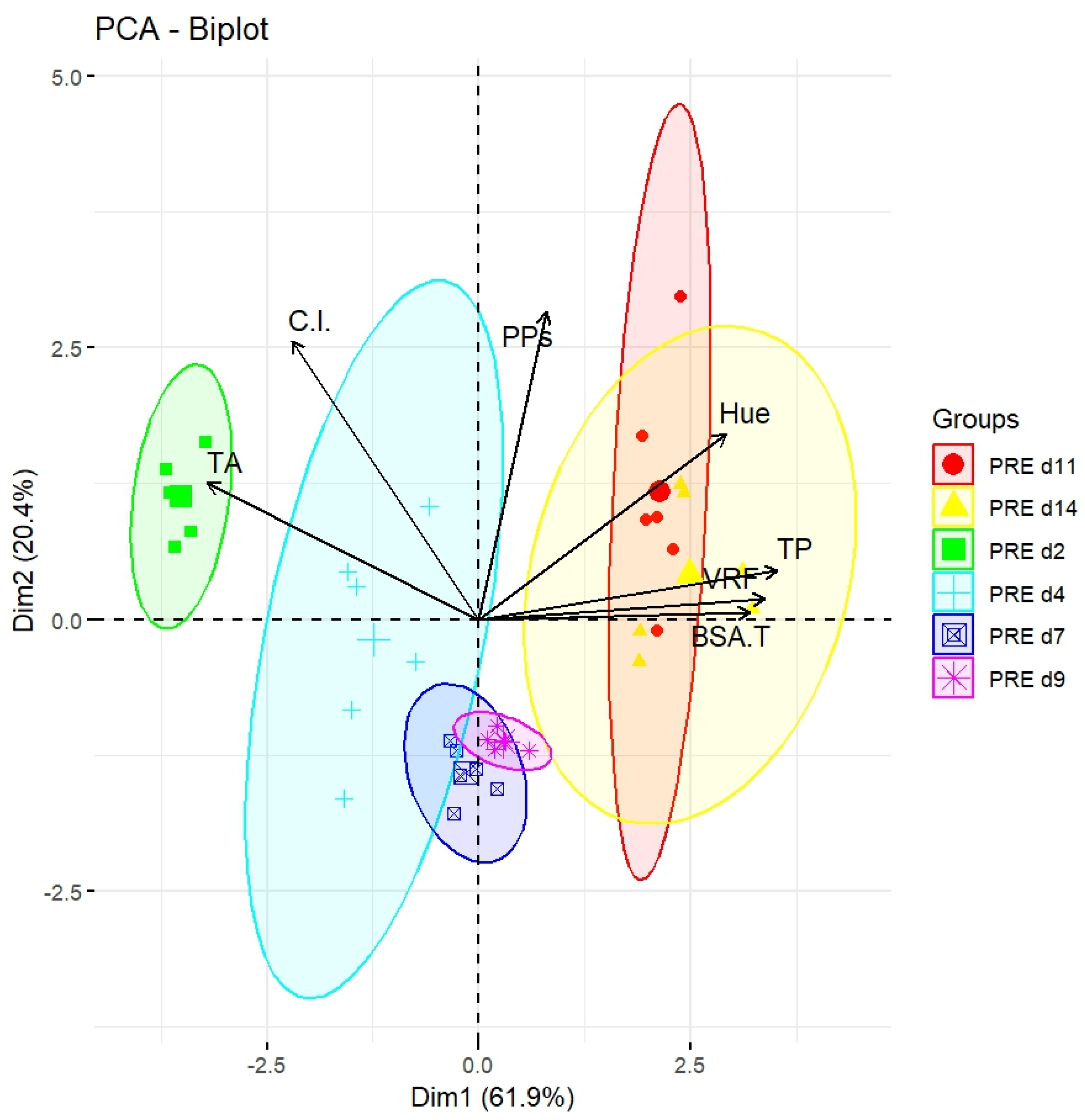
2.3. Analysis of Polyphenol in the Liquid Obtained by Pressing Wine Fermentation Cap (PRE)
2.4. Analysis of Polyphenol Extracted from Residual Grape Pomace (EXH)
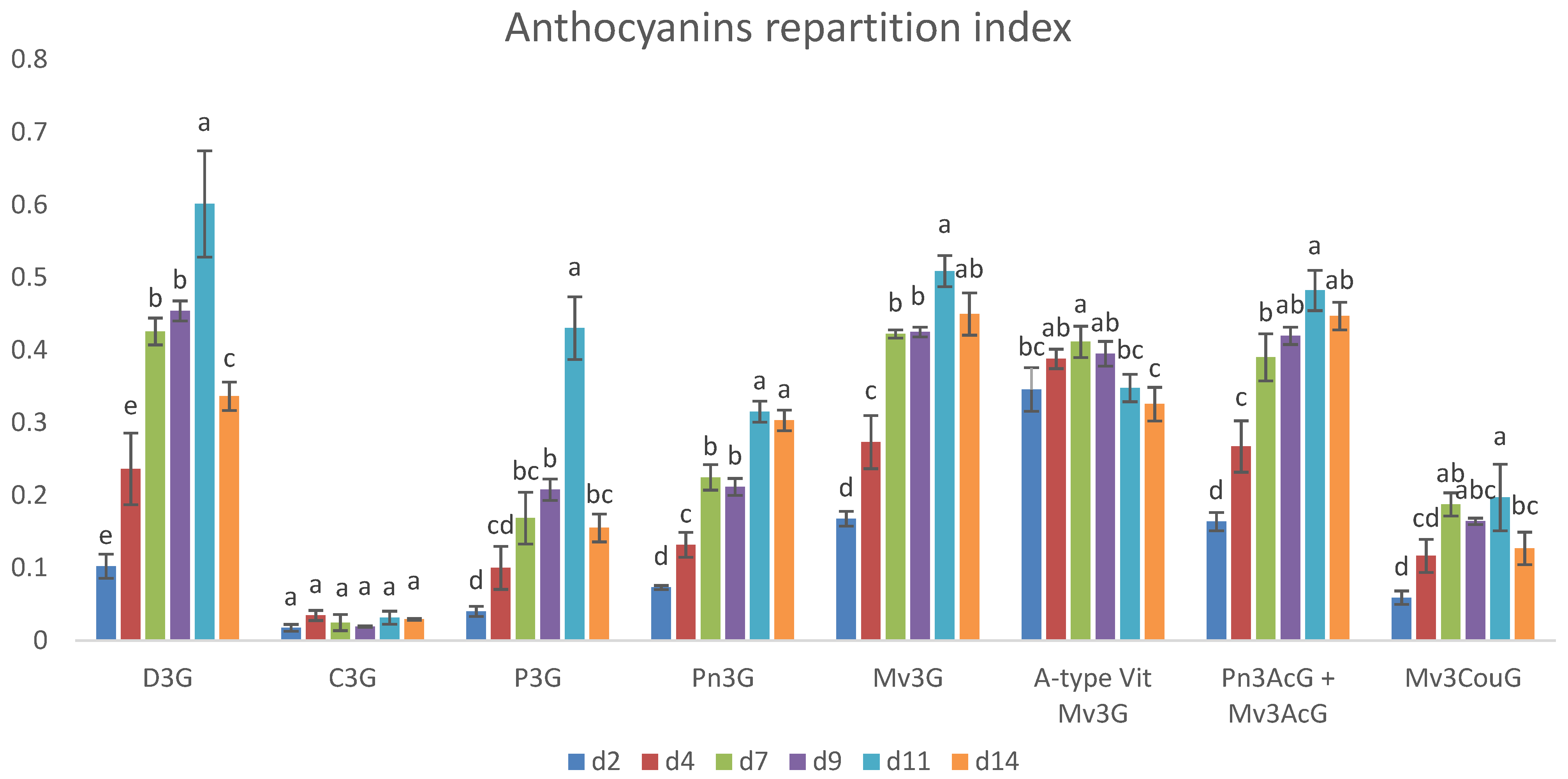
2.5. Evolution of Phenol Extraction During Maceration
3. Materials and Methods
3.1. Chemicals
3.2. Red Wine Fermentation Trial
3.3. Sampling Procedures and Experimental Plan
3.4. Wine Color Evaluation and Spectrophotometry-Based Analyses of Phenols
3.5. High-Performance Liquid Chromatography (HPLC) Analyses of Anthocyanins
3.6. Phenolic Evolution Index
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aleixandre-Tudo, J.L.; Buica, A.; Nieuwoudt, H.; Aleixandre, J.L.; du Toit, W. Spectrophotometric analysis of phenolic compounds in grapes and wines. J. Agric. Food Chem. 2017, 65, 4009–4026. [Google Scholar] [CrossRef]
- Casassa, L.F.; Harbertson, J.F. Extraction, evolution, and sensory impact of phenolic compounds during red wine maceration. Annu. Rev. Food Sci. Technol. 2014, 5, 83–109. [Google Scholar] [CrossRef]
- Barbará, J.A.; Nicolli, K.P.; Souza-Silva, É.A.; Biasoto, A.C.; Welke, J.E.; Zini, C.A. Volatile profile and aroma potential of tropical Syrah wines elaborated in different maturation and maceration times using comprehensive two-dimensional gas chromatography and olfactometry. Food Chem. 2020, 308, 125552. [Google Scholar] [CrossRef]
- Cheynier, V.; Duenas-Paton, M.; Salas, E.; Maury, C.; Souquet, J.M.; Sarni-Manchado, P.; Fulcrand, H. Structure and properties of wine pigments and tannins. Am. J. Enol. Vitic. 2006, 57, 298–305. [Google Scholar] [CrossRef]
- Bindon, K.; Kassara, S.; Hayasaka, Y.; Schulkin, A.; Smith, P. Properties of wine polymeric pigments formed from anthocyanin and tannins differing in size distribution and subunit composition. J. Agric. Food Chem. 2014, 62, 11582–11593. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Plaza, E.; Gil-Muñoz, R.; López-Roca, J.M.; Martínez-Cutillas, A.; Fernández-Fernández, J.I. Phenolic compounds and color stability of red wines: Effect of skin maceration time. Am. J. Enol. Vitic. 2001, 52, 266–270. [Google Scholar] [CrossRef]
- Canals, R.; Llaudy, M.C.; Valls, J.; Canals, J.M.; Zamora, F. Influence of ethanol concentration on the extraction of color and phenolic compounds from the skin and seeds of Tempranillo grapes at different stages of ripening. J. Agric. Food Chem. 2005, 53, 4019–4025. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Jiménez, A.; Kennedy, J.A.; Bautista-Ortín, A.B.; Gómez-Plaza, E. Effect of ethanol on grape seed proanthocyanidin extraction. Am. J. Enol. Vitic. 2012, 63, 57–61. [Google Scholar] [CrossRef]
- Busse-Valverde, N.; Gomez-Plaza, E.; Lopez-Roca, J.M.; Gil-Munoz, R.; Bautista-Ortin, A.B. The extraction of anthocyanins and proanthocyanidins from grapes to wine during fermentative maceration is affected by the enological technique. J. Agric. Food Chem. 2011, 59, 5450–5455. [Google Scholar] [CrossRef] [PubMed]
- Vidal, S.; Francis, L.; Guyot, S.; Marnet, N.; Kwiatkowski, M.; Gawel, R.; Cheynier, V.; Waters, E.J. The mouth-feel properties of grape and apple proanthocyanidins in a wine-like medium. J. Sci. Food Agric. 2003, 83, 564–573. [Google Scholar] [CrossRef]
- Cerpa-Calderón, F.K.; Kennedy, J.A. Berry integrity and extraction of skin and seed proanthocyanidins during red wine fermentation. J. Agric. Food Chem. 2008, 56, 9006–9014. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, A.M.; Smart, R.E.; Dambergs, R.G.; Close, D.C. Skin particle size affects the phenolic attributes of Pinot noir wine: Proof of concept. Am. J. Enol. Vitic. 2016, 67, 29–37. [Google Scholar] [CrossRef]
- Setford, P.C.; Jeffery, D.W.; Grbin, P.R.; Muhlack, R.A. Factors affecting extraction and evolution of phenolic compounds during red wine maceration and the role of process modelling. Trends Food Sci. Technol. 2017, 69, 106–117. [Google Scholar] [CrossRef]
- Nagel, C.W.; Wulf, L.W. Changes in the anthocyanins, flavonoids and hydroxycinnamic acid esters during fermentation and aging of Merlot and Cabernet Sauvignon. Am. J. Enol. Vitic. 1979, 30, 111–116. [Google Scholar] [CrossRef]
- Chris Somers, T.; Evans, M.E. Grape pigment phenomena: Interpretation of major colour losses during vinification. J. Sci. Food Agric. 1979, 30, 623–633. [Google Scholar] [CrossRef]
- Federico Casassa, L.; Beaver, C.W.; Mireles, M.S.; Harbertson, J.F. Effect of extended maceration and ethanol concentration on the extraction and evolution of phenolics, colour components and sensory attributes of Merlot wines. Aust. J. Grape Wine Res. 2013, 19, 25–39. [Google Scholar] [CrossRef]
- Casassa, L.F.; Larsen, R.C.; Beaver, C.W.; Mireles, M.S.; Keller, M.; Riley, W.R.; Smithyman, R.; Harbertson, J.F. Impact of extended maceration and regulated deficit irrigation (RDI) in Cabernet Sauvignon wines: Characterization of proanthocyanidin distribution, anthocyanin extraction, and chromatic properties. J. Agric. Food Chem. 2013, 61, 6446–6457. [Google Scholar] [CrossRef] [PubMed]
- Somers, T.C. The polymeric nature of wine pigments. Phytochemistry 1971, 10, 2175–2186. [Google Scholar] [CrossRef]
- Chris Somers, T.; Evans, M.E. Spectral evaluation of young red wines: Anthocyanin equilibria, total phenolics, free and molecular SO2, “chemical age”. J. Sci. Food Agric. 1977, 28, 279–287. [Google Scholar] [CrossRef]
- Es-Safi, N.E.; Fulcrand, H.; Cheynier, V.; Moutounet, M. Competition between (+)-catechin and (−)-epicatechin in acetaldehyde-induced polymerization of flavanols. J. Agric. Food Chem. 1999, 47, 2088–2095. [Google Scholar] [CrossRef] [PubMed]
- Vidal, S.; Francis, L.; Noble, A.; Kwiatkowski, M.; Cheynier, V.; Waters, E. Taste and mouth-feel properties of different types of tannin-like polyphenolic compounds and anthocyanins in wine. Anal. Chim. Acta. 2004, 513, 57–65. [Google Scholar] [CrossRef]
- Hanlin, R.L.; Hrmova, M.; Harbertson, J.F.; Downey, M.O. Condensed tannin and grape cell wall interactions and their impact on tannin extractability into wine. Aust. J. Grape Wine Res. 2010, 16, 173–188. [Google Scholar] [CrossRef]
- Hernández-Hierro, J.M.; Quijada-Morín, N.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Influence of the physiological stage and the content of soluble solids on the anthocyanin extractability of Vitis vinifera L. cv. Tempranillo grapes. Anal. Chim. Acta. 2012, 732, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Understanding Wine Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2024. [Google Scholar]
- Sun, B.S.; Pinto, T.; Leandro, M.C.; Ricardo-Da-Silva, J.M.; Spranger, M.I. Transfer of catechins and proanthocyanidins from solid parts of the grape cluster into wine. Am. J. Enol. Vitic. 1999, 50, 179–184. [Google Scholar] [CrossRef]
- González-Manzano, S.; Rivas-Gonzalo, J.C.; Santos-Buelga, C. Extraction of flavan-3-ols from grape seed and skin into wine using simulated maceration. Anal. Chim. Acta. 2004, 513, 283–289. [Google Scholar] [CrossRef]
- González-Manzano, S.; Santos-Buelga, C.; Pérez-Alonso, J.J.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Characterization of the mean degree of polymerization of proanthocyanidins in red wines using liquid chromatography–mass spectrometry (LC-MS). J. Agric. Food Chem. 2006, 54, 4326–4332. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, A.; Iturmendi, N.; Jourdes, M.; Teissedre, P.L.; Moio, L. Transfer of tannin characteristics from grape skins or seeds to wine-like solutions and their impact on potential astringency. LWT Food Sci. Technol. 2015, 63, 667–676. [Google Scholar] [CrossRef]
- Giacosa, S.; Ferrero, L.; Paissoni, M.A.; Segade, S.R.; Gerbi, V.; Rolle, L. Grape skin anthocyanin extraction from red varieties during simulated maceration: Influence of grape seeds and pigments adsorption on their surface. Food Chem. 2023, 424, 136463. [Google Scholar] [CrossRef] [PubMed]
- Danilewicz, J.C. Reaction of oxygen and sulfite in wine. Am. J. Enol. Vitic. 2016, 67, 13–17. [Google Scholar] [CrossRef]
- Harbertson, J.F.; Mireles, M.; Yu, Y. Improvement of BSA tannin precipitation assay by reformulation of resuspension buffer. Am. J. Enol. Vitic. 2015, 66, 95–99. [Google Scholar] [CrossRef]
- Harbertson, J.F.; Kilmister, R.L.; Kelm, M.A.; Downey, M.O. Impact of condensed tannin size as individual and mixed polymers on bovine serum albumin precipitation. Food Chem. 2014, 160, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Liu, X.; Jin, X.; Li, C.; Wu, X.; Yang, S.; Ning, J.; Yanne, P. Determination of total iron-reactive phenolics, anthocyanins and tannins in wine grapes of skins and seeds based on near-infrared hyperspectral imaging. Food Chem. 2017, 237, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Harbertson, J.F.; Picciotto, E.A.; Adams, D.O. Measurement of polymeric pigments in grape berry extract sand wines using a protein precipitation assay combined with bisulfite bleaching. Am. J. Enol. Vitic. 2003, 54, 301–306. [Google Scholar] [CrossRef]
- Peleg, H.; Gacon, K.; Schlich, P.; Noble, A.C. Bitterness and astringency of flavan-3-ol monomers, dimers and trimers. J. Sci. Food Agric. 1999, 79, 1123–1128. [Google Scholar] [CrossRef]
- Fulcrand, H.; Dueñas, M.; Salas, E.; Cheynier, V. Phenolic reactions during winemaking and aging. Am. J. Enol. Vitic. 2006, 57, 289–297. [Google Scholar] [CrossRef]
- Singleton, V.L.; Trousdale, E.K. Anthocyanin-tannin interactions explaining differences in polymeric phenols between white and red wines. Am. J. Enol. Vitic. 1992, 43, 63–70. [Google Scholar] [CrossRef]
- Timberlake, C.F.; Bridle, P. Interactions between anthocyanins, phenolic compounds, and acetaldehyde and their significance in red wines. Am. J. Enol. Vitic. 1976, 27, 97–105. [Google Scholar] [CrossRef]
- Heras-Roger, J.; Díaz-Romero, C.; Darias-Martín, J. What gives a wine its strong red color? Main correlations affecting copigmentation. J. Agric. Food Chem. 2016, 64, 6567–6574. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Liang, N.N.; Mu, L.; Pan, Q.H.; Wang, J.; Reeves, M.J.; Duan, C.Q. Anthocyanins and their variation in red wines I. Monomeric anthocyanins and their color expression. Molecules 2012, 17, 1571–1601. [Google Scholar] [CrossRef]
- Merrell, C.P.; Larsen, R.C.; Harbertson, J.F. Effects of berry maturity and wine alcohol on phenolic content during winemaking and aging. Am. J. Enol. Vitic. 2018, 69, 1. [Google Scholar] [CrossRef]
- Gambuti, A.; Picariello, L.; Rinaldi, A.; Moio, L. Evolution of Sangiovese wines with varied tannin and anthocyanin ratios during oxidative aging. Front. Chem. 2018, 6, 63. [Google Scholar] [CrossRef]
- Weilack, I.; Schmitz, C.; Harbertson, J.F.; Weber, F. Effect of structural transformations on precipitability and polarity of red wine phenolic polymers. Am. J. Enol. Vitic. 2021, 72, 230–239. [Google Scholar] [CrossRef]
- Harbertson, J.F.; Spayd, S. Measuring phenolics in the winery. Am. J. Enol. Vitic. 2006, 57, 280–288. [Google Scholar] [CrossRef]
- Gambuti, A.; Capuano, R.; Lecce, L.; Fragasso, M.G.; Moio, L. Extraction of phenolic compounds from ‘Aglianico’ and ‘Uva di Troia’ grape skins and seeds in model solutions: Influence of ethanol and maceration time. Vitis 2009, 48, 193–200. [Google Scholar]
- Errichiello, F.; Cucciniello, R.; Tomasini, M.; Falivene, L.; Gambuti, A.; Cassiano, C.; Forino, M. Efficient and selective extraction of oleanolic acid from grape pomace with dimethyl carbonate. Green. Chem. 2024, 26, 10177–10188. [Google Scholar] [CrossRef]
- Castillo-Sánchez, J.J.; Mejuto, J.C.; Garrido, J.; García-Falcón, S. Influence of wine-making protocol and fining agents on the evolution of the anthocyanin content, colour and general organoleptic quality of Vinhão wines. Food Chem. 2006, 97, 130–136. [Google Scholar] [CrossRef]
- Romero-Cascales, I.; Fernández-Fernández, J.I.; López-Roca, J.M.; Gómez-Plaza, E. The maceration process during winemaking extraction of anthocyanins from grape skins into wine. Eur. Food Res. Technol. 2005, 221, 163–167. [Google Scholar] [CrossRef]
- Quirós, M.; Morales, P.; Pérez-Través, L.; Barcenilla, J.M.; Gonzalez, R. A new methodology to determine cell wall mannoprotein content and release in wine yeasts. Food Chem. 2011, 125, 760–766. [Google Scholar] [CrossRef]
- Monagas, M.; Gómez-Cordovés, C.; Bartolomé, B. Evaluation of different Saccharomyces cerevisiae strains for red winemaking. Influence on the anthocyanin, pyranoanthocyanin and non-anthocyanin phenolic content and colour characteristics of wines. Food Chem. 2007, 104, 814–823. [Google Scholar] [CrossRef]
- Valentão, P.; Seabra, R.M.; Lopes, G.; Silva, L.R.; Martins, V.; Trujillo, M.E.; Velázquez, E.; Andrade, P.B. Influence of Dekkera bruxellensis on the contents of anthocyanins, organic acids and volatile phenols of Dão red wine. Food Chem. 2007, 100, 64–70. [Google Scholar] [CrossRef]
- Morata, A.; Gómez-Cordovés, M.C.; Colomo, B.; Suárez, J.A. Pyruvic acid and acetaldehyde production by different strains of Saccharomyces cerevisiae: Relationship with vitisin A and B formation in red wines. J. Agric. Food Chem. 2003, 51, 7402–7409. [Google Scholar] [CrossRef] [PubMed]
- Morata, A.; Gómez-Cordovés, M.C.; Colomo, B.; Suárez, J.A. Cell wall anthocyanin adsorption by different Saccharomyces strains during the fermentation of Vitis vinifera L. cv Graciano grapes. Eur. Food Res. Technol. 2005, 220, 341–346. [Google Scholar] [CrossRef]
- Favre, G.; Hermosín-Gutiérrez, I.; Piccardo, D.; Gómez-Alonso, S.; González-Neves, G. Selectivity of pigments extraction from grapes and their partial retention in the pomace during red-winemaking. Food Chem. 2019, 277, 391–397. [Google Scholar] [CrossRef]
- Marangon, M.; De Iseppi, A.; Gerbi, V.; Mattivi, F.; Moio, L.; Piombino, P.; Parpinello, G.P.; Rolle, L.; Slaghenaufi, D.; Versari, A.; et al. The macromolecular diversity of Italian monovarietal red wines. OENO ONE 2022, 56, 81–90. [Google Scholar] [CrossRef]
- Seddon, T.J.; Downey, M.O. Comparison of analytical methods for the determination of condensed tannins in grape skin. Aust. J. Grape Wine Res. 2008, 14, 54–61. [Google Scholar] [CrossRef]
- Chauhan, S.; Pandit, N.K.; Mohanty, A.; Meena, S.S. Resource recovery of bioactive compounds from food waste and their diverse industrial applications. Biomass Convers. Biorefin. 2023, 1–21. [Google Scholar] [CrossRef]
- Malinowska, M.; Ferrier, M.; Sharafan, M.; Szopa, A.; Gémin, M.P.; Miastkowska, M.A.; Bialik-Wąs, K.; Dziki, A.K.; Sikora, E.; Kiszka, A.; et al. The cosmetic potential evaluation of the fungus resistant grapevine extracts using metabolomic approaches. Sci. Radices 2024, 3, 187–211. [Google Scholar] [CrossRef]
- Glories, Y. La couleur des vins rouges. Conn. Vigne Vin. 1984, 18, 253–271. [Google Scholar]
- Di Stefano, R.; Guidoni, S. La determinazione dei polifenoli totali nei mosti e nei vini. Vignevini 1989, 1, 47–52. [Google Scholar]
- Organisation of Vine and Wine (OIV). Compendium of International Methods of Wine and Must Analysis; International Organization of Vine and Wine: Paris, France, 2009; pp. 154–196. [Google Scholar]
- Errichiello, F.; D’Amato, M.; Gambuti, A.; Moio, L.; Pastore, A.; Hekmat, A.H.; Stornaiuolo, M.; Serino, E.; Taglialatela-Scafati, O.; Forino, M. Oleanolic acid: A promising antidiabetic metabolite detected in Aglianico grape pomace. J. Funct. Foods. 2023, 104, 105548. [Google Scholar] [CrossRef]

| Iron-Reactive Phenols | BSA-Reactive Tannins | Vanillin-Reactive Flavans | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SUR d2 | 707.13 | ± | 60.49 | d | 173.62 | ± | 7.37 | e | 23.15 | ± | 3.96 | d |
| SUR d4 | 1086.70 | ± | 78.70 | c | 288.93 | ± | 9.54 | d | 33.19 | ± | 1.60 | c |
| SUR d7 | 1350.81 | ± | 60.43 | b | 333.88 | ± | 12.10 | c | 46.20 | ± | 4.61 | b |
| SUR d9 | 1419.86 | ± | 52.12 | b | 368.72 | ± | 11.51 | b | 45.96 | ± | 2.23 | b |
| SUR d11 | 1603.61 | ± | 51.24 | a | 392.07 | ± | 15.71 | a | 68.04 | ± | 10.61 | a |
| SUR d14 | 1673.77 | ± | 92.30 | a | 412.47 | ± | 11.84 | a | 65.77 | ± | 9.12 | a |
| Total Anthocyanins | Sum of PPs | Color Intensity | Hue | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SUR d2 | 269.02 | ± | 13.13 | c | 0.40 | ± | 0.04 | d | 4.37 | ± | 0.03 | d | 0.78 | ± | 0.01 | a |
| SUR d4 | 389.57 | ± | 20.47 | b | 0.81 | ± | 0.07 | c | 5.37 | ± | 0.17 | c | 0.66 | ± | 0.01 | d |
| SUR d7 | 452.32 | ± | 9.72 | a | 1.13 | ± | 0.04 | b | 5.48 | ± | 0.10 | c | 0.64 | ± | 0.01 | e |
| SUR d9 | 451.70 | ± | 12.00 | a | 1.10 | ± | 0.06 | b | 6.20 | ± | 0.14 | b | 0.64 | ± | 0.00 | e |
| SUR d11 | 458.68 | ± | 8.39 | a | 1.39 | ± | 0.14 | a | 6.58 | ± | 0.31 | ab | 0.68 | ± | 0.01 | c |
| SUR d14 | 391.94 | ± | 3.51 | b | 1.22 | ± | 0.10 | a | 6.61 | ± | 0.12 | a | 0.72 | ± | 0.01 | b |
| Iron-Reactive Phenols | BSA-Reactive Tannins | Vanillin-Reactive Flavans | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PRE d2 | 1651.23 | ± | 149.92 | d | 462.44 | ± | 10.58 | c | 86.94 | ± | 3.35 | c |
| PRE d4 | 2600.40 | ± | 138.28 | c | 627.31 | ± | 164.78 | b | 151.87 | ± | 22.84 | b |
| PRE d7 | 2441.73 | ± | 83.39 | c | 799.46 | ± | 97.65 | b | 130.61 | ± | 11.11 | b |
| PRE d9 | 3140.18 | ± | 80.91 | b | 776.58 | ± | 123.72 | b | 153.68 | ± | 2.56 | b |
| PRE d11 | 3910.89 | ± | 39.00 | a | 886.20 | ± | 82.47 | b | 184.50 | ± | 17.95 | a |
| PRE d14 | 4086.52 | ± | 400.96 | a | 1263.55 | ± | 98.30 | a | 205.35 | ± | 20.66 | a |
| Total Anthocyanins | Sum of PPs | Color Intensity | Hue | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PRE d2 | 288.00 | ± | 26.67 | a | 0.73 | ± | 0.14 | a | 0.43 | ± | 0.01 | a | 0.31 | ± | 0.00 | d |
| PRE d4 | 225.53 | ± | 13.64 | b | 0.74 | ± | 0.20 | a | 0.34 | ± | 0.03 | b | 0.28 | ± | 0.03 | e |
| PRE d7 | 153.84 | ± | 8.47 | cd | 0.64 | ± | 0.08 | a | 0.27 | ± | 0.01 | d | 0.32 | ± | 0.01 | c |
| PRE d9 | 162.58 | ± | 2.59 | c | 0.58 | ± | 0.02 | a | 0.29 | ± | 0.00 | c | 0.33 | ± | 0.01 | b |
| PRE d11 | 142.88 | ± | 6.78 | d | 0.97 | ± | 0.27 | a | 0.31 | ± | 0.02 | b | 0.43 | ± | 0.02 | a |
| PRE d14 | 162.98 | ± | 20.03 | cd | 0.71 | ± | 0.12 | a | 0.33 | ± | 0.03 | b | 0.41 | ± | 0.03 | a |
| Iron-Reactive Phenols | BSA-Reactive Tannins | Vanillin-Reactive Flavans | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EXH d2 | 1158.83 | ± | 685.40 | c | 6063.56 | ± | 476.97 | b | 837.73 | ± | 38.99 | b |
| EXH d4 | 27,513.37 | ± | 3126.95 | a | 8772.25 | ± | 268.69 | a | 1538.69 | ± | 79.35 | a |
| EXH d7 | 11,463.78 | ± | 1394.68 | c | 3770.57 | ± | 153.05 | d | 642.31 | ± | 75.28 | c |
| EXH d9 | 17,713.85 | ± | 918.96 | b | 6067.92 | ± | 619.10 | b | 832.49 | ± | 118.79 | b |
| EXH d11 | 8899.53 | ± | 2649.36 | c | 4010.90 | ± | 298.61 | d | 720.15 | ± | 45.86 | bc |
| EXH d14 | 12,149.15 | ± | 832.69 | c | 4679.75 | ± | 310.43 | c | 712.74 | ± | 80.40 | bc |
| Total Anthocyanins | Sum of PPs | Color Intensity | Hue | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EXH d2 | 8237.62 | ± | 323.76 | a | 19.73 | ± | 0.63 | a | 54.50 | ± | 2.70 | a | 0.96 | ± | 0.04 | b |
| EXH d4 | 4215.16 | ± | 313.90 | b | 18.81 | ± | 1.60 | a | 49.14 | ± | 2.76 | b | 1.14 | ± | 0.11 | ab |
| EXH d7 | 2940.15 | ± | 244.94 | cd | 15.01 | ± | 0.69 | d | 32.29 | ± | 0.11 | e | 1.28 | ± | 0.05 | ab |
| EXH d9 | 3009.97 | ± | 187.81 | c | 16.57 | ± | 0.34 | b | 36.31 | ± | 0.41 | cd | 1.30 | ± | 0.03 | ab |
| EXH d11 | 2490.25 | ± | 139.19 | d | 15.90 | ± | 0.21 | c | 35.49 | ± | 0.80 | d | 1.33 | ± | 0.04 | a |
| EXH d14 | 2442.29 | ± | 321.90 | d | 17.69 | ± | 0.62 | a | 37.57 | ± | 0.84 | c | 1.28 | ± | 0.09 | ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Errichiello, F.; Forino, M.; Picariello, L.; Moio, L.; Gambuti, A. Analysis of Polyphenols During Alcoholic Fermentation of Red Grape Aglianico (Vitis vinifera L.): Potential Winemaking Optimization and Pomace Valorization. Molecules 2024, 29, 5962. https://doi.org/10.3390/molecules29245962
Errichiello F, Forino M, Picariello L, Moio L, Gambuti A. Analysis of Polyphenols During Alcoholic Fermentation of Red Grape Aglianico (Vitis vinifera L.): Potential Winemaking Optimization and Pomace Valorization. Molecules. 2024; 29(24):5962. https://doi.org/10.3390/molecules29245962
Chicago/Turabian StyleErrichiello, Francesco, Martino Forino, Luigi Picariello, Luigi Moio, and Angelita Gambuti. 2024. "Analysis of Polyphenols During Alcoholic Fermentation of Red Grape Aglianico (Vitis vinifera L.): Potential Winemaking Optimization and Pomace Valorization" Molecules 29, no. 24: 5962. https://doi.org/10.3390/molecules29245962
APA StyleErrichiello, F., Forino, M., Picariello, L., Moio, L., & Gambuti, A. (2024). Analysis of Polyphenols During Alcoholic Fermentation of Red Grape Aglianico (Vitis vinifera L.): Potential Winemaking Optimization and Pomace Valorization. Molecules, 29(24), 5962. https://doi.org/10.3390/molecules29245962







