Abstract
The development of lanthanide–organic frameworks (Ln-MOFs) using for luminescence sensing and selective gas adsorption applications is of great significance from an energy and environmental perspective. This study reports the solvothermal synthesis of a fluorine-functionalized 3D microporous Tb-MOF with a face-centered cubic (fcu) topology constructed from hexanuclear clusters (Tb6O30) bridged by fdpdc ligands, formulated as {[Tb6(fdpdc)6(μ3-OH)8(H2O)6]·4DMF}n (1), (fdpdc = 3-fluorobiphenyl-4,4′-dicarboxylate). Complex 1 displays a 3D framework with the channel of 7.2 × 7.2 Å2 (measured between opposite atoms) perpendicular to the a-axis. With respect to Ba2+ cation, the framework of activated 1 (1a) exhibits high selectivity and reversibility in luminescence sensing function, with an LOD of 4.34665 mM. According to the results of simulations, compared to other small gas molecules (CO2, N2, H2, CO, and CH4), activated 1 (1a) shows a high adsorption selectivity for C2H2 at 298 K.
1. Introduction
Water has become increasingly polluted as a result of the rapid expansion of agriculture and industry. It is well known that harmful ions can cause irreversible harm to humans and aquatic life, and they are a major contributor to climate change [1]. Ba2+ ions are heavy metal ions that cause protein denaturation as well as enzyme inactivation in most human organs. Additionally, it is extremely difficult to remove Ba2+ ions from the human body. Therefore, it is crucial to develop a material that is capable of sensing Ba2+ ions with high specificity [2]. Ba2+ is traditionally detected using flameless atomic absorption scatterometry (ICP-AES, ICP-MS, ETAAS, NAA, and HPLC). However, these methods have several limitations, including sophisticated instrumentation, complicated pre-treatment procedures, and time-consuming procedures, which makes them inefficient for fast and easy determinations of Ba2+ [3]. Luminescent-based probes have garnered significant interest for sensing applications due to their selectivity, sensitivity, cost-effectiveness, visualizability, and rapid response [4].
There is no doubt that carbon dioxide causes some environmental problems, such as global warming. Therefore, it is vital that CO2 is captured and separated from gas mixtures in order to solve the aforementioned environmental issues, which continues to be a challenge for scientists [5]. As the simplest alkyne in the petrochemical industry and an important monomer in petrochemicals, C2H2 has been widely used in manufacturing industrial chemicals such as acetaldehyde, acetic acid, benzene, synthetic rubber, and synthetic fiber. C2H2 can be produced in industry by cracking hydrocarbons with oxygen or by combusting methane with oxygen, in which CO2 or some other impurity will be absorbed [6]. So, it is important to find suitable porous materials to selectively uptake C2H2 from the mixtures of C2H2/CO2 to obtain C2H2 with high purity.
Lanthanide–organic frameworks (Ln-MOFs) have garnered significant attention in recent decades because of their unique coordination modes, high coordination numbers, distinctive luminescence properties, and rapid response. Ln-MOFs have been widely used in gas storage/separation, catalysis, luminescent sensing, biomedicine, and so on [7,8,9]. Ln-MOFs generally consist of lanthanide ions/clusters and organic linkers. Lanthanide ions are recognized for their intrinsic Lewis acidity and notable optical properties from 4f-4f electronic transitions, potentially forming robust frameworks with distinct emission peaks. Meanwhile, as a result of the π-conjugated organic ligands, energy was transferred from the ligand-excited state to the lanthanide-excited state, increasing emission intensity [10]. The red and green emissions of Eu3+ and Tb3+ ions can be observed by the naked eye, making them great sensing materials. Furthermore, recent studies indicate that MOFs, featuring open metal sites and fluorine-decorated binding sites, exhibit significant potential for selective C2H2 sorption, characterized by high IAST selectivity and substantial C2H2 sorption heat [11,12]. The open metal sites are susceptible to both C2H2 absorption and water molecule attack. Excessive C2H2 binding energy increases the energy required for adsorbent regeneration.
It has been found that in addition to enhancing hydrophobicity, fluorine atoms may also influence the MOF structure in other ways. Small atom radius, high electronegativity, and low electric polarization of fluorine atoms allow them to create specific adsorption sites for sorbed molecules, resulting in an increase in gas absorption capacity [13]. Furthermore, fluorine-functionalized MOFs makes them more resistant to oxidation and light, improving the stability of these materials against environmental influences [14].
Based on the above considerations, the organic ligand 3-fluorobiphenyl-4,4′-dicarboxylic acid (H2fdpdc) containing the fluorine group was chosen to construct a bifunctional Tb(III)–organic framework, {[Tb6(fdpdc)6(μ3-OH)8(H2O)6]·4DMF}n (1), with a face-centered cubic (fcu) topology built from hexanuclear clusters (Tb6O30) bridged by fdpdc ligands. Despite the presence of other metal ions, complex 1 is highly selective for the detection of Ba2+ in solution. Moreover, in order to better understand the role of OH groups and fluorine atoms in activated 1, simulations of the adsorption isotherms of C2H2, CO2, N2, H2, CO, and CH4 at 298 K were conducted. As far as we know, although complex 1 has been reported by xue et al. and used for CO2 adsorption [15], 1 is the initial instance of lanthanide-based MOFs built from fdpdc ligands and used for Ba2+ sensing.
2. Results and Discussion
2.1. The Crystal Structure of Complex 1
Single-crystal X-ray diffraction analysis indicates that 1 crystallizes in the cubic space group Fm-3m and exhibits a 3D channel-type framework constructed by fdpdc ligands and hexanuclear clusters. Tb1 is nine-coordinated by four carboxylate oxygen atoms, four μ3-OH group, and one coordinated water molecule, and displays a single-capped tetragonal prism (Figure 1a). The bond lengths surrounding the Tb(III) centers (Td-O bond distances) are in the range of 2.3335(8)–2.652(11) Å, and the bond angles are in the range of 64.94(9)–139.80(5)°. As with other Tb(III) complexes containing oxygen-donating ligands, the values are consistent [16]. In the crystal structure of 1, the fdpdc2- anions act in a bridging-μ4 mode to link four metal ions, whereas the OH group adopts the μ3 bridging mode to connect three Tb(III) ions. The ten fdpdc2- anions and eight μ3-OH groups link six Tb(III) ions to construct hexanuclear clusters (Tb6O30), which can be regarded as a supramolecular secondary building unit (SBU) or cluster node (Figure 1b). These units are interconnected through carboxylate groups of fdpdc2- anions to generate a 3D framework with the channel of 7.2 × 7.2 Å2 (measured between opposite atoms) perpendicular to the a-axis (Figure 1c). Using PLATON software (for Windows Taskbar V1.10), the accessible volume in activated 1 (1a) after removing the guest molecules is 58.2%, with a potential void of 12,095.9 Å3.
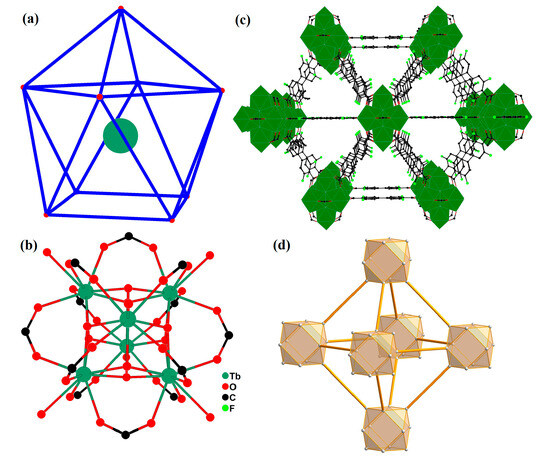
Figure 1.
(a) Single-capped tetragonal prism of Tb(III) in 1, blue line, configuration bar; red bullet, oxygen atoms; green circle, Tb. The coordination environment of Zn(II) ions in 1. (b) Hexanuclear Tb6O30 unit of 1. (c) View of the 3D framework packing perpendicular to the a-axis. (d) The fcu topology network of 1.
In order to gain a deeper understanding of this framework, topology analysis is necessary [17]. In this structure, each hexanuclear unit (Tb6O30) acts as a 12-connected node, fdpdc2- anions act as linker, and complex 1 can be represented as a face-centered cubic (fcu) net with a Schlafli symbol of {62·82·92}2(62·8}4(62·9}2{63·8·102} (Figure 1d).
2.2. IR, TGA, and PXRD Analyses
A KBr pellet was used to record the FT-IR spectra. The IR spectra show an adsorption band at 3422 cm−1, which may be assigned to the ʋ(O-H) stretching vibrations of the water molecules. The adsorption bands observed at 2964 and 2902 cm−1 are attributed to the ʋ(C-H) vibrations of fdpdc anions. The strong and sharp bands at 1585 and 1400 cm−1 are associated with the asymmetric (C-O-C) and symmetric (C-O-C) stretching vibrations.
TGA was used to determine the thermal stability of compound 1. Compound 1 was heated to 800 °C at a rate of 10 °C/min in an N2 atmosphere. The TG curve is shown in Figure S2. The TG curve for 1 shows the following three weight loss steps: The first, corresponding to the release of four guest DMF molecules and six aqua ligands, is observed from 50 to 300 °C (calcd.14.56%, obsd. 15.15%). The second, which corresponds to the release of eight OH groups, is observed from 300 to 450 °C (calcd. 4.95%, obsd. 5.20%). In the third, weight loss occurred above 450 °C due to the decomposition of the structure.
At room temperature, bulk samples of 1 were also analyzed using X-ray diffraction. PXRD patterns for as-synthesized and activated 1 match well with their simulated counterparts, which indicates the same structure as the one solved with single-crystal diffraction (Figure 2). Although there were some peaks in the range of 9.5–10.5° after detection in water for one day, the positions and intensities of the major peaks (5.5° and 6.4°) were unchanged, indicating the stability of the structure. The water stability of compound 1 was evaluated by immersing 20 mg of the samples in water for one day. The PXRD patterns remained unchanged, indicating good water stability.
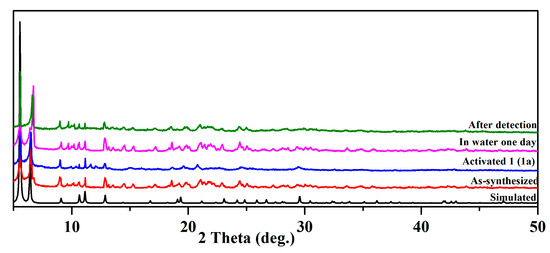
Figure 2.
PXRD patterns of 1.
2.3. Sensing of 1 Toward Ba2+ Ion
Under ambient temperatures, we determined the luminescent property of 1a (activated 1) and H2fdpdc ligand using an excitation wavelength of 328 nm and 278 nm, respectively. Complex 1a exhibits characteristic transitions of the Tb(III) ion at 489, 545, 588, and 622 nm, which correspond to 5D4→7Fn (n = 3–6) transitions, respectively (Figure 3a). When excited at 278 nm, the H2fdpdc ligand’s photoluminescence spectrum shows emission peaks at 395 nm (Figure 3a). It is probable that the emission band of the free ligand is caused by π*−π transitions. Upon excitation at 328 nm, the emission of 1 exhibits ligand-based emission between 300 and 400 nm and metal-based emission between 400 and 650 nm. It is important to note that H2fdpdc emission (395 nm) is absent in the luminescent spectrum of 1, suggesting that the fdpdc2- ligand can efficiently sensitize Tb3+ through the antenna effect. Compared to the free ligand H2fdpdc, the emission spectra of 1 exhibit a clear red shift. This occurrence can be explained by the interaction between lanthanide metal ions and ligands. It is interesting that complex 1 has a long fluorescence lifetime (τ = 120 ms at 545 nm) (Figure S3). To examine the potential of 1a for sensing of cations (Ba2+, Mg2+, Ag+, Cd2+, Ca2+, Na+, Co2+, Ni2+, K+, Zn2+, Pb2+, Eu3+, Al3+, Cr3+, Cu2+, and Fe3+), the complex was immersed in different cations (chloride salts) aqueous solutions for luminescence studies. In order to evaluate the sensing performance, emission intensity at the peak of 545 nm was observed. The results revealed that photoluminescence (PL) is strongly dependent on the cations. As shown in Figure 3b,c, the addition of Ba2+ causes a significant enhancement of luminescence, while the addition of other metal ions causes the fluorescence intensity to weaken or quench. To further investigate the sensing performance of 1 for Ba2+ ion, the fluorescence titrations were performed by gradually adding the ions to a H2O suspension of 1. Figure 3d demonstrates that emission was rapidly enhanced, with the Ba2+ concentration increasing from 0 to 600 μM. As a result, Ba2+ can be sensed selectively using compound 1. The PXRD patterns of 1 after Ba2+ ion detection was collected, which reveals a similar profile with that of the as-synthesized one; the results indicated that the structure transformations/collapses were not responsible for changing luminescence signal. Furthermore, the reaction of luminescence to Ba2+ ion is instantaneous, reaching a maximum in only about 6 s at 545 nm (Figure 4a). The limit of detection (LOD) value was obtained based on the equation of LOD = 3σ/Ksv [18,19]. The result revealed that 1 has good selectivity for luminescent enhancement of Ba2+ ions, with a low detection limit (LOD) of 4.34665 mM in aqueous solution (Figure 4b). During the time-dependent intensity measurements, the whole fluorescence enhancement process takes only 6 s. The results suggest that 1 could be an efficient sensor for Ba2+ targets [19].
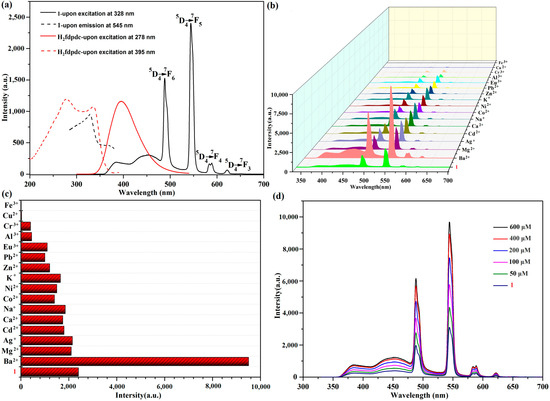
Figure 3.
(a) The fluorescence excitation and emission of 1 and H2fdpdc ligand in solid state. (b) The cation-dependent fluorescence emission of 1. (c) The luminescence intensity at 545 nm with the presence of different cations. (d) The luminescent emission spectra of 1 in Ba2+ solutions with different concentrations.
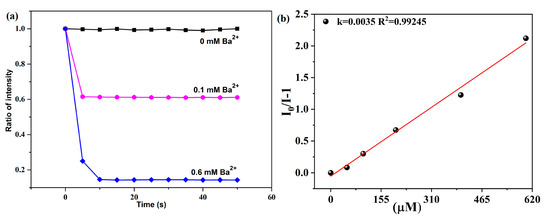
Figure 4.
(a) The correlation between relative intensity and the addition of Ba2+ ion at different concentrations over time at 545 nm. (b) Calculated LOD of 1 in Ba2+ solutions.
One of the most important characteristics of sensors is their selectivity. Using mixed metal ions as an additional test, we evaluated the selectivity of 1 to Ba2+. As shown in Figure 5a, the enhancement efficiency of Ba2+ in the presence of other metal ions does not differ much from that in their absence. Despite Fe3+ and Cu2+ ions quenching luminescence significantly, the luminescence intensities remain higher than blanks. In the study, 1 was found to have high Ba2+ sensitivity and to be able to avoid interference from other metal ions.
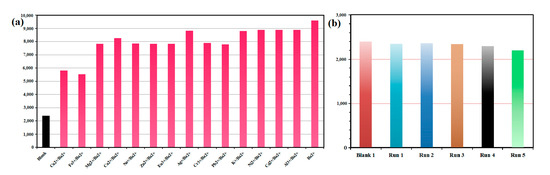
Figure 5.
(a) Luminescence intensity of 1 at 545 nm dispersed in water with the addition of different metal ions. The concentration of interferential metal ions and Ba2+ ion are both 1 mM. (b) Luminescence intensity (545 nm) of 1 during five recycling.
We investigated the recycling performance of 1 as a Ba2+ sensor. The same recycling experiment was conducted five times (Figure 5b). The PXRD patterns and luminescence intensity of the recycled samples are in accordance with the blank of complex 1.
The mechanism for 1’s high sensitivity and selectivity to Ba2+ is discussed. There are many factors that influence the fluorescence intensity variations in MOFs, such as structural collapse, resonance energy transfer (RET), competitive absorption, and ionic exchange [20]. As one of these ions, Ba2+ exhibits such a high charge/size ratio that it is predicted to cause rupture of the C-H bond from the fdpdc ligands, resulting in hydronium ions (H3O+) [21], and the O atoms of μ3-OH group act as simple electron donor to Ba2+ ions through Lewis acid-base interactions. The luminescence enhancement of 1 toward Ba2+ may be caused by strong electrostatic interactions between Ba2+ ions and μ3-OH [22,23].
2.4. Sorption Performances
We modified the outgassing temperature to 120 °C for 8 h to remove the guest molecules. At 77 K, N2 adsorption of 1a was carried out and exhibits a type I isotherm (Figure 6a). The BET surface area of 1a was calculated to be 1905 m2/g, which is in good agreement with the theoretical value (1986.63 m2/g). The Atom Volumes & Surfaces tool in MS software (v3.2) was used to calculate the theoretical specific surface areas of 1a, utilizing N2 as the probe molecule. According to the NLDFT model, the pore volume and pore size width of 1a are 0.52 cm³/g and 7.2Å, respectively.
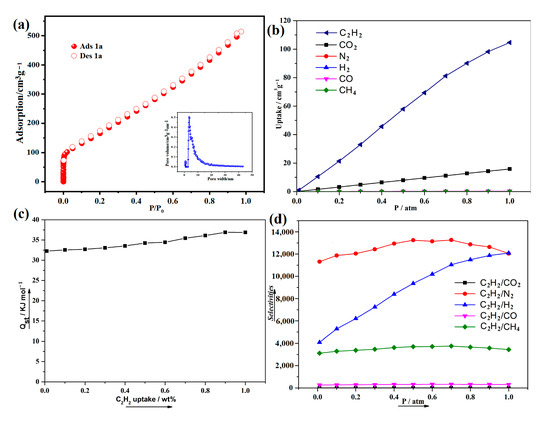
Figure 6.
(a) N2 sorption isotherm of 1a at 77 K (the inset shows the pore size distribution). (b) Room temperature adsorption isotherms of C2H2, CO2, N2, H2, CO, and CH4 based on the simulation. (c) Isosteric heat of adsorption of C2H2 for 1 calculated based on molecular simulation isotherms at 298 K. (d) Selectivities for C2H2 in the equimolar C2H2/CO2, C2H2/N2, C2H2/H2, C2H2/CO, and C2H2/CH4 at 298 K from the simulation.
In recent years, it has becoming increasingly common to use simulation to model the sorption properties of MOFs. A study of the adsorption properties of C2H2, CO2, N2, H2, CO, and CH4 has been conducted in 1a (Figure 6b). C2H2 is preferentially adsorbable in 1a due to the more intense dispersion interactions with the pores, as the pores of 1 are decorated by OH groups and fluorine atoms, and these groups point toward the center of the channels within complex 1, enhancing the binding affinity between C2H2 and framework. We calculated the isosteric heat (Qst) of C2H2 adsorption based on the molecular simulation adsorption isotherm at 298 K. As shown in Figure 6c, Qst values range from 32.3–36.9 kJ·mol−1 for compound 1. The Qst value for 1 at zero loading and 298 K is 32.3 kJ·mol−1. This value is higher than other reported fluorine-decorated MOFs, such as [In(TBOT)(2,3-FDA)](DEF)(H2O)2}n (25.9 kJ·mol−1), UTSA-121 (22.6 kJ·mol−1), SIFSIX-3-Ni (25.9 kJ·mol−1), ELM-12 (25.4 kJ·mol−1), UPC-106 (19.0 kJ·mol−1), Iso-MOF-2 (17.4 kJ·mol−1), Iso-MOF-4 (27.8 kJ·mol−1), UiO-67-F8 (17.6 kJ·mol−1), UPC-200(Fe)-F-BIM (20.43 kJ·mol−1), UPC-200(Al)-F-BIM (18.9–20.5 kJ·mol−1), {(Et2NH2)[In(TBOT)(2,3-FDA)](DEF)(H2O)2}n (25.9 kJ·mol−1), {[(CH3)2NH2]2[Dy6(m3-OH)8(FTZB)6(H2O)6](solvent)}n (26.7 kJ·mol−1), and Cu-FINA-2 (12.3 kJ·mol−1) [24,25,26,27,28,29,30,31,32]. The findings demonstrate a significant interaction between C2H2 and 1a. The introduction of fluorine atoms on the organic ligands and OH group can significantly enhance the H-bond interaction (C-H···F, C-H···O) [33].
Furthermore, the selective adsorptions of C2H2 from the equimolar C2H2/CO2, C2H2/N2, C2H2/H2, C2H2/CO, and C2H2/CH4 from 0 to 1 atm are illustrated in Figure 6d. Selectivities were calculated by using the thermodynamic parameter A/B = xA.yB/xB.yA, where x represents the adsorbed molar fraction and y represents the gas molar fraction of the compound. Obviously, C2H2 is more preferentially adsorbed than these gas molecules, including CO2, N2, H2, CO, and CH4, which may be due to the stronger dispersion interactions toward the pores of 1a. Their pressure-dependent selectivity is similar, i.e., slightly/obviously decreasing with increasing pressure. It can be attributed to the packing effect that makes CO2, N2, H2, CO, and CH4 adsorption less significant, resulting in an obvious increase in C2H2 selectivity as pressure increases [34].
3. Materials and Methods
3.1. Materials and Physical Measurements
Reagent grade H2fdpdc ligand (98%) and Tb(NO3)3·6H2O (99.999% trace) were procured from Aladdin and used as received. Solvents involving DMF were obtained from Aladdin. The metal salts utilized in the sensing experiments were procured from Aladdin.
On a PerkinElmer 240 CHN elemental analyzer, C, H, and N were analyzed. KBr disks were used to measure infrared spectra with a Shimadzu IR-440 spectrometer. Thermogravimetric analyses (TGA) were performed using a Shimadzu DTG-60 simultaneous thermal analyzer under a nitrogen atmosphere with a heating rate of 10 °C /min. An AXS D8-Advance diffractometer with Cu-Kα (λ = 1.5418 Å) radiation was used to collect powder X-ray diffraction patterns (PXRD). At room temperature, an EdinburghFLS920 fluorescence spectrometer was used to measure the luminescence spectra and lifetime of crystalline samples. The experiments of N2 adsorption were carried out using a Quantachrome Autosorb-iQ facility.
3.2. Synthesis of {[Tb6(fdpdc)6(μ3-OH)8(H2O)6]·4DMF}n (1)
3-fluorobiphenyl-4,4′-dicarboxylic acid (0.78 g, 3 mmol), Tb(NO3)3·6H2O (1.36 g, 3 mmol), DMF (5 mL), and H2O (5 mL) were added to a 23 mL Teflon reactor, and the resulting solution was heated (150 °C, 72 h). The mixture was cooled to room temperature at a rate of 5 °C/h. Light-yellow crystals were obtained in a yield of 67% based on Tb. Calcd for Tb6C76H82O42F6N4 (1): C, 30.0; N, 1.8; H, 2.7. Found: C, 31.9; N, 2.2; H, 2.6. IR (KBr pellet) (cm−1): 3422(vs, ʋ(O-H)), 2964(w), 2902(w), 1646 (m), 1585(s), 1510(m), 1400(vs), 1361(w), 1263(m, ʋ(C-F)), 1171(w), 1056(w), 1042(w), 844(w), 776(vs), 648(s), 586(m), 437(w) (Figure S1).
3.3. X-Ray Crystal Structural Determination
Complex 1 data were collected using a Bruker Apex II CCD diffractometer with a 50 kV and 30 mA MoK radiation source (λ = 0.71073 Å). The APEX II software (v2.0) was used to collect and reduce data [35]. Direct methods were used to solve the structure, followed by least-squares method on F2 using SHELXTL [36]. Anisotropic displacement parameters were taken into account for non-hydrogen atoms, and the riding model was used to refine hydrogen atoms attached to carbon and oxygen. By applying SQUEEZE (PLATON), we removed diffuse electron density caused by poorly disordered guest DMF molecules [37]. Infrared, thermogravimetric, and elemental analyses were used to determine the formula unit of 1. An analysis of the topology of the title compound was carried out using the TOPOS 4.0 software [17]. More detailed information can be found in the CIF file. Table 1 shows crystallographic data and refinement details for compound 1. Table 2 presents selected bond lengths and bond angles.

Table 1.
Crystallographic data of complex 1.

Table 2.
Selected bond length and angles of 1 (Å, °).
3.4. Preparation of Ba2+ Aqueous Solution
Standard solution of BaCl2 (0.01 M) was obtained by dissolving BaCl2 (208.23 mg, 1.0 mmol) in 50 mL deionized water and then continuously adding deionized water to 100 mL. Different concentrations of Ba2+ solutions were obtained by diluting the standard solution (0.01 M) with deionized water (50 μM, 100 μM, 200 μM, 400 μM, and 600 μM).
3.5. Fluorescent Studies
An ultrasonic dispersion method was used to combine 5.0 mg of 1 with 1 mL of deionized water and disperse it. Then, different concentrations of Ba2+ solutions prepared in deionized water were added into the 1 original suspension (one solution for each suspension). Upon excitation at 328 nm, luminescence was observed. The homogeneity of solutions was maintained by stirring them continuously throughout the experiment. A triplicate of each experiment was conducted, and consistent results were obtained. After the experiment, we washed the samples three times with deionized water and dried them at 100 °C for 30 min.
3.6. Theoretical Simulations Section
Force field. CO2 was regarded as a rigid linear molecule with 0.116 nm C-O bond length. The LJ potential parameters of O atom (σO = 0.305 nm and εO/κB = 79.0 K) and C atom (σC = 0.280 nm and εC/κB = 27.0 K) in CO2 molecule were taken from the TraPPE force field [38]. Partial point charges are qO = −0.35e and qC = 0.70e. CH4 was represented by united-atom model (σ = 0.373 nm and ε/κB = 148.0 K) [39]. N2 molecule was treated as a three-site model with three sites located at two N atoms and its center of mass COM (σN = 0.331 nm, εN/κB = 36.0 K, qN = −0.482e and qCOM = 0.964e) with the N-N bond length of 0.110 nm [38]. The SK model with three sites developed by Straub and Karplusa was employed to describe the CO molecule. Similar to N2, the SK model is operated based on the combination of three Lennard-Jones pair potentials and three partial point charges, which are located at the LJ centers (i.e., the carbon and oxygen atoms) and center of mass (COM) site (σC = 0.383 nm, εC/κB = 13.18 K, σO = 0.312 nm, εO/κB = 80.06 K, qO = −0.85e, qC = 0.75e and qCOM = 1.60e). H2 molecule was regarded as a two-site LJ molecule as in our previous works (σH = 0.272 nm and εH/κB = 10 K) [40]. To represent vdW interactions, the C2H2 molecule was treated as a two-site model, with the LJ positions located on the carbon atoms. The LJ parameters were originally proposed for the central CH groups of 2-butene [41], which have been successfully used in earlier MD studies of acetylene diffusion [42].
Monte Carlo simulation details. For the determination of single gas adsorption isotherms (C2H2, CO2, N2, H2, CO, and CH4) at 298 K, GCMC simulations were used. The material framework has been modeled as rigid in our simulations [43]. The simulation box contains 2 × 1 × 4 unit cells. A cutoff radius of 12.8 Å was applied to the Lennard-Jones interactions. Additionally, long-range electrostatic interactions with periodic boundary conditions were handled using the Ewald summation technique. For each state point, the number of steps in GCMC simulation was 2 × 107, where the first 107 steps were used to guarantee the equilibration, and the subsequent 107 steps were used for sampling the desired thermodynamics properties [44]. The following is the equation for calculating the isosteric heat of adsorption, Qst:
where R is the gas constant, N is the number of molecules adsorbed, and < > indicates the ensemble average [45]. The first and second terms are the contributions from the molecular thermal energy and adsorbate–adsorbate interaction energy Uff, respectively, while the remaining term is the contribution from the adsorbent–adsorbate interaction energy Usf [46].
4. Conclusions
In summary, a dual-functional Tb-MOF has been successfully synthesized by using a fluorine-functionalized ligand strategy. Due to the fact that a fluorine-containing system was incorporated into the title compound, abundant F and O donor sites can be utilized for selective C2H2 binding. Complex 1 demonstrated high selectivity and sensitivity for the detection of Ba2+ in aqueous media through luminescence enhancement (turn-on), with a LOD of 4.34665 mM. Furthermore, in contrast to other small gas molecules, including CO2, N2, H2, CO, and CH4, 1 performs a highly selective adsorption of C2H2.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29245903/s1, Figure S1: IR spectra of 1. Figure S2: TGA curves of 1 and activated 1 (1a). Figure S3: Luminescent lifetime for 1 in solid. CCDC: 2402714. Copies of this information may be obtained free of charge from the Director, CCDC, 12 Union Road, Cambridge, CB21EZ, UK (fax: +44 1223 336 033; e-mail: deposit@ccdc.cam.ac.uk or (http://www.ccdc.cam.ac.uk (accessed on 14 November 2024)) or are also available from the author on request.
Author Contributions
Conceptualization, Y.Z., H.T., J.Z., and L.D.; methodology, Y.D. and Y.L.; software, X.P.; validation, R.J., J.Y., and J.F.; formal analysis, Y.C. and R.C.; investigation, F.L.; resources, D.M.; data curation, F.L.; writing—original draft preparation, Y.Z. and F.L.; writing—review and editing, D.M.; visualization, Y. Zhang; supervision, F.L.; project administration, D.M.; funding acquisition, D.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the characteristics of innovative projects in Colleges and Universities in Guangdong Province (Nos. 202310580019, 202310580048, and 2024014747), Zhaoqing University-Zhangjiang Institute for Food and Drug Control Joint Laboratory (No. 51), the National Natural Science Foundation of China (No. 22478135), and Guangdong Science and Technology Innovation Strategy Special Foundation (No. 240718108184842).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The detail crystallographic data of 1 are provided in CIF files.
Acknowledgments
It is a pleasure to thank Peng Yan for the characterization of PXRD results.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yu, H.H.; Chi, J.Q.; Su, Z.M.; Li, X.; Sun, J.; Zhou, C.; Hu, X.L.; Liu, Q. A water-stable terbium metal-organic framework with functionalized ligands for the detection of Fe3+ and Cr2O72− ions in water and picric acid in seawater. CrystEngComm 2020, 22, 3638–3643. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, F.; Chen, L.; Pang, J.; Wu, M.; Wan, X.; Pan, J.; Qian, J.; Hong, M. An unusual bifunctional Tb-MOF for highly sensitive sensing of Ba2+ ions and with remarkable selectivities for CO2-N2 and CO2-CH4. J. Mater. Chem. A 2015, 3, 13526–13532. [Google Scholar] [CrossRef]
- Ma, D.; Chen, C.; Chen, M.; Zhu, S.; Wu, Y.; Li, Z.; Li, Y.; Zhou, L. A hydrostable Cadmium-Organic Framework for Highly Selective and Sensitive Luminescence Sensing of Al3+ Ion. J. Inorg. Organomet. Poly. Mater. 2019, 29, 1829–1837. [Google Scholar] [CrossRef]
- Carter, K.; Young, A.; Palmer, A. Fluorescent sensors for measuring metal ions in living systems. Chem. Rev. 2014, 114, 4564–4601. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, D.M.; Smit, B.; Long, J.R. Carbon dioxide capture: Prospects for new materials. Angew. Chem. Int. Ed. 2010, 49, 6058–6082. [Google Scholar] [CrossRef]
- Sahoo, S.; Mondal, S.; Sarma, D. Luminescent Lanthanide Metal Organic Frameworks (LnMOFs): A Versatile Platform towards Organomolecule Sensing. Coord. Chem. Rev. 2022, 470, 214707. [Google Scholar] [CrossRef]
- Pal, S.C.; Ahmed, R.; Manna, A.K.; Das, M.C. Potential of a pH-Stable Microporous MOF for C2H2/C2H4 and C2H2/CO2 Gas Separations under Ambient Conditions. Inorg. Chem. 2022, 61, 18293–18302. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Shi, W.J.; Wang, G.D.; Hou, L.; Wang, Y.Y. One Co-MOF with F Active Sites for Separation of C2H2 from CO2, C2H4, and CH4. Inorg. Chem. 2023, 62, 16574–16581. [Google Scholar] [CrossRef]
- Wang, G.D.; Li, Y.Z.; Zhang, W.F.; Hou, L.; Wang, Y.Y.; Zhu, Z. Acetylene Separation by a Ca-MOF Containing Accessible Sites of Open Metal Centers and Organic Groups. ACS Appl. Mater. Interfaces 2021, 13, 58862–58870. [Google Scholar] [CrossRef]
- Zhang, R.; Zhu, L.; Yue, B. Luminescent properties and recent progress in applications of lanthanide metal-organic frameworks. Chin. Chem. Lett. 2023, 34, 108009. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, J.; Dong, X.; Li, M.; He, Q.; Zhao, S.; Xie, L. Fluorinated metal–organic frameworks for enhanced stability and iodine adsorption selectivity under humid conditions. Chem. Eng. J. 2023, 461, 142058. [Google Scholar] [CrossRef]
- Xie, J.; Sun, F.; Wang, C.; Pan, Q. Stability and hydrocarbon/fluorocarbon sorption of a metal-organic framework with fluorinated channels. Materials 2016, 9, 327. [Google Scholar] [CrossRef] [PubMed]
- Noro, S.; Nakamura, T. Fluorine-functionalized metal-organic frameworks and porous coordination polymers. NPG Asia Mater. 2017, 9, e433. [Google Scholar] [CrossRef]
- Santra, A.; Lah, M.S.; Bharadwaj, P.K. A partially fluorinated three-fold interpenetrated stable metal-organic framework with selective CO2 uptake. Z. Anorg. Allg. Chem. 2014, 640, 1134–1140. [Google Scholar] [CrossRef]
- Xue, D.X.; Cairns, A.J.; Belmabkhout, Y.; Wojtas, L.; Liu, Y.; Alkordi, M.H.; Eddaoudi, M. Tunable Rare-Earth fcu-MOFs: A Platform for Systematic Enhancement of CO2 Adsorption Energetics and Uptake. J. Am. Chem. Soc. 2013, 135, 7660–7667. [Google Scholar] [CrossRef]
- Huheey, J.; Keiter, E.A.; Keiter, R.L. Inorganic Chemistry: Principles of Structure and Reactivity, 4th ed.; Pearson Education: New York, NY, USA, 2000. [Google Scholar]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied Topological Analysis of Crystal Structures with the Program Package ToposPro. Cryst. Growth Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Ma, K.; Li, J.; Ma, H.; Yang, Y.; Yang, H.; Lu, J.; Li, Y.; Dou, J.; Wang, S.; Liu, S. 2D Cd-MOF and its mixed-matrix membranes for luminescence sensing antibiotics in various aqueous systems and visible fingerprint identifying. Chin. Chem. Lett. 2023, 34, 108227. [Google Scholar] [CrossRef]
- Yang, G.L.; Jiang, X.L.; Xu, H.; Zhao, B. Applications of MOFs as Luminescent Sensors for Environmental Pollutants. Small 2021, 17, 2005327. [Google Scholar] [CrossRef]
- Chen, D.M.; Qiao, H.D.; Fang, S.M. Introduction of functionality into a Tb(III)-organic framework via mixed-ligand strategy for selective C2H2 capture and ratiometric Cr(VI) detection. J. Rare Earths, 2024; in press. [Google Scholar] [CrossRef]
- Xu, Q.; Guo, Z.; Zhang, M.; Hu, Z.; Qian, Y.; Zhao, D. Highly efficient photocatalysts by pyrolyzing a Zn-Ti heterometallic metal-organic framework. CrystEngComm 2016, 18, 4046–4052. [Google Scholar] [CrossRef]
- Singha, D.K.; Mahata, P. Highly Selective and Sensitive Luminescence Turn-On-Based Sensing of Al3+ Ions in Aqueous Medium Using a MOF with Free Functional Sites. Inorg. Chem. 2015, 54, 6373–6379. [Google Scholar] [CrossRef]
- Zhang, M.; Qi, Z.; Feng, Y.; Guo, B.; Hao, Y.; Xu, Z.; Zhang, L.; Sun, D. A 2D porous pentiptycene-based MOF for efficient detection of Ba2+ and selective adsorption of dyes from water. Inorg. Chem. Front. 2018, 5, 1314–1320. [Google Scholar] [CrossRef]
- Chen, D.M.; Zheng, Y.P.; Fang, S.M. Microporous mixed-ligand metal-organic framework with fluorine-decorated pores for efficient C2H2/C2H4 separation. J. Solid State Chem. 2021, 296, 121990. [Google Scholar] [CrossRef]
- Alduhaish, O.; Lin, R.-B.; Wang, H.; Li, B.; Arman, H.D.; Hu, T.-L.; Chen, B. Metal-Organic Framework with Trifluoromethyl Groups for Selective C2H2 and CO2 Adsorption. Cryst. Growth Des. 2018, 18, 4522–4527. [Google Scholar] [CrossRef]
- Zheng, J.; Cui, X.; Yang, Q.; Ren, Q.; Yang, Y.; Xing, H. Shaping of ultrahigh-loading MOF pellet with a strongly anti-tearing binder for gas separation and storage. Chem. Eng. J. 2018, 354, 1075–1082. [Google Scholar] [CrossRef]
- Li, L.; Lin, R.-B.; Krishna, R.; Wang, X.; Li, B.; Wu, H.; Li, J.; Zhou, W.; Chen, B. Efficient separation of ethylene from acetylene/ethylene mixtures by a flexible-robust metal-organic framework. J. Mater. Chem. A 2017, 5, 18984–18988. [Google Scholar] [CrossRef]
- Fan, W.; Wang, X.; Liu, X.; Xu, B.; Zhang, X.; Wang, W.; Wang, X.; Wang, Y.; Dai, F.; Yuan, D.; et al. Regulating C2H2 and CO2 Storage and Separation through Pore Environment Modification in a Microporous Ni-MOF. ACS Sustain. Chem. Eng. 2019, 7, 2134–2140. [Google Scholar] [CrossRef]
- Fan, W.; Wang, X.; Zhang, X.; Liu, X.; Wang, Y.; Kang, Z.; Dai, F.; Xu, B.; Wang, R.; Sun, D. Fine-tuning the pore environment of the microporous Cu-MOF for high propylene storage and efficient separation of light hydrocarbons. ACS Cent. Sci. 2019, 5, 1261–1268. [Google Scholar] [CrossRef]
- Cheplakova, A.M.; Kovalenko, K.A.; Vinogradov, A.S.; Karpov, V.M.; Platonov, V.E.; Fedin, V.P. A comparative study of perfluorinated and non-fluorinated UiO-67 in gas adsorption. J. Porous Mater. 2020, 27, 1773–1782. [Google Scholar] [CrossRef]
- Fan, W.; Yuan, S.; Wang, W.; Feng, L.; Liu, X.; Zhang, X.; Wang, X.; Kang, Z.; Dai, F.; Yuan, D.; et al. Optimizing Multivariate Metal-Organic Frameworks for Efficient C2H2/CO2 Separation. J. Am. Chem. Soc. 2020, 142, 8728–8737. [Google Scholar] [CrossRef]
- Li, Y.-Z.; Wang, H.-H.; Wang, G.-D.; Hou, L.; Wang, Y.Y.; Zhu, Z. A Dy 6-cluster-based fcu-MOF with efficient separation of C2H2/C2H4 and selective adsorption of benzene. Inorg. Chem. Front. 2021, 8, 376–382. [Google Scholar] [CrossRef]
- Cui, X.; Chen, K.; Xing, H.; Yang, Q.; Krishna, R.; Bao, Z.; Wu, H.; Zhou, W.; Dong, X.; Han, Y.; et al. Pore chemistry and size control in hybrid porous materials for acetylene capture from ethylene. Science 2016, 353, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Li, Z.; Jiang, F.; Ma, Y.; Lin, W.; Wen, M.; Liu, B.; Xu, J. Selective CO2 Adsorption By Methyl-Functionalized Metal-Organic Framework. ChemistrySelect 2017, 2, 7821–7826. [Google Scholar] [CrossRef]
- Bruker. APEXII Software; Version 6.3.1; Bruker AXS Inc.: Madison, WI, USA, 2004. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON, A Multipurpose Crystallographic Tool; Utrecht University: Utrecht, The Netherlands, 2005. [Google Scholar]
- Potoff, J.J.; Siepmann, J.I. Vapor-liquid equilibria of mixtures containing alkanes, carbon dioxide and nitrogen. AIChE 2001, 47, 1676–1682. [Google Scholar] [CrossRef]
- Martin, M.G.; Siepmann, J.I. Transferable Potentials for Phase Equilibria. 1. United-Atom Description of n-Alkanes. J. Phys. B 1998, 102, 2569–2577. [Google Scholar] [CrossRef]
- Chen, B.; Liang, C.; Yang, J.; Contreras, D.S.; Clancy, Y.L.; Lobkovsky, E.B.; Yaghi, O.M.; Dai, S. A microporous metal-organic framework for gas-chromatographic separation of alkanes. Angew. Chem. Int. Ed. 2006, 45, 1390–1393. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Madura, J.D.; Swenson, C.J. Optimized intermolecular potential functions for liquid hydrocarbons. J. Am. Chem. Soc. 1984, 106, 6638–6646. [Google Scholar] [CrossRef]
- Gautam, S.; Mitra, S.; Mukhopadhyay, R.; Chaplot, S.L. Diffusion of acetylene inside Na-Y zeolite: Molecular dynamics simulation studies. Phys. Rev. E 2006, 74, 041202. [Google Scholar] [CrossRef]
- Yang, Q.; Zhong, C. Molecular simulation of adsorption and diffusion of hydrogen in metal-organic frameworks. J. Phys. B 2005, 24, 11862–11864. [Google Scholar] [CrossRef]
- Wu, D.; Wang, C.; Liu, B.; Liu, D.; Yang, Q.; Zhong, C. Large-scale computational screening of metal-organic frameworks for CH4/H2 separation. AIChE 2012, 58, 2078–2084. [Google Scholar] [CrossRef]
- Yang, Q.; Zhong, C.; Chen, J. Computational Study of CO2 Storage in Metal-Organic Frameworks. J. Phys. Chem. C 2008, 112, 1562–1569. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, H.; Zhong, C.; Liu, D. Cooperative effect of temperature and linker functionality on CO2 capture from industrial gas mixtures in metal–organic frameworks: A combined experimental and molecular simulation study. Phys. Chem. Chem. Phys. 2012, 14, 2317–2325. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).