Quercetin Ameliorates Acute Lethal Sepsis in Mice by Inhibiting Caspase-11 Noncanonical Inflammasome in Macrophages
Abstract
1. Introduction
2. Results
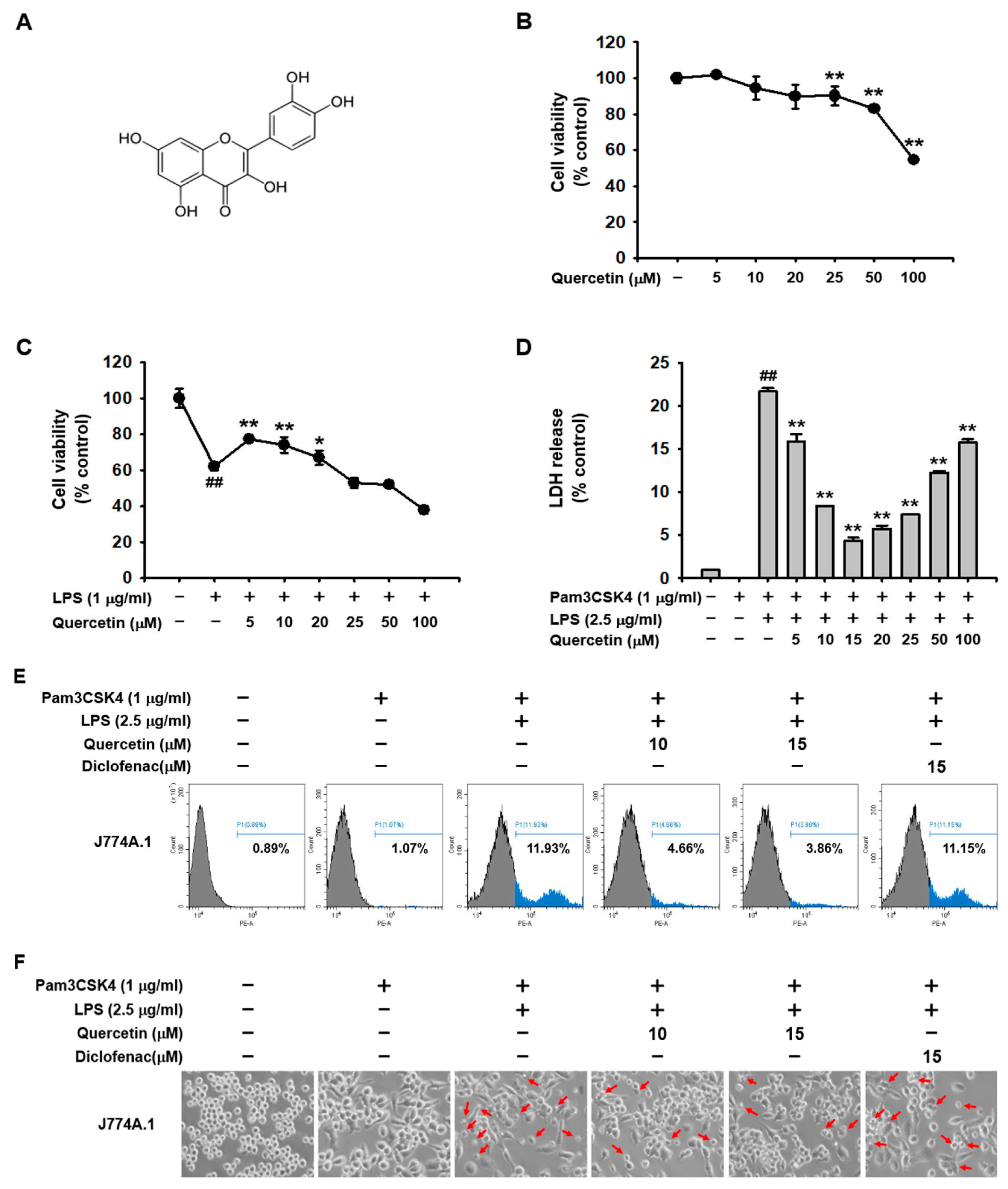
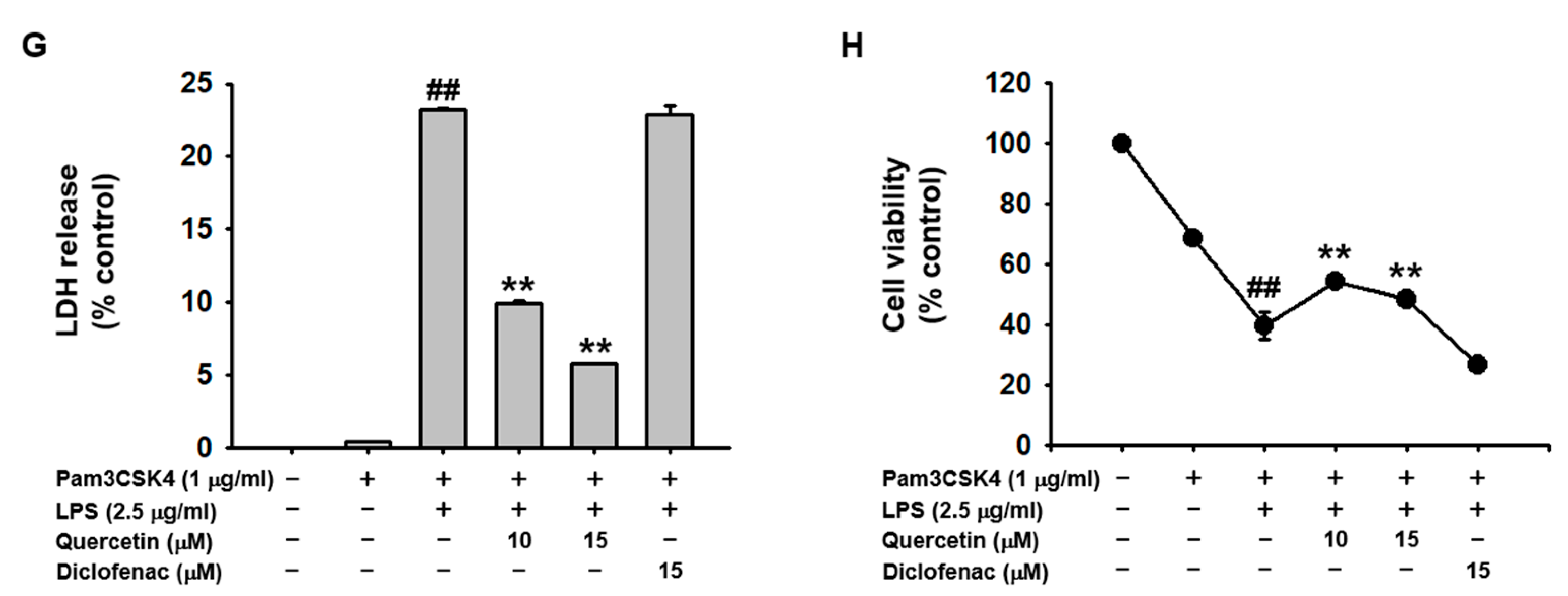
2.1. Quercetin Prevented Pyroptosis Induced by Caspase-11 Noncanonical Inflammasome Activation in Macrophages
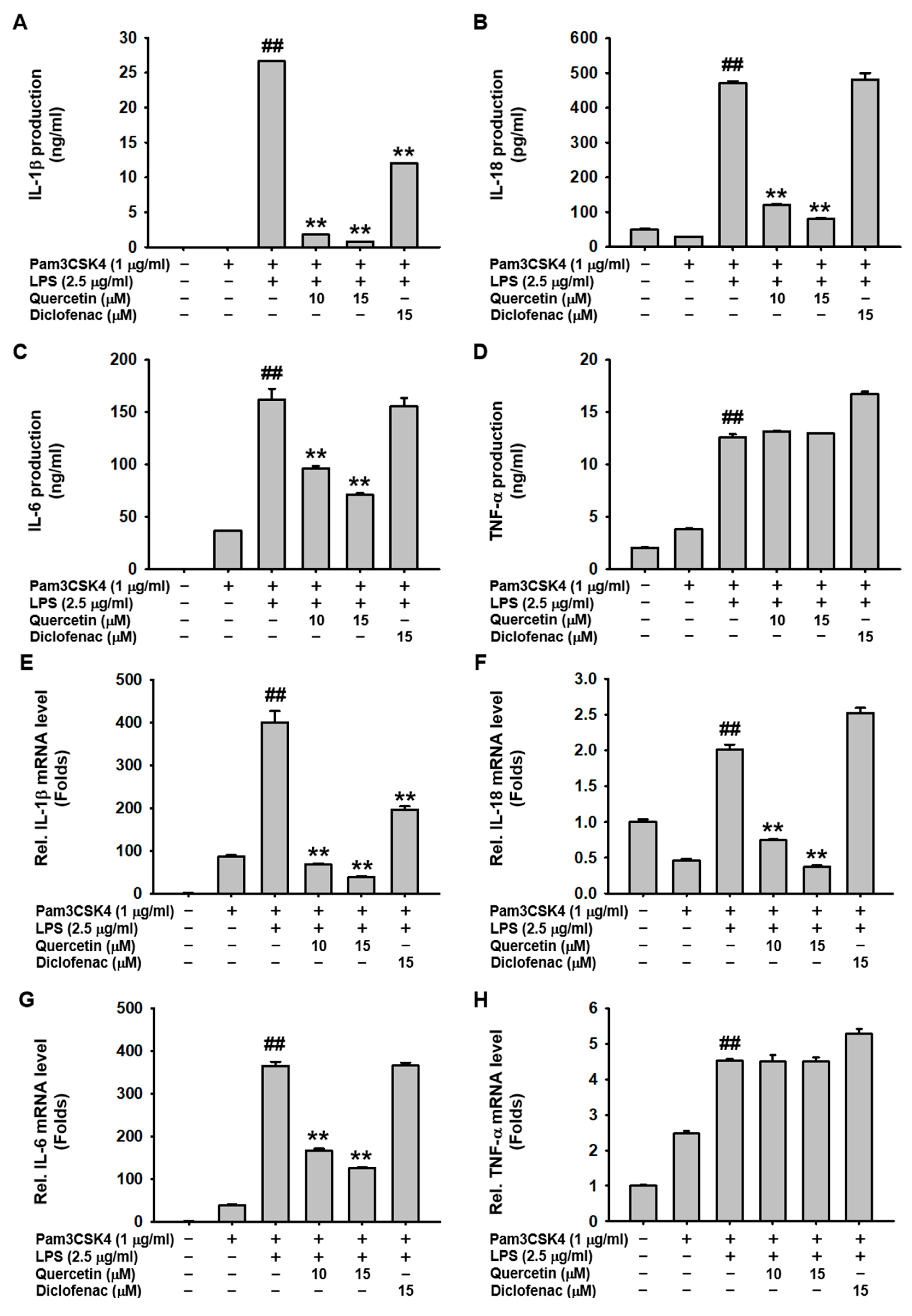
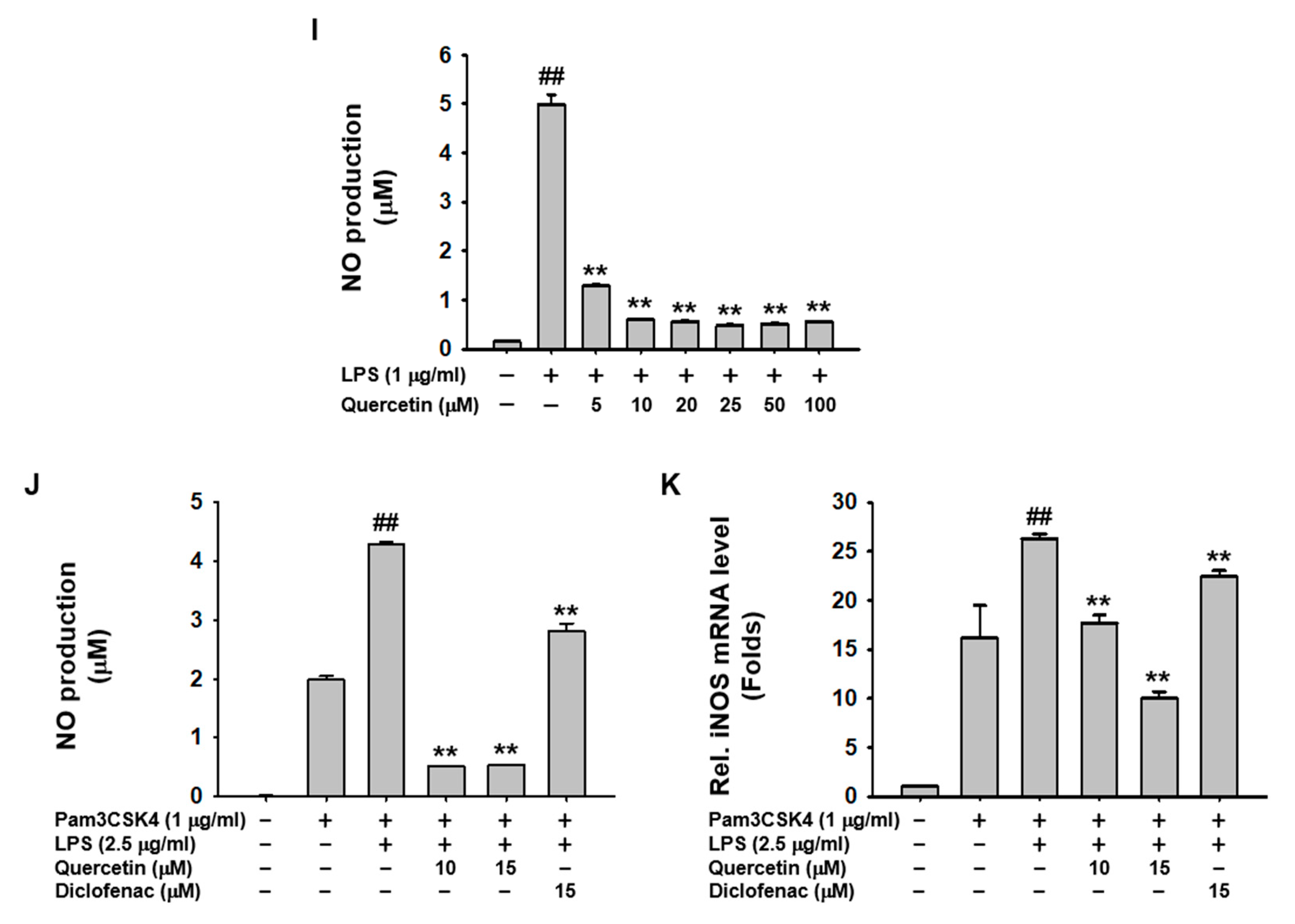
2.2. Quercetin Suppressed the Production of Inflammatory Mediators Induced by Caspase-11 Noncanonical Inflammasome Activation in Macrophages
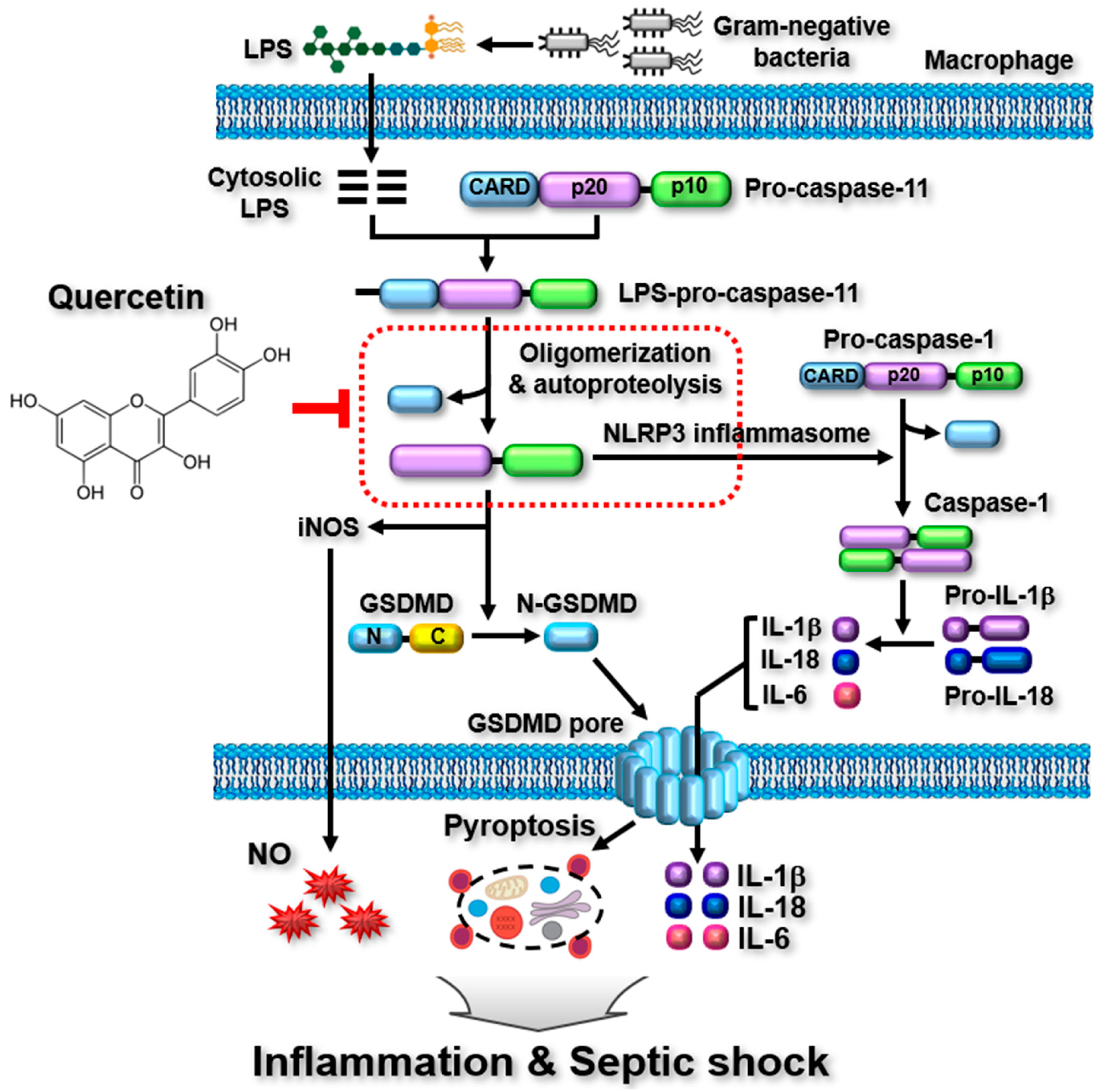
2.3. Quercetin-Mediated Inhibitory Mechanisms of Caspase-11 Noncanonical Inflammasome in Macrophages
2.4. Quercetin Ameliorated LPS-Induced Acute Lethal Sepsis in Mice
3. Materials and Methods
3.1. Cell Culture
3.2. Cell Viability Assay
3.3. Lactate Dehydrogenase (LDH) Assay
3.4. Flow Cytometry
3.5. Enzyme-Linked Immunosorbent Assay (ELISA)
3.6. Quantitative Real-Time PCR (qPCR)
3.7. Nitric Oxide (NO) Production Assay
3.8. Western Blot Analysis
3.9. LPS-Caspase-11 Binding Pull-Down Assay
3.10. Acute Lethal Sepsis in Mice
3.11. Statistical Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Janeway, C.A., Jr.; Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 2002, 20, 197–216. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.S. Functional crosstalk between non-canonical caspase-11 and canonical NLRP3 inflammasomes during infection-mediated inflammation. Immunology 2020, 159, 142–155. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liwinski, T.; Elinav, E. Inflammasome activation and regulation: Toward a better understanding of complex mechanisms. Cell Discov. 2020, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Enosi Tuipulotu, D.; Tan, W.H.; Kay, C.; Man, S.M. Emerging Activators and Regulators of Inflammasomes and Pyroptosis. Trends Immunol. 2019, 40, 1035–1052. [Google Scholar] [CrossRef]
- Kayagaki, N.; Wong, M.T.; Stowe, I.B.; Ramani, S.R.; Gonzalez, L.C.; Akashi-Takamura, S.; Miyake, K.; Zhang, J.; Lee, W.P.; Muszynski, A.; et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 2013, 341, 1246–1249. [Google Scholar] [CrossRef]
- Hagar, J.A.; Powell, D.A.; Aachoui, Y.; Ernst, R.K.; Miao, E.A. Cytoplasmic LPS activates caspase-11: Implications in TLR4-independent endotoxic shock. Science 2013, 341, 1250–1253. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, Y.; Wang, Y.; Gao, W.; Ding, J.; Li, P.; Hu, L.; Shao, F. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 2014, 514, 187–192. [Google Scholar] [CrossRef]
- Yi, Y.S. Caspase-11 non-canonical inflammasome: A critical sensor of intracellular lipopolysaccharide in macrophage-mediated inflammatory responses. Immunology 2017, 152, 207–217. [Google Scholar] [CrossRef]
- Yi, Y.S. Regulatory Roles of the Caspase-11 Non-Canonical Inflammasome in Inflammatory Diseases. Immune Netw. 2018, 18, e41. [Google Scholar] [CrossRef]
- Li, Y.; Huang, H.; Liu, B.; Zhang, Y.; Pan, X.; Yu, X.Y.; Shen, Z.; Song, Y.H. Inflammasomes as therapeutic targets in human diseases. Signal Transduct. Target. Ther. 2021, 6, 247. [Google Scholar] [CrossRef]
- Bulte, D.; Rigamonti, C.; Romano, A.; Mortellaro, A. Inflammasomes: Mechanisms of Action and Involvement in Human Diseases. Cells 2023, 12, 1766. [Google Scholar] [CrossRef] [PubMed]
- Byun, D.J.; Lee, J.; Yu, J.W.; Hyun, Y.M. NLRP3 Exacerbate NETosis-Associated Neuroinflammation in an LPS-Induced Inflamed Brain. Immune Netw. 2023, 23, e27. [Google Scholar] [CrossRef] [PubMed]
- Chae, B.J.; Lee, K.S.; Hwang, I.; Yu, J.W. Extracellular Acidification Augments NLRP3-Mediated Inflammasome Signaling in Macrophages. Immune Netw. 2023, 23, e23. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.S. MicroRNA-mediated epigenetic regulation of inflammasomes in inflammatory responses and immunopathologies. Semin. Cell Dev. Biol. 2024, 154, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.S. Potential benefits of ginseng against COVID-19 by targeting inflammasomes. J. Ginseng Res. 2022, 46, 722–730. [Google Scholar] [CrossRef]
- Ye, J.; Zeng, B.; Zhong, M.; Li, H.; Xu, L.; Shu, J.; Wang, Y.; Yang, F.; Zhong, C.; Ye, X.; et al. Scutellarin inhibits caspase-11 activation and pyroptosis in macrophages via regulating PKA signaling. Acta Pharm. Sin. B 2021, 11, 112–126. [Google Scholar] [CrossRef]
- Cho, H.J.; Kim, E.; Yi, Y.S. Korean Red Ginseng Saponins Play an Anti-Inflammatory Role by Targeting Caspase-11 Non-Canonical Inflammasome in Macrophages. Int. J. Mol. Sci. 2023, 24, 1077. [Google Scholar] [CrossRef]
- Cho, H.J.; Lee, D.J.; Yi, Y.S. Anti-inflammatory activity of calmodulin-lysine N-methyltransferase through suppressing the caspase-11 non-canonical inflammasome. Immunobiology 2023, 228, 152758. [Google Scholar] [CrossRef]
- Kim, Y.B.; Cho, H.J.; Yi, Y.S. Anti-inflammatory role of Artemisia argyi methanol extract by targeting the caspase-11 non-canonical inflammasome in macrophages. J. Ethnopharmacol. 2023, 307, 116231. [Google Scholar] [CrossRef]
- Yi, Y.S. Regulatory Roles of Flavonoids in Caspase-11 Non-Canonical Inflammasome-Mediated Inflammatory Responses and Diseases. Int. J. Mol. Sci. 2023, 24, 10402. [Google Scholar] [CrossRef]
- Joon Lee, D.; Yeol Lee, S.; Yi, Y.S. Maclurin inhibits caspase-11 non-canonical inflammasome in macrophages and ameliorates acute lethal sepsis in mice. Int. Immunopharmacol. 2024, 129, 111615. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.S. Roles of the Caspase-11 Non-Canonical Inflammasome in Rheumatic Diseases. Int. J. Mol. Sci. 2024, 25, 2091. [Google Scholar] [CrossRef] [PubMed]
- Min, J.H.; Cho, H.J.; Yi, Y.S. A novel mechanism of Korean Red Ginseng-mediated anti-inflammatory action via targeting caspase-11 non-canonical inflammasome in macrophages. J. Ginseng Res. 2022, 46, 675–682. [Google Scholar] [CrossRef]
- Yi, Y.S. Dual roles of the caspase-11 non-canonical inflammasome in inflammatory bowel disease. Int. Immunopharmacol. 2022, 108, 108739. [Google Scholar] [CrossRef]
- Yi, Y.S. Regulatory Roles of Caspase-11 Non-Canonical Inflammasome in Inflammatory Liver Diseases. Int. J. Mol. Sci. 2022, 23, 4986. [Google Scholar] [CrossRef]
- Singh, P.; Arif, Y.; Bajguz, A.; Hayat, S. The role of quercetin in plants. Plant Physiol. Biochem. 2021, 166, 10–19. [Google Scholar] [CrossRef]
- Batiha, G.E.; Beshbishy, A.M.; Ikram, M.; Mulla, Z.S.; El-Hack, M.E.A.; Taha, A.E.; Algammal, A.M.; Elewa, Y.H.A. The Pharmacological Activity, Biochemical Properties, and Pharmacokinetics of the Major Natural Polyphenolic Flavonoid: Quercetin. Foods 2020, 9, 374. [Google Scholar] [CrossRef]
- Carrillo-Martinez, E.J.; Flores-Hernandez, F.Y.; Salazar-Montes, A.M.; Nario-Chaidez, H.F.; Hernandez-Ortega, L.D. Quercetin, a Flavonoid with Great Pharmacological Capacity. Molecules 2024, 29, 1000. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Shen, P.; Lin, W.; Deng, X.; Ba, X.; Han, L.; Chen, Z.; Qin, K.; Huang, Y.; Tu, S. Potential Implications of Quercetin in Autoimmune Diseases. Front. Immunol. 2021, 12, 689044. [Google Scholar] [CrossRef]
- Blevins, H.M.; Xu, Y.; Biby, S.; Zhang, S. The NLRP3 Inflammasome Pathway: A Review of Mechanisms and Inhibitors for the Treatment of Inflammatory Diseases. Front. Aging Neurosci. 2022, 14, 879021. [Google Scholar] [CrossRef] [PubMed]
- Aghababaei, F.; Hadidi, M. Recent Advances in Potential Health Benefits of Quercetin. Pharmaceuticals 2023, 16, 1020. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Guevara, I.; Iwanejko, J.; Dembinska-Kiec, A.; Pankiewicz, J.; Wanat, A.; Anna, P.; Golabek, I.; Bartus, S.; Malczewska-Malec, M.; Szczudlik, A. Determination of nitrite/nitrate in human biological material by the simple Griess reaction. Clin. Chim. Acta 1998, 274, 177–188. [Google Scholar] [CrossRef]
- Koontz, L. TCA precipitation. Methods Enzymol. 2014, 541, 3–10. [Google Scholar] [CrossRef]
- Yang, W.S.; Jeong, D.; Yi, Y.S.; Lee, B.H.; Kim, T.W.; Htwe, K.M.; Kim, Y.D.; Yoon, K.D.; Hong, S.; Lee, W.S.; et al. Myrsine seguinii ethanolic extract and its active component quercetin inhibit macrophage activation and peritonitis induced by LPS by targeting to Syk/Src/IRAK-1. J. Ethnopharmacol. 2014, 151, 1165–1174. [Google Scholar] [CrossRef]
- Endale, M.; Park, S.C.; Kim, S.; Kim, S.H.; Yang, Y.; Cho, J.Y.; Rhee, M.H. Quercetin disrupts tyrosine-phosphorylated phosphatidylinositol 3-kinase and myeloid differentiation factor-88 association, and inhibits MAPK/AP-1 and IKK/NF-kappaB-induced inflammatory mediators production in RAW 264.7 cells. Immunobiology 2013, 218, 1452–1467. [Google Scholar] [CrossRef]
- Yi, Y.S.; Kim, H.G.; Kim, J.H.; Yang, W.S.; Kim, E.; Jeong, D.; Park, J.G.; Aziz, N.; Kim, S.; Parameswaran, N.; et al. Syk-MyD88 Axis Is a Critical Determinant of Inflammatory-Response in Activated Macrophages. Front. Immunol. 2021, 12, 767366. [Google Scholar] [CrossRef]
- Yi, Y.S.; Son, Y.J.; Ryou, C.; Sung, G.H.; Kim, J.H.; Cho, J.Y. Functional roles of Syk in macrophage-mediated inflammatory responses. Mediat. Inflamm. 2014, 2014, 270302. [Google Scholar] [CrossRef]
- Kwon, K.W.; Jang, W.Y.; Kim, J.W.; Noh, J.K.; Yi, D.K.; Cho, J.Y. Anti-Inflammatory Effect of Meriania hexamera Sprague by Targeting Syk Kinase in NF-kappaB Signaling. Plants 2023, 12, 3044. [Google Scholar] [CrossRef]
- Lee, B.L.; Stowe, I.B.; Gupta, A.; Kornfeld, O.S.; Roose-Girma, M.; Anderson, K.; Warming, S.; Zhang, J.; Lee, W.P.; Kayagaki, N. Caspase-11 auto-proteolysis is crucial for noncanonical inflammasome activation. J. Exp. Med. 2018, 215, 2279–2288. [Google Scholar] [CrossRef]
- Kobori, M.; Yang, Z.; Gong, D.; Heissmeyer, V.; Zhu, H.; Jung, Y.K.; Gakidis, M.A.; Rao, A.; Sekine, T.; Ikegami, F.; et al. Wedelolactone suppresses LPS-induced caspase-11 expression by directly inhibiting the IKK complex. Cell Death Differ. 2004, 11, 123–130. [Google Scholar] [CrossRef] [PubMed]

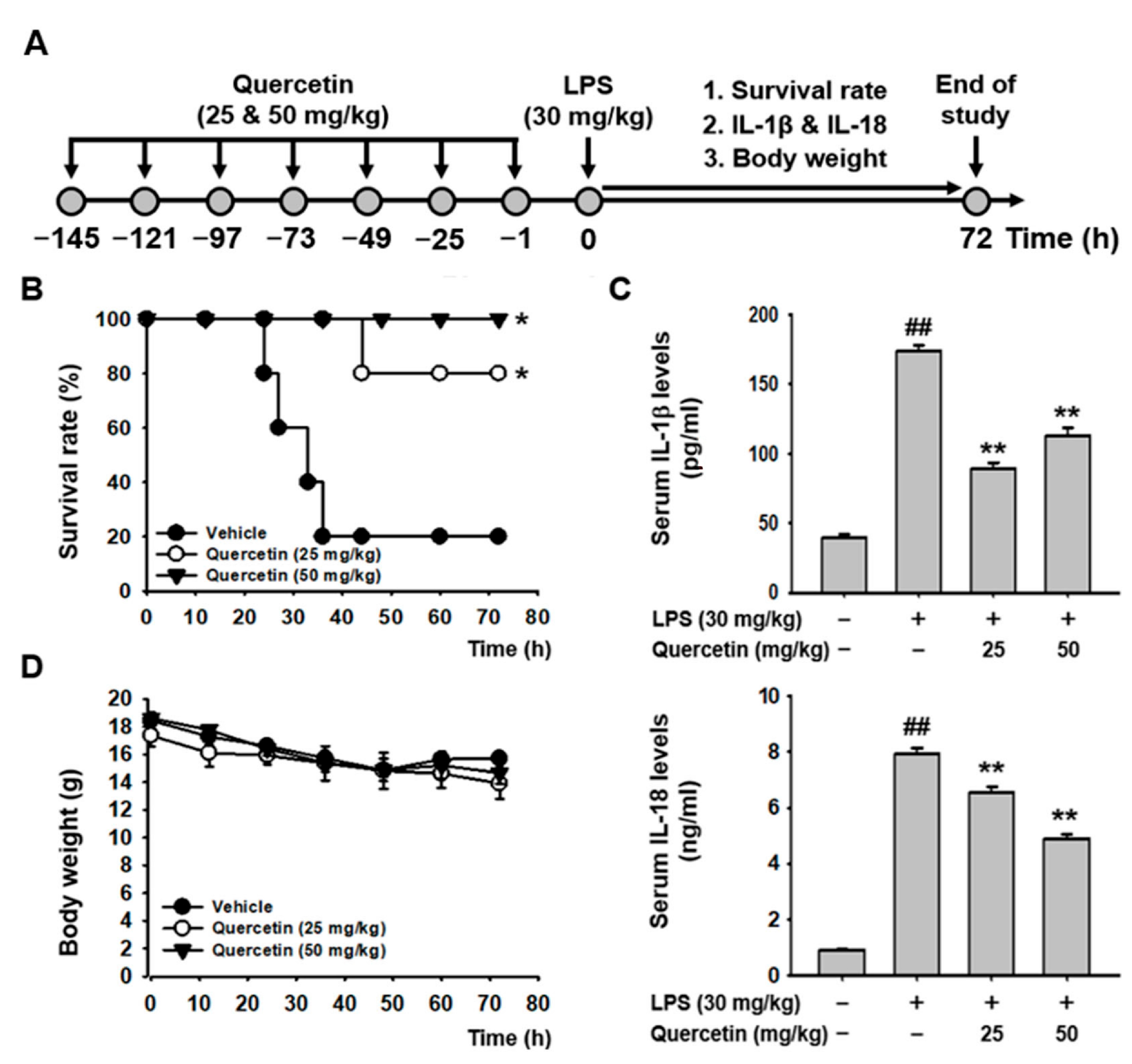
| Group | Vs. | Group | p Value |
|---|---|---|---|
| Vehicle | Quercetin (25 mg/kg) | 0.031572 * | |
| Vehicle | Quercetin (50 mg/kg) | 0.013279 * | |
| Quercetin (25 mg/kg) | Quercetin (50 mg/kg) | 0.317311 |
| Target | Sequence (5’ to 3’) | ||
|---|---|---|---|
| Mouse | IL-1β | For | GTGAAATGCCACCTTTTGACAGTG |
| Rev | CCTGCCTGAAGCTCTTGTTG | ||
| Mouse | IL-18 | For | CAGCCTGTGTTCGAGGATATG |
| Rev | TCACAGCCAGTCCTCTTACT | ||
| Mouse | TNF-α | For | TGCCTATGTCTCAGCCTCTTC |
| Rev | GAGGCCATTTGGGAACTTCT | ||
| Mouse | IL-6 | For | GACAAAGCCAGAGTCCTTCAGAGA |
| Rev | CTAGGTTTGCCGAGTAGATCTC | ||
| Mouse | iNOS | For | GCCACCAACAATGGCAACAT |
| Rev | TCGATGCACAACTGGGTGAA | ||
| Mouse | GAPDH | For | CAATGAATACGGCTACAGCAAC |
| Rev | AGGGAGATGCTCAGTGTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, E.; Choi, D.-H.; Yi, Y.-S. Quercetin Ameliorates Acute Lethal Sepsis in Mice by Inhibiting Caspase-11 Noncanonical Inflammasome in Macrophages. Molecules 2024, 29, 5900. https://doi.org/10.3390/molecules29245900
Kim E, Choi D-H, Yi Y-S. Quercetin Ameliorates Acute Lethal Sepsis in Mice by Inhibiting Caspase-11 Noncanonical Inflammasome in Macrophages. Molecules. 2024; 29(24):5900. https://doi.org/10.3390/molecules29245900
Chicago/Turabian StyleKim, Eojin, Deok-Hyeong Choi, and Young-Su Yi. 2024. "Quercetin Ameliorates Acute Lethal Sepsis in Mice by Inhibiting Caspase-11 Noncanonical Inflammasome in Macrophages" Molecules 29, no. 24: 5900. https://doi.org/10.3390/molecules29245900
APA StyleKim, E., Choi, D.-H., & Yi, Y.-S. (2024). Quercetin Ameliorates Acute Lethal Sepsis in Mice by Inhibiting Caspase-11 Noncanonical Inflammasome in Macrophages. Molecules, 29(24), 5900. https://doi.org/10.3390/molecules29245900










