Biological Properties and Phytochemicals of Multipurpose Tree Plant Hagenia abyssinica
Abstract
1. Introduction
2. Electronic Literature Search Strategy
3. Botanical Description
4. Toxicity Studies
5. Phytochemicals of HA
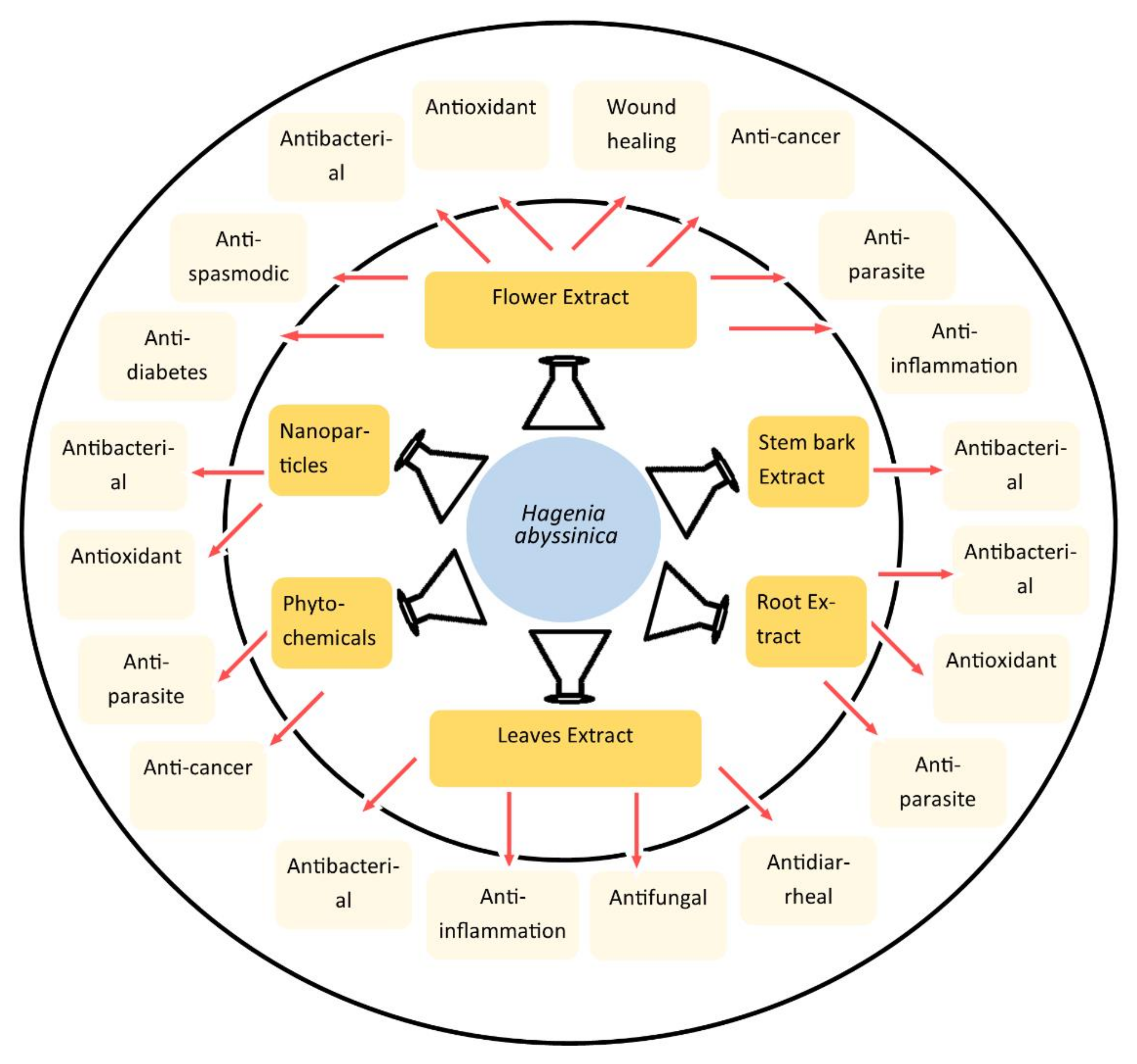
6. Pharmacological Activities of HA
6.1. Anti-Microbial Activity
6.2. Anti-Parasite Activity of HA
6.2.1. In Vitro Anti-Parasite Activity of HA
6.2.2. In Vivo Anti-Parasite Activity of HA
6.2.3. Anti-Parasite Activity on Human Subjects
6.3. Antidiarrheal Activity
6.4. The Anti-Spasmodic Activity of HA
6.5. Anti-Cancer Effects of HA
6.5.1. In Vitro Anti-Cancer Effects of HA
6.5.2. In Vivo Anti-Cancer Study of HA
6.6. Antioxidant Activity of HA
6.7. Antidiabetic Activity of HA
6.7.1. In Vitro Anti-Diabetes Studies on HA
6.7.2. In Vivo Anti-Diabetes Studies on HA
6.8. Wound Healing Effects of HA
6.9. Anti-Inflammatory Activity of HA
7. Results Gaps and Future Direction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Assefa, B.; Glatzel, G.; Buchmann, C. Ethnomedicinal uses of Hagenia abyssinica (Bruce) J.F. Gmel. among rural communities of Ethiopia. J. Ethnobiol. Ethnomed. 2010, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Chen, G.; Zhang, Y.; Nahar, L.; Sarker, S.D.; Hu, G.; Guo, M. Antioxidant and anti-proliferative properties of Hagenia abyssinica roots and their potentially active components. Antioxidants 2020, 9, 143. [Google Scholar] [CrossRef] [PubMed]
- Low, G.; Rogers, L.; Brumley, S.; Ehrlich, D. Visual deficits and retinotoxicity caused by the naturally occurring anthelmintics, Embelia ribes and Hagenia abyssinica. Toxicol. Appl. Pharmacol. 1985, 81, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Feyissa, T.; Welander, M.; Negash, L. Micropropagation of Hagenia abyssinica: A multipurpose tree. Plant Cell Tissue Organ Cult. 2005, 80, 119–127. [Google Scholar] [CrossRef]
- Kifle, Z.D.; Debeb, S.G.; Belayneh, Y.M. In Vitro α-Amylase and α-Glucosidase Inhibitory and Antioxidant Activities of the Crude Extract and Solvent Fractions of Hagenia abyssinica Leaves. BioMed Res. Int. 2021, 2021, 6652777. [Google Scholar] [CrossRef]
- Belachew, T.F.; Asrade, S.; Geta, M.; Fentahun, E. In Vivo Evaluation of Wound Healing and Anti-Inflammatory Activity of 80% Methanol Crude Flower Extract of Hagenia abyssinica (Bruce) JF Gmel in Mice. Evid.-Based Complement. Altern. Med. 2020, 2020, 9645792. [Google Scholar] [CrossRef]
- Jaiswal, V.; Lee, H.-J. Pharmacological Properties of Shionone: Potential Anti-Inflammatory Phytochemical against Different Diseases. Molecules 2023, 29, 189. [Google Scholar] [CrossRef]
- Jaiswal, V.; Lee, H.-J. Pharmacological Activities of Mogrol: Potential Phytochemical against Different Diseases. Life 2023, 13, 555. [Google Scholar] [CrossRef]
- Jaiswal, V.; Lee, H.-J. The Bioactivity and Phytochemicals of Muscari comosum (Leopoldia comosa), a Plant of Multiple Pharmacological Activities. Int. J. Mol. Sci. 2024, 25, 2592. [Google Scholar] [CrossRef]
- Phull, A.-R.; Ali, A.; Rafiq, M.; Tahir, T.; Majid, A.; Seo, S.-Y.; Park, H.-J. Antioxidant potential, urease and acetylcholine esterase inhibitory activity and phytochemical analysis of selected medicinal plants from the Republic of Korea. Explor. Res. Hypothesis Med. 2021, 6, 51–59. [Google Scholar] [CrossRef]
- Ayele, T.B.; Gailing, O.; Finkeldey, R. Assessment and integration of genetic, morphological and demographic variation in Hagenia abyssinica (Bruce) J.F. Gmel to guide its conservation. J. Nat. Conserv. 2011, 19, 8–17. [Google Scholar] [CrossRef]
- Ngeny, L.C.; Magiri, E.; Mutai, C.; Mwikwabe, N.; Bii, C. Antimicrobial properties and toxicity of Hagenia abyssinica (Bruce) JF Gmel, Fuerstia africana TCE Fries, Asparagus racemosus (Willd.) and Ekebergia capensis Sparrm. Afr. J. Pharmacol. Ther. 2013, 2, 76–82. [Google Scholar]
- Kifle, Z.D.; Atnafie, S.A.; Yimer Tadesse, T.; Belachew, T.F.; Kidanu, B.B. Methanolic crude extract of hagenia abyssinica possesses significant antidiarrheal effect: Evidence for in vivo antidiarrheal activity. Evid.-Based Complement. Altern. Med. 2021, 2021, 9944629. [Google Scholar] [CrossRef] [PubMed]
- Desta, B. Ethiopian traditional herbal drugs. Part I: Studies on the toxicity and therapeutic activity of local taenicidal medications. J. Ethnopharmacol. 1995, 45, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Abegaz, B.M.; Mdee, L.K.; Bezabih, M.; Ngadjui, B. Novel natural products from marketed plants of eastern and southern Africa. Int. Union Pure Appl. Chem. 1999, 71, 919–926. [Google Scholar] [CrossRef]
- Kifle, Z.D.; Yesuf, J.S.; Atnafie, S.A. Evaluation of in vitro and in vivo anti-diabetic, anti-hyperlipidemic and anti-oxidant activity of flower crude extract and solvent fractions of hagenia abyssinica (rosaceae). J. Exp. Pharmacol. 2020, 12, 151–167. [Google Scholar] [CrossRef]
- Woldemariam, T.Z.; Fell, A.F.; Linley, P.A. Chromatographic and spectroscopic studies on the constituents in male and female flowers of Hagenia abyssinica. J. Pharm. Biomed. Anal. 1990, 8, 859–865. [Google Scholar] [CrossRef]
- Nibret, E.; Wink, M. Trypanocidal and antileukaemic effects of the essential oils of Hagenia abyssinica, Leonotis ocymifolia, Moringa stenopetala, and their main individual constituents. Phytomedicine 2010, 17, 911–920. [Google Scholar] [CrossRef]
- Dampc, A.; Luczkiewicz, M. Rhododendron tomentosum (Ledum palustre). A review of traditional use based on current research. Fitoterapia 2013, 85, 130–143. [Google Scholar] [CrossRef]
- Behiry, S.I.; El-Hefny, M.; Salem, M.Z. Toxicity effects of Eriocephalus africanus L. leaf essential oil against some molecularly identified phytopathogenic bacterial strains. Nat. Prod. Res. 2020, 34, 3394–3398. [Google Scholar] [CrossRef]
- Fan, M.; Guo, M.; Chen, G.; Rakotondrabe, T.F.; Muema, F.W.; Hu, G. Exploring potential inhibitors of acetylcholinesterase, lactate dehydrogenases, and glutathione reductase from Hagenia abyssinica (Bruce) JF Gmel. based on multi-target ultrafiltration-liquid chromatography-mass spectrometry and molecular docking. J. Ethnopharmacol. 2024, 332, 118356. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, H.; Reider, K.; Franke, K.; Wessjohann, L.A.; Keiser, J.; Dagne, E.; Arnold, N. Characterization of constituents and anthelmintic properties of Hagenia abyssinica. Sci. Pharm. 2012, 80, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Kifle, Z.D.; Belayneh, Y.M. Antidiabetic and anti-hyperlipidemic effects of the crude hydromethanol extract of Hagenia abyssinica (Rosaceae) leaves in streptozotocin-induced diabetic mice. Diabetes Metab. Syndr. Obes. 2020, 13, 4085–4094. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, V.; Lee, H.-J. Conservation and evolution of antigenic determinants of SARS-CoV-2: An insight for immune escape and vaccine design. Front. Immunol. 2022, 13, 832106. [Google Scholar] [CrossRef] [PubMed]
- Naghavi, M.; Vollset, S.E.; Ikuta, K.S.; Swetschinski, L.R.; Gray, A.P.; Wool, E.E.; Aguilar, G.R.; Mestrovic, T.; Smith, G.; Han, C. Global burden of bacterial antimicrobial resistance 1990–2021: A systematic analysis with forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef]
- Jung, I.-G.; Jeong, J.-Y.; Yum, S.-H.; Hwang, Y.-J. Inhibitory Effects of Selected Medicinal Plants on Bacterial Growth of Methicillin-Resistant Staphylococcus aureus. Molecules 2022, 27, 7780. [Google Scholar] [CrossRef]
- Jeong, J.-Y.; Jung, I.-G.; Yum, S.-H.; Hwang, Y.-J. In Vitro Synergistic Inhibitory Effects of Plant Extract Combinations on Bacterial Growth of Methicillin-Resistant Staphylococcus aureus. Pharmaceuticals 2023, 16, 1491. [Google Scholar] [CrossRef]
- Wolde, T.; Bizuayehu, B.; Hailemariam, T.; Tiruha, K. Phytochemical analysis and antimicrobial activity of Hagenia abyssinica. Indian J. Pharm. Pharmacol. 2016, 3, 127–134. [Google Scholar] [CrossRef]
- Abebe, G.; Birhanu, G. A comparative study on antibacterial effects of Hagenia abyssinica oil extracted from different parts of the plant using different solvents against two selected and standardized human pathogens. Afr. J. Microbiol. Res. 2019, 13, 99–105. [Google Scholar]
- Melkamu, W.W.; Bitew, L.T. Green synthesis of silver nanoparticles using Hagenia abyssinica (Bruce) JF Gmel plant leaf extract and their antibacterial and anti-oxidant activities. Heliyon 2021, 7, e08459. [Google Scholar] [CrossRef]
- Zewde, D.; Geremew, B. Biosynthesis of ZnO nanoparticles using Hagenia abyssinica leaf extracts; their photocatalytic and antibacterial activities. Environ. Pollut. Bioavailab. 2022, 34, 224–235. [Google Scholar] [CrossRef]
- Zewudie, A.G.; Zereffa, E.A.; Segne, T.A.; Murthy, H.A.; Ravikumar, C.; Muniswamy, D.; Binagdie, B.B. Biosynthesis of Ag/bentonite, ZnO/bentonite, and Ag/ZnO/bentonite nanocomposites by aqueous leaf extract of Hagenia abyssinica for antibacterial activities. Rev. Adv. Mater. Sci. 2023, 62, 20220307. [Google Scholar] [CrossRef]
- Murthy, H.A.; Desalegn, T.; Kassa, M.; Abebe, B.; Assefa, T. Synthesis of green copper nanoparticles using medicinal plant Hagenia abyssinica (Brace) JF. Gmel. leaf extract: Antimicrobial properties. J. Nanomater. 2020, 2020, 3924081. [Google Scholar] [CrossRef]
- Hirphaye, B.Y.; Bonka, N.B.; Tura, A.M.; Fanta, G.M. Biosynthesis of magnesium oxide nanoparticles using Hagenia abyssinica female flower aqueous extract for characterization and antibacterial activity. Appl. Water Sci. 2023, 13, 175. [Google Scholar] [CrossRef]
- Amsalu Abera, A.A.; Fikre Lemessa, F.L.; Diriba Muleta, D.M. The antifungal activity of some medicinal plants against coffee berry disease caused by Colletotrichum kahawae. Int. J. Agric. Res. 2011, 6, 268–279. [Google Scholar] [CrossRef]
- Chakka, S.V.; Thanjavur, N.; Lee, S.; Kim, S. Synthesis and characterization of lanthanum-doped curcumin-functionalized antimicrobial copper oxide nanoparticles. J. Rare Earths 2023, 41, 1606–1615. [Google Scholar] [CrossRef]
- Huq, M.A.; Ashrafudoulla, M.; Parvez, M.A.K.; Balusamy, S.R.; Rahman, M.M.; Kim, J.H.; Akter, S. Chitosan-Coated Polymeric Silver and Gold Nanoparticles: Biosynthesis, Characterization and Potential Antibacterial Applications: A Review. Polymers 2022, 14, 5302. [Google Scholar] [CrossRef]
- Kim, C.; Kim, J.-G.; Kim, K.-Y. Anti-Candida Potential of Sclareol in Inhibiting Growth, Biofilm Formation, and Yeast–Hyphal Transition. J. Fungi 2023, 9, 98. [Google Scholar] [CrossRef]
- Scantlebury, C.E.; Peachey, L.; Hodgkinson, J.; Matthews, J.B.; Trawford, A.; Mulugeta, G.; Tefera, G.; Pinchbeck, G.L. Participatory study of medicinal plants used in the control of gastrointestinal parasites in donkeys in Eastern Shewa and Arsi zones of Oromia region, Ethiopia. BMC Vet. Res. 2013, 9, 179. [Google Scholar] [CrossRef]
- Hulme, J. COVID-19 and Diarylamidines: The Parasitic Connection. Int. J. Mol. Sci. 2023, 24, 6583. [Google Scholar] [CrossRef]
- Oliveira, F.M.S.; Cruz, R.E.; Pinheiro, G.R.G.; Caliari, M.V. Comorbidities involving parasitic diseases: A look at the benefits and complications. Exp. Biol. Med. 2022, 247, 1819–1826. [Google Scholar] [CrossRef] [PubMed]
- Abebe, G.; Dawson, L.; Detweiler, G.; Gipson, T.; Sahlu, T. Hagenia abyssinica (Kosso) for internal parasite control in goats. In Proceedings of the Opportunities and Challenges of Enhancing Goat Production in East Africa, Awassa, Ethiopia, 10–12 November 2000; E (Kika) de la Garza Institute for Goat Research: Langston, OK, USA, 2000; pp. 190–195. [Google Scholar]
- Kifle, Z.D.; Kidanu, B.B.; Tadesse, T.Y.; Belachew, T.F.; Atnafie, S.A. Evaluation of in vivo antidiarrheal activity of solvent fractions of Hagenia abyssinica (Rosaceae) in Swiss albino mice. Evid.-Based Complement. Altern. Med. 2021, 2021, 8828331. [Google Scholar] [CrossRef] [PubMed]
- Arragie, M.; Metzner, J.; Bekemeier, H. Antispasmodic effect of Hagenia abyssinica. Planta Medica 1983, 47, 240–241. [Google Scholar] [CrossRef] [PubMed]
- Woldemariam, T.Z.; Fell, A.F.; Linley, P.A.; Bibby, M.C.; Phillips, R.M. Evaluation of the anti-tumour action and acute toxicity of kosins from Hagenia abyssinica. J. Pharm. Biomed. Anal. 1992, 10, 555–560. [Google Scholar] [CrossRef]
- Kim, S.J.; Shin, M.-S.; Choi, Y.-K. Ameliorative Effects of Zingiber officinale Rosc on Antibiotic-Associated Diarrhea and Improvement in Intestinal Function. Molecules 2024, 29, 732. [Google Scholar] [CrossRef]
- Ahn, H.-R.; Park, D.H.; Shin, M.-S.; Nguyen, Q.N.; Park, J.Y.; Kim, D.-W.; Kang, K.S.; Lee, H.L. The Ameliorating Effect of Lizhong-Tang on Antibiotic-Associated Imbalance in the Gut Microbiota in Mouse. Appl. Sci. 2022, 12, 6943. [Google Scholar] [CrossRef]
- Hashem, S.; Ali, T.A.; Akhtar, S.; Nisar, S.; Sageena, G.; Ali, S.; Al-Mannai, S.; Therachiyil, L.; Mir, R.; Elfaki, I. Targeting cancer signaling pathways by natural products: Exploring promising anti-cancer agents. Biomed. Pharmacother. 2022, 150, 113054. [Google Scholar] [CrossRef]
- Kim, S.J.; Nah, S.-Y.; Park, I.-H.; Shin, M.-S.; Kang, K.S. Gintonin Isolated from Ginseng Inhibits the Epithelial—Mesenchymal Transition Induced by TGF-β in A549 Lung Cancer Cells. Plants 2023, 12, 2013. [Google Scholar] [CrossRef]
- Alshehri, A.; Ahmad, A.; Tiwari, R.K.; Ahmad, I.; Alkhathami, A.G.; Alshahrani, M.Y.; Asiri, M.A.; Almeleebia, T.M.; Saeed, M.; Yadav, D.K. In vitro evaluation of antioxidant, anticancer, and anti-inflammatory activities of ethanolic leaf extract of Adenium obesum. Front. Pharmacol. 2022, 13, 847534. [Google Scholar] [CrossRef]
- Ong, K.L.; Stafford, L.K.; McLaughlin, S.A.; Boyko, E.J.; Vollset, S.E.; Smith, A.E.; Dalton, B.E.; Duprey, J.; Cruz, J.A.; Hagins, H. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef]
- Mekuria, A.B.; Belachew, S.A.; Tegegn, H.G.; Ali, D.S.; Netere, A.K.; Lemlemu, E.; Erku, D.A. Prevalence and correlates of herbal medicine use among type 2 diabetic patients in Teaching Hospital in Ethiopia: A cross-sectional study. BMC Complement. Altern. Med. 2018, 18, 85. [Google Scholar] [CrossRef]
- Kim, H.-S.; Lee, D.; Seo, Y.-H.; Ryu, S.-M.; Lee, A.-Y.; Moon, B.-C.; Kim, W.-J.; Kang, K.-S.; Lee, J. Chemical Constituents from the Roots of Angelica reflexa That Improve Glucose-Stimulated Insulin Secretion by Regulating Pancreatic β-Cell Metabolism. Pharmaceutics 2023, 15, 1239. [Google Scholar] [CrossRef]
- Razia, S.; Park, H.; Shin, E.; Shim, K.-S.; Cho, E.; Kang, M.C.; Kim, S.Y. Synergistic effect of Aloe vera flower and Aloe gel on cutaneous wound healing targeting MFAP4 and its associated signaling pathway: In-vitro study. J. Ethnopharmacol. 2022, 290, 115096. [Google Scholar] [CrossRef]
- Park, D.H.; Park, J.Y.; Shin, M.-S.; Hwang, G.S. Wound Healing Effect of 20(S)-Protopanaxadiol of Ginseng Involves VEGF-ERK Pathways in HUVECs and Diabetic Mice. Processes 2023, 11, 692. [Google Scholar] [CrossRef]
- Jaiswal, V.; Lee, H.-J. Antioxidant activity of Urtica dioica: An important property contributing to multiple biological activities. Antioxidants 2022, 11, 2494. [Google Scholar] [CrossRef]
- Bhola, S.; Jaiswal, V.; Lee, H.-J. Investigation of molecular mechanisms of Ulmus davidiana against gastric cancer: A network pharmacology study incorporating gene expression data and molecular docking analysis. S. Afr. J. Bot. 2023, 159, 51–60. [Google Scholar] [CrossRef]
- Ferenczyova, K.; Kalocayova, B.; Bartekova, M. Potential implications of quercetin and its derivatives in cardioprotection. Int. J. Mol. Sci. 2020, 21, 1585. [Google Scholar] [CrossRef]
- Suganthy, N.; Devi, K.P.; Nabavi, S.F.; Braidy, N.; Nabavi, S.M. Bioactive effects of quercetin in the central nervous system: Focusing on the mechanisms of actions. Biomed. Pharmacother. 2016, 84, 892–908. [Google Scholar] [CrossRef]
- Jaiswal, V.; Park, M.; Lee, H.-J. Comparative transcriptome analysis of the expression of antioxidant and immunity genes in the spleen of a Cyanidin 3-O-Glucoside-treated alzheimer’s mouse model. Antioxidants 2021, 10, 1435. [Google Scholar] [CrossRef]
| Sr. No. | Compound Name/Molecular Formula | Amount (µg/g) | Compound Type | Type of Extract/Fraction | Part of Plant | References |
|---|---|---|---|---|---|---|
| 1 | p-Hydroxybenzoic acid/C7H6O3 | 8.92 | Phenolic acids | Ethyl acetate | Male Flower | [17] |
| 4.27 | Phenolic acids | Ethyl acetate | Female flower | [17] | ||
| 2 | Protocatechuic acid/C7H6O4 | 4.51 | Phenolic acids | Ethyl acetate | Male Flower | [17] |
| 4.86 | Phenolic acids | Ethyl acetate | Female flower | [17] | ||
| 3 | Vanillic acid/C8H8O4 | 9.93 | Phenolic acids | Ethyl acetate | Male Flower | [17] |
| 5.39 | Phenolic acids | Ethyl acetate | Female flower | [17] | ||
| 4 | α-Kosin/C25H32O8 | 220 | Phenol derivative | Diethyl ether | Male Flower | [17] |
| 240 | Phenol derivative | Diethyl ether | Female flower | [17] | ||
| 5 | Kosotoxin/C25H32O8 | 1080 | Phenol derivative | Diethyl ether | Male Flower | [17] |
| 960 | Phenol derivative | Diethyl ether | Female flower | [17] | ||
| 6 | Protokosin/C37H46O12 | 760 | Phenol derivative | Diethyl ether | Male Flower | [17] |
| 720 | Phenol derivative | Diethyl ether | Female flower | [17] | ||
| 7 | Corilagin/C27H22O18 | NP | Tannin | Ethyl acetate fraction | Root | [21] |
| 8 | Brevifolin carboxylic acid/C13H8O8 | NP | Phenol derivative | Ethyl acetate fraction | Root | [21] |
| 9 | Brevifolin/C10H12O4 | NP | Phenolic compound | Ethyl acetate fraction | Root | [21] |
| 10 | Methyl brevifolin carboxylate/C14H10O8 | NP | Phenol derivative | Ethyl acetate fraction | Root | [21] |
| 11 | Quercetin/C15H10O7 | NP | Flavonoid | Ethyl acetate fraction | Root | [21] |
| 12 | Methyl ellagic acid/C15H8O8 | NP | Polyphenol | Ethyl acetate fraction | Root | [21] |
| 13 | Yomogi alcohol/C10H18O | 1.11 # | Terpenoid | Essential oil | Female flower | [18] |
| 14 | Camphenone/C10H14O | 0.32 # | Monoterpenoid ketone | Essential oil | Female flower | [18] |
| 15 | L-Camphor/C10H16O | 2.16 # | Monoterpenoid ketone | Essential oil | Female flower | [18] |
| 16 | Limonene oxide/C10H16O | 0.68 # | Monoterpenoid epoxide | Essential oil | Female flower | [18] |
| 17 | Cis-verbenol/C10H16O | 3.27 # | Monoterpenoid alcohol | Essential oil | Female flower | [18] |
| 18 | Trans-verbenone/C10H14O | 0.38 # | Monoterpenoid ketone | Essential oil | Female flower | [18] |
| 19 | α-Phellandren-8-ol/C10H16O | 3.68 # | Monoterpenoid alcohol | Essential oil | Female flower | [18] |
| 20 | Gurjunene/C15H24 | 1.15 # | Sesquiterpene | Essential oil | Female flower | [18] |
| 21 | Curcumene/C15H22 | 0.58 # | Sesquiterpene | Essential oil | Female flower | [18] |
| 22 | α-Selinene/C15H24 | 0.45 # | Sesquiterpene | Essential oil | Female flower | [18] |
| 23 | Valeranone/C15H26O | 10.58 # | Sesquiterpenoid ketone | Essential oil | Female flower | [18] |
| 24 | Palustrol/C15H26O | 5.70 # | Sesquiterpenoid alcohol | Essential oil | Female flower | [18] |
| 25 | Ledol/C15H26O | 58.57 # | Sesquiterpenoid alcohol | Essential oil | Female flower | [18] |
| 26 | Hexadecen-1-ol/C16H32O | 2.59 # | Fatty alcohol (Lipid) | Essential oil | Female flower | [18] |
| 27 | Trans-9-E-15-Heptadecenal/C17H32O | 4.45 # | Fatty aldehyde | Essential oil | Female flower | [18] |
| 28 | Tetracosane/C24H50 | 2.07 # | Alkane | Essential oil | Female flower | [18] |
| 29 | Diallyl methyl carbinol/C8H14O | 0.99 # | Terpenoid alcohol | Essential oil | Female flower | [18] |
| 30 | 3-Pinanylamine/C10H19N | 0.46 # | Terpene amine | Essential oil | Female flower | [18] |
| 31 | 2-Furanmethanol/C5H6O2 | 0.44 # | Alcohol | Essential oil | Female flower | [18] |
| 32 | Dihydroquercetin/C15H12O7 | NP | Flavonoid | Ethanol extract | Root | [2] |
| 33 | Acacetin/C16H12O5 | NP | Flavonoid | Ethanol extract | Root | [2] |
| 34 | Isoquercitin/C21H20O12 | NP | Flavonoid glycocide | Ethanol extract | Root | [2] |
| 35 | Dehydrodicatechin A/C30H24O12 | NP | Flavonoid | Ethanol extract | Root | [2] |
| 36 | Trans-ferulic acid/C10H10O4 | NP | Phenol | Ethanol extract | Root | [2] |
| 37 | Caffeic acid/C9H8O4 | NP | Polyphenol | Ethanol extract | Root | [2] |
| 38 | 3,4-Dihydroxybenzoic acid/C7H6O4 | NP | Polyphenol | Ethanol extract | Root | [2] |
| 39 | 2-Methoxyterephthalic/C9H8O5 | NP | Aromatic acid | Ethanol extract | Root | [2] |
| 40 | Quercetin 3-O-β-glucuronide/C21H18O13 | NP | Flavonoid glycoside | Methanol extract | Female flower | [22] |
| 41 | Quercetin 3-O-β-glucoside/C21H20O12 | NP | Flavonoid glycoside | Methanol extract | Female flower | [22] |
| 42 | Rutin/C27H30O16 | NP | Polyphenolflavonoid glycoside | Methanol extract | Female flower | [22] |
| 43 | Quercetin glycuronide/C21H18O13 | NP | Flavonol glucuronide | Methanol extract | Female flower | [22] |
| 44 | Ellagic acid/C14H6O8 | NP | Polyphenol | Methanol extract | Female flower | [22] |
| Sr. No. | Activity | Plant Part/Extract Type | Method | Result | Ref. |
|---|---|---|---|---|---|
| 1 | Antibacterial | Organic (hexane, DCM and methanol) and aqueous extract of leaves | Mean inhibition zones (MIZ) caculation through agar diffusion method against different bacteria | MIZ in hexane: 6.67 ± 0.67, 19.33 ± 1.33, 13.00 ± 1.00, and 6.83 ± 0.17 mm against SA, MRSA, PA, and TM, respectively. MIZ in DCM: 20.00 ± 1.15, 19.50 ± 1.50, 15.5 ± 1.50, and 7.17 ± 0.44 mm against SA, MRSA, PA, and TM, respectively. MIZ in methanol: 7.75 ± 0.25, 7.63 ± 0.24, and 12.00 ± 0.82 mm against SA, MRSA, and PA, respectively. | [12] |
| Minimum inhibitory concentration (MIC) against different bacteria | MIC in hexane: 0.195, 0.195, and 3.125 against SA, MRSA, and PA, respectively. MIC in DCM: 0.195, 0.391, and 0.195 mg/mL against SA, MRSA, and PA, respectively. MIC in Methanol: 12.5 mg/mL against PA; | ||||
| Organic (hexane and methonol) and aqueous extract of stem bark | MIC against different bacteria | MIC in hexane: 6.25 and 6.25 mg/mL against SA and MRSA, respectively. MIC in DCM: 100 and 100 mg/mL against SA and MRSA, respectively. MIC in methanol: 25 and 50 mg/mL against SA and MRSA, respectively. | |||
| MIZ against different bacteria | MIZ in hexane: 9.00 ± 1.00, 9.50 ± 0.96, and 7.00 ± 0.33 mm against SA, MRSA, and TM, respectively. MIZ in DCM: 8.67 ± 0.33, and 9.33 ± 0.67 mm against SA and MRSA, respectively. MIZ in methanol: 11.00 ± 0.41, 10.75 ± 0.25, 12.25 ± 1.75, and 7.33 ± 0.33 mm SA, MRSA, PA, and MG, respectively. Water: 11.33 ± 0.67 mm against PA; | ||||
| Extract of female flowers in methanol, ethanol, n-hexane, and petroleum ether | Agar well diffusion method. | SA: 21, 20, 15, and 14 mm for methanol, ethanol, n-hexane, and petroleum ether, respectively. ST: 15, 14, 14, and 12 mm for methanol, ethanol, n-hexane, and petroleum ether, respectively. | [28] | ||
| Oil extracted from the root through methanol, ethyl acetate, and hexane. | Antibiotics diffusion method against SA and EC | Ethyl acetate: 8.87 and 8.75 mm in case of SA and EC, respectively. Hexane: 9.87 and 5.87 in case of SA and EC, respectively. Methanol: 24.38 ad 27.13 in case of SA and EC, respectively. | [29] | ||
| Oil extracted from the leaves through methanol, ethyl acetate and hexane | Antibiotics diffusion method against SA and EC | Ethy acetate: 8.38 and 9 mm in case of SA and EC, respectively. Hexane: 20 and 6.5 in case of SA and EC, respectively. Methanol: 12 ad 12.5 in case of SA and EC, respectively. | |||
| Oil extracted from the root bark through methanol, ethyl acetate and hexane | Antibiotics diffusion method against SA and EC. | Ethyl acetate: 8.75 and 6.75 mm in case of SA and EC, respectively. Hexane: 7.5 and 4.88 in case of SA and EC, respectively. Methanol: 15.37 ad 1.125 in case of SA and EC, respectively. | |||
| extract of leaves and Silver nanoparticles synthesized from the extract (50, 100, 150, and 200 μg/mL) | Agar well diffusion method. | AgNPs: 18.3, 14, and 8.6 in case of ST, KP, and SP, respectively. Leave extract: 13, 10, and 6.3 in case of ST, KP, and SP, respectively. | [30] | ||
| Zinc oxide nanoparticles from the aqueous extract of leaves. (10, 20, and 30 mg/mL) | Disc diffusion method 2,3,5-triphenyltetrazolium chloride | 19 ± 1.0, 18 ± 1.0 19.33 ± 0.58, and 21 ± 1.0 mm in case of EC, KP, SA, and SE, respectively. | [31] | ||
| Ag NPs, Ag/bentonite NCs, Ag/ZnO/bentonite, ZnO NPs, and ZnO/bentonite nanocomposites prepared from the leaves extract | Disc diffusion method | Ag NPs: 11.6 ± 0.3 and 14.3 ± 0.3 mm in case of EC and SA, respectively. Ag/bentonite NCs: 14.3 ± 0.1 and 14.7 ± 0.3 mm in case of EC and SA, respectively. Ag/ZnO/bentonite:14.3 ± 0.3 and 17.3 ± 0.2 mm in case of EC and SA, respectively. ZnO NPs: 10.3 ± 0.3 and 11 ± 0.6 mm in case of EC and SA, respectively. ZnO/bentonite: 12.3 ± 0.3 and 12.3 ± 0.3 mm in case of EC and SA, respectively. Plant extract: 9.1 ± 0.3 and 10 ± 0.0 mm in case of EC and SA, respectively. | [32] | ||
| Copper nanoparticles synthesized from the extract of leaves | Agar disc-diffusion method Ampicillin | 12.7 ± 0.4, 14.7 ± 0.2, 14.2 ± 0.8, and 12.7 ± 1.1 mm in case of EC, SA, BS, and PA, respectively. | [33] | ||
| Ag/ZnO/bentonite nanocomposite prepared from the leaves extract | MIC and MBC were calculated through Broth dilution methods | MIC: 156.25 and 78.125 μg/mL for EC and SA, respectively. MBC: 312.5 and 156.25 for EC and SA, respectively. | [32] | ||
| MgO nanoparticles synthesized from the aqueous extract of the flowers | Agar-well-diffusion method (0.8 mg/mL) chloramphenicol (30 μg) | 15 ± 0 and 27 ± 0.28 mm for EC and SA, respectively. | [34] | ||
| 2 | Antioxidant | Methanol extract of HA roots and its fraction in water, n-hexane, dichloromethane, and ethyl acetate | DPPH | IC50 values 99.700 ± 0.013 g/mL (Ethyl acetate) and 98.680 ± 0.010 (Trolox) | [2] |
| ABTS | 31.200 ± 0.001 g/mL (Ethyl acetate) and 64.760 ± 0.003 g/mL (Trolox) | ||||
| FRAP | 3.478 mg Fe2+/g (ethanol extract) | ||||
| Silver nanoparticles synthesized from the extract of leaves and extract of leaves (10–320 μg/mL) | Percentage inhibition DPPH radical scavenging activity (ascorbic acid as control) | 66% in AgNPs and 95.9% in control | [30] | ||
| Methanol extract of leaves and solvent fractions (water, ethyl acetate, and chloroform) 15.6–500 μg/mL | percentage inhibition DPPH radical scavenging activity (ascorbic acid as control) | 86.36% (IC50, 10.25 μg/mL) followed by water fraction 78.59% (IC50, 13.86 μg/mL), ethyl acetate fraction 71.58% (IC50, 16.34 μg/mL), andchloroform fraction 63.65% (IC50, 18.83 μg/mL). | [5] | ||
| 3 | Antifungal | Aqueous and ethanol extracts of leaves | In vitro assay of radial growth in petri dish of Colletotrichum kahawae. | 60% and 40% inhibition of growth in aqueous and ethanol extracts, respectively. | [35] |
| 4 | Anti-diabetes | Methanol extract of leaves and solvent fractions (water, ethyl acetate, and chloroform) 15.6–500 μg/mL | α-amylase inhibition using 3,5-dinitrosalicylic acid (DNSA) assays | 74.52% (IC50, 14.52 μg/mL) followed by water fraction 68.24% (IC50, 16.31 μg/mL), ethyl acetate fraction 61.57% (IC50, 18.73 μg/mL), and chloroform fraction 56.87% (IC50, 21.57 μg/mL) of H. abyssinica leaves. | [5] |
| α-glucosidase inhibiton of the plant extract were assessed p-nitro-phenyl-a-D glucopyranoside (p-NPG) assays | Aqueous fraction 62.54% (IC50, 11.67 μg/mL) followed by ethyl acetate fraction 54.97% (IC50, 15.89 μg/mL), crude extract 46.79% (IC50, >16.5 μg/mL), and chloroform fraction 36.44% (IC50, >16.5 μg/mL). | ||||
| Methanol crude extract and solvent fractions (water, ethyl acetate, and chloroform) 25 to 800 μg/mL. | In vitro α-amylase inhibition assay was conducted through DNSA method | 26.18 ± 0.88 (water) 28.27 ± 0.74 (chloroform) 43.38 ± 0.78 (crude extract) 54.23 ± 0.53 (ethyl acetate) 91.87 ± 0.54 (Acarbose). | [16] | ||
| 5 | Anticancer activity | Essential oil extracted from the female flowers of HA through hydo-distillation | Anti-proliferative assay on HL-60 Cell line | IC50: 50.07 μg/mL | [18] |
| Methanol extract of HA roots and its fraction in water, n-hexane, dichloromethane, and ethyl acetate | MTT assay conducted on HepG2, cell line | # IC50 values were 162, 30, and 41 for ethanol extract, n-hexane, and ethyl acetate fractions. | [2] | ||
| MTT assay conducted on HT-29, cell line | # IC50 values were 102, 50, and 59 for ethanol extract, n-hexane, and ethyl acetate fraction, respectively. | ||||
| MTT assay conducted on SGC-7901 cell line | # IC50 values were 137, 58, and 46 for ethanol extract, n-hexane, and ethyl acetate fractions. | ||||
| 6 | Anti-parasite | Essential oil extracted from the female flowers of HA through hydo-distillation | Antitrypanosomal activity against Trypanosoma brucei | IC50 = 42.30 μg/mL | [18] |
| Ethanol crude extract of root and fractions in n-hexane, dichloromethane, ethyl acetate, and water. | Inhibition rates (IRs) of sample after cultured with Trypanosoma brucei for 24 h. | Crude extract 51% inhibition at low dose and 100% inhibition at high dose. Ethyl acetate fraction 100% inhibition at both low and high dose. | [21] | ||
| Acetylcholinesterase inhibitory activity by Ellman’s method | IC50 values for ethyl acetate, crude extract, n-hexane, dichloromethane, and water fractions were 12.85 ± 1.82, 144.05 ± 20.58, 632.80 ± 1.00, 250.15 ± 20.44, and 211.50 ± 11.17 μg/mL, respectively. | ||||
| Phytochemical isolated from the roots of HA | Inhibition of acetylcholinesterase through ultrafiltration-liquid chromatography–mass spectrometry based assay. | Binding degree % of phytochemicsals were 33.26, 49.81, 27.99, 33.74, 27.11, 40.34, and 26.57 for protocatechuic acid, corilagin, brevifolin carboxylic acid, brevifolin, methyl brevifolin carboxylate, quercetin, and methyl ellagic acid, respectively | |||
| Inhibition of glutathione reductase through ultrafiltration-liquid chromatography–mass spectrometry based assay. | Binding degree % of phytochemicsals were 15.55, 10.02, 18.43, 20.96, 1.32, and 17.26 for protocatechuic acid, brevifolin carboxylic acid, brevifolin, methyl brevifolin carboxylate, quercetin, and methyl ellagic acid, respectively. | ||||
| Inhibition of lactate dehydrogenases through ultrafiltration-liquid chromatography–mass spectrometry based assay. | Binding degree % of phytochemicsals were 8.27, 4.94, 29.73, 8.17, and 12.16 for brevifolin carboxylic acid, brevifolin, methyl brevifolin carboxylate, quercetin, and methyl ellagic acid, respectively. | ||||
| Female flowers of HA and its fraction in n-heptane, ethyl acetate, and methanol | 100 μg/mL Schistosoma mansoni | Time of death were 3, 3, 3, and 166 h in crude extract, n-heptane, ethyl acetate, and methanol fractions. respectively. | [22] | ||
| Clonorchis sinensis | Time of death were 5, 5, 5, and 8 h in crude extract, n-heptane, ethyl acetate, and methanol fractions. respectively. | ||||
| Fasciola hepatica | Time of death were 51, 17, 41, and >72 h in crude extract, n-heptane, ethyl acetate, and methanol fractions. respectively. | ||||
| Echinostoma caproni | Time of death were 1, 1, 18, and 1 h in crude extract, n-heptane, ethyl acetate, and methanol fractions. respectively. |
| Sr. No. | Activity | Material | Method and Model | Results | Reference |
|---|---|---|---|---|---|
| 1 | Antidiarrheal | Fraction of leaves extract in aqueous, ethyl acetate, and chloroform and crude extract | Inhibition of defecation in castor oil-induced diarrhea in Swiss albino mice (Loperamide 3 mg/kg used as positive control) | 84.60, 78.00, 57.91, 38.46, and 73.85% inhibtion of defecation in positive control, aqueous, ethyl acetate, chloroform, and crude extract groups respectivly. | [13,43] |
| Castor oil-induced gastrointestinal motility in Swiss albino mice | 59.80, 58.83, 51.92, 31.70, and 54.00% inhibtion of gastrointestinal motility in positive control, aqueous, ethyl acetate, chloroform, and crude extract groups respectivly. | ||||
| Castor oil-induced enteropooling in Swiss albino mice | 50.60, 47.00, 53.00, 40.70, and 46.00% inhibtion of enteropooling in positive control, aqueous, ethyl acetate, chloroform, and crude extract respectivly. | ||||
| Antidiarrheal index (ADI) in Swiss albino mice | 97.13, 74.14, 72.17, 52.37, and 81.24 in vivo ADI in positive control, aqueous, ethyl acetate, chloroform, and crude extract groups respectivly | ||||
| 2 | Anti-spasmodic activity | Water extract of the female flowers | Guinea pig ileum induce by the selected spamogens (acetyl choline, histamine, and barium chloride) | level of contraction ↓ amplitude of contraction of the guinea pig ileum ↓ induced by histamine. | [44] |
| 3 | Anticancer | α-kosin, kosotoxin, and protokosin isolated from the female flowers The single dose of 50 mg/kg and split doses of 12.5 mg/kg (for 4 days) | NMRI mice after transplantation of MAC tumors | Survival time ↑ in case of split doses of (protokosin and kosotoxin) | [45] |
| 4 | Anti-inflammatory activity | Methanol extract fraction in ethyl acetate, chloroform and water; Oral doses: 100, 200, and 400 mg/kg | Carrageenan-induced hind paw edema model in mice. Indomethacin (positive control) | Paw edema ↓ (in all 3 doses) | [6] |
| 5 | Wound-healing | Methanol (80%) extract of flowers and fractions in water, ethyl acetate and chloroform used to prepared ointment (5 and 10% w/w) | Swiss albino mice excision wound model | Time of contraction of wound ↓ and epithelization of period ↓ | |
| Methanol (80%) extract of flowers (5 and 10% w/w) | Swiss albino mice incision wound model | Tensile strength ↑ (In both doses) | |||
| 6 | Anti-diabetes | Doses: 100, 200, and 400 mg/kg of crude extract of flower | Hypoglycemic activity at baseline, 1, 2, 4, and 6 h in healthy male Swiss albino mice (Normoglycemic mice model) | BGL ↓ (in 200 and 400 mg/kg groups on the 6th hour after the administration of extracts) | [16] |
| Oral glucose tolerance test on healthy male Swiss albino mice oral administration of glucose (2 g/kg) after 30 min of extract administration. | BGL ↓ (in 200 and 400 mg/kg groups after 120 min of the oral administration of glucose) | ||||
| Doses: 100, 200, and 400 mg/kg of crude methanol extract of flower and its fraction ethyl acetate. | Anti-hyperglycemic activity in STZ-induced male Swiss albino mice for a single dose of extracts. | BGL ↓ (at the 8th hour after treatment of 400 mg/kg crude extract and ethyl acetate fraction) | |||
| Anti-hyperglycemic activity in STZ-induced male Swiss albino mice for daily doses of extracts. | BGL ↓ (at 7th and 14th days after the start of daily treatment of 200 and 400 mg/kg crude extract and ethyl acetate fraction) | ||||
| Body weight in STZ-induced male Swiss albino mice for daily doses of extracts. | BW ↑ (at the 7th day after the start of daily treatment of 200 and 400 mg/kg doses of crude extract and 400 mg/kg dose of ethyl acetate); BW ↑ (on the 14th day all doses of crude and ethyl acetate fraction) | ||||
| Serum lipid profile in STZ-induced male Swiss albino mice for daily doses of extracts. | TC ↓, TG ↓, LDL ↓, and VLDL ↓ (all doses of crude extract and 400 mg/kg ethyl acetate fraction groups);HDL-c ↑ (all doses of crude extract and 200 and 400 mg/kg of ethyl acetated fractions) | ||||
| Doses: 100, 200, and 400 mg/kg of crude methanol extract of leaves (100, 200, and 400 mg/kg) | Anti-hyperglycemic activity in STZ-induced male Swiss albino mice for daily doses of extracts. | BGL ↓ (at 7th and 14th days after the start of daily treatment of all doses). | [23] | ||
| Body weight in STZ-induced male Swiss albino mice for daily doses of extracts. | BW ↑ (7th day after the start of daily treatment of 400 mg/kg dose) BW ↑ (on the 14th day in 200 and 400 mg/kg doses groups) | ||||
| Serum lipidprofiles in STZ-induced male Swiss albino mice for daily doses of extracts. | Serum levels of TC ↓, TG ↓, VLDL-c ↓, LDL-c ↓, and HDL-c ↑ (15th day after the start of daily treatment on all doses). | ||||
| 7 | Anti-parasite activity | Dried HA material at 20, 40, and 60 mg doses | Cestode egg count in feces of Alpine goats. | Egg count ↓ (in all 3 doses) | [42] |
| Powdered plant material (flowers) with honey one time in the morning | Group of 6 humans (worm-infested subjects) in each dose group | MESD was 12.5 ± 2.2 g and WET was 11.3 ± 1.4 (hour) | [14] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaiswal, V.; Lee, H.-J. Biological Properties and Phytochemicals of Multipurpose Tree Plant Hagenia abyssinica. Molecules 2024, 29, 5871. https://doi.org/10.3390/molecules29245871
Jaiswal V, Lee H-J. Biological Properties and Phytochemicals of Multipurpose Tree Plant Hagenia abyssinica. Molecules. 2024; 29(24):5871. https://doi.org/10.3390/molecules29245871
Chicago/Turabian StyleJaiswal, Varun, and Hae-Jeung Lee. 2024. "Biological Properties and Phytochemicals of Multipurpose Tree Plant Hagenia abyssinica" Molecules 29, no. 24: 5871. https://doi.org/10.3390/molecules29245871
APA StyleJaiswal, V., & Lee, H.-J. (2024). Biological Properties and Phytochemicals of Multipurpose Tree Plant Hagenia abyssinica. Molecules, 29(24), 5871. https://doi.org/10.3390/molecules29245871









