Phenolic Compounds of the Medicinal Plants in an Anthropogenically Transformed Environment
Abstract
1. Introduction
2. Phenolic Compounds
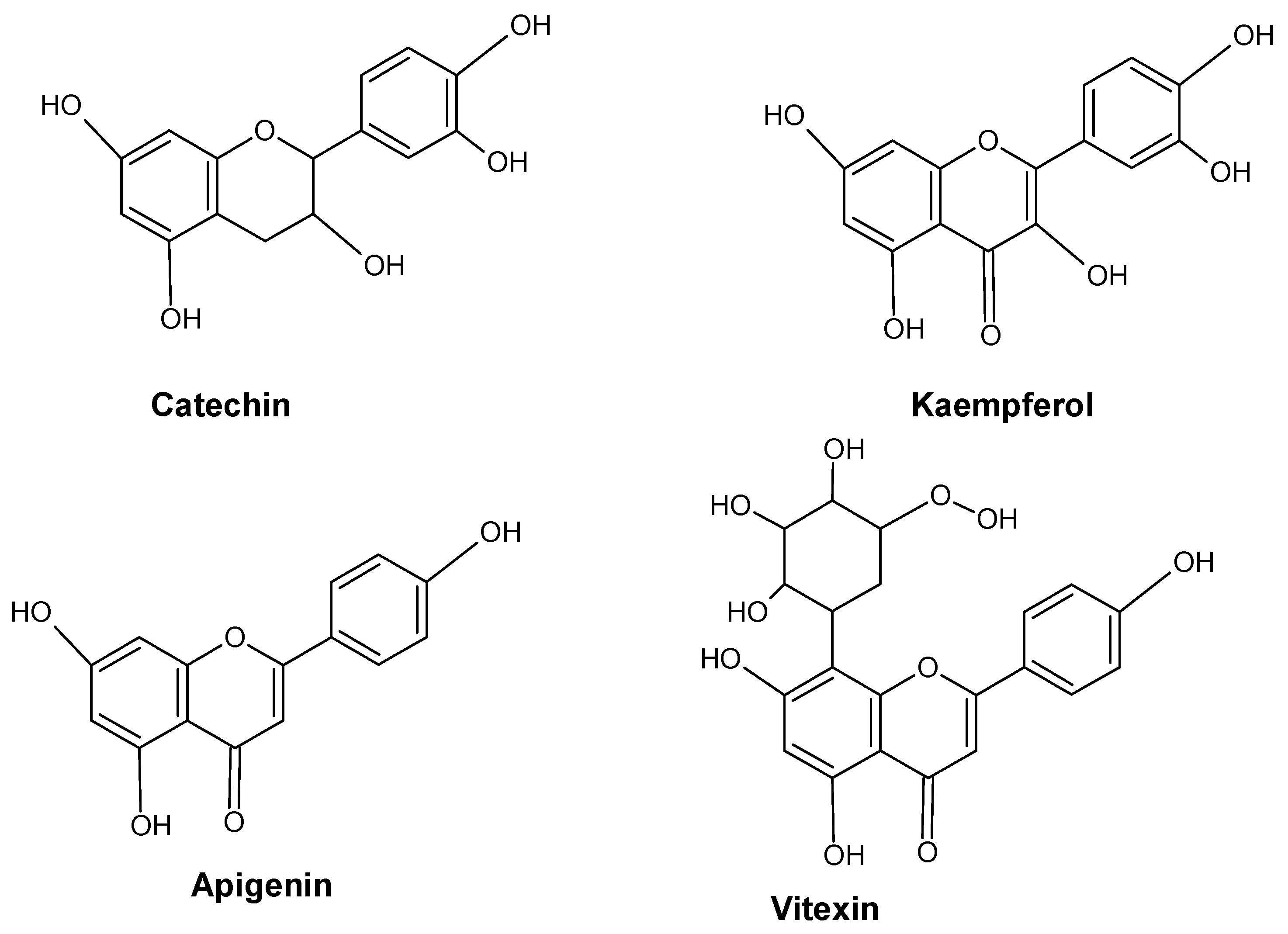
2.1. Flavonoids
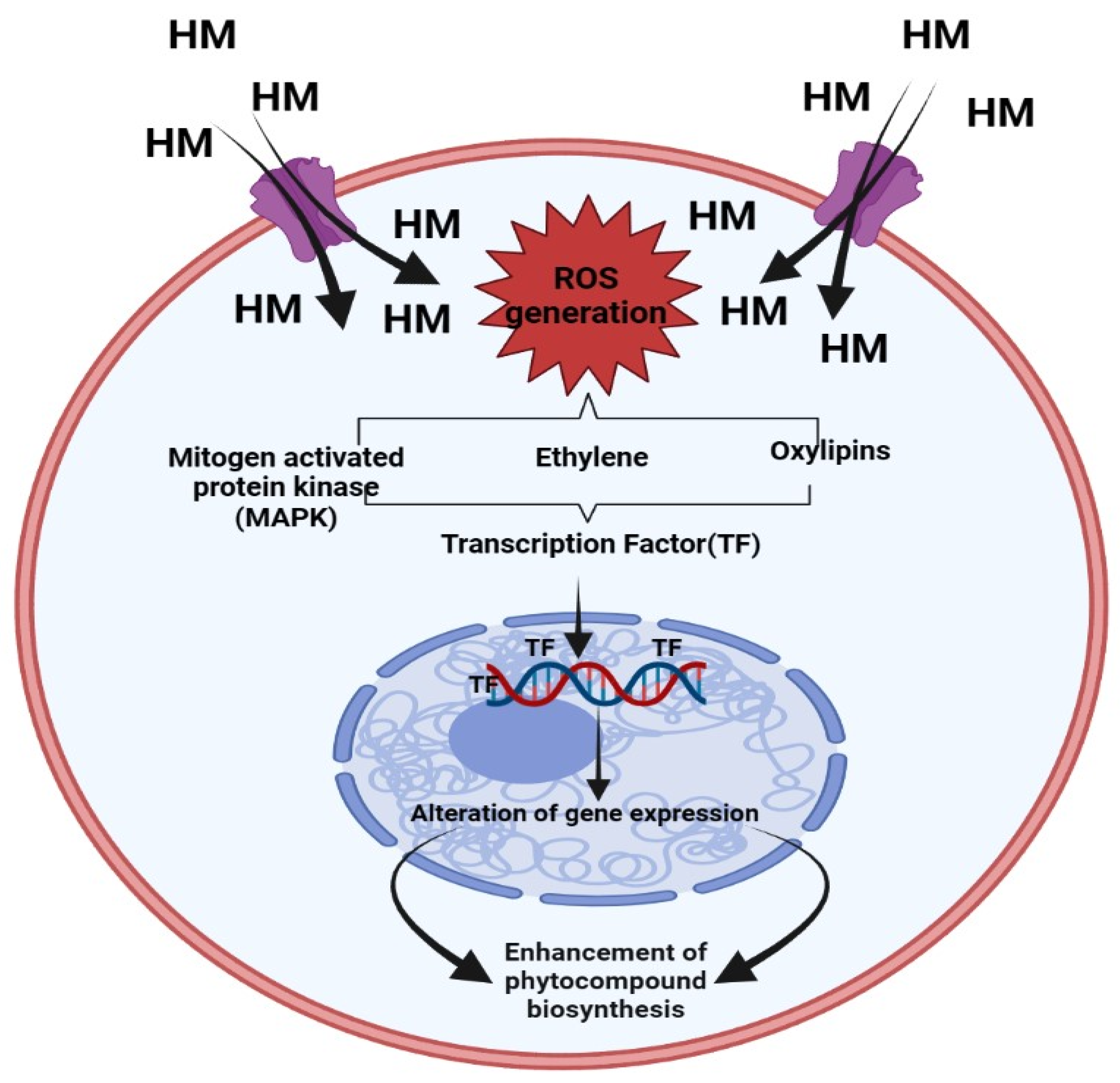
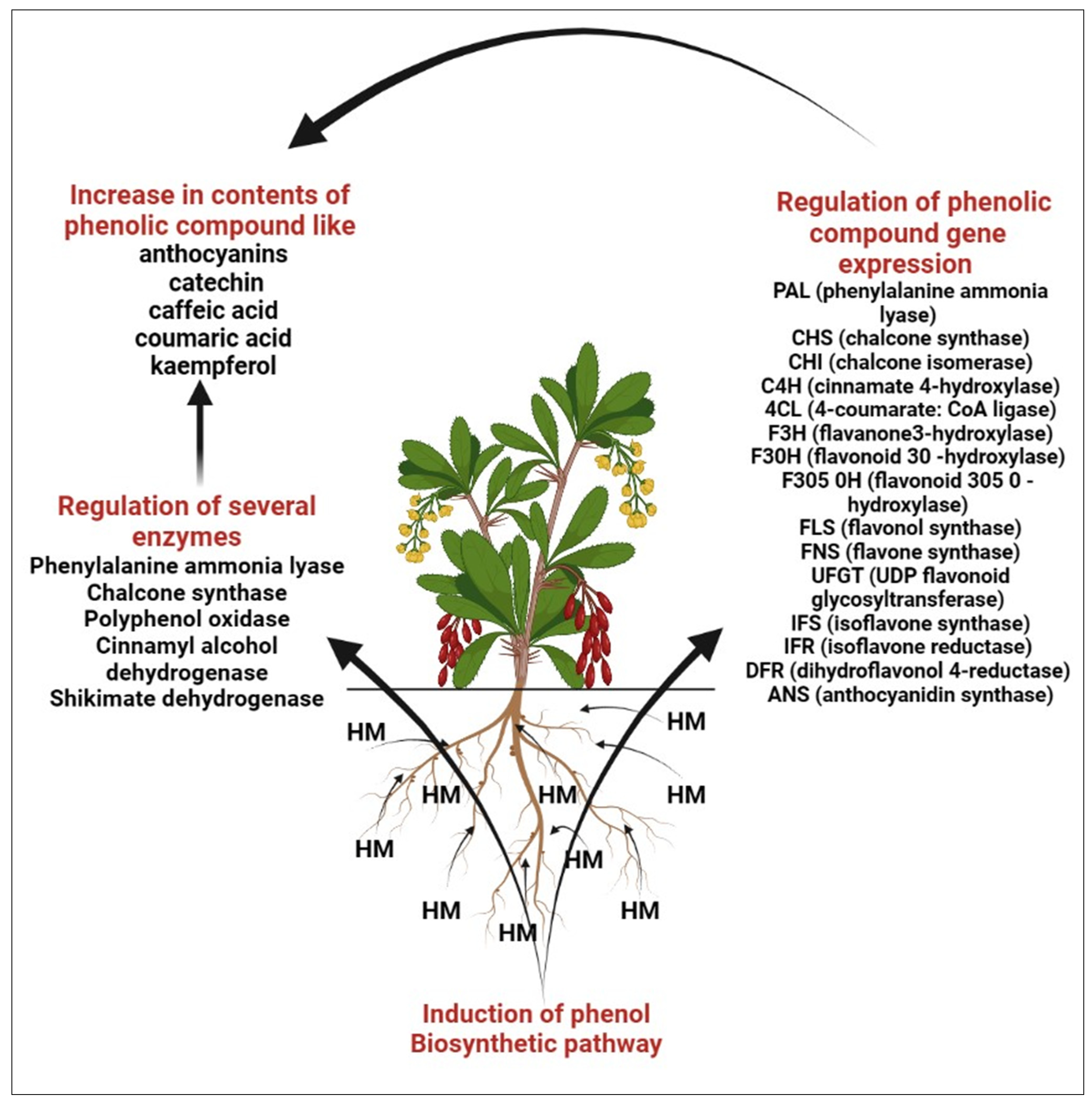
Mechanism of Heavy Metals in Enhancement of Phenolic Compound
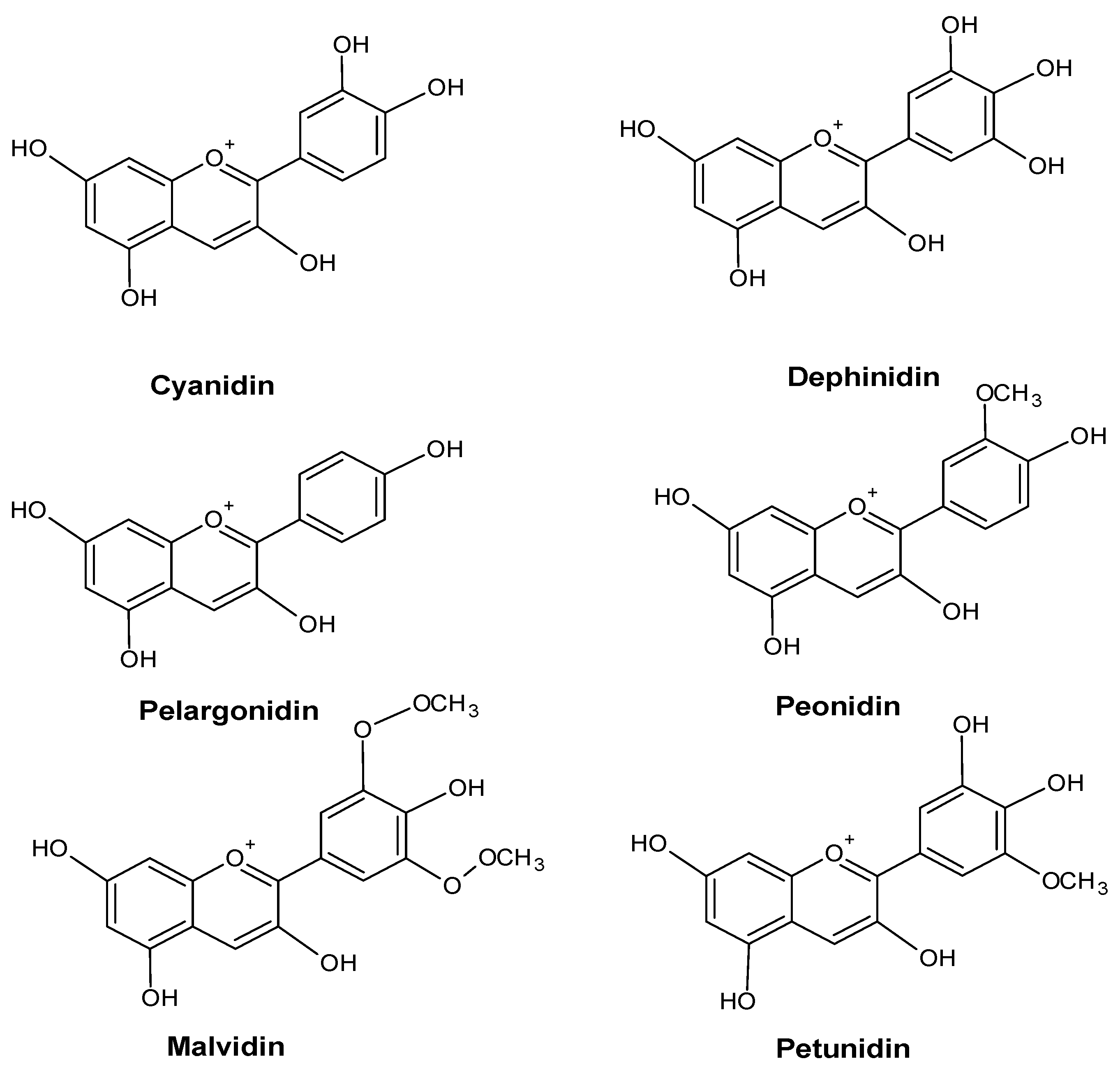
2.2. Anthocyanins
2.3. Tannins
2.4. Phenolcarboxylic Acids
3. Nonmonotonic Dependences of the Content of Phenolic Compounds on the Technogenic Load
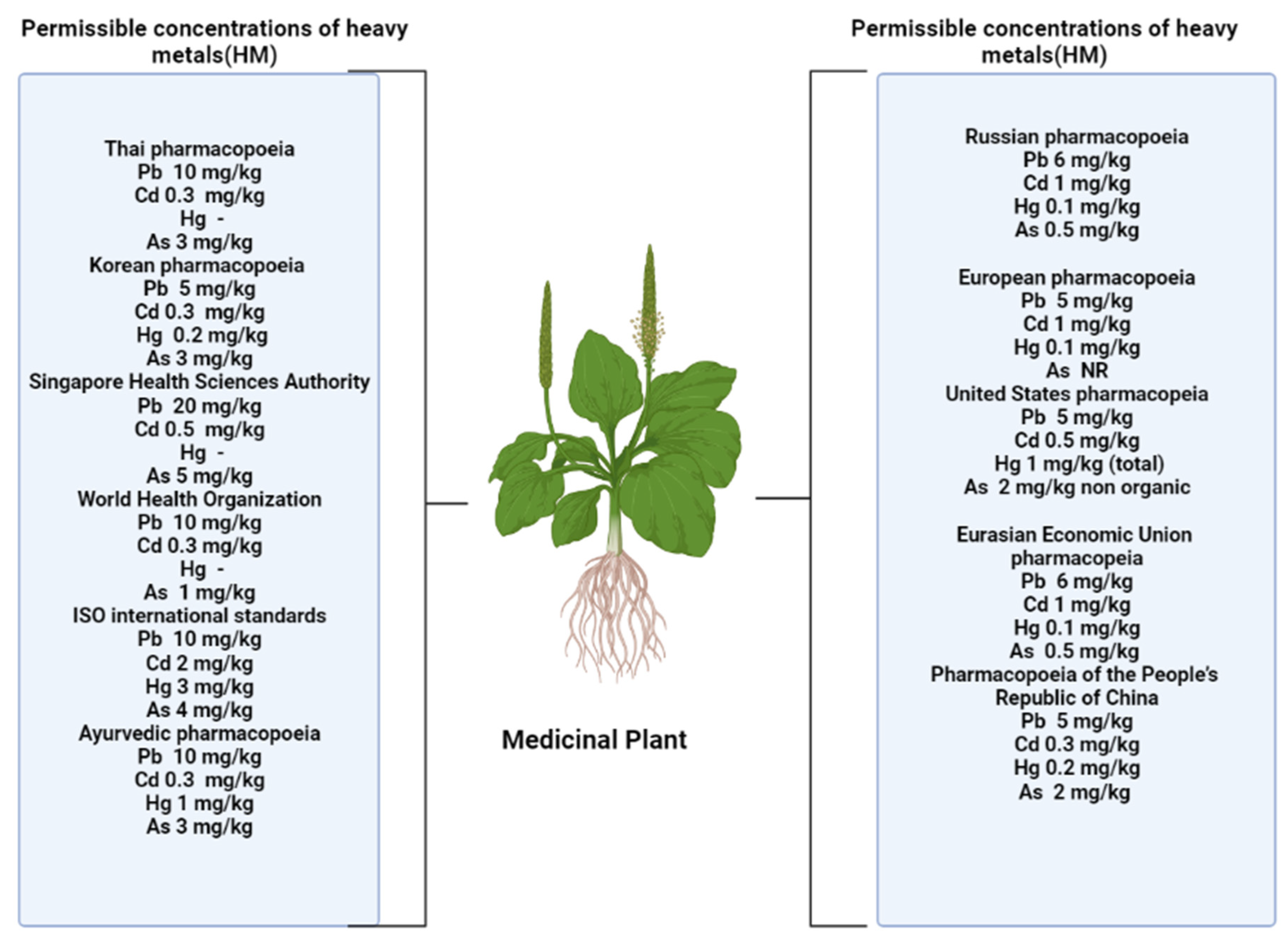
4. Safety of Medicinal Plants, Growing under Technogenic Pressure
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mafimisebi, T.E.; Oguntade, A.E.; Ajibefun, I.A.; Mafimisebi, O.E.; Ikuemonisan, E.S. The expanding market for herbal, medicinal and aromatic plants in Nigeria and the international scene. Med. Aromat. Plants 2013, 144, 2167–2412. [Google Scholar]
- Vinogradova, N.; Glukhov, A.; Chaplygin, V.; Kumar, P.; Mandzieva, S.; Minkina, T.; Rajput, V.D. The content of heavy metals in medicinal plants in various environmental conditions: A review. Horticulturae 2023, 9, 239–253. [Google Scholar] [CrossRef]
- Santiago, L.J.; Louro, R.P.; Olivera, D.E. Compartmentation of phenolic compounds and phenylalanine ammonia-lyase in leaves of Phyllanthus tenellus Roxb. and their Induction by copper sulphate. Ann. Bot. 2000, 86, 1023–1032. [Google Scholar] [CrossRef]
- Karpova, E.A.; Khramova, E.P. Phenolic composition and content of representetives of genus Spirea L. under industrial pollution in Novosibirsk. Contemp. Probl. Ecol. 2014, 7, 228–236. [Google Scholar] [CrossRef]
- Adelisardou, F.; Mederly, P.; Minkina, T. Assessment of soil- and water-related ecosystem services with coupling the factors of climate and land-use change (Example of the Nitra region, Slovakia). Environ. Geochem. Health 2023, 45, 6605–6620. [Google Scholar] [CrossRef]
- Ali, M.A.; Fahad, S.; Haider, I.; Ahmed, N.; Ahmad, S.; Hussain, S.; Arshad, M. Oxidative stress and antioxidant defense in plants exposed to metal/metalloid toxicity. In Reactive Oxygen, Nitrogen and Sulfur Species in Plants: Production, Metabolism, Signaling and Defense Mechanisms; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2019; Volume 1, pp. 353–370. [Google Scholar]
- Anahita, A.; Asmah, R.; Fauziah, O. Evaluation of total phenolic content, total antioxidant activity, and antioxidant vitamin composition of pomegranate seed and juice. Int. Food Res. J. 2015, 22, 1212–1217. [Google Scholar]
- Lukaszewicz, M.; Matysiak-Kata, I.; Skala, J.; Fecka, I.; Cisowski, W.; Szopa, J. Antioxidant capacity manipulation in transgenic Potato tuber by changes in phenolic compounds content. J. Agric. Food Chem. 2004, 52, 1526–1533. [Google Scholar] [CrossRef]
- Guangqiu, Q.; Chongling, Y.; Haoliang, L. Influence of heavy metals on the carbohydrate and phenolics in Mangrove, Aegiceras corniculatum L.; seedlings. Bull. Environ. Contam. Toxicol. 2007, 78, 440–444. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Belghith, T.; Athmouni, K.; Bellassoued, K.; El Feki, A.; Ayadi, H. Physiological and biochemical response of Dunaliella salina to cadmium pollution. J. Appl. Phycol. 2015, 28, 991–999. [Google Scholar] [CrossRef]
- Bialonska, D.; Zobel, A.M.; Kuras, M.; Tykarska, T.; Sawicka-Kapusta, K. Phenolic compounds and cell structure in bilberry leaves affected by Emissions from a Zn–Pb smelter. Water Air Soil. Pollut. 2007, 181, 123–133. [Google Scholar] [CrossRef]
- Pasqualinia, V.; Roblesb, C.; Garzinob, S.; Greffb, S.; Bousquet-Meloub, A.; Bonin, G. Phenolic compounds content in Pinus halepensis Mill. needles: A bioindicator of air pollution. Chemosphere 2003, 52, 239–248. [Google Scholar] [CrossRef]
- Petukhov, A.S.; Petukhova, G.A. Biochemical protective mechanisms in the accumulation of heavy metals in organisms. Gig. I Sanit. 2017, 96, 114–117. [Google Scholar] [CrossRef]
- Furlan, C.M.; Santos, D.Y.; Motta, L.B.; Domingos, M.; Salatino, A. Guava flavonoids and the effects of industrial air pollutants. Atmos. Pollut. Res. 2010, 1, 30–35. [Google Scholar] [CrossRef]
- Yakovleva, A.I.; Okhlopkova, E.D.; Olesova, L.D.; Krivoshapkina, Z.N.; Konstantinova, L.I.; Grigorieva, A.A.; Semenova, E.I.; Efremova, A.V.; Mironova, G.E. Influence of cement plant emissions on medicinal properties of medicinal plants in central Yakutia. Yakutia Med. J. 2019, 2, 38–41. [Google Scholar] [CrossRef]
- Zagurskaya, Y.V.; Siromlya, T.I. Chemical elemental composition of Htpericum perforatum—Standardized chemical elements (As, Cd, Hg, Pb). Khimiya Rastit. Syr’ya 2020, 3, 163–170. [Google Scholar]
- Azzazy, M.F. Plant bioindicators of pollution in Sadat City, Western Nile Delta, Egypt. PLoS ONE 2019, 15, e0226315. [Google Scholar] [CrossRef] [PubMed]
- Rakhmankulova, Z.F.; Shuyskaya, E.V.; Shcherbakov, A.V.; Fedyaev, V.V.; Biktimerova, G.Y.; Khafisova, R.R.; Usmanov, I.Y. Content of proline and flavonoids in the shoots of halophytes inhabiting the South Urals. Russ. J. Plant Physiol. 2015, 62, 71–79. [Google Scholar] [CrossRef]
- Nihal, A.; Mithun, P.R.; Praveen, N. Effect of heavy metals (Hg, As and La) on biochemical constituents of Spinacia oleracea. J. pharmacogn. Phytochem. 2019, 8, 669–674. [Google Scholar]
- Kalugina, O.V.; Afanasyeva, L.V.; Mikhailova, T.A.; Filinova, N.V. Activity of low-molecular weight components of Larix sibirica antioxidant system under exposure to technogenic pollution. Ecotoxicology 2022, 31, 1492–1505. [Google Scholar] [CrossRef] [PubMed]
- Vinogradova, N.A.; Glukhov, A.Z. Ecological and phytochemical features of Crataegus fallacina Klokov under conditions of technogenic pollution. Contemp. Probl. Ecol. 2021, 14, 90–97. [Google Scholar] [CrossRef]
- Furlan, C.M.; Salatino, A.; Domingos, M. Leaf contents of nitrogen and phenolic compounds and their bearing with the herbivore damage to Tibouchina pulchra Cogn. (Melastomataceae), under the influence of air pollutants from industries of Cubatao, Sao Paulo. Braz. J. Bot. 1999, 22, 317–323. [Google Scholar] [CrossRef][Green Version]
- Vinogradova, N.A.; Glukhov, A.Z. Ecological valence of Rosa corymbifera Borkh. to conditions of the technogenic environment. Contemp. Probl. Ecol. 2022, 15, 521–527. [Google Scholar] [CrossRef]
- Nikitina, V.S.; Kuz’mina, L.Y.; Melent’ev, A.I.; Shendel’, G.V. Antibacterial activity of polyphenolic compounds isolated from plants of Geraniaceae and Rosaceae families. Appl. Biochem. Microbiol. 2007, 43, 629–634. [Google Scholar] [CrossRef]
- Lajayer, B.A.; Ghorbanpour, M.; Nikabadi, S. Heavy Metals in Contaminated Environment: Destiny of Secondary Metabolite Biosynthesis, Oxidative Status and Phytoextraction in Medicinal Plants. Ecotoxicol. Environ. Saf. 2017, 145, 377–390. [Google Scholar] [CrossRef]
- Pandey, A.; Agrawal, M.; Agrawal, S.B. Ultraviolet-B and Heavy Metal-Induced Regulation of Secondary Metabolites in Medicinal Plants: A Review. Metabolites 2023, 13, 341. [Google Scholar] [CrossRef]
- Paeizi, M.; Karimi, F.; Razavi, K. Changes in Medicinal Alkaloids Production and Expression of Related Regulatory and Biosynthetic Genes in Response to Silver Nitrate Combined with Methyl Jasmonate in Catharanthus roseus in vitro Propagated Shoots. Plant Physiol. Biochem. 2018, 132, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Nasim, S.A.; Dhir, B. Heavy metals alter the potency of medicinal plants. Rev. Environ. Contam. Toxicol. 2010, 203, 139–149. [Google Scholar]
- AbdElgawad, H.; Zinta, G.; Hamed, B.A.; Selim, S.; Beemster, G.; Hozzein, W.N.; Wadaan, M.A.M.; Asard, H.; Abuelsoud, W. Maize roots and shoots show distinct profiles of oxidative stress and antioxidant defense under heavy metal toxicity. Environ. Pollut. 2020, 258, 113705. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, B.; Tanveer, M.; Che, Z.; Rehman, A.; Cheema, S.A.; Sharma, A.; Song, H.; Rehman, S.U.; Zhaorong, D. Role of 24-epibrassinolide (EBL) in mediating heavy metal and pesticide induced oxidative stress in plants: A review. Ecotoxicol. Environ. Saf. 2018, 147, 935–944. [Google Scholar] [CrossRef]
- Pandey, N.; Sharma, C.P. Effect of heavy metals Co2+, Ni2+ and Cd2+ on growth and metabolism of cabbage. Plant Sci. 2002, 163, 753–758. [Google Scholar] [CrossRef]
- Villiers, F.; Ducruix, C.; Hugouvieux, V.; Jarno, N.; Ezan, E.; Garin, J.; Junot, C.; Bourguignon, J. Investigating the plant response to cadmium exposure by proteomic and metabolomic approaches. Proteomics 2011, 11, 1650–1663. [Google Scholar] [CrossRef]
- Handa, N.; Kohli, S.K.; Sharma, A.; Thukral, A.K.; Bhardwaj, R.; Abd_Allah, E.F.; Alqarawi, A.A.; Ahmad, P. Selenium modulates dynamics of antioxidative defence expression, photosynthetic attributes and secondary metabolites to mitigate chromium toxicity in Brassica juncea L. plants. Environ. Exp. Bot. 2019, 161, 180–192. [Google Scholar] [CrossRef]
- Kohli, S.K.; Handa, N.; Sharma, A.; Gautam, V.; Arora, S.; Bhardwaj, R.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Interaction of 24-epibrassinolide and salicylic acid regulates pigment contents, antioxidative defense responses, and gene expression in Brassica juncea L. seedlings under Pb stress. Environ. Sci. Pollut. Res. 2018, 25, 15159–15173. [Google Scholar] [CrossRef]
- Kaur, R.; Yadav, P.; Sharma, A.; Kumar Thukral, A.; Kumar, V.; Kaur Kohli, S.; Bhardwaj, R. Castasterone and citric acid treatment restores photosynthetic attributes in Brassica juncea L. under Cd(II) toxicity. Ecotoxicol. Environ. Saf. 2017, 145, 466–475. [Google Scholar] [CrossRef]
- Handa, N.; Kohli, S.K.; Sharma, A.; Thukral, A.K.; Bhardwaj, R.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P. Selenium ameliorates chromium toxicity through modifications in pigment system, antioxidative capacity, osmotic system, and metal chelators in Brassica juncea seedlings. S. Afr. J. Bot. 2018, 119, 1–10. [Google Scholar] [CrossRef]
- Mira, L.; Fernandez, M.T.; Santos, M.; Rocha, R.; Florencio, M.H.; Jennings, K.R. Interactions of flavonoids with iron and copper ions: A mechanism for their antioxidant activity. Free. Radic. Res. 2002, 36, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J.; Spencer, J.P.; Rice-Evans, C. Flavonoids: Antioxidants or signalling molecules? Free. Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Cardenas-Sandoval, B.A.; López-Laredo, A.R.; Martínez-Bonfil, B.P.; Bermudez-Torres, K.; Trejo-Tapia, G. Avances en la fitoquímica de Cuphea aequipetala, C. aequipetala var. hispida y C. lanceolata: Extracción y cuantificación de los compuestos fenólicos y actividad antioxidante. Rev. Mex. Ing. Quim. 2012, 11, 401–413. [Google Scholar]
- Leng, X.; Jia, H.; Sun, X.; Shangguan, L.; Mu, Q.; Wang, B.; Fang, J. Comparative transcriptome analysis of grapevine in response to copper stress. Sci. Rep. 2015, 5, 17749. [Google Scholar] [CrossRef] [PubMed]
- Zafari, S.; Sharifi, M.; Ahmadian Chashmi, N.; Mur, L.A. Modulation of Pb-induced stress in Prosopis shoots through an interconnected network of signaling molecules, phenolic compounds and amino acids. Plant Physiol. Biochem. 2016, 99, 11–20. [Google Scholar] [CrossRef]
- Michalak, A. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol. J. Environ. Stud. 2016, 15, 523–530. [Google Scholar]
- Keilig, K.; Ludwig-Mueller, J. Effect of flavonoids on heavy metal tolerance in Arabidopsis thaliana seedlings. Bot. Stud. 2009, 50, 311–318. [Google Scholar]
- Kovácik, J.; Klejdus, B.; Hedbavny, J.; Štork, F.; Bačkor, M. Comparison of cadmium and copper effect on phenolic metabolism, mineral nutrients and stress-related parameters in Matricaria chamomilla plants. Plant Soil. 2009, 320, 231. [Google Scholar] [CrossRef]
- Mishra, B.; Sangwan, N.S. Amelioration of cadmium stress in Withania somnifera by ROS management: Active participation of primary and secondary metabolism. Plant Growth Regul. 2019, 87, 403–412. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [PubMed]
- Oren-Shamir, M.; Levi-Nissim, A. Temperature effects on the leaf pigmentation of Cotinus coggygria ‘Royal Purple’. J. Horti. Sci. 2015, 72, 425–432. [Google Scholar] [CrossRef]
- Kapoor, D.; Singh, M.P.; Kaur, S.; Bhardwaj, R.; Zheng, B.; Sharma, A.M. Modulation of the functional components of growth, photosynthesis, and anti-oxidant stress markers in cadmium exposed Brassica juncea L. Plants 2019, 8, 26–39. [Google Scholar] [CrossRef]
- Tchoupakhina, G.N.; Maslennikov, P.V.; Skrypnik, L.N.; Besserezhnova, M. Reaction of pigmental and antioxidant systems of plant on environmental pollution of Kaliningrad by motor transport emission. Vestn. Tomsk. Gos. Univ. Biol. 2012, 2, 171–185. [Google Scholar]
- Deeva, A.M.; Zubarev, A.V.; Reshetnikov, V.N.; Spiridovich, E.V.; Shabunya, P.S.; Fatykhova, S.A. Anthocyanin content in the flowers of common Lilic varieties (Syringa vulgaris L.). Russ. J. Plant Physiol. 2015, 69, 161–170. [Google Scholar]
- Vasconcelos, M.J.V.; Figueiredo, J.E.F.; Raghothama, K.G. Expression analysis of anthocyanin gene induced under phosphorus starvation in maize genotypes with contrasting phosphorus use efficiency. Genet. Mol. Res. 2018, 17, GMR18036. [Google Scholar] [CrossRef]
- Matos, L.P.; Andrade, H.M.; Marinato, C.S.; Prado, I.G.; Coelho, D.G.; Montoya, D.G.; Kasuya, M.C.; Oliveira, J.A. Limitations to use of Cassia grandis L. in the revegetation of the Areas Impacted with mining tailings from Fundão Dam. Water Air Soil Pollut. 2020, 231, 127–136. [Google Scholar] [CrossRef]
- Feduraev, P.V.; Skrypnik, L.N.; Nebreeva, S.; Dzhobdze, G.; Vatagina, A.; Pungin, A.; Maslennikov, P.V.; Chupahina, G. Variability of phenolic compound accumulation and antioxidant activity in the wild plants of some Rumex species (Poligonaceae). Antioxidants 2022, 11, 311. [Google Scholar] [CrossRef]
- Sandre, A.A.; Pina, J.M.; Moraes, R.M.; Furlan, C.M. Anthocyanins and tannins: Is the urban air pollution an elicitor factor? Braz. J. Bot. 2014, 37, 9–18. [Google Scholar] [CrossRef]
- Lyamzin, V.I.; Bukharina, I.L.; Zdobyakhina, O.V.; Isupova, A.A. Influence of plants in consortium with microorganisms on agrochemical indicators of oil-contaminated soils. Theor. Appl. Ecol. 2022, 4, 166–171. [Google Scholar] [CrossRef]
- Mirzaeva, H.A.; Yunusova, F.M. Content of main active substances in herbs and extraction preparations herbs Thymus marshallianus Willd. Khimiya Rastit. Syr’ya 2017, 2, 75–79. [Google Scholar]
- Das, A.K.; Islam, M.N.; Faruk, O.; Ashaduzzaman, M.; Dungani, R.; Rosamah, E.; Hartati, S.; Rumidatul, A. Hardwood tannin: Sources, utilizations and prospects. In Tannins-Structural Properties, Biological Properties and Current Knowledge; IntechOpen: London, UK, 2019; pp. 1–18. [Google Scholar]
- Katoh, T.; Kasuya, M.; Kagamimori, S.; Kozuka, H.; Kawano, S. Effects of air pollution on tannin biosynthesis and predation damage in Cryptomeria japonica. Phytochemistry 1989, 28, 439–445. [Google Scholar] [CrossRef]
- Nemereshina, O.N.; Shaykhutdinova, A.A. Assessment of heavy metals content in tissues of the Poligonum aviculare L. on technogenically contaminated territories. Ecol. Ind. Russ. 2012, 9, 46–49. [Google Scholar]
- Berni, R.; Luyckx, M.; Xu, X.; Legay, S.; Sergeant, K.; Hausman, J.; Lutts, S.; Cai, G.; Guerriero, G. Reactive oxygen species and heavy metal stress in plants: Impact on the cell wall and secondary metabolism. Environ. Exp. Bot. 2019, 161, 98–106. [Google Scholar] [CrossRef]
- Kang, Y.; Yang, L.; Dai, H.; Xie, M.; Wang, Y.; Peng, J.; Sun, H.; Ao, T.; Chen, W. Antioxidant system response, mineral element uptake and safe utilization of Polygonatum sibiricum in cadmium-contaminated soil. Sci. Rep. 2021, 11, 18737. [Google Scholar] [CrossRef]
- Mengdi, X.; Wenqing, C.; Haibo, D.; Xiaoqing, W.; Li, Y.; Yuchen, K.; Lei, W. Cadmium-induced hormesis effect in medicinal herbs improves the efficiency of safe utilization for low cadmium-contaminated farmland soil. Ecotoxicol. Environ. Saf. 2021, 225, 112724. [Google Scholar] [CrossRef]
- Guo, C.; Lv, L.; Liu, Y.; Ji, M.; Zang, E.; Liu, Q.; Zhang, M.; Li, M. Applied analytical methods for detecting heavy metals in medicinal plants. Crit. Rev. Anal. 2023, 53, 339–359. [Google Scholar] [CrossRef]
- Gautam, M.; Agrawal, M. Influence of metals on essential oil content and composition of lemongrass (Cymbopogon citratus (D.C.) Stapf.) grown under different levels of red mud in sewage sludge amended soil. Chemosphere 2017, 175, 315–322. [Google Scholar] [CrossRef]
- Adamakis, I.S.; Sperdouli, I.; Hanc, A.; Dobrikova, A.; Apostolova, E.; Moustakas, M. Rapid Hormetic Responses of Photosystem II Photochemistry of Clary Sage to Cadmium Exposure. Int. J. Mol. Sci. 2021, 22, 41. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Robyn, B.; Blain, R.B. Hormesis and plant biology. Environ. Pollut. 2009, 157, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Erofeeva, E.A. Estimating the frequency of hormesis and other non-monotonic responses in plants experiencing road traffic pollution in urban areas and experimental pollutant exposure. Environ. Monit. Assess. 2020, 192, 460–477. [Google Scholar] [CrossRef] [PubMed]
- Emshanova, S.V.; Potanina, O.G.; Budanova, E.V.; Chistyakov, V.V. State Pharmacopoeia of the Russian Federation, XIV ed.; Ministry of Health of the Russian Federation: Moscow, Russia, 2018; Volume II, p. 1449.
- Council of Europe. European Directorate for the Quality of Medicines & HealthCare (EDQM); Council of Europe: Strasbourg, France, 2019; Volume 7. [Google Scholar]
- USP44–NF39; 561 Articles of Botanical Origin. United States Pharmacopeia: Rockville, MD, USA, 2020; 15p.
- D’yakova, N.A.; Samylina, I.A.; Slivkin, A.I.; Gaponov, S.P.; Myndra, A.A. Estimated heavy-metal and Arsenic contents in medicinal plant raw materials of the Voronezh region. Pharm. Chem. J. 2018, 52, 220–223. [Google Scholar] [CrossRef]
- Kuzovkova, A.A.; Drebenkova, I.V.; Velentei, Y.N.; Pleshkova, A.A.; Bychok, G.E.; Chernik, D.V.; Maskalevich, N.V. Heavy metal contamination of wild and cultivated medicinal plants in the Republic of Belarus. Occup. Med. Human Ecol. 2020, 4, 112–117. [Google Scholar]
- Babkina, L.A.; Lukyanchikov, D.S.; Lukyanchikova, O.V. Features of the accumulation of heavy metals by plantain leaves. Samara Sci. Bull. 2018, 7, 19–24. [Google Scholar] [CrossRef]
- Siromlya, T.I. Influence of traffic pollution on ecological state of Plantago major L. Contemp. Probl. Ecol. 2011, 4, 499–507. [Google Scholar] [CrossRef]
- Nikulin, A.; Potanina, O.; Alyussef, M.; Vasil’ev, V.; Abramovich, R.; Novikov, O.; Boyko, N.; Khromov, A.; Platonov, E. Development of a technique for determining cadmium, lead, arsenic with the etaas method in medicinal plant raw materials. Farmacia 2021, 69, 566–575. [Google Scholar] [CrossRef]
- Shikov, A.N.; Shikova, V.A.; Whaley, A.O.; Burakova, M.A.; Flisyuk, E.V.; Whaley, A.K.; Terninko, I.I.; Generalova, Y.E.; Gravel, I.V.; Pozharitskaya, O.N. The ability of acid-based natural deep eutectic solvents to co-extract elements from the roots of Glycyrrhiza glabra L. and associated health risks. Molecules 2022, 27, 7690. [Google Scholar] [CrossRef] [PubMed]
- Shchukin, V.M.; Kuzmina, N.E.; Erina, A.A.; Yashkir, V.A.; Merkulov, V.A. Comparative analysis of the heavy metal, Aluminum, and Arsenic contents in brown algae of various origins. Pharm. Chem. J. 2018, 52, 627–634. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinogradova, N.; Vinogradova, E.; Chaplygin, V.; Mandzhieva, S.; Kumar, P.; Rajput, V.D.; Minkina, T.; Seth, C.S.; Burachevskaya, M.; Lysenko, D.; et al. Phenolic Compounds of the Medicinal Plants in an Anthropogenically Transformed Environment. Molecules 2023, 28, 6322. https://doi.org/10.3390/molecules28176322
Vinogradova N, Vinogradova E, Chaplygin V, Mandzhieva S, Kumar P, Rajput VD, Minkina T, Seth CS, Burachevskaya M, Lysenko D, et al. Phenolic Compounds of the Medicinal Plants in an Anthropogenically Transformed Environment. Molecules. 2023; 28(17):6322. https://doi.org/10.3390/molecules28176322
Chicago/Turabian StyleVinogradova, Natalya, Elena Vinogradova, Victor Chaplygin, Saglara Mandzhieva, Pradeep Kumar, Vishnu D. Rajput, Tatiana Minkina, Chandra Shekhar Seth, Marina Burachevskaya, Dionise Lysenko, and et al. 2023. "Phenolic Compounds of the Medicinal Plants in an Anthropogenically Transformed Environment" Molecules 28, no. 17: 6322. https://doi.org/10.3390/molecules28176322
APA StyleVinogradova, N., Vinogradova, E., Chaplygin, V., Mandzhieva, S., Kumar, P., Rajput, V. D., Minkina, T., Seth, C. S., Burachevskaya, M., Lysenko, D., & Singh, R. K. (2023). Phenolic Compounds of the Medicinal Plants in an Anthropogenically Transformed Environment. Molecules, 28(17), 6322. https://doi.org/10.3390/molecules28176322










