Adsorption Behaviors of ctDNA and Biological Activities Based on Polyvinyl Alcohol/Polyethylene Glycol/Quaternized Chitosan Composite Hydrogel
Abstract
1. Introduction
2. Experimental Sections
2.1. Materials
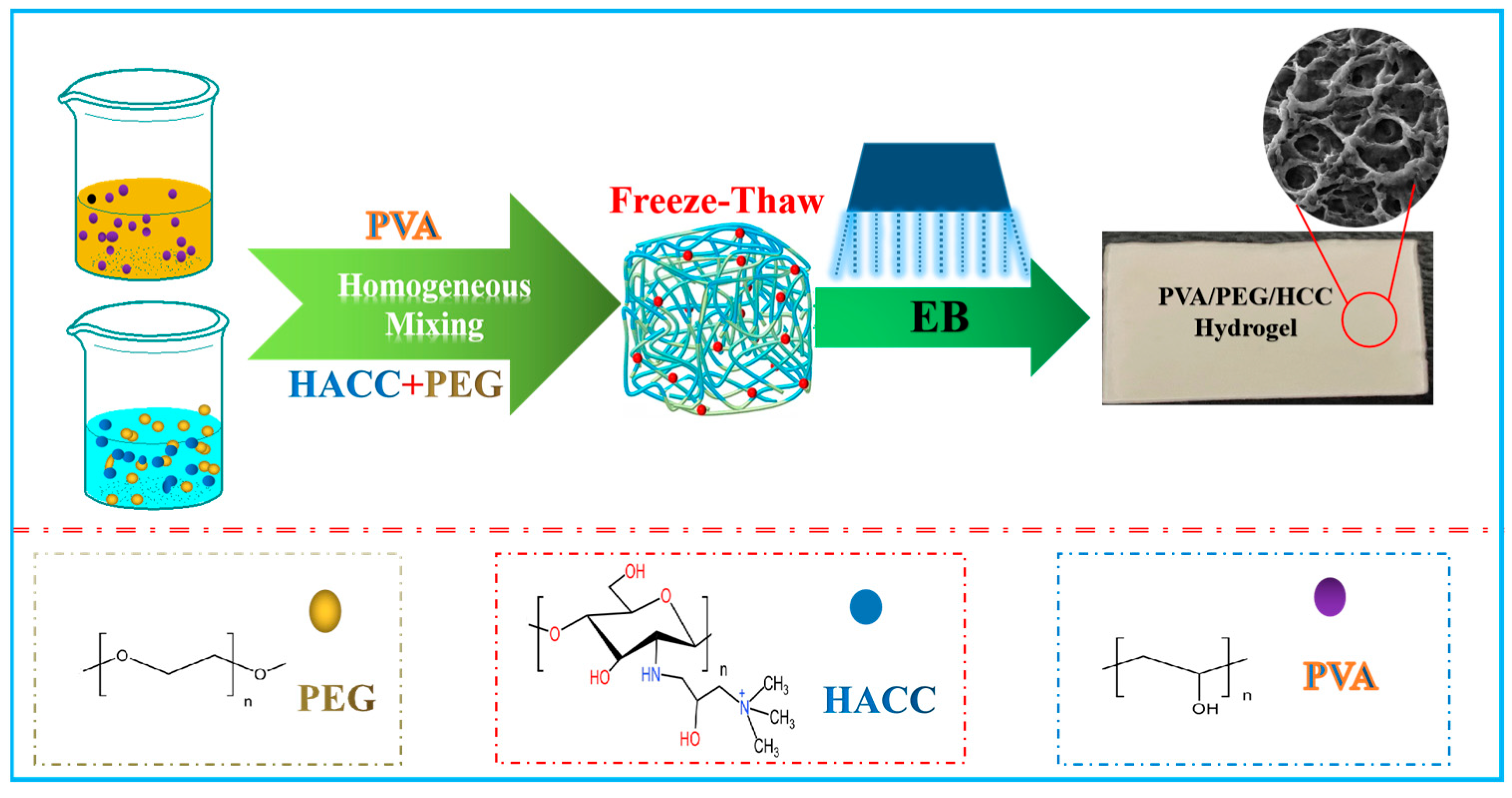
2.2. Radiation Construction of PVA/PEG/HACC Composite Hydrogel
2.3. Material Characterization
2.4. Study on the Adsorption Properties of Hydrogel to ctDNA
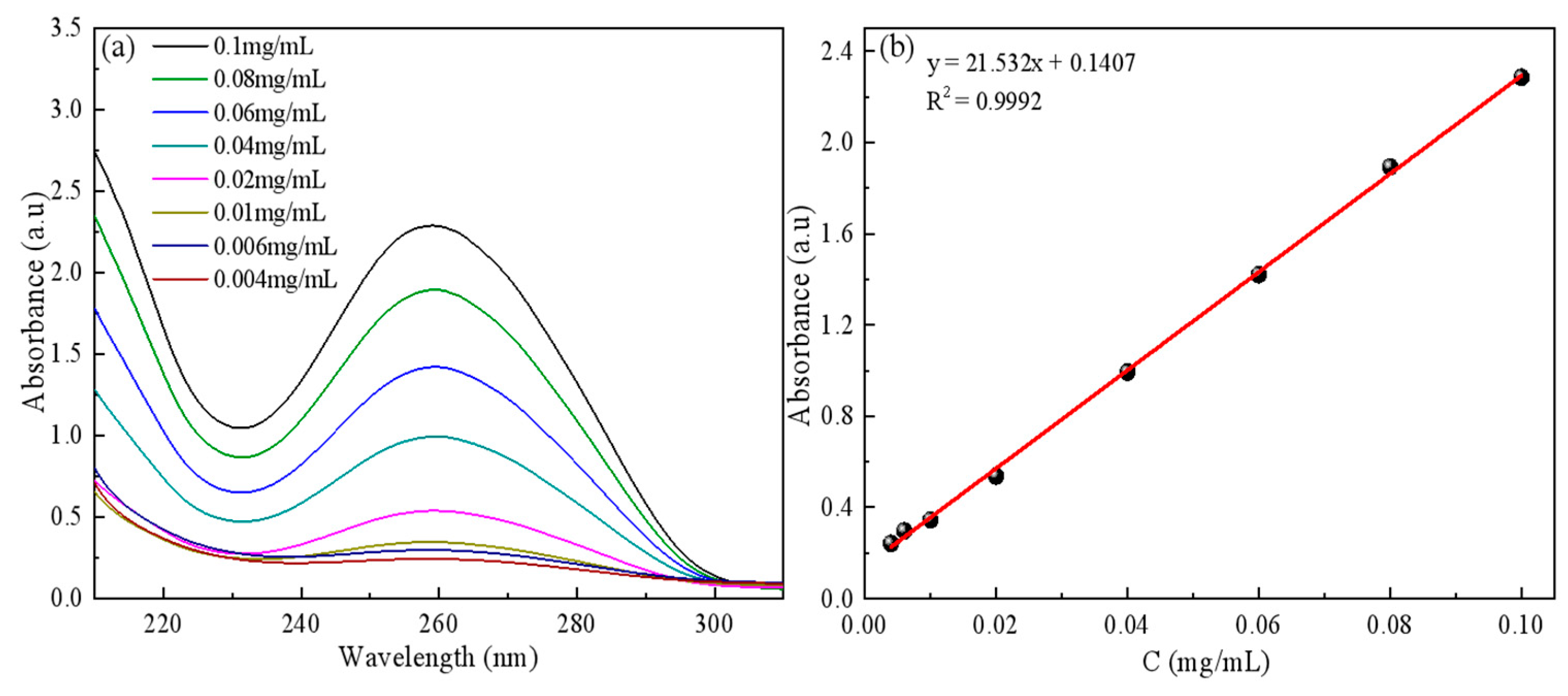
2.4.1. Calf Thymus ctDNA Standard Curves Were Drawn
2.4.2. Adsorption Experiments of ctDNA
2.5. Biological Activity of PVA/PEG/HACC Ternary Composite Hydrogel
2.5.1. Bacterial Culture
2.5.2. Preparation of the Gel Extract
2.5.3. Determination of Antibacterial Properties of Gels
2.5.4. Cell Toxicity Test, Cell Apoptosis Test
2.5.5. Statistical Analysis
3. Results and Discussion
3.1. Radiation Preparation of Composite Hydrogel Proof
3.1.1. FTIR Analysis
3.1.2. XRD Analysis
3.1.3. Specific Surface Area Test (BET)
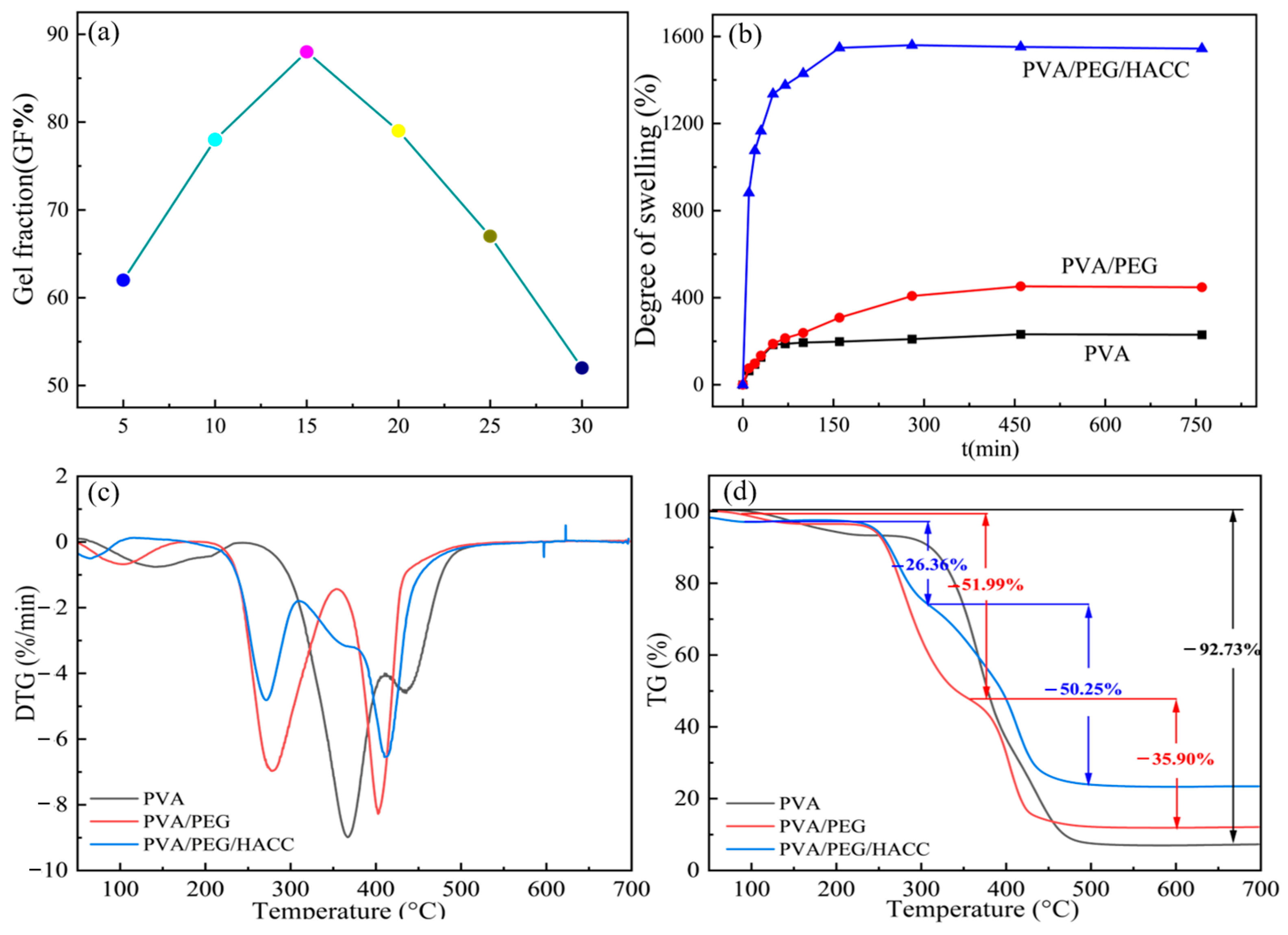
3.1.4. Gel Fraction and Swelling Performance Test
3.1.5. Thermogravimetric Analysis (TGA)
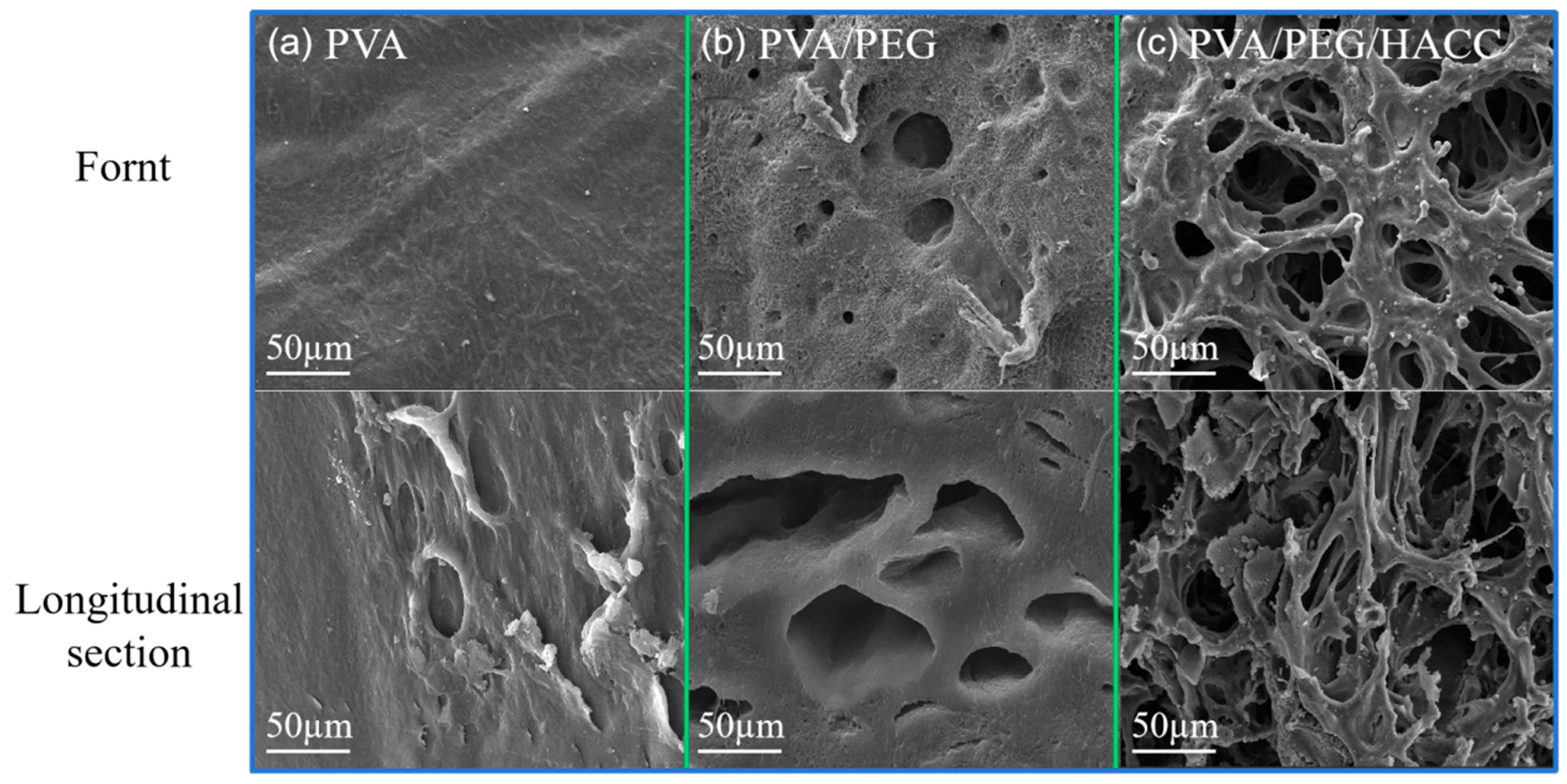
3.1.6. SEM Analysis
3.2. Adsorption Properties of HACC/PVA/PEG Hydrogel
3.2.1. Data Analysis Methods
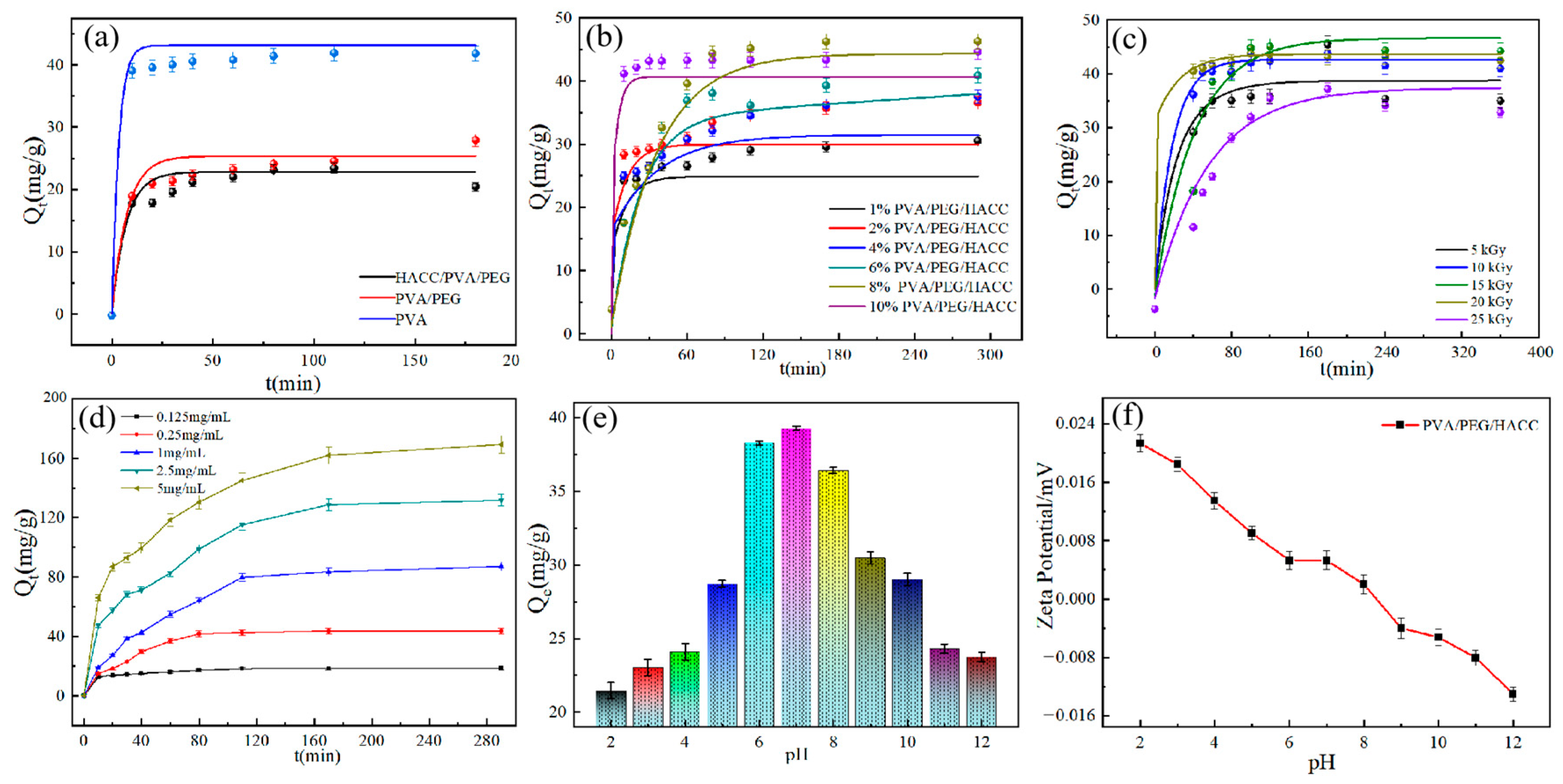
3.2.2. Effect of Different Samples on the Adsorption Efficiency of ctDNA
3.2.3. The Effect of Different Radiation Doses on the Adsorption of ctDNA
3.2.4. Effect of pH on Adsorption and Zeta Potential Determination
3.2.5. Adsorption Kinetics Studies
3.2.6. Adsorption Isotherm Studies
3.2.7. Adsorption Thermodynamic Studies
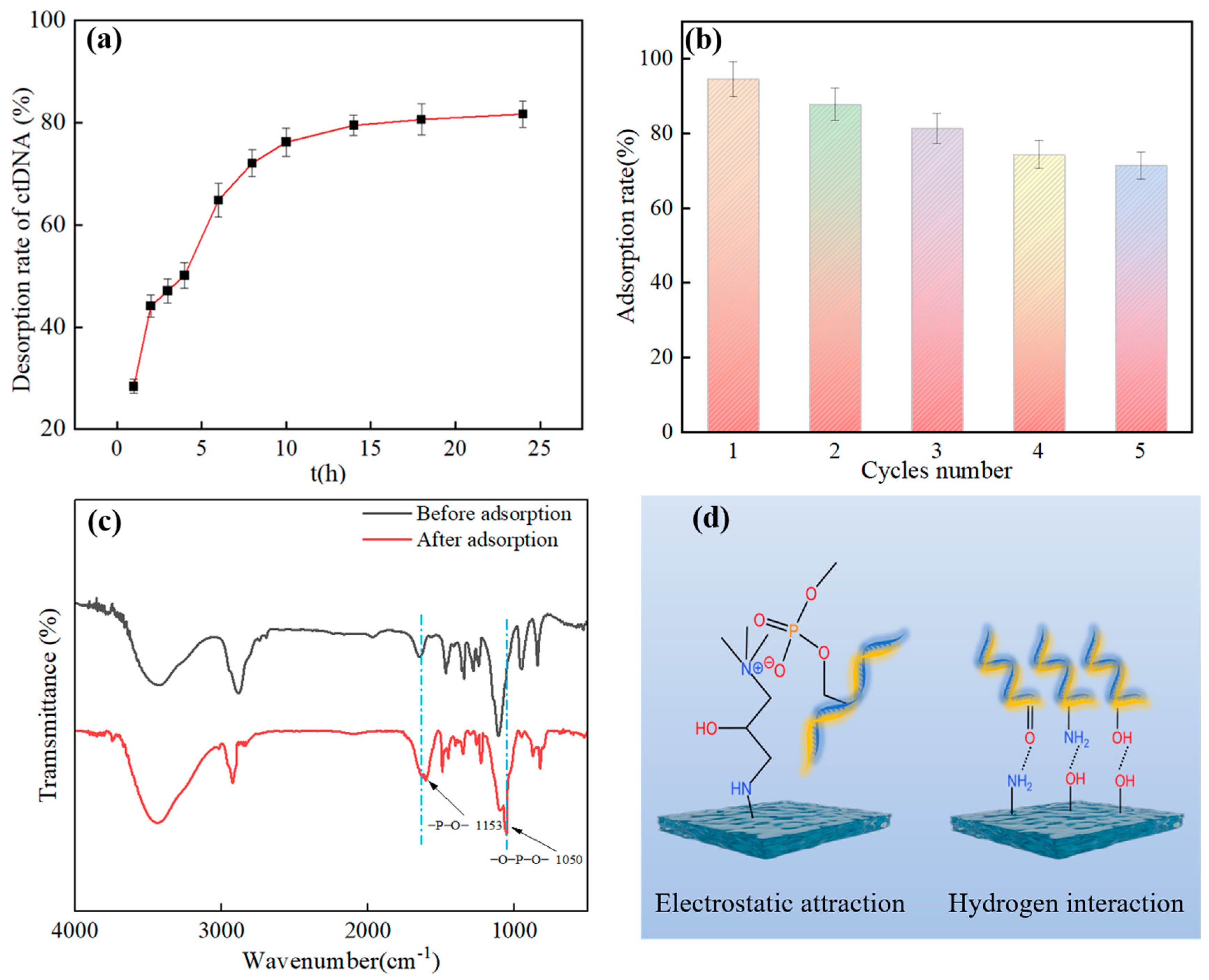
3.2.8. ctDNA Desorption and Hydrogel Regeneration Performance Tests
3.2.9. Adsorption Mechanism Analysis
3.3. Study of the Bioactivity of PVA/PEG/HACC Hydrogel
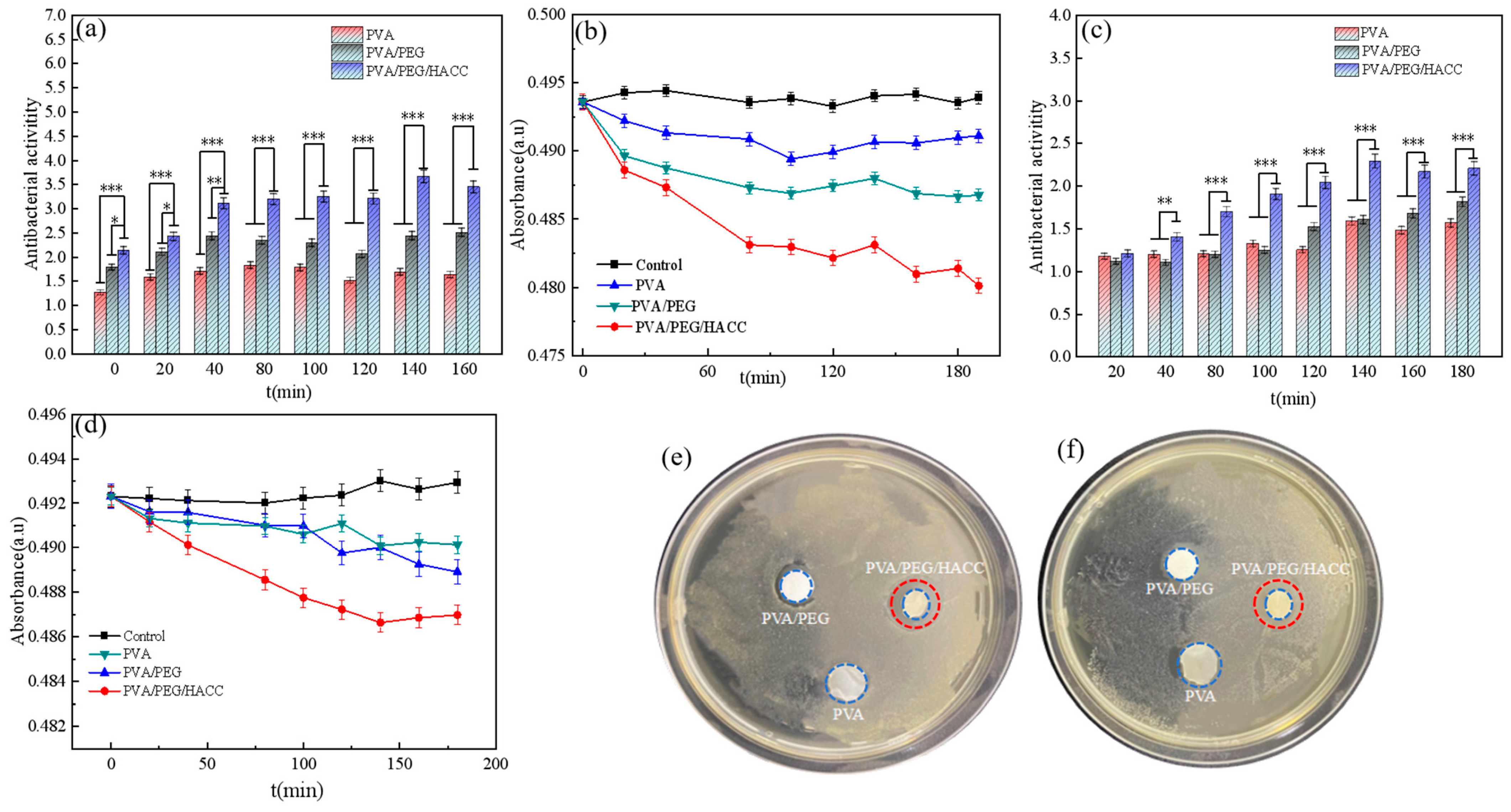
3.3.1. Hydrogel Antibacterial Experiment
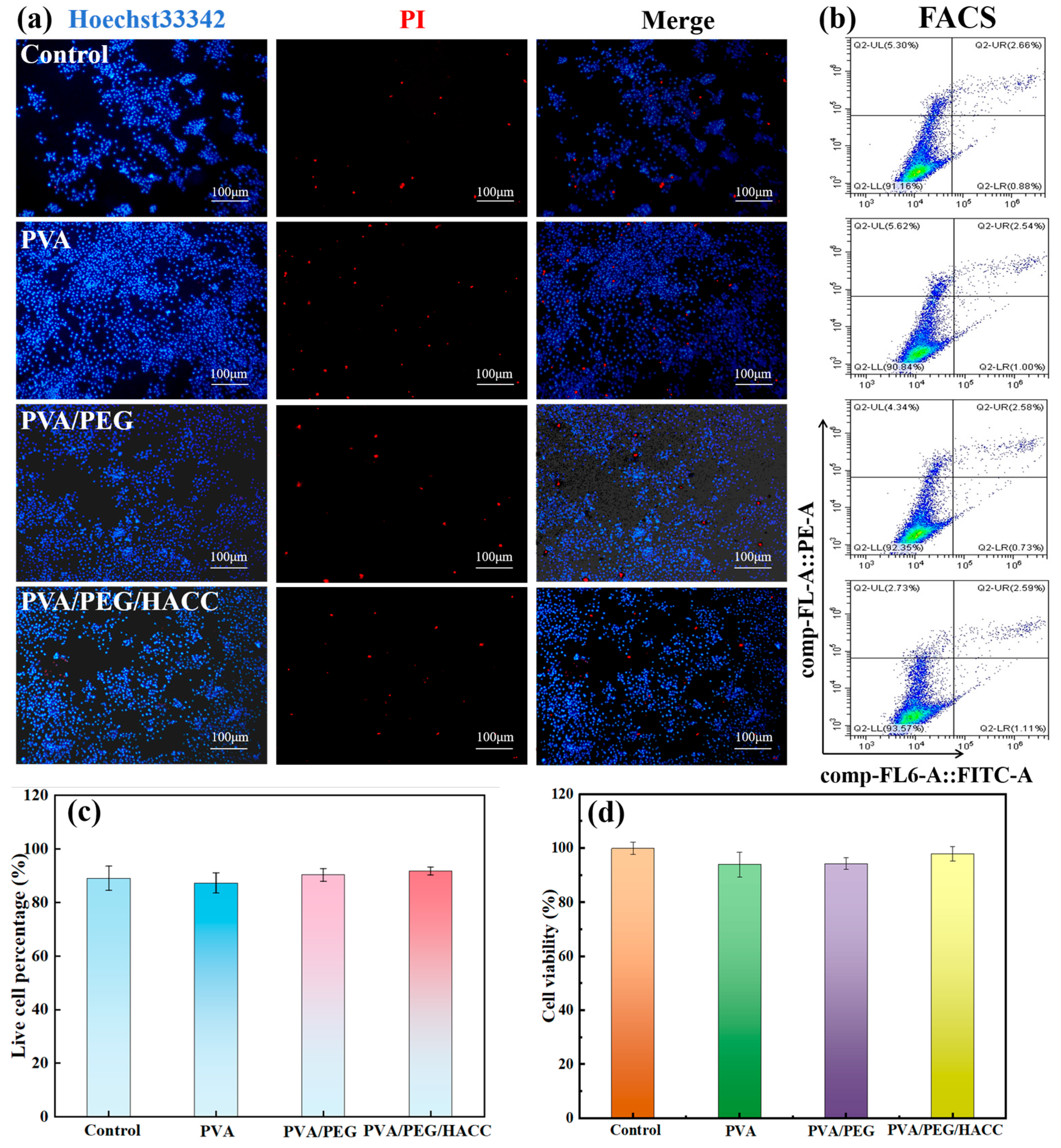
3.3.2. Cell Compatibility
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dao, J.; Conway, P.J.; Subramani, B.; Meyyappan, D.; Russell, S.; Mahadevan, D. Using cfDNA and ctDNA as oncologic markers: A path to clinical validation. Int. J. Mol. Sci. 2023, 17, 13219. [Google Scholar] [CrossRef] [PubMed]
- Eturi, A.; Bhasin, A.; Zarrabi, K.K.; Tester, W.J. Predictive and prognostic biomarkers and tumor antigens for targeted therapy in urothelial carcinoma. Molecules 2024, 29, 1896. [Google Scholar] [CrossRef] [PubMed]
- Temperley, H.C.; Fannon, T.; O’Sullivan, N.J.; O’Neill, M.; Mac Curtain, B.M.; Gilham, C.; Kelly, M.E. Assessing circulating tumour DNA (ctDNA) as a biomarker for anal cancer management: A systematic review. Int. J. Mol. Sci. 2024, 25, 4005. [Google Scholar] [CrossRef] [PubMed]
- Shereef, H.A.; Moemen, Y.S.; Elshami, F.I.; El-Nahas, A.M.; Shaban, S.Y.; Eldik, R. DNA binding and cleavage, stopped-flow kinetic, mechanistic, and molecular docking studies of cationic ruthenium (II) nitrosyl complexes containing “NS4” core. Molecules 2023, 28, 3028. [Google Scholar] [CrossRef]
- Murray, J.C.; Sivapalan, L.; Hummelink, K.; Balan, A.; White, J.R.; Niknafs, N.; Anagnostou, V. Elucidating the heterogeneity of immunotherapy response and immune-related toxicities by longitudinal ctDNA and immune cell compartment tracking in lung cancer. Clin. Cancer Res. 2024, 30, 389–403. [Google Scholar] [CrossRef]
- Abdelrahim, M.; Esmail, A.; Saharia, A.; McMillan, R.; He, A.R.; Starr, J.S.; Ghobrial, R.M. Feasibility of disease recurrence monitoring in liver post-transplantation for patients with hepatocellular carcinoma via personalized and tumor-informed ctDNA test. J. Clin. Oncol. 2022, 40, e16123. [Google Scholar] [CrossRef]
- Han, X.; Tang, X.; Zhu, H.; Zhu, D.; Zhang, X.; Meng, X.; Wang, Z. Short-term dynamics of circulating tumor DNA predicting efficacy of sintilimab plus docetaxel in second-line treatment of advanced NSCLC: Biomarker analysis from a single-arm, phase 2 trial. Immunother. Cancer 2022, 10, e004952. [Google Scholar] [CrossRef]
- Nabet, B.Y.; Esfahani, M.S.; Moding, E.J.; Hamilton, E.G. Noninvasive early identification of therapeutic benefit from immune checkpoint inhibition. Cell 2020, 183, 363–376. [Google Scholar] [CrossRef]
- Cinar, M.; Martinez-Medina, L.; Puvvula, P.K.; Arakelyan, A.; Vardarajan, B.N.; Anthony, N.; Bernal-Mizrachi, L. Retrotransposons facilitate the tissue-specific horizontal transfer of circulating tumor DNA between human cells. bioRxiv 2022, 11, 2022-08. [Google Scholar] [CrossRef]
- Tsai, C.J.; Yang, J.T.; Guttmann, D.M.; Shaverdian, N.; Eng, J.; Yeh, R.; Powell, S.N. Final analysis of consolidative use of radiotherapy to block (CURB) oligoprogression trial-a randomized study of stereotactic body radiotherapy for oligoprogressive metastatic lung and breast cancers. Int. J. Radiat. Oncol. Biol. Phys. 2022, 114, 1061. [Google Scholar] [CrossRef]
- Arisi, M.F.; Dotan, E.; Fernandez, S.V. Circulating tumor DNA in precision oncology and its applications in colorectal cancer. Int. J. Mol. Sci. 2022, 23, 4441. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Mei, C.; Nan, X.; Hui, L. Evaluation and comparison of in vitro degradation kinetics of DNA in serum, urine and saliva: A qualitative study. Gene 2016, 590, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Janabi, A.H.; Kerkhof, L.J.; McGuinness, L.R.; Biddle, A.S.; McKeever, K.H. Comparison of a modified phenol/chloroform and commercial-kit methods for extracting DNA from horse fecal material. J. Microbiol. Methods 2016, 129, 14–19. [Google Scholar] [CrossRef]
- Shi, J.J.; Wu, D.; Liu, T.Z.; Hao, S.J.; Meng, B.C.; Li, S.L.; Liu, Y.N. Comparative of forensic DNA iIdentification using Cell lysis method and magnetic beads method. Fa Yi Xue Za Zhi 2022, 39, 45–49. [Google Scholar] [CrossRef]
- Chakka, R.; Vadaguru Dakshinamurthy, R.; Rawal, P.; Belladamadagu Appajappa, S.; Pramanik, S. Gallic acid a flavonoid isolated from euphorbia hirta antagonizes gamma radiation induced radiotoxicity in lymphocytes in vitro. Complement. Integr. Med. 2023, 20, 146–152. [Google Scholar] [CrossRef]
- Yu, J.; Zhu, J.; Chen, L.; Chao, Y.; Zhu, W.; Liu, Z. A review of adsorption materials and their application of 3D printing technology in the separation process. Chem. Eng. J. 2023, 475, 146247. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Q.; Zhang, G.; Zhao, Y.; Liu, X. Evaluation of separation effect for CH4 enrichment from coalbed methane (CBM) under the synergistic action of temperature and pressure based on IAST theory. GHG Sci. Technol. 2021, 11, 590–605. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, T.; Liu, J.; Huang, J.; Zhou, G.; Hu, S. Synthesis of microgel-reinforced double network hydrogel adsorbent and its adsorption on heavy metals. J. Chem. Technol. Biotechnol. 2023, 98, 1260–1268. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Liu, B.; Liu, J. Adsorption of DNA oligonucleotides by boronic acid-functionalized hydrogel nanoparticles. Langmuir 2024, 35, 13727–13734. [Google Scholar] [CrossRef]
- Wang, Z.J.; Fu, L.L.; Liu, D.L.; Tang, D.X.; Liu, K.; Rao, L.; Yang, J.Y.; Liu, Y.; Li, Y.S.; Chen, H.Q.; et al. Controllable preparation and research progress of photosensitive antibacterial complex hydrogels. Gels 2023, 9, 571. [Google Scholar] [CrossRef]
- Yang, J.Y.; Tang, D.X.; Liu, D.L.; Liu, K.; Yang, X.J.; Li, Y.S.; Liu, Y. Excellent dark/light dual-Mode photoresponsive activities based on g-C3N4/CMCh/PVA nanocomposite hydrogel using electron beam radiation method. Molecules 2023, 28, 7544. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ren, K.; Wang, H.; Wu, L.; Wang, D.; Xu, L. Ga3+ loaded radiation crosslinked gel-Alg-CMC hydrogels for promoting diabetic wound healing. J. Biomater. Appl. 2023, 37, 1676–1686. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.X.; Liu, K.; Yang, J.Y.; Wang, Z.J.; Fu, L.L.; Yang, X.J.; Li, Y.S.; Huang, B.; Liu, Y. Artificial nonenzymatic antioxidant Prussian blue/KGM-BSA nanocomposite hydrogel dressing as ROS scavenging for diabetic wound healing. Int. J. Biol. Macromol. 2024, 266, 131106. [Google Scholar] [CrossRef]
- Ansari, M.J.; Jasim, S.A.; Bokov, D.O.; Thangavelu, L.; Yasin, G.; Khalaji, A.D. Preparation of new bio-based chitosan/Fe2O3/NiFe2O4 as an efficient removal of methyl green from aqueous solution. Int. J. Biol. Macromol. 2022, 198, 128–134. [Google Scholar] [CrossRef]
- Bandura, L.; Panek, R.; Madej, J.; Franus, W. Synthesis of zeolite-carbon composites using high-carbon fly ash and their adsorption abilities towards petroleum substances. Fuel 2021, 283, 119173. [Google Scholar] [CrossRef]
- Jung, K.W.; Lee, S.Y.; Choi, J.W.; Hwang, M.J.; Shim, W.G. Synthesis of Mg-Al layered double hydroxides-functionalized hydrochar composite via an in situ one-pot hydrothermal method for arsenate and phosphate removal: Structural characterization and adsorption performance. Chem. Eng. J. 2021, 420, 129775. [Google Scholar] [CrossRef]
- Rostami, N.; Faridghiasi, F.; Ghebleh, A.; Noei, H.; Samadzadeh, M.; Gomari, M.M.; Smith, B.R. Design, synthesis, and comparison of PLA-PEG-PLA and PEG-PLA-PEG copolymers for curcumin delivery to cancer cells. Polymers 2023, 15, 3133. [Google Scholar] [CrossRef]
- Zhou, W.; Hu, Z.; Wei, J.; Dai, H.; Chen, Y.; Liu, S.; Guo, R. Quantum dots-hydrogel composites for biomedical applications. Chin. Chem. Lett. 2022, 33, 1245–1253. [Google Scholar] [CrossRef]
- Yang, J.Y.; Liu, K.; Wang, Z.J.; Rao, L.; Fu, L.L.; Tang, D.X.; Yang, X.J.; Li, Y.S.; Chen, H.Q.; Liu, Y. Electron beam radiation constructed and improved bone repairing performance based on hierarchical thermoplastic polyurethane/graphene oxide/hydroxyapatite hot melt adhesive with high flow ductility. Polym. Compos. 2024, 23, 4961–4972. [Google Scholar] [CrossRef]
- Demeter, M.; Meltzer, V.; Călina, I.; Scărișoreanu, A.; Micutz, M.; Kaya, M.G.A. Highly elastic superabsorbent collagen/PVP/PAA/PEO hydrogels crosslinked via e-beam radiation. Rad. Phys. Chem. 2020, 174, 108898. [Google Scholar] [CrossRef]
- Torkaman, R.; Maleki, F.; Gholami, M.; Torab-Mostaedi, M.; Asadollahzadeh, M. Assessing the radiation-induced graft polymeric adsorbents with emphasis on heavy metals removing: A systematic literature review. J. Water Process. Eng. 2021, 44, 102371. [Google Scholar] [CrossRef]
- Lahsmin, Y.K.; Heryanto, H.; Ilyas, S.; Fahri, A.N.; Abdullah, B.; Tahir, D. Optical properties determined from infrared spectroscopy and structural properties from diffraction spectroscopy of composites Fe/CNs/PVA for electromagnetic wave absorption. Opt. Mater. 2021, 111, 110639. [Google Scholar] [CrossRef]
- Singh, W.I.; Sinha, S.; Devi, N.A.; Nongthombam, S.; Laha, S.; Swain, B.P. Investigation of chemical bonding and electronic network of rGO/PANI/PVA electrospun nanofiber. Polym. Bull. 2021, 78, 6613–6629. [Google Scholar] [CrossRef]
- Li, S.; Luo, C.; Tang, F.; Xiao, W.; Fang, M.; Sun, J.; Chen, W. Effect of polyethylene glycol modified MWCNTs-OH on the crystallization of PLLA and its stereocomplex. J. Polym. Res. 2022, 29, 162. [Google Scholar] [CrossRef]
- Furer, V.L.; Vandyukov, A.E.; Tripathi, V.; Majoral, J.P.; Caminade, A.M.; Kovalenko, V.I. Vibrational spectroscopic study on polycationic phosphorus dendrimers. Vib. Spectrosc. 2017, 93, 65–77. [Google Scholar] [CrossRef]
- Sanjay, V.; Rajashekara, K.M.; Pattar, V.; Murugendrappa, M.V. Effect on electrical and dielectric properties of Te nanoparticle-doped PVA composite. J. Mater. Sci. Mater. Electron. 2022, 33, 17382–17394. [Google Scholar] [CrossRef]
- Yang, Y.; Kong, W.; Cai, X. Synthesis and characterization of comb-like crosslinking polyurethane based form-stable phase-change materials for thermal energy storage. Polym. Advan. Technol. 2021, 32, 4162–4170. [Google Scholar] [CrossRef]
- Tang, R.; Zhang, Y.; Zhang, Y.; Yu, Z. Synthesis and characterization of chitosan based dye containing quaternary ammonium group. Carbohyd. Polym. 2016, 139, 191–196. [Google Scholar] [CrossRef]
- Ezzati, R.; Azizi, M.; Ezzati, S. A theoretical approach for evaluating the contributions of pseudo-first-order and pseudo-second-order kinetics models in the Langmuir rate equation. Vacuum 2024, 222, 113018. [Google Scholar] [CrossRef]
- Moussavi, S.P.; Kadier, A.; Zaidi, N.S.; Aryanti, P.T.P.; Nugroho, F.A.; Wang, J.; Ma, P.C. Removal of phosphorus from aqueous solution using multi-wall carbon nanotube (MWCNT) as adsorbent: Kinetics and isotherms. Fuller. Nanotub. Carbon Nanostruct. 2022, 30, 589–595. [Google Scholar] [CrossRef]
- Miglioranza, G.; Schwaab, M. Generalization and evaluation of the analytical solution of intraparticle diffusion models in finite batch adsorption. Macromol. React. Eng. 2023, 17, 2300018. [Google Scholar] [CrossRef]
- Ojeme, V.C.; Ayodele, O.; Oluwasina, O.O.; Okoronkwo, E.A. Adsorption of Pb (II) ions from aqueous solutions using chemically treated and untreated cow dung ash. BioResources 2019, 14, 2622–2641. [Google Scholar] [CrossRef]
- Sen, T.K. Adsorptive removal of dye (Methylene blue) organic pollutant from water by pine tree leaf biomass adsorbent. Processes 2023, 11, 1877. [Google Scholar] [CrossRef]
- Zou, M.; Zhang, H.; Miyamoto, N.; Kano, N.; Okawa, H. Adsorption of an anionic surfactant (sodium dodecyl sulfate) from an aqueous solution by modified cellulose with quaternary ammonium. Polymers 2022, 14, 1473. [Google Scholar] [CrossRef]
- Pan, X.H.; Zu, J.H.; Diao, J.J.; Xue, Y.; Liu, S.Y. Rapid and selective recovery of Ag (I) from simulative electroplating effluents by sulfydryl-rich covalent organic framework (COF-SH) with high adsorption capacity. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129156. [Google Scholar] [CrossRef]
- Zeng, L.; Zhou, F.; Hu, R.; Xie, W.; Wang, G.; Yang, C.; Van der Bruggen, B. Synthesis of cross-linked carboxyl modified polyvinyl alcohol and its application in selective adsorption separation of Cu (II) from Cd (II) and Ni (II). J. Polym. Environ. 2021, 29, 28–37. [Google Scholar] [CrossRef]
- Dai, S.; Ye, R.; Huang, J.; Wang, B.; Xie, Z.; Ou, X.; Tian, B. Distinct lipid membrane interaction and uptake of differentially charged nanoplastics in bacteria. J. Nanobiotechnol. 2022, 20, 191. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, C.; Deng, X.; Zhang, J.; Xue, Y.; Zhang, J.; Chen, J. Designing Superhydrophilic Hydrogels as Binder-free catalysts for enhanced oxygen evolution performance. Ind. Eng. Chem. Res. 2023, 62, 5543–5551. [Google Scholar] [CrossRef]
- Murugan, E.; Akshata, C.R.; Ilangovan, R.; Mohan, M. Evaluation of quaternization effect on chitosan-HAP composite for bone tissue engineering application. Colloids Surf. B Biointerfaces 2022, 218, 112767. [Google Scholar] [CrossRef]
- Huang, P.; Zhang, Y.; Wang, B.; Zhu, X.; He, Y.; Song, P.; Wang, R. Synthesis of acryloyl copolymer core-shell microspheres with antibacterial activity and surface cationic effects. J. Mater. Sci. 2023, 58, 7718–7730. [Google Scholar] [CrossRef]
- Logigan, C.L.; Delaite, C.; Popa, M.; Băcăiță, E.S.; Tiron, C.E.; Peptu, C.; Peptu, C.A. Poly (ethylene glycol) methyl ether acrylate-grafted chitosan-based micro-and nanoparticles as a drug delivery system for antibiotics. Polymers 2024, 16, 144. [Google Scholar] [CrossRef]
- Guo, L.; Hu, K.; Wang, H. Antimicrobial and mechanical properties of Ag@Ti3C2Tx-modified PVA composite hydrogels enhanced with quaternary ammonium chitosan. Polymers 2023, 15, 2352. [Google Scholar] [CrossRef]


| Samples | Surface Area (m2/g) | Pore Volume (mL/g) | Pore Size (nm) | Adsorption Average Pore (nm) |
|---|---|---|---|---|
| PVA | 1.0222 | 0.000952 | 117.233 | 7.5252 |
| PVA/PEG | 1.7001 | 0.001947 | 68.499 | 4.3378 |
| PVA/PEG/HACC | 1.9510 | 0.012090 | 48.622 | 4.6639 |
| Kinetic Models | Parameters | Values |
|---|---|---|
| Pseudo-first order | K1 | 0.0349 |
| Qe | 47.8609 | |
| R2 | 0.976 | |
| Pseudo-second order | K2 | 9.1 × 104 |
| Qe | 48.3092 | |
| R2 | 0.9938 | |
| Intra-particle diffusion | Kid1 | 4.625 |
| C1 | 0.564 | |
| R2 | 0.9930 | |
| Kid2 | 3.834 | |
| C2 | 2.334 | |
| R2 | 0.9633 | |
| Kid3 | 0.159 | |
| C3 | 43.131 | |
| R2 | 0.3228 |
| Kinetic models | Parameters | T (k) | ||
|---|---|---|---|---|
| 293.15 | 298.15 | 310.15 | ||
| Langmuir | Qm (mg/g) | 135.135 | 149.254 | 163.934 |
| KL (L/mg) | 0.0555 | 0.0558 | 0.074 | |
| R2 | 0.9968 | 0.9981 | 0.9984 | |
| Freundlich | KF (mg1−1/n·L1/n.g−1) | 109.793 | 103.203 | 117.296 |
| n | 2.606 | 2.795 | 2.894 | |
| R2 | 0.7771 | 0.7349 | 0.7285 | |
| Temperature (K) | ΔG (KJ/mol) | ΔH (KJ/mol) | ΔS (J/mol·K) |
|---|---|---|---|
| 293.15 | −16.564 | 68.238 | 289.280 |
| 298.15 | −18.011 | ||
| 310.15 | −21.482 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, L.; Liu, K.; Yang, J.; Zhao, Y.; Wang, Z.; Tang, D.; Li, Y.; Chen, H. Adsorption Behaviors of ctDNA and Biological Activities Based on Polyvinyl Alcohol/Polyethylene Glycol/Quaternized Chitosan Composite Hydrogel. Molecules 2024, 29, 5770. https://doi.org/10.3390/molecules29235770
Fu L, Liu K, Yang J, Zhao Y, Wang Z, Tang D, Li Y, Chen H. Adsorption Behaviors of ctDNA and Biological Activities Based on Polyvinyl Alcohol/Polyethylene Glycol/Quaternized Chitosan Composite Hydrogel. Molecules. 2024; 29(23):5770. https://doi.org/10.3390/molecules29235770
Chicago/Turabian StyleFu, Lili, Kun Liu, Jinyu Yang, Yuan Zhao, Zhijun Wang, Dongxu Tang, Yuesheng Li, and Huangqin Chen. 2024. "Adsorption Behaviors of ctDNA and Biological Activities Based on Polyvinyl Alcohol/Polyethylene Glycol/Quaternized Chitosan Composite Hydrogel" Molecules 29, no. 23: 5770. https://doi.org/10.3390/molecules29235770
APA StyleFu, L., Liu, K., Yang, J., Zhao, Y., Wang, Z., Tang, D., Li, Y., & Chen, H. (2024). Adsorption Behaviors of ctDNA and Biological Activities Based on Polyvinyl Alcohol/Polyethylene Glycol/Quaternized Chitosan Composite Hydrogel. Molecules, 29(23), 5770. https://doi.org/10.3390/molecules29235770







