Methylophiopogonanone A Inhibits Ferroptosis in H9c2 Cells: An Experimental and Molecular Simulation Study
Abstract
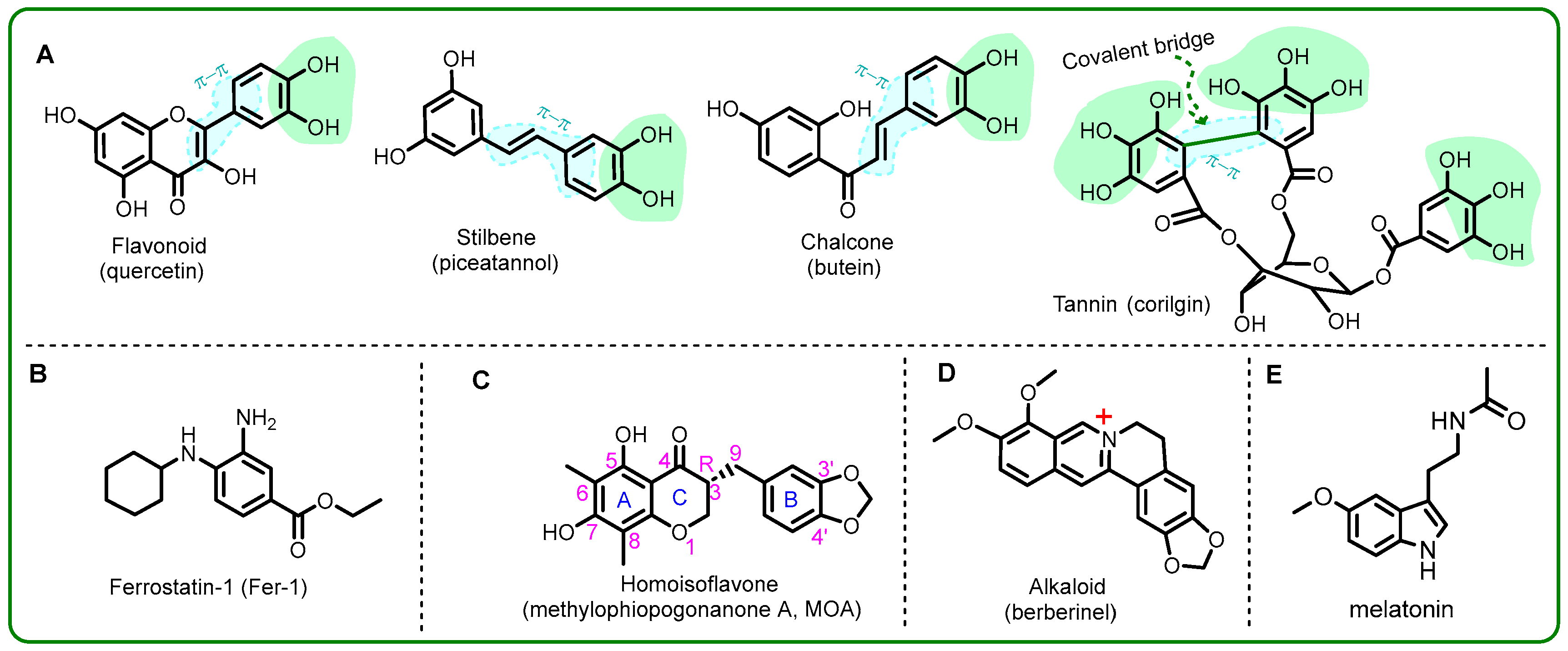
1. Introduction
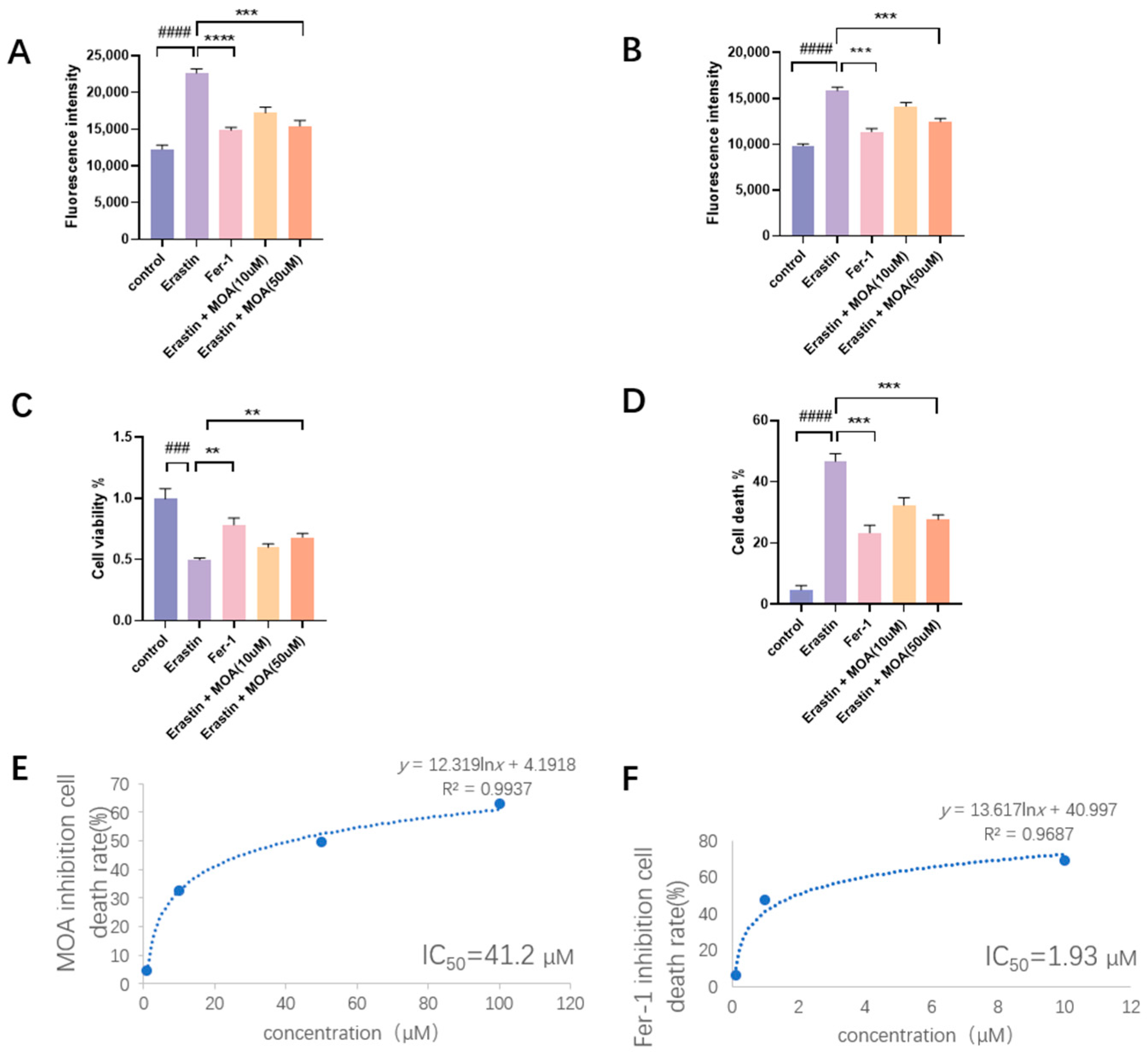
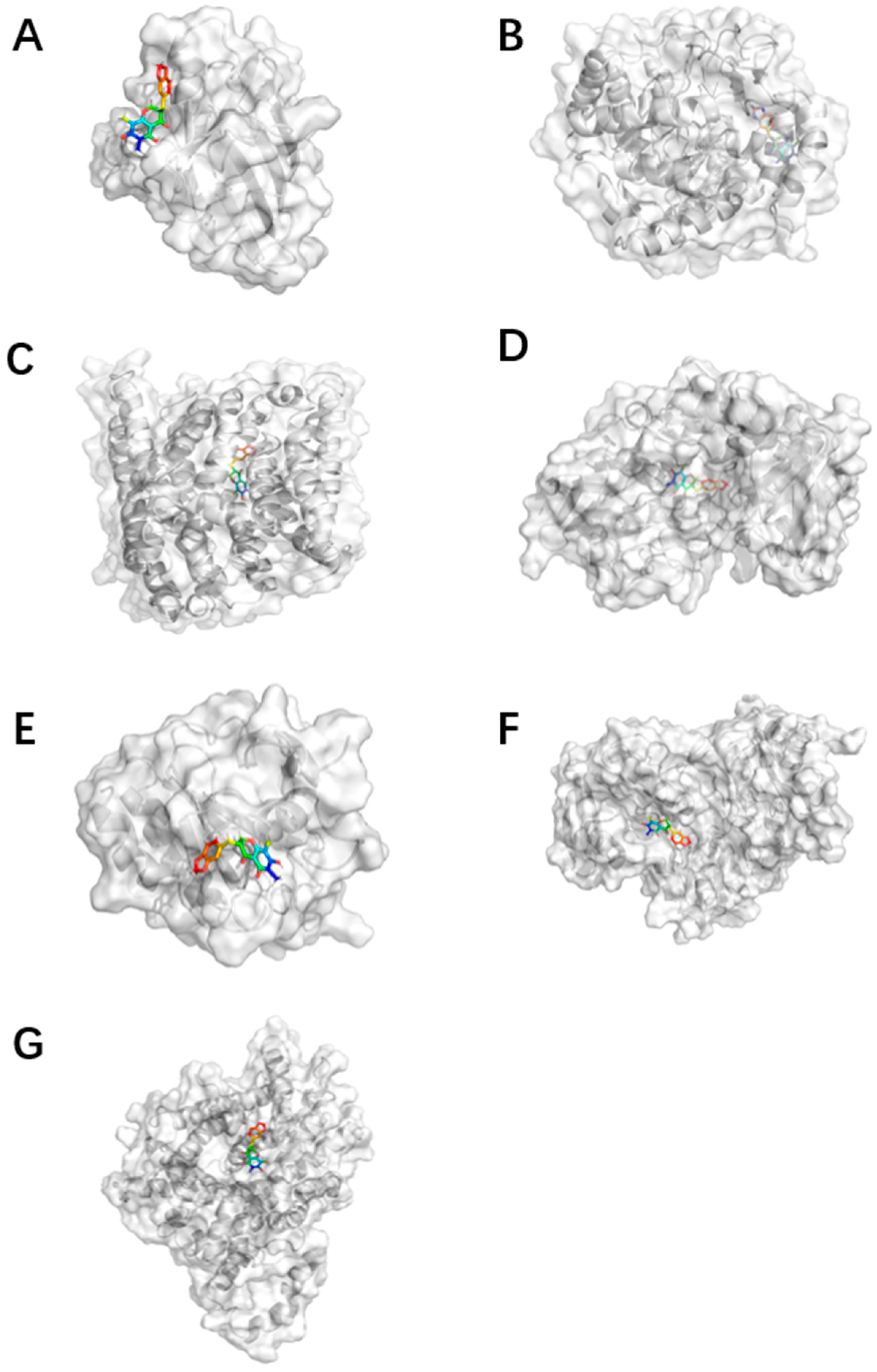
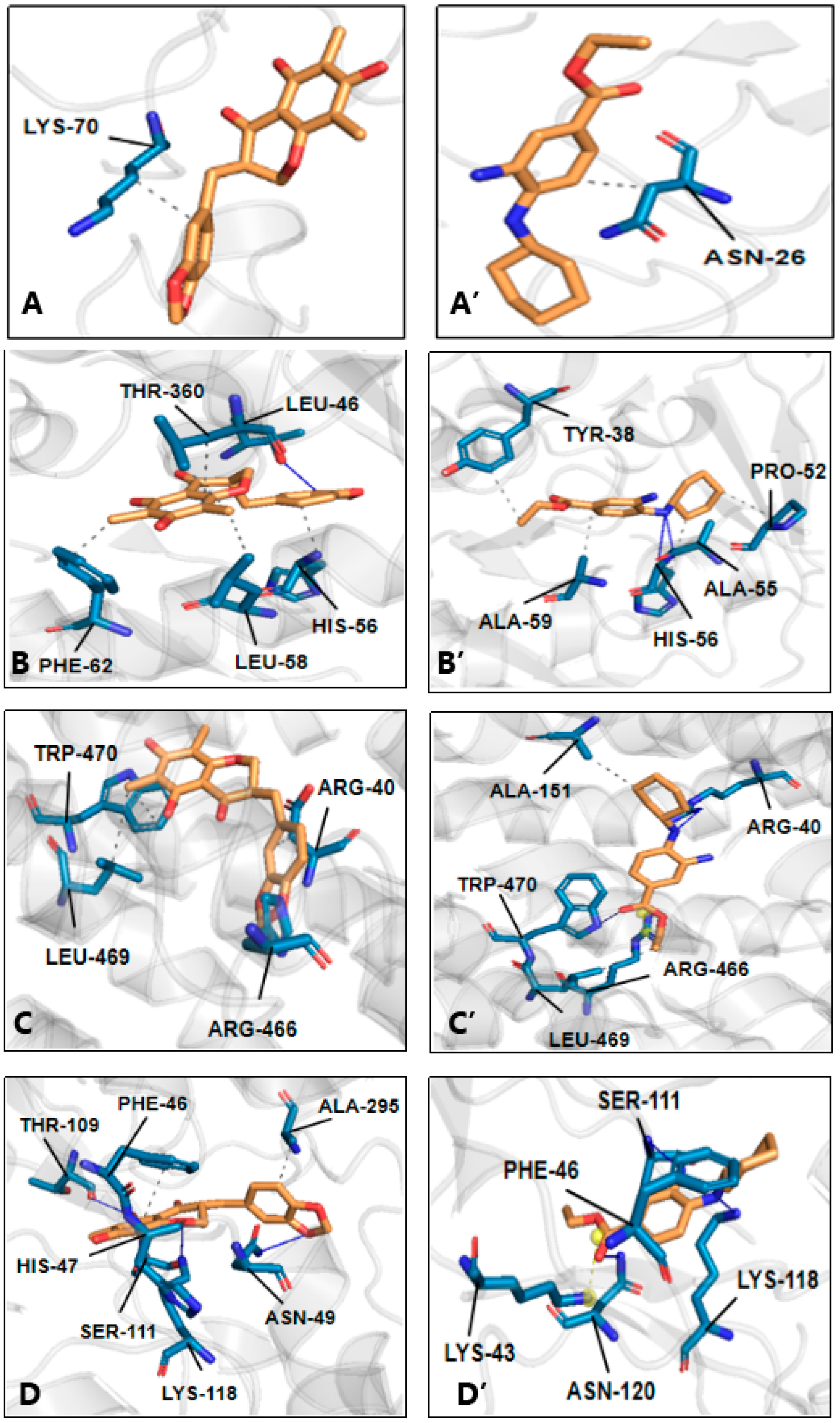
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals and Biological Kits
3.2. Flow Cytometry for the Assessment of LPO Accumulation Using a C11-BODIPY Probe
3.3. Lactate Dehydrogenase (LDH) and CCK-8 Determination
3.4. Characterization of Mitochondrial ROS in Ferroptotic H9c2 Cells
3.5. Flow Cytometry for Determination of the Total Intracellular ROS Using a H2DCFDA Probe
3.6. PTIO•-Scavenging Assay
3.7. ABTS•+-Scavenging Assay
3.8. Molecular Docking Simulation, Molecular Dynamics Simulation, and Molecular Mechanics with Generalized Born and Surface Area Solvation (MM/PBSA) Calculations
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Bai, T.; Li, M.; Liu, Y.; Qiao, Z.; Wang, Z. Inhibition of ferroptosis alleviates atherosclerosis through attenuating lipid peroxidation and endothelial dysfunction in mouse aortic endothelial cell. Free Radi. Biol. Med. 2020, 160, 92–102. [Google Scholar] [CrossRef]
- Chen, X.; Xu, S.; Zhao, C.; Liu, B. Role of TLR4/NADPH oxidase 4 pathway in promoting cell death through autophagy and ferroptosis during heart failure. Biochem. Biophys. Res. Commun. 2019, 516, 37–43. [Google Scholar] [CrossRef]
- Feng, Y.; Madungwe, N.B.; Imam Aliagan, A.D.; Tombo, N.; Bopassa, J.C. Liproxstatin-1 protects the mouse myocardium against ischemia/reperfusion injury by decreasing VDAC1 levels and restoring GPX4 levels. Biochem. Biophys. Res. Commun. 2019, 520, 606–611. [Google Scholar] [CrossRef]
- Li, N.; Wang, W.; Zhou, H.; Wu, Q.; Duan, M.; Liu, C.; Wu, H.; Deng, W.; Shen, D.; Tang, Q. Ferritinophagy-mediated ferroptosis is involved in sepsis-induced cardiac injury. Free Radic. Biol. Med. 2020, 160, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Kong, B.; Qin, T.; Xiao, Z.; Fang, J.; Gong, Y.; Zhu, J.; Liu, Q.; Fu, H.; Meng, H.; et al. Inhibition of ferroptosis reduces susceptibility to frequent excessive alcohol consumption-induced atrial fibrillation. Toxicology 2022, 465, 153055. [Google Scholar] [CrossRef]
- Wang, X.; Chen, X.; Zhou, W.; Men, H.; Bao, T.; Sun, Y.; Wang, Q.; Tan, Y.; Keller, B.B.; Tong, Q.; et al. Ferroptosis is essential for diabetic cardiomyopathy and is prevented by sulforaphane via AMPK/NRF2 pathways. Acta Pharm. Sin. B 2022, 12, 708–722. [Google Scholar] [CrossRef]
- Li, X.; Zeng, J.; Liu, Y.; Liang, M.; Liu, Q.; Li, Z.; Zhao, X.; Chen, D. Inhibitory Effect and Mechanism of Action of Quercetin and Quercetin Diels-Alder anti-Dimer on Erastin-Induced Ferroptosis in Bone Marrow-Derived Mesenchymal Stem Cells. Antioxidants 2020, 9, 205. [Google Scholar] [CrossRef]
- Guo, M.; Du, X.; Wang, X. Inhibition of ferroptosis: A new direction in the treatment of ulcerative colitis by traditional Chinese medicine. J. Ethnopharmacol. 2024, 324, 117787. [Google Scholar] [CrossRef]
- Chen, B.; Li, X.; Ouyang, X.; Liu, J.; Liu, Y.; Chen, D. Comparison of Ferroptosis-inhibitory Mechanisms between Ferrostatin-1 and Dietary Stilbenes (Piceatannol and Astringin). Molecules 2021, 26, 1029. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Cai, R.; Ren, Z.; Zhang, A.; Deng, F.; Chen, D. Simultaneous Study of Anti-Ferroptosis and Antioxidant Mechanisms of Butein and (S)-Butin. Molecules 2020, 25, 674. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Chen, B.; Chen, Y.; Dai, W.; Li, Y.; Zhu, M. Covalent Bridging of Corilagin Improves Antiferroptosis Activity: Comparison with 1,3,6-Tri-O-galloyl-β-D-glucopyranose. ACS Med. Chem. Lett. 2020, 11, 2232–2237. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Hua, Y.; Zhang, W.; Zhou, X.; He, J.; Chen, D. Tannic Acid as a Natural Ferroptosis Inhibitor: Mechanisms and Beneficial Role of 3′-O-Galloylation. ChemistrySelect 2021, 6, 1562–1569. [Google Scholar] [CrossRef]
- Chen, B.; Li, X.; Liu, J.; Li, Y.; Dai, W.; Chen, Y.; Chen, D. Ferroptosis-Inhibitory Effect and Possible Mechanisms of Ellagitannin Geraniin. ChemistryOpen 2021, 10, 737–739. [Google Scholar] [CrossRef]
- Li, X.; Xie, Y.; Xie, H.; Yang, J.; Chen, D. π-π Conjugation Enhances Oligostilbene’s Antioxidant Capacity: Evidence from α-Viniferin and Caraphenol A. Molecules 2018, 23, 694. [Google Scholar] [CrossRef] [PubMed]
- Li, X.C.; Xie, H.; Zhan, R.C.; Chen, D.F. Effect of Double Bond Position on 2-Phenyl-benzofuran Antioxidants: A Comparative Study of Moracin C and Iso-Moracin C. Molecules 2018, 23, 754. [Google Scholar] [CrossRef]
- Li, N.; Zhang, J.-Y.; Zeng, K.-W.; Zhang, L.; Che, Y.-Y.; Tu, P.-F. Anti-inflammatory homoisoflavonoids from the tuberous roots of Ophiopogon japonicus. Fitoterapia 2012, 83, 1042–1045. [Google Scholar] [CrossRef]
- Tharakan, B.; Lin, M.; Sun, W.; Gong, W.; Zhou, Z.; Ding, Y.; Hou, Q. Methylophiopogonanone A Protects against Cerebral Ischemia/Reperfusion Injury and Attenuates Blood-Brain Barrier Disruption In Vitro. PLoS ONE 2015, 10, e0124558. [Google Scholar]
- He, F.; Xu, B.-l.; Chen, C.; Jia, H.-j.; Wu, J.-x.; Wang, X.-c.; Sheng, J.-l.; Huang, L.; Cheng, J. Methylophiopogonanone A suppresses ischemia/reperfusion-induced myocardial apoptosis in mice via activating PI3K/Akt/eNOS signaling pathway. Acta Pharmacol. Sin. 2016, 37, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, M.; Liao, R.; Han, Q. Clinical Significance and Potential Mechanism of Circ_00008842 in Acute Myocardial Infarction. Int. Heart J. 2024, 65, 703–712. [Google Scholar] [CrossRef]
- Lu, T.; Yuan, X.; Sui, C.; Yang, C.; Li, D.; Liu, H.; Zhang, G.; Li, G.; Li, S.; Zhang, J.; et al. Exposure to Polypropylene Microplastics Causes Cardiomyocyte Apoptosis Through Oxidative Stress and Activation of the MAPK-Nrf2 Signaling Pathway. Environ. Toxicol. 2024, 39, 5371–5381. [Google Scholar] [CrossRef]
- Hu, K.; Wang, H.; Wang, H.; Li, T.; Liu, L.; Zhang, H.; Li, Z.; Wang, S.; Han, L. Lipid discovered in American ginseng alleviates doxorubicin-induced cardiotoxicity by inhibiting cardiomyocyte ferroptosis. Fitoterapia 2024, 177, 106097. [Google Scholar] [CrossRef]
- Ying-hao, P.; Yu-shan, Y.; Song-yi, C.; Hua, J.; Peng, Y.; Xiao-hu, C. Time of day-dependent alterations of ferroptosis in LPS-induced myocardial injury via Bmal-1/AKT/Nrf2 in rat and H9c2 cell. Heliyon 2024, 10, e37088. [Google Scholar] [CrossRef]
- Chen, B.; Ouyang, X.; Cheng, C.; Chen, D.; Su, J.; Hua, Y.; Li, X. Bioactive peptides derived from Radix Angelicae sinensis inhibit ferroptosis in HT22 cells through direct Keap1–Nrf2 PPI inhibition. RSC Adv. 2023, 13, 22148–22157. [Google Scholar] [CrossRef]
- Blot, C.; Lavernhe, M.; Lugo-Villarino, G.; Coulson, K.; Salon, M.; Tertrais, M.; Planès, R.; Santoni, K.; Authier, H.; Jacquemin, G.; et al. Leishmania infantum exploits the anti-ferroptosis effects of Nrf2 to escape cell death in macrophages. Cell Rep. 2024, 43, 114720. [Google Scholar] [CrossRef]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of Ferroptotic Cancer Cell Death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef]
- Doll, S.; Freitas, F.P.; Shah, R.; Aldrovandi, M.; da Silva, M.C.; Ingold, I.; Goya Grocin, A.; Xavier da Silva, T.N.; Panzilius, E.; Scheel, C.H.; et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature 2019, 575, 693–698. [Google Scholar] [CrossRef]
- Yuan, H.; Li, X.; Zhang, X.; Kang, R.; Tang, D. Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem. Biophys. Res. Commun. 2016, 478, 1338–1343. [Google Scholar] [CrossRef]
- Han, H.; Zhang, G.; Zhang, X.; Zhao, Q. Nrf2-mediated ferroptosis inhibition: A novel approach for managing inflammatory diseases. Inflammopharmacology 2024, 32, 2961–2986. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Huang, S.; Liu, Y.; Liu, L.; Guo, F.; Guo, Y.; Li, D.; Cen, X.; Chen, Y.; Zhang, M.; et al. Idebenone alleviates doxorubicin-induced cardiotoxicity by stabilizing FSP1 to inhibit ferroptosis. Acta Pharm. Sin. B 2024, 14, 2581–2597. [Google Scholar] [CrossRef]
- Laynes, L.; Raghavamenon, A.C.; Atkins-Ball, D.S.; Uppu, R.M. NADPH Oxidase System Mediates Cholesterol Secoaldehyde-Induced Oxidative Stress and Cytotoxicity in H9c2 Cardiomyocytes. In Cardiovascular Signaling in Health and Disease; Parinandi, N.L., Hund, T.J., Eds.; Springer: Cham, Switzerland, 2022; pp. 213–234. [Google Scholar]
- Krähenbühl, S.; Paech, F.; Abegg, V.; Panajatovic, M.; Alshaikhali, A.; Bouitbir, J. Mechanisms of Cardiotoxicity Associated with Tyrosine Kinase Inhibitors in H9c2 Cells and Mice. Eur. Cardiol. Rev. 2020, 15, e33. [Google Scholar]
- Ouyang, X.; Li, X.; Liu, J.; Liu, Y.; Xie, Y.; Du, Z.; Xie, H.; Ban, C.; Lu, W.; Chen, D. Structure–activity relationship and mechanism of four monostilbenes with respect to ferroptosis inhibition. RSC Adv. 2020, 10, 31171. [Google Scholar] [CrossRef] [PubMed]
- Li, X.C. Improved pyrogallol autoxidation method: A reliable and cheap superoxide-scavenging assay suitable for all antioxidants. J. Agric. Food Chem. 2012, 60, 6418–6424. [Google Scholar] [CrossRef] [PubMed]
- Li, X.C. Solvent effects and improvements in the deoxyribose degradation assay for hydroxyl radical-scavenging. Food Chem. 2013, 141, 2083–2088. [Google Scholar] [CrossRef]
- Wang, Z.-Q.; Li, Y.-Q.; Wang, D.-Y.; Shen, Y.-Q. Natural product piperlongumine inhibits proliferation of oral squamous carcinoma cells by inducing ferroptosis and inhibiting intracellular antioxidant capacity. Transl. Cancer Res. 2023, 12, 2911–2922. [Google Scholar] [CrossRef] [PubMed]
- Li, X. Comparative study of 1,1-Diphenyl-2-picryl-hydrazl Radical (DPPH•) Scavenging Capacity of Antioxidant Xanthones Family. ChemistrySelect 2018, 2, 13081–13085. [Google Scholar] [CrossRef]
- Li, X.; Chen, B.; Zhao, X.; Chen, D. 2-Phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide Radical (PTIO•) Trapping Activity and Mechanisms of 16 Phenolic Xanthones. Molecules 2018, 23, 1692. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.B.; Zhang, L.H.; Yuan, D.P.; Wang, W.; Su, X.M.; Weng, W.X.; Miao, R.; Xu, J.Y.; Long, J.; Song, Y.H. Methylophiopogonanone A Inhibits LPS/ATP-Induced Macrophage Pyroptosis via ROS/NLRP3 Pathway. Mol. Biol. 2023, 57, 106–108. [Google Scholar] [CrossRef]
- Liu, L.; Wang, M.; Gong, N.; Tian, P.; Deng, H. Se improves GPX4 expression and SOD activity to alleviate heat-stress-induced ferroptosis-like death in goat mammary epithelial cells. Anim. Cells Systems 2021, 25, 283–295. [Google Scholar] [CrossRef]
- Ye, Y.; Chen, A.; Li, L.; Liang, Q.; Wang, S.; Dong, Q.; Fu, M.; Lan, Z.; Li, Y.; Liu, X.; et al. Repression of the antiporter SLC7A11/glutathione/glutathione peroxidase 4 axis drives ferroptosis of vascular smooth muscle cells to facilitate vascular calcification. Kidney Int. 2022, 102, 1259–1275. [Google Scholar] [CrossRef]
- Nguyen, K.T.; Mun, S.-H.; Yang, J.; Lee, J.; Seok, O.-H.; Kim, E.; Kim, D.; An, S.Y.; Seo, D.-Y.; Suh, J.-Y.; et al. The MARCHF6 E3 ubiquitin ligase acts as an NADPH sensor for the regulation of ferroptosis. Nat. Cell Biol. 2022, 24, 1239–1251. [Google Scholar] [CrossRef]
- Fang, Y.; Chen, X.; Tan, Q.; Zhou, H.; Xu, J.; Gu, Q. Inhibiting Ferroptosis through Disrupting the NCOA4–FTH1 Interaction: A New Mechanism of Action. ACS Cent. Sci. 2021, 7, 980–989. [Google Scholar] [CrossRef]
- Bersuker, K.; Hendricks, J.M.; Li, Z.; Magtanong, L.; Ford, B.; Tang, P.H.; Roberts, M.A.; Tong, B.; Maimone, T.J.; Zoncu, R.; et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 2019, 575, 688–692. [Google Scholar] [CrossRef]
- Bao, W.-D.; Pang, P.; Zhou, X.-T.; Hu, F.; Xiong, W.; Chen, K.; Wang, J.; Wang, F.; Xie, D.; Hu, Y.-Z.; et al. Loss of ferroportin induces memory impairment by promoting ferroptosis in Alzheimer’s disease. Cell Death Differ. 2021, 28, 1548–1562. [Google Scholar] [CrossRef]
- Mao, C.; Liu, X.; Zhang, Y.; Lei, G.; Yan, Y.; Lee, H.; Koppula, P.; Wu, S.; Zhuang, L.; Fang, B.; et al. DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature 2021, 593, 586–590. [Google Scholar] [CrossRef]
- Wang, B.; Jin, Y.; Liu, J.; Liu, Q.; Shen, Y.; Zuo, S.; Yu, Y. EP1 activation inhibits doxorubicin-cardiomyocyte ferroptosis via Nrf2. Redox Biol. 2023, 65, 102825. [Google Scholar] [CrossRef]
- Hiromatsu, M.; Toshida, K.; Itoh, S.; Harada, N.; Kohashi, K.; Oda, Y.; Yoshizumi, T. Transferrin Receptor is Associated with Sensitivity to Ferroptosis Inducers in Hepatocellular Carcinoma. Ann. Sur. Oncol. 2023, 30, 8675–8689. [Google Scholar] [CrossRef]
- Ma, Z.-G.; Kong, C.-Y.; Wu, H.-M.; Song, P.; Zhang, X.; Yuan, Y.-P.; Deng, W.; Tang, Q.-Z. Toll-like receptor 5 deficiency diminishes doxorubicin-induced acute cardiotoxicity in mice. Theranostics 2020, 10, 11013–11025. [Google Scholar] [CrossRef] [PubMed]
- Fourre, I.; Di Meo, F.; Podloucka, P.; Otyepka, M.; Trouillas, P. Dimerization of quercetin, Diels-Alder vs. radical-coupling approach: A joint thermodynamics, kinetics, and topological study. J. Mol. Model. 2016, 22, 190. [Google Scholar] [CrossRef]
- Ingold, K.U.; Pratt, D.A. Advances in radical-trapping antioxidant chemistry in the 21st century: A kinetics and mechanisms perspective. Chem. Rev. 2014, 114, 9022–9046. [Google Scholar] [CrossRef]
- Zhong, Z.; Zhang, C.; Ni, S.; Ma, M.; Zhang, X.; Sang, W.; Lv, T.; Qian, Z.; Yi, C.; Yu, B. NFATc1-mediated expression of SLC7A11 drives sensitivity to TXNRD1 inhibitors in osteoclast precursors. Redox Biol. 2023, 63, 102711. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Y.; Zhang, W.; Hua, Y.; Chen, B.; Wu, Q.; Chen, D.; Liu, S.; Li, X. Ferroptosis-Inhibitory Difference between Chebulagic Acid and Chebulinic Acid Indicates Beneficial Role of HHDP. Molecules 2021, 26, 4300. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, X.; Cai, J.; Li, Y.; Zou, Y.; Wang, Y.; Xie, C.; Xu, S.; Wang, Y.; Zheng, Y.; et al. Atractylenolide-III alleviates osteoarthritis and chondrocyte senescence by targeting NF-κB signaling. Phytother. Res. 2023, 37, 4607–4620. [Google Scholar] [CrossRef]
- Li, X.C. 2-Phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide (PTIO•) Radical Scavenging: A New and Simple Antioxidant Assay In Vitro. J. Agric. Food Chem. 2017, 65, 6288–6297. [Google Scholar] [CrossRef]
- Chen, B.; Li, X.; Liu, J.; Qin, W.; Liang, M.; Liu, Q.; Chen, D. Antioxidant and Cytoprotective effects of Pyrola decorata H. Andres and its five phenolic components. BMC Complement. Altern. Med. 2019, 19, 275. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Kim, S.; Thiessen, P.A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.; He, J.; He, S.; Shoemaker, B.A.; et al. PubChem Substance and Compound databases. Nucleic Acids Res. 2016, 44, D1202–D1213. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 2002, 98, 11623–11627. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurateab initioparametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Rose, P.W.; Prlić, A.; Altunkaya, A.; Bi, C.; Bradley, A.R.; Christie, C.H.; Costanzo, L.D.; Duarte, J.M.; Dutta, S.; Feng, Z.; et al. The RCSB protein data bank: Integrative view of protein, gene and 3D structural information. Nucleic Acids Res. 2017, 45, D271–D281. [Google Scholar]
- Meller, A.; Ward, M.; Borowsky, J.; Kshirsagar, M.; Lotthammer, J.M.; Oviedo, F.; Ferres, J.L.; Bowman, G.R. Predicting locations of cryptic pockets from single protein structures using the PocketMiner graph neural network. Nat. Commun. 2023, 14, 445a. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Nguyen, T.H.; Pham, T.N.H.; Huy, N.T.; Bay, M.V.; Pham, M.Q.; Nam, P.C.; Vu, V.V.; Ngo, S.T. Autodock Vina Adopts More Accurate Binding Poses but Autodock4 Forms Better Binding Affinity. J. Chem. Inf. Mod. 2019, 60, 204–211. [Google Scholar] [CrossRef]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J.C. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef]
- Wang, J.; Cieplak, P.; Kollman, P.A. How well does a restrained electrostatic potential (RESP) model perform in calculating conformational energies of organic and biological molecules? J. Comput. Chem. 2000, 21, 1049–1074. [Google Scholar] [CrossRef]
- Valdés-Tresanco, M.S.; Valdés-Tresanco, M.E.; Valiente, P.A.; Moreno, E. gmx_MMPBSA: A New Tool to Perform End-State Free Energy Calculations with GROMACS. J. Chem. Theory Comput. 2021, 17, 6281–6291. [Google Scholar] [CrossRef]

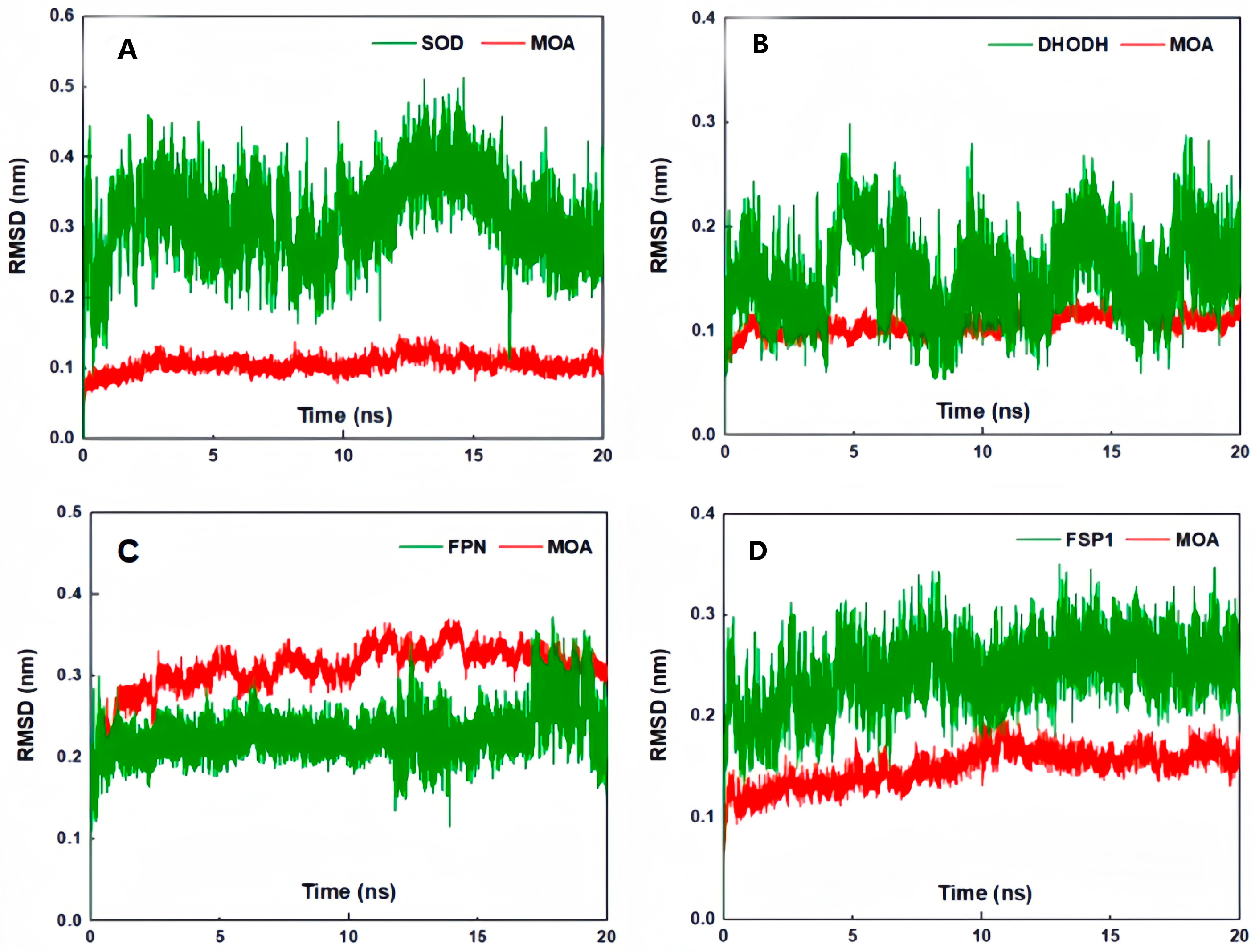
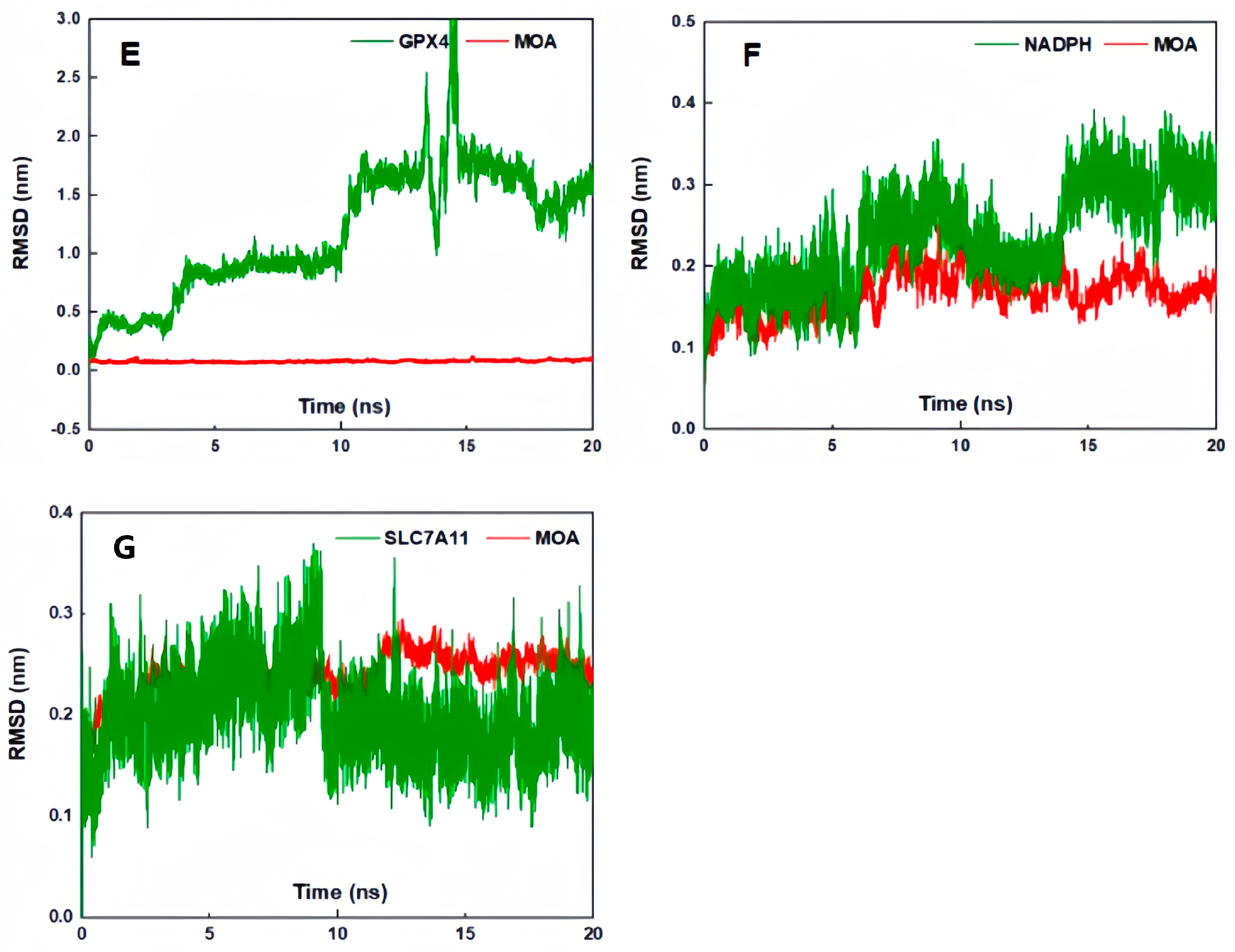

| Enzyme | Binding Energy of MOA | Binding Energy of Fer-1 |
|---|---|---|
| SOD | −6.5 | −5.4 |
| GPX4 | −4.0 | −4.7 |
| DHODH | −10.1 | −8.1 |
| FPN | −8.2 | −6.3 |
| FSP1 | −8.0 | −7.8 |
| NADPH | −9.4 | −8.0 |
| SLC7A11 | −7.1 | −5.7 |
| NRF2 | Unavailable | Unavailable |
| FTH1 | Unavailable | Unavailable |
| System Name | Total | |||||||
|---|---|---|---|---|---|---|---|---|
| SOD and Fer_1 | 0.00 ± 1.14 | 0.00 ± 2.11 | 0.00 ± 1.53 | −0.96 ± 2.73 | −0.18 ± 1.11 | 2.48 ± 2.55 | 0.00 ± 0.01 | 1.34 ± 3.90 |
| SOD and MOA | 0.00 ± 1.60 | 0.00 ± 0.68 | 0.00 ± 1.13 | −54.92 ± 0.55 | −17.37 ± 0.22 | 70.95 ± 1.08 | −2.48 ± 0.02 | −3.82 ± 1.23 |
| GPX4 and Fer_1 | 0.00 ± 2.24 | 0.00 ± 1.75 | 0.00 ± 1.00 | −25.29 ± 0.54 | −15.88 ± 0.47 | 42.95 ± 2.17 | −1.91 ± 0.04 | −0.12 ± 2.28 |
| GPX4 and MOA | 0.00 ± 1.30 | 0.00 ± 1.81 | 0.00 ± 2.37 | −49.11 ± 0.07 | −13.22 ± 0.77 | 46.57 ± 2.39 | −2.14 ± 0.02 | −17.90 ± 2.52 |
| DHODH and Fer_1 | 0.00 ± 1.50 | 0.00 ± 0.77 | 0.00 ± 1.29 | −62.67 ± 2.17 | −30.30 ± 0.24 | 59.71 ± 2.85 | −4.99 ± 0.00 | −38.25 ± 3.59 |
| DHODH and MOA | 0.00 ± 1.77 | 0.00 ± 1.91 | 0.00 ± 0.76 | −41.08 ± 1.62 | −47.53 ± 0.10 | 63.67 ± 1.22 | −5.68 ± 0.06 | −30.63 ± 2.03 |
| FPN and Fer_1 | 0.00 ± 1.17 | 0.00 ± 0.71 | 0.00 ± 1.12 | −153.82 ± 1.93 | −2.19 ± 0.58 | 65.47 ± 1.66 | −4.26 ± 0.01 | −94.80 ± 2.61 |
| FPN and MOA | 0.00 ± 2.25 | 0.00 ± 2.27 | 0.00 ± 1.85 | −17.36 ± 1.36 | −38.60 ± 0.80 | 48.63 ± 1.26 | −4.81 ± 0.01 | −12.14 ± 2.02 |
| FSP1 and Fer_1 | 0.00 ± 1.80 | 0.00 ± 1.56 | 0.00 ± 1.13 | −119.96 ± 2.67 | −18.05 ± 0.65 | 74.53 ± 2.47 | −4.84 ± 0.02 | −68.32 ± 3.70 |
| FSP1 and MOA | 0.00 ± 2.25 | 0.00 ± 2.27 | 0.00 ± 1.85 | −17.36 ± 1.36 | −38.60 ± 0.80 | 48.63 ± 1.26 | −4.81 ± 0.01 | −12.14 ± 2.02 |
| NADPH and Fer_1 | 0.00 ± 2.27 | 0.00 ± 0.82 | 0.00 ± 1.11 | −136.37 ± 0.20 | −24.91 ± 0.58 | 97.85 ± 1.66 | −4.99 ± 0.01 | −68.43 ± 1.77 |
| NADPH and MOA | 0.00 ± 2.10 | 0.00 ± 1.48 | 0.00 ± 1.33 | −57.87 ± 2.42 | −42.39 ± 0.53 | 86.46 ± 2.05 | −4.98 ± 0.01 | −18.78 ± 3.22 |
| SLC7A11 and Fer_1 | 0.00 ± 1.42 | 0.00 ± 4.45 | 0.00 ± 1.55 | −89.79 ± 2.68 | −28.67 ± 0.41 | 61.66 ± 3.63 | −5.22 ± 0.01 | −62.02 ± 4.52 |
| SLC7A11 and MOA | 0.00 ± 2.50 | 0.00 ± 3.07 | 0.00 ± 1.96 | −36.54 ± 0.79 | −30.31 ± 0.41 | 58.20 ± 0.26 | −4.51 ± 0.00 | −13.16 ± 0.93 |
| Target Names | PDB Codes | Pocket Coordinates | Pocket Points | Spacing | Docking Runs |
|---|---|---|---|---|---|
| SOD | 1PU0 | 67.79, 49.86, 12.09 | 40 × 40 × 40 | 0.5 | 100 |
| GPX4 | 5H5Q | 3.28, 24.73, −18.10 | |||
| NADPH | 2CDU | 2.00, −1.25, −0.30 | |||
| FSP1 | 8WIK | 15.62, −4.96, −21.41 | |||
| DHODH | 6LP7 | −33.29, −11.83, 1.31 | |||
| DMT1 | 4YJ0 | −29.95, −17.72, −26.31 | |||
| FTH1 | 4OYN | 37.10, 19.79, 29.48 | |||
| FPN | 8C03 | 117.51, 120.35, 121.50 | |||
| SLC7A11 | 7EPZ | 148.71, 147.14, 119.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhao, X.; Chen, B.; Chen, S.; Liang, Y.; Chen, D.; Li, X. Methylophiopogonanone A Inhibits Ferroptosis in H9c2 Cells: An Experimental and Molecular Simulation Study. Molecules 2024, 29, 5764. https://doi.org/10.3390/molecules29235764
Wang Y, Zhao X, Chen B, Chen S, Liang Y, Chen D, Li X. Methylophiopogonanone A Inhibits Ferroptosis in H9c2 Cells: An Experimental and Molecular Simulation Study. Molecules. 2024; 29(23):5764. https://doi.org/10.3390/molecules29235764
Chicago/Turabian StyleWang, Yanqing, Xi Zhao, Ban Chen, Shaoman Chen, Yongbai Liang, Dongfeng Chen, and Xican Li. 2024. "Methylophiopogonanone A Inhibits Ferroptosis in H9c2 Cells: An Experimental and Molecular Simulation Study" Molecules 29, no. 23: 5764. https://doi.org/10.3390/molecules29235764
APA StyleWang, Y., Zhao, X., Chen, B., Chen, S., Liang, Y., Chen, D., & Li, X. (2024). Methylophiopogonanone A Inhibits Ferroptosis in H9c2 Cells: An Experimental and Molecular Simulation Study. Molecules, 29(23), 5764. https://doi.org/10.3390/molecules29235764






