Mechanical Synthesis and Calorimetric Studies of the Enthalpies of Formation of Chosen Mg-Pd Alloys
Abstract
1. Introduction
2. Results and Discussion
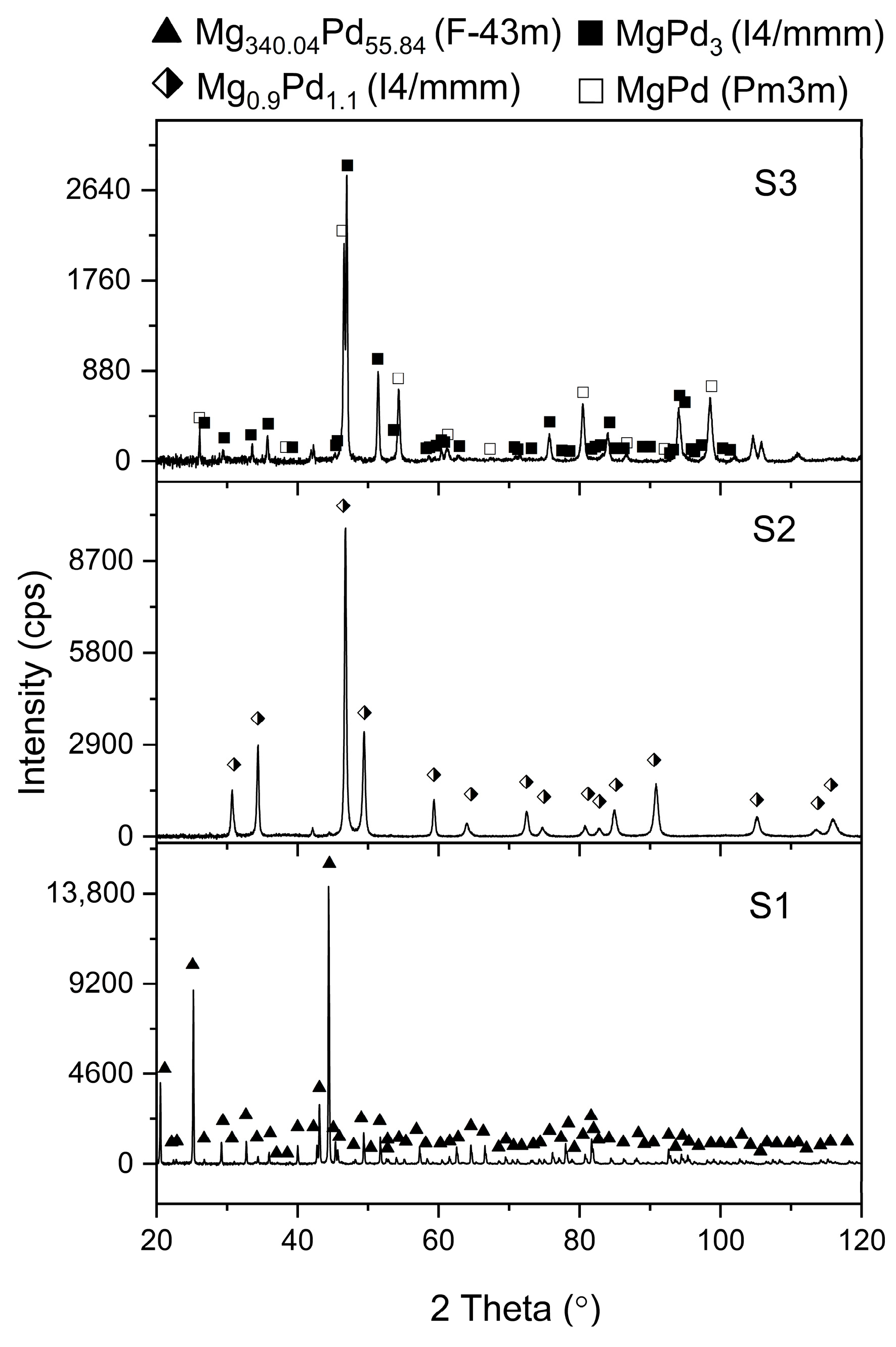
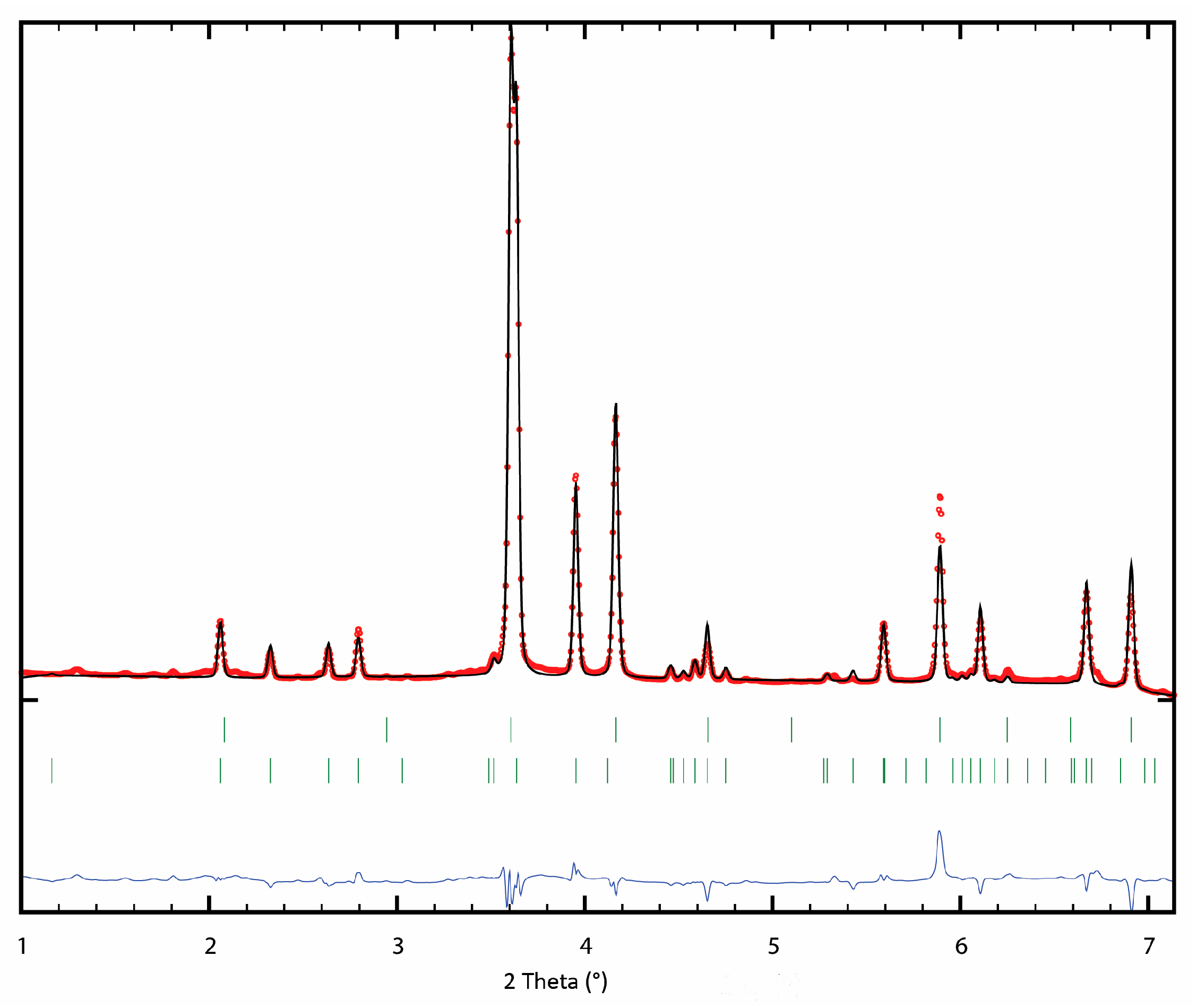
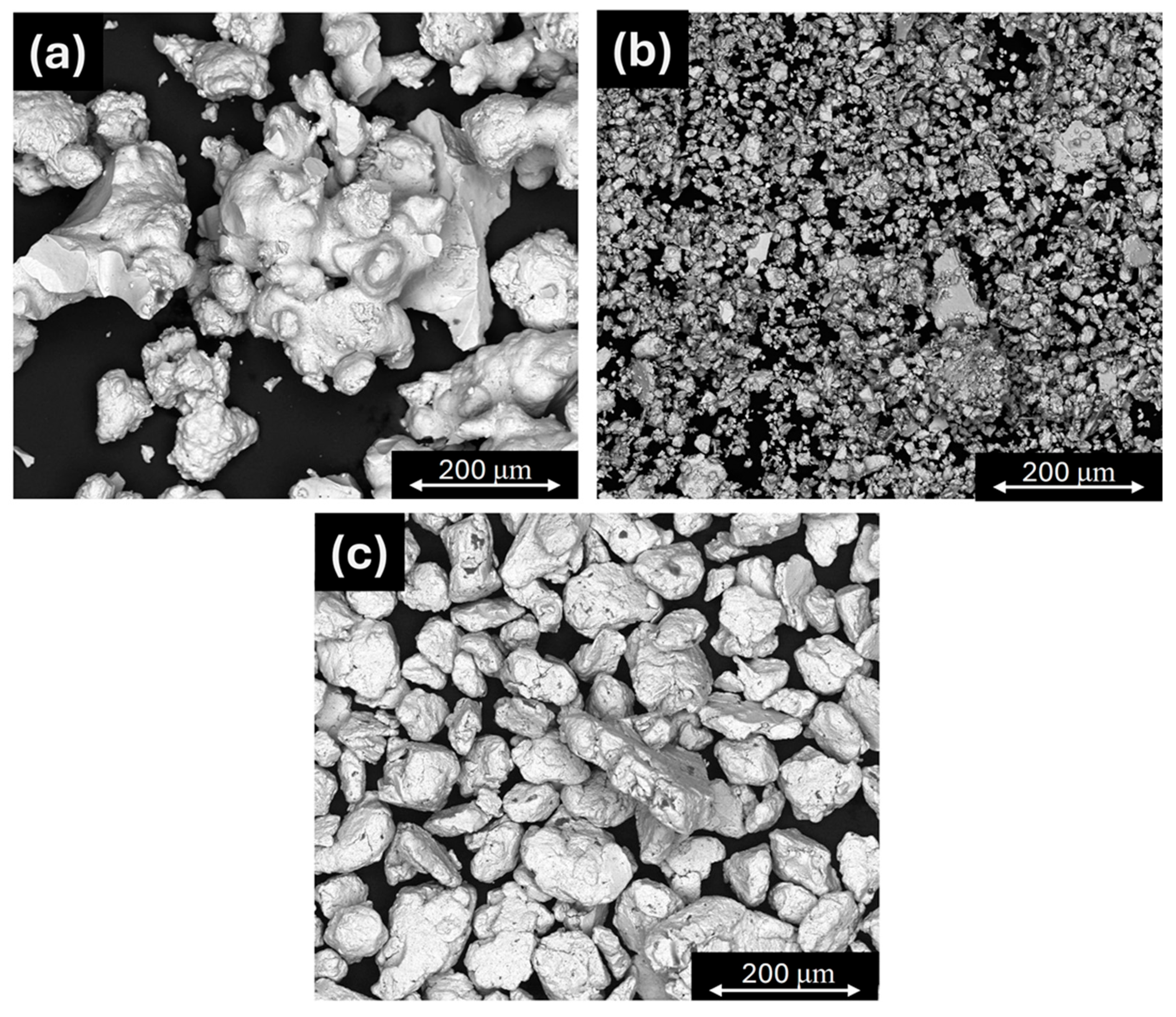
2.1. Phase Analysis and Microstructural Characterization
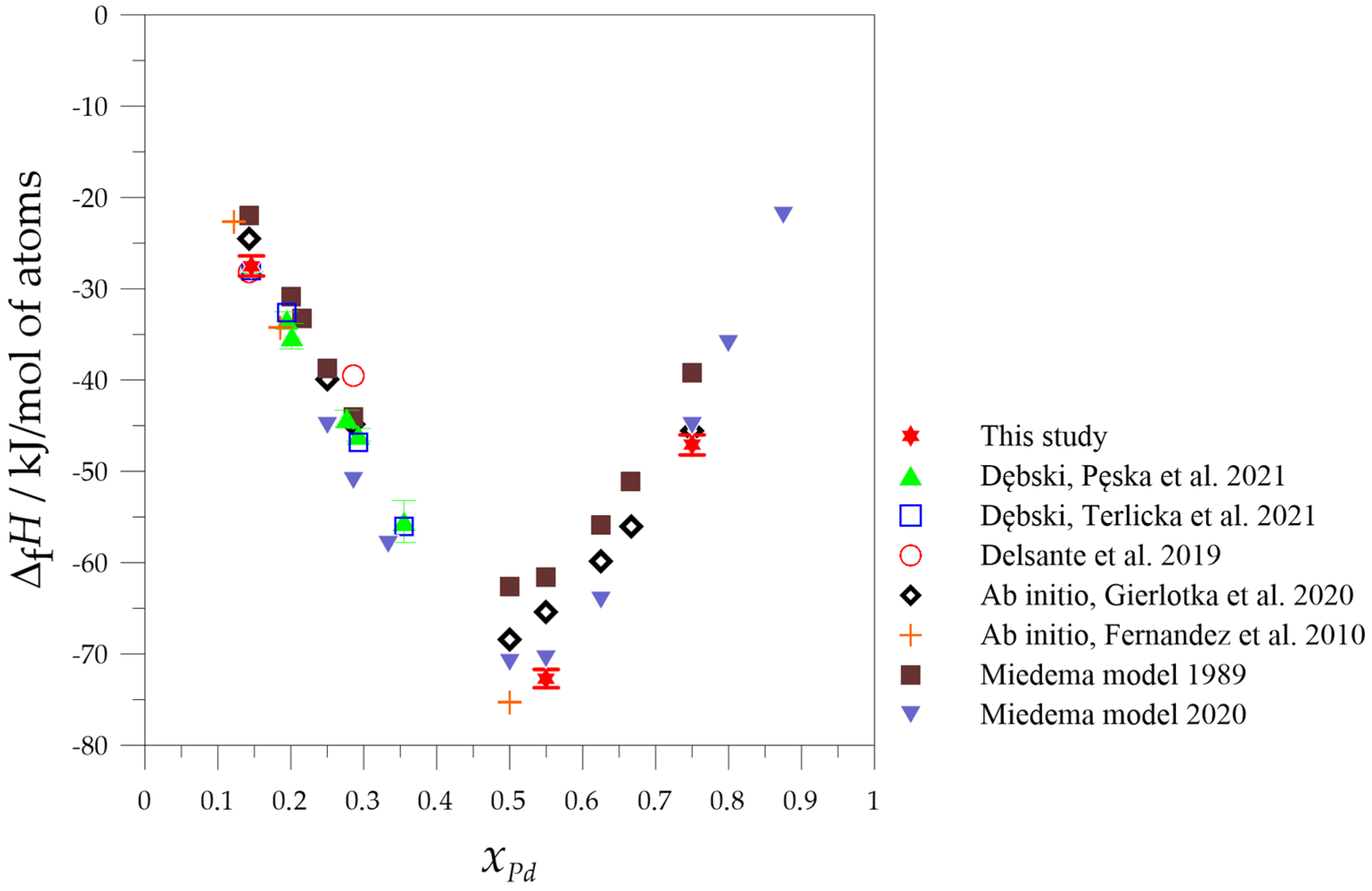
2.2. Calorimetric Studies
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.; Yan, S.; Qu, H. Recent progress in magnesium hydride modified through catalysis and nanoconfinement. Int. J. Hydrogen Energy 2018, 43, 1545–1565. [Google Scholar] [CrossRef]
- Tian, M.; Shang, C. Mg-based composites for enhanced hydrogen storage performance. Int. J. Hydrogen Energy 2018, 44, 338–344. [Google Scholar] [CrossRef]
- Wang, H.; Lin, H.J.; Cai, W.T.; Ouyang, L.Z.; Zhu, M. Tuning kinetics and thermodynamics of hydrogen storage in light metal element based systems—A review of recent progress. J. Alloys Compd. 2016, 658, 280–300. [Google Scholar] [CrossRef]
- Webb, C.J. A review of catalyst-enhanced magnesium hydride as a hydrogen storage material. J. Phys. Chem. Solids 2015, 84, 96–106. [Google Scholar] [CrossRef]
- Wiberg, E.; Goeltzer, H.; Bauer, R. Synthese von Magnesiumhydrid aus den Elementen. Z. Naturforsch.–B J. Chem. 1951, 6, 394–395. [Google Scholar] [CrossRef]
- Ellinger, F.H.; Holley, C.E.J.; McInteer, B.B.; Pavone, D.; Potter, R.M.; Staritzky, E.; Zachariasen, W.H. The preparation and some properties of magnesium hydride. J. Am. Chem. Soc. 1955, 77, 2647–2648. [Google Scholar] [CrossRef]
- Kennelley, J.A.; Varwig, J.W.; Myers, H.W. Magnesium-Hydrogen Relationships. J. Phys. Chem. 1960, 64, 703–704. [Google Scholar] [CrossRef]
- Stampfer, J.F.J.; Holley, C.E.J.; Suttle, J.F. The magnesium-hydrogen system. J. Am. Chem. Soc. 1960, 82, 3504–3508. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Hisamoto, T.; Ura, M.; Nakamura, R. Hydrogen absorption characteristics of Pd1−xLix (x = 0.055 and 0.072) alloys and ordered Pd7Li. J. Alloys Compd. 1993, 200, 141–146. [Google Scholar] [CrossRef]
- Steinlechner, S.; Antrekowitsch, J. Potential of a Hydrometallurgical Recycling Process for Catalysts to Cover the Demand for Critical Metals, Like PGMs and Cerium. J. JOM 2015, 67, 406–411. [Google Scholar] [CrossRef]
- Dekura, S.; Kobayashi, H.; Kusada, K.; Kitagawa, H. Hydrogen in Palladium and Storage Properties of Related Nanomaterials: Size, Shape, Alloying, and Metal-Organic Framework Coating Effects. ChemPhysChem 2019, 20, 1158–1176. [Google Scholar] [CrossRef] [PubMed]
- Gierlotka, W.; Dębski, A.; Terlicka, S.; Gąsior, W.; Pęska, M.; Polański, M. Insight into Phase Stability in the Mg-Pd System: The Ab Initio Calculations. J. Phase Equilib. Diffus. 2020, 41, 681–686. [Google Scholar] [CrossRef]
- Dębski, A.; Pęska, M.; Dworecka-Wójcik, J.; Terlicka, S.; Gąsior, W.; Gierlotka, W.; Polański, M. Structural and calorimetric studies of magnesium-rich Mg-Pd alloys. J. Alloys Compd. 2021, 858, 158085. [Google Scholar] [CrossRef]
- Dębski, A.; Terlicka, S.; Gąsior, W.; Gierlotka, W.; Pęska, M.; Dworecka-Wójcik, J.; Polański, M. Calorimetric Studies of Magnesium-Rich Mg-Pd Alloys. Materials 2021, 14, 680. [Google Scholar] [CrossRef]
- Gierlotka, W.; Terlicka, S.; Gąsior, W.; Dębski, A.; Pęska, M.; Polański, M. Electrochemical investigation and thermodynamic assessment of the Mg-Pd system. J. Min. Metall. B 2024, 60, 105–116. [Google Scholar] [CrossRef]
- Eisheh, J.-T. Untersuchungen zur Thermodynamik, Konstitution und Diffusion an den Systemen Magnesium Platin und Magnesium Palladium. Ph.D. Thesis, Fakultät Christian Albrechts Universität zu Kiel, Kiel, Germany, 2006. Available online: https://macau.uni-kiel.de/receive/diss_mods_00001798 (accessed on 7 July 2022).
- Chen, S.L.; Daniel, S.; Zhang, F.; Chang, Y.A.; Yan, X.-Y.; Xie, F.-Y.; Schmid-Fetzer, R.; Oates, W.A. The PANDAT software package and its applications. Calphad 2002, 26, 175–188. [Google Scholar] [CrossRef]
- Dinsdale, A.T. SGTE data for pure elements. Calphad 1991, 15, 317–425. [Google Scholar] [CrossRef]
- Delsante, S.; Novakovic, R.; Gagliolo, A.; Borzone, G. Thermodynamic investigation on the Mg–Pd intermetallic phases. J. Chem. Thermodyn. 2019, 139, 105890. [Google Scholar] [CrossRef]
- Fernandez, J.F.; Ares, J.R.; Cuevas, F.; Bodega, J.; Leardini, F.; Sanchez, C. A thermodynamic study of the hydrogenation of the pseudo-binary Mg6Pd0.5Ni0.5 intermetallic compound. Intermetallics 2010, 18, 233–241. [Google Scholar] [CrossRef]
- de Boer, F.R.; Boom, R.; Mattens, W.C.M.; Miedema, A.R.; Niessen, A.K. Cohesion in Metals: Transition Metal Alloys; North-Holland: Amsterdam, The Netherlands, 1989; pp. 1–758. [Google Scholar]
- Boom, R.; de Boer, F.R. Enthalpy of formation of binary solid and liquid Mg alloys—Comparison of Miedema-model calculations with data reported in literature. Calphad 2020, 68, 101647. [Google Scholar] [CrossRef]
- Lapshin, O.V.; Boldyreva, E.V.; Boldyrev, V.V. Role of Mixing and Milling in Mechanochemical Synthesis (Review). Russ. J. Inorg. Chem. 2021, 66, 433–453. [Google Scholar] [CrossRef]
- Osman, A.I.; Ayati, A.; Farrokhi, M.; Khadempir, S.; Rajabzadeh, A.R.; Farghali, M.; Krivoshapkin, P.; Tanhaei, B.; Rooney, D.W.; Yap, P.S. Innovations in hydrogen storage materials: Synthesis, applications, and prospects. J. Energy Storage 2024, 95, 112376. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B 1993, 192, 55–69. [Google Scholar] [CrossRef]

| Bath | Metal | Temperature [K] | Limiting Partial Enthalpy of Solution [kJ/mol at.] | References |
|---|---|---|---|---|
| Sn | Mg | 736 | −33.03 | [13] |
| Pd | −126.3 | |||
| Al | Mg | 1033 | −8.6 | [14] |
| Pd | −186.8 |
| Sample | T [K] | Sample No. | ΔHef [kJ/mol at.] | ΔfH [kJ/mol at.] |
|---|---|---|---|---|
| Dissolution in liquid tin. Temperature of the Sn bath: 733 K. | ||||
| S1 (Mg6Pd) | 298 | 1 | 2.4 | −28.5 |
| 2 | 1.0 | −27.1 | ||
| 3 | 1.7 | −27.7 | ||
| 4 | 0.8 | −26.9 | ||
| Average | 1.5 | −27.5 | ||
| Standard error | 1.1 | 1.1 | ||
| S2 (Mg0.9Pd1.1) | 298 | 1 | 10.6 | −73.2 |
| 2 | 10.1 | −72.8 | ||
| 3 | 9.2 | −71.8 | ||
| 4 | 10.4 | −73.1 | ||
| Average | 10.1 | −72.7 | ||
| Standard error | 1.0 | 1.0 | ||
| Dissolution in liquid aluminum. Temperature of the Al bath: 1033 K. | ||||
| S3 (MgPd3, MgPd) | 298 | 1 | −64.6 | −45.8 |
| 2 | −62.5 | −47.9 | ||
| 3 | −62.9 | −47.5 | ||
| Average | −63.4 | −47.1 | ||
| Standard error | 1.1 | 1.1 | ||
| Chemical Name | Source | Purity [Mass%] |
|---|---|---|
| Magnesium powder | Sigma Aldrich, Poznań, Poland | >99% |
| Palladium wire | Safina a.s., Vestec, Czech Republic | 99.95 |
| Argon | Pioniergas, Krakow, Poland | 99.9999 |
| No. | Alloys (Phases) | Magnesium | Palladium | ||
|---|---|---|---|---|---|
| At.% | Mass% | At.% | Mass% | ||
| 1 | S1 (Mg6Pd) | 85.71 | 57.81 | 14.29 | 42.19 |
| 2 | S2 (Mg0.9Pd1.1) | 45.01 | 15.75 | 54.99 | 84.25 |
| 3 | S3 (MgPd3, MgPd) | 24.99 | 7.07 | 75.01 | 92.92 |
| No. | Alloys (Phases) | Annealing Temperature [K] | Annealing Time [h] |
|---|---|---|---|
| 1 | S1 (Mg6Pd) | 773 | 5 |
| 2 | S2 (Mg0.9Pd1.1) | 773 | 5 |
| 3 | S3 (MgPd3, MgPd) | 973 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dębski, A.; Pęska, M.; Dworecka-Wójcik, J.; Gąsior, W.; Gierlotka, W.; Chulist, R.; Cerny, R.; Wyrębska, I.; Terlicka, S.; Polański, M. Mechanical Synthesis and Calorimetric Studies of the Enthalpies of Formation of Chosen Mg-Pd Alloys. Molecules 2024, 29, 5734. https://doi.org/10.3390/molecules29235734
Dębski A, Pęska M, Dworecka-Wójcik J, Gąsior W, Gierlotka W, Chulist R, Cerny R, Wyrębska I, Terlicka S, Polański M. Mechanical Synthesis and Calorimetric Studies of the Enthalpies of Formation of Chosen Mg-Pd Alloys. Molecules. 2024; 29(23):5734. https://doi.org/10.3390/molecules29235734
Chicago/Turabian StyleDębski, Adam, Magda Pęska, Julita Dworecka-Wójcik, Władysław Gąsior, Wojciech Gierlotka, Robert Chulist, Radovan Cerny, Iwona Wyrębska, Sylwia Terlicka, and Marek Polański. 2024. "Mechanical Synthesis and Calorimetric Studies of the Enthalpies of Formation of Chosen Mg-Pd Alloys" Molecules 29, no. 23: 5734. https://doi.org/10.3390/molecules29235734
APA StyleDębski, A., Pęska, M., Dworecka-Wójcik, J., Gąsior, W., Gierlotka, W., Chulist, R., Cerny, R., Wyrębska, I., Terlicka, S., & Polański, M. (2024). Mechanical Synthesis and Calorimetric Studies of the Enthalpies of Formation of Chosen Mg-Pd Alloys. Molecules, 29(23), 5734. https://doi.org/10.3390/molecules29235734






