Effect of Pyrolysis Temperature on the Carbon Sequestration Capacity of Spent Mushroom Substrate Biochar in the Presence of Mineral Iron
Abstract
1. Introduction
2. Results and Discussion
2.1. Basic Properties of the Biochars
2.2. Effect of Pyrolysis Temperature and Fe Addition on Carbon Retention
2.2.1. Effect of Pyrolysis Temperature on Carbon Retention
2.2.2. Effect of Fe Addition on Carbon Retention
2.3. Effect of Pyrolysis Temperature and Fe Addition on Carbon Stability
2.3.1. Effect of Pyrolysis Temperature on Carbon Stability
2.3.2. Effect of Fe Addition on Carbon Stability
2.4. Pyrolysis Temperature for Maximum Carbon Sequestration
3. Environmental Application
4. Materials and Methods
4.1. Pre-Treatment and Modification Methods for Mushroom Substrate
4.2. Biochar Production and Characterization
4.2.1. Biochar Preparation
4.2.2. Elemental and Ash Analysis
4.2.3. Structure Characterization
4.3. Biochar Carbon Retention and Stability
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bijla, S. Status of mushroom production: Global and national scenario. Mushroom Res. 2023, 32, 91–98. [Google Scholar] [CrossRef]
- Liu, X.; Yu, J.; Lu, X.; Yang, M.; Liu, Y.; Sun, H.; Tang, J. Tracing potentially toxic elements in button mushroom cultivation and environmental implications: Insights via stable lead (Pb) isotope analysis. J. Clean. Prod. 2024, 479, 144079. [Google Scholar] [CrossRef]
- Babu, S.; Rathore, S.S.; Singh, R.; Kumar, S.; Singh, V.K.; Yadav, S.K.; Wani, O.A. Exploring agricultural waste biomass for energy, food and feed production and pollution mitigation: A review. Bioresource Technol. 2022, 360, 127566. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.S.; Lee, X.Y.; Nam, W.L.; Phang, X.Y.; Liew, R.K.; Yek, P.N.; Rosli, M.H. Microwave vacuum pyrolysis conversion of waste mushroom substrate into biochar for use as growth medium in mushroom cultivation. J. Chem. Technol. Biotechnol. 2019, 94, 1406–1415. [Google Scholar] [CrossRef]
- Bo, X.; Zhang, Z.; Wang, J.; Guo, S.; Li, Z.; Lin, H.; Zou, J. Benefits and limitations of biochar for climate-smart agriculture: A review and case study from China. Biochar 2023, 5, 77. [Google Scholar] [CrossRef]
- Ayaz, M.; Feizienė, D.; Tilvikienė, V.; Akhtar, K.; Stulpinaitė, U.; Iqbal, R. Biochar role in the sustainability of agriculture and environment. Sustainability 2021, 13, 1330. [Google Scholar] [CrossRef]
- Pyshyev, S.; Miroshnichenko, D.; Malik, I.; Contreras, A.B.; Hassan, N.; ElRasoul, A.A.; Carbosur, C.M.F. State of the art in the production of charcoal: A review. Chem. Chem. Technol. 2021, 15, 61–73. [Google Scholar] [CrossRef]
- Zhou, Y.; Qin, S.; Verma, S.; Sar, T.; Sarsaiya, S.; Ravindran, B.; Awasthi, M.K. Production and beneficial impact of biochar for environmental application: A comprehensive review. Bioresource Technol. 2021, 337, 125451. [Google Scholar] [CrossRef]
- Qiu, B.; Shao, Q.; Shi, J.; Yang, C.; Chu, H. Application of biochar for the adsorption of organic pollutants from wastewater: Modification strategies, mechanisms and challenges. Sep. Purif. Technol. 2022, 300, 121925. [Google Scholar] [CrossRef]
- Wu, Q.; Xian, Y.; He, Z.; Zhang, Q.; Wu, J.; Yang, G.; Long, L. Adsorption characteristics of Pb (II) using biochar derived from spent mushroom substrate. Sci. Rep. 2019, 9, 15999. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, X.; Zhang, P.; Zhao, L.; Qiu, H.; Cao, X. Pyrolysis-temperature depended quinone and carbonyl groups as the electron accepting sites in barley grass derived biochar. Chemosphere 2019, 232, 273–280. [Google Scholar] [CrossRef]
- Chen, W.S.; Tsai, W.T.; Lin, Y.Q.; Tsai, C.H.; Chang, Y.T. Production of highly porous biochar materials from spent mushroom composts. Horticulturae 2022, 8, 46. [Google Scholar] [CrossRef]
- Sewu, D.D.; Boakye, P.; Jung, H.; Woo, S.H. Synergistic dye adsorption by biochar from co-pyrolysis of spent mushroom substrate and Saccharina japonica. Bioresource Technol. 2017, 244, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Qin, K.; Li, J.; Yang, W.; Wang, Z.; Zhang, H. Role of minerals in mushroom residue on its adsorption capability to Cd (II) from aqueous solution. Chemosphere 2023, 324, 138290. [Google Scholar] [CrossRef] [PubMed]
- Mattila, P.; Könkö, K.; Eurola, M.; Pihlava, J.M.; Astola, J.; Vahteristo, L.; Piironen, V. Contents of vitamins, mineral elements, and some phenolic compounds in cultivated mushrooms. J. Agr. Food Chem. 2001, 49, 2343–2348. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Wang, Q.; Liu, Y.; Zhu, J.; Deng, Y.; Fu, Q.; Hu, H. Co-pyrolysis biochar derived from rape straw and phosphate rock: Carbon retention, aromaticity, and Pb removal capacity. Energ. Fuel. 2018, 33, 413–419. [Google Scholar] [CrossRef]
- Ahmad, M.; Ahmad, M.; Usman, A.R.; Al-Faraj, A.S.; Abduljabbar, A.; Ok, Y.S.; Al-Wabel, M.I. Date palm waste-derived biochar composites with silica and zeolite: Synthesis, characterization and implication for carbon stability and recalcitrant potential. Environ. Geochem. Health 2019, 41, 1687–1704. [Google Scholar] [CrossRef]
- Nan, H.; Zhao, L.; Yang, F.; Liu, Y.; Xiao, Z.; Cao, X.; Qiu, H. Different alkaline minerals interacted with biomass carbon during pyrolysis: Which one improved biochar carbon sequestration? J. Clean. Prod. 2020, 255, 120162. [Google Scholar] [CrossRef]
- Carneiro, J.S.D.S.; Lustosa-Filho, J.F.; Nardis, B.O.; Ribeiro-Soares, J.; Zinn, Y.L.; Melo, L.C.A. Carbon stability of engineered biochar-based phosphate fertilizers. ACS Sustain. Chem. Eng. 2018, 6, 14203–14212. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, C.; Wang, Y.; He, L.; Lu, H.; Yang, S. Vermiculite modification increases carbon retention and stability of rice straw biochar at different carbonization temperatures. J. Clean. Prod. 2020, 254, 120111. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, G.; Wu, W.; Ali, A.; Wang, H.; Wang, Q.; Zhang, Z. Magnetic biochar composite decorated with amino-containing biopolymer for phosphorus recovery from swine wastewater. Colloids Surf. A: Physicochem. Eng. Asp. 2022, 634, 127980. [Google Scholar] [CrossRef]
- Tomin, O.; Yazdani, M.R. Production and characterization of porous magnetic biochar: Before and after phosphate adsorption insights. J. Porous Mat. 2022, 29, 849–859. [Google Scholar] [CrossRef]
- Shen, M.; Zhu, X.; Zhang, S. Extraneous Fe increased the carbon retention of sludge-based biochar. B Environ. Contam. Tox. 2021, 106, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Yang, Y.; Liu, P.; Li, Y.; Huang, F.; Zeng, L.; Hou, B. Iron-montmorillonite treated corn straw biochar: Interfacial chemical behavior and stability. Sci. Total Environ. 2020, 708, 134773. [Google Scholar] [CrossRef]
- Clemente, J.S.; Beauchemin, S.; Thibault, Y.; MacKinnon, T.; Smith, D. Differentiating inorganics in biochars produced at commercial scale using principal component analysis. ACS Omega 2018, 3, 6931–6944. [Google Scholar] [CrossRef]
- Zhao, L.; Cao, X.; Zheng, W.; Kan, Y. Phosphorus-assisted biomass thermal conversion: Reducing carbon loss and improving biochar stability. PLoS ONE 2014, 9, e115373. [Google Scholar] [CrossRef]
- Spokas, K.A. Review of the stability of biochar in soils: Predictability of O: C molar ratios. Carbon. Manag. 2010, 1, 289–303. [Google Scholar] [CrossRef]
- Budai, A.; Calucci, L.; Rasse, D.P.; Strand, L.T.; Pengerud, A.; Wiedemeier, D.; Forte, C. Effects of pyrolysis conditions on Miscanthus and corncob chars: Characterization by IR, solid state NMR and BPCA analysis. J. Anal. Appl. Pyrol. 2017, 128, 335–345. [Google Scholar] [CrossRef]
- Wang, H.; Nan, Q.; Waqas, M.; Wu, W. Stability of biochar in mineral soils: Assessment methods, influencing factors and potential problems. Sci. Total Environ. 2022, 806, 150789. [Google Scholar] [CrossRef]
- Hu, Y.; Lu, Y.; Ma, W.; Wang, L.; Wibowo, H.; Huang, Z.; Yu, F. Thermal transformation of carbon and oxygen-containing organic compounds in sewage sludge during pyrolysis treatment. Energies 2019, 12, 2258. [Google Scholar] [CrossRef]
- Zornoza, R.; Moreno-Barriga, F.; Acosta, J.A.; Muñoz, M.A.; Faz, A. Stability, nutrient availability and hydrophobicity of biochars derived from manure, crop residues, and municipal solid waste for their use as soil amendments. Chemosphere 2016, 144, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Wang, J.J.; Gaston, L.A.; Zhou, B.; Park, J.H.; Li, R.; Zhang, Z. Biochar produced from mineral salt-impregnated chicken manure: Fertility properties and potential for carbon sequestration. Waste Manag. 2018, 78, 802–810. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, K.; Han, L.; Jin, J.; Sun, H.; Yang, Y.; Xing, B. Effect of minerals on the stability of biochar. Chemosphere 2018, 204, 310–317. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Q.; Sun, X.; Wang, X. A novel magnetic biochar from spent shiitake substrate: Characterization and analysis of pyrolysis process. Biomass Convers. Bior. 2015, 5, 339–346. [Google Scholar] [CrossRef]
- Manyà, J.J.; Ortigosa, M.A.; Laguarta, S.; Manso, J.A. Experimental study on the effect of pyrolysis pressure, peak temperature, and particle size on the potential stability of vine shoots-derived biochar. Fuel 2014, 133, 163–172. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Resonant Raman spectroscopy of disordered, amorphous, and diamondlike carbon. Phys. Rev. B 2001, 64, 075414. [Google Scholar] [CrossRef]
- Chen, J.; Mao, Z.; Zhang, L.; Tang, Y.; Wang, D.; Bie, L.; Fahlman, B.D. Direct production of nitrogen-doped porous carbon from urea via magnesiothermic reduction. Carbon 2018, 130, 41–47. [Google Scholar] [CrossRef]
- Mikutta, R.; Kleber, M.; Kaiser, K.; Jahn, R. Organic matter removal from soils using hydrogen peroxide, sodium hypochlorite, and disodium peroxodisulfate. Soil Sci. Soc. Am. J. 2005, 69, 120–135. [Google Scholar] [CrossRef]
- Jiang, Y.; Ren, C.; Guo, H.; Guo, M.; Li, W. Speciation transformation of phosphorus in poultry litter during pyrolysis: Insights from X-ray diffraction, Fourier transform infrared, and solid-state NMR spectroscopy. Environ. Sci. Technol. 2019, 53, 13841–13849. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Liu, G.; He, Y.; Chen, C.; Liu, X.; Zhao, Y. Mixed heavy metal removal from wastewater by using discarded mushroom-stick biochar: Adsorption properties and mechanisms. Environ. Sci. Proc. Imp. 2019, 21, 584–592. [Google Scholar] [CrossRef]
- Li, X.; Hayashi, J.I.; Li, C.Z. FT-Raman spectroscopic study of the evolution of char structure during the pyrolysis of a Victorian brown coal. Fuel 2006, 85, 1700–1707. [Google Scholar] [CrossRef]
- Estrade-Szwarckopf, H. XPS photoemission in carbonaceous materials: A “defect” peak beside the graphitic asymmetric peak. Carbon 2004, 42, 1713–1721. [Google Scholar] [CrossRef]
- Czernik, S.; Bridgwater, A.V. Overview of applications of biomass fast pyrolysis oil. Energ. Fuel. 2004, 18, 590–598. [Google Scholar] [CrossRef]
- Feng, D.; Zhao, Y.; Zhang, Y.; Zhang, Z.; Zhang, L.; Sun, S. In-situ steam reforming of biomass tar over sawdust biochar in mild catalytic temperature. Biomass Bioenerg. 2017, 107, 261–270. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.F.R. The role of surface chemistry in catalysis with carbons. Catal. Today 2010, 150, 2–7. [Google Scholar] [CrossRef]
- Zhao, Y.; Feng, D.; Zhang, Y.; Huang, Y.; Sun, S. Effect of pyrolysis temperature on char structure and chemical speciation of alkali and alkaline earth metallic species in biochar. Fuel Process Technol. 2016, 141, 54–60. [Google Scholar] [CrossRef]
- Jiang, S.; Hu, X.; Xia, D.; Li, C.Z. Formation of aromatic ring structures during the thermal treatment of mallee wood cylinders at low temperature. Appl. Energ. 2016, 183, 542–551. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U., Jr.; Steele, P.H. Pyrolysis of wood/biomass for bio-oil: A critical review. Energ. Fuel. 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Guizani, C.; Haddad, K.; Limousy, L.; Jeguirim, M. New insights on the structural evolution of biomass char upon pyrolysis as revealed by the Raman spectroscopy and elemental analysis. Carbon 2017, 119, 519–521. [Google Scholar] [CrossRef]
- Liu, S.; Kong, F.; Li, Y.; Jiang, Z.; Xi, M.; Wu, J. Mineral-ions modified biochars enhance the stability of soil aggregate and soil carbon sequestration in a coastal wetland soil. Catena 2020, 193, 104618. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, S.; Wang, Q.; Hu, K.; Zhang, H.; Chang, J.; Cheng, H. Tetracycline removal from aqueous solution by magnetic biochar modified with different iron valences: A comparative study. Sep. Purif. Technol. 2024, 339, 126614. [Google Scholar] [CrossRef]
- Mei, Y.; Xu, J.; Zhang, Y.; Li, B.; Fan, S.; Xu, H. Effect of Fe–N modification on the properties of biochars and their adsorption behavior on tetracycline removal from aqueous solution. Bioresource Technol. 2021, 325, 124732. [Google Scholar] [CrossRef]
- Nan, H.; Yin, J.; Yang, F.; Luo, Y.; Zhao, L.; Cao, X. Pyrolysis temperature-dependent carbon retention and stability of biochar with participation of calcium: Implications to carbon sequestration. Environ. Pollut. 2021, 287, 117566. [Google Scholar] [CrossRef]
- Pstrowska, K.; Łużny, R.; Fałtynowicz, H.; Jaroszewska, K.; Postawa, K.; Pyshyev, S.; Witek-Krowiak, A. Unlocking Sustainability: A Comprehensive Review of Up-Recycling Biomass Waste into Biochar for Environmental Solutions. Chem. Chem. Technol. 2024, 18, 211–231. [Google Scholar] [CrossRef]
- Cowie, A.; Azzi, E.; Weng, Z.H.; Woolf, D. Biochar, Greenhouse Gas Accounting, and Climate Change Mitigation. In Biochar for Environmental Management; Routledge: Abingdon, UK, 2024; pp. 759–784. [Google Scholar] [CrossRef]
- Friedlingstein, P.; O’Sullivan, M.; Jones, M.W.; Andrew, R.M.; Hauck, J.; Landschützer, P.; Zeng, J. Global Carbon Budget 2024. Earth Syst. Sci. Data. 2024, 2024, 1–133. [Google Scholar] [CrossRef]
- Chen, X.; Dai, Y.; Fan, J.; Xu, X.; Cao, X. Application of iron-biochar composite in topsoil for simultaneous remediation of chromium-contaminated soil and groundwater: Immobilization mechanism and long-term stability. J. Hazard. Mater. 2021, 405, 124226. [Google Scholar] [CrossRef] [PubMed]
- Irshad, M.K.; Noman, A.; Alhaithloul, H.A.; Adeel, M.; Rui, Y.; Shah, T.; Shang, J. Goethite-modified biochar ameliorates the growth of rice (Oryza sativa L.) plants by suppressing Cd and As-induced oxidative stress in Cd and As co-contaminated paddy soil. Sci. Total Environ. 2020, 717, 137086. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, X.; Ma, J.; Li, P.; Chen, Y.; Weng, L.; Li, Y. Comparison of the effects of large-grained and nano-sized biochar, ferrihydrite, and complexes thereof on Cd and As in a contaminated soil–plant system. Chemosphere 2021, 280, 130731. [Google Scholar] [CrossRef]
- Gillingham, M.D.; Gomes, R.L.; Ferrari, R.; West, H.M. Sorption, separation and recycling of ammonium in agricultural soils: A viable application for magnetic biochar? Sci. Total Environ. 2022, 812, 151440. [Google Scholar] [CrossRef]
- Silva, T.C.F.; VergÜtz, L.; Pacheco, A.A.; Melo, L.F.; Renato, N.S.; Melo, L.C. Characterization and application of magnetic biochar for the removal of phosphorus from water. An. Da Acad. Bras. De Ciências 2020, 92, e20190440. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, Y.; Shao, S.; Xiao, R. The effects of potassium on distributions of bio-oils obtained from fast pyrolysis of agricultural and forest biomass in a fluidized bed. Appl. Energ. 2017, 208, 867–877. [Google Scholar] [CrossRef]
- Tirol-Padre, A.; Ladha, J.K. Assessing the reliability of permanganate-oxidizable carbon as an index of soil labile carbon. Soil Sci. Soc. Am. J. 2004, 68, 969–978. [Google Scholar] [CrossRef]

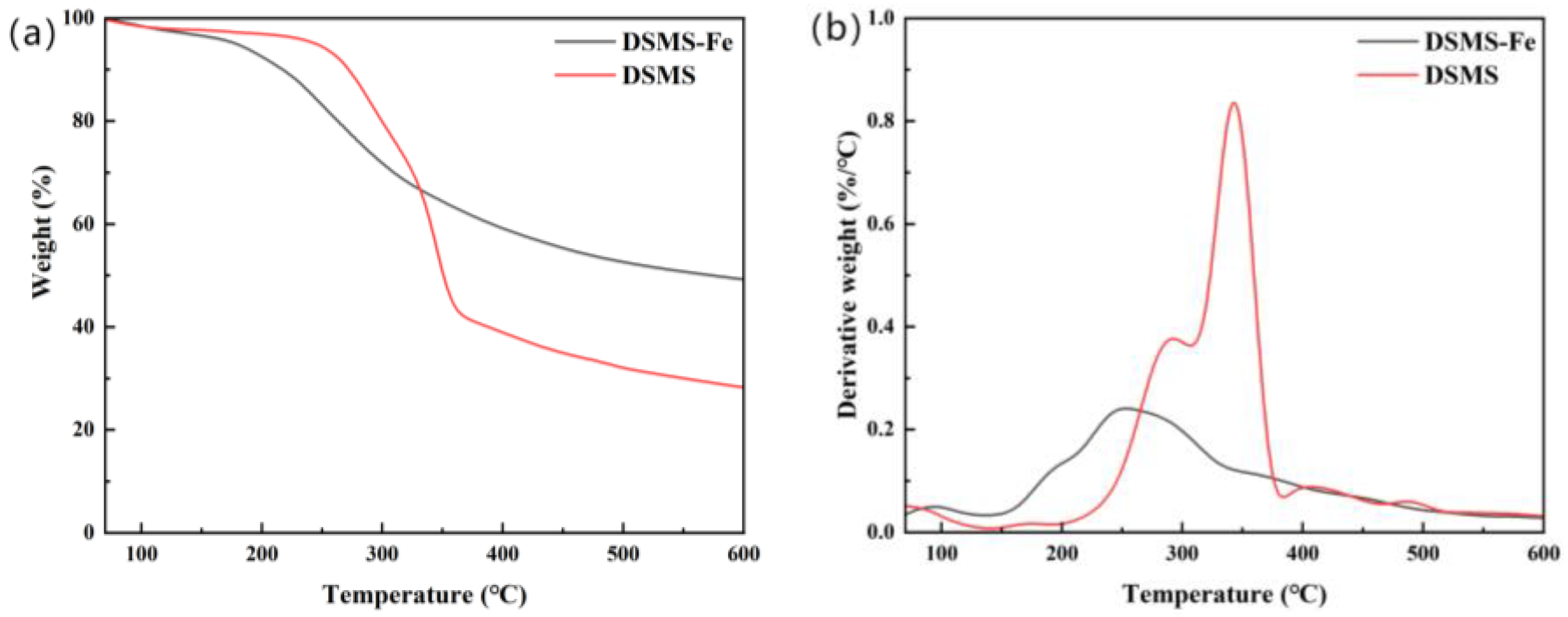
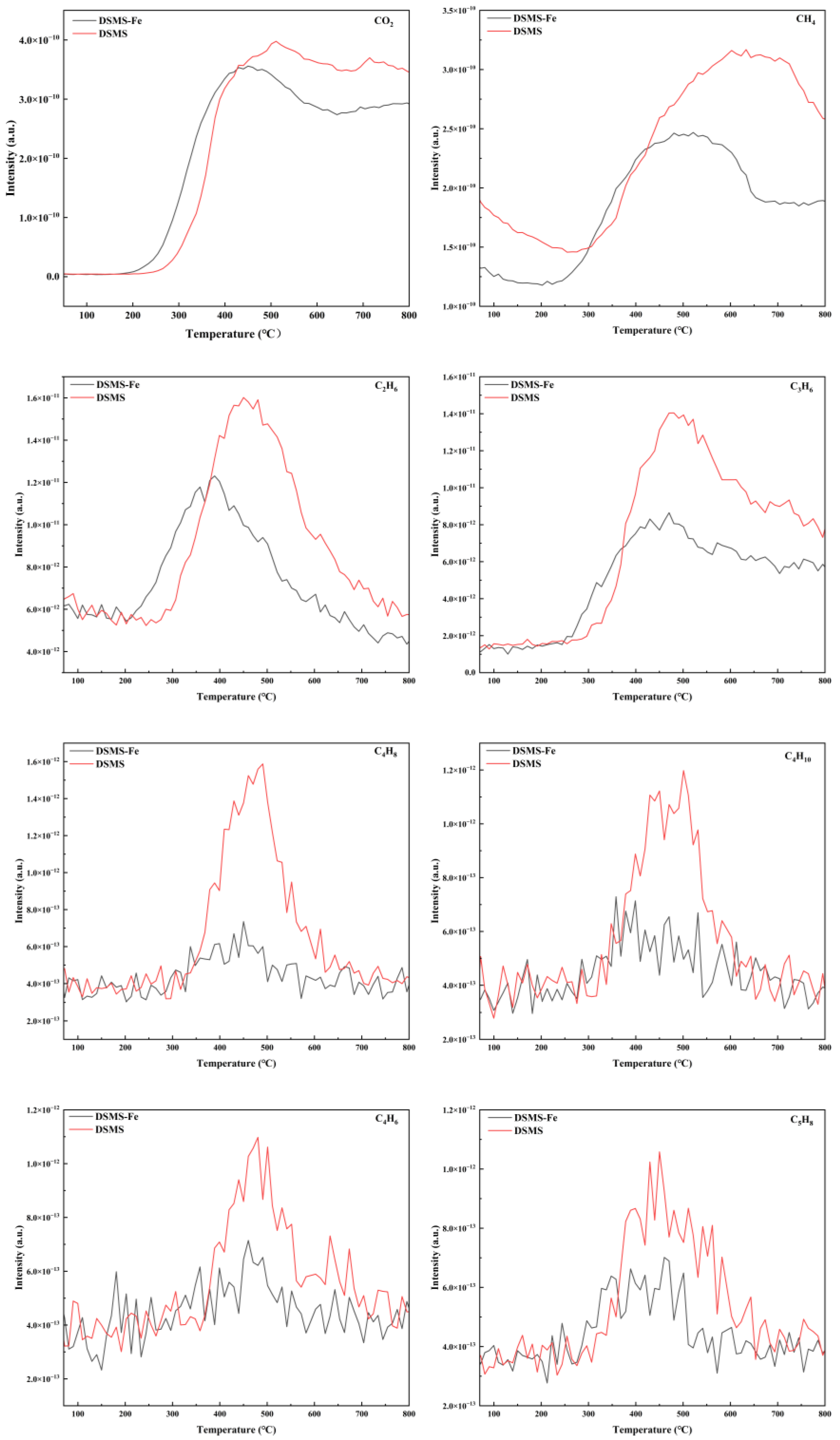

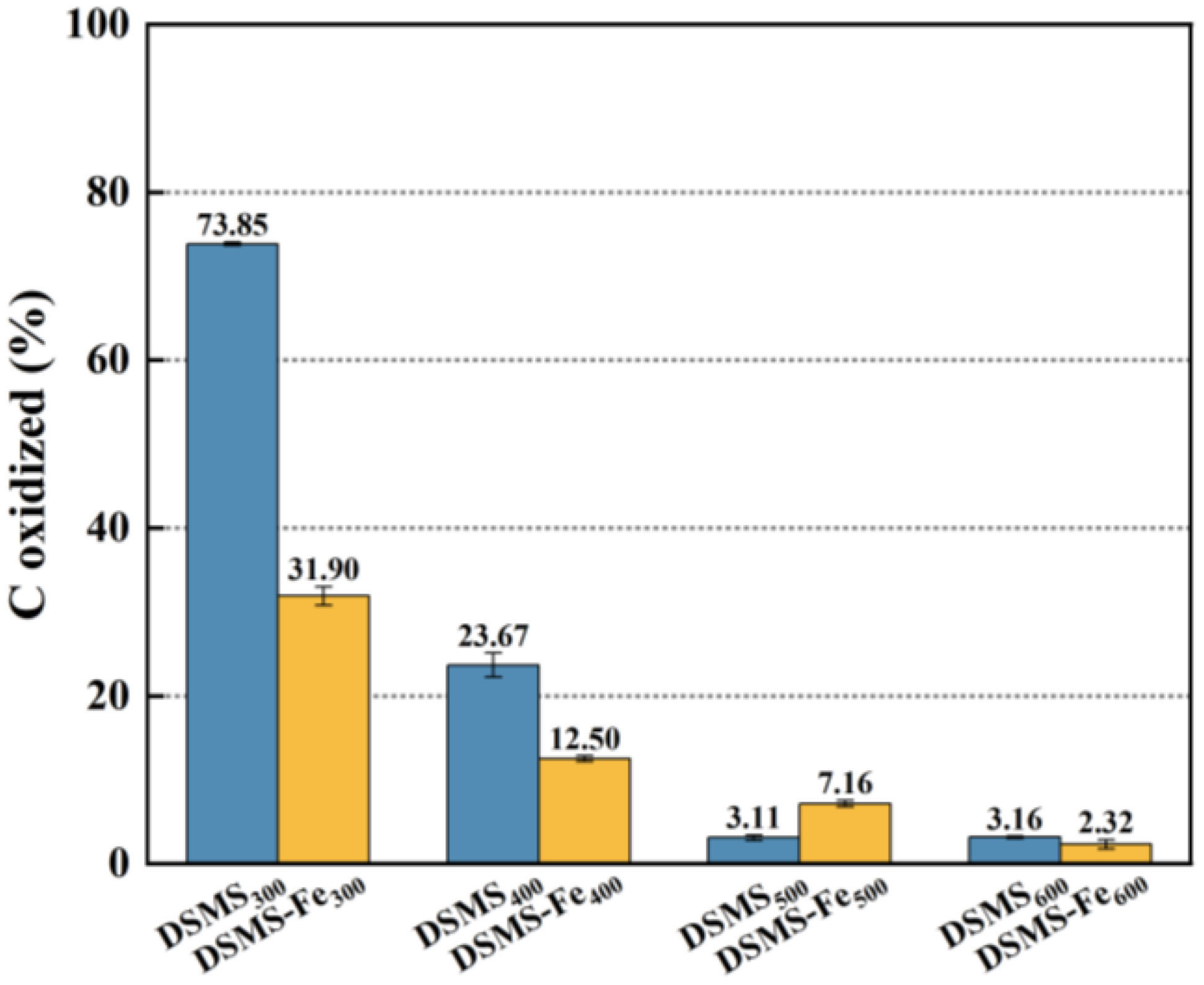
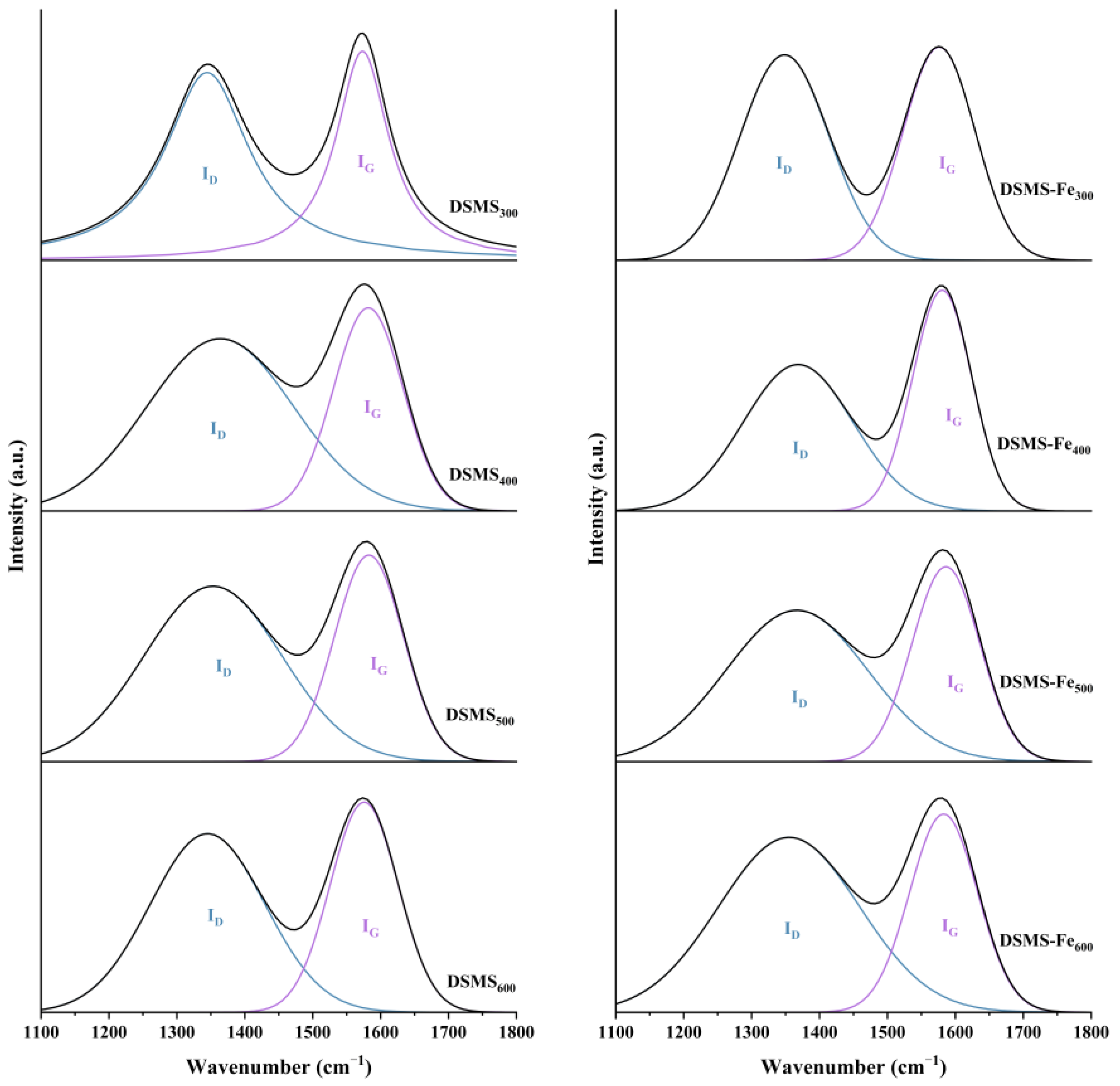
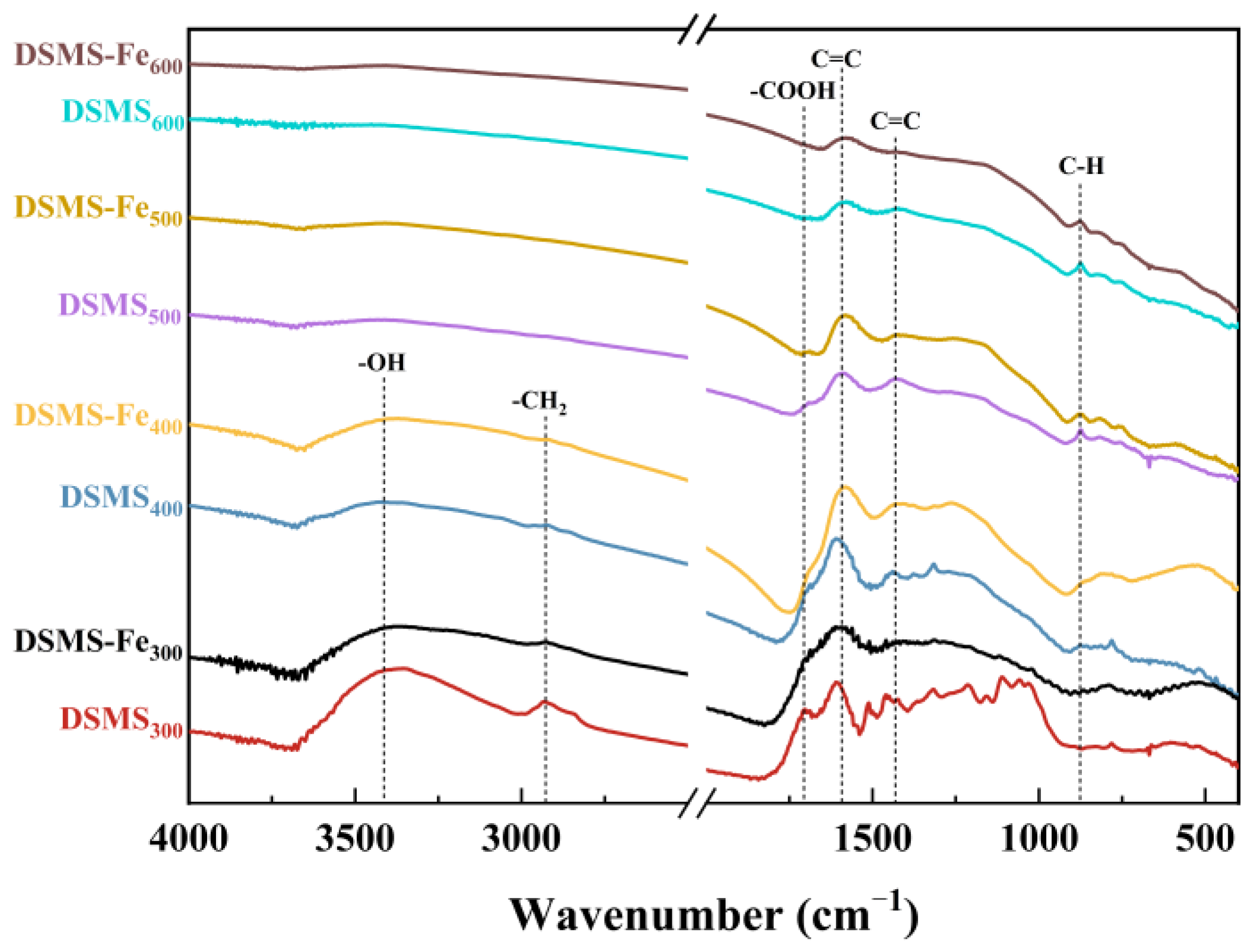


| Samples | Yield | Ash | C | H | N | S | O | Fe | H/C | O/C |
|---|---|---|---|---|---|---|---|---|---|---|
| % | Wt % | |||||||||
| SMS | / | 4.25 | 44.79 | 6.64 | 0.50 | 0.231 | 43.59 | 0.020 | 1.78 | 0.730 |
| DSMS | / | 4.01 | 47.42 | 7.87 | 0.45 | 0.255 | 39.99 | 0.011 | 1.99 | 0.633 |
| DSMS300 | 48.1 | 5.69 | 61.24 | 5.33 | 0.59 | 0.260 | 26.89 | 0.030 | 1.04 | 0.329 |
| DSMS400 | 31.4 | 6.62 | 67.37 | 2.30 | 0.92 | 0.112 | 22.68 | 0.047 | 0.41 | 0.252 |
| DSMS500 | 27.6 | 9.9 | 74.25 | 1.63 | 0.71 | 0.326 | 22.68 | 0.040 | 0.26 | 0.229 |
| DSMS600 | 25.2 | 8.52 | 80.73 | 1.11 | 0.77 | 0.238 | 8.63 | 0.047 | 0.16 | 0.08 |
| DSMS-Fe | / | 9.59 | 40.81 | 5.33 | 0.41 | 0.206 | 43.65 | 3.784 | 1.57 | 0.802 |
| DSMS-Fe300 | 57.0 | 13.64 | 58.14 | 3.21 | 0.59 | 0.311 | 24.11 | 6.100 | 0.66 | 0.311 |
| DSMS-Fe400 | 45.8 | 17.6 | 59.61 | 2.73 | 0.58 | 0.306 | 19.17 | 7.830 | 0.55 | 0.241 |
| DSMS-Fe500 | 41.4 | 17.11 | 68.66 | 1.78 | 0.54 | 0.227 | 11.69 | 9.492 | 0.31 | 0.128 |
| DSMS-Fe600 | 38.8 | 16.99 | 75.82 | 0.30 | 0.55 | 0.380 | 5.96 | 7.958 | 0.05 | 0.059 |
| I(D)/I(G) | I(G)/I(All) | |
|---|---|---|
| DSMS300 | 1.44 | 0.41 |
| DSMS400 | 1.74 | 0.37 |
| DSMS500 | 1.73 | 0.37 |
| DSMS600 | 1.42 | 0.41 |
| DSMS-Fe300 | 1.26 | 0.45 |
| DSMS-Fe400 | 1.44 | 0.41 |
| DSMS-Fe500 | 1.64 | 0.38 |
| DSMS-Fe600 | 1.80 | 0.36 |
| C-C/C=C/% | C-O/% | C=O/% | O-C=O/% | |
|---|---|---|---|---|
| DSMS300 | 74.37 | 15.64 | 4.41 | 5.58 |
| DSMS400 | 77.58 | 8.15 | 6.80 | 7.46 |
| DSMS500 | 78.05 | 9.00 | 5.76 | 7.19 |
| DSMS600 | 78.10 | 12.472 | 1.72 | 7.49 |
| Increase | Decrease | Decrease | Increase | |
| DSMS-Fe300 | 82.44 | 7.66 | 3.74 | 6.15 |
| DSMS-Fe400 | 76.65 | 11.72 | 3.52 | 8.10 |
| DSMS-Fe500 | 79.74 | 9.23 | 8.21 | 2.82 |
| DSMS-Fe600 | 79.47 | 6.16 | 3.85 | 10.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Xing, Z.; Xue, Y.; Zhang, J.; Zhai, J. Effect of Pyrolysis Temperature on the Carbon Sequestration Capacity of Spent Mushroom Substrate Biochar in the Presence of Mineral Iron. Molecules 2024, 29, 5712. https://doi.org/10.3390/molecules29235712
Liu B, Xing Z, Xue Y, Zhang J, Zhai J. Effect of Pyrolysis Temperature on the Carbon Sequestration Capacity of Spent Mushroom Substrate Biochar in the Presence of Mineral Iron. Molecules. 2024; 29(23):5712. https://doi.org/10.3390/molecules29235712
Chicago/Turabian StyleLiu, Bin, Zebing Xing, Yuxin Xue, Ji Zhang, and Junlin Zhai. 2024. "Effect of Pyrolysis Temperature on the Carbon Sequestration Capacity of Spent Mushroom Substrate Biochar in the Presence of Mineral Iron" Molecules 29, no. 23: 5712. https://doi.org/10.3390/molecules29235712
APA StyleLiu, B., Xing, Z., Xue, Y., Zhang, J., & Zhai, J. (2024). Effect of Pyrolysis Temperature on the Carbon Sequestration Capacity of Spent Mushroom Substrate Biochar in the Presence of Mineral Iron. Molecules, 29(23), 5712. https://doi.org/10.3390/molecules29235712







