Changes in Ibuprofen Toxicity and Degradation in Response to Immobilization of Bacillus thuringiensis B1(2015b)
Abstract
1. Introduction
2. Results and Discussion
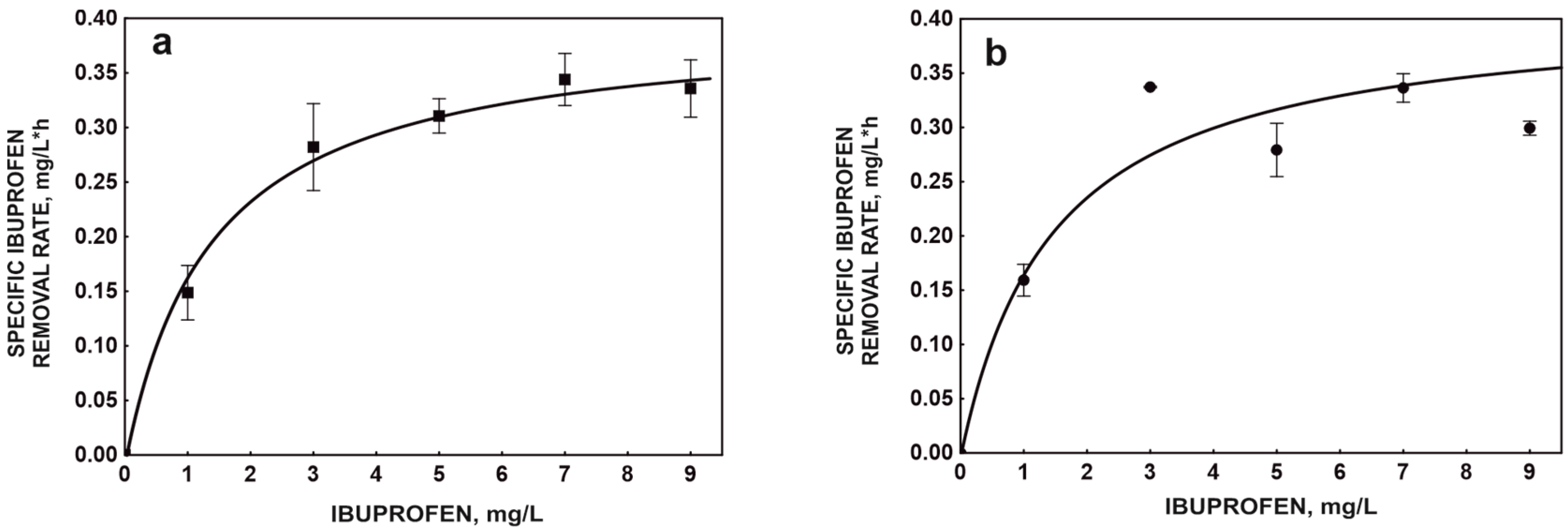
2.1. Kinetic Degradation by Free and Immobilized Bacteria
2.2. Analysis of the Effect of Ibuprofen on the Metabolic Profile of B1(2015b) Strain
2.3. Impact of Immobilization on the Changes in Intermediate Toxicity During Ibuprofen Degradation
3. Materials and Methods
3.1. Immobilization of B. thuringiensis B1(2015b) Strain
3.2. Biochemical Analysis
3.2.1. Assay of Total Enzymatic Activity Free and Immobilized Bacteria
3.2.2. Determination of Ibuprofen Concentration
3.2.3. Metabolic Profile Analysis
3.3. Ibuprofen Degradation Kinetics Study
3.4. Toxicity Study
3.4.1. Ibuprofen and Its Metabolite Toxicity Against to B1(2015b) Strain
3.4.2. Test Thamnotox
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, C.Y.; Fu, L.H.; Sung, M.H.; Huang, C.F.; Wu, J.P.; Kuo, H.W. Ibuprofen biodegradation by hospital, municipal, and distillery activated sludges. Environ. Technol. 2020, 41, 171–180. [Google Scholar]
- Ogunwole, G.A.; Saliu, J.K. Seasonal occurence of ibuprofen in sediment, water, and biota in river Owena and Ogbese, and its ecological risk assessment. Ann. Sci. Technol. 2020, 5, 11–19. [Google Scholar]
- Salgado, R.; Brito, D.; Noronha, J.P.; Almeida, B.; Bronze, M.R.; Oehmen, A.; Carvalho, G.; Crespo, M.T.B. Metabolite identification of ibuprofen biodegradation by Patulibacter medicamentivorans under aerobic conditions. Environ. Technol. 2020, 41, 450–465. [Google Scholar] [CrossRef] [PubMed]
- Aulestia, M.; Flores, A.; Acosta-Jurado, S.; Santero, E.; Camacho, E.M. Genetic characterization of the ibuprofen-degradative pathway of Rhizorhabdus wittichii MPO218. Appl. Environ. Micorbiol. 2022, 88, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Özgüven, A.; Öztürk, D.; Bayram, T. An investigation based on removal of ibuprofen and its transformation products by a batch activated sludge procesess: A kinetic study. Environ. Res. Tec. 2021, 4, 329–341. [Google Scholar] [CrossRef]
- Ossowicz-Rupniewska, P.E.; Kucharska, E.; Klebeko, J.; Kopciuch, E.; Bilska, K.; Janus, E. Effect of the type of amino acid on the biodegradation of ibuprofen derivatives. Arch. Environ. Protect. 2023, 49, 46–69. [Google Scholar]
- Balciunas, E.M.; Kappelmeyer, U.; Harms, H.; Heipieper, H.J. Increasing ibuprofen degradation in constructed wetlands by bioaugmentation with gravel containing biofilms od an ibuprofen-degrading Sphingobium yanoikuyae. Eng. Life Sci. 2020, 20, 160–167. [Google Scholar] [CrossRef]
- Ivshina, I.B.; Tyumina, E.A.; Bazhutin, G.A.; Vikhareva, E.V. Response of Rhodococcus cerastii IEGM 1278 to toxic effects of ibuprofen. PLoS ONE 2021, 16, e0260032. [Google Scholar] [CrossRef] [PubMed]
- Blasco, J.; Trombini, C. Ibuprofen and diclofenac in the marine environment—A critical review of their occurrence and potential risk for invertebrate species. Water Emerg. Contam. Nanoplastics 2023, 2, 14. [Google Scholar] [CrossRef]
- Jan-Roblero, J.; Cruz-Maya, J.A. Ibuprofen: Toxicology and biodegradation of an emerging contaminant. Molecules 2023, 28, 2097. [Google Scholar] [CrossRef]
- Sanchez-Aceves, L.; Perez-Alvarez, I.; Gomez-Olivan, L.M.; Islas-Flores, H.; Barcelo, D. Long-term exposure to environmentally relevant concentrations of ibuprofen and aluminium alters oxidative stress status on Danio rerio. Comp. Biochem. Physiol. C 2021, 248, 109071. [Google Scholar]
- Marchlewicz, A.; Guzik, U.; Hupert-Kocurek, K.; Wojcieszyńska, D. Evaluation of the defined bacterial consortium efficacy in the biodegradation of NSAIDs. Molecules 2023, 28, 2185. [Google Scholar] [CrossRef]
- Najim, A.A.; Radeef, A.Y.; al-Doori, I.; Jabbar, Z.H. Immobilization: The promising technique to protect and increase the efficiency of microorganisms to remove contaminants. J. Chem. Technol. Biotechnol. 2024, 99, 1707–1733. [Google Scholar] [CrossRef]
- Dzionek, A.; Wojcieszyńska, D.; Marchlewicz, A.; Smułek, W.; Potocka, I.; Jałowiecki, Ł.; Borgulat, J.; Płaza, G.; Guzik, U. Naproxen as environmental pollution, its effect on bacteria metabolism and degradation mechanism in immobilized Planococcus sp. S5. Chem. Eng. J. 2024, 481, 148174. [Google Scholar] [CrossRef]
- Marchlewicz, A.; Guzik, U.; Hupert-Kocurek, K.; Nowak, A.; Wilczyńska, S.; Wojcieszyńska, D. Toxicity and biodegradation of ibuprofen by Bacillus thuringiensis B1(2015b). Environ. Sci. Pollut. Res. 2017, 24, 7572–7584. [Google Scholar] [CrossRef]
- Dzionek, A.; Wojcieszyńska, D.; Adamczyk-Habrajska, M.; Guzik, U. Enhanced degradation of naproxen by immobilization of Bacillus thuringiensis B1(2015b) on Loofah sponge. Molecules 2020, 25, 872. [Google Scholar] [CrossRef]
- Sutherland, I.W. The biofilm matrix—An immobilized but dynamic microbial environment. Trends Microbiol. 2001, 9, 222–227. [Google Scholar] [CrossRef]
- Bochner, B.R. Phenomics and Phenotype Microarrays: Applications Complementing Metagenomics. In Handbook of Molecular Microbial Ecology I: Metagenomics and Complementary Approaches; de Bruijn, F.J., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2011; pp. 533–540. [Google Scholar]
- Marchlewicz, A.; Guzik, U.; Smułek, W.; Wojcieszyńska, D. Exploring the degradation of ibuprofen by Bacillus thuringiensis B1(2015b): The new pathway and factors affecting degradation. Molecules 2017, 22, 1676. [Google Scholar] [CrossRef]
- Otto, M.; Dickey, S.W.; Wolz, C. Editorial: Quorum-sensing in gram-positive pathogens—Mechanisms, role in infection, and potential as a therapeutic target. Front. Cell. Infect. Microbiol. 2023, 13, 1236705. [Google Scholar] [CrossRef]
- Prazdnova, E.V.; Gorovtsov, a.v.; Vasilchenko, n.g.; Kulikov, M.P.; Statsenko, V.N.; Bogdanova, A.A.; Refeld, A.G.; Brislavskiy, Y.A.; Chistyakov, V.A.; Chikindas, M.L. Quorum-sensing inhibition by gram-positive bacteria. Microorganisms 2022, 10, 350. [Google Scholar] [CrossRef]
- Asma, S.T.; Imre, K.; Morar, A.; Herman, V.; Acaroz, U.; Mukhtar, H.; Arslan-Acaroz, D.; Shah, S.R.A.; Gerlach, R. An overview of biofilm formation-combating strategies and mechanisms of action of antibiofilm agents. Life 2022, 12, 1110. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 2018, 9, 522–554. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Liu, H.; Nanayakkara, N.P.D.; Khan, I.A.; Tekwani, B.L.; Walker, L.A.; Doerksen, R.J. Hydroxylated derivatives of NPC1161: Theoretical insights into their potential toxicity and the feasibility and regioselectivity of their formation. J. Phys. Chem. A 2014, 118, 5501–5507. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, R.; Tehrani, R.; Van Aken, B. Toxicity of hydroxylated polychlorinated biphenyls (HO-PCBs) using the bioluminescent assay Microtox. Ecotoxicology 2016, 25, 1438–1444. [Google Scholar] [CrossRef] [PubMed]
- Dzionek, A.; Dzik, J.; Wojcieszyńska, D.; Guzik, U. Fluorescein diacetate hydrolysis using the whole biofilm as a sensitive tool to evaluate the physiological state of immobilized bacterial cells. Catalysts 2018, 8, 434. [Google Scholar] [CrossRef]
- Mara, K.; Decorosi, F.; Viti, C.; Giovannetti, L.; Papaleo, M.C.; Maida, I.; Perrin, E.; Fondi, M.; Vaneechoutte, M.; Nemec, A.; et al. Molecular and phenotypic characterization of Acinetobacter strains able to degrade diesel fuel. Res. Microbiol. 2012, 163, 161e172. [Google Scholar] [CrossRef]
- Galardini, M.; Mengoni, A.; Biondi, E.G.; Semeraro, R.; Florio, A.; Bazzicalupo, M.; Benedetti, A.; Mocali, S. DuctApe: A suite for the analysis and correlation of genomic and OmniLog Phenotype Microarray data. Genomics 2014, 103, 1e10. [Google Scholar] [CrossRef] [PubMed]
- Greń, I.; Wojcieszyńska, D.; Guzik, U.; Perkosz, M.; Hupert-Kocurek, K. Enhanced biotransformation of mononitrophenols by Stenotrophomonas maltophilia KB2 in the presence of aromatic compounds of plant origin. World J. Microbiol. Biotechnol. 2010, 26, 289–295. [Google Scholar] [CrossRef]

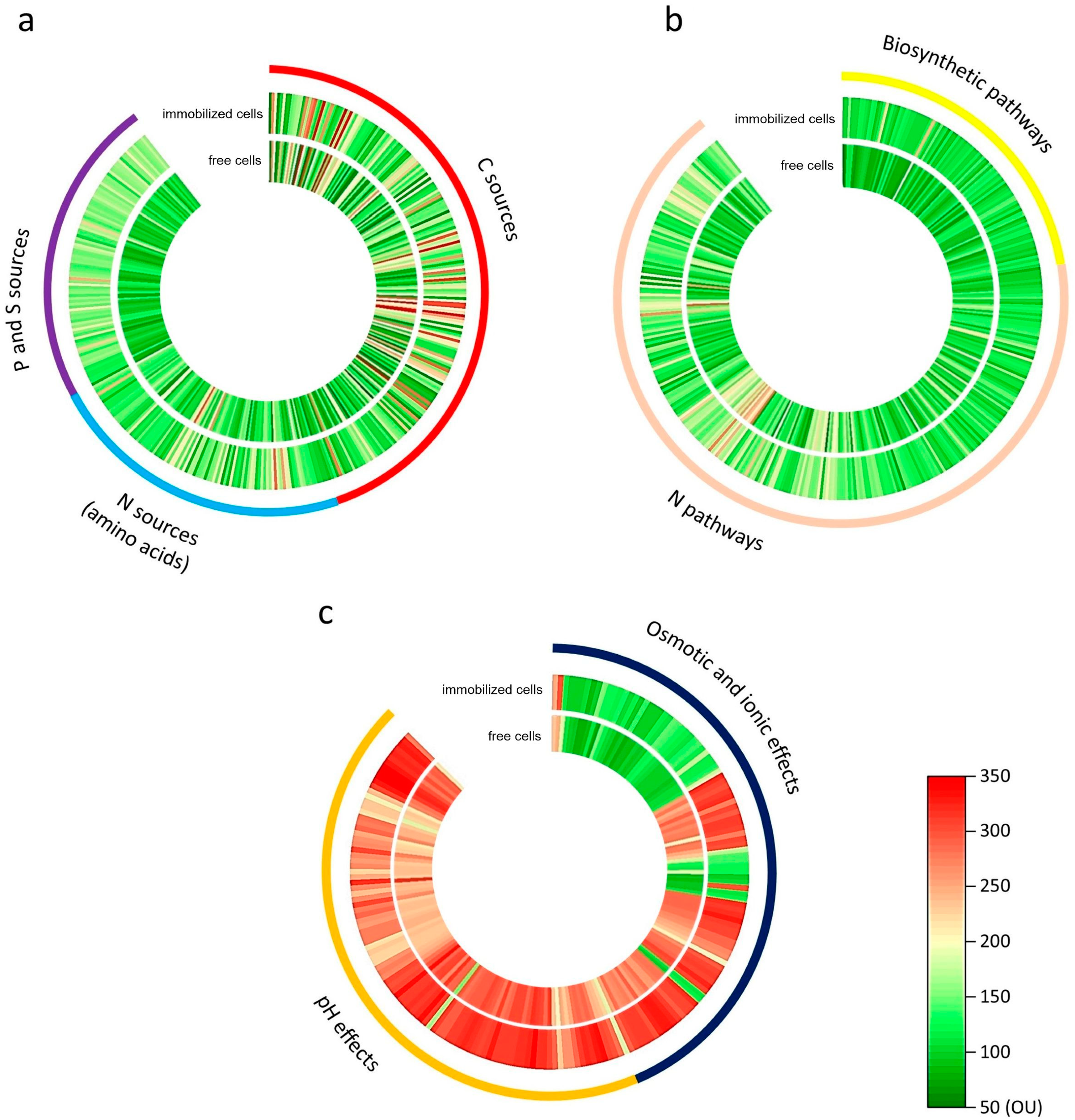
| Functional Group | Free Cells * | Immobilized Cells * |
|---|---|---|
| C sources | 121 ± 52.4 a | 147 ± 52.2 b |
| N sources | 108 ± 29.7 a | 127 ± 37.9 b |
| S and P sources | 94 ± 14.2 a | 138 ± 18.8 b |
| Biosynthetic pathways | 94 ± 14.8 a | 107 ± 15.9 b |
| N pathways | 121 ± 30.2 a | 138 ± 42.4 b |
| Osmotic and ion effects | 193 ± 78.9 a | 216 ± 91.4 b |
| pH effects | 258 ± 35.5 a | 286 ± 40.2 b |
| Free Cells | Immobilized Cells | |||||
|---|---|---|---|---|---|---|
| Compounds | EC50 mg/L | MIC mg/L | NIC mg/L | EC50 mg/L | MIC mg/L | NIC mg/L |
| Ibuprofen | 1175.0 | 1909.0 | 533.0 | 1190.0 | 1798.0 | 603.7 |
| Catechol | 36.9 | 67.2 | 21.7 | 39.5 | 62.2 | 26.4 |
| 1-hydroxyibuprofen | 809.3 | 1103.0 | 577.2 | 787.5 | 1102.0 | 547.1 |
| 1,2,4-benzenetriol | 35.5 | 371.7 | 3.6 | 27.5 | 60.0 | 13.2 |
| 2-(4-hydroxyphenyl)propionic acid | Lack of toxicity to 2 g/L | Lack of toxicity to 2 g/L | ||||
| Concentration [mg/L] | ||||||
| 0 | 0.625 | 1.25 | 2.50 | 5.00 | 10.00 | |
| Survival rate [%] * | ||||||
| Ibuprofen | 100.0 a ± 0.0 | 100.0 a ± 0.0 | 100.0 a ± 0.0 | 100.0 a ± 0.0 | 100.0 a ± 0.0 | 96.7 a ± 0.0 |
| Concentration [%] | ||||||
| 0 | 0.625 | 12.50 | 25.00 | 50.00 | 100.00 | |
| Survival rate [%] * | ||||||
| Ibuprofen’s post-breeding broth (free cells system) | 100.0 ± 0.0 a | 100.0 ± 0.0 a | 100.0 ± 0.0 a | 100.0 ± 0.0 a | 96.7 ± 0.0 a | 86.7 ± 0.0 a |
| Ibuprofen’s post-breeding broth (immobilized cells system) [%] | 100.0 ± 0.0 a | 100.0 ± 0.0 a | 100.0 ± 0.0 a | 100.0 ± 0.0 a | 100.0 ± 0.0 a | 90.0 ± 10.0 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchlewicz, A.; Dzionek, A.; Wojcieszyńska, D.; Borgulat, J.; Jałowiecki, Ł.; Guzik, U. Changes in Ibuprofen Toxicity and Degradation in Response to Immobilization of Bacillus thuringiensis B1(2015b). Molecules 2024, 29, 5680. https://doi.org/10.3390/molecules29235680
Marchlewicz A, Dzionek A, Wojcieszyńska D, Borgulat J, Jałowiecki Ł, Guzik U. Changes in Ibuprofen Toxicity and Degradation in Response to Immobilization of Bacillus thuringiensis B1(2015b). Molecules. 2024; 29(23):5680. https://doi.org/10.3390/molecules29235680
Chicago/Turabian StyleMarchlewicz, Ariel, Anna Dzionek, Danuta Wojcieszyńska, Jacek Borgulat, Łukasz Jałowiecki, and Urszula Guzik. 2024. "Changes in Ibuprofen Toxicity and Degradation in Response to Immobilization of Bacillus thuringiensis B1(2015b)" Molecules 29, no. 23: 5680. https://doi.org/10.3390/molecules29235680
APA StyleMarchlewicz, A., Dzionek, A., Wojcieszyńska, D., Borgulat, J., Jałowiecki, Ł., & Guzik, U. (2024). Changes in Ibuprofen Toxicity and Degradation in Response to Immobilization of Bacillus thuringiensis B1(2015b). Molecules, 29(23), 5680. https://doi.org/10.3390/molecules29235680










