Laser-Induced Graphene Decorated with MOF-Derived NiCo-LDH for Highly Sensitive Non-Enzymatic Glucose Sensor
Abstract
1. Introduction
2. Results and Discussion
2.1. Fabrication and Performance Optimization of LIG on PI
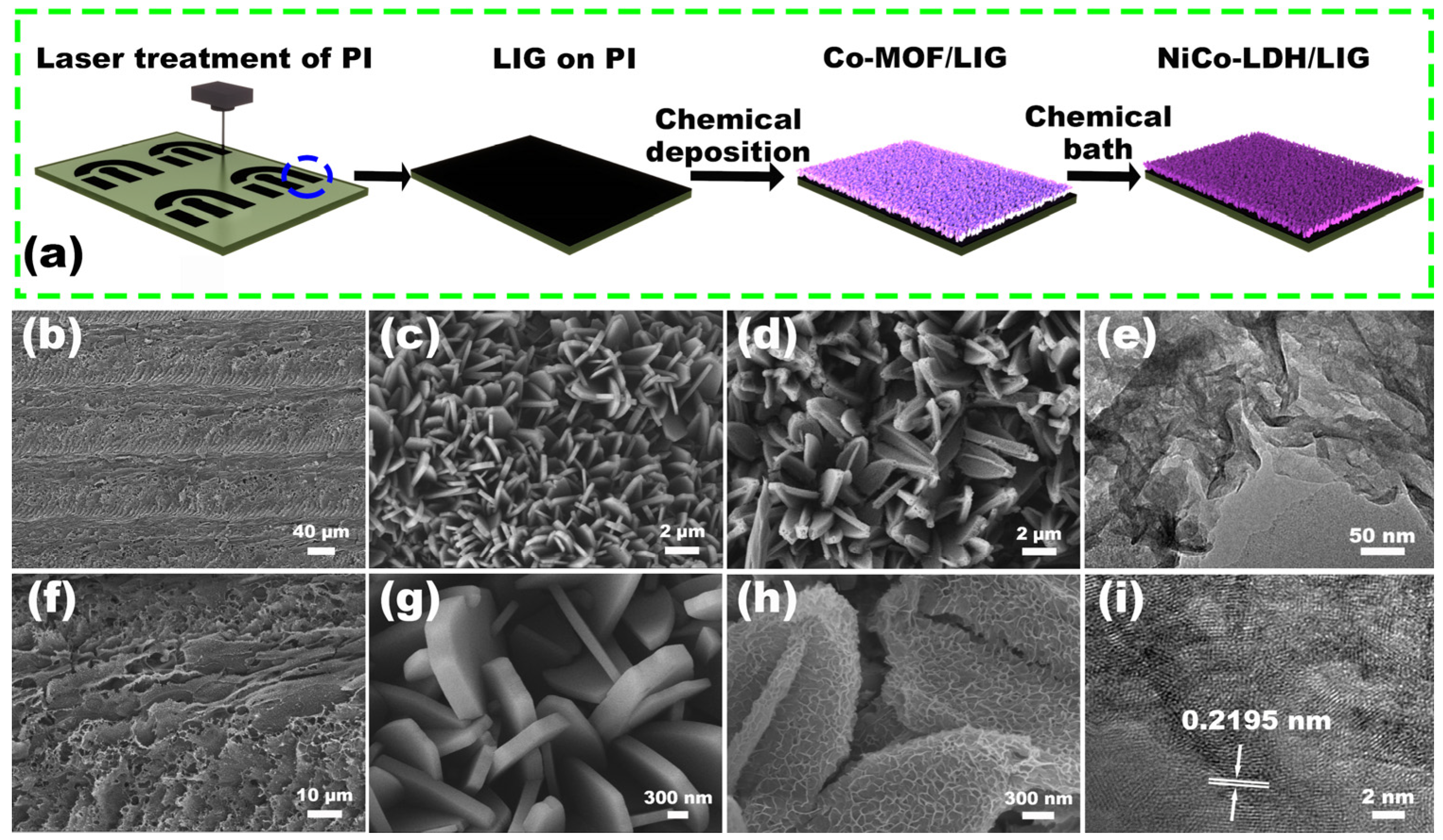
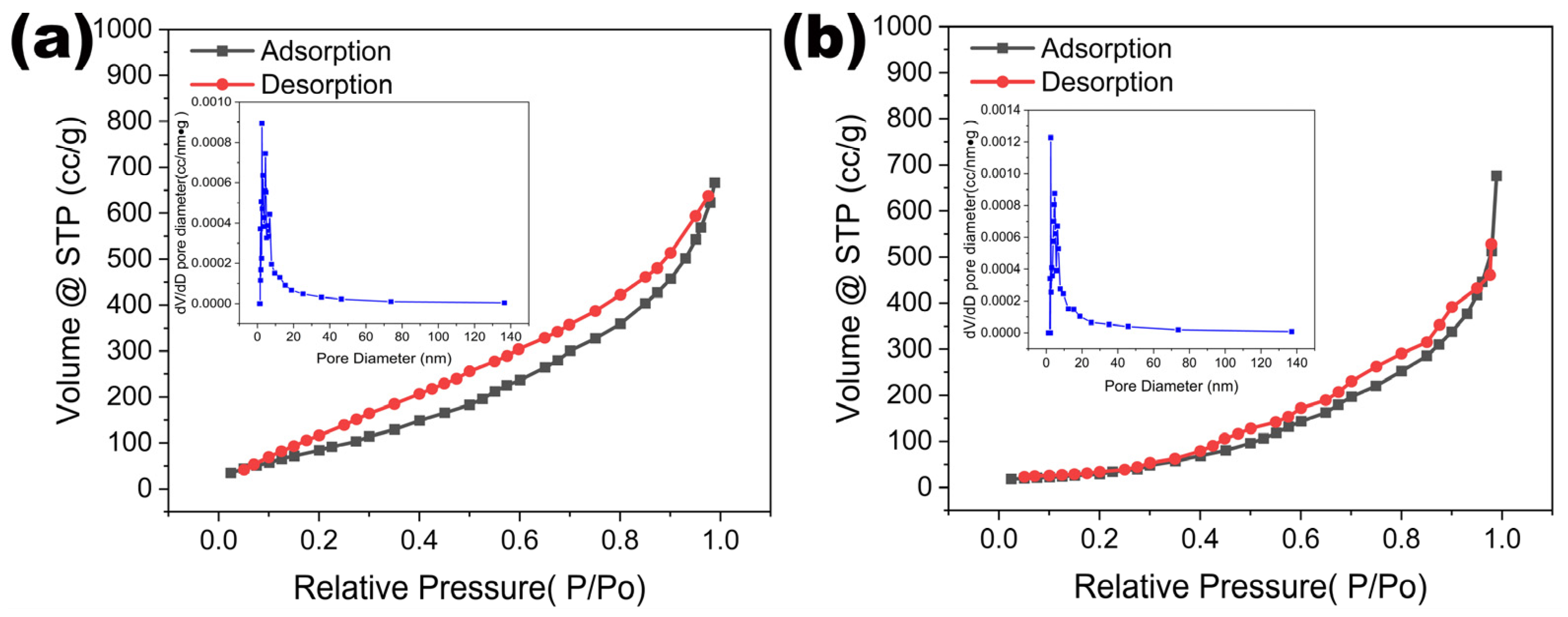
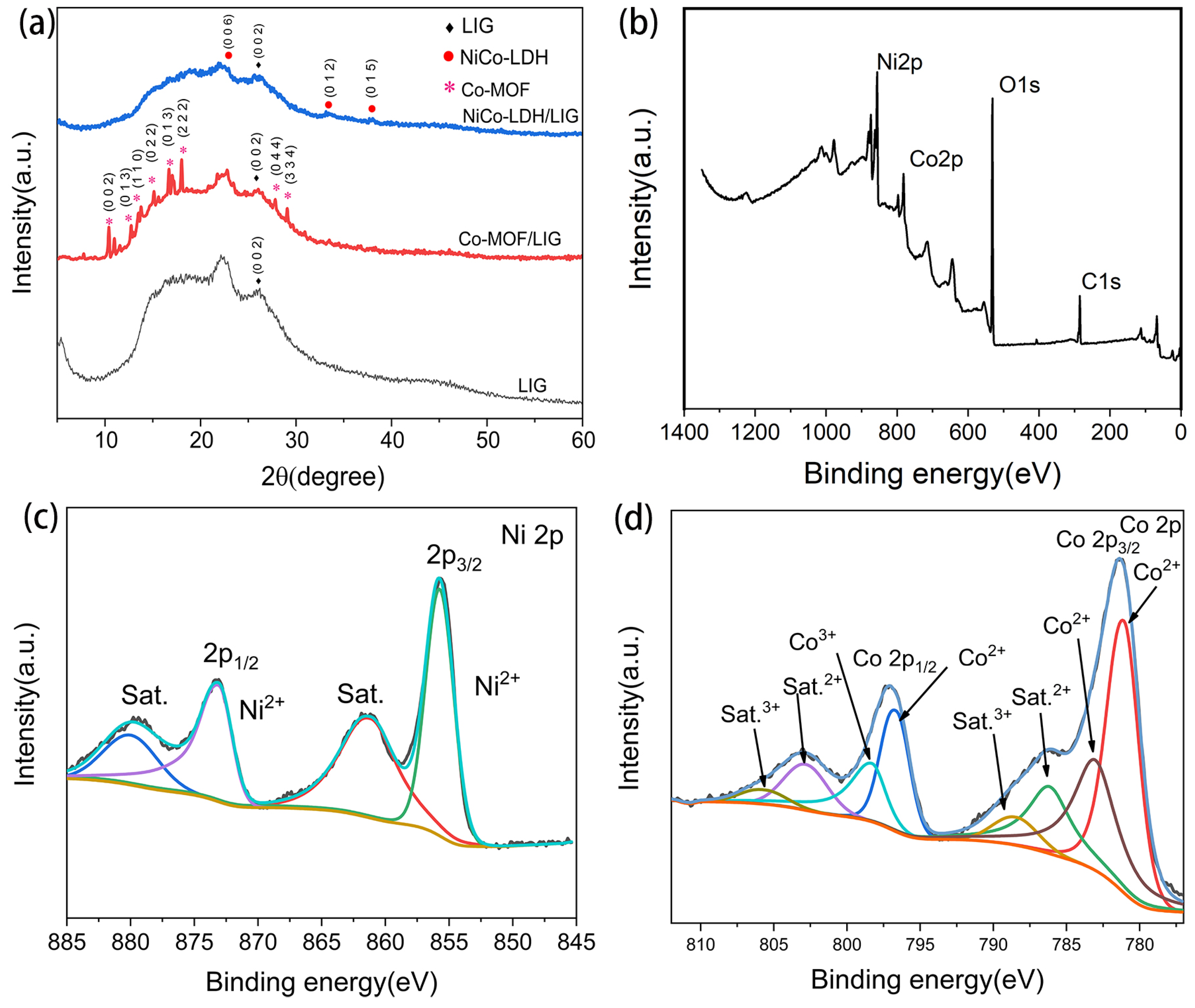
2.2. Fabrication and Structure Characterizations of NiCo-LDH/LIG
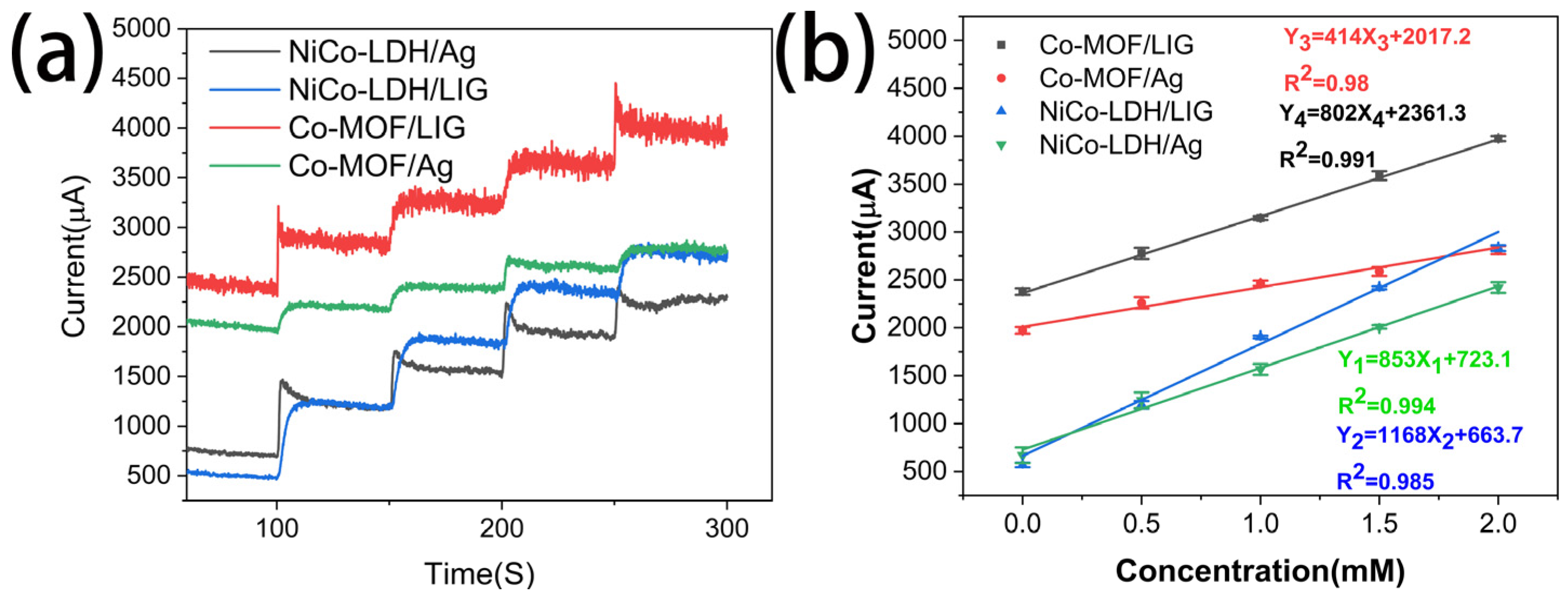
2.3. Electrochemical Measurements of the NiCo-LDH/LIG Electrode
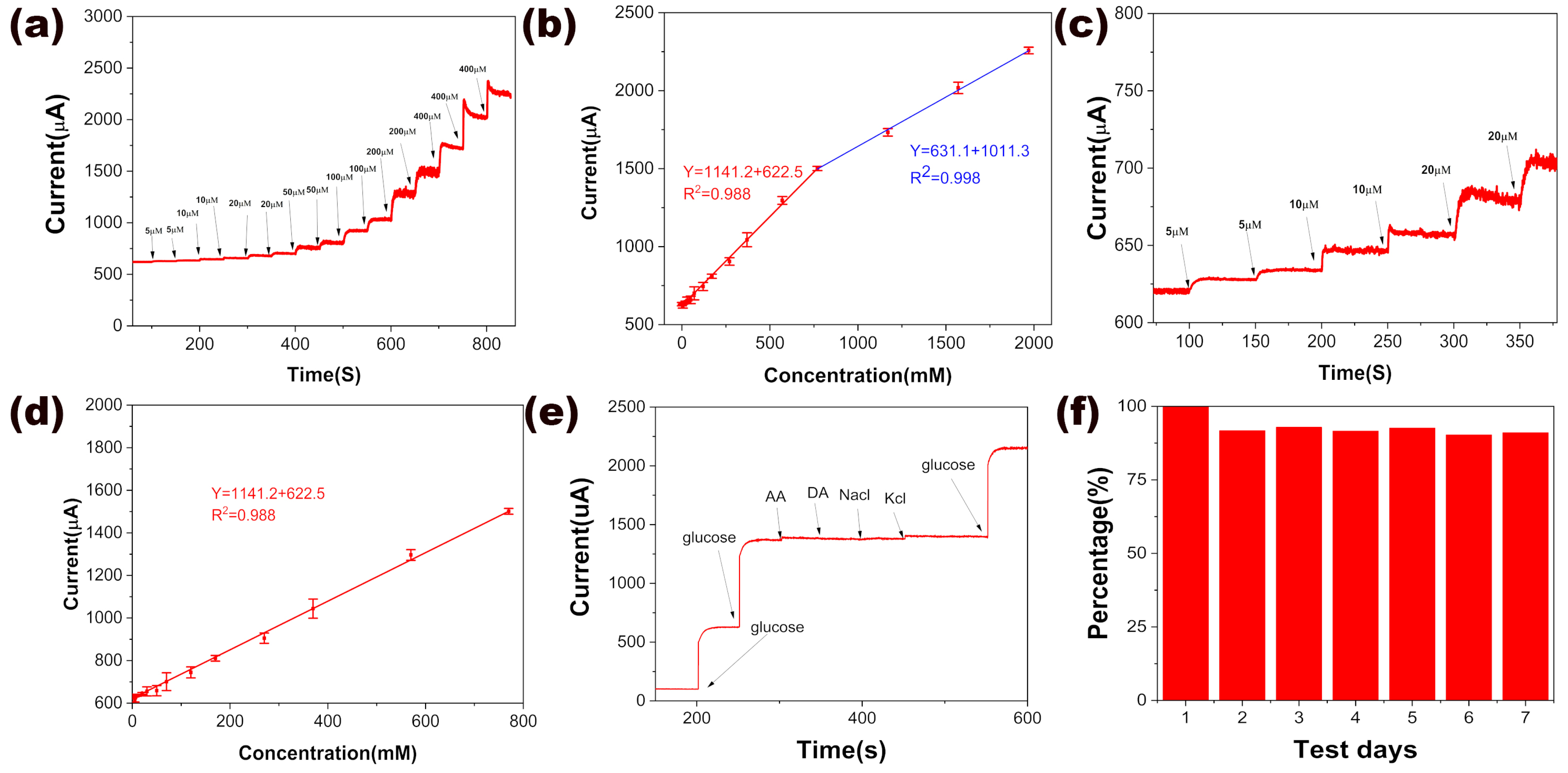
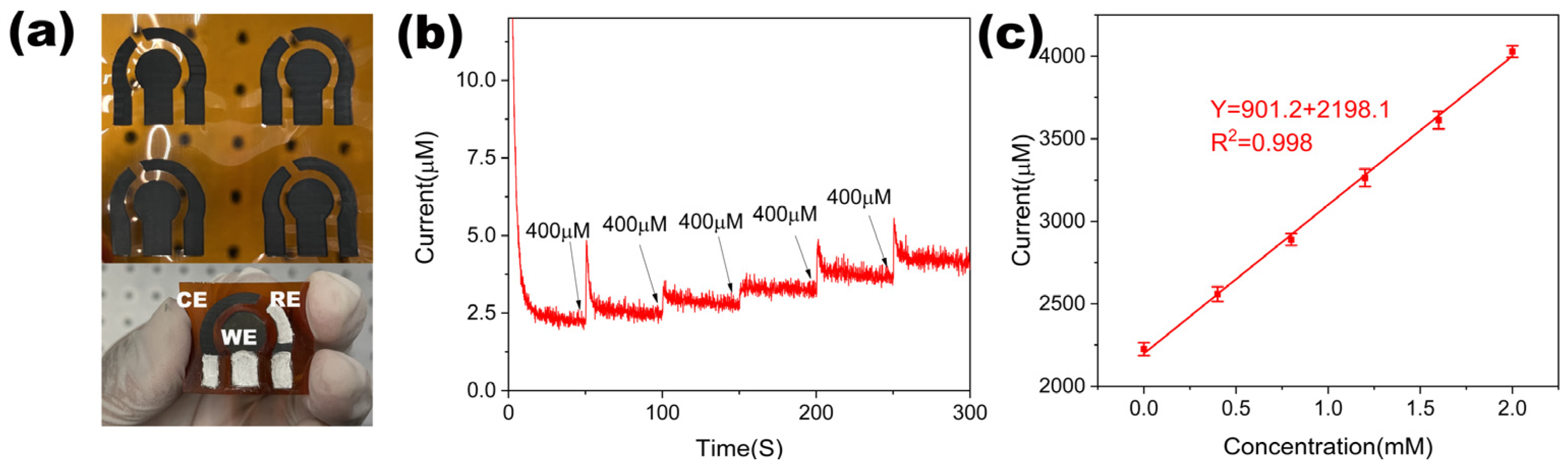
2.4. Electrochemical Measurements of the NiCo-LDH/LIG-Based Glucose Sensor
3. Materials and Methods
3.1. Materials Preparation
3.2. Synthesis of NiCo-LDH Sensing Electrode
3.3. Fabrication of NiCo-LDH/LIG-Based Glucose Sensor
3.4. Material Characterizations
3.5. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, W.; Qi, H.; Wang, B.; Wang, Q.; Wei, S.; Zhang, X.; Wang, Y.; Zhang, L.; Cui, X. Ultrathin NiCo2O4 nanowalls supported on a 3D nanoporous gold coated needle for non-enzymatic amperometric sensing of glucose. Microchim. Acta 2018, 185, 124. [Google Scholar] [CrossRef]
- Xiong, L.-Y.; Kim, Y.-J.; Seo, W.-C.; Lee, H.-K.; Yang, W.-C.; Xie, W.-F. High-performance non-enzymatic glucose sensor based on Co3O4/rGO nanohybrid. Rare Met. 2023, 42, 3046–3053. [Google Scholar] [CrossRef]
- Zhang, H.; Hua, S.F.; Zhang, L. Co-immobilization of cellulase and glucose oxidase on graphene oxide by covalent bonds: A biocatalytic system for one-pot conversion of gluconic acid from carboxymethyl cellulose. J. Chem. Technol. Biotechnol. 2020, 95, 1116–1125. [Google Scholar] [CrossRef]
- Kim, K.M.; Nguyen, P.T.; Kim, J.; Song, S.H.; Park, J.W.; Kim, M.I. Chemiluminescence Immunoassay for Sensitive Detection of C-reactive Protein Using Graphene Oxide–Gold Nanoparticle–Luminol Hybrids as Enhanced Luminogenic Molecules. Chemosensors 2024, 12, 193. [Google Scholar] [CrossRef]
- Rabchinskii, M.K.; Besedina, N.A.; Brzhezinskaya, M.; Stolyarova, D.Y.; Ryzhkov, S.A.; Saveliev, S.D.; Antonov, G.A.; Baidakova, M.V.; Pavlov, S.I.; Kirilenko, D.A.; et al. Graphene Amination towards Its Grafting by Antibodies for Biosensing Applications. Nanomaterials 2023, 13, 1730. [Google Scholar] [CrossRef]
- Moon, J.S.; Seo, H.C.; Stratan, F.; Antcliffe, M.; Schmitz, A.; Ross, R.S.; Kiselev, A.A.; Wheeler, V.D.; Nyakiti, L.O.; Gaskill, D.K.; et al. Lateral Graphene Heterostructure Field-Effect Transistor. IEEE Electron Device Lett. 2013, 34, 1190–1192. [Google Scholar] [CrossRef]
- Safari, A.; Dousti, M.; Tavakoli, M.B. Monolayer Graphene Field Effect Transistor-Based Operational Amplifier. J. Circuits Syst. Comput. 2019, 28, 1950052. [Google Scholar] [CrossRef]
- Sotoudeh, A.; Amirmazlaghani, M. Graphene-based Field Effect Diode. Superlattices Microstruct. 2018, 120, 828–836. [Google Scholar] [CrossRef]
- Tian, J.F.; Jauregui, L.A.; Lopez, G.; Cao, H.; Chen, Y.P. Ambipolar graphene field effect transistors by local metal side gates. Appl. Phys. Lett. 2010, 96, 263110. [Google Scholar] [CrossRef]
- Ishikawa, R.; Kurokawa, Y.; Miyajima, S.; Konagai, M. Graphene transparent electrode for thin-film solar cells. In Proceedings of the 19th International Conference on Ternary and Multinary Compounds (ICTMC), Niigata, Japan, 1–5 September 2014; pp. 777–780. [Google Scholar]
- Mankowski, T.S.; Zhu, Z.Z.; Balakrishnan, K.; Shikoh, A.S.; Touati, F.; Benammar, M.; Mansuripur, M.; Falco, C.M. Metal nanowire-graphene composite transparent electrodes. In Proceedings of the Conference on Thin Films for Solar and Energy Technology VI, San Diego, CA, USA, 17–20 August 2014. [Google Scholar]
- Woo, Y.S. Transparent Conductive Electrodes Based on Graphene-Related Materials. Micromachines 2018, 10, 13. [Google Scholar] [CrossRef]
- Chen, K.N.; Wang, Q.R.; Niu, Z.Q.; Chen, J. Graphene-based materials for flexible energy storage devices. J. Energy Chem. 2018, 27, 12–24. [Google Scholar] [CrossRef]
- Fan, X.L.; Chen, X.L.; Dai, L.M. 3D graphene based materials for energy storage. Curr. Opin. Colloid Interface Sci. 2015, 20, 429–438. [Google Scholar] [CrossRef]
- Poorna, A.R.; Saravanathamizhan, R.; Balasubramanian, N. Graphene and graphene-like structure from biomass for Electrochemical Energy Storage application- A Review. Electrochem. Sci. Adv. 2021, 1, e2000028. [Google Scholar] [CrossRef]
- Wang, B.; Ruan, T.T.; Chen, Y.; Jin, F.; Peng, L.; Zhou, Y.; Wang, D.L.; Dou, S.X. Graphene-based composites for electrochemical energy storage. Energy Storage Mater. 2020, 24, 22–51. [Google Scholar] [CrossRef]
- Bollella, P.; Fusco, G.; Tortolini, C.; Sanzò, G.; Favero, G.; Gorton, L.; Antiochia, R. Beyond graphene: Electrochemical sensors and biosensors for biomarkers detection. Biosens. Bioelectron. 2017, 89, 152–166. [Google Scholar] [CrossRef]
- Justino, C.I.L.; Gomes, A.R.; Freitas, A.C.; Duarte, A.C.; Rocha-Santos, T.A.P. Graphene based sensors and biosensors. TrAC Trends Anal. Chem. 2017, 91, 53–66. [Google Scholar] [CrossRef]
- Reddy, Y.V.M.; Shin, J.H.; Palakollu, V.N.; Sravani, B.; Choi, C.-H.; Park, K.; Kim, S.-K.; Madhavi, G.; Park, J.P.; Shetti, N.P. Strategies, advances, and challenges associated with the use of graphene-based nanocomposites for electrochemical biosensors. Adv. Colloid Interface Sci. 2022, 304, 102664. [Google Scholar] [CrossRef]
- Warner, J.H.; Schaffel, F.; Rummeli, M.; Bachmatiuk, A. Graphene: Fundamentals and Emergent Applications; Newnes: London, UK, 2012. [Google Scholar]
- Tung, V.C.; Allen, M.J.; Yang, Y.; Kaner, R.B. High-throughput solution processing of large-scale graphene. Nat. Nanotechnol. 2009, 4, 25–29. [Google Scholar] [CrossRef]
- Ullah, S.; Hasan, M.; Ta, H.Q.; Zhao, L.; Shi, Q.T.; Fu, L.; Choi, J.; Yang, R.Z.; Liu, Z.F.; Rümmeli, M.H.; et al. Synthesis of Doped Porous 3D Graphene Structures by Chemical Vapor Deposition and Its Applications. Adv. Funct. Mater. 2019, 29, 1904457. [Google Scholar] [CrossRef]
- Lin, J.; Peng, Z.W.; Liu, Y.Y.; Ruiz-Zepeda, F.; Ye, R.Q.; Samuel, E.L.G.; Yacaman, M.J.; Yakobson, B.I.; Tour, J.M. Laser-induced porous graphene films from commercial polymers. Nat. Commun. 2014, 5, 5714. [Google Scholar] [CrossRef]
- Thaweeskulchai, T.; Sakdaphetsiri, K.; Schulte, A. Ten years of laser-induced graphene: Impact and future prospect on biomedical, healthcare, and wearable technology. Microchim. Acta 2024, 191, 292. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Huang, X.; Song, W. Physical and chemical sensors on the basis of laser-induced graphene: Mechanisms, applications, and perspectives. ACS Nano 2021, 15, 18708–18741. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Astruc, D. State of the art and prospects in metal–organic framework (MOF)-based and MOF-derived nanocatalysis. Chem. Rev. 2020, 120, 1438–1511. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-G.; Liang, Z.B.; Gao, S.; Qu, C.; Zou, R.G. Metal-organic framework-based materials for hybrid supercapacitor application. Coord. Chem. Rev. 2020, 404, 213093. [Google Scholar] [CrossRef]
- Sheberla, D.; Bachman, J.C.; Elias, J.S.; Sun, C.-J.; Shao-Horn, Y.; Dinca, M. Conductive MOF electrodes for stable supercapacitors with high areal capacitance. Nat. Mater. 2017, 16, 220–224. [Google Scholar] [CrossRef]
- Salunkhe, R.R.; Kaneti, Y.V.; Kim, J.; Kim, J.H.; Yamauchi, Y. Nanoarchitectures for Metal-Organic Framework-Derived Nanoporous Carbons toward Supercapacitor Applications. Acc. Chem. Res. 2016, 49, 2796–2806. [Google Scholar] [CrossRef]
- Worrall, S.D.; Mann, H.; Rogers, A.; Bissett, M.A.; Attfield, M.P.; Dryfe, R.A.W. Electrochemical deposition of zeolitic imidazolate framework electrode coatings for supercapacitor electrodes. Electrochim. Acta 2016, 197, 228–240. [Google Scholar] [CrossRef]
- Wang, B.; Shang, J.; Guo, C.; Zhang, J.; Zhu, F.; Han, A.; Liu, J. A General Method to Ultrathin Bimetal-MOF Nanosheets Arrays via In Situ Transformation of Layered Double Hydroxides Arrays. Small 2019, 15, e1804761. [Google Scholar] [CrossRef]
- Aziz, A.; Asif, M.; Ashraf, G.; Azeem, M.; Majeed, I.; Ajmal, M.; Wang, J.; Liu, H. Advancements in electrochemical sensing of hydrogen peroxide, glucose and dopamine by using 2D nanoarchitectures of layered double hydroxides or metal dichalcogenides. A review. Microchim. Acta 2019, 186, 671. [Google Scholar] [CrossRef]
- Lv, J.; Wu, M.; Fan, M.; Zhang, Q.; Chang, Z.; Wang, X.; Zhou, Q.; Wang, L.; Chong, R.; Zhang, L. Insights into the multirole CoAl layered double hydroxide on boosting photoelectrochemical activity of hematite: Application to hydrogen peroxide sensing. Talanta 2023, 262, 124681. [Google Scholar] [CrossRef]
- Rafique, N.; Asif, A.H.; Hirani, R.A.K.; Wu, H.; Shi, L.; Zhang, S.; Sun, H. Binder free 3D core–shell NiFe layered double hydroxide (LDH) nanosheets (NSs) supported on Cu foam as a highly efficient non-enzymatic glucose sensor. J. Colloid Interface Sci. 2022, 615, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.H.; Hui, Y.X.; Lu, X.J.; Liu, C.Z.; Zhang, Y.L.; Fan, Y.J.; Chen, W. One-pot preparation of NiMn layered double hydroxide-MOF material for highly sensitive electrochemical sensing of glucose. J. Electroanal. Chem. 2023, 933, 117276. [Google Scholar] [CrossRef]
- Riaz, S.; ur Rehman, A.; Akhter, Z.; Najam, T.; Hossain, I.; Karim, M.R.; Assiri, M.A.; Shah, S.S.A.; Nazir, M.A. Recent advancement in synthesis and applications of layered double hydroxides (LDHs) composites. Mater. Today Sustain. 2024, 27, 100897. [Google Scholar] [CrossRef]
- Liu, L.L.; Guan, T.; Fang, L.; Wu, F.; Lu, Y.; Luo, H.J.; Song, X.F.; Zhou, M.; Hu, B.S.; Wei, D.P.; et al. Self-supported 3D NiCo-LDH/Gr composite nanosheets array electrode for high-performance supercapacitor. J. Alloys Compd. 2018, 763, 926–934. [Google Scholar] [CrossRef]
- Lin, Y.P.; Li, Y.M.; Lan, Q.Y.; Lv, X.-J.; Liu, S.M.; Liu, D.; Hu, W.T. A novel strategy for preparing layered double hydroxide/exfoliated carbon nanostructures composites as superior electrochemical catalysts with respect to oxygen evolution and methanol oxidation. J. Alloys Compd. 2018, 744, 347–356. [Google Scholar] [CrossRef]
- Ni, G.; Cheng, J.; Dai, X.; Guo, Z.H.; Ling, X.; Yu, T.; Sun, Z.J. Integrating Ultrathin Polypyrrole Framework on Nickel-Cobalt Layered Double Hydroxide as an Amperometric Sensor for Non-enzymatic Glucose Determination. Electroanalysis 2018, 30, 2366–2373. [Google Scholar] [CrossRef]
- Wang, L.L.; Miao, X.L.; Qu, Y.N.; Duan, C.P.; Wang, B.; Yu, Q.L.; Gao, J.; Song, D.D.; Li, Y.T.; Yin, Z. Rattle-type Au@NiCo LDH hollow core-shell nanostructures for nonenzymatic glucose sensing. J. Electroanal. Chem. 2020, 858, 113810. [Google Scholar] [CrossRef]
- Zhao, J.; Zheng, C.; Gao, J.; Gui, J.; Deng, L.; Wang, Y.; Xu, R. Co3O4 nanoparticles embedded in laser-induced graphene for a flexible and highly sensitive enzyme-free glucose biosensor. Sens. Actuators B Chem. 2021, 347, 130653. [Google Scholar] [CrossRef]
- Cui, F.; Sun, H.; Yang, X.; Zhou, H.; Wu, Y.; Li, J.; Li, H.; Liu, J.; Zeng, C.; Qu, B. Laser-induced graphene (LIG)-based Au@ CuO/V2CTx MXene non-enzymatic electrochemical sensors for the urine glucose test. Chem. Eng. J. 2023, 457, 141303. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, Y.; Song, J.; Li, Y.; Xie, Y.; Cui, H.; Gong, W.; Hu, J.; Chen, Y. Highly sensitive nonenzymetic glucose sensing based on multicomponent hierarchical NiCo-LDH/CCCH/CuF nanostructures. Sens. Actuators B Chem. 2021, 326, 128811. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, Y.; Wu, J.; He, Z.; Xing, Y.; Yang, J.; Zou, Z.; Huang, K.; Yu, H.; Xiong, X. Ultrafast construction of 3D ultrathin NiCo-LDH@Cu heteronanosheet array by plasma magnetron sputtering for non-enzymatic glucose sensing in beverage and human serum. Food Chem. 2022, 393, 133399. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tian, W.; Wan, C.; Liu, S.; Liu, X.; Su, J.; Chai, H.; Wu, Y. Hierarchically porous cellulose nanofibril aerogel decorated with polypyrrole and nickel-cobalt layered double hydroxide for high-performance nonenzymatic glucose sensors. Front. Chem. Sci. Eng. 2023, 17, 1593–1607. [Google Scholar] [CrossRef]
- Chen, H.; Mei, Z.; Qi, K.; Wang, Y.; Chen, R. A wearable enzyme-free glucose sensor based on nickel nanoparticles decorated laser-induced graphene. J. Electroanal. Chem. 2022, 920, 116585. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, N.; Xiang, Y.; Wang, D.; Zhang, P.; Wang, Y.; Lu, S.; Xu, R.; Zhao, J. A flexible non-enzymatic glucose sensor based on copper nanoparticles anchored on laser-induced graphene. Carbon 2020, 156, 506–513. [Google Scholar] [CrossRef]
- Li, C.; Xiong, J.; Zheng, C.; Zhao, J. Screen-printing preparation of high-performance nonenzymatic glucose sensors based on Co3O4 nanoparticles-embedded N-doped laser-induced graphene. ACS Appl. Nano Mater. 2022, 5, 16655–16663. [Google Scholar] [CrossRef]
- Aparicio-Martínez, E.P.; Vega-Rios, A.; Osuna, V.; Dominguez, R.B. Salivary Glucose Detection with Laser Induced Graphene/AgNPs Non-Enzymatic Sensor. Biosensors 2023, 13, 207. [Google Scholar] [CrossRef]
- Prabhakaran, A.; Nayak, P. Surface engineering of laser-scribed graphene sensor enables non-enzymatic glucose detection in human body fluids. ACS Appl. Nano Mater. 2019, 3, 391–398. [Google Scholar] [CrossRef]
- Misra, U.; Dixit, N.; Singh, S.P. Effect of laser parameters on laser-induced graphene filter fabrication and its performance for desalination and water purification. ACS Appl. Mater. Interfaces 2023, 15, 7899–7910. [Google Scholar] [CrossRef]
- de la Roche, J.; López-Cifuentes, I.; Jaramillo-Botero, A. Influence of lasing parameters on the morphology and electrical resistance of polyimide-based laser-induced graphene (LIG). Carbon Lett. 2023, 33, 587–595. [Google Scholar] [CrossRef]
- Patella, B.; Parisi, A.; Moukri, N.; Gitto, F.; Busacca, A.; Aiello, G.; Russo, M.; O’Riordan, A.; Inguanta, R. Phosphate ions detection by using an electrochemical sensor based on laser-scribed graphene oxide on paper. Electrochim. Acta 2023, 461, 142600. [Google Scholar] [CrossRef]
- Lee, J.-U.; Lee, C.-W.; Cho, S.-C.; Shin, B.-S. Laser-induced graphene heater pad for de-icing. Nanomaterials 2021, 11, 3093. [Google Scholar] [CrossRef]
- Ray, A.; Roth, J.; Saruhan, B. Laser-induced interdigital structured graphene electrodes based flexible micro-supercapacitor for efficient peak energy storage. Molecules 2022, 27, 329. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Du, K.; Wang, X.; Han, Y.; Li, L.; Wen, G. Fabrication of Hierarchical MOF-Derived NiCo2S4@ Mo-Doped Co-LDH Arrays for High-Energy-Density Asymmetric Supercapacitors. Nanomaterials 2023, 13, 2663. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Li, J.; Lu, J.; Luo, S.; Wan, J.; Li, B.; Hu, C.; Cheng, X. High mass-loading NiCo-LDH nanosheet arrays grown on carbon cloth by electrodeposition for excellent electrochemical energy storage. Nano Energy 2021, 86, 106079. [Google Scholar] [CrossRef]
- Huang, X.; Chu, B.; Han, B.; Wu, Q.; Yang, T.; Xu, X.; Wang, F.; Li, B. 2D-on-2D Al-Doped NiCo LDH Nanosheet Arrays for Fabricating High-Energy-Density, Wide Voltage Window, and Ultralong-Lifespan Supercapacitors. Small 2024, 20, 2401315. [Google Scholar] [CrossRef]
- Xuan, X.Y.; Qian, M.; Han, L.; Wan, L.J.; Li, Y.Q.; Lu, T.; Pan, L.K.; Niu, Y.P.; Gong, S.Q. In-situ growth of hollow NiCo layered double hydroxide on carbon substrate for flexible supercapacitor. Electrochim. Acta 2019, 321, 134710. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Shi, C.; Chen, Y.; Li, D.; He, Z.; Wang, C.; Guo, L.; Ma, J. Co-ZIF derived porous NiCo-LDH nanosheets/N doped carbon foam for high-performance supercapacitor. Carbon 2020, 165, 129–138. [Google Scholar] [CrossRef]
- Zhou, M.; Xu, F.; Zhang, S.; Liu, B.; Yang, Y.; Chen, K.; Qi, J. Low consumption design of hollow NiCo-LDH nanoflakes derived from MOFs for high-capacity electrode materials. J. Mater. Sci. Mater. Electron. 2020, 31, 3281–3288. [Google Scholar] [CrossRef]
- Jia, H.; Wang, Z.; Zheng, X.; Lin, J.; Liang, H.; Cai, Y.; Qi, J.; Cao, J.; Feng, J.; Fei, W. Interlaced Ni-Co LDH nanosheets wrapped Co9S8 nanotube with hierarchical structure toward high performance supercapacitors. Chem. Eng. J. 2018, 351, 348–355. [Google Scholar] [CrossRef]
- Shen, M.; Li, W.; Chen, L.; Chen, Y.X.; Ren, S.B.; Han, D.M. NiCo-LDH nanoflake arrays-supported Au nanoparticles on copper foam as a highly sensitive electrochemical non-enzymatic glucose sensor. Anal. Chim. Acta 2021, 1177, 338787. [Google Scholar] [CrossRef]
- Zahed, M.A.; Barman, S.C.; Das, P.S.; Sharifuzzaman, M.; Yoon, H.S.; Yoon, S.H.; Park, J.Y. Highly flexible and conductive poly (3,4-ethylene dioxythiophene)-poly (styrene sulfonate) anchored 3-dimensional porous graphene network-based electrochemical biosensor for glucose and pH detection in human perspiration. Biosens. Bioelectron. 2020, 160, 112220. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-C.; Kim, K.-B.; Gurudatt, N.G.; Hussain, K.K.; Choi, C.S.; Park, D.-S.; Shim, Y.-B. Comparison of enzymatic and non-enzymatic glucose sensors based on hierarchical Au-Ni alloy with conductive polymer. Biosens. Bioelectron. 2019, 130, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, F.; Vesali-Naseh, M.; Khodadadi, A.A.; Mortazavi, Y. A Comparison of a Nanostructured Enzymeless Au/Fe2O3/MWCNTs/GCE Electrode and a GOx Modified One in Electrocatalytic Detection of Glucose. Electroanalysis 2018, 30, 2044–2052. [Google Scholar] [CrossRef]
- Meng, W.; Wen, Y.Y.; Dai, L.; He, Z.X.; Wang, L. A novel electrochemical sensor for glucose detection based on Ag@ZIF-67 nanocomposite. Sens. Actuators B Chem. 2018, 260, 852–860. [Google Scholar] [CrossRef]
- Asadian, E.; Shahrokhian, S.; Zad, A.I. Highly sensitive nonenzymetic glucose sensing platform based on MOF-derived NiCo LDH nanosheets/graphene nanoribbons composite. J. Electroanal. Chem. 2018, 808, 114–123. [Google Scholar] [CrossRef]
- Xu, J.Q.; Qiao, X.J.; Arsalan, M.; Cheng, N.; Cao, W.; Yue, T.L.; Sheng, Q.L.; Zheng, J.B. Preparation of one dimensional silver nanowire/nickel-cobalt layered double hydroxide and its electrocatalysis of glucose. J. Electroanal. Chem. 2018, 823, 315–321. [Google Scholar] [CrossRef]
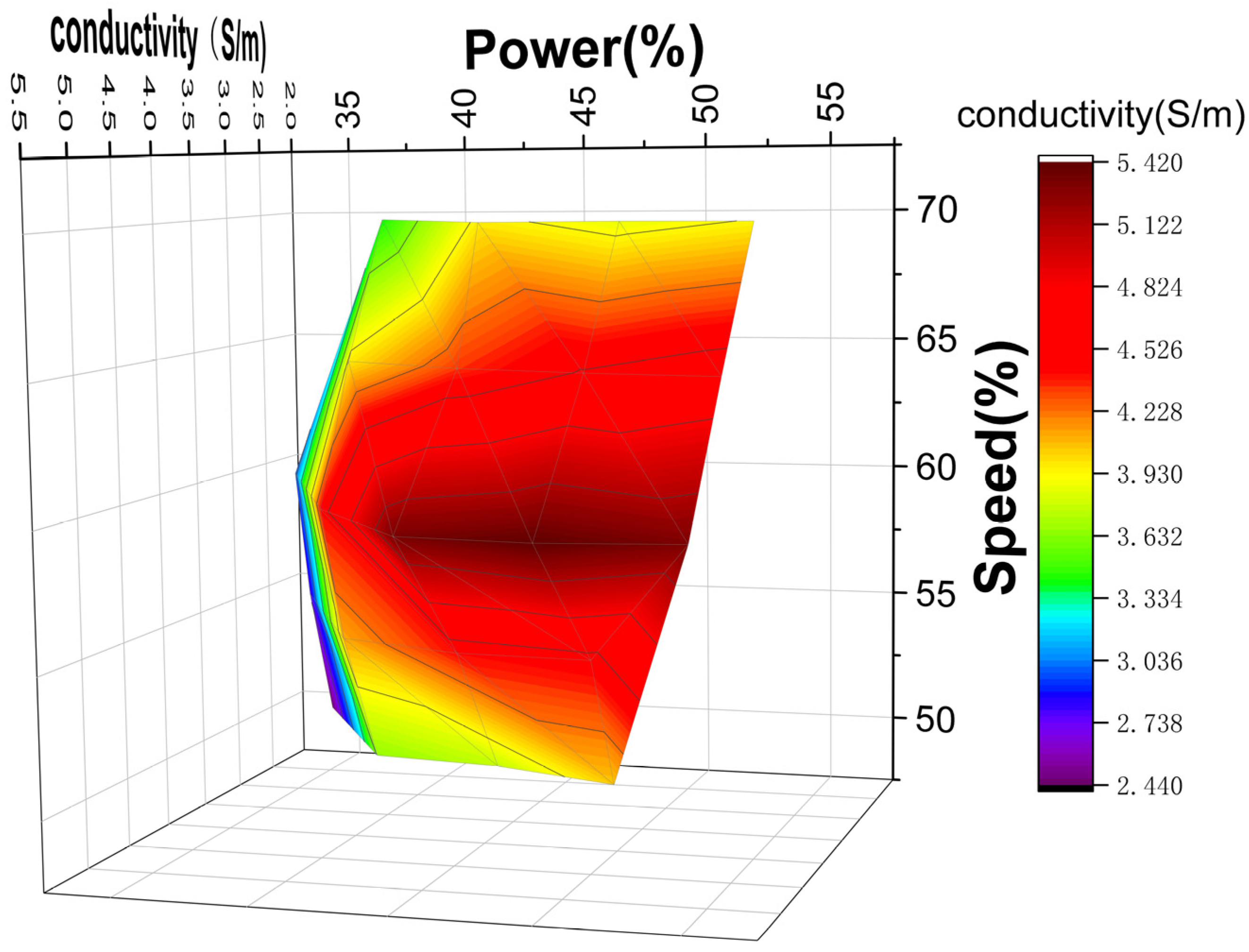


| Power (%) | Speed (%) | Conductivity (S/m) |
|---|---|---|
| 35 | 50, 55, 60 | 2.44, 2.76, 2.95 |
| 40 | 50, 55, 60, 65, 70 | 3.65, 4.12, 4.42, 3.97, 3.42 |
| 45 | 50, 55, 60, 65, 70 | 3.76, 4.41, 5.33, 4.34, 3.97 |
| 50 | 50, 55, 60, 65, 70 | 4.05, 4.46, 5.42, 4.54, 3.86 |
| 55 | 60, 65, 70 | 5.32, 4.65, 3.94 |
| Materials | Linear Range (mM) | Sensitivity (μA Mm−1 cm−2) | LOD (μM) | Reference |
|---|---|---|---|---|
| Cu NPS/LIG | — | 495 | 0.39 | [47] |
| Pt@Pd/PP/LIG | 0.01–9.2 | 247.3 | 3 | [64] |
| Au/Ni | 0–30 | 30.58 | 5.84 | [65] |
| Au/Fe2O3/f-MWCNTs | 0.01–0.08 | 512.4 | 1.71 | [66] |
| Ag@ZIF-67/GCE | 0.002–1 | 379 | 0.66 | [67] |
| NiCo-LDH/GNR/GCE | 0.005–0.8 | 344 | 0.60 | [68] |
| AgNW/NiCo LDH | 0.002–6 | 71.4 | 0.66 | [69] |
| NiCo-LDH/LIG | 0.05–1.97 | 1141.2 | 0.43 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Han, Y.; Zhang, Y.; Wu, W.; Du, W.; Wen, G.; Cheng, S. Laser-Induced Graphene Decorated with MOF-Derived NiCo-LDH for Highly Sensitive Non-Enzymatic Glucose Sensor. Molecules 2024, 29, 5662. https://doi.org/10.3390/molecules29235662
Li L, Han Y, Zhang Y, Wu W, Du W, Wen G, Cheng S. Laser-Induced Graphene Decorated with MOF-Derived NiCo-LDH for Highly Sensitive Non-Enzymatic Glucose Sensor. Molecules. 2024; 29(23):5662. https://doi.org/10.3390/molecules29235662
Chicago/Turabian StyleLi, Longxiao, Yufei Han, Yuzhe Zhang, Weijia Wu, Wei Du, Guojun Wen, and Siyi Cheng. 2024. "Laser-Induced Graphene Decorated with MOF-Derived NiCo-LDH for Highly Sensitive Non-Enzymatic Glucose Sensor" Molecules 29, no. 23: 5662. https://doi.org/10.3390/molecules29235662
APA StyleLi, L., Han, Y., Zhang, Y., Wu, W., Du, W., Wen, G., & Cheng, S. (2024). Laser-Induced Graphene Decorated with MOF-Derived NiCo-LDH for Highly Sensitive Non-Enzymatic Glucose Sensor. Molecules, 29(23), 5662. https://doi.org/10.3390/molecules29235662







