Influence of Hydrogen and Ethanol Addition in Methanogen-Free Mixed Culture Syngas Fermentations in Trickle Bed Reactors
Abstract
1. Introduction
2. Results and Discussion
2.1. Experimental Overview
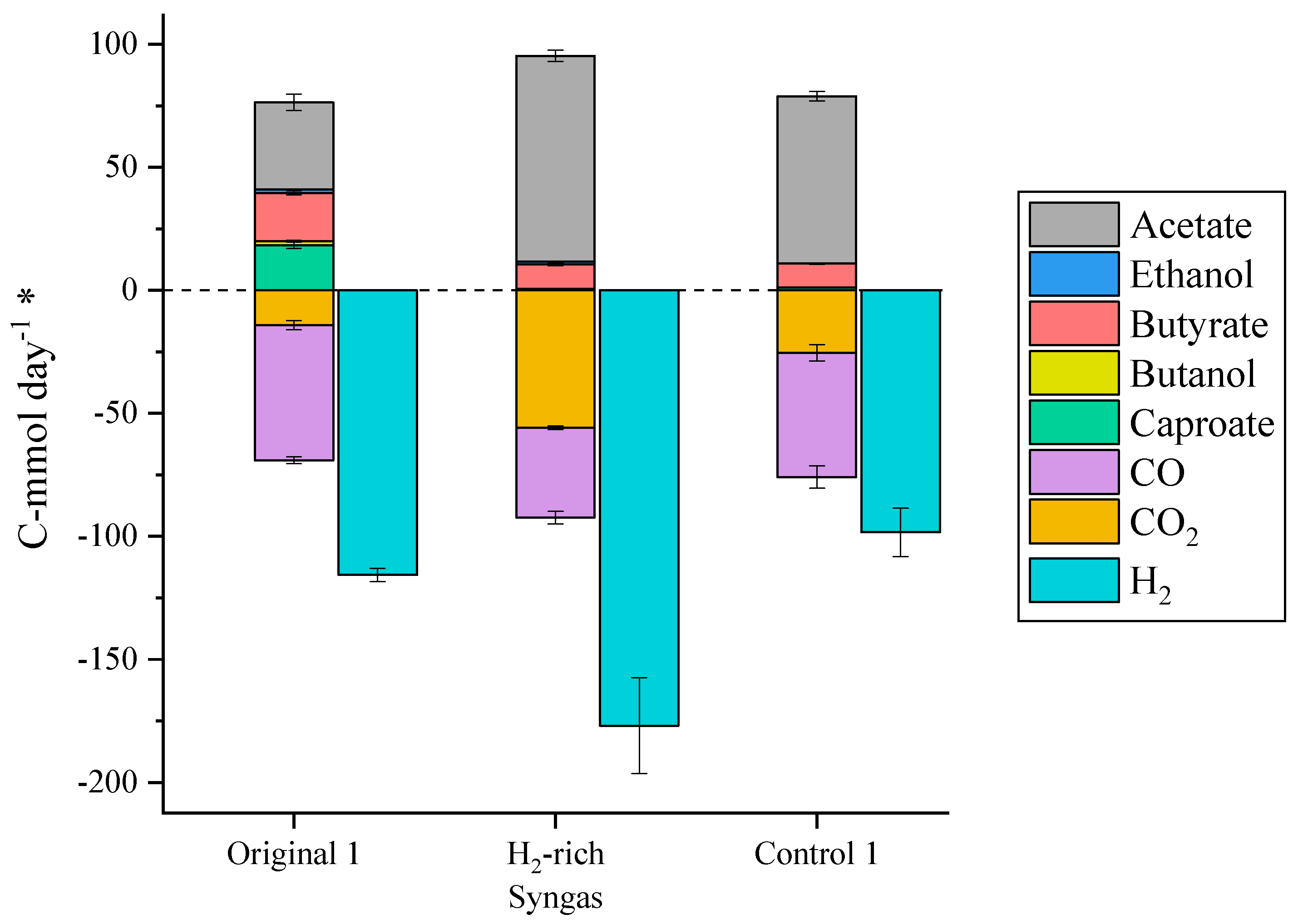
2.2. Gaseous Electron Donors
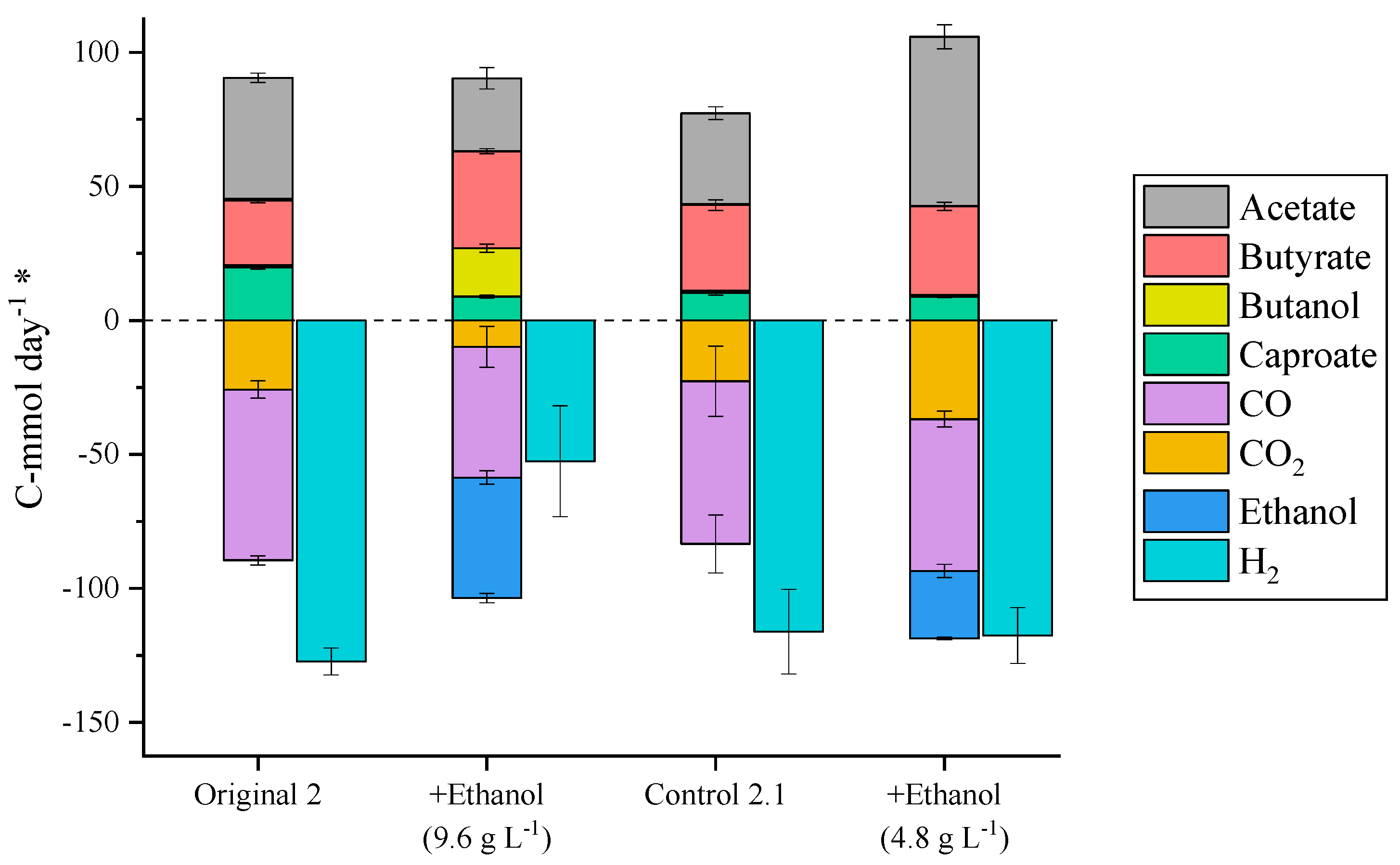
2.3. Exogenous Ethanol Addition
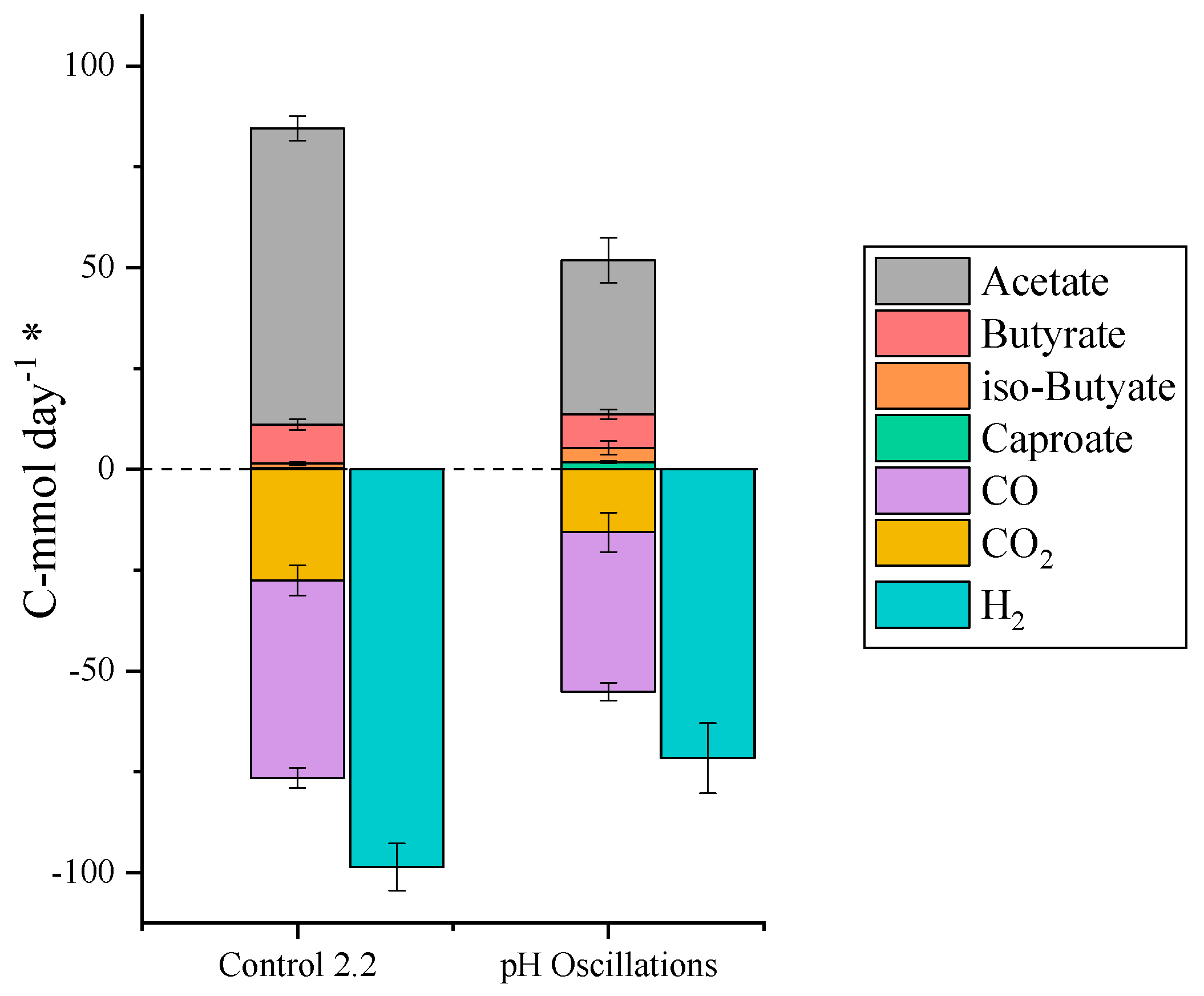
2.4. pH Oscillations to Improve Endogenous Ethanol Production
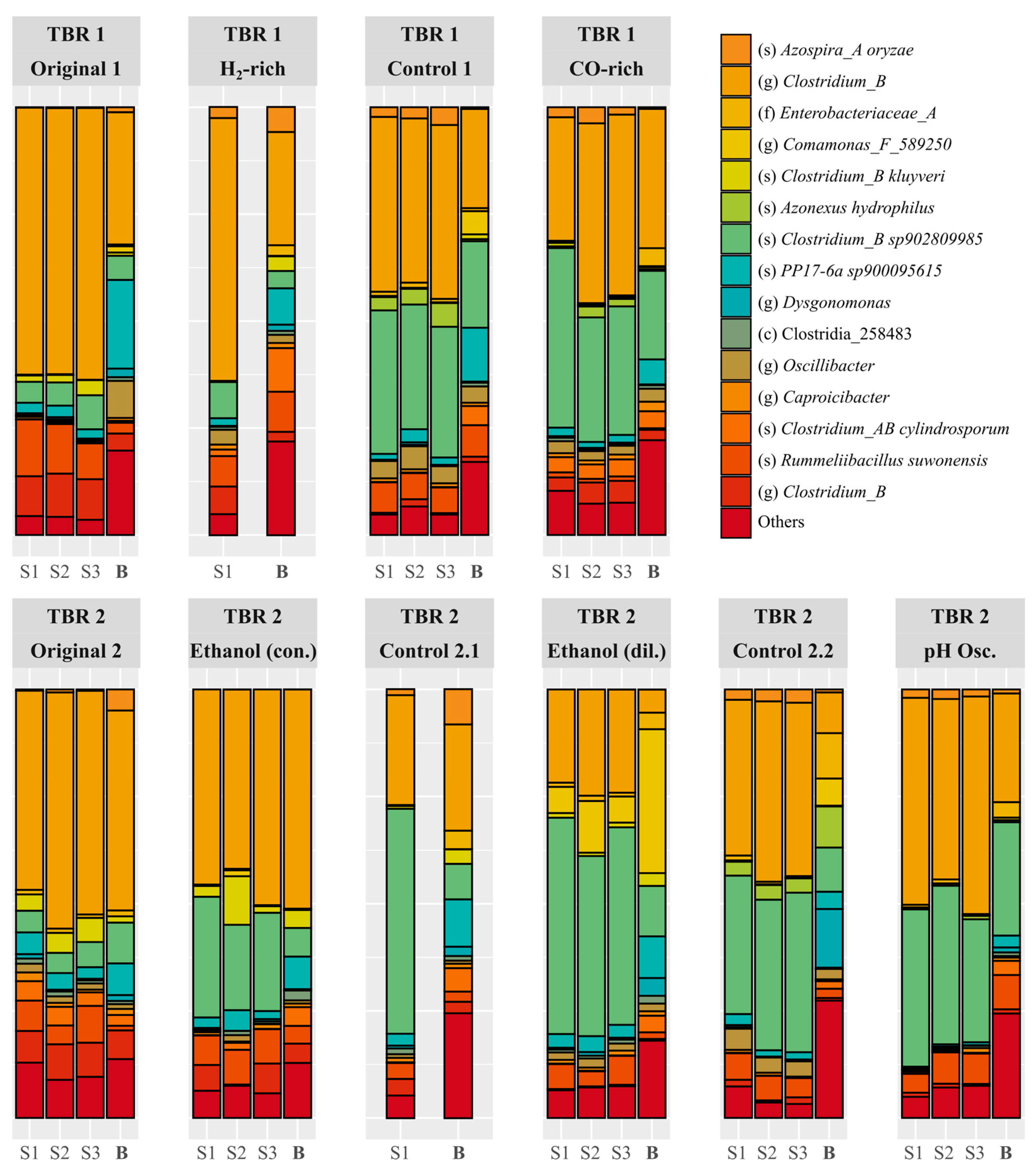
2.5. Evolution of the Microbial Community Through the Study
3. Materials and Methods
3.1. Growth Medium
3.2. Experimental Set-Up
3.3. Analytical Techniques and Stoichiometric Calculations
3.4. Community Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Fackler, N.; Heijstra, B.D.; Rasor, B.J.; Brown, H.; Martin, J.; Ni, Z.; Shebek, K.M.; Rosin, R.R.; Simpson, S.D.; Tyo, K.E.; et al. Stepping on the Gas to a Circular Economy: Accelerating Development of Carbon-Negative Chemical Production from Gas Fermentation. Annu. Rev. Chem. Biomol. Eng. 2021, 12, 439–470. [Google Scholar] [CrossRef]
- Gavala, H.N.; Grimalt-Alemany, A.; Asimakopoulos, K.; Skiadas, I.V. Gas Biological Conversions: The Potential of Syngas and Carbon Dioxide as Production Platforms. Waste Biomass Valorization 2021, 12, 5303–5328. [Google Scholar] [CrossRef]
- Kleerebezem, R.; van Loosdrecht, M.C. Mixed Culture Biotechnology for Bioenergy Production. Curr. Opin. Biotechnol. 2007, 18, 207–212. [Google Scholar] [CrossRef]
- Esquivel-Elizondo, S.; Delgado, A.G.; Rittmann, B.E.; Krajmalnik-Brown, R. The Effects of CO2 and H2 on CO Metabolism by Pure and Mixed Microbial Cultures. Biotechnol. Biofuels 2017, 10, 220. [Google Scholar] [CrossRef]
- Quintela, C.; Grimalt-Alemany, A.; Modin, O.; Nygård, Y.; Olsson, L.; Skiadas, I.V.; Gavala, H.N. Effect of PH in Syngas Conversion to C4 & C6 Acids in Mixed-Culture Trickle Bed Reactors. Biomass Bioenergy 2024, 187, 107292. [Google Scholar] [CrossRef]
- Asimakopoulos, K.; Kaufmann-Elfang, M.; Lundholm-Høffner, C.; Rasmussen, N.B.K.; Grimalt-Alemany, A.; Gavala, H.N.; Skiadas, I.V. Scale up Study of a Thermophilic Trickle Bed Reactor Performing Syngas Biomethanation. Appl. Energy 2021, 290, 116771. [Google Scholar] [CrossRef]
- Laguillaumie, L.; Peyre-Lavigne, M.; Grimalt-Alemany, A.; Gavala, H.N.; Skiadas, I.V.; Paul, E.; Dumas, C. Controlling the Microbial Competition between Hydrogenotrophic Methanogens and Homoacetogens Using Mass Transfer and Thermodynamic Constraints. J. Clean. Prod. 2023, 414, 137549. [Google Scholar] [CrossRef]
- Ganigué, R.; Sánchez-Paredes, P.; Bañeras, L.; Colprim, J. Low Fermentation PH Is a Trigger to Alcohol Production, but a Killer to Chain Elongation. Front. Microbiol. 2016, 7, 702. [Google Scholar] [CrossRef] [PubMed]
- Arslan, D.; Steinbusch, K.J.J.; Diels, L.; Hamelers, H.V.M.; Strik, D.P.B.T.B.; Buisman, C.J.N.; De Wever, H. Selective Short-Chain Carboxylates Production: A Review of Control Mechanisms to Direct Mixed Culture Fermentations. Crit. Rev. Environ. Sci. Technol. 2016, 46, 592–634. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Yu, S.J.; Zhang, F.; Xia, X.Y.; Zeng, R.J. Enhancement of Acetate Productivity in a Thermophilic (55 °C) Hollow-Fiber Membrane Biofilm Reactor with Mixed Culture Syngas (H2/CO2) Fermentation. Appl. Microbiol. Biotechnol. 2017, 101, 2619–2627. [Google Scholar] [CrossRef] [PubMed]
- Grimalt-Alemany, A.; Łȩzyk, M.; Lange, L.; Skiadas, I.V.; Gavala, H.N. Enrichment of Syngas-Converting Mixed Microbial Consortia for Ethanol Production and Thermodynamics-Based Design of Enrichment Strategies. Biotechnol. Biofuels 2018, 11, 198. [Google Scholar] [CrossRef]
- Deerberg, G.; Oles, M.; Schlögl, R. The Project Carbon2Chem®. Chem. Ing. Tech. 2018, 90, 1365–1368. [Google Scholar] [CrossRef]
- Slivka, R.M.; Chinn, M.S.; Grunden, A.M. Gasification and Synthesis Gas Fermentation: An Alternative Route to Biofuel Production. Biofuels 2011, 2, 405–419. [Google Scholar] [CrossRef]
- Schuchmann, K.; Müller, V. Autotrophy at the Thermodynamic Limit of Life: A Model for Energy Conservation in Acetogenic Bacteria. Nat. Rev. Microbiol. 2014, 12, 809–821. [Google Scholar] [CrossRef]
- Bertsch, J.; Müller, V. Bioenergetic Constraints for Conversion of Syngas to Biofuels in Acetogenic Bacteria. Biotechnol. Biofuels 2015, 8, 210. [Google Scholar] [CrossRef] [PubMed]
- Liew, F.; Henstra, A.M.; Köpke, M.; Winzer, K.; Simpson, S.D.; Minton, N.P. Metabolic Engineering of Clostridium Autoethanogenum for Selective Alcohol Production. Metab. Eng. 2017, 40, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Allaart, M.T.; Diender, M.; Sousa, D.Z.; Kleerebezem, R. Overflow Metabolism at the Thermodynamic Limit of Life: How Carboxydotrophic Acetogens Mitigate Carbon Monoxide Toxicity. Microb. Biotechnol. 2023, 16, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Campanaro, S.; Kougias, P.; Treu, L.; Angelidaki, I.; Zhang, S.; Luo, G. Anaerobic Granular Sludge for Simultaneous Biomethanation of Synthetic Wastewater and CO with Focus on the Identification of CO-Converting Microorganisms. Water Res. 2017, 126, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Valgepea, K.; De Souza Pinto Lemgruber, R.; Abdalla, T.; Binos, S.; Takemori, N.; Takemori, A.; Tanaka, Y.; Tappel, R.; Köpke, M.; Simpson, S.D.; et al. H2 Drives Metabolic Rearrangements in Gas-Fermenting Clostridium Autoethanogenum. Biotechnol. Biofuels 2018, 11, 55. [Google Scholar] [CrossRef]
- Richter, H.; Molitor, B.; Diender, M.; Sousa, D.Z.; Angenent, L.T. A Narrow PH Range Supports Butanol, Hexanol, and Octanol Production from Syngas in a Continuous Co-Culture of Clostridium Ljungdahlii and Clostridium Kluyveri with in-Line Product Extraction. Front. Microbiol. 2016, 7, 1773. [Google Scholar] [CrossRef]
- Shen, N.; Dai, K.; Xia, X.Y.; Zeng, R.J.; Zhang, F. Conversion of Syngas (CO and H2) to Biochemicals by Mixed Culture Fermentation in Mesophilic and Thermophilic Hollow-Fiber Membrane Biofilm Reactors. J. Clean. Prod. 2018, 202, 536–542. [Google Scholar] [CrossRef]
- Fernández-Blanco, C.; Veiga, M.C.; Kennes, C. Efficient Production of N-Caproate from Syngas by a Co-Culture of Clostridium Aceticum and Clostridium Kluyveri. J. Environ. Manag. 2022, 302, 113992. [Google Scholar] [CrossRef]
- Quintela, C.; Peshkepia, E.; Grimalt-Alemany, A.; Nygård, Y.; Olsson, L.; Skiadas, I.V.; Gavala, H. Excessive Ethanol Oxidation versus Efficient Chain Elongation Processes. Waste Biomass Valorization 2023, 15, 2545–2558. [Google Scholar] [CrossRef]
- Diender, M.; Parera Olm, I.; Gelderloos, M.; Koehorst, J.J.; Schaap, P.J.; Stams, A.J.M.; Sousa, D.Z. Metabolic Shift Induced by Synthetic Co-Cultivation Promotes High Yield of Chain Elongated Acids from Syngas. Sci. Rep. 2019, 9, 18081. [Google Scholar] [CrossRef] [PubMed]
- Abubackar, H.N.; Fernández-Naveira, Á.; Veiga, M.C.; Kennes, C. Impact of Cyclic PH Shifts on Carbon Monoxide Fermentation to Ethanol by Clostridium Autoethanogenum. Fuel 2016, 178, 56–62. [Google Scholar] [CrossRef]
- Sallam, A.; Steinbüchel, A. Clostridium sulfidigenes Sp. Nov., a Mesophilic, Proteolytic, Thiosulfate- and Sulfur-Reducing Bacterium Isolated from Pond Sediment. Int. J. Syst. Evol. Microbiol. 2009, 59, 1661–1665. [Google Scholar] [CrossRef] [PubMed]
- Quintela, C.; Bountzis, P.; Rezaei, B.; Im, C.; Modin, O.; Nygård, Y.; Olsson, L.; Skiadas, I.V.; Gavala, H.N. Chain Elongation in Continuous Microbial Electrosynthesis Cells: The Effect of PH and Precursors Supply. J. CO2 Util. 2024, 83, 102789. [Google Scholar] [CrossRef]
- Spirito, C.M.; Richter, H.; Rabaey, K.; Stams, A.J.M.; Angenent, L.T. Chain Elongation in Anaerobic Reactor Microbiomes to Recover Resources from Waste. Curr. Opin. Biotechnol. 2014, 27, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Walters, W.; Hyde, E.R.; Berg-Lyons, D.; Ackermann, G.; Humphrey, G.; Parada, A.; Gilbert, J.A.; Jansson, J.K.; Caporaso, J.G.; Fuhrman, J.A. Improved Bacterial 16S RRNA Gene (V4 and V4-5) and Fungal Internal Transcribed Spacer Marker Gene Primers for Microbial Community Surveys. Msystems 2016, 1, e00009-15. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]

| Reactor | Condition | Description | Days |
|---|---|---|---|
| TBR 1 | Original 1 | Starting conditions (45% H2, 25% CO2, 20% CO, 10% N2) | 0–24 |
| TBR 1 | H2-rich Syngas | Syngas composition changed to 68% H2, 15% CO2, 12% CO, 6% N2 | 24–124 |
| TBR 1 | Control 1 | Starting conditions | 124–215 |
| TBR 1 | CO-rich syngas | Syngas composition changed to 67% H2, 33% CO | 215–294 |
| TBR 2 | Original 2 | Starting conditions | 0–11 |
| TBR 2 | +Ethanol (9.6 g L−1) | Ethanol supplementation in liquid media (9.6 g L−1) | 11–100 |
| TBR 2 | Control 2.1 | Starting conditions | 100–273 |
| TBR 2 | +Ethanol (4.8 g L−1) | Ethanol supplementation in liquid media (4.8 g L−1) | 273–319 |
| TBR 2 | Control 2.2 | Starting conditions | 319–371 |
| TBR 2 | pH Oscillations | 24 h cycles comprised of: 18 h uncontrolled pH, 6 h of pH 6 setting | 371–438 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quintela, C.; Alexe, I.-G.; Nygård, Y.; Olsson, L.; Skiadas, I.V.; Gavala, H.N. Influence of Hydrogen and Ethanol Addition in Methanogen-Free Mixed Culture Syngas Fermentations in Trickle Bed Reactors. Molecules 2024, 29, 5653. https://doi.org/10.3390/molecules29235653
Quintela C, Alexe I-G, Nygård Y, Olsson L, Skiadas IV, Gavala HN. Influence of Hydrogen and Ethanol Addition in Methanogen-Free Mixed Culture Syngas Fermentations in Trickle Bed Reactors. Molecules. 2024; 29(23):5653. https://doi.org/10.3390/molecules29235653
Chicago/Turabian StyleQuintela, Cesar, Iulian-Gabriel Alexe, Yvonne Nygård, Lisbeth Olsson, Ioannis V. Skiadas, and Hariklia N. Gavala. 2024. "Influence of Hydrogen and Ethanol Addition in Methanogen-Free Mixed Culture Syngas Fermentations in Trickle Bed Reactors" Molecules 29, no. 23: 5653. https://doi.org/10.3390/molecules29235653
APA StyleQuintela, C., Alexe, I.-G., Nygård, Y., Olsson, L., Skiadas, I. V., & Gavala, H. N. (2024). Influence of Hydrogen and Ethanol Addition in Methanogen-Free Mixed Culture Syngas Fermentations in Trickle Bed Reactors. Molecules, 29(23), 5653. https://doi.org/10.3390/molecules29235653







