M2BPgs-HCC: An Automated Multilectin Bead Array Indicating Aberrant Glycosylation Signatures Toward Hepatitis C Virus-Associated Hepatocellular Carcinoma Prognosis
Abstract
1. Introduction
2. Results
2.1. Patient Flow and Baseline Characteristics
2.2. Clinical Characteristics of the HCC Group at the HCC Observation Point
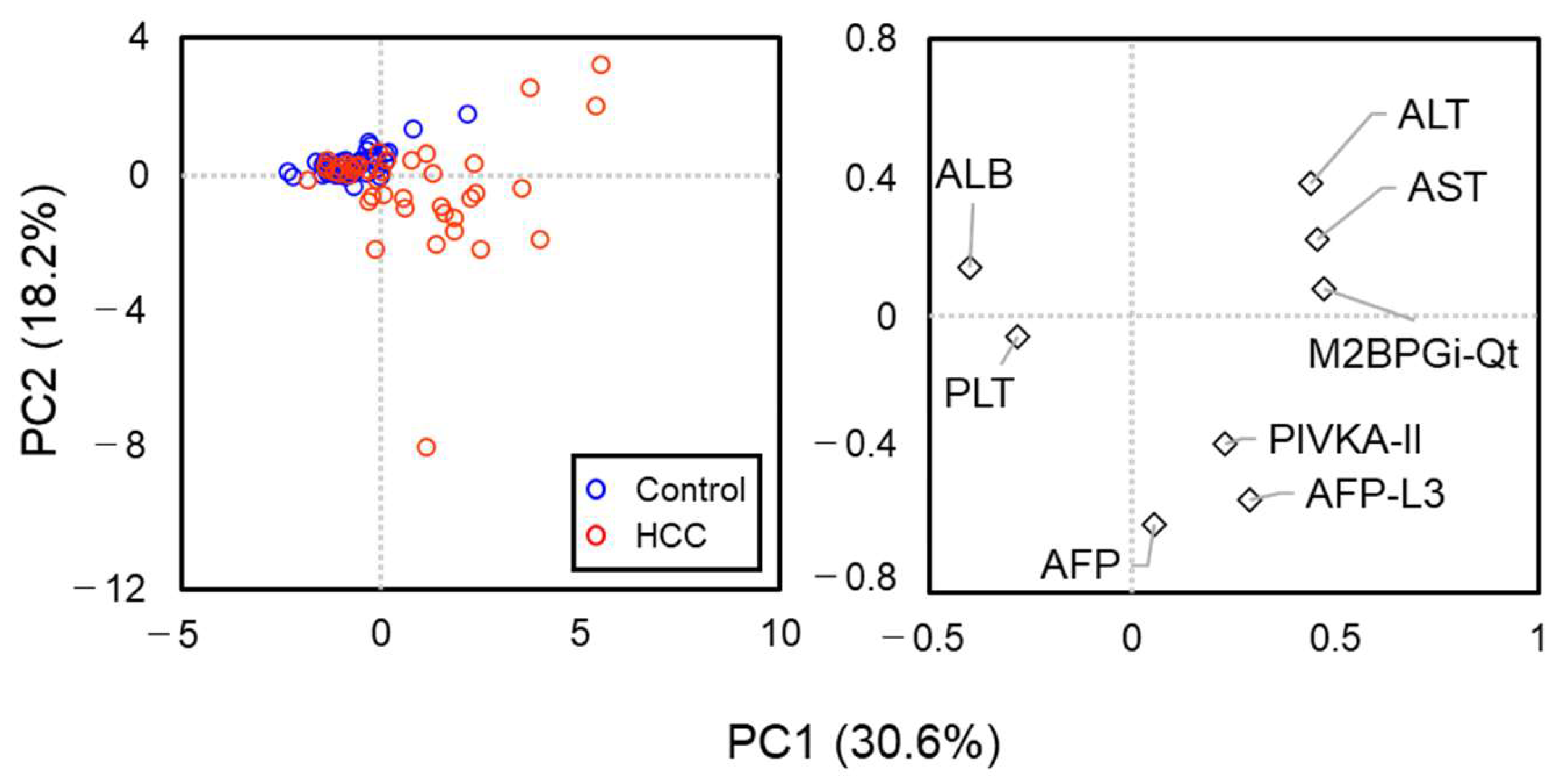
2.3. HCC-Related Glycan Feature Extraction of M2BP in Serum Samples from Patients
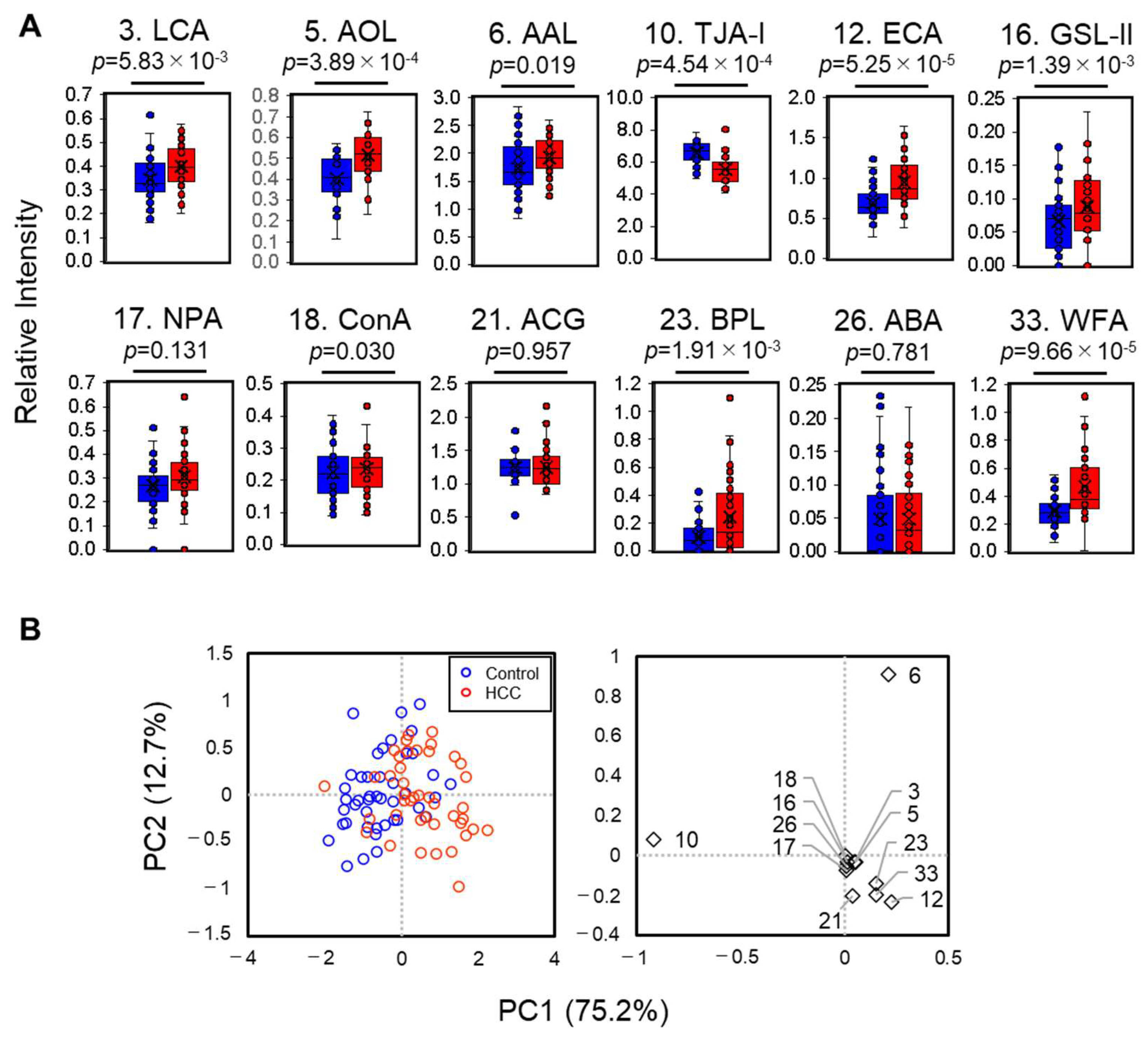
2.4. Automatic Glycofeature Detection of Serum M2BP Using a 12-Plexed Lectin Bead Array
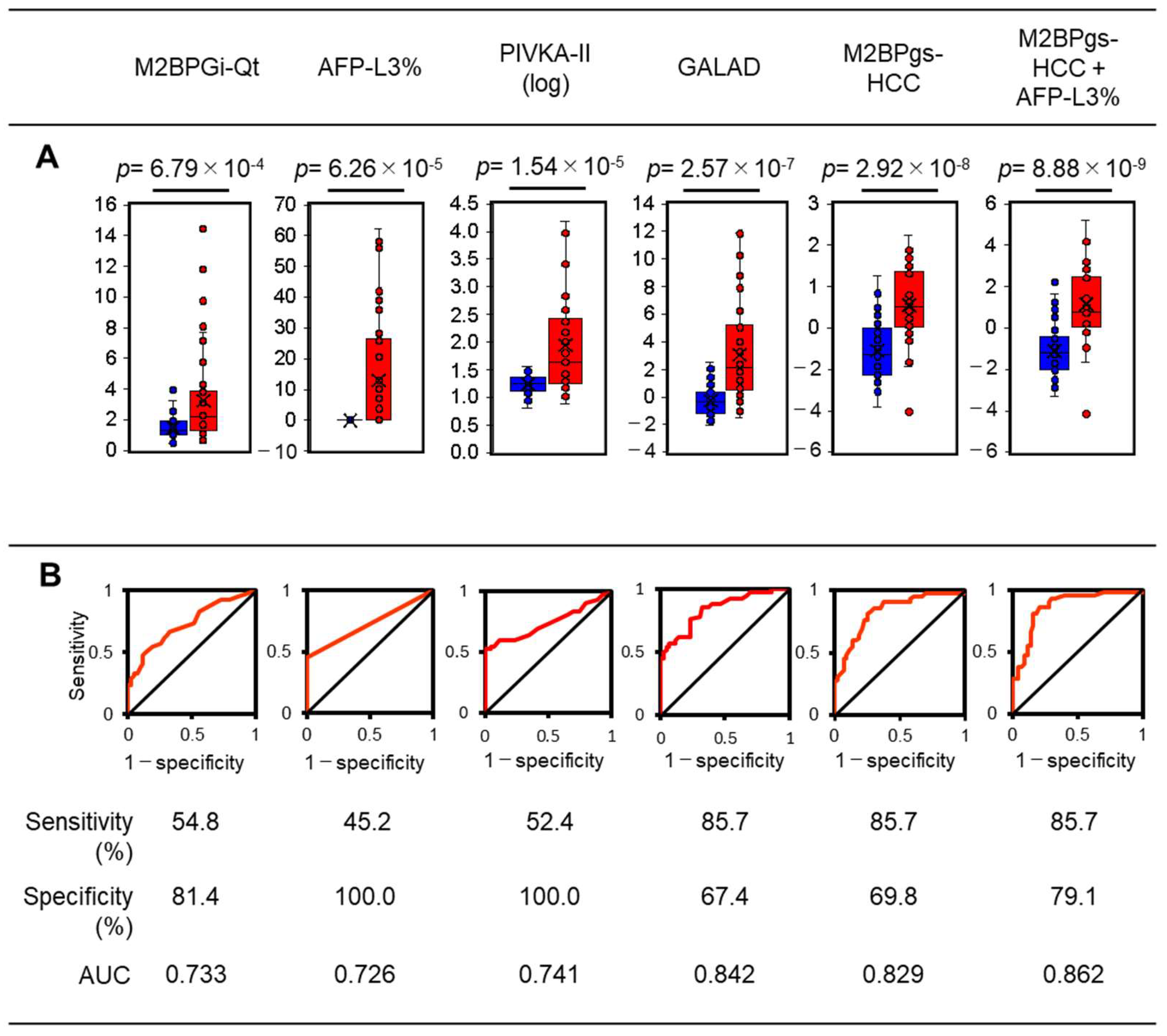
2.5. Statistical Characterization of the 12-Plexed Lectin Bead Array
3. Discussion
4. Materials and Methods
4.1. Patients and Study Design
4.1.1. Study Population
4.1.2. Study Design
4.2. Immunoprecipitation of M2BP
4.3. Differential Glycan Analysis of M2BP
4.4. Multilectin Bead Array Analysis
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Cucarull, B.; Tutusaus, A.; Rider, P.; Hernáez-Alsina, T.; Cuño, C.; de Frutos, P.G.; Colell, A.; Marí, M.; Morales, A. Hepatocellular carcinoma: Molecular pathogenesis and therapeutic advances. Cancers 2022, 14, 621. [Google Scholar] [CrossRef] [PubMed]
- Ginès, P.; Krag, A.; Abraldes, J.G.; Solà, E.; Fabrellas, N.; Kamath, P.S. Liver cirrhosis. Lancet 2021, 398, 1359–1376. [Google Scholar] [CrossRef] [PubMed]
- Na, S.K.; Song, B.C. Development and surveillance of hepatocellular carcinoma in patients with sustained virologic response after antiviral therapy for chronic hepatitis C. Clin. Mol. Hepatol. 2019, 25, 234–244. [Google Scholar] [CrossRef]
- Toyoda, H.; Kumada, T.; Tada, T.; Sone, Y.; Kaneoka, Y.; Maeda, A. Tumor markers for hepatocellular carcinoma: Simple and significant predictors of outcome in patients with HCC. Liver Cancer 2015, 4, 126–136. [Google Scholar] [CrossRef]
- Hanif, H.; Ali, M.J.; Susheela, A.T.; Khan, I.W.; Luna-Cuadros, M.A.; Khan, M.M.; Tan-Yeung Lau, D.; Susheela, A.T. Update on the applications and limitations of alpha-fetoprotein for hepatocellular carcinoma. World J. Gastroenterol. 2022, 28, 216–229. [Google Scholar] [CrossRef]
- Berhane, S.; Toyoda, H.; Tada, T.; Kumada, T.; Kagebayashi, C.; Satomura, S.; Schweitzer, N.; Vogel, A.; Manns, M.P.; Benckert, J.; et al. Role of the GALAD and BALAD-2 serologic models in diagnosis of hepatocellular carcinoma and prediction of survival in patients. Clin. Gastroenterol. Hepatol. 2016, 14, 875–886. [Google Scholar] [CrossRef]
- Piratvisuth, T.; Hou, J.; Tanwandee, T.; Berg, T.; Vogel, A.; Trojan, J.; De Toni, E.N.; Kudo, M.; Eiblmaier, A.; Klein, H.G.; et al. Development and clinical validation of a novel algorithmic score (GAAD) for detecting HCC in prospective cohort studies. Hepatol. Commun. 2023, 7, e0317. [Google Scholar] [CrossRef]
- Marsh, T.L.; Parikh, N.D.; Roberts, L.R.; Schwartz, M.E.; Nguyen, M.H.; Befeler, A.; Page-Lester, S.; Tayob, N.; Srivastava, S.; Rinaudo, J.A.; et al. A phase 3 biomarker validation of GALAD for the detection of hepatocellular carcinoma in cirrhosis. Gastroenterology 2024. Ahead of print. [Google Scholar] [CrossRef]
- Kuno, A.; Ikehara, Y.; Tanaka, Y.; Ito, K.; Matsuda, A.; Sekiya, S.; Hige, S.; Sakamoto, M.; Kage, M.; Mizokami, M.; et al. A serum sweet-doughnut protein facilitates fibrosis evaluation and therapy assessment in patients with viral hepatitis. Sci. Rep. 2013, 3, 1065. [Google Scholar] [CrossRef]
- Feng, S.; Wang, Z.; Zhao, Y.; Tao, C. Wisteria floribunda agglutinin-positive Mac-2-binding protein as a diagnostic biomarker in liver cirrhosis: An updated meta-analysis. Sci. Rep. 2020, 10, 10582. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Enomoto, H.; Iwata, Y.; Kishino, K.; Shimono, Y.; Hasegawa, K.; Nakano, C.; Takata, R.; Yoh, K.; Nishimura, T.; et al. Clinical significance of serum Wisteria floribunda agglutinin positive Mac-2-binding protein level in non-alcoholic steatohepatitis. Hepatol. Res. 2016, 46, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Saleh, S.A.; Salama, M.M.; Alhusseini, M.M.; Mohamed, G.A. M2BPGi for assessing liver fibrosis in patients with hepatitis C treated with direct-acting antivirals. World J. Gastroenterol. 2020, 26, 2864–2876. [Google Scholar] [CrossRef] [PubMed]
- Bui, H.H.; Nguyen, S.T.B.; Phan, S.T.; Nguyen, K.M.; Nguyen, C.D. Evaluating M2BPGi as a marker for liver fibrosis in patients with chronic hepatitis B. Dig. Dis. Sci. 2023, 68, 4407–4417. [Google Scholar] [CrossRef]
- Yamasaki, K.; Tateyama, M.; Abiru, S.; Komori, A.; Nagaoka, S.; Saeki, A.; Hashimoto, S.; Sasaki, R.; Bekki, S.; Kugiyama, Y.; et al. Elevated serum levels of Wisteria floribunda agglutinin-positive human Mac-2 binding protein predict the development of hepatocellular carcinoma in hepatitis C patients. Hepatology 2014, 60, 1563–1570. [Google Scholar] [CrossRef]
- Lin, Y.J.; Chang, C.L.; Chen, L.C.; Hu, H.H.; Liu, J.; Korenaga, M.; Huang, Y.H.; Jen, C.L.; Su, C.Y.; Nishida, N.; et al. A glycomarker for short-term prediction of hepatocellular carcinoma: A longitudinal study with serial measurements. Clin. Transl. Gastroenterol. 2018, 9, 183. [Google Scholar] [CrossRef]
- Tamaki, N.; Kurosaki, M.; Loomba, R.; Izumi, N. Clinical utility of Mac-2 binding protein glycosylation isomer in chronic liver diseases. Ann. Lab. Med. 2020, 41, 16–24. [Google Scholar] [CrossRef]
- Somers, N.; Butaye, E.; Grossar, L.; Pauwels, N.; Geerts, A.; Raevens, S.; Lefere, S.; Devisscher, L.; Meuris, L.; Callewaert, N.; et al. Glycomics as prognostic biomarkers of hepatocellular carcinoma: A systematic review. Oncol. Lett. 2024, 29, 24. [Google Scholar] [CrossRef]
- Sasaki, R.; Yamasaki, K.; Abiru, S.; Komori, A.; Nagaoka, S.; Saeki, A.; Hashimoto, S.; Bekki, S.; Kugiyama, Y.; Kuno, A.; et al. Serum Wisteria Floribunda Agglutinin-Positive Mac-2 Binding Protein Values Predict the Development of Hepatocellular Carcinoma among Patients with Chronic Hepatitis C after Sustained Virological Response. PLoS ONE 2015, 10, e0129053. [Google Scholar] [CrossRef]
- Harimoto, N.; Itoh, S.; Yamanaka, T.; Hagiwara, K.; Ishii, N.; Tsukagoshi, M.; Watanabe, A.; Araki, K.; Yoshizumi, T.; Shirabe, K. Mac-2 Binding Protein Glycosylation Isomer as a Prognostic Marker for Hepatocellular Carcinoma with Sustained Virological Response. Anticancer Res. 2022, 42, 245–251. [Google Scholar] [CrossRef]
- Varki, A.; Cummings, R.D.; Esko, J.D.; Stanley, P.; Hart, G.W.; Aebi, M.; Mohnen, D. Essentials of Glycobiology, 4th ed.Cold Spring Harbor Laboratory Press: New York, NY, USA, 2022; ISBN 9781621824213. [Google Scholar]
- Zou, X.; Lu, J.; Deng, Y.; Liu, Q.; Yan, X.; Cui, Y.; Xiao, X.; Fang, M.; Yang, F.; Sawaki, H.; et al. ST6GAL1 inhibits metastasis of hepatocellular carcinoma via modulating sialylation of MCAM on cell surface. Oncogene 2023, 42, 516–529. [Google Scholar] [CrossRef] [PubMed]
- Dolgormaa, G.; Harimoto, N.; Ishii, N.; Yamanaka, T.; Hagiwara, K.; Tsukagoshi, M.; Igarashi, T.; Watanabe, A.; Kubo, N.; Araki, K.; et al. Mac-2-binding protein glycan isomer enhances the aggressiveness of hepatocellular carcinoma by activating mTOR signaling. Br. J. Cancer 2020, 123, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Masuda, M.; Asuka, T.; Terao, N.; Nishino, S.; Ikeda, S.; Takamatsu, S.; Kondo, J.; Miyoshi, E. Establishment of a novel 70K Mac-2 binding protein antibody through screening of fucosylation-related antibodies. J. Biochem. 2023, 173, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Bojar, D.; Meche, L.; Meng, G.; Eng, W.; Smith, D.F.; Cummings, R.D.; Mahal, L.K. A useful guide to lectin binding: Machine-learning directed annotation of 57 unique lectin specificities. ACS Chem. Biol. 2022, 17, 2993–3012. [Google Scholar] [CrossRef] [PubMed]
- Shilova, N.V.; Galanina, O.E.; Polyakova, S.M.; Nokel, A.Y.; Pazynina, G.V.; Golovchenko, V.V.; Patova, O.A.; Mikshina, P.V.; Gorshkova, T.A.; Bovin, N.V. Specificity of widely used lectins as probed with oligosaccharide and plant polysaccharide arrays. Histochem. Cell Biol. 2024, 162, 495–510. [Google Scholar] [CrossRef]
- Shimazaki, H.; Saito, K.; Matsuda, A.; Sawakami, K.; Kariya, M.; Segawa, O.; Miyashita, Y.; Ueda, T.; Koizuka, M.; Nakamura, K.; et al. Lectin bead array in a single tip facilitates fully automatic glycoprotein profiling. Anal. Chem. 2019, 91, 11162–11169. [Google Scholar] [CrossRef]
- Fuseya, S.; Ono, A.; Ootani, H.; Mizukado, S.; Obayashi, T.; Tanaka, N.; Shimazaki, H.; Kajiyama, K.; Ashitomi, M.; Yasuda, S.; et al. Rapid glyco-qualitative assessment of recombinant proteins using a fully automated system. J. Vis. Exp. 2024, 2024, e66571. [Google Scholar] [CrossRef]
- Miyoshi, E.; Moriwaki, K.; Nakagawa, T. Biological function of fucosylation in cancer biology. J. Biochem. 2008, 143, 725–729. [Google Scholar] [CrossRef]
- Terao, N.; Takamatsu, S.; Minehira, T.; Sobajima, T.; Nakayama, K.; Kamada, Y.; Miyoshi, E. Fucosylation is a common glycosylation type in pancreatic cancer stem cell-like phenotypes. World J. Gastroenterol. 2015, 21, 3876–3887. [Google Scholar] [CrossRef]
- Noro, E.; Matsuda, A.; Kyoutou, T.; Sato, T.; Tomioka, A.; Nagai, M.; Sogabe, M.; Tsuruno, C.; Takahama, Y.; Kuno, A.; et al. N-glycan structures of Wisteria floribunda agglutinin-positive Mac2 binding protein in the serum of patients with liver fibrosis. Glycobiology 2021, 31, 1268–1278. [Google Scholar] [CrossRef]
- Bekki, Y.; Yoshizumi, T.; Shimoda, S.; Itoh, S.; Harimoto, N.; Ikegami, T.; Kuno, A.; Narimatsu, H.; Shirabe, K.; Maehara, Y. Hepatic stellate cells secreting WFA+-M2BP: Its role in biological interactions with Kupffer cells. J. Gastroenterol. Hepatol. 2017, 32, 1387–1393. [Google Scholar] [CrossRef] [PubMed]
- Yano, H.; Kojiro, M.; Nakashima, T. A new human hepatocellular carcinoma cell line (KYN-1) with a transformation to adenocarcinoma. Vitr. Cell Dev. Biol. 1986, 22, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Yano, H.; Iemura, A.; Fukuda, K.; Mizoguchi, A.; Haramaki, M.; Kojiro, M. Establishment of two distinct human hepatocellular carcinoma cell lines from a single nodule showing clonal dedifferentiation of cancer cells. Hepatology 1993, 18, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Dang, K.; Zhang, W.; Jiang, S.; Lin, X.; Qian, A. Application of Lectin Microarrays for Biomarker Discovery. ChemistryOpen 2020, 9, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Uojima, H.; Yamasaki, K.; Sugiyama, M.; Kage, M.; Ishii, N.; Shirabe, K.; Hidaka, H.; Kusano, C.; Murakawa, M.; Asahina, Y.; et al. Quantitative measurements of M2BPGi depend on liver fibrosis and inflammation. J. Gastroenterol. 2024, 59, 598–608. [Google Scholar] [CrossRef]
- Kuno, A.; Kato, Y.; Matsuda, A.; Kaneko, M.K.; Ito, H.; Amano, K.; Chiba, Y.; Narimatsu, H.; Hirabayashi, J. Focused differential glycan analysis with the platform antibody-assisted lectin profiling for glycan-related biomarker verification. Mol. Cell. Proteom. 2009, 8, 99–108. [Google Scholar] [CrossRef]

| Clinical Characteristics | HCC | Control | p-Value | |
|---|---|---|---|---|
| Number of patients | 42 | 43 | ||
| Age | Years | 69.9 ± 9.8 | 69.1 ± 9.9 | 0.713 |
| Gender: Male | n (%) | 21 (50.0) | 23 (53.5) | 0.829 |
| Liver cirrhosis | n (%) | 26 (61.9) | 25 (58.1) | 0.826 |
| DAA a: DCA b + ASV c/SOF d + RBV e/SOF + LDV f/OBV g + PTV h-r i/EG j/GP k | n | 9/1/28/0/0/4 | 4/3/31/1/2/2 | 0.241 |
| DM | n (%) | 7 (16.7) | 9 (20.9) | 0.787 |
| Alcohol | n (%) | 6 (14.3) | 6 (14.0) | 0.782 |
| Aspartate aminotransferase (AST) | IU/L | 59.0 ± 31.9 | 54.5 ± 24.6 | 0.474 |
| Alanine aminotransferase (ALT) | IU/L | 47.5 ± 28.1 | 55.1 ± 27.0 | 0.204 |
| Platelet (PLT) | 103/μL | 12.0 ± 5.7 | 12.8 ± 5.7 | 0.506 |
| Serum albumin (ALB) | g/dL | 3.7 ± 0.5 | 3.8 ± 0.3 | 0.404 |
| AFP | ng/mL | 17.2 ± 27.1 | 11.5 ± 15.3 | 0.239 |
| AFP-L3% | % | 1.5 ± 2.7 | 1.0 ± 1.9 | 0.374 |
| M2BPGi-Qt | AU/mL | 5.75 ± 4.1 | 5.80 ± 4.5 | 0.964 |
| Clinical Characteristics | HCC | Control | p-Value | |
|---|---|---|---|---|
| Number of patients | 42 | 43 | ||
| Time of HCC development after SVR | Days | 1202 ± 697 | 1272 ± 635 | 0.803 |
| AST | IU/L | 35.7 ± 24.2 | 27.4 ± 21.6 | 0.117 |
| ALT | IU/L | 25.0 ± 24.3 | 19.1 ± 10.7 | 0.150 |
| PLT | 103/μL | 13.5 ± 5.7 | 15.6 ± 5.1 | 0.082 |
| ALB | g/dL | 4.0 ± 0.5 | 4.3 ± 0.3 | <0.001 |
| AFP | ng/mL | 1896 ± 11.8 | 2.5 ± 2.1 | 0.304 |
| AFP-L3% | % | 12.9 ± 18.7 | 0 | <0.001 |
| M2BPGi-Qt | AU/mL | 3.24 ± 3.1 | 1.44 ± 0,7 | 0.001 |
| PIVKA-II | mAU/mL | 1196 ± 3280 | 18 ± 6.8 | 0.021 |
| ID | Lectin | Rough Lectin Binding a | Relative Intensity b | For GlycoBIST d | ||
|---|---|---|---|---|---|---|
| Control (95% CI) | HCC (95% CI) | p-Value c | ||||
| 1 | LTL | fucose | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 1.22 × 10−2 | |
| 2 | PSA | fucose | 0.21 (0.20–0.23) | 0.27 (0.25–0.29) | 1.71 × 10−4 | |
| 3 | LCA | fucose | 0.37 (0.35–0.40) | 0.47 (0.43–0.50) | 6.32 × 10−5 | ⭘ |
| 4 | UEA-I | fucose | 0.02 (0.02–0.03) | 0.03 (0.02–0.04) | 1.26 × 10−1 | |
| 5 | AOL | fucose | 0.22 (0.20–0.24) | 0.38 (0.34–0.42) | 6.34 × 10−10 | ⭘ |
| 6 | AAL | fucose | 1.43 (1.33–1.53) | 1.82 (1.71–1.94) | 1.74 × 10−6 | ⭘ |
| 7 | MAL-I | sialic acid | 0.71 (0.65–0.78) | 0.78 (0.71–0.85) | 1.70 × 10−1 | |
| 8 | SNA | sialic acid | 3.37 (3.26–3.47) | 3.04 (2.88–3.20) | 8.30 × 10−4 | |
| 9 | SSA | sialic acid | 3.39 (3.29–3.49) | 3.21 (3.03–3.39) | 7.62 × 10−2 | |
| 10 | TJA-I | sialic acid | 4.22 (4.08–4.36) | 3.70 (3.51–3.90) | 5.41 × 10−5 | ⭘ |
| 11 | PHA(L) | complex N-glycan | 0.70 (0.64–0.77) | 0.85 (0.76–0.95) | 9.94 × 10−3 | |
| 12 | ECA | LacNAc | 0.39 (0.36–0.42) | 0.55 (0.48–0.62) | 2.32 × 10−4 | ⭘ |
| 13 | RCA120 | LacNAc | 4.19 (4.12–4.27) | 4.03 (3.94–4.12) | 7.34 × 10−3 | |
| 14 | PHA(E) | complex N-glycan | 3.60 (3.44–3.77) | 3.46 (3.31–3.61) | 1.95 × 10−1 | |
| 15 | DSA | complex N-glycan | 5.65 (5.48–5.81) | 5.06 (4.92–5.20) | 8.39 × 10−7 | |
| 16 | GSL-II | GlcNAc | 0.05 (0.04–0.05) | 0.06 (0.05–0.06) | 6.55 × 10−4 | ⭘ |
| 17 | NPA | mannose | 0.08 (0.07–0.08) | 0.09 (0.08–0.10) | 7.09 × 10−4 | ⭘ |
| 18 | ConA | mannose | 0.14 (0.13–0.16) | 0.31 (0.27–0.35) | 2.14 × 10−10 | ⭘ |
| 19 | GNA | mannose | 0.02 (0.02–0.03) | 0.02 (0.02–0.02) | 9.54 × 10−1 | |
| 20 | HHL | mannose | 0.03 (0.02–0.03) | 0.04 (0.03–0.04) | 1.11 × 10−2 | |
| 21 | ACG | Gal | 3.24 (3.11–3.37) | 2.82 (2.69–2.95) | 1.22 × 10−5 | ⭘ |
| 22 | TxLC-I | complex N-glycan | 2.54 (2.39–2.69) | 2.54 (2.40–2.68) | 9.90 × 10−1 | |
| 23 | BPL | Gal | 0.16 (0.14–0.19) | 0.36 (0.26–0.46) | 4.27 × 10−4 | ⭘ |
| 24 | TJA-II | fucose | 0.19 (0.17–0.20) | 0.25 (0.22–0.29) | 4.29 × 10−4 | |
| 25 | EEL | Gal | 0.23 (0.12–0.34) | 0.20 (0.09–0.31) | 6.80 × 10−1 | |
| 26 | ABA | complex N-glycan | 0.20 (0.17–0.22) | 0.12 (0.11–0.13) | 3.43 × 10−8 | ⭘ |
| 27 | LEL | LacNAc | 4.65 (4.44–4.85) | 4.50 (4.28–4.73) | 3.48 × 10−1 | |
| 28 | STL | LacNAc | 2.59 (2.46–2.71) | 2.70 (2.58–2.83) | 1.72 × 10−1 | |
| 29 | UDA | GlcNAc | 0.42 (0.38–0.45) | 0.53 (0.50–0.57) | 1.02 × 10−5 | |
| 30 | PWM | GlcNAc | 0.00 (0.00–0.01) | 0.01 (0.00–0.01) | 3.96 × 10−2 | |
| 31 | Jacalin | O-glycan | 0.01 (0.01–0.02) | 0.01 (0.01–0.01) | 5.34 × 10−1 | |
| 32 | PNA | O-glycan | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | ND | |
| 33 | WFA | GalNAc | 0.43 (0.37–0.49) | 0.83 (0.64–1.02) | 2.14 × 10−4 | ⭘ |
| 34 | ACA | O-glycan | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 2.82 × 10−1 | |
| 35 | MPA | O-glycan | 0.00 (0.00–0.01) | 0.00 (0.00–0.01) | 2.90 × 10−1 | |
| 36 | HPA | O-glycan | 0.05 (0.02–0.07) | 0.11 (0.04–0.19) | 1.04 × 10−1 | |
| 37 | VVA | GalNAc | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 4.27 × 10−2 | |
| 38 | DBA | GalNAc | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 3.38 × 10−1 | |
| 39 | SBA | GalNAc | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | ND | |
| 40 | Calsepa | Mannose | 0.51 (0.46–0.55) | 0.62 (0.56–0.68) | 3.89 × 10−3 | |
| 41 | PTL-I | fucose | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | ND | |
| 42 | MAH | sialic acid | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | ND | |
| 43 | WGA | GlcNAc | 0.99 (0.86–1.12) | 1.21 (1.11–1.31) | 8.58 × 10−3 | |
| 44 | GSL-IA4 | Gal | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | ND | |
| 45 | GSL-IB4 | Gal | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | ND | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimazaki, H.; Uojima, H.; Yamasaki, K.; Obayashi, T.; Fuseya, S.; Sato, T.; Mizokami, M.; Kuno, A. M2BPgs-HCC: An Automated Multilectin Bead Array Indicating Aberrant Glycosylation Signatures Toward Hepatitis C Virus-Associated Hepatocellular Carcinoma Prognosis. Molecules 2024, 29, 5640. https://doi.org/10.3390/molecules29235640
Shimazaki H, Uojima H, Yamasaki K, Obayashi T, Fuseya S, Sato T, Mizokami M, Kuno A. M2BPgs-HCC: An Automated Multilectin Bead Array Indicating Aberrant Glycosylation Signatures Toward Hepatitis C Virus-Associated Hepatocellular Carcinoma Prognosis. Molecules. 2024; 29(23):5640. https://doi.org/10.3390/molecules29235640
Chicago/Turabian StyleShimazaki, Hiroko, Haruki Uojima, Kazumi Yamasaki, Tomomi Obayashi, Sayaka Fuseya, Takashi Sato, Masashi Mizokami, and Atsushi Kuno. 2024. "M2BPgs-HCC: An Automated Multilectin Bead Array Indicating Aberrant Glycosylation Signatures Toward Hepatitis C Virus-Associated Hepatocellular Carcinoma Prognosis" Molecules 29, no. 23: 5640. https://doi.org/10.3390/molecules29235640
APA StyleShimazaki, H., Uojima, H., Yamasaki, K., Obayashi, T., Fuseya, S., Sato, T., Mizokami, M., & Kuno, A. (2024). M2BPgs-HCC: An Automated Multilectin Bead Array Indicating Aberrant Glycosylation Signatures Toward Hepatitis C Virus-Associated Hepatocellular Carcinoma Prognosis. Molecules, 29(23), 5640. https://doi.org/10.3390/molecules29235640







