1,2-Oxidative Trifluoromethylation of Olefin with Ag(O2CCF2SO2F) and O2: Synthesis of α-Trifluoromethyl Ketones
Abstract
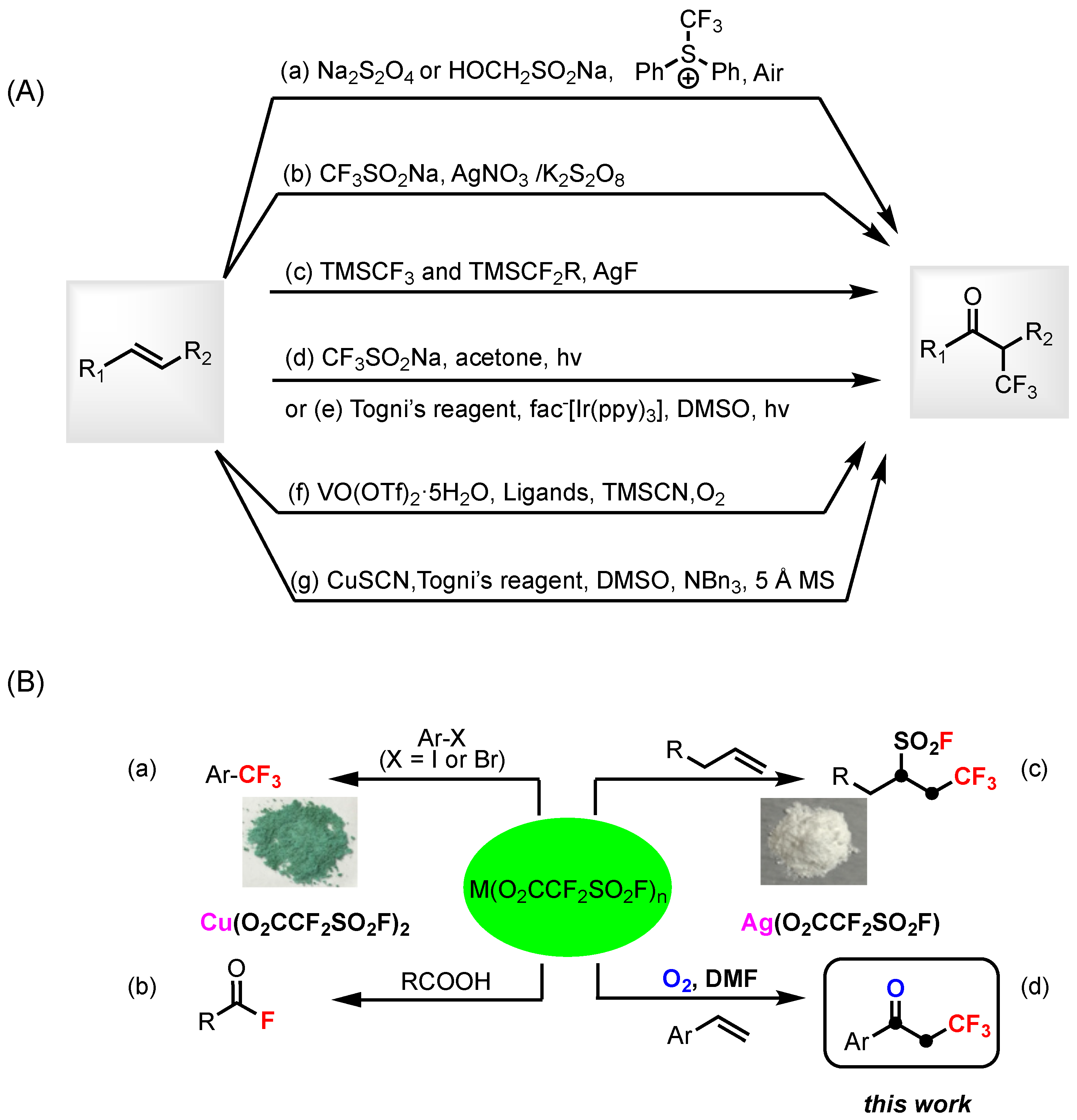
1. Introduction
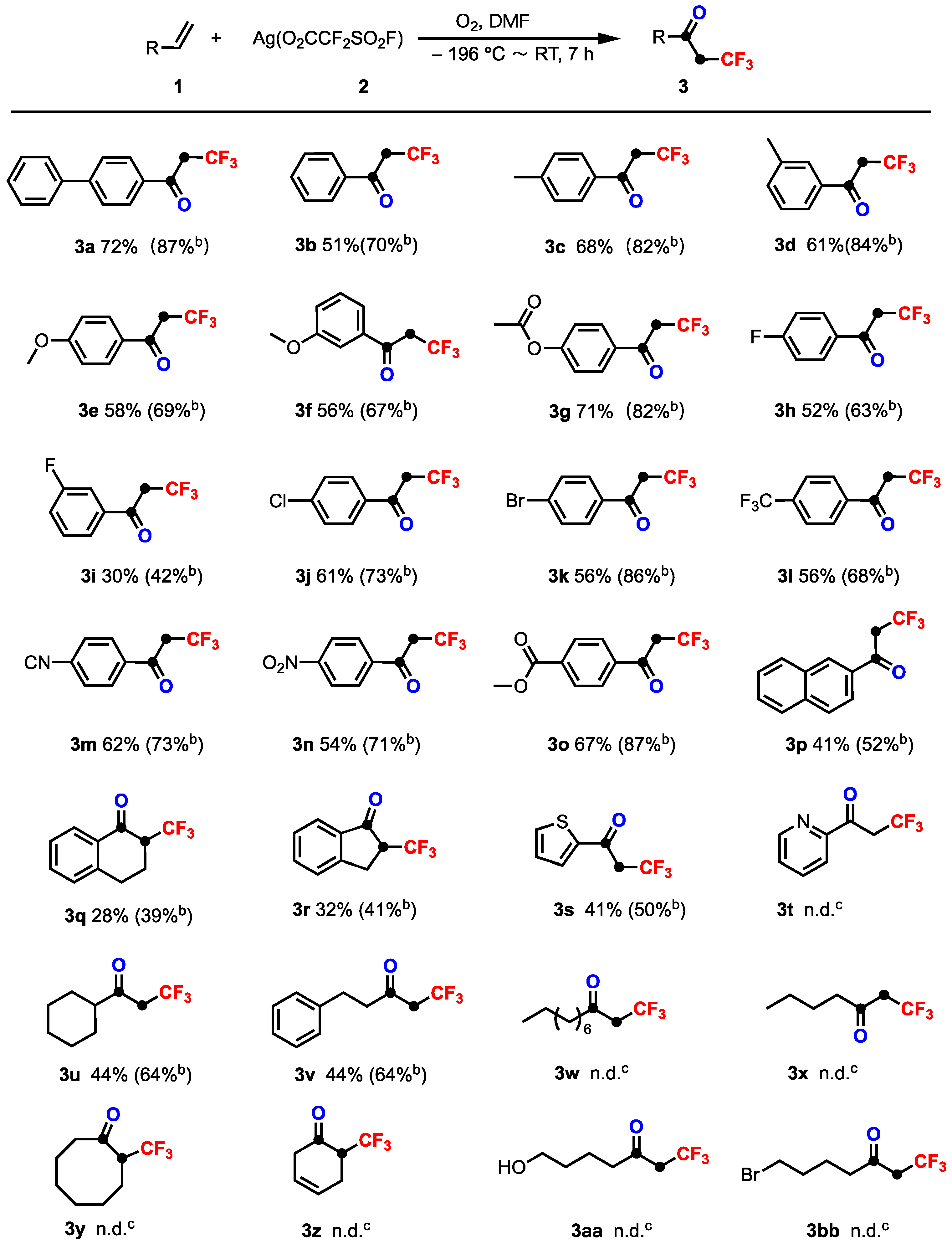
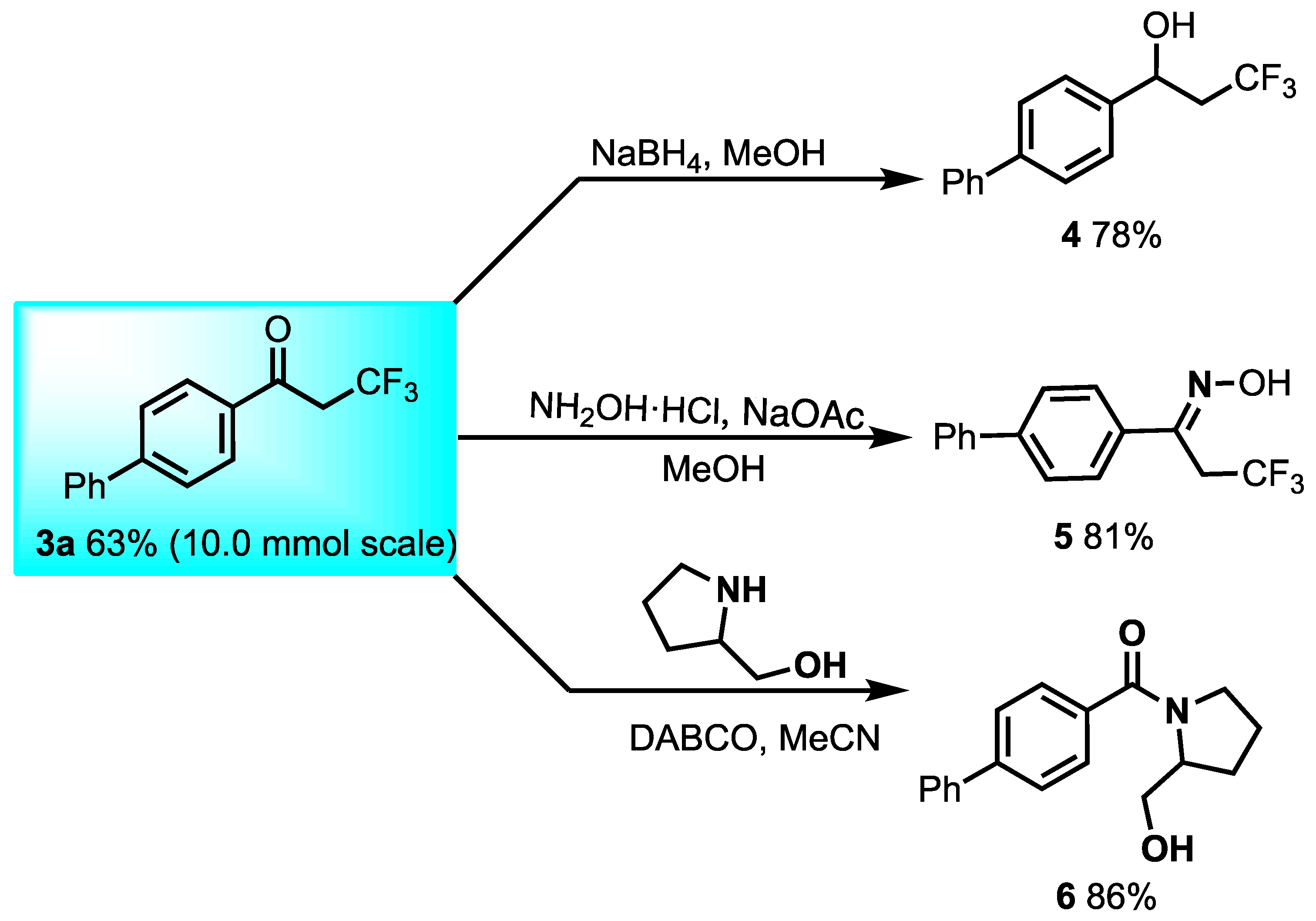
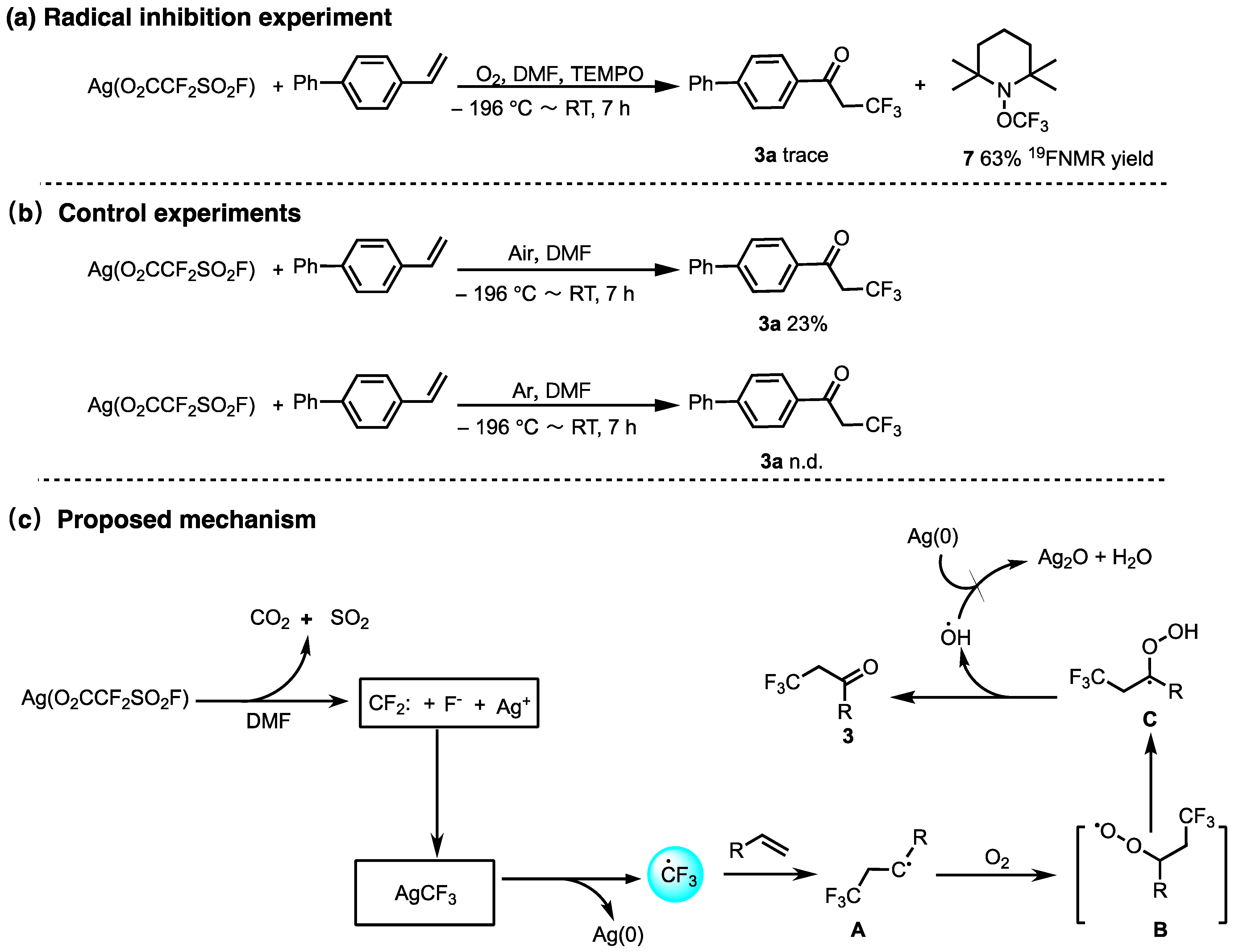
2. Results and Discussion
3. Materials and Methods
3.1. General Information of Materials and Instruments
3.2. General Procedure for the Synthesis of Compounds (3a–3t)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Houdhury-Mukherjee, I.; Schenck, H.A.; Cechova, S.; Pajewski, T.N.; Kapur, J.; Ellena, J.; Cafiso, D.S.; Brown, M.L. Design, Synthesis, and Evaluation of Analogues of 3,3,3-Trifluoro-2-Hydroxy-2-Phenyl-Propionamide as Orally Available General Anesthetics. J. Med. Chem. 2003, 46, 2494–2501. [Google Scholar] [CrossRef]
- Schenck, H.A.; Lenkowski, P.W.; Choudhury-Mukherjee, I.; Ko, S.-H.; Stables, J.P.; Patel, M.K.; Brown, M.L. Design, synthesis and evaluation of novel hydroxyamides as orally available anticonvulsants. Bioorg. Med. Chem. 2004, 12, 979–993. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Q.; Xie, Q.; Huang, B.; Dai, J.-J.; Xu, J.; Xu, H.-J. Pd-catalyzed defluorination/arylation of α-trifluoromethyl ketones via consecutive β-F elimination and C–F bond activation. Chem. Commun. 2018, 54, 4406–4409. [Google Scholar] [CrossRef]
- Lv, L.; Gao, G.; Luo, Y.; Mao, K.; Li, Z. Three-Component Reactions of α-CF3 Carbonyls, NaN3, and Amines for the Synthesis of NH-1,2,3-Triazoles. J. Org. Chem. 2021, 86, 17197–17212. [Google Scholar] [CrossRef] [PubMed]
- Mao, K.; Lv, L.; Li, Z. Amine-Induced Selective C–C Bond Cleavage of 2,2,2-Trifluoroethyl Carbonyls for the Synthesis of Ureas and Amides. J. Org. Chem. 2023, 88, 10137–10146. [Google Scholar] [CrossRef] [PubMed]
- Shu, S.; Wang, L.; Lv, L.; Li, Z. Copper-Catalyzed [3+2] Annulation of Pyridinium Ylides with α-CF3 Ketones: Synthesis of Functionalized 2-Fluoroindolizines. Asian J. Org. Chem. 2023, 12, e202300075. [Google Scholar] [CrossRef]
- Itoh, Y.; Mikami, K. Facile Radical Trifluoromethylation of Lithium Enolates. Org. Lett. 2005, 7, 4883–4885. [Google Scholar] [CrossRef]
- Itoh, Y.; Mikami, K. Radical Trifluoromethylation of Titanium Ate Enolate. Org. Lett. 2005, 7, 649–651. [Google Scholar] [CrossRef]
- Nagib, D.A.; Scott, M.E.; MacMillan, D.W.C. Enantioselective α-Trifluoromethylation of Aldehydes via Photoredox Organocatalysis. J. Am. Chem. Soc. 2009, 131, 10875–10877. [Google Scholar] [CrossRef]
- Allen, A.E.; MacMillan, D.W.C. The Productive Merger of Iodonium Salts and Organocatalysis: A Non-photolytic Approach to the Enantioselective α-Trifluoromethylation of Aldehydes. J. Am. Chem. Soc. 2010, 132, 4986–4987. [Google Scholar] [CrossRef]
- Matoušek, V.; Togni, A.; Bizet, V.; Cahard, D. Synthesis of α-CF3-Substituted Carbonyl Compounds with Relative and Absolute Stereocontrol Using Electrophilic CF3-Transfer Reagents. Org. Lett. 2011, 13, 5762–5765. [Google Scholar] [CrossRef] [PubMed]
- Katayev, D.; Matoušek, V.; Koller, R.; Togni, A. Lewis Acid Catalyzed Synthesis of α-Trifluoromethyl Esters and Lactones by Electrophilic Trifluoromethylation. Org. Lett. 2015, 17, 5898–5901. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Deng, Z.; Wang, K.-H.; Wang, J.; Huang, D.; Su, Y.; Hu, Y.; Lv, X. Photoinduced Trifluoromethylation with CF3Br as a Trifluoromethyl Source: Synthesis of α-CF3-Substituted Ketones. ACS Omega 2022, 7, 14357–14362. [Google Scholar] [CrossRef]
- Novák, P.; Lishchynskyi, A.; Grushin, V.V. Trifluoromethylation of α-Haloketones. J. Am. Chem. Soc. 2012, 134, 16167–16170. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Huang, H.; Yuan, Y.; Li, Y. Radical Desulfur-Fragmentation and Reconstruction of Enol Triflates: Facile Access to alpha-Trifluoromethyl Ketones. Angew. Chem. Int. Ed. 2017, 56, 1338–1341. [Google Scholar] [CrossRef]
- Yu, X.; Maity, A.; Studer, A. Cooperative Photoredox and N-Heterocyclic Carbene Catalyzed Fluoroaroylation for the Synthesis of alpha-Trifluoromethyl-Substituted Ketones. Angew. Chem. Int. Ed. 2023, 62, e202310288. [Google Scholar] [CrossRef]
- Zhang, C.-P.; Wang, Z.-L.; Chen, Q.-Y.; Zhang, C.-T.; Gu, Y.-C.; Xiao, J.-C. Generation of the CF3 radical from trifluoromethylsulfonium triflate and its trifluoromethylation of styrenes. Chem. Commun. 2011, 47, 6632–6634. [Google Scholar] [CrossRef]
- Deb, A.; Manna, S.; Modak, A.; Patra, T.; Maity, S.; Maiti, D. Oxidative trifluoromethylation of unactivated olefins: An efficient and practical synthesis of alpha-trifluoromethyl-substituted ketones. Angew. Chem. Int. Ed. 2013, 52, 9747–9750. [Google Scholar] [CrossRef]
- Wu, Y.B.; Lu, G.P.; Yuan, T.; Xu, Z.B.; Wan, L.; Cai, C. Oxidative trifluoromethylation and fluoroolefination of unactivated olefins. Chem. Commun. 2016, 52, 13668–13670. [Google Scholar] [CrossRef]
- Tomita, R.; Yasu, Y.; Koike, T.; Akita, M. Combining photoredox-catalyzed trifluoromethylation and oxidation with DMSO: Facile synthesis of alpha-trifluoromethylated ketones from aromatic alkenes. Angew. Chem. Int. Ed. 2014, 53, 7144–7148. [Google Scholar] [CrossRef]
- Zhao, L.; Li, P.; Zhang, H.; Wang, L. Photoinduced synthesis of α-trifluoromethylated ketones through the oxidative trifluoromethylation of styrenes using CF3SO2Na as a trifluoromethyl reagent without an external photoredox catalyst. Org. Chem. Front. 2019, 6, 87–93. [Google Scholar] [CrossRef]
- Chen, C.-T.; Chen, Y.-P.; Tsai, B.-Y.; Liao, Y.-Y.; Su, Y.-C.; Chen, T.-C.; Lu, C.-H.; Fujii, R.; Kawashima, K.; Mori, S. Vanadyl Species Catalyzed 1,2-Oxidative Trifluoromethylation of Unactivated Olefins. ACS Catal. 2020, 10, 3676–3683. [Google Scholar] [CrossRef]
- Wang, J.; Liu, H.; Qi, C. Copper-Catalyzed Oxidative Trifluoromethylation of Aromatic Alkenes for the Synthesis of α-Trifluoromethylated Ketones. Asian J. Org. Chem. 2024, 13, e202300638. [Google Scholar] [CrossRef]
- Zhao, G.; Wu, H.; Xiao, Z.; Chen, Q.-Y.; Liu, C. Trifluoromethylation of haloarenes with a new trifluoro-methylating reagent Cu(O2CCF2SO2F)2. RSC Adv. 2016, 6, 50250–50254. [Google Scholar] [CrossRef]
- Wu, X.; Qiu, X.; Lou, W.; Zhang, S.; Zhang, C.; Ma, X.; Liu, C. Efficient Trifluoromethylation of Halogenated Hydrocarbons Using Novel [(bpy)Cu(O2CCF2SO2F)2] Reagent. Molecules 2024, 29, 2849. [Google Scholar] [CrossRef]
- Le, B.; Wu, H.; Hu, X.; Zhou, X.; Guo, Y.; Chen, Q.-Y.; Liu, C. Rapid synthesis of acyl fluorides from carboxylic acids with Cu(O2CCF2SO2F)2. Tetrahedron Lett. 2020, 61, 152624. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, H.; Guo, Y.; Xiao, J.C.; Chen, Q.Y.; Liu, C. Trifluoromethylfluorosulfonylation of Unactivated Alkenes Using Readily Available Ag(O2CCF2SO2F) and N-Fluorobenzenesulfonimide. Angew. Chem. Int. Ed. 2017, 56, 15432–15435. [Google Scholar] [CrossRef]
- Li, Q.; Fan, W.; Peng, D.; Meng, B.; Wang, S.; Huang, R.; Liu, S.; Li, S. Cobalt–Tertiary-Amine-Mediated Hydroxytrifluoromethylation of Alkenes with CF3Br and Atmospheric Oxygen. ACS Catal. 2020, 10, 4012–4018. [Google Scholar] [CrossRef]
- Lu, K.; Wei, X.; Li, Q.; Li, Y.; Ji, L.; Hua, E.; Dai, Y.; Zhao, X. Synthesis of α-trifluoromethyl ethanone oximes via the three-component reaction of aryl-substituted ethylenes, tert-butyl nitrite, and the Langlois reagent. Org. Chem. Front. 2019, 6, 3766–3770. [Google Scholar] [CrossRef]
- Xu, J.; Li, Y.; Zhu, X.; Lv, S.; Xu, Y.; Cheng, T.; Liu, G.; Liu, R. Pyridinium-Masked Enol as a Precursor for Constructing Alpha-Fluoromethyl Ketones. Org. Lett. 2023, 25, 6211–6216. [Google Scholar] [CrossRef]
- Li, Y.-J.; Liu, D.-G.; Ren, J.-H.; Gong, T.-J.; Fu, Y. Photocatalytic Alkyl Radical Addition Tandem Oxidation of Alkenyl Borates. J. Org. Chem. 2023, 88, 4325–4333. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, R.; Tzouras, N.V.; Nolan, S.P.; Paquin, J.-F. Exploiting the inductive effect of the trifluoromethyl group: Regioselective gold-catalyzed hydration of 2,2,2-trifluoroethyl-substituted alkynes. Chem. Commun. 2023, 59, 9138–9141. [Google Scholar] [CrossRef] [PubMed]
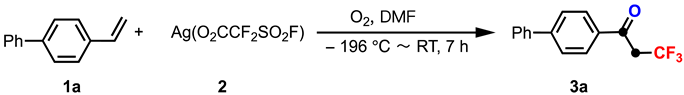 | ||
| Entry | Variation from Standard Conditions | Yield b (%) |
|---|---|---|
| 1 | None | 87 |
| 2 | MeCN, NMP, DMAc instead of DMF | <81 |
| 3 | DCE, EtOAc, THF instead of DMF | n.d. c |
| 4 | Cu(O2CCF2SO2F)2 instead of Ag(O2CCF2SO2F) | n.d. c |
| 5 | TMSCF3/AgF system instead of Ag(O2CCF2SO2F) | 33 |
| 6 | 2.0 equiv of Ag(O2CCF2SO2F) | 40 |
| 7 | 3.0 equiv of Ag(O2CCF2SO2F) | 49 |
| 8 | 5.0 equiv of Ag(O2CCF2SO2F) | 89 |
| 9 | −15 °C | 23 |
| 10 | 0 °C | 76 |
| 11 | 40 °C | 56 |
| 12 | 60 °C | 47 |
| 13 | m-CPBA, NaIO4, K2S2O8, MnO2, DMP instead of O2 | <45 |
| 14 | Air | 23 |
| 15 | Ar | n.d. c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Xiao, W.; Wu, J.; Wu, F.; Huang, H.; Ma, X.; Shi, Y.; Liu, C. 1,2-Oxidative Trifluoromethylation of Olefin with Ag(O2CCF2SO2F) and O2: Synthesis of α-Trifluoromethyl Ketones. Molecules 2024, 29, 5622. https://doi.org/10.3390/molecules29235622
Zhang S, Xiao W, Wu J, Wu F, Huang H, Ma X, Shi Y, Liu C. 1,2-Oxidative Trifluoromethylation of Olefin with Ag(O2CCF2SO2F) and O2: Synthesis of α-Trifluoromethyl Ketones. Molecules. 2024; 29(23):5622. https://doi.org/10.3390/molecules29235622
Chicago/Turabian StyleZhang, Shengxue, Wangchuan Xiao, Jingjing Wu, Fanhong Wu, Houjin Huang, Xiaoyu Ma, Yafei Shi, and Chao Liu. 2024. "1,2-Oxidative Trifluoromethylation of Olefin with Ag(O2CCF2SO2F) and O2: Synthesis of α-Trifluoromethyl Ketones" Molecules 29, no. 23: 5622. https://doi.org/10.3390/molecules29235622
APA StyleZhang, S., Xiao, W., Wu, J., Wu, F., Huang, H., Ma, X., Shi, Y., & Liu, C. (2024). 1,2-Oxidative Trifluoromethylation of Olefin with Ag(O2CCF2SO2F) and O2: Synthesis of α-Trifluoromethyl Ketones. Molecules, 29(23), 5622. https://doi.org/10.3390/molecules29235622







