A New Demand for Improved Selectivity and Potency of Cyanine Dyes as Antiproliferative Agents Against Colorectal Cancer Cells
Abstract
1. Introduction
2. Results and Discussion
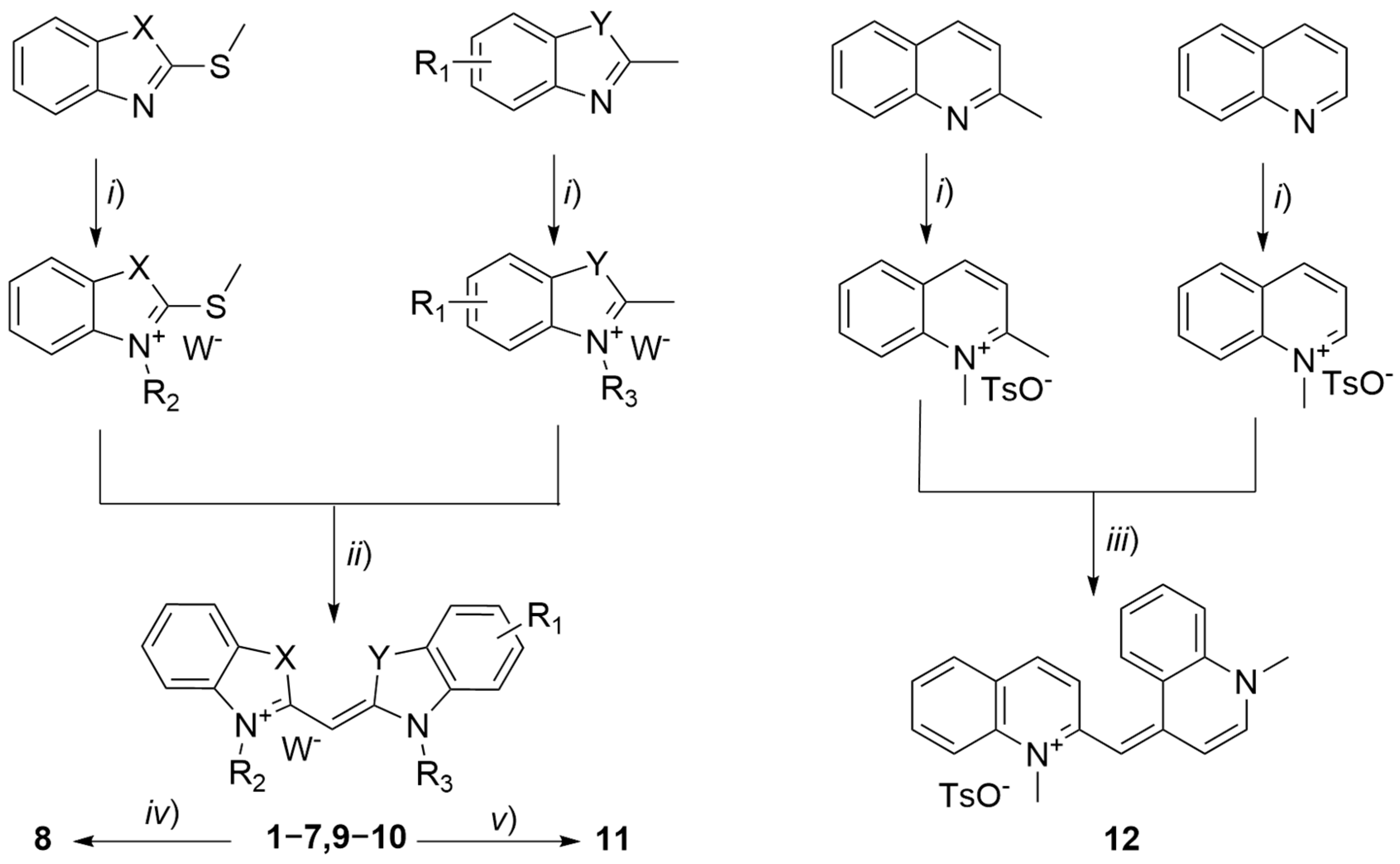
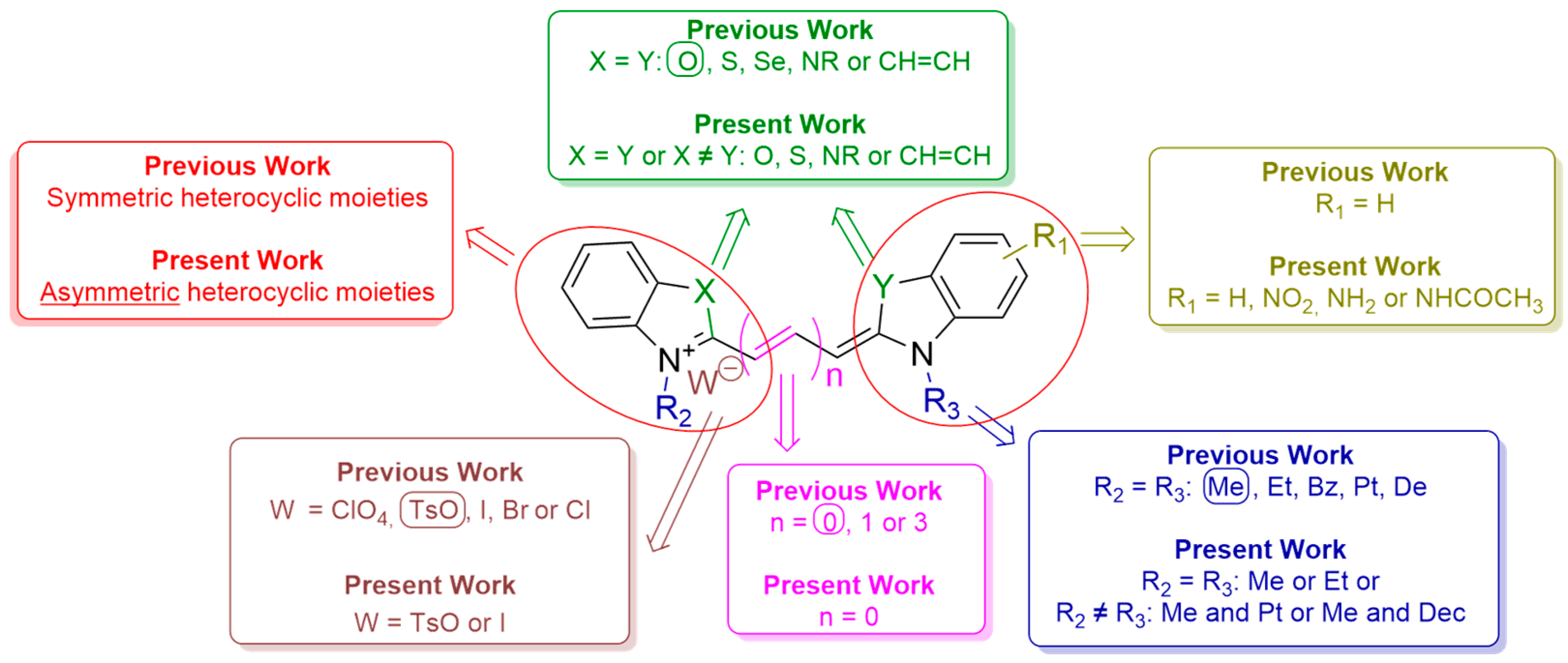
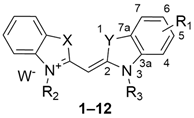
2.1. Synthesis and Structural Characterisation
2.2. In Vitro Studies
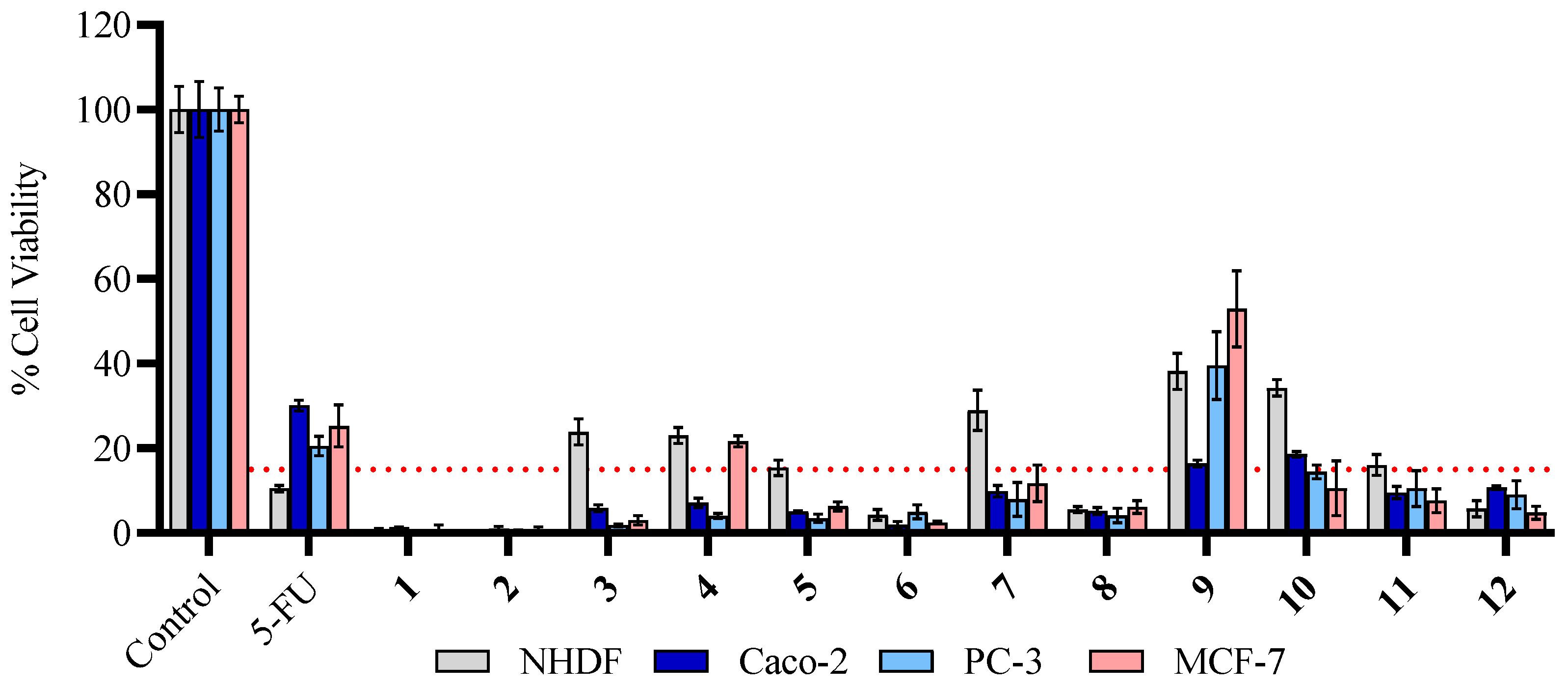
2.2.1. Antiproliferative Effects in Human Cell Lines
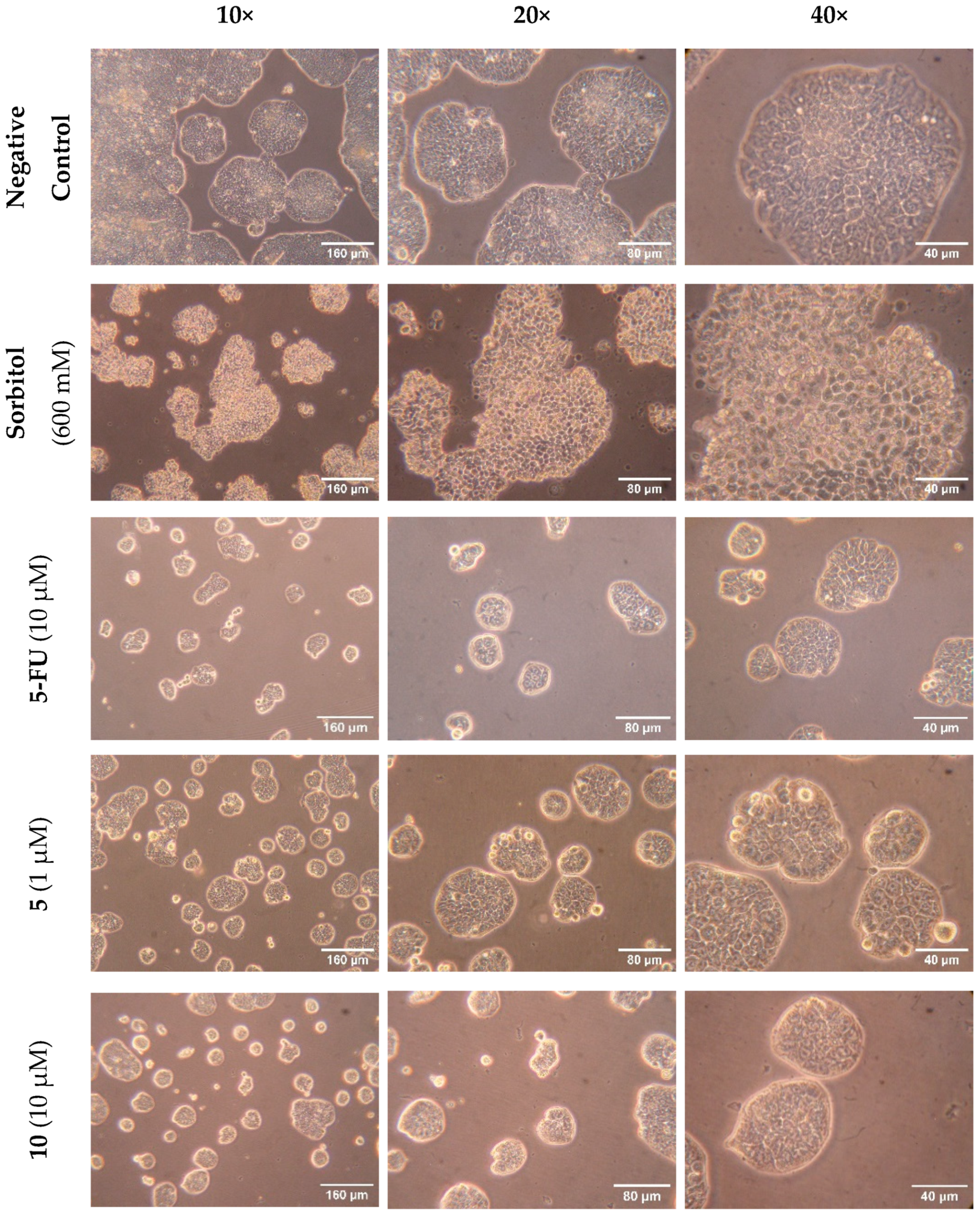
2.2.2. Caco-2 Morphological Analysis
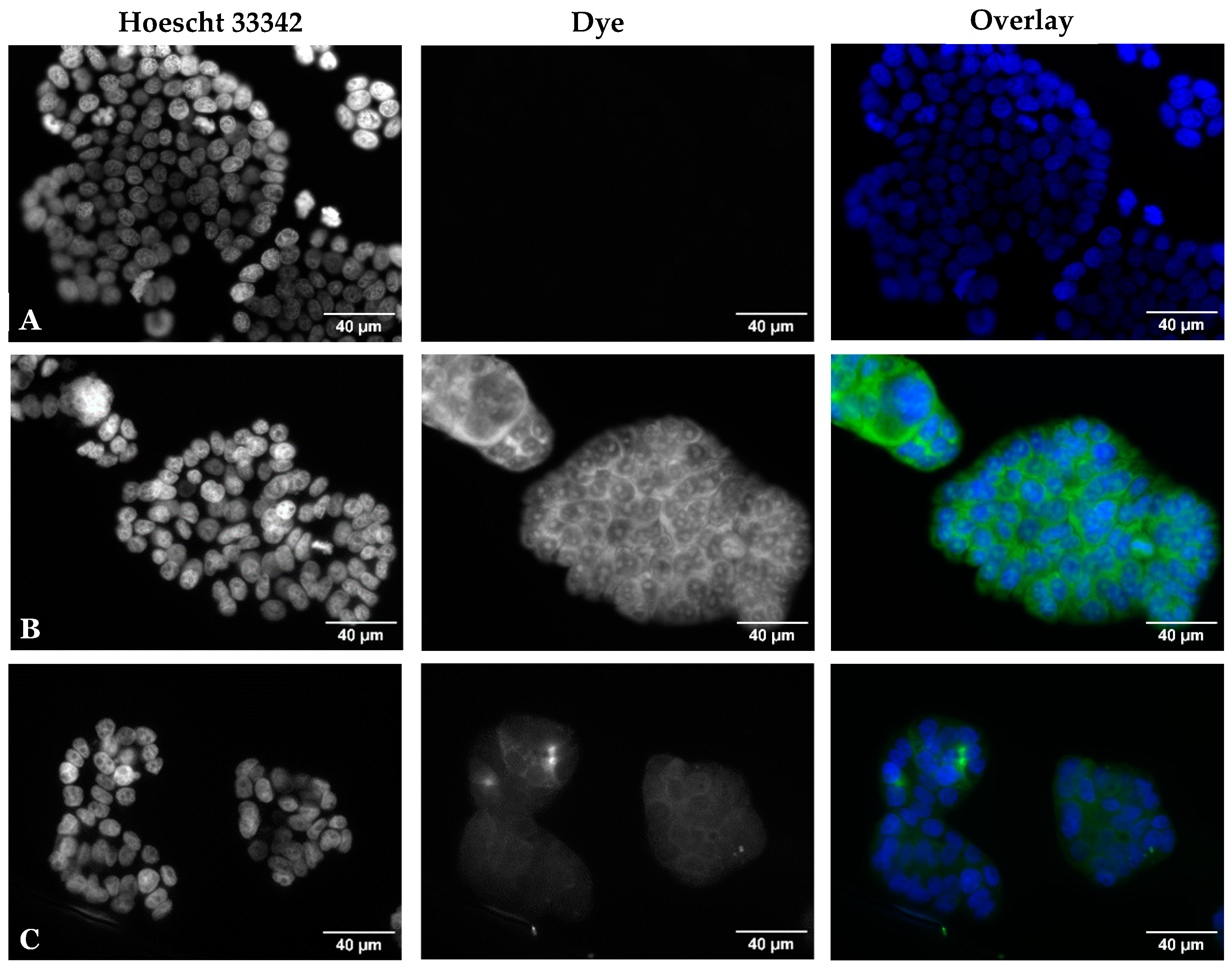
2.2.3. Fluorescence Microscopy
2.2.4. Apoptosis Induction and Cell Cycle Arrest Studies by Flow Cytometry
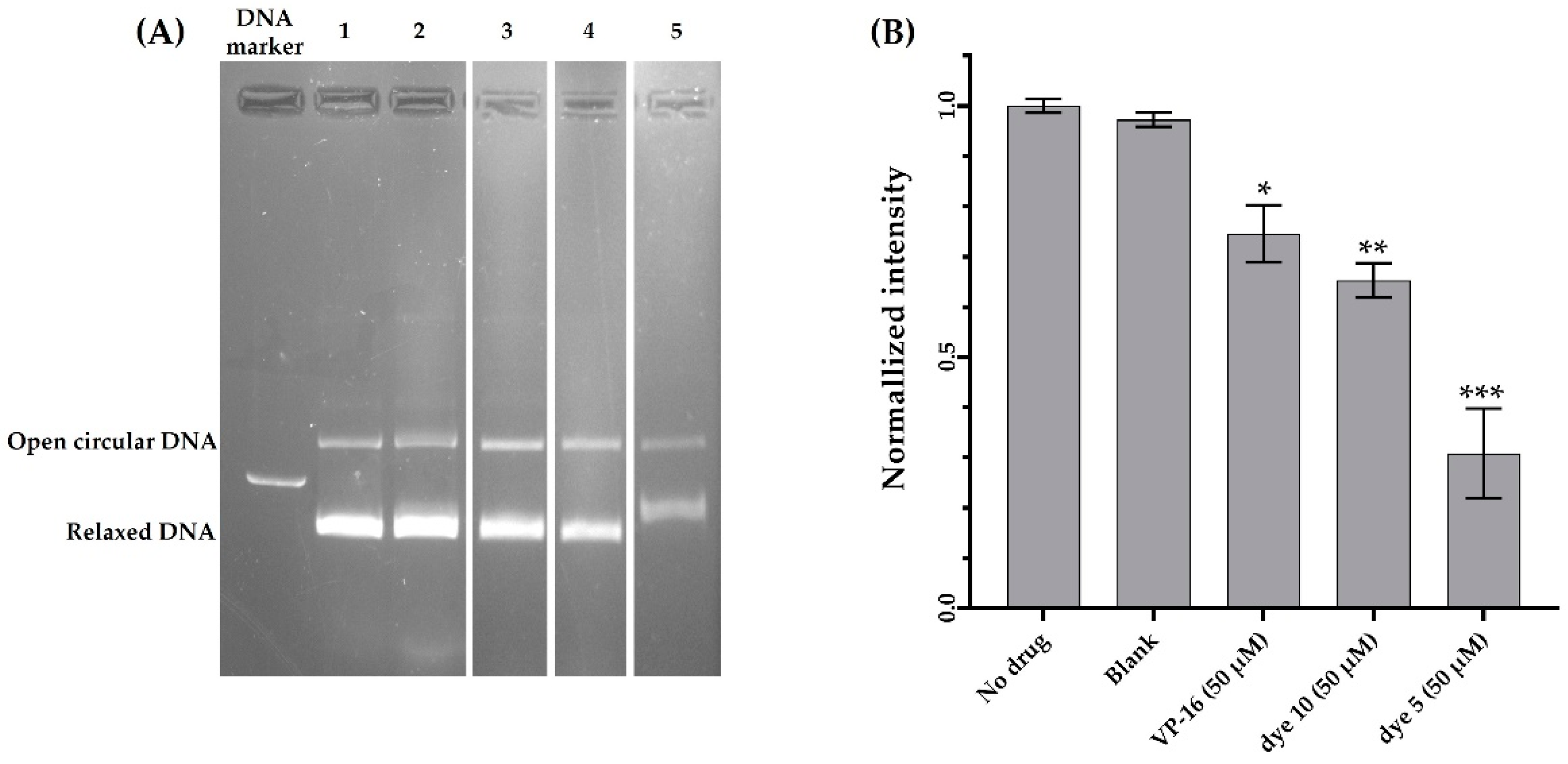
2.2.5. Topoisomerase II Inhibition
2.3. In Silico Drug-Likeness Predictions
3. Materials and Methods
3.1. Chemistry
- 3-Methyl-2-((3-pentylbenzo[d]thiazol-2(3H)-ylidene)methyl)benzo[d]thiazol-3-ium iodide (1), GP2 method, from 3-methyl-2-(methylthio)benzo[d]thiazol-3-ium iodide (323 mg) and 2-methyl-3-pentylbenzo[d]thiazol-3-ium iodide (347 mg) in ethanol. Yield: 72%; yellow crystals; m.p. 254–258 °C; Vis λmax (EtOH): 425 nm, log ε = 4.85; 1H NMR (400 MHz, DMSO-d6): δ 8.21 (d, J = 8.0 Hz, 2H), 7.88 (d, J = 8.4 Hz, 2H), 7.68 (t, J = 7.8 Hz, 2H), 7.50 (t, J = 7.6 Hz, 2H), 6.70 (s, 1H), 4.62 (t, J = 7.5 Hz, 2H), 4.03 (s, 3H), 1.81 (p, J = 7.7 Hz, 2H), 1.54–1.28 (m, 4H), 0.88 (t, J = 7.0 Hz, 3H) ppm; 13C NMR (101 MHz, DMSO-d6): δ 162.0, 161.4, 140.6, 140.1, 128.5, 128.4, 124.9, 124.8, 124.8, 124.7, 123.5, 123.4, 113.8, 82.6, 46.2, 34.3, 28.1, 26.5, 21.9, 13.8 ppm; ESI-HRMS: Calculated for [M − I]+ C21H23N2S2+ 367.1297, found 367.1289.
- 3-Methyl-2-((3-decylbenzo[d]thiazol-2(3H)-ylidene)methyl)benzo[d]thiazol-3-ium iodide (2), GP2 method, from 3-methyl-2-(methylthio)benzo[d]thiazol-3-ium iodide (323 mg) and 3-decyl-2-methylbenzo[d]thiazol-3-ium iodide (417 mg) in ethanol. Yield: 68%; yellow crystals; m.p. 217–218 °C; Vis λmax (EtOH): 427 nm, log ε = 4.95; 1H NMR (400 MHz, DMSO-d6): δ 8.22 (d, J = 8.1 Hz, 2H), 7.89 (d, J = 8.3 Hz, 2H), 7.69 (t, J = 7.7 Hz, 1H), 7.68 (t, J = 7.7 Hz, 1H), 7.50 (t, J = 7.6 Hz, 2H), 6.70 (s, 1H), 4.63 (t, J = 7.4 Hz, 2H), 4.02 (s, 3H), 1.80 (p, J = 7.8 Hz, 2H), 1.43 (p, J = 7.0 Hz, 2H), 1.32 (p, J = 6.3 Hz, 2H), 1.36–1.15 (m, 10H), 0.82 (t, J = 6.8 Hz, 3H) ppm; 13C NMR (101 MHz, DMSO-d6): δ 162.1, 161.4, 140.6, 140.1, 128.5, 128.4, 124.9, 124.8, 124.8, 124.7, 123.5, 123.4, 113.8, 113.8, 82.7, 46.2, 34.2, 31.2, 28.9, 28.8, 28.7, 28.6, 26.8, 25.9, 22.0, 13.9 ppm; ESI-HRMS: Calculated for [M − I]+ C26H33N2S2+ 437.2080, found 437.2071.
- 3-Methyl-2-((3-methylbenzo[d]oxazol-2(3H)-ylidene)methyl)benzo[d]thiazol-3-ium 4-methylbenzenesulfonate (3), GP1 method, from 2-(methylthio)benzothiazole (181 mg), 2-methylbenzoxazole (133 mg) and methyl p-toluenesulfonate (409 mg). Yield: 47%; yellow crystals; m.p. 270–273 °C (lit. 301–304 °C [19]); Vis λmax (EtOH): 401 nm, log ε = 4.88; 1H NMR (400 MHz, DMSO-d6): δ 8.11 (d, J = 7.9 Hz, 1H), 7.81 (d, J = 9.2 Hz, 2H), 7.71 (d, J = 7.8 Hz, 1H), 7.64 (t, J = 7.8 Hz, 1H), 7.54 (t, J = 7.7 Hz, 1H), 7.50–7.42 (m, 4H), 7.10 (d, J = 7.8 Hz, 2H), 6.28 (s, 1H), 3.98 (s, 3H), 3.84 (s, 3H), 2.28 (s, 3H) ppm; 13C NMR (101 MHz, DMSO-d6): δ 163.4, 161.3, 146.1, 145.7, 140.5, 137.7, 131.4, 128.1, 128.0, 126.3, 125.5, 125.1, 124.9, 124.8, 123.0, 113.5, 111.4, 110.8, 69.8, 34.0, 30.6, 20.8 ppm.
- 1,3-Dimethyl-2-((3-methylbenzo[d]thiazol-2(3H)-ylidene)methyl)-1H-benzo[d]imidazol-3-ium iodide (4), GP1 method, from 2-(methylthio)benzothiazole (352 mg), 2-methyl-1H-benzo[d]imidazole (132 mg) and methyl p-toluenesulfonate (596 mg). Anion exchange was made by reflux in a saturated potassium iodide solution (10 mL). Yield: 84%; brown powder; m.p. 270–272 °C (287–289 °C in the form of perchlorate anion [36]); Vis λmax (EtOH): 385 nm, log ε = 5.26; 1H NMR (400 MHz, DMSO-d6): δ 7.91–7.82 (m, 2H), 7.75 (dd, J = 7.6, 1.0 Hz, 1H), 7.63–7.54 (m, 2H), 7.52–7.41 (m, 2H), 7.22 (ddd, J = 8.3, 6.9, 1.6 Hz, 1H), 5.79 (s, 1H), 3.90 (s, 6H), 3.70 (s, 3H) ppm; 13C NMR (101 MHz, DMSO-d6): δ 158.6, 151.4, 141.4, 132.4, 127.3, 125.3, 123.0, 122.6, 122.3, 112.1, 111.3, 68.5, 33.3, 32.8 ppm.
- 3-Methyl-2-((1-methylquinolin-2(1H)-ylidene)methyl)benzo[d]oxazol-3-ium 4-methylbenzenesulfonate (5), GP1 method, from 2-(methylthio)benzoxazole (165 mg), quinaldine (143 mg) and methyl p-toluenesulfonate (409 mg). Yield: 63%; Orange crystals; m.p. 243–246 °C; Vis λmax (EtOH): 436 nm, log ε = 4.72; 1H NMR (400 MHz, DMSO-d6): δ 8.39–8.31 (m, 2H), 8.10 (d, J = 8.8 Hz, 1H), 7.99 (dd, J = 7.8, 1.6 Hz, 1H), 7.87 (ddd, J = 8.8, 7.1, 1.6 Hz, 1H), 7.81 (d, J = 8.0 Hz, 1H), 7.67 (dd, J = 8.0, 1.2 Hz, 1H), 7.58 (t, J = 7.4 Hz, 1H), 7.54–7.44 (m, 1H), 7.47 (d, J = 8.1 Hz, 2H), 7.41 (td, J = 7.8, 1.3 Hz, 1H), 7.10 (d, J = 7.8 Hz, 2H), 5.62 (s, 1H), 4.10 (s, 3H), 3.83 (s, 3H), 2.28 (s, 3H) ppm; 13C NMR (101 MHz, DMSO-d6): δ 161.3, 153.3, 145.8, 145.7, 139.3, 138.6, 137.6, 132.8, 131.2, 129.2, 128.0, 125.9, 125.5, 125.3, 124.5, 123.7, 119.8, 117.1, 111.0, 110.8, 72.4, 37.5, 30.6, 20.8 ppm.
- 3-Methyl-2-((1-methylquinolin-2(1H)-ylidene)methyl)benzo[d]thiazol-3-ium 4-methylbenzenesulfonate (6), by GP1 method, from 2-(methylthio)benzothiazole (181 mg), quinaldine (143 mg) and methyl p-toluenesulfonate (409 mg). Yield: 72%; orange reddish crystals; m.p. 255–257 °C (lit. 272–276 °C in the form of iodide anion [37]); Vis λmax (EtOH): 483 nm, log ε = 4.53; 1H NMR (400 MHz, DMSO-d6): δ 8.44 (d, J = 9.4 Hz, 1H), 8.13 (d, J = 8.8 Hz, 1H), 8.07 (d, J = 9.2 Hz, 1H), 8.02 (d, J = 7.9 Hz, 2H), 7.90 (t, J = 8.1 Hz, 1H), 7.78 (d, J = 8.4 Hz, 1H), 7.61 (t, J = 8.0 Hz, 2H), 7.47 (d, J = 7.7 Hz, 2H), 7.42 (t, J = 7.6 Hz, 1H), 7.10 (d, J = 7.7 Hz, 2H), 6.16 (s, 1H), 4.12 (s, 3H), 3.94 (s, 3H), 2.28 (s, 3H) ppm; 13C NMR (101 MHz, DMSO-d6): δ 161.4, 153.1, 145.7, 140.6, 139.7, 139.6, 137.6, 133.3, 129.4, 128.1, 128.0, 125.6, 125.5, 124.8, 123.9, 123.4, 122.8, 118.4, 117.3, 113.0, 86.5, 37.6, 33.9, 20.8 ppm.
- 5-Acetamido-3-ethyl-2-((3-ethylbenzo[d]thiazol-2(3H)-ylidene)methyl)benzo[d]thiazol-3-ium 4-methylbenzenesulfonate (7), GP1 method, from 2-(ethylthio)benzothiazole (195 mg), 5-acetamido-2-methylbenzothiazole (206 mg) and ethyl p-toluenesulfonate (440 mg). Yield: 29%; yellow crystals; m.p. 278–279 °C; Vis λmax (EtOH): 432 nm, log ε = 4.92; 1H NMR (400 MHz, DMSO-d6): δ 10.40 (s, 1H), 8.25 (d, J = 1.8 Hz, 1H), 8.21 (dd, J = 8.1, 1.2 Hz, 1H), 8.10 (d, J = 8.7 Hz, 1H), 7.89 (d, J = 8.4 Hz, 1H), 7.68 (ddd, J = 8.5, 7.3, 1.2 Hz, 1H), 7.54–7.45 (m, 2H), 7.47 (d, J = 8.1 Hz, 2H), 7.10 (d, J = 7.7 Hz, 2H), 6.74 (s, 1H), 4.69 (q, J = 7.2 Hz, 2H), 4.58 (q, J = 7.2 Hz, 2H), 2.28 (s, 3H), 2.11 (s, 3H), 1.39 (t, J = 7.4 Hz, 3H), 1.37 (t, J = 7.5 Hz, 3H) ppm; 13C NMR (101 MHz, DMSO-d6): δ 169.1, 162.2, 161.2, 145.8, 140.2, 140.2, 139.8, 137.5, 128.7, 128.0, 125.5, 125.1, 125.0, 123.8, 123.6, 118.7, 116.4, 113.6, 103.2, 82.1, 41.7, 41.6, 24.2, 20.8, 12.3, 12.1 ppm; ESI-HRMS: Calculated for [M − TsO]+ C21H22N3OS2+ 396.1199, found 396.1194.
- 5-Amino-3-ethyl-2-((3-ethylbenzo[d]thiazol-2(3H)-ylidene)methyl)benzo[d]thiazol-3-ium iodide (8), 5-acetamidocyanine dye 7 (1.0 mmol, 568 mg) was refluxed in a 25% hydrochloric acid solution (5 mL) for a duration of 30 min. The mixture was cooled to room temperature and neutralized with a 25% ammonia solution. The obtained solid was filtered under reduced pressure and anion exchange was made by reflux in a saturated potassium iodide solution (10 mL). Yield: 31%; green crystals; m.p. 288–289 °C; Vis λmax (EtOH): 454 nm, log ε = 4.67; 1H NMR (400 MHz, DMSO-d6): δ 8.18 (d, J = 7.9 Hz, 1H), 7.84 (d, J = 8.4 Hz, 1H), 7.79 (d, J = 8.6 Hz, 1H), 7.66 (t, J = 7.8 Hz, 1H), 7.46 (t, J = 7.7 Hz, 1H), 6.89 (s, 1H), 6.77 (d, J = 8.4 Hz, 1H), 6.69 (s, 1H), 5.80 (s, 2H), 4.66 (q, J = 7.1 Hz, 2H), 4.53 (q, J = 7.4 Hz, 2H), 1.37 (t, J = 7.2 Hz, 3H), 1.36 (t, J = 7.3 Hz, 3H) ppm; 13C NMR (101 MHz, DMSO-d6): δ 161.8, 160.0, 150.3, 141.2, 139.8, 128.5, 125.0, 124.6, 123.6, 123.5, 113.3, 113.0, 110.7, 96.6, 81.6, 41.5, 41.3, 12.2 ppm; ESI-HRMS: Calculated for [M − I]+ C19H20N3S2+ 354.1093, found 354.1086.
- 1,3-Diethyl-2-((3-ethylbenzo[d]thiazol-2(3H)-ylidene)methyl)-5-nitro-1H-benzo[d]imidazol-3-ium iodide (9), GP1 method, from 2-(ethylthio)benzothiazole (195 mg), 2-methyl-5-nitrobenzimidazole (177 mg) and ethyl p-toluenesulfonate (700 mg). Anion exchange was made by reflux in a saturated potassium iodide solution (10 mL). Yield: 26%; Red crystals; m.p. 219–220 °C; Vis λmax (EtOH): 422 nm, log ε = 4.56; 1H NMR (400 MHz, DMSO-d6): δ 8.92 (d, J = 2.1 Hz, 1H), 8.44 (dd, J = 9.0, 2.2 Hz, 1H), 8.17 (d, J = 9.0 Hz, 1H), 7.81 (dd, J = 7.8, 0.9 Hz, 1H), 7.54 (d, J = 8.1 Hz, 1H), 7.48 (td, J = 8.2, 7.4, 0.9 Hz, 1H), 7.26 (td, J = 7.6, 0.8 Hz, 1H), 5.88 (s, 1H), 4.59 (q, J = 7.1 Hz, 2H), 4.52 (q, J = 7.2 Hz, 2H), 4.35 (q, J = 7.0 Hz, 2H), 1.39–1.30 (m, 9H) ppm; 13C NMR (101 MHz, DMSO-d6): δ 158.5, 153.8, 144.7, 140.4, 135.9, 131.6, 127.6, 123.4, 122.9, 122.6, 120.8, 113.3, 111.6, 109.1, 67.6, 42.2, 42.1, 40.4, 14.0, 13.8, 11.5 ppm; ESI-HRMS: Calculated for [M − I]+ C21H23N4O2S+ 395.1536, found 395.1527.
- 3-Ethyl-2-((3-ethylbenzo[d]thiazol-2(3H)-ylidene)methyl)-6-nitrobenzo[d]thiazol-3-ium 4-methylbenzenesulfonate (10), by GP1 method, from 2-(ethylthio)benzothiazole (195 mg), 2-methyl-6-nitrobenzothiazole (177 mg) and ethyl p-toluenesulfonate (700 mg). Yield: 53%; orange crystals; m.p. 313–314 °C; Vis λmax (EtOH): 439 nm, log ε = 4.81; 1H NMR (400 MHz, DMSO-d6): δ 9.21 (d, J = 2.4 Hz, 1H), 8.49 (dd, J = 9.1, 2.4 Hz, 1H), 8.33 (dd, J = 8.1, 1.2 Hz, 1H), 8.05–7.95 (m, 2H), 7.75 (ddd, J = 8.4, 7.2, 1.3 Hz, 1H), 7.58 (t, J = 7.6 Hz, 1H), 7.47 (d, J = 8.1 Hz, 2H), 7.10 (d, J = 7.8 Hz, 2H), 6.85 (s, 1H), 4.77 (q, J = 7.5 Hz, 2H), 4.71 (q, J = 7.5 Hz, 2H), 2.28 (s, 3H), 1.39 (t, J = 7.1 Hz, 3H), 1.38 (t, J = 7.1 Hz, 3H) ppm; 13C NMR (101 MHz, DMSO-d6): δ 162.8, 162.7, 145.8, 144.6, 143.4, 139.8, 137.6, 129.0, 128.1, 126.1, 125.7, 125.6, 125.5, 124.4, 124.0, 120.0, 114.3, 113.5, 83.9, 42.2, 42.0, 20.8, 12.6, 12.2 ppm; ESI-HRMS: Calculated for [M–TsO]+ C19H18N3O2S2+ 384.0835, found 384.0828.
- 6-Amino-3-ethyl-2-((3-ethylbenzo[d]thiazol-2(3H)-ylidene)methyl)benzo[d]thiazol-3-ium iodide (11), hydrazine hydrate (3 mmol, 148 µL) was added to a solution of 6-nitrocyanine 10 (1 mmol, 556 mg) in a minimal amount of etilenoglicol,. After heating at 80 °C for 5 min, a few drops of a 50% Raney nickel solution were added. The resulting mixture was refluxed for an additional 6 h, hot filtered, and a saturated potassium iodide solution was added to the filtrate. After cooling, the obtained solid was filtered, dried, and recrystallized from methanol. Yield: 81%; Brown crystals; m.p. 233–235 °C (described without m.p. [18]); Vis λmax (EtOH): 444 nm, ε = 4.62; 1H NMR (400 MHz, DMSO-d6): δ 8.10 (d, J = 7.8 Hz, 1H), 7.75 (d, J = 8.2 Hz, 1H), 7.67–7.49 (m, 2H), 7.39 (t, J = 7.6 Hz, 1H), 7.22 (d, J = 2.4 Hz, 1H), 6.88 (dd, J = 8.7, 2.3 Hz, 1H), 6.54 (s, 1H), 5.67 (s, 2H), 4.57 (q, J = 7.4 Hz, 4H), 1.33 (t, J = 7.0 Hz, 6H) ppm; 13C NMR (101 MHz, DMSO-d6): δ 159.2, 158.5, 147.4, 139.9, 130.1, 128.4, 126.8, 124.5, 124.3, 123.3, 115.6, 114.4, 112.9, 105.5, 81.1, 41.7, 41.1, 12.5, 12.0 ppm.
- 1-Methyl-4-((1-methylquinolin-2(1H)-ylidene)methyl)quinolin-1-ium 4-methylbenzenesulfonate (12), GP3 method, from 1,2-dimethylquinolin-1-ium 4-methylbenzenesulfonate (329 mg), and 1-methylquinolin-1-ium 4-methylbenzenesulfonate (315 mg). Yield: 68%; dark blue crystals; m.p. 199–201 °C (lit. 268 °C in the form of iodide anion [38]); Vis λmax (EtOH): 558 nm, log ε = 5.26; 1H NMR (400 MHz, DMSO-d6): δ 8.56 (d, J = 8.5 Hz, 1H), 8.23 (d, J = 7.2 Hz, 1H), 8.08 (d, J = 9.4 Hz, 1H), 8.02–7.92 (m, 4H), 7.89 (dd, J = 7.9, 1.6 Hz, 1H), 7.79 (ddd, J = 8.8, 7.1, 1.6 Hz, 1H), 7.67 (dt, J = 8.3, 4.1 Hz, 1H), 7.50 (t, J = 7.2 Hz, 1H), 7.47 (d, J = 8.1 Hz, 2H), 7.40 (d, J = 7.2 Hz, 1H), 7.10 (d, J = 7.8 Hz, 2H), 6.49 (s, 1H), 4.07 (s, 3H), 4.01 (s, 3H), 2.28 (s, 3H) ppm; 13C NMR (101 MHz, DMSO-d6): δ 153.9, 149.3, 145.8, 143.5, 140.0, 138.4, 137.6, 137.1, 132.8, 132.4, 128.8, 128.0, 126.3, 125.8, 125.5, 124.8, 124.4, 124.3, 121.2, 117.6, 116.6, 108.5, 94.4, 41.7, 37.7, 20.8 ppm.
3.2. In Vitro Studies
3.2.1. Cytotoxic Effects in Human Cell Lines
Cell Culture
MTT Assay
Phototoxicity Assay
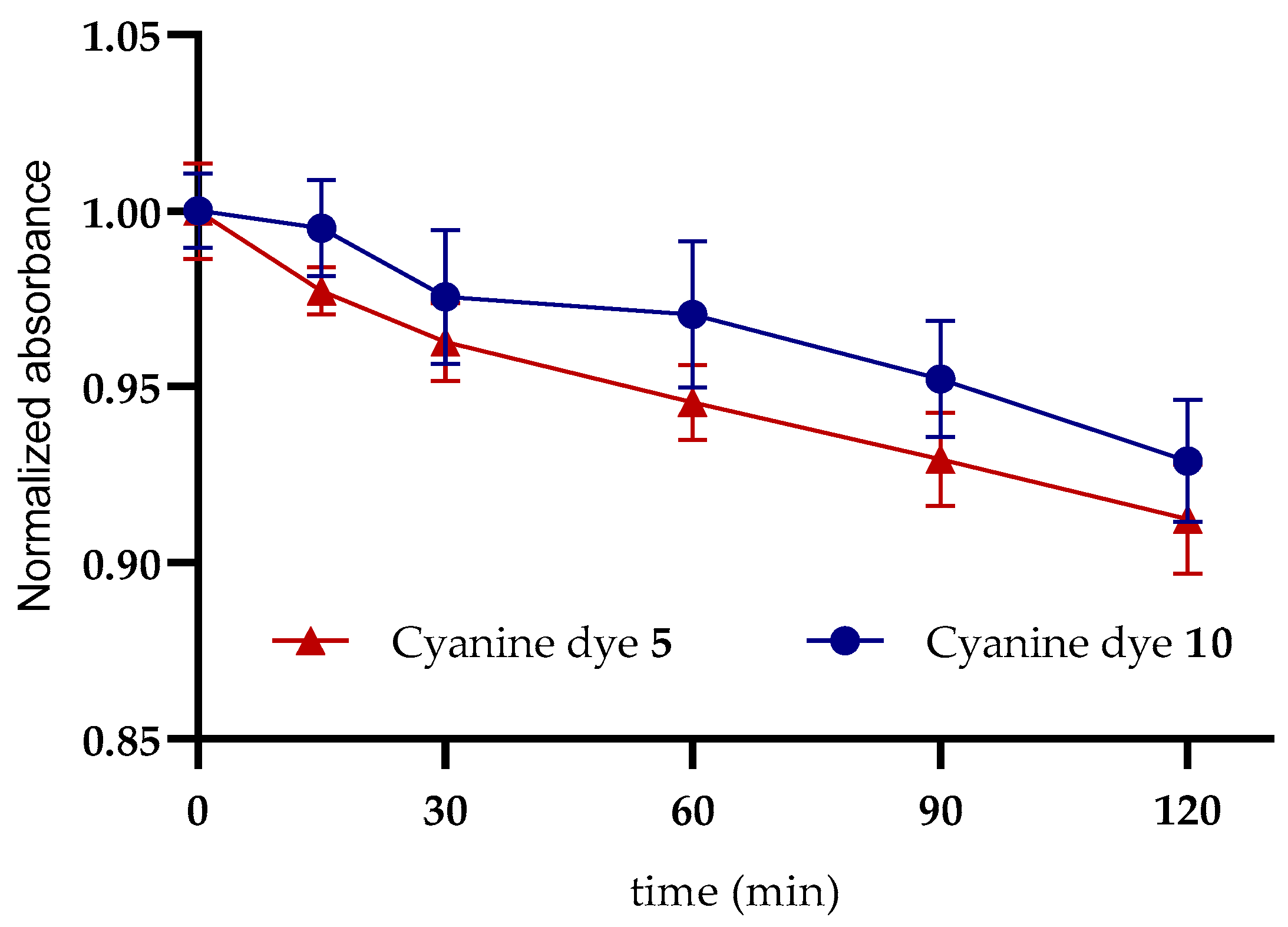
Photostability Assay
Morphological Analysis
3.2.2. Fluorescence Microscopy
3.2.3. Flow Cytometry
3.2.4. Topoisomerase II Inhibitory Assay
3.3. Computational Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dichiara, M.; Prezzavento, O.; Marrazzo, A.; Pittalà, V.; Salerno, L.; Rescifina, A.; Amata, E. Recent advances in drug discovery of phototherapeutic non-porphyrinic anticancer agents. Eur. J. Med. Chem. 2017, 142, 459–485. [Google Scholar] [CrossRef] [PubMed]
- Shindy, H.A. Fundamentals in the chemistry of cyanine dyes: A review. Dye. Pigment. 2017, 145, 505–513. [Google Scholar] [CrossRef]
- Ilina, K.; Henary, M. Cyanine dyes containing quinoline moieties: History, synthesis, optical properties, and applications. Chem. Eur. J. 2021, 27, 4230–4248. [Google Scholar] [CrossRef] [PubMed]
- Gopika, G.S.; Prasad, P.M.H.; Lekshmi, A.G.; Lekshmypriya, S.; Sreesaila, S.; Arunima, C.; Kumar, M.S.; Anil, A.; Sreekumar, A.; Pillai, Z.S. Chemistry of cyanine dyes—A review. Mater. Today Proc. 2021, 46, 3102–3108. [Google Scholar] [CrossRef]
- Bilici, K.; Cetin, S.; Aydındogan, E.; Acar, H.Y.; Kolemen, S. Recent advances in cyanine-based phototherapy agents. Front. Chem. 2021, 9, 707876. [Google Scholar] [CrossRef]
- Dereje, D.M.; Pontremoli, C.; Plata, M.J.M.; Visentin, S.; Barbero, N. Polymethine dyes for PDT: Recent advances and perspectives to drive future applications. Photochem. Photobiol. Sci. 2022, 21, 397–419. [Google Scholar] [CrossRef]
- Shindy, H.A. Characterization, mechanisms and applications in the chemistry of cyanine dyes: A review. Eur. J. Mol. Biotechnol. 2016, 14, 158–170. [Google Scholar] [CrossRef]
- Li, B.; Lin, L.; Lin, H.; Wilson, B.C. Photosensitized singlet oxygen generation and detection: Recent advances and future perspectives in cancer photodynamic therapy. J. Biophotonics 2016, 9, 1314–1325. [Google Scholar] [CrossRef]
- Conceição, S.D.; Ferreira, P.D.; Ferreira, F.V.L. Photochemistry and cytotoxicity evaluation of heptamethinecyanine near infrared (NIR) dyes. Int. J. Mol. Sci. 2013, 14, 18557–18571. [Google Scholar] [CrossRef]
- Li, L.; Chen, Y.; Chen, W.; Tan, Y.; Chen, H.; Yin, J. Photodynamic therapy based on organic small molecular fluorescent dyes. Chin. Chem. Lett. 2019, 30, 1689–1703. [Google Scholar] [CrossRef]
- Lima, E.; Reis, L.V. ‘Lights, squaraines, action!’—The role of squaraine dyes in photodynamic therapy. Future Med. Chem. 2022, 14, 1375–1402. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, Y.; Sato, Y.; Nishizawa, S. Deep-red light-up signaling of benzo[c,d]indole–quinoline monomethine cyanine for imaging of nucleolar RNA in living cells and for sequence-selective RNA analysis. Anal. Chem. 2019, 91, 14254–14260. [Google Scholar] [CrossRef] [PubMed]
- Kurutos, A.; Orehovec, I.; Paić, A.T.; Crnolatac, I.; Horvat, L.; Gadjev, N.; Piantanida, I.; Deligeorgiev, T. New series of non-toxic DNA intercalators, mitochondria targeting fluorescent dyes. Dye. Pigment. 2018, 148, 452–459. [Google Scholar] [CrossRef]
- Crnolatac, I.; Tumir, L.-M.; Lesev, N.Y.; Vasilev, A.A.; Deligeorgiev, T.G.; Mišković, K.; Glavaš-Obrovac, L.; Vugrek, O.; Piantanida, I. Probing the structural properties of DNA/RNA grooves with sterically restricted phosphonium dyes: Screening of dye cytotoxicity and uptake. ChemMedChem 2013, 8, 1093–1103. [Google Scholar] [CrossRef]
- Glavaš-Obrovac, L.; Piantanida, I.; Marczi, S.; Mašić, L.; Timcheva, I.I.; Deligeorgiev, T.G. Minor structural differences of monomethine cyanine derivatives yield strong variation in their interactions with DNA, RNA as well as on their in vitro antiproliferative activity. Bioorganic Med. Chem. 2009, 17, 4747–4755. [Google Scholar] [CrossRef]
- Kaloyanova, S.; Crnolatac, I.; Lesev, N.; Piantanida, I.; Deligeorgiev, T. Synthesis and study of nucleic acids interactions of novel monomethine cyanine dyes. Dye. Pigment. 2012, 92, 1184–1191. [Google Scholar] [CrossRef]
- Serrano, J.L.; Maia, A.; Santos, A.O.; Lima, E.; Reis, L.V.; Nunes, M.J.; Boto, R.E.F.; Silvestre, S.; Almeida, P. An insight into symmetrical cyanine dyes as promising selective antiproliferative agents in Caco-2 colorectal cancer cells. Molecules 2022, 27, 5779. [Google Scholar] [CrossRef]
- Volkova, K.D.; Kovalska, V.B.; Balanda, A.O.; Losytskyy, M.Y.; Golub, A.G.; Vermeij, R.J.; Subramaniam, V.; Tolmachev, O.I.; Yarmoluk, S.M. Specific fluorescent detection of fibrillar α-synuclein using mono- and trimethine cyanine dyes. Bioorganic Med. Chem. 2008, 16, 1452–1459. [Google Scholar] [CrossRef]
- Geigy, J.R. Process for the Production of Cyanine Compounds. GB1167110A. N.S.a.C.I.H. Corp. 1969. Available online: https://worldwide.espacenet.com/patent/search/family/004215648/publication/GB1167110A?q=pn%3DGB1167110A (accessed on 1 October 2024).
- Riccardi, C.; Nicoletti, I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat. Protoc. 2006, 1, 1458–1461. [Google Scholar] [CrossRef]
- Guloglu, S.; Kirmaci, N.F.; Çetinkol, P.Ö.; Forough, M.; Gulkaya, A. Azacyanines as novel topoisomerase II alpha inhibitors. Lett. Drug Des. Discov. 2020, 17, 666–671. [Google Scholar] [CrossRef]
- Okoro, C.O.; Fatoki, T.H. A mini review of novel topoisomerase II inhibitors as future anticancer agents. Int. J. Mol. Sci. 2023, 24, 2532. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Wang, J.; Li, Y.; Li, D.; Xu, L.; Hou, T. The application of in silico drug-likeness predictions in pharmaceutical research. Adv. Drug Deliv. Rev. 2015, 86, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J.J. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J. Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Egan, W.J.; Merz, K.M.; Baldwin, J.J. Prediction of drug absorption using multivariate statistics. J. Med. Chem. 2000, 43, 3867–3877. [Google Scholar] [CrossRef]
- Muegge, I.; Heald, S.L.; Brittelli, D. Simple selection criteria for drug-like chemical matter. J. Med. Chem. 2001, 44, 1841–1846. [Google Scholar] [CrossRef]
- Baell, J.B.; Holloway, G.A. New substructure filters for removal of pan assay interference compounds (PAINS) from screening libraries and for their exclusion in bioassays. J. Med. Chem. 2010, 53, 2719–2740. [Google Scholar] [CrossRef]
- Brenk, R.; Schipani, A.; James, D.; Krasowski, A.; Gilbert, I.H.; Frearson, J.; Wyatt, P.G. Lessons learnt from assembling screening libraries for drug discovery for neglected diseases. ChemMedChem 2008, 3, 435–444. [Google Scholar] [CrossRef]
- Arnold, W.A.; Blum, A.; Branyan, J.; Bruton, T.A.; Carignan, C.C.; Cortopassi, G.; Datta, S.; DeWitt, J.; Doherty, A.C.; Halden, R.U.; et al. Quaternary ammonium compounds: A chemical class of emerging concern. Environ. Sci. Technol. 2023, 57, 7645–7665. [Google Scholar] [CrossRef] [PubMed]
- Kovacic, P.; Somanathan, R. Nitroaromatic compounds: Environmental toxicity, carcinogenicity, mutagenicity, therapy and mechanism. J. Appl. Toxicol. 2014, 34, 810–824. [Google Scholar] [CrossRef] [PubMed]
- Catarro, M.; Serrano, J.; Cavalheiro, E.; Ramos, S.; Santos, A.O.; Silvestre, S. Novel 4-acetamide-2-alkylthio-N-acetanilides resembling nimesulide: Synthesis, Cell viability evaluation and in silico studies. Bioorganic Med. Chem. 2017, 25, 4304–4313. [Google Scholar] [CrossRef] [PubMed]
- Pardal, A.C.; Ramos, S.S.; Santos, P.F.; Reis, L.V.; Almeida, P. Synthesis and spectroscopic characterisation of N-alkyl quaternary ammonium salts typical precursors of cyanines. Molecules 2002, 7, 320–330. [Google Scholar] [CrossRef]
- Dimroth, K.; Grief, N.; Klapproth, A. Phosphamethincyanin-Farbstoffe mit Benzimidazolylsubstituenten. Justus Liebigs Ann. Chem. 1975, 1975, 373–386. [Google Scholar] [CrossRef]
- Hamer, F.M. XXXI.—The ψ-cyanine condensation. J. Chem. Soc. (Resumed) 1928, 206–215. [Google Scholar] [CrossRef]
- Hamer, F.M. CLXVI.—A comparison of some isomeric isocyanines. J. Chem. Soc. Trans. 1921, 119, 1432–1444. [Google Scholar] [CrossRef]
- Lima, E.; Ferreira, O.; Silva, J.F.; Santos, A.O.; Boto, R.E.; Fernandes, J.R.; Almeida, P.; Silvestre, S.M.; Reis, L.V. Photodynamic activity of indolenine-based aminosquaraine cyanine dyes: Synthesis and in vitro photobiological evaluation. Dye. Pigment. 2020, 174, 108024. [Google Scholar] [CrossRef]


 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Dye—Method | R1 | R2 | R3 | X | Y | W | Yield (%) | λmax (nm) (log ε) |
| 1 a | H | CH3 | C5H11 | S | S | I | 72 | 425 (4.85) |
| 2 a | H | CH3 | C10H21 | S | S | I | 68 | 427 (4.95) |
| 3 b | H | CH3 | CH3 | S | O | TsO | 47 | 401 (4.88) |
| 4 b | H | CH3 | CH3 | S | NCH3 | I | 84 | 385 (5.26) |
| 5 b | H | CH3 | CH3 | O | CH=CH | TsO | 63 | 436 (4.72) |
| 6 b | H | CH3 | CH3 | S | CH=CH | TsO | 72 | 483 (4.53) |
| 7 b | 5-NHCOCH3 | C2H5 | C2H5 | S | S | TsO | 29 | 432 (4.92) |
| 8 c | 5-NH2 | C2H5 | C2H5 | S | S | I | 31 | 454 (4.67) |
| 9 b | 5-NO2 | C2H5 | C2H5 | S | NCH2CH3 | I | 26 | 422 (4.56) |
| 10 b | 6-NO2 | C2H5 | C2H5 | S | S | TsO | 53 | 439 (4.81) |
| 11 d | 6-NH2 | C2H5 | C2H5 | S | S | I | 81 | 444 (4.62) |
| 12 e |  | 68 | 558 (5.26) | |||||
| Dye | NHDF | Caco-2 | PC-3 | MCF-7 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IC50 | 95% CI | IC50 | 95% CI | SI | IC50 | 95% CI | SI | IC50 | 95% CI | SI | |
| 5-FU | 4.53 | 2.20–9.32 | 1.11 | 0.66–1.89 | 4.1 | 2.43 | 1.10–5.38 | 1.9 | 2.12 | 1.22–3.70 | 2.1 |
| 1 | 0.55 | 0.42–0.70 | 0.36 | 0.28–0.46 | 1.5 | - | - | - | - | - | - |
| 2 | 0.27 | 0.19–0.39 | 0.24 | 0.17–0.35 | 1.1 | - | - | - | - | - | - |
| 3 | 7.53 | 4.09–13.84 | 0.09 | 0.06–0.14 | 81.2 | 0.43 | 0.28–0.67 | 17.2 | 4.04 | 1.78–9.15 | 1.9 |
| 4 | 7.93 | 4.44–14.14 | 0.23 | 0.15–0.35 | 33.6 | 2.85 | 1.35–6.01 | 2.8 | - | - | - |
| 5 | 2.79 | 1.68–4.65 | 0.010 | 0.006–0.016 | 278.3 | 1.43 | 0.99–2.09 | 1.9 | 3.83 | 1.74–8.44 | 0.7 |
| 6 | 0.79 | 0.59–1.05 | 0.27 | 0.14–0.54 | 2.9 | - | - | - | - | - | - |
| 7 | 11.63 | 7.11–19.0 | 0.14 | 0.10–0.19 | 80.5 | 3.56 | 1.55–8.16 | 3.3 | 0.38 | 0.10–1.38 | 30.2 |
| 8 | 0.95 | 0.77–1.17 | 0.74 | 0.44–1.20 | 1.3 | - | - | - | - | - | - |
| 9 | 24.54 | 11.16–53.96 | 1.40 | 0.86–2.28 | 17.5 | - | - | - | - | - | - |
| 10 | 22.38 | 10.47–47-85 | 0.14 | 0.08–0.25 | 151.4 | 5.93 | 2.65–13.28 | 3.8 | 13.30 | 6.84–25.86 | 1.7 |
| 11 | 2.997 | 1.82–4.92 | 0.17 | 0.13–0.23 | 17.1 | 2.10 | 1.30–3.38 | 1.4 | 0.33 | 0.16–0.65 | 9.0 |
| 12 | 0.63 | 0.43–0.89 | 0.73 | 0.58–0.96 | 0.9 | - | - | - | - | - | - |
| Descriptor | Dye 5 | Dye 10 | |
|---|---|---|---|
| Physicochemical Properties | H-bond donors | 0 | 0 |
| H-bond acceptors | 1 | 2 | |
| Molecular weight (g/mol) | 289.35 | 384.50 | |
| Consensus CLogP | 2.74 | 3.36 | |
| Molar Refractivity | 90.60 | 112.53 | |
| Rotatable bonds | 1 | 4 | |
| Fraction Csp3 | 0.11 | 0.21 | |
| TPSA (Å2) | 21.95 | 111.11 | |
| Log S (ESOL) | −5.07 | −5.94 | |
| Drug-likeness | Lipinski | Yes; 0 violations | Yes; 0 violations |
| Ghose | Yes | Yes | |
| Veber | Yes | Yes | |
| Egan | Yes | Yes | |
| Muegge | Yes | Yes | |
| Bioavailability Score | 0.55 | 0.55 | |
| Medicinal Chemistry | PAINS | 0 alert | 0 alert |
| Brenk | 1 alert; Quaternary nitrogen | 2 alerts; Quaternary nitrogen; nitro group | |
| Leadlikeness | Yes | No; 1 violation; MW > 350 | |
| Synthetic accessibility | 3.56 | 3.95 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maia, A.; Ventura, C.; Santos, A.O.; Nunes, M.J.; Boto, R.E.F.; Sousa, Â.; Silvestre, S.M.; Almeida, P.; Serrano, J.L. A New Demand for Improved Selectivity and Potency of Cyanine Dyes as Antiproliferative Agents Against Colorectal Cancer Cells. Molecules 2024, 29, 5581. https://doi.org/10.3390/molecules29235581
Maia A, Ventura C, Santos AO, Nunes MJ, Boto REF, Sousa Â, Silvestre SM, Almeida P, Serrano JL. A New Demand for Improved Selectivity and Potency of Cyanine Dyes as Antiproliferative Agents Against Colorectal Cancer Cells. Molecules. 2024; 29(23):5581. https://doi.org/10.3390/molecules29235581
Chicago/Turabian StyleMaia, Ana, Cathy Ventura, Adriana O. Santos, Maria J. Nunes, Renato E. F. Boto, Ângela Sousa, Samuel M. Silvestre, Paulo Almeida, and João L. Serrano. 2024. "A New Demand for Improved Selectivity and Potency of Cyanine Dyes as Antiproliferative Agents Against Colorectal Cancer Cells" Molecules 29, no. 23: 5581. https://doi.org/10.3390/molecules29235581
APA StyleMaia, A., Ventura, C., Santos, A. O., Nunes, M. J., Boto, R. E. F., Sousa, Â., Silvestre, S. M., Almeida, P., & Serrano, J. L. (2024). A New Demand for Improved Selectivity and Potency of Cyanine Dyes as Antiproliferative Agents Against Colorectal Cancer Cells. Molecules, 29(23), 5581. https://doi.org/10.3390/molecules29235581











