Preparation and Evaluation of a Novel Branched Polymer as Thickener for Calcium Chloride-Based Drilling and Completion Fluids
Abstract
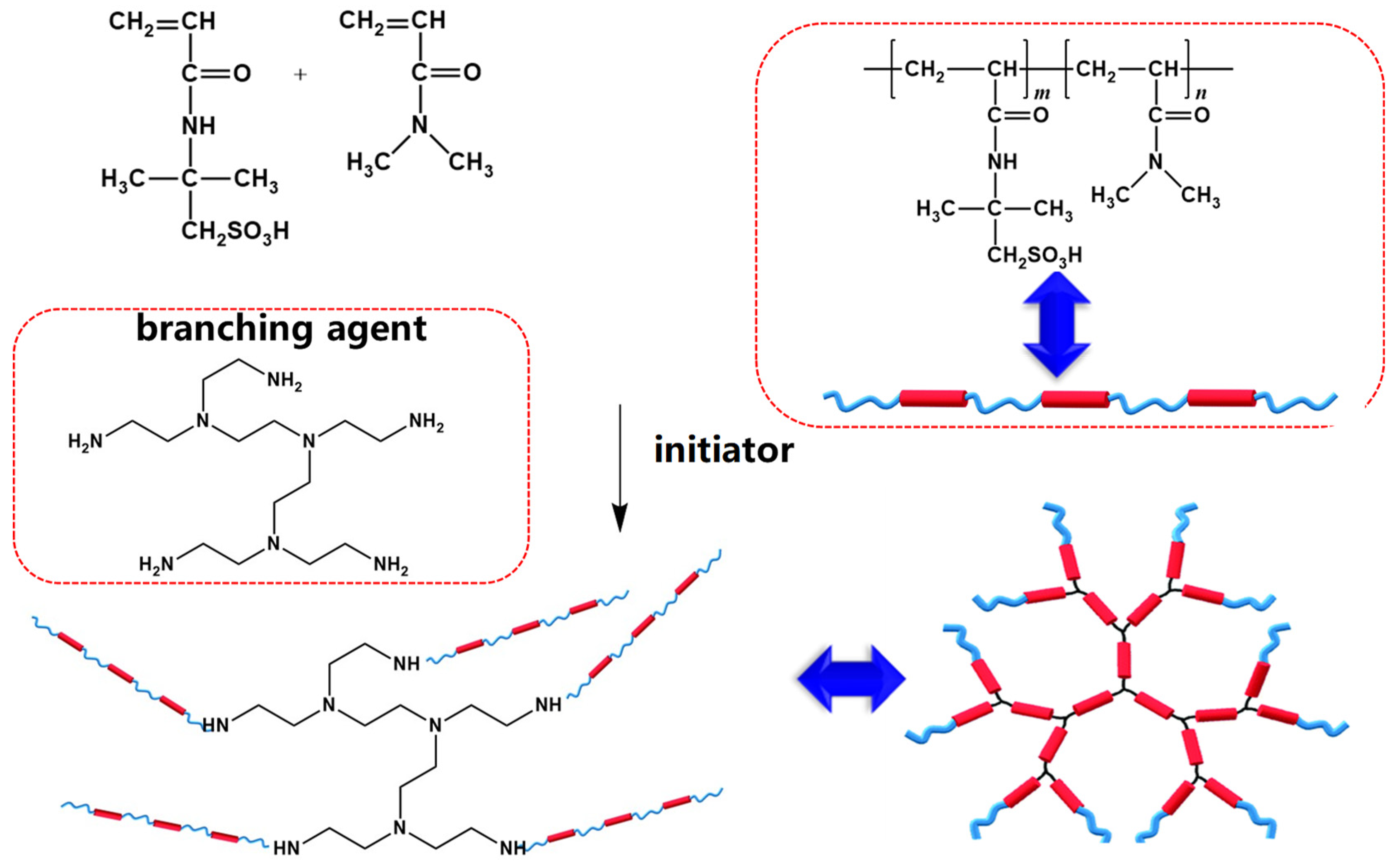
1. Introduction
2. Results and Discussion
2.1. Structural Analysis of PAD-B
2.2. Rheological Properties of PAD-B/PAD-L
2.3. The Effect of CaCl2 on the Rheological Measurements of PAD-B
2.4. The Effect of CaCl2 on YP and PV of PAD-B
2.5. Comparative Evaluation Experiment
2.5.1. Comparison of YP and PV with PAD-L
2.5.2. Comparison of Rheological Measurements with PAD-L
2.6. Morphology Characterization
3. Materials and Methods
3.1. Materials
3.2. Preparation of Polymers
3.3. Preparation of Sample Solutions
3.4. Instruments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Livescu, S. Mathematical modeling of thixotropic drilling mud and crude oil flow in wells and pipelines—A review. J. Petrol. Sci. Eng. 2012, 98, 174–184. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, Y.; Zhou, F.; Chu, P.K. Effects of carbon ash on rheological properties of water-based drilling fluids. J. Petrol. Sci. Eng. 2012, 100, 1–8. [Google Scholar] [CrossRef]
- Dua, H.; Wang, G.; Deng, G.; Cao, C. Modelling the effect of mudstone cuttings on rheological properties of KCl/Polymer water-based drilling fluid. J. Petrol. Sci. Eng. 2018, 170, 422–429. [Google Scholar] [CrossRef]
- Yan, X.; Lan, H.; Moore, I.D.; Ma, B. Flow properties of fresh mud (drilling fluid) used in horizontal directional drilling. J. Pipeline Syst. Eng. Pract. 2021, 12, 04020063. [Google Scholar] [CrossRef]
- Mohammadi, M.; Kouhi, M.; Sarrafi, A.; Schaffie, M. Studying rheological behavior of nanoclay as oil well drilling fluid. Res. Chem. Intermed. 2015, 41, 2823–2831. [Google Scholar] [CrossRef]
- Khalil, M.; Jan, B.M. Herschel-Bulkley rheological parameters of a novel environmentally friendly lightweight biopolymer drilling fluid from xanthan gum and starch. J. Appl. Polym. Sci. 2012, 124, 595–606. [Google Scholar] [CrossRef]
- Barry, M.M.; Jung, Y.; Lee, J.K.; Phuoc, T.X.; Chyu, M.K. Fluid filtration and rheological properties of nanoparticle additive and intercalated clay hybrid bentonite drilling fluids. J. Petrol. Sci. Eng. 2015, 127, 338–346. [Google Scholar] [CrossRef]
- Kelessidis, V.C.; Poulakakis, E.; Chatzistamou, V. Use of Carbopol 980 and carboxymethyl cellulose polymers as rheology modifiers of sodium-bentonite water dispersions. Appl. Clay Sci. 2011, 54, 63–69. [Google Scholar] [CrossRef]
- Abbas, A.K.; Dahm, H.H.; Hadi, F.A. Experimental study of cuttings transport efficiency of low solid bentonite drilling fluids. Arab. J. Sci. Eng. 2022, 47, 11863–11878. [Google Scholar] [CrossRef]
- Song, K.; Wu, Q.; Li, M.C.; Wojtanowicz, A.K.; Dong, L.L.; Zhang, X.Q.; Ren, S.X.; Lei, T.Z. Performance of low solid bentonite drilling fluids modified by cellulose nanoparticles. J. Nat. Gas Sci. Eng. 2016, 34, 1403–1411. [Google Scholar] [CrossRef]
- Baba Hamed, S.; Belhadri, M. Rheological properties of biopolymers drilling fluids. J. Petrol. Sci. Eng. 2009, 67, 84–90. [Google Scholar] [CrossRef]
- Temraz, M.G.; Hassanien, I. Mineralogy and rheological properties of some Egyptian bentonite for drilling fluids. J. Nat. Gas Sci. Eng. 2016, 31, 791–799. [Google Scholar] [CrossRef]
- Fereydouni, M.; Sabbaghi, S.; Saboori, R.; Zeinali, S. Effect of polyanionic cellulose polymer nanoparticles on rheological properties of drilling mud. Int. J. Nanosci. Nanotechnol. 2012, 8, 171–174. [Google Scholar]
- Jain, R.; Mahto, V. Evaluation of polyacrylamide/clay composite as a potential drilling fluid additive in inhibitive water based drilling fluid system. J. Pet. Sci. Eng. 2015, 133, 612–621. [Google Scholar] [CrossRef]
- Qu, Y.Z.; Lai, X.Q.; Zou, L.F.; Su, Y.N. Polyoxyalkyleneamine as shale inhibitor in water-based drilling fluids. Appl. Clay Sci. 2009, 44, 265–268. [Google Scholar] [CrossRef]
- Mortimer, D.A. Synthetic polyelectrolytes—A review. Polym. Int. 1991, 25, 29–41. [Google Scholar] [CrossRef]
- Nee, L.S.; Khalil, M.; Jan, B.M. Lightweight biopolymer drilling fluid for underbalanced drilling: An optimization study. J. Pet. Sci. Eng. 2015, 129, 178–188. [Google Scholar]
- Gao, C.; Yan, D. Hyperbranched polymers: From synthesis to applications. Prog. Pol. Sci. 2004, 29, 183–275. [Google Scholar] [CrossRef]
- Kim, Y.H.; Webster, O.W. Water soluble hyperbranched polyphenylene: “A unimolecular micelle? ” J. Am. Chem. Soc. 1990, 112, 4592–4593. [Google Scholar] [CrossRef]
- Huang, H.H.; Yao, Q.; Chen, H.L.; Liu, B.L. Scale inhibitors with a hyper-branched structure: Preparation, characterization and scale inhibition mechanism. RSC Adv. 2016, 6, 92943–92952. [Google Scholar] [CrossRef]
- Zhang, D.S.; Toh, G.W.; Lin, H.; Chen, Y.Y. In situ synthesis of silver nanoparticles on silk fabric with PNP for antibacterial finishing. J. Mater. Sci. 2012, 47, 5721–5728. [Google Scholar] [CrossRef]
- Xie, G.; Luo, P.Y.; Deng, M.Y.; Wang, Z. Nanoplugging performance of hyperbranched polyamine as nanoplugging agent in oil-based drilling fluid. J. Nanomater. 2015, 2015, 821910. [Google Scholar] [CrossRef]
- Cao, J.; Xu, G.; Wang, X.; Wang, H.; Zhang, J.; Liu, C. Tug-of-war between hydrogen bond and hydrophobic interaction of bisfunctionalized graphene oxide/hydrolyzed polyacrylamide allows thickening and salt-resistance in enhanced oil recovery. Colloid Surf. A Physicochem. Eng. Asp. 2022, 653, 129909. [Google Scholar] [CrossRef]
- Huang, H.; Yao, Q.; Liu, B.; Shan, N.; Chen, H.L. Synthesis and characterization of scale and corrosion inhibitors with hyper-branched structure and the mechanism. New J. Chem. 2017, 41, 12205–12217. [Google Scholar] [CrossRef]
- Zhong, H.Y.; Qiu, Z.S.; Sun, D.; Zhang, D.M.; Huang, W.A. Inhibitive properties comparison of different polyetheramines in water-based drilling fluid. J. Nat. Gas Sci. Eng. 2015, 26, 99–107. [Google Scholar] [CrossRef]
- Xin, H.P.; Ao, D.; Wang, X.J.; Zhu, Y.J.; Zhang, J.; Tan, Y.B. Synthesis, characterization, and properties of copolymers of acrylamide with sodium 2-acrylamido-2-methylpropane sulfonate with nano silica structure. Colloid. Polym. Sci. 2015, 293, 1307–1316. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, G.; An, Y. Research and evaluation of supramolecular tackifier and shear-strength improving agent ZJA for drilling fluids. Mod. Chem. Ind. 2016, 36, 131–135. [Google Scholar]
- Routledge, K.E.; Tartaglia, G.G.; Platt, G.W.; Vendruscolo, M.; Radford, S.E. Competition between Intramolecular and Intermolecular Interactions in an Amyloid-Forming Protein. J. Mol. Biol. 2009, 389, 776–786. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, C.; Wang, Z. Force spectroscopy of polymers: Studying on intramolecular and intermolecular interactions in single molecular level. Polymer 2008, 49, 3353–3361. [Google Scholar] [CrossRef]
- Ilyin, S.O. Structural Rheology in the Development and Study of Complex Polymer Materials. Polymers 2024, 16, 2458. [Google Scholar] [CrossRef]
- Xin, X.; Xu, G.Y.; Wu, D.; Li, Y.M.; Cao, X.R. The effect of CaCl2 on the interaction between hydrolyzed polyacrylamide and sodium stearate: Rheological property study. Colloids Surf. A Physicochem. Eng. Asp. 2007, 305, 138–144. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Kulichikhin, V.G. Rheology and Miscibility of Linear/Hyperbranched Polydimethylsiloxane Blends and an Abnormal Decrease in Their Viscosity. Macromolecules 2023, 56, 6818–6833. [Google Scholar] [CrossRef]
- Ding, T.J.; Wang, R.H.; Xu, J.F.; Ma, J.Y.; Wang, X.H.; Xue, J.W. Synthesis and application of a temperature sensitive poly (N-vinylcaprolactam-co-N,N-diethyl acrylamide) for low-temperature rheology control of water-based drilling fluid. Colloids Surf. A Physicochem. Eng. Asp. 2022, 644, 128855. [Google Scholar]
- Song, T.; Feng, Q.; Schuman, T.; Cao, J.; Bai, B. A novel branched polymer gel system with delayed gelation property for conformance control. SPE J. 2022, 27, 105–115. [Google Scholar] [CrossRef]
- Song, T.; Zhai, Z.; Liu, J.; Eriyagama, Y.; Ahdaya, M.; Alotibi, A.; Wang, Z.; Schuman, T.; Bai, B. Laboratory evaluation of a novel self-healable polymer gel for CO2 leakage remediation during CO2 storage and CO2 flooding. Chem. Eng. J. 2022, 444, 136635. [Google Scholar] [CrossRef]








| Samples | YP (τ0, Pa) | PV (μp, mPa·s) |
|---|---|---|
| PAD-B at 25 °C | 306.47 | 285 |
| PAD-B at 50 °C | 163.62 | 143.72 |
| PAD-B/CaCl2 at 25 °C | 128.40 | 134.45 |
| PAD-B/CaCl2 at 50 °C | 73.04 | 112.96 |
| PAD-L(+1×/CaCl2 at 25 °C | 10.04 | 43.70 |
| PAD-L(+1×)/CaCl2 at 50 °C | 3.41 | 33.66 |
| PAD-L(+2.5×)/CaCl2 at 25 °C | 82.56 | 143.42 |
| PAD-L(+2.5×)/CaCl2 at 50 °C | 48.94 | 112.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Yang, Z.; Wang, Q.; Chen, W.; Liu, T.; Zhou, T.; Li, S.; Xin, T.; Cao, J.; Xin, X. Preparation and Evaluation of a Novel Branched Polymer as Thickener for Calcium Chloride-Based Drilling and Completion Fluids. Molecules 2024, 29, 5542. https://doi.org/10.3390/molecules29235542
Zhang X, Yang Z, Wang Q, Chen W, Liu T, Zhou T, Li S, Xin T, Cao J, Xin X. Preparation and Evaluation of a Novel Branched Polymer as Thickener for Calcium Chloride-Based Drilling and Completion Fluids. Molecules. 2024; 29(23):5542. https://doi.org/10.3390/molecules29235542
Chicago/Turabian StyleZhang, Xianbin, Zhongfeng Yang, Qian Wang, Weijie Chen, Tengjiao Liu, Tao Zhou, Shulin Li, Tongle Xin, Jie Cao, and Xia Xin. 2024. "Preparation and Evaluation of a Novel Branched Polymer as Thickener for Calcium Chloride-Based Drilling and Completion Fluids" Molecules 29, no. 23: 5542. https://doi.org/10.3390/molecules29235542
APA StyleZhang, X., Yang, Z., Wang, Q., Chen, W., Liu, T., Zhou, T., Li, S., Xin, T., Cao, J., & Xin, X. (2024). Preparation and Evaluation of a Novel Branched Polymer as Thickener for Calcium Chloride-Based Drilling and Completion Fluids. Molecules, 29(23), 5542. https://doi.org/10.3390/molecules29235542






