Lignin Isolated by Microwave-Assisted Acid-Catalyzed Solvolysis Induced Cell Death in Mammalian Tumor Cells by Modulating Apoptotic Pathways
Abstract
1. Introduction
2. Results
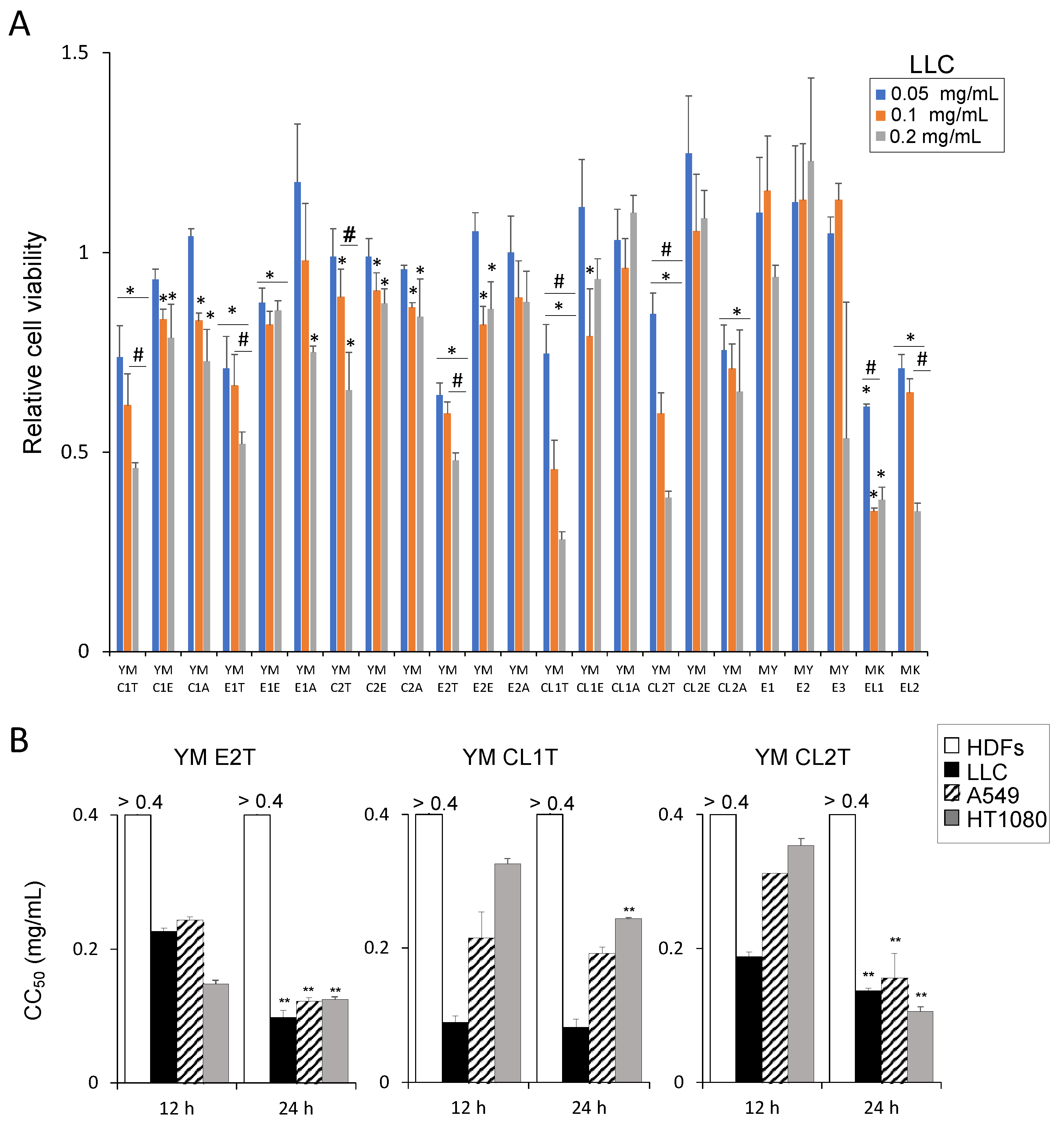
2.1. MASL Treatments Reduced the Viability of Tumor Cells, Whereas Normal Cells Were Less Remarkably Affected
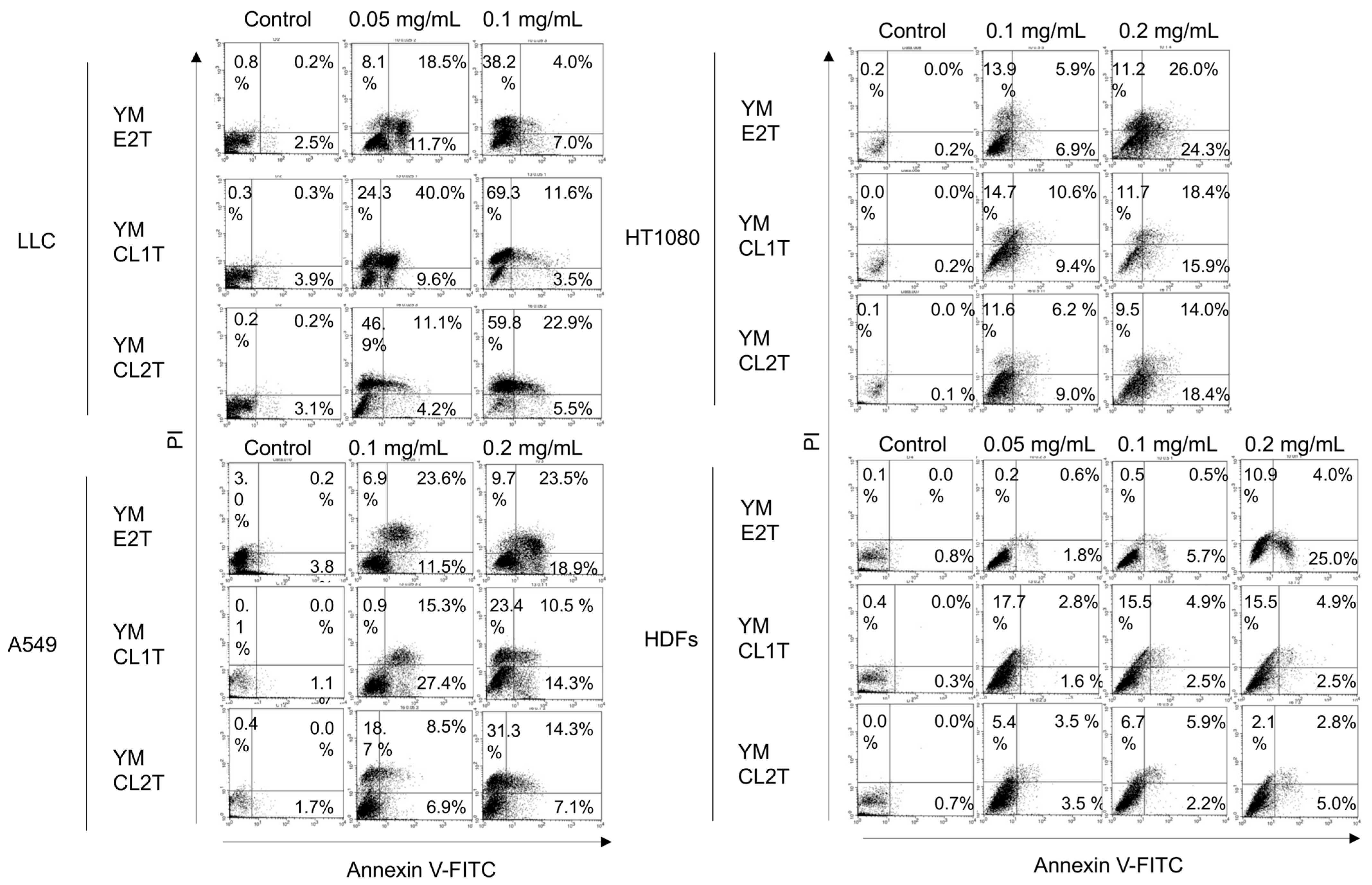
2.2. Tumor Cells Underwent Apoptosis After MASL Treatment
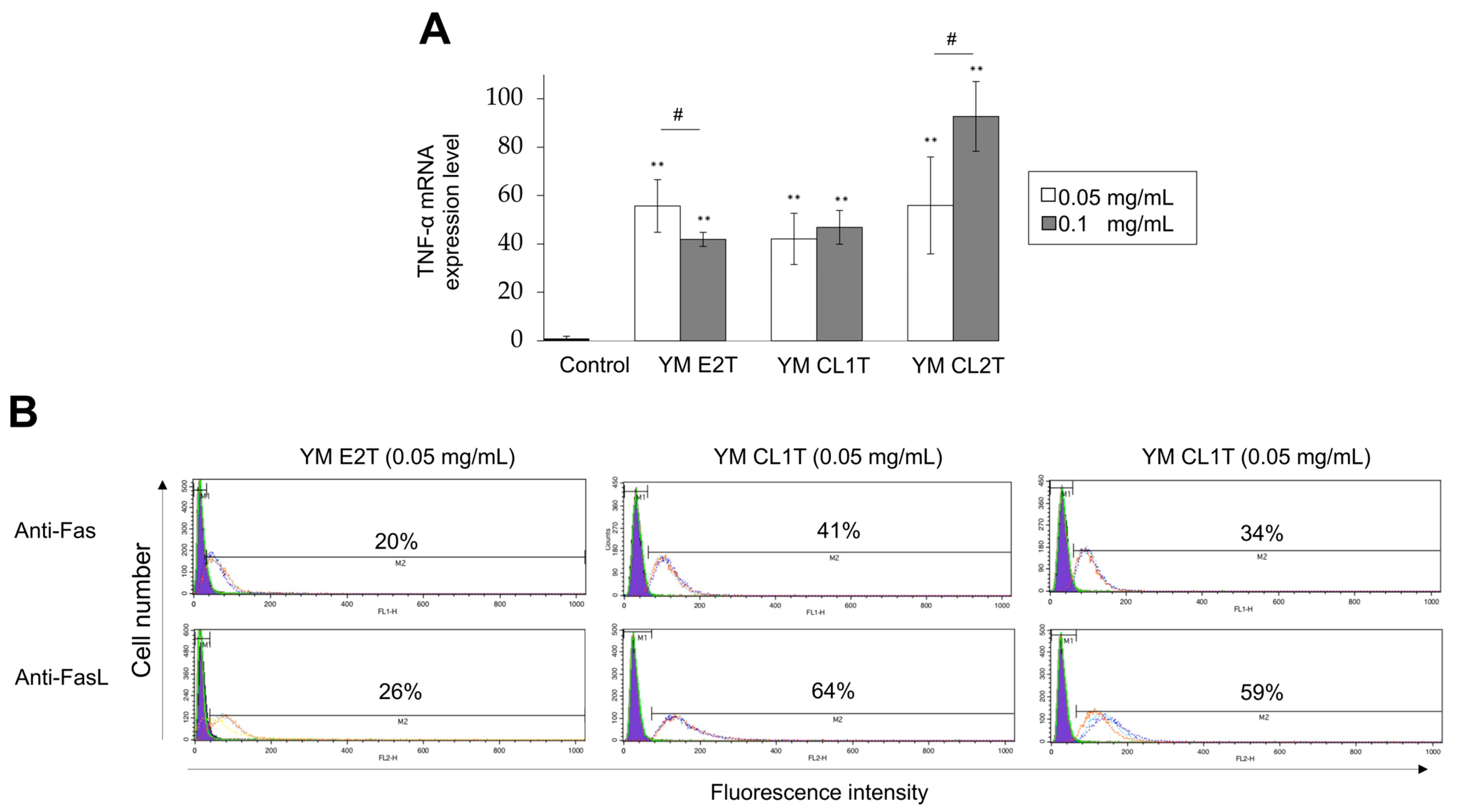
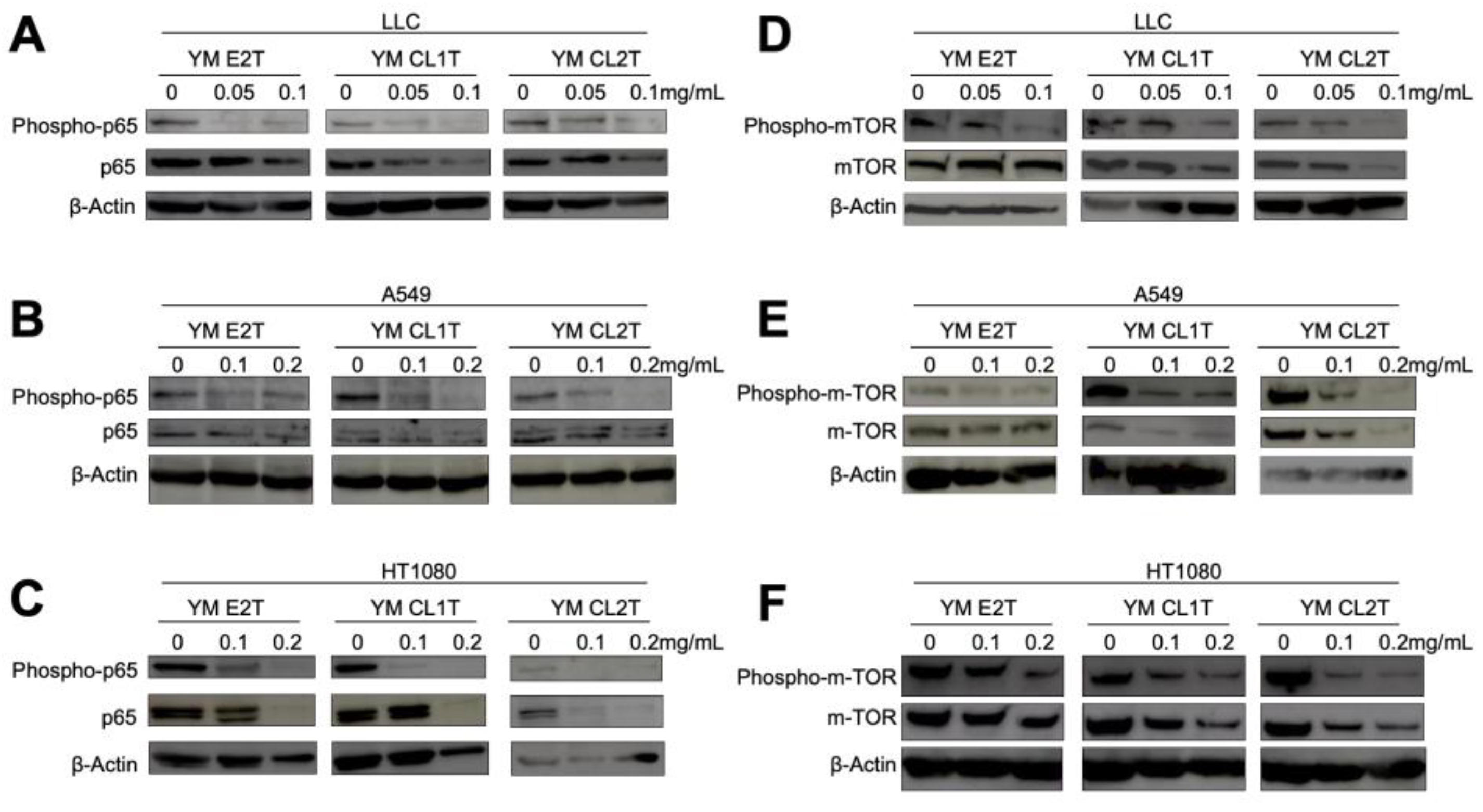
2.3. MASL Treatment Modulated Apoptotic Pathways in Tumor Cells
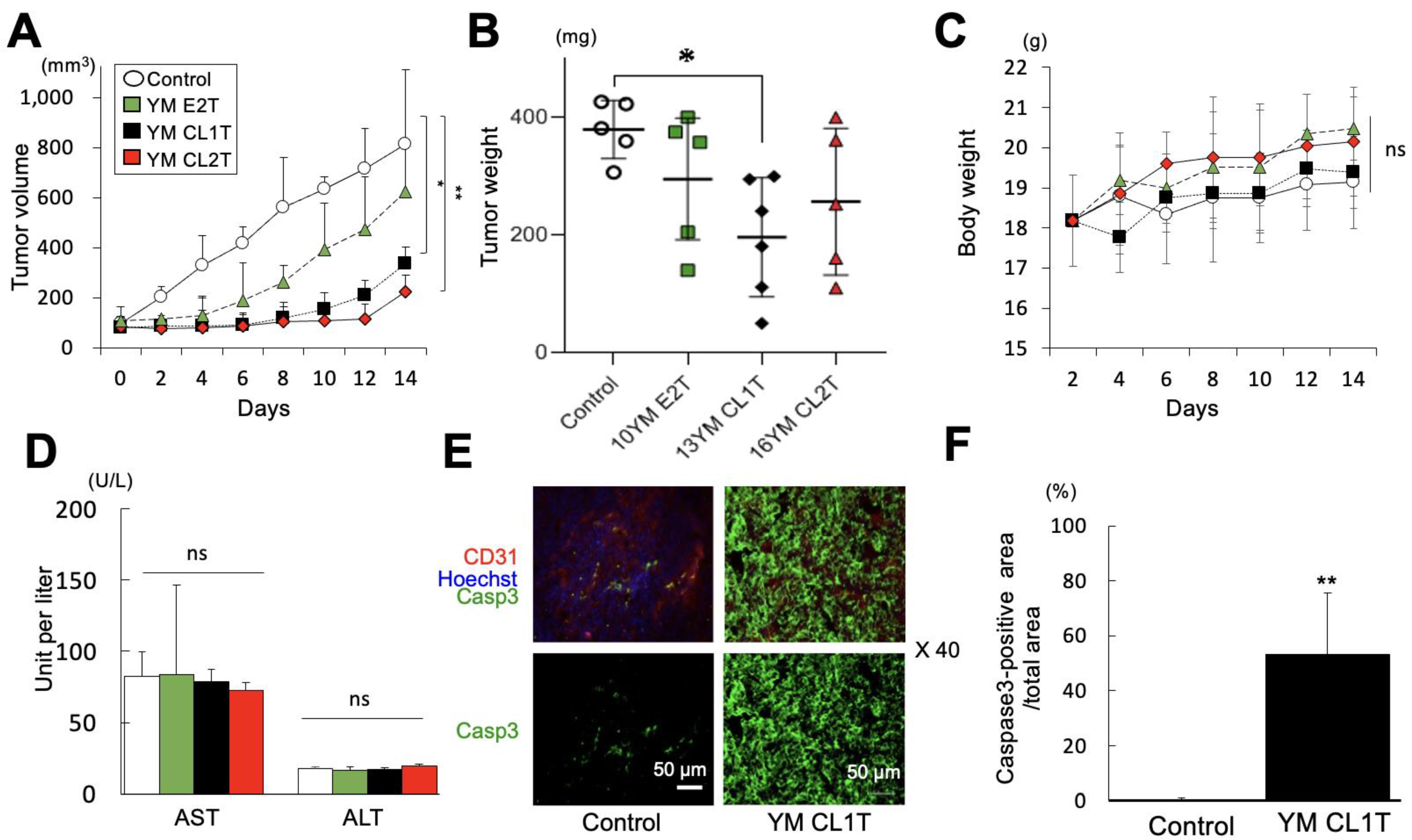
2.4. YM CL1T Administration Inhibited Tumor Growth In Vivo
3. Discussion
4. Materials and Methods
4.1. MASL Samples
4.2. Cell Lines and Culture Conditions
4.3. Cell Viability Assay and Estimation of CC50 Values
4.4. Flowcytometric Analysis
4.5. Real-Time RT-PCR
4.6. Western Blotting
4.7. LLC Tumor Mouse Model
4.8. Immunohistochemistry
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bidlack, J.; Malone, M.; Benson, R. Molecular Structure and Component Integration of Secondary Cell Walls in Plants. Proc. Okla. Acad. Sci. USA 1992, 72, 51–56. [Google Scholar]
- Siddiqui, L.; Mishra, H.; Mishra, P.K.; Iqbal, Z.; Talegaonkar, S. Novel 4-in-1 Strategy to Combat Colon Cancer, Drug Resistance and Cancer Relapse Utilizing Functionalized Bioinspiring Lignin Nanoparticle. Med. Hypotheses 2018, 121, 10–14. [Google Scholar] [CrossRef]
- Li, Y.; Qiu, X.; Qian, Y.; Xiong, W.; Yang, D. PH-Responsive Lignin-Based Complex Micelles: Preparation, Characterization and Application in Oral Drug Delivery. Chem. Eng. J. 2017, 327, 1176–1183. [Google Scholar] [CrossRef]
- Sakagami, H.; Hashimoto, K.; Suzuki, F.; Ogiwara, T.; Satoh, K.; Ito, H.; Hatano, T.; Takashi, Y.; Fujisawa, S. Molecular Requirements of Lignin-Carbohydrate Complexes for Expression of Unique Biological Activities. Phytochemistry 2005, 66, 2108–2120. [Google Scholar] [CrossRef]
- Tsuji, R.; Koizumi, H.; Aoki, D.; Watanabe, Y.; Sugihara, Y.; Matsushita, Y.; Fukushima, K.; Fujiwara, D. Lignin-Rich Enzyme Lignin (LREL), a Cellulase-Treated Lignin-Carbohydrate Derived from Plants, Activates Myeloid Dendritic Cells via Toll-like Receptor 4 (TLR4). J. Biol. Chem. 2015, 290, 4410–4421. [Google Scholar] [CrossRef]
- Mori, Y.; Shirai, T.; Terauchi, R.; Tsuchida, S.; Mizoshiri, N.; Hayashi, D.; Arai, Y.; Kishida, T.; Mazda, O.; Kubo, T. Antitumor Effects of Pristimerin on Human Osteosarcoma Cells In Vitro and In Vivo. Onco Targets Ther. 2017, 10, 5703–5710. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Tchounwou, P.B. Cisplatin in Cancer Therapy: Molecular Mechanisms of Action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Ilieva, Y.; Zhelezova, I.; Atanasova, T.; Zaharieva, M.M.; Sasheva, P.; Ionkova, I.; Konstantinov, S. Cytotoxic Effect of the Biotechnologically-Derived Justicidin B on Human Lymphoma Cells. Biotechnol. Lett. 2014, 36, 2177–2183. [Google Scholar] [CrossRef]
- Arumugam, S.; Bandil, K.; Proksch, P.; Murugiyan, K.; Bharadwaj, M. Effects of A.Marina-Derived Isoquercitrin on TNF-Related Apoptosis-Inducing Ligand Receptor (TRAIL-R) Expression and Apoptosis Induction in Cervical Cancer Cells. Appl. Biochem. Biotechnol. 2017, 182, 697–707. [Google Scholar] [CrossRef]
- Skórkowska-Telichowska, K.; Kulma, A.; Gębarowski, T.; Wojtasik, W.; Kostyn, K.; Moreira, H.; Szyjka, A.; Boba, A.; Preisner, M.; Mierziak, J.; et al. V79 Fibroblasts Are Protected Against Reactive Oxygen Species by Flax Fabric. Appl. Biochem. Biotechnol. 2018, 184, 366–385. [Google Scholar] [CrossRef] [PubMed]
- Kaiho, A.; Kogo, M.; Sakai, R.; Saito, K.; Watanabe, T. In Situ Trapping of Enol Intermediates with Alcohol during Acid-Catalysed de-Polymerisation of Lignin in a Nonpolar Solvent. Green Chem. 2015, 17, 2780–2783. [Google Scholar] [CrossRef]
- Saito, K.; Kaiho, A.; Sakai, R.; Nishimura, H.; Okada, H.; Watanabe, T. Characterization of the Interunit Bonds of Lignin Oligomers Released by Acid-Catalyzed Selective Solvolysis of Cryptomeria Japonica and Eucalyptus Globulus Woods via Thioacidolysis and 2D-NMR. J. Agric. Food Chem. 2016, 64, 9152–9160. [Google Scholar] [CrossRef]
- Rivas, S.; Parajó, J.C. Single-Stage Fractionation of Vine Shoots Using Microwave Heating. Appl. Sci. 2021, 11, 7954. [Google Scholar] [CrossRef]
- Schutyser, W.; Renders, T.; Bosch, S.V.d.; Koelewijn, S.-F.; Beckham, G.T.; Sels, B.F. Chemicals from Lignin: An Interplay of Lignocellulose Fractionation, Depolymerisation, and Upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Fridrich, B.; de Santi, A.; Elangovan, S.; Barta, K. Bright Side of Lignin Depolymerization: Toward New Platform Chemicals. Chem. Rev. 2018, 118, 614–678. [Google Scholar] [CrossRef]
- Guo, M.; Jin, T.; Nghiem, N.P.; Fan, X.; Qi, P.X.; Jang, C.H.; Shao, L.; Wu, C. Assessment of Antioxidant and Antimicrobial Properties of Lignin from Corn Stover Residue Pretreated with Low-Moisture Anhydrous Ammonia and Enzymatic Hydrolysis Process. Appl. Biochem. Biotechnol. 2018, 184, 350–365. [Google Scholar] [CrossRef] [PubMed]
- Mahata, D.; Jana, M.; Jana, A.; Mukherjee, A.; Mondal, N.; Saha, T.; Sen, S.; Nando, G.B.; Mukhopadhyay, C.K.; Chakraborty, R.; et al. Lignin-Graft-Polyoxazoline Conjugated Triazole a Novel Anti-Infective Ointment to Control Persistent Inflammation. Sci. Rep. 2017, 7, 46412. [Google Scholar] [CrossRef]
- Kocab, A.J.; Duckett, C.S. Inhibitor of Apoptosis Proteins as Intracellular Signaling Intermediates. FEBS J. 2016, 283, 221–231. [Google Scholar] [CrossRef]
- Wallach, D.; Boldin, M.; Varfolomeev, E.; Beyaert, R.; Vandenabeele, P.; Fiers, W. Cell Death Induction by Receptors of the TNF Family: Towards a Molecular Understanding. FEBS Lett. 1997, 410, 96–106. [Google Scholar] [CrossRef]
- Lorenzo, H.K.; Susin, S.A. Mitochondrial Effectors in Caspase-Independent Cell Death. FEBS Lett. 2004, 557, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Dan, H.C.; Cooper, M.J.; Cogswell, P.C.; Duncan, J.A.; Ting, J.P.-Y.; Baldwin, A.S. Akt-Dependent Regulation of NF-κB Is Controlled by mTOR and Raptor in Association with IKK. Genes Dev. 2008, 22, 1490–1500. [Google Scholar] [CrossRef]
- Morrison, D.K. MAP Kinase Pathways. Cold Spring Harb. Perspect. Biol. 2012, 4, a011254. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jiao, D. Necroptosis, Tumor Necrosis and Tumorigenesis. Cell Stress 2020, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhabg, H.; Xu, Z.; Wu, L.P. Advanced materials of lignin-based materials. In Functional Materials from Lignin Methods and Advances; Loh, X.J., Kai, D., Li, Z., Eds.; World Scientific Publishing Europe: London, UK, 2018; pp. 169–205. [Google Scholar]
- Yoshioka, K.; Daidai, M.; Matsumoto, Y. Self-Sufficient Bioethanol Production System Using a Lignin-Derived Adsorbent of Fermentation Inhibitors. ACS Sustain. Chem. Eng. 2018, 6, 3070–3078. [Google Scholar] [CrossRef]
- Tokunaga, Y.; Nagata, T.; Kondo, K.; Katahira, M.; Watanabe, T. Complete NMR Assignment and Analysis of Molecular Structural Changes of β-O-4 Lignin Oligomer Model Compounds in Organic Media with Different Water Content. Holzforshung 2020, 75, 379–389. [Google Scholar] [CrossRef]
| Sample | Resources | Reagents for Microwave Solvolysis |
|---|---|---|
| YM C1T | Japanese cedar wood particles | H2O, EtOH, toluene (6:6:8), 0.75 g H2SO4 |
| YM C1E | Japanese cedar wood particles | H2O, EtOH, toluene (6:6:8), 0.75 g H2SO4 |
| YM C1A | Japanese cedar wood particles | H2O, EtOH, toluene (6:6:8), 0.75 g H2SO4 |
| YM E1T | E. globulus wood particles | H2O, EtOH, toluene (6:6:8), 0.75 g H2SO4 |
| YM E1E | E. globulus wood particles | H2O, EtOH, toluene (6:6:8), 0.75 g H2SO4 |
| YM E1A | E. globulus wood particles | H2O, EtOH, toluene (6:6:8), 0.25 g H2SO4 |
| YM C2T | Japanese cedar wood particles | H2O, EtOH, toluene (6:6:8), 0.25 g H2SO4 |
| YM C2E | Japanese cedar wood particles | H2O, EtOH, toluene (6:6:8), 0.25 g H2SO4 |
| YM C2A | Japanese cedar wood particles | H2O, EtOH, toluene (6:6:8), 0.25 g H2SO4 |
| YM E2T | E. globulus wood particles | H2O, EtOH, toluene (6:6:8), 0.25 g H2SO4 |
| YM E2E | E. globulus wood particles | H2O, EtOH, toluene (6:6:8), 0.25 g H2SO4 |
| YM E2A | E. globulus wood particles | H2O, EtOH, toluene (6:6:8), 7.5 g H2SO4 |
| YM CL1T | Japanese cedar alkali lignin | H2O, MeOH, toluene (6:6:8), 7.5 g H2SO4 |
| YM CL1E | Japanese cedar alkali lignin | H2O, MeOH, toluene (6:6:8), 7.5 g H2SO4 |
| YM CL1A | Japanese cedar alkali lignin | H2O, MeOH, toluene (6:6:8), 7.5 g H2SO4 |
| YM CL2T | Japanese cedar alkali lignin | H2O, EtOH, toluene (6:6:8), 7.5 g H2SO4 |
| YM CL2E | Japanese cedar alkali lignin | H2O, EtOH, toluene (6:6:8), 7.5 g H2SO4 |
| YM CL2A | Japanese cedar alkali lignin | H2O, EtOH, toluene (6:6:8), 7.5 g H2SO4 |
| MY E1 | Ball-milled E. globulus wood flour | AcOH containing 1% peracetic acid |
| MY E2 | Ball-milled E. globulus wood flour | AcOH containing 1% peracetic acid |
| MY E3 | Ball-milled E. globulus wood flour | AcOH containing 1% peracetic acid |
| MK EL1 | E. globulus alkali lignin | D2O, CD3COOD (2:1), 1.67 mM TsCl |
| MK EL2 | E. globulus celulase-treated residual lignin | D2O, CD3COOD (2:1), 1.67 mM TsCl |
| Reaction | |||||
|---|---|---|---|---|---|
| Sample | Volume | Temp | Time | Extraction Solvent | Microwave Reactor |
| YM C1T | 20 mL | 180 °C | 30 min | Toluene | Biotage Initiator+ |
| YM C1E | 20 mL | 180 °C | 30 min | Ethyl acetate | Biotage Initiator+ |
| YM C1A | 20 mL | 180 °C | 30 min | Acetone | Biotage Initiator+ |
| YM E1T | 20 mL | 180 °C | 30 min | Toluene | Biotage Initiator+ |
| YM E1E | 20 mL | 180 °C | 30 min | Ethyl acetate | Biotage Initiator+ |
| YM E1A | 20 mL | 180 °C | 30 min | Acetone | Biotage Initiator+ |
| YM C2T | 20 mL | 180 °C | 30 min | Toluene | Biotage Initiator+ |
| YM C2E | 20 mL | 180 °C | 30 min | Ethyl acetate | Biotage Initiator+ |
| YM C2A | 20 mL | 180 °C | 30 min | Acetone | Biotage Initiator+ |
| YM E2T | 20 mL | 180 °C | 30 min | Toluene | Biotage Initiator+ |
| YM E2E | 20 mL | 180 °C | 30 min | Ethyl acetate | Biotage Initiator+ |
| YM E2A | 200 mL | 180 °C | 30 min | Acetone | Milestone StartSYNTH |
| YM CL1T | 200 mL | 180 °C | 30 min | Toluene | Milestone StartSYNTH |
| YM CL1E | 200 mL | 180 °C | 30 min | Ethyl acetate | Milestone StartSYNTH |
| YM CL1A | 200 mL | 180 °C | 30 min | Acetone | Milestone StartSYNTH |
| YM CL2T | 200 mL | 180 °C | 30 min | Toluene | Milestone StartSYNTH |
| YM CL2E | 200 mL | 180 °C | 30 min | Ethyl acetate | Milestone StartSYNTH |
| YM CL2A | 20 mL | 180 °C | 30 min | Acetone | Milestone StartSYNTH |
| MY E1 | 20 mL | 50 °C | 10 min | Ethyl acetate | Biotage Initiator+ |
| MY E2 | 20 mL | 100 °C | 10 min | Ethyl acetate | Biotage Initiator+ |
| MY E3 | 20 mL | 140 °C | 10 min | Ethyl acetate | Biotage Initiator+ |
| MK EL1 | 15 mL | 160 °C | 30 min | Ethyl acetate | Biotage Initiator+ |
| MK EL2 | 15 mL | 160 °C | 60 min | Ethyl acetate | Milestone StartSYNTH |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kashimoto, R.; Ohgitani, E.; Makimura, Y.; Miyazaki, T.; Kimura, C.; Shin-Ya, M.; Nishimura, H.; Pezzotti, G.; Watanabe, T.; Mazda, O. Lignin Isolated by Microwave-Assisted Acid-Catalyzed Solvolysis Induced Cell Death in Mammalian Tumor Cells by Modulating Apoptotic Pathways. Molecules 2024, 29, 5490. https://doi.org/10.3390/molecules29235490
Kashimoto R, Ohgitani E, Makimura Y, Miyazaki T, Kimura C, Shin-Ya M, Nishimura H, Pezzotti G, Watanabe T, Mazda O. Lignin Isolated by Microwave-Assisted Acid-Catalyzed Solvolysis Induced Cell Death in Mammalian Tumor Cells by Modulating Apoptotic Pathways. Molecules. 2024; 29(23):5490. https://doi.org/10.3390/molecules29235490
Chicago/Turabian StyleKashimoto, Rio, Eriko Ohgitani, Yutaka Makimura, Tatsuya Miyazaki, Chihiro Kimura, Masaharu Shin-Ya, Hiroshi Nishimura, Giuseppe Pezzotti, Takashi Watanabe, and Osam Mazda. 2024. "Lignin Isolated by Microwave-Assisted Acid-Catalyzed Solvolysis Induced Cell Death in Mammalian Tumor Cells by Modulating Apoptotic Pathways" Molecules 29, no. 23: 5490. https://doi.org/10.3390/molecules29235490
APA StyleKashimoto, R., Ohgitani, E., Makimura, Y., Miyazaki, T., Kimura, C., Shin-Ya, M., Nishimura, H., Pezzotti, G., Watanabe, T., & Mazda, O. (2024). Lignin Isolated by Microwave-Assisted Acid-Catalyzed Solvolysis Induced Cell Death in Mammalian Tumor Cells by Modulating Apoptotic Pathways. Molecules, 29(23), 5490. https://doi.org/10.3390/molecules29235490








