Insight into the Local Surface Plasmon Resonance Effect of Pt-SnS2 Nanosheets in Tetracycline Photodegradation
Abstract
1. Introduction
2. Results and Discussion
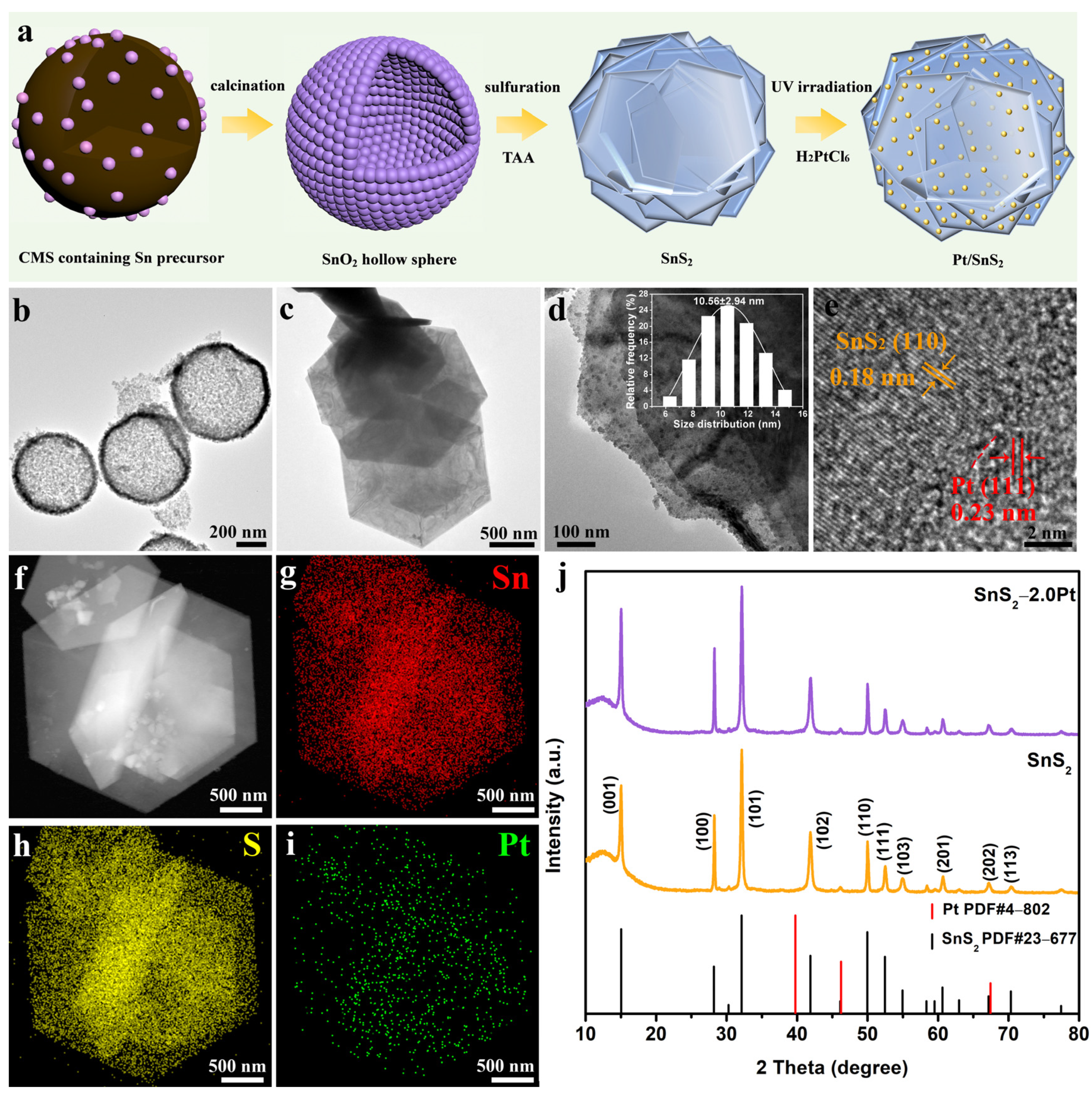
2.1. Morphological and Structural Characterization
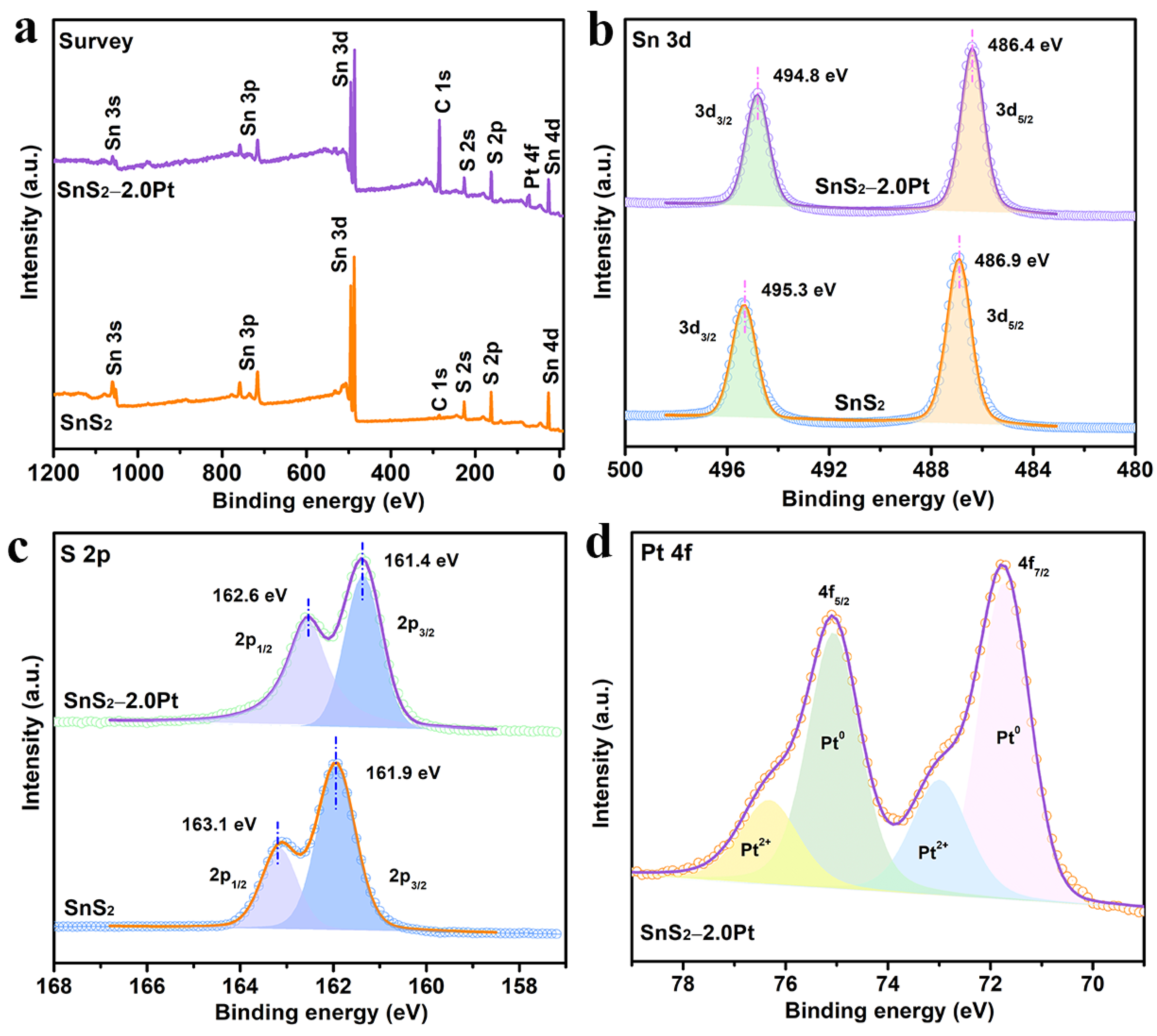
2.2. XPS Analysis
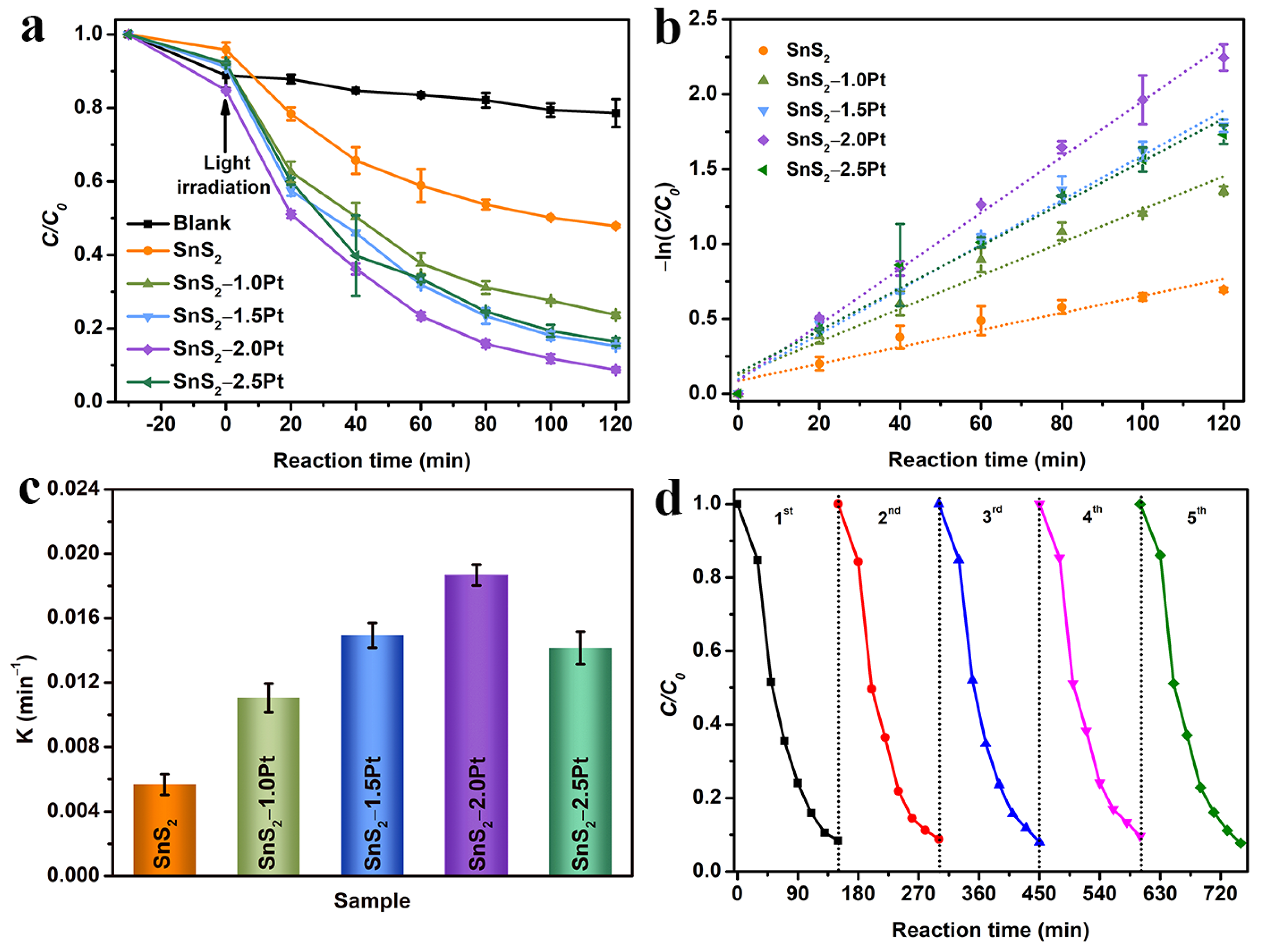
2.3. Photocatalytic TC Degradation Evolution
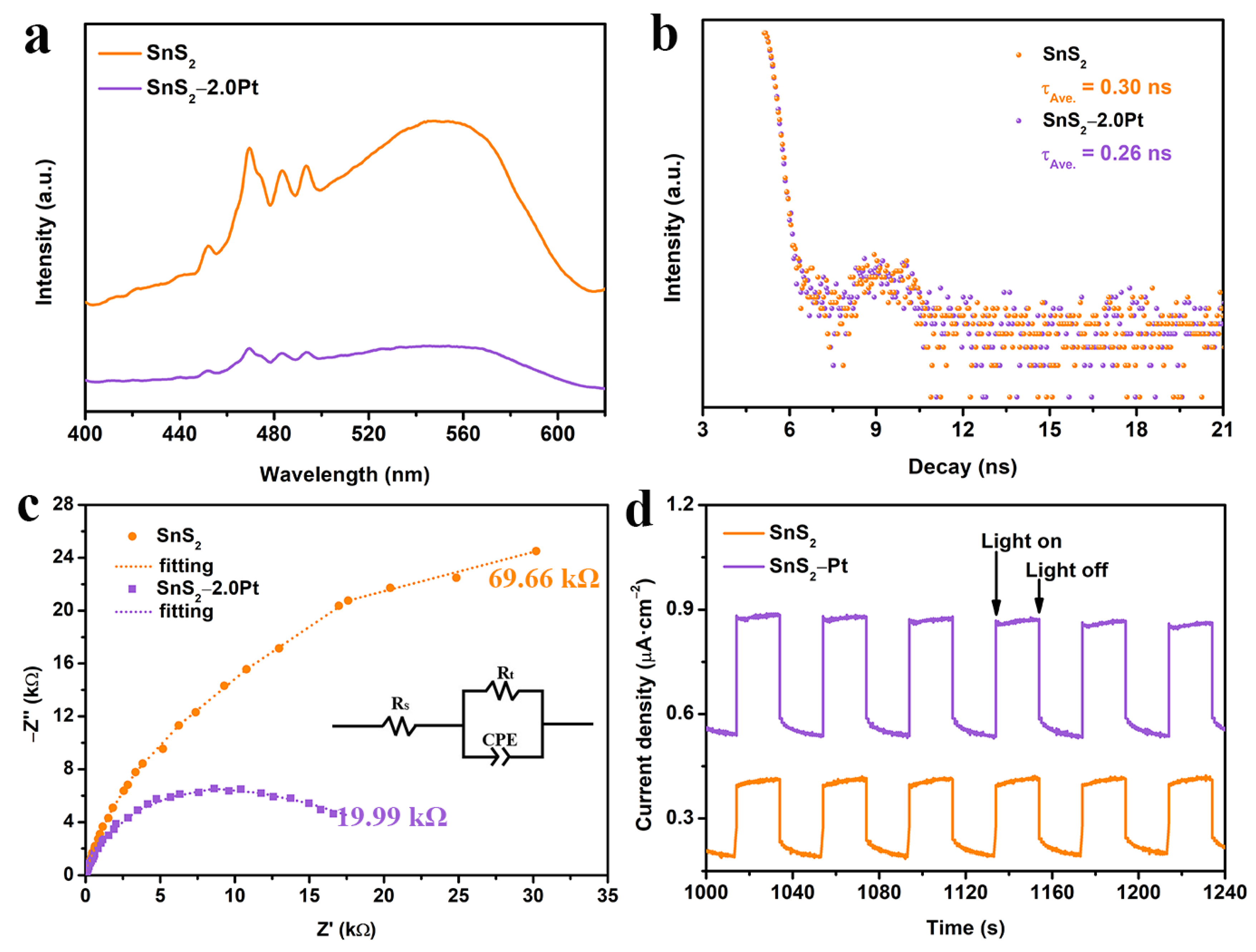
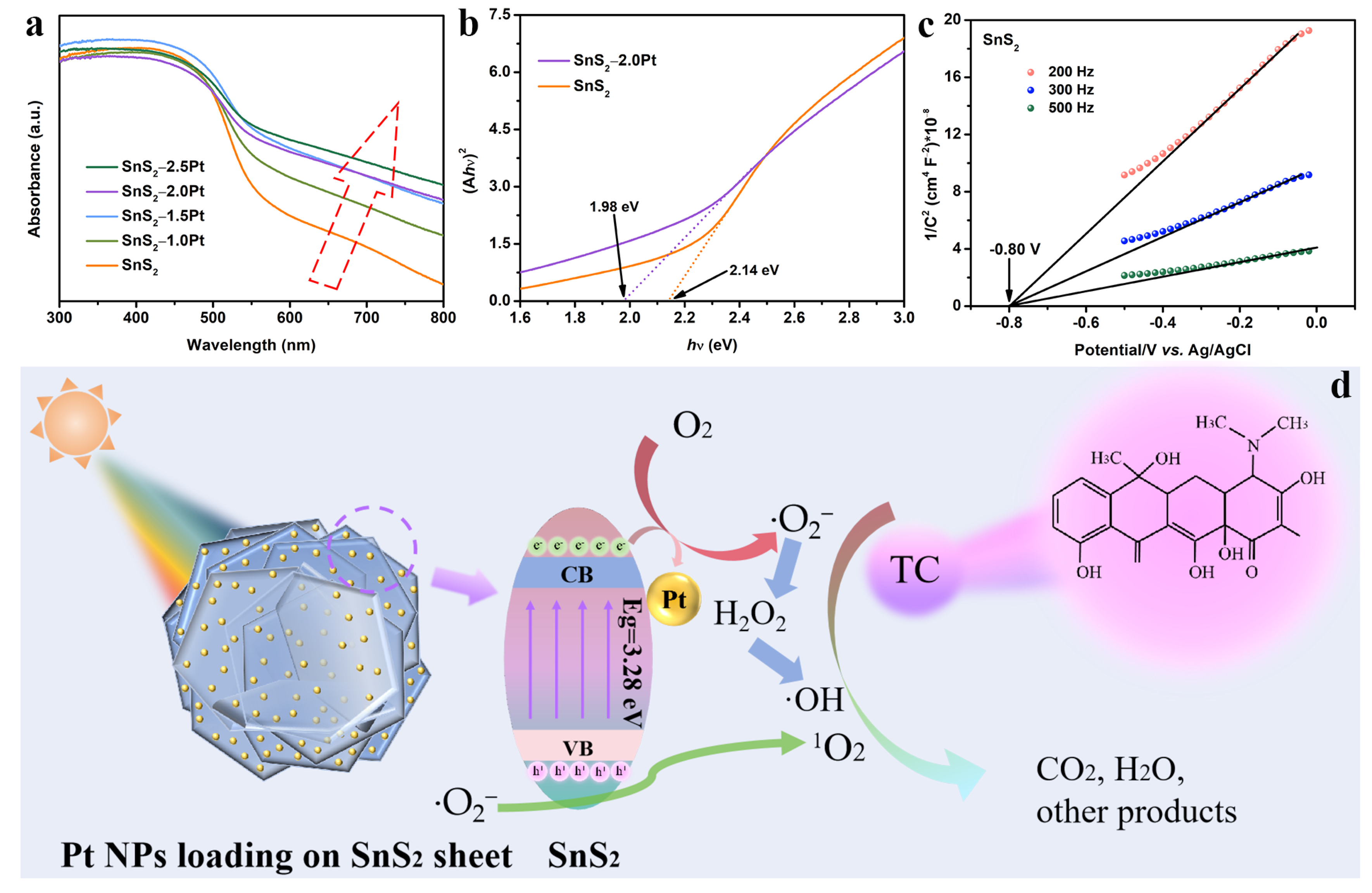
2.4. Photoelectronic Tests
2.5. Mechanism Analysis
3. Materials and Methods
3.1. Materials
3.2. Preparation of Photocatalysts
3.2.1. Preparation of Carbonaceous Microsphere (CMS) Templates
3.2.2. Preparation of SnO2 Hollow-Structured Microspheres
3.2.3. Preparation of Sheet-Like SnS2
3.2.4. Synthesis of Pt/SnS2 Hybrids
3.3. Characterization
3.4. Evolution of Photocatalytic TC Degradation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kalelkar, P.P.; Riddick, M.; García, A.J. Biomaterial-Based Antimicrobial Therapies for the Treatment of Bacterial Infections. Nat. Rev. Mater. 2022, 7, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Hao, W.; Wang, X.; Ouyang, J.; Deng, X.; Yu, H.; Wang, Y. Antimicrobial Peptides, Conventional Antibiotics, and Their Synergistic Utility for the Treatment of Drug-Resistant Infections. Med. Res. Rev. 2022, 42, 1377–1422. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Pan, Y.; Zhong, J.; Wang, J.; Lu, Z.; He, Q.; Zhou, S.; Liao, X.; Liu, Y.; An, T. An Antibiotic-Destructase-Activated Fenton-Like Catalyst for Synergistic Removal of Tetracycline Residues from Aquatic Environment. Chem. Eng. J. 2023, 459, 141576. [Google Scholar] [CrossRef]
- Tang, H.; Liu, Z.; Hu, B.; Zhu, L. D-Ring Modifications of Tetracyclines Determine Their Ability to Induce Resistance Genes in the Environment. Environ. Sci. Technol. 2023, 58, 1338–1348. [Google Scholar] [CrossRef]
- Stapleton, M.J.; Ansari, A.J.; Hai, F.I. Antibiotic Sorption onto Microplastics in Water: A Critical Review of the Factors, Mechanisms and Implications. Water Res. 2023, 233, 119790. [Google Scholar] [CrossRef]
- Chen, X.; Yang, Y.; Ke, Y.; Chen, C.; Xie, S. A Comprehensive Review on Biodegradation of Tetracyclines: Current Research Progress and Prospect. Sci. Total Environ. 2022, 814, 152852. [Google Scholar] [CrossRef]
- Liao, Q.; Rong, H.; Zhao, M.; Luo, H.; Chu, Z.; Wang, R. Interaction between Tetracycline and Microorganisms During Wastewater Treatment: A Review. Sci. Total Environ. 2021, 757, 143981. [Google Scholar] [CrossRef]
- Zhang, X.; Cai, T.; Zhang, S.; Hou, J.; Cheng, L.; Chen, W.; Zhang, Q. Contamination Distribution and Non-Biological Removal Pathways of Typical Tetracycline Antibiotics in the Environment: A Review. J. Hazard. Mater. 2024, 463, 132862. [Google Scholar] [CrossRef]
- Nasrollahi, N.; Vatanpour, V.; Khataee, A. Removal of Antibiotics from Wastewaters by Membrane Technology: Limitations, Successes, and Future Improvements. Sci. Total Environ. 2022, 838, 156010. [Google Scholar] [CrossRef]
- He, Z.; Siddique, M.S.; Yang, H.; Xia, Y.; Su, J.; Tang, B.; Wang, L.; Kang, L.; Huang, Z. Novel Z-Scheme In2S3/Bi2WO6 Core-Shell Heterojunctions with Synergistic Enhanced Photocatalytic Degradation of Tetracycline Hydrochloride. J. Clean. Prod. 2022, 339, 130634. [Google Scholar] [CrossRef]
- Zhu, D.; Xue, S.; Yang, S.; Zuo, Q.; Wang, H.; Lu, Q.; Ruan, G.; Zhao, C.; Du, F. Layered Bimetallic Oxide Composite Film: A Highly Efficient and Reusable Photocatalytic Film for Removal of Tetracycline Antibiotics. Chem. Eng. J. 2023, 476, 146681. [Google Scholar] [CrossRef]
- Yang, J. FeOOH Nanosheets Coupled with ZnCdS Nanoparticles for Highly Improved Photocatalytic Degradation of Organic Dyes and Tetracycline in Water. Molecules 2024, 29, 2913. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Hou, W.; Fang, C.; Huang, Y.; Liu, X. Sulfite Activation by Cobaltosic Oxide Nanohydrangeas for Tetracycline Degradation: Performance, Degradation Pathways and Mechanism. J. Hazard. Mater. 2022, 439, 129618. [Google Scholar] [CrossRef] [PubMed]
- El Messaoudi, N.; Ciğeroğlu, Z.; Şenol, Z.M.; Elhajam, M.; Noureen, L. A Comparative Review of the Adsorption and Photocatalytic Degradation of Tetracycline in Aquatic Environment by g-C3N4-Based Materials. J. Water Process Eng. 2023, 55, 104150. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, J.; Li, J.; Wang, X.; Song, H. Coupling Agent Grafting Assisted Synthesis of C3N4-ZrO2 Heterojunction Composites with Enhanced Photocatalytic Performance. China Pet. Process. Petrochem. Technol. 2023, 25, 123–132. [Google Scholar]
- Liu, X.; Cheng, H.; Wang, Z.; Zhang, J.; Lan, Y.; Wang, X. Assembling Superfine Bi3TaO7 Particles into 2D Fe2O3 Nanosheets for Enhanced Usability to Aqueous Tetracycline Residues. China Pet. Process. Petrochem. Technol. 2022, 24, 147–158. [Google Scholar]
- Liu, Y.; Zhao, H.; Li, H.; Cai, T. One-step Synthesis and Photocatalytic Degradation Performance of Sulfur-doped Porous g-C3N4 Nanosheets. China Pet. Process. Petrochem. Technol. 2022, 24, 81–89. [Google Scholar]
- He, J.; Shi, C.; Yang, Z.; Hou, Q.; Zhang, R.; Zhu, T.; Pan, P.; Zhang, P. Visible-Light Photocatalytic Activity of TiO2 Nanorods and Its Application to Degrading Organic Pollutants. China Pet. Process. Petrochem. Technol. 2022, 24, 138–146. [Google Scholar]
- Liu, C.; Jin, T.; Qian, J.; Xu, X.; Meng, X.; Chen, Z. Synthesis and Photocatalytic Property of CuS-Ag/g-C3N4. Chin. J. Rare Met. 2023, 47, 1104–1112. [Google Scholar]
- Yu, F.; Li, Y.; Liu, Z.; Cui, J.; Zhou, Y. Synthesis and Photocatalytic Properties of Na Doped g-C3N4 Nanotubes. Chin. J. Rare Met. 2022, 46, 889–895. [Google Scholar]
- Xu, Y.; Liu, C.; Jin, T.; Chen, F.; Qian, J.; Qiu, Y.; Meng, X.; Chen, Z. Preparation and Photocatalytic Properties of MoS2/CQDs/g-C3N4 Composites. Chin. J. Rare Met. 2024, 48, 682–694. [Google Scholar]
- Miao, H.; Zhang, W.; Wang, T.; Yang, Z.; Kong, C. g-C3N4-based Nanocomposites for the Photocatalytic Degradation of VOCs: A review. Prog. Nat. Sci. Mater. Int. 2023, 33, 407–424. [Google Scholar] [CrossRef]
- Ye, L.; Peng, X.; Wen, Z.; Huang, H. Solid-state Z-scheme Assisted Hydrated Tungsten Trioxide/ZnIn2S4 Photocatalyst for Efficient Photocatalytic H2 Production. Mater. Futures 2022, 1, 035103. [Google Scholar] [CrossRef]
- Gao, R.; Bai, J.; Shen, R.; Hao, L.; Huang, C.; Wang, L.; Liang, G.; Zhang, P.; Li, X. 2D/2D Covalent Organic Framework/CdS Z-scheme Heterojunction for Enhanced Photocatalytic H2 Evolution: Insights into Interfacial Charge Transfer Mechanism. J. Mater. Sci. Technol. 2023, 137, 223–231. [Google Scholar] [CrossRef]
- Shang, W.; Liu, W.; Cai, X.; Hu, J.; Guo, J.; Xin, C.; Li, Y.; Zhang, N.; Wang, N.; Hao, C.; et al. Insights into Atomically Dispersed Reactive Centers on g-C3N4 Photocatalysts for Water Splitting. Adv. Powder Mater. 2023, 2, 100094. [Google Scholar] [CrossRef]
- Mo, X.; Zhang, X.; Lin, B.; Ning, C.; Li, M.; Liao, H.; Chen, Z.; Wang, X. Boosting Interfacial S-scheme Charge Transfer and Photocatalytic H2-production Activity of 1D/2D WO3/g-C3N4 Heterojunction by Molecular Benzene-rings Integration. J. Mater. Sci. Technol. 2023, 145, 174–184. [Google Scholar] [CrossRef]
- Zhao, G.; Ma, W.; Wang, X.; Xing, Y.; Hao, S.; Xu, X. Self-water-absorption-type Two-dimensional Composite Photocatalyst with High-efficiency Water Absorption and Overall Water-splitting Performance. Adv. Powder Mater. 2022, 1, 100008. [Google Scholar] [CrossRef]
- Chu, X.; Sathish, C.; Li, M.; Yang, J.; Li, W.; Qi, D.; Chu, D.; Vinu, A.; Yi, J. Anti-Stoke Effect Induced Enhanced Photocatalytic Hydrogen Production. Battery Energy. 2023, 2, 20220041. [Google Scholar] [CrossRef]
- Yang, N.; He, T.; Chen, X.; He, Y.; Zhou, T.; Zhang, G.; Liu, Q. TiO2-based Heterojunctions for Photocatalytic Hydrogen Evolution Reaction. Microstructures 2024, 4, 2024042. [Google Scholar] [CrossRef]
- He, J.; Zhang, G.; Jiang, Y.; Jia, J.; Cao, J. Design and Synthesis of Two Dimensional-ZnIn2S4 Nanosheets with Sulfur Vacancies for Improving Photocatalytic Hydrogen Production Performance. Prog. Nat. Sci. Mater. Int. 2023, 33, 607–615. [Google Scholar] [CrossRef]
- Li, L.; Dai, X.; Lu, M.; Guo, C.; Wabaidur, S.; Wu, X.; Lou, Z.; Zhong, Y.; Hu, Y. Electron-enriched Single-Pd-sites on g-C3N4 Nanosheets Achieved by in-situ Anchoring Twinned Pd Nanoparticles for Efficient CO2 Photoreduction. Adv. Powder Mater. 2024, 3, 100170. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Wang, Y.; Guo, Y.; Zhang, S.; Song, H.; Li, X.; Gao, Q.; Shang, W.; Hu, S.; et al. MXene Ti3C2 Decorated g-C3N4/ZnO Photocatalysts with Improved Photocatalytic Performance for CO2 Reduction. Nano Mater. Sci. 2023, 5, 237–245. [Google Scholar] [CrossRef]
- Wei, H.; Meng, F.; Zhang, H.; Yu, W.; Li, J.; Yao, S. Novel Oxygen Vacancy-enriched Bi2MoO6-x/MoS2 S-scheme Heterojunction for Strengthening Photocatalytic Reduction CO2 and Efficient Degradation of Levofloxacin Hydrochloride: Mechanism, DFT Calculations. J. Mater. Sci. Technol. 2024, 185, 107–120. [Google Scholar] [CrossRef]
- Asrami, M.; Jourshabani, M.; Park, M.; Shin, D.; Lee, B. A Unique and Well-designed 2D Graphitic Carbon Nitride with Sponge-like Architecture for Enhanced Visible-light Photocatalytic Activity. J. Mater. Sci. Technol. 2023, 159, 99–111. [Google Scholar] [CrossRef]
- Chen, L.; Maigbay, M.; Li, M.; Qiu, M. Synthesis and Modification Strategies of g-C3N4 Nanosheets for Photocatalytic Applications. Adv. Powder Mater. 2024, 3, 100150. [Google Scholar] [CrossRef]
- Raza, S.; Ghasali, E.; Orooji, Y.; Lin, H.; Karaman, C.; Dragoi, E.N.; Erk, N. Two Dimensional (2D) Materials and Biomaterials for Water Desalination; Structure, Properties, and Recent Advances. Environ. Res. 2023, 219, 114998. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, D.; Tao, X.; Ouyang, Y.; Duan, C.; Liang, G. Two-Dimensional Polarized Blue P/SiS Heterostructures as Promising Photocatalysts for Water Splitting. Molecules 2024, 29, 4355. [Google Scholar] [CrossRef]
- Guo, Z.; Zheng, J.; Li, B.; Da, Z.; Meng, M. Fabrication of Mixed Matrix Membranes Blending with the TiO2/Bi3O4Cl 2D/2D Heterojunction for Photocatalytic Degradation of Tetracycline. Appl. Surf. Sci. 2022, 574, 151549. [Google Scholar] [CrossRef]
- Gan, W.; Fu, X.; Guo, J.; Zhang, M.; Li, D.; Ding, C.; Lu, Y.; Wang, P.; Sun, Z. Ag Nanoparticles Decorated 2D/2D TiO2/g-C3N4 Heterojunction for Efficient Removal of Tetracycline Hydrochloride: Synthesis, Degradation Pathways, and Mechanism. Appl. Surf. Sci. 2022, 606, 154837. [Google Scholar] [CrossRef]
- Fang, H.; Zhou, C.; Xu, S.; Shi, J.; Hu, Y.; Liu, G. S-Scheme Photocatalyst Mo2C/α-Fe2O3 with Vacant Oxygen for Highly Efficient Tetracycline Degradation in Peroxymonosulfate-Mediated Photocatalytic System. J. Alloys Compd. 2023, 958, 170547. [Google Scholar] [CrossRef]
- Yazdi, A.A.; Pirbazari, A.E.; Saraei, F.E.K.; Esmaeili, A.; Pirbazari, A.E.; Kohnehsari, A.A.; Derakhshesh, A. Design of 2D/2D β-Ni(OH)2/ZnO Heterostructures Via Photocatalytic Deposition of Nickel for Sonophotocatalytic Degradation of Tetracycline and Modeling with Three Supervised Machine Learning Algorithms. Chemosphere 2024, 352, 141328. [Google Scholar] [CrossRef] [PubMed]
- Raja, A.; Son, N.; Pandey, S.; Kang, M. Fabrication of Solar-Driven Hierarchical ZnIn2S4/rGO/SnS2 Heterojunction Photocatalyst for Hydrogen Generation and Environmental Pollutant Elimination. Sep. Purif. Technol. 2022, 293, 121119. [Google Scholar] [CrossRef]
- Sun, H.; Tillotson, M.R.; Wang, D.; Zhao, X. A Novel Z-Scheme SnS/NiAl-LDH/g-C3N4 Heterojunction for Piezo-Photocatalytic Degradation of Tetracycline: Performance and Mechanism. Surf. Interfaces 2024, 52, 104861. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, Y.; Sun, X.; Zhang, Q.; Cheng, Z.; Xue, W.; Zeng, Q. Resourceful Treatment of Complex Uranium-Organic Wastewater by a Hybrid Tandem Photocatalytic Fuel Cell with SnS2 Nanoplate Modified Carbon Felt Cathode. J. Hazard. Mater. 2024, 480, 135861. [Google Scholar] [CrossRef]
- Chen, M.; Ye, Z.; Wei, L.; Yuan, J.; Xiao, L. Shining at the Tips: Anisotropic Deposition of Pt Nanoparticles Boosting Hot Carrier Utilization for Plasmon-Driven Photocatalysis. J. Am. Chem. Soc. 2022, 144, 12842–12849. [Google Scholar] [CrossRef]
- Gadore, V.; Mishra, S.R.; Ahmaruzzaman, M. Bandgap Engineering Approach for Synthesising Photoactive Novel Ag/HAp/SnS2 for Removing Toxic Anti-Fungal Pharmaceutical from Aqueous Environment. J. Hazard. Mater. 2024, 461, 132458. [Google Scholar] [CrossRef]
- Li, D.; Li, Y.; Liao, D.; Cao, M.; Zhang, L.; Zhang, S.; Chen, L.; Chen, Y.; Wang, H.; Qi, J. Enhanced Light Harvesting Ability in Hollow Pt/TiO2 Nanoreactor for Boosting Tetracycline Photodegradation. Prog. Nat. Sci. Mater. Int. 2024, 34, 767–775. [Google Scholar] [CrossRef]
- Li, J.; Cao, Z.; Wang, Q.; Cheng, H. Highly Dispersed Fe/SnS2/Kaolinite Composite for the Enhanced Photo-Fenton Degradation of Tetracycline Hydrochloride. J. Alloys Compd. 2024, 976, 173061. [Google Scholar] [CrossRef]
- Wang, J.-T.; Cai, Y.-L.; Liu, X.-J.; Zhang, X.-D.; Cai, F.-Y.; Cao, H.-L.; Zhong, Z.; Li, Y.-F.; Lü, J. Unveiling the Visible–Light–Driven Photodegradation Pathway and Products Toxicity of Tetracycline in the System of Pt/BiVO4 Nanosheets. J. Hazard. Mater. 2022, 424, 127596. [Google Scholar] [CrossRef]
- Kumar, G.; Kumar, J.; Bag, M.; Dutta, R.K. Solar Light Induced Photocatalytic Process for Reduction of Hexavalent Chromium and Degradation of Tetracycline and Methylene Blue by Heterostructures Made of SnS2 Nanoplates Surface Modified by ZnWO4 Nanorods. Sep. Purif. Technol. 2022, 292, 121040. [Google Scholar] [CrossRef]
- Yan, J.; Wu, R.; Jin, G.; Jia, L.; Feng, G.; Tong, X. The Hybrid Pt Nanoclusters/Ru Nanowires Catalysts Accelerating Alkaline Hydrogen Evolution Reaction. Adv. Powder Mater. 2024, 3, 100214. [Google Scholar] [CrossRef]
- Gao, M.; Zhou, W.; Mo, Y.; Sheng, T.; Deng, Y.; Chen, L.; Wang, K.; Tan, Y.; Zhou, H. Outstanding Long-cycling Lithium-sulfur Batteries by Core-shell Structure of S@Pt Composite with Ultrahigh Sulfur Content. Adv. Powder Mater. 2022, 1, 100006. [Google Scholar] [CrossRef]
- Shi, Y.; Feng, Y.; Wang, Z.; Wang, X.; Jiang, Y.; Deng, J.; Dai, H.; Liu, Y. Light-driven Photothermocatalytic Performance of the Pt/Co3O4 Catalyst for Toluene Oxidation. Prog. Nat. Sci. Mater. Int. 2023, 33, 526–533. [Google Scholar] [CrossRef]
- Shu, C.; Cao, J.; Gan, Z.; Qiu, P.; Chen, Z.; Lian, G.; Chen, Z.; Deng, C.; Tang, W. Synergistic Effect between Co Single Atoms and Pt Nanoparticles for Efficient Alkaline Hydrogen Evolution. Mater. Futur. 2024, 3, 035101. [Google Scholar] [CrossRef]
- Ma, X.; Li, D.; Jiang, Y.; Jin, H.; Bai, L.; Qi, J.; You, F.; Yuan, F. Fiber-Like ZnO with Highly Dispersed Pt Nanoparticles for Enhanced Photocatalytic CO2 Reduction. J. Colloid Interf. Sci. 2022, 628, 768–776. [Google Scholar] [CrossRef]
- Huang, P.; Chen, F.; Tang, Y.; Sun, W.; Song, Y.; Sun, Y. Zn2SnO4 Decorated SnS2 Flower-Like Ball for Enhanced Photocatalytic Degradation of Tetracycline under Visible Irradiation: The Role of Zn2SnO4 in Photoinduced Electrons Transfer. Mat. Sci. Semicon. Proc. 2024, 173, 108182. [Google Scholar] [CrossRef]
- Singla, S.; Basu, S.; Devi, P. Solar Light Responsive 2D/2D BiVO4/SnS2 Nanocomposite for Photocatalytic Elimination of Recalcitrant Antibiotics and Photoelectrocatalytic Water Splitting with High Performance. J. Ind. Eng. Chem. 2023, 118, 119–131. [Google Scholar] [CrossRef]
- Luo, J.; Li, R.; Chen, Y.; Zhou, X.; Ning, X.; Zhan, L.; Ma, L.; Xu, X.; Xu, L.; Zhang, L. Rational Design of Z-Scheme LaFeO3/SnS2 Hybrid with Boosted Visible Light Photocatalytic Activity Towards Tetracycline Degradation. Sep. Purif. Technol. 2019, 210, 417–430. [Google Scholar] [CrossRef]
- Zou, X.; Sun, B.; Wang, L.; Bai, H.; Meng, X.; Li, C.; Li, Z. Enhanced Photocatalytic Degradation of Tetracycline by SnS2/Bi2MoO6-X Heterojunction: Multi-Electric Field Modulation through Oxygen Vacancies and Z-Scheme Charge Transfer. Chem. Eng. J. 2024, 482, 148818. [Google Scholar] [CrossRef]
- Bao, Y.; Liu, Y.; Zhang, Z.; Pan, J.; Li, X.; Zhao, B.; Wang, R.; Liu, J. Constructing 2D/2D Ultrathin Ti3C2/SnS2 Schottky Heterojunctions toward Efficient Tetracycline Degradation. Chemosphere 2022, 307, 136118. [Google Scholar] [CrossRef]
- Chen, X.; Han, Z.; Lu, Z.; Qu, T.; Liang, C.; Wang, Y.; Zhang, B.; Han, X.; Xu, P. Construction of Strongly Coupled 2D-2D SnS2/CdS S-Scheme Heterostructures for Photocatalytic Hydrogen Evolution. Sustain. Energy Fuels 2023, 7, 1311–1321. [Google Scholar] [CrossRef]
- Zhang, J.; Fang, Y.; Zhang, Y.; Lin, Y.; Gui, Y.; Liu, L. Photocatalytic Degradation of Volatile Organic Compounds over WO2/SnS2 Nanofibers. ACS Appl. Nano Mater. 2023, 6, 22301–22310. [Google Scholar] [CrossRef]
- Qiang, T.; Chen, L.; Xia, Y.; Qin, X. Dual Modified MoS2/SnS2 Photocatalyst with Z-Scheme Heterojunction and Vacancies Defects to Achieve a Superior Performance in Cr (VI) Reduction and Dyes Degradation. J. Clean. Prod. 2021, 291, 125213. [Google Scholar] [CrossRef]
- Gadore, V.; Mishra, S.R.; Ahmaruzzaman, M. Green and Environmentally Sustainable Fabrication of SnS2 Quantum Dots/Chitosan Nanocomposite for Enhanced Photocatalytic Performance: Effect of Process Variables, and Water Matrices. J. Hazard. Mater. 2023, 444, 130301. [Google Scholar] [CrossRef]
- Wu, S.; Hu, H.; Lin, Y.; Zhang, J.; Hu, Y.H. Visible Light Photocatalytic Degradation of Tetracycline over TiO2. Chem. Eng. J. 2020, 382, 122842. [Google Scholar] [CrossRef]
- Liu, C.; Dai, H.; Tan, C.; Pan, Q.; Hu, F.; Peng, X. Photo-Fenton Degradation of Tetracycline over Z-Scheme Fe-g-C3N4/Bi2WO6 Heterojunctions: Mechanism Insight, Degradation Pathways and DFT Calculation. Appl. Catal. B Environ. 2022, 310, 121326. [Google Scholar] [CrossRef]
- You, F.; Zhou, T.; Li, J.; Huang, S.; Chang, C.; Fan, X.; Zhang, H.; Ma, X.; Gao, D.; Qi, J. Rich Oxygen Vacancies in Confined Heterostructured TiO2@In2S3 Hybrid for Boosting Solar-Driven CO2 Reduction. J. Colloid Interf. Sci. 2024, 660, 77–86. [Google Scholar] [CrossRef]
- Li, D.; Zhang, H.; Xie, S.; Zhang, H.; Wang, H.; Ma, X.; Gao, D.; Qi, J.; You, F. Lattice Distortion in a Confined Structured ZnS/ZnO Heterojunction for Efficient Photocatalytic CO2 Reduction. ACS Appl. Mater. Interfaces 2023, 15, 36324–36333. [Google Scholar] [CrossRef]

| Catalyst | Initial Concentration of TC (mg/L) | Dosage (g/L) | Reaction Time (min) | Degradation (%) | Kinetic Constant (min−1) | Ref. |
|---|---|---|---|---|---|---|
| Pt/SnS2 | 20 | 0.33 | 120 | 91.27 | 0.0187 | This work |
| Fe/SnS2/Kaolinite a | 40 | 0.50 | 60 | 80.38 | 0.0257 | 48 |
| Zn2SnO4/SnS2 | 10 | 0.10 | 120 | 83.00 | 0.0176 | 56 |
| BiVO4/SnS2 | 10 | 0.20 | 150 | 80.80 | 0.0100 | 57 |
| LaFeO3/SnS2 | 50 | 0.33 | 120 | 28.80 | 0.0028 | 58 |
| Bi2MoO6-x/SnS2 | 20 | 0.20 | 90 | 89.00 | 0.0139 | 59 |
| Ti3C2/SnS2 | 10 | 0.50 | 90 | 87.70 | 0.0156 | 60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, M.; Zhou, T.; Li, J.; Cao, M.; Cheng, J.; Li, D.; Qi, J.; You, F. Insight into the Local Surface Plasmon Resonance Effect of Pt-SnS2 Nanosheets in Tetracycline Photodegradation. Molecules 2024, 29, 5423. https://doi.org/10.3390/molecules29225423
Feng M, Zhou T, Li J, Cao M, Cheng J, Li D, Qi J, You F. Insight into the Local Surface Plasmon Resonance Effect of Pt-SnS2 Nanosheets in Tetracycline Photodegradation. Molecules. 2024; 29(22):5423. https://doi.org/10.3390/molecules29225423
Chicago/Turabian StyleFeng, Mao, Tianhao Zhou, Jiaxin Li, Mengqing Cao, Jing Cheng, Danyang Li, Jian Qi, and Feifei You. 2024. "Insight into the Local Surface Plasmon Resonance Effect of Pt-SnS2 Nanosheets in Tetracycline Photodegradation" Molecules 29, no. 22: 5423. https://doi.org/10.3390/molecules29225423
APA StyleFeng, M., Zhou, T., Li, J., Cao, M., Cheng, J., Li, D., Qi, J., & You, F. (2024). Insight into the Local Surface Plasmon Resonance Effect of Pt-SnS2 Nanosheets in Tetracycline Photodegradation. Molecules, 29(22), 5423. https://doi.org/10.3390/molecules29225423








