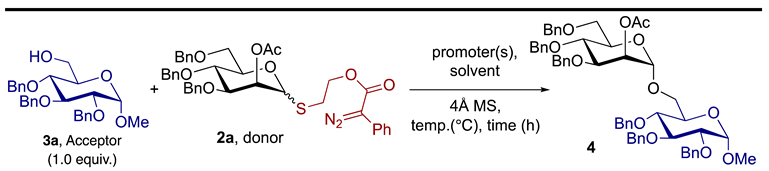
Abstract
Traditional glycosylation methods using thioglycosides often require harsh conditions or expensive metal catalysts. This study presents a more sustainable alternative by employing copper, an earth-abundant catalyst. We developed diazo-based thioglycoside donors that, through copper catalysis, undergo intramolecular activation to form glycosyl sulfonium ions, leading to the generation of oxocarbenium ions. This versatile approach efficiently accommodates a variety of O-nucleophiles, including primary, secondary, and tertiary, as well as complex bioactive molecules. It is compatible with various glycosyl donors and protecting groups, including superarmed, armed, and disarmed systems. Notably, the methodology operates orthogonally to traditional thioglycoside and alkyne donors and has been successfully applied to the orthogonal iterative synthesis of trisaccharides. Mechanistic insights were gained by studying the electronic effects of electron-donating (OMe) and electron-withdrawing (NO2) groups on the donors, offering a valuable understanding of the intramolecular reaction pathway.
1. Introduction
Carbohydrates are essential biological molecules in many physiological processes, including cell–cell recognition, signaling, and immune responses [1,2]. The synthesis of complex carbohydrates and glycoconjugates is crucial in understanding these processes and developing therapeutic agents [3]. Glycosylation, the process of attaching sugar moieties to other molecules, is critical in synthesizing these intricate structures [2,3]. Among various glycosyl donors, thioglycosides have gained prominence due to their stability and ease of handling, making them ideal candidates for glycosylation reactions [4,5].
The activation of thioglycoside donors is a crucial aspect of glycosylation chemistry. Typically, this activation involves promoters like N-iodosuccinimide (NIS) [6,7], tri-chloroisocyanuric acid (TCICA) [8], silver salts [9], and TMSOTf [10], which generate a reactive glycosyl oxocarbenium ion to facilitate glycosidic bond formation. Other methods have been recently reported, utilizing triperoxide/TMSOTf [11], electrophilic nitrogen mesitylenesulfonyl-hydroxylamine (MSH), and in situ benzyne for glycosylation [12]. While effective, these traditional and recent methods often require harsh conditions and stoichiometric amounts of promoters, limiting their applicability and functional group compatibility. Activating thioglycoside donors under catalytic conditions has been challenging for synthetic organic chemists. In 2012, Yu and coworkers first reported on the gold (Au(I))-catalyzed activation of well-established alkyne-based glycosyl donors [13]. Shortly after, in 2013, Adhikari et al. activated propargyl thioglycosides using Au–Ag dual-metal catalysts [14]. Recently, Sun and coworkers achieved Au- and Cu-catalyzed activation of ortho-methoxycarbonyl ethynyl phenyl thioglycosides [15], and very recently, Liu and coworkers harnessed the potential of the strained cyclopropyl ring in glycosylation activation using catalytic Sc(OTf)3 (Figure 1A) [16,17]. Apart from these reports, few known reports perform superacid-catalyzed activation of thioglycosides [18,19,20]. In 2019, the Wan group reported the catalytic activation of thioglycosides using rhodium carbenes [21]. They demonstrated the formation of glycosyl sulfonium ylide upon the reaction of thioglycoside with rhodium carbene, followed by Brønsted acid-derived cascade formation of a glycosyl sulfonium ion, which further transforms into a glycosyl oxocarbenium ion, leading to glycosidic linkages (Figure 1B).
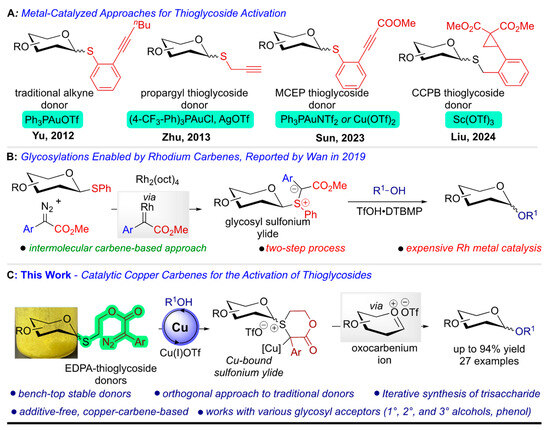
Figure 1.
Catalytic activation of thioglycosides and our work. (A) Previous approaches for the catalytic thioglycoside activation [13,14,15,16]. (B) Catalytic thioglycoside activation with rhodium carbenes [21]. (C) Copper-carbene mediated catalytic activation of thioglycoside (this work).
There has been a remarkable development in the catalytic activation of thioglycoside, but it has noticeable shortcomings. More specifically, expensive metals, including Au, Ag, Rh, Sc, and harsh reaction conditions are used. To overcome these limitations, a methodology for activating thioglycosides using earth-abundant metal catalysts, which can proceed through mild reaction conditions and offer the orthogonal reactivity needed to establish traditional donors, must be developed.
We aim to develop a copper-catalyzed, carbene-based method using earth-abundant copper to activate thioglycosides, addressing the current challenges in this area. Our hypothesis is centered on the intramolecular activation of thioglycosides through copper carbenes (Figure 1C). The designed donor can be easily synthesized in a single step by coupling well-established anomeric thiols with linear donor–acceptor diazo linkers. This approach will introduce orthogonal reactivity compared to traditional thioglycoside and alkyne donors. We plan to screen a variety of nucleophiles, including primary, secondary, and tertiary alcohols, phenols, and biologically relevant molecules like cholesterol. Additionally, iterative oligosaccharide synthesis will be conducted to explore the broader applications of this methodology.
2. Results and Discussion
We initiated our studies by synthesizing a designed thioglycoside donor. To access these donors, we coupled benzylated/benzoylated glycosyl thiols [22], which were used without purification. The crude thiols were coupled with a 2-bromomethyl 2-diazo-2-phenylacetate (EDPA) linker [23] using triethylamine as a base and acetonitrile as a solvent (Scheme 1) [16]. These donors were yellow, solid, and benchtop stable, showcasing the ease of practical utility. Using the same strategy, we synthesized both superarmed (2-O-Ac-3,4,6-tri-O-Bn) and disarmed (perbenzoylated) protecting groups with different classes of carbohydrates (glucose and mannose). Overall, synthesizing newly designed donors was simple and could be applied to various sugars.
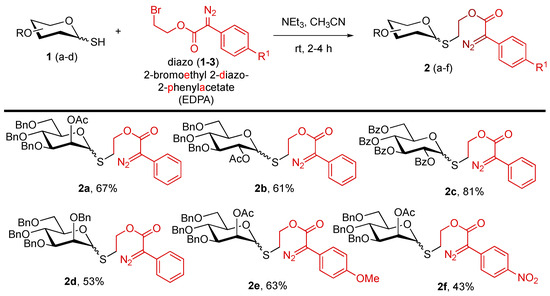
Scheme 1.
Synthesis of novel EDPA thioglycoside donors a. a Reactions were performed with glycosyl thiol (1.0 equiv.), diazo linker 1-3 (1.2 equiv.), NEt3 (1.5 equiv.), and CH3CN (0.5 M). EDPA = 2-bromoethyl 2-diazo-2-phenylacetate.
In our efforts to optimize the reaction conditions, we chose 2-O-Ac-3,4,6-tri-O-Bn mannose (superarmed) EDPA thioglycoside 2a as our model donor and methyl 6-OH, 2,3,4-tri-O-Bn-glucopyranoside 3a as the model glycosyl acceptor. In our first attempt, Cu(I)OTf was used as a catalyst, and 2-ethyl 2-diazo-2-phenylacetate thioglycoside (EDPAT) donor 2a was coupled with a glycosyl acceptor using CH2Cl2 as a solvent. To our delight, after 5 h, both starting materials were consumed, and the desired product was observed at a 68% yield (entry 1, Table 1). After well-crafted initial success, we turned our attention to screening other metal catalysts. In continuation of that, we screened Fe(BF4)2·6H2O (entry 2), ZnCl2 (entry 3), Zn(OTf)2 (entry 4), and Rh2OAc4 (entry 5). None of these catalysts were observed to produce the desired disaccharide in significant amounts. These screenings indicated that copper, along with the triflate counter-anion, is the best metal to provide a good yield of the desired product. To probe the role of the counter-anion, we tested other counter-anions, such as PF6 (entry 6), BF4 (entry 7), and I (entry 8); still, the triflate anion was the most optimal, further showcasing the importance of OTf in carbohydrate chemistry [24]. Our studies also found that Cu(OTf)2 was equally efficient in catalyzing the reaction (entry 9). Apart from the metal catalysts, we also screened strong Brønsted acids (TfOH), which are known to decompose donor-acceptor diazos. The diazo remained unreacted, and TfOH was quenched by molecular sieves (entry 10), indicating no potential TfOH generated in situ from the metal triflates responsible for the transformation. The reaction was performed at 0 °C, which further led to a slight increase in the yield but with a prolonged reaction time (entry 11). Increasing the amount of donor to 1.5 equivalent produces slightly higher yields (entry 12), while performing the reaction at room temperature produces a similar yield but with a lower reaction time (entry 13). We further reduced the catalyst loading (10 mol%), resulting in the exact yield as the desired product (entry 14). We further attempted to increase the yield and charged donors in portions. Adding donors in portions led to a significant increase in the product (entry 15). The portion-wise addition of Cu(OTf)2 also led to a considerable increment in the product yield (entry 16). We also screened various solvents, such as CH3CN (entry17), THF (entry 18), toluene (entry 19), and EtOAc (entry 20). Toluene provided better yields from these solvents, but CH2Cl2 was still the most optimal solvent.

Table 1.
Optimization of the reaction conditions for EDPAT donor 2a a.
In the end, we performed a reaction without the catalyst to further probe the role of the copper catalyst, leading to no decomposition of the donor. Finally, Cu(I)OTf in 10 mol% was the optimum catalyst, and the portion-wise addition of the donor was the most suitable condition for the best results.
After optimizing the reaction conditions with superarmed donor 2a, we sought to broaden the scope of glycosyl donors and acceptors. We initially explored a range of primary glycosyl acceptors, including benzyl mannopyranoside and benzoyl-protected gluco- and mannopyranosides, coupling them with EDPAT donor 2a. These acceptors all provided excellent yields of the desired disaccharides (5–7, Scheme 2).
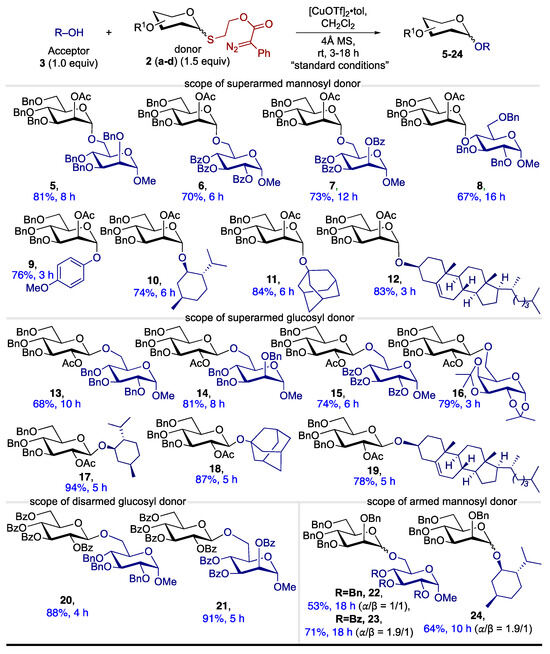
Scheme 2.
Substrate scope of our novel EDPAT donors a. a Reactions were conducted with an acceptor (0.050 mmol), donor (0.075 mmol), promoters (0.005 mmol, 10 mol%), and CH2Cl2 (0.05 M).
Next, we tested a 2°-glycosyl acceptor, which also delivered a good yield of disaccharide 8, although requiring a longer reaction time for completion. With these successful glycosyl acceptor screenings, we turned our attention to other nucleophiles. As a nucleophile, phenol gave a good yield of the desired product 9. We also tested a secondary alcohol (l-menthol) and tertiary alcohol (1-adamantanol), both of which produced the desired products (10 and 11) in excellent yields. Furthermore, we demonstrated the tolerance of donor 2a by glycosylating the bioactive molecule cholesterol 12, again achieving excellent yields. The success of mannosyl-superarmed EDPAT donor 2a with a broad range of O-nucleophiles motivated us to investigate other glycosyl donors.
Next, we screened glucosyl EDPAT donor 2b with various glycosyl acceptors, including benzyl-protected glucose and mannose, benzoyl-protected glucose, and acetonide-protected galactose. All these acceptors reacted well with donor 2b, yielding the desired disaccharides (13–16) in excellent yields. Similarly, 2°- and 3°-nucleophiles such as chiral menthol, adamantanol, and bioactive cholesterol provided the expected products (17–19) with good yields. After successfully testing the superarmed donors (2a, 2b) with a wide range of nucleophiles, we focused on screening a disarmed glycosyl donor. Using our synthesized glucosyl, EDPAT disarmed donor 2c, we coupled it with various glycosyl acceptors, achieving excellent results and yielding the desired products (20, 21). Finally, we also screened the armed protection group (benzyl) with mannosyl EDPAT donor 2d, which successfully coupled with glycosyl acceptors, including menthol, yielding good products (22–24) and a mixture of diastereomers. Here, we observed an interesting reactivity pattern, where disarmed donors provided the disaccharides in less reaction time as compared to the armed donors. These experimental observations can be explained by neighboring group participation (NGP) in disarmed donor 2c and superarmed donor 2b. Both donors have similar reactivity and reaction times under our optimized conditions. More specifically, the benzoyl group stabilizes the oxocarbenium ion, leading to the efficient formation of glycosidic bonds. In the case of an armed glycosyl donor, there is no NGP to stabilize the oxocarbenium ion, which could be the reason for the low yields and prolonged reaction time. Overall, our methodology demonstrates robust compatibility with various glycosyl EDPAT donors and acceptors, highlighting its versatility. This approach offers a compelling catalytic glycosylation strategy compared to other precious metal-catalyzed thioglycoside activation methods.
After successfully screening a myriad of glycosyl donors and acceptors, we focused on accessing the oligosaccharides utilizing the orthogonal reactivity of our EDPAT donors. We synthesize literature-known acceptors bearing traditional alkyne 3j and thioglycoside 3k functional groups at the anomeric position. We coupled these acceptors with EDPAT donor 2a under our optimized conditions (Figure 2), and ecstatically, both couplings work with excellent yields of the disaccharides (25, 27), further providing evidence for the orthogonal reactivity. Furthermore, these disaccharides were activated under well-established reaction conditions (Figure 2) to access the trisaccharide 26. After this orthogonal iterative synthesis of trisaccharide, we also synthesized disaccharide acceptor 3l and coupled it with EDPAT donor 2a to afford the same trisaccharide 26 (Figure 2). Overall, these studies further supported our claim that this methodology is one of the best approaches for the catalytic activation of thioglycosides for oligosaccharide synthesis.
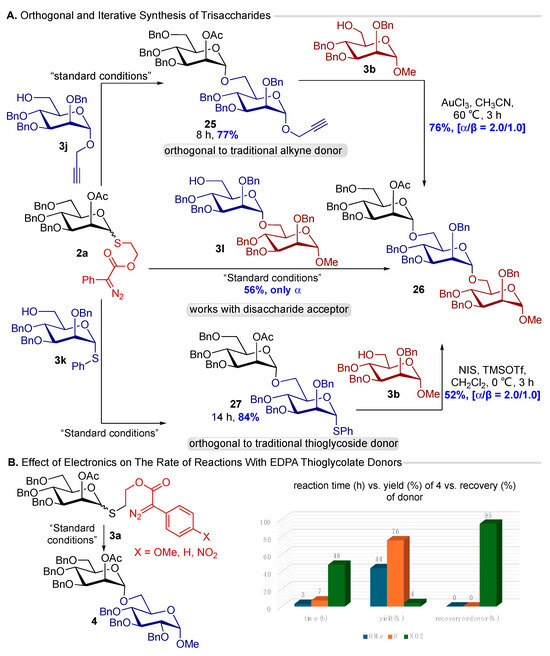
Figure 2.
Applications of our EDPAT donor and control experiment. (A) Orthogonal and iterative synthesis of trisaccharide. (B) Effect of electronics on the rate and yield of the reaction.
In the end, we also synthesized EDPAT donors having different electronic functional groups OMe (2e) [25] and NO2 (2f) at the para position of the phenyl ring of diazo functionality. The coupling of these three donors (2a, 2e, and 2f) with the glycosyl acceptor 3a demonstrates the effect of electronics. Specifically, donor 2e decomposes rapidly, only providing 44% yield of the desired product, while 2f was found to be less reactive under these conditions, providing the recovered yield of unreacted donors in 95%. The diazo compounds used in this study are donor-acceptor diazos. Their stability is enhanced by adding electron-withdrawing groups, while electron-donating groups destabilize the diazo compounds. An electron-donating group at the para position of the phenyl ring makes the diazo less stable. In contrast, an electron-withdrawing group (NO2) at the para position of the phenyl ring further stabilizes the diazo, making it hard to decompose to carbene under mild Cu-catalytic conditions. Ultimately, the OMe group will form copper carbenoid very quickly, increasing the concentration of highly reactive carbene in the reaction medium. This further leads to extensive side reactions. Overall, having no substitution at the para positions (parent donor 2a) remains the best option to achieve the desired reactivity. These studies provide us with insights into the reaction pathway.
After completing the experiments, we proposed plausible reaction pathways for this transformation (Figure 3). The reaction begins with the formation of copper-bound diazo complex I, which provides the copper carbenoid II intermediate upon extrusion of the dinitrogen molecule. The copper carbenoid coordinates with the sulfur atom, bringing it near the in situ-generated carbene through chelation. Inserting the sulfur atom into copper carbene produces the copper-bound glycosyl sulfonium ion III. The copper-bound C-enolate then tautomerizes into the corresponding more stable metal-bound O-enolate IV, as described in our previous work [26]. This intermediate releases ((3-argio-5,6-dihydro-1,4-oxathiin-2-yl)oxy)copper as the possible byproduct V to generate the oxocarbenium ion VII, a reactive intermediate in glycosylation chemistry. Simultaneously, the byproduct V undergoes protodemetallation to generate copper-bound activated glycosyl acceptor (O-nucleophiles) and 3-argio-5,6-dihydro-1,4-oxathiin-2-ol as a plausible byproduct (VI), as described in our related work on copper carbene-mediated 1,2-cis-furanosylations [27]. Finally, the oxocarbenium ion reacts with the activated copper nucleophile to generate the major glycoside product VIII. The findings from all our studies support this proposed reaction pathway.
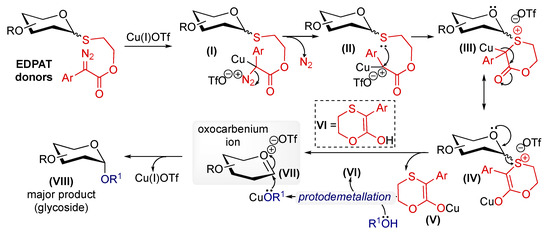
Figure 3.
Plausible reaction mechanism for activating EDPAT donors using Cu(I)OTf for stereoselective glycosylation.
3. Experimental Section: Materials and Methods
3.1. General Considerations
Reagents: The reagents and solvents were obtained from Sigma-Aldrich (www.sigma-aldrich.com, St. Louis, MO, USA), Chem-Impex (www.chemimpex.com, Wood Dale, IL, USA), Acros Organics (www.fishersci.com, Geel, Belgium), or AmBeed (Arlington Heights, IL, USA) and used without further purification unless otherwise indicated. Dry solvents (acetonitrile) were obtained from Acros Organics (www.fishersci.com), and dichloromethane was distilled over CaH2 under N2 unless stated otherwise. THF purchased from Sigma-Aldrich was distilled over Na metal with a benzophenone indicator. Toluene was obtained from Sigma-Aldrich. Reactions: All reactions were performed in flame-dried glassware under positive N2 pressure with magnetic stirring unless otherwise noted. Liquid reagents and solutions were transferred through rubber septa via syringes flushed with N2 before use. Cold baths were generated as follows: 0 °C with wet ice/water and −78 °C using a Julabo chiller. Chromatography: TLC was performed on 0.25 mm E. Merck silica gel 60 F254 plates (Rahway, NJ, USA) and visualized under UV light (254 nm) or by staining with potassium permanganate (KMnO4), cerium ammonium molybdate (CAM), phosphomolybdic acid (PMA), and ninhydrin. Silica flash chromatography was performed on Sorbtech 230–400 mesh silica gel 60 (Norcross, Georgia). Analytical instrumentation: NMR spectra were recorded on a Varian VNMRS (Palo Alto, CA, USA) at 300, 400, 500, and 600 MHz and a Jeol 400 and 500 NMR spectrometer (Tokyo, Japan) in CDCl3 unless otherwise indicated. Chemical shifts are expressed in ppm relative to solvent signals: CDCl3 (1H, 7.26 ppm; 13C, 77.0 ppm); coupling constants are expressed in Hz. NMR spectra were processed using Mnova 14.2.1-27684 (www.mestrelab.com/software/mnova-nmr, accessed on 20 June 2023). Mass spectra were obtained at the OU Analytical Core Facility on an Agilent 6538 High-Mass-Resolution QTOF Mass Spectrometer (Santa Clara, CA, USA) and an Agilent 1290 UPLC. We utilized Kessil blue light lamps at four distinct wavelengths (nm)—PR160-390, PR160-440, PR160-456, and PR160-467—as the blue light source, accompanied by a cooling fan to regulate the ambient reaction temperature.
3.2. General Procedure A for the Synthesis of EDPA Thioglycoside Donor 3 (a–f)
To a solution of thiol, 1 (a–d) (1.0 equiv.) and a diazo linker (1–3) (1.15 equiv.) in anhydrous CH3CN (0.3 M) were added to triethylamine (1.5 equiv.). The mixture was stirred at room temperature for two hours before being concentrated in vacuo. The residue was purified using silica gel column chromatography (hexane:EtOAc = 5:1) to afford the desired EDPA thioglycoside donor 2 (a–f).
- 2-O-acetyl-3,4,6-tri-O-benzyl-α/β-d-mannopyranoside EDPA thioglycoside donor (2a): This was prepared according to general procedure A: a solution of 2-O-acetyl-3,4,6-tri-O-benzyl-d-mannopyranosyl thiol 1a [28] (1 g, 1.98 mmol, 1.0 equiv.) was coupled with diazo linker-1 [23] (0.608 g, 2.26 mmol, 1.15 equiv.) to afford EDPA thioglycoside donor 2a (0.93 g, 67%, α:β = 10:1). Rf = 0.3 (hexane:ethyl acetate = 80:20). 1H NMR (400 MHz, CDCl3) δ 7.48 (d, J = 8.0 Hz, 2H), 7.39 (t, J = 7.8 Hz, 2H), 7.31 (dq, J = 11.5, 5.9 Hz, 13H), 7.22–7.15 (m, 3H), 5.46 (s, 1H), 5.37 (s, 1H), 4.86 (d, J = 10.7 Hz, 1H), 4.69 (dd, J = 11.6, 7.3 Hz, 2H), 4.51 (dd, J = 20.5, 11.3 Hz, 4H), 4.38 (dt, J = 11.4, 6.9 Hz, 1H), 4.17 (dd, J = 8.7, 3.2 Hz, 1H), 3.97–3.88 (m, 2H), 3.84 (dd, J = 10.5, 4.5 Hz, 1H), 3.71 (d, J = 10.7 Hz, 1H), 2.99 (dt, J = 13.8, 6.9 Hz, 1H), 2.88 (dt, J = 13.7, 6.5 Hz, 1H), 2.17 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 170.44, 138.31, 138.18, 137.65, 129.07, 128.57, 128.46, 128.42, 128.30, 128.02, 127.99, 127.87, 127.80, 127.71, 126.01, 124.11, 83.31, 78.50, 75.35, 74.49, 73.52, 72.16, 72.05, 70.27, 68.77, 63.51, 30.37, 21.26. HRMS (ESI): calc. for C39H40N2NaO8S (M + Na): 719.2403; found: 719.2420.
- 2-O-acetyl-3,4,6-tri-O-benzyl-α/β-d-glucopyranoside EDPA thioglycoside donor (2b): This was prepared according to general procedure A: a solution of 2-O-acetyl-3,4,6-tri-O-benzyl-d-glucoopyranosyl thiol 1b [28] (0.5 g, 0.99 mmol, 1.0 equiv.) was coupled with diazo linker-1 [23] (0.291 g, 1.13 mmol, 1.15 equiv.) to afford EDPA thioglycoside donor 2b (0.415 g, 61%, α:β = 1:10). Rf = 0.3 (hexane:ethyl acetate = 80:20). 1H NMR (400 MHz, CDCl3) δ 7.47 (d, J = 7.8 Hz, 2H), 7.39 (t, J = 7.7 Hz, 2H), 7.33–7.27 (m, 13H), 7.21–7.18 (m, 3H), 5.04 (t, J = 9.4 Hz, 1H), 4.81 (dd, J = 11.1, 9.1 Hz, 2H), 4.69 (d, J = 11.5 Hz, 1H), 4.59 (t, J = 11.7 Hz, 2H), 4.54 (d, J = 12.1 Hz, 1H), 4.48–4.40 (m, 3H), 3.77–3.66 (m, 5H), 3.52 (dq, J = 7.4, 2.2 Hz, 1H), 3.05 (ddd, J = 14.0, 7.7, 6.3 Hz, 1H), 2.88 (ddd, J = 13.9, 7.7, 6.2 Hz, 1H), 1.96 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 169.64, 164.72, 138.13, 138.07, 137.86, 128.94, 128.43, 128.39, 128.34, 128.02, 127.88, 127.87, 127.80, 127.74, 127.70, 127.59, 125.87, 125.38, 124.02, 84.21, 83.54, 79.47, 77.74, 75.23, 75.11, 73.51, 73.49, 71.62, 68.81, 64.17, 28.59, 20.89. HRMS (ESI): calc. for C39H40N2NaO8S (M + Na): 719.2403; found: 719.2421.
- 2,3,4,6-tetra-O-benzoyl-α/β-d-glucopyranoside EDPA thioglycoside donor (2c): This was prepared according to general procedure A: a solution of 2,3,4,6-tetra-O-benzoyl-d-glucopyranosyl thiol 1c [22] (0.25 g, 0.408 mmol, 1.0 equiv.) was coupled with diazo linker-1 [23] (0.132 g, 0.49 mmol, 1.15 equiv.) to afford EDPA thioglycoside donor 2c (0.265 g, 81%, α:β = 1:8). Rf = 0.2 (hexane:ethyl acetate = 80:20). 1H NMR (400 MHz, CDCl3) δ 8.02 (dd, J = 8.5, 1.4 Hz, 2H), 7.98–7.90 (m, 5H), 7.82 (dd, J = 8.5, 1.4 Hz, 2H), 7.44 (d, J = 1.4 Hz, 1H), 7.42 (d, J = 1.9 Hz, 2H), 7.40 (s, 1H), 7.39–7.35 (m, 8H), 7.29 (d, J = 8.2 Hz, 2H), 7.22–7.17 (m, 1H), 5.93 (t, J = 9.5 Hz, 1H), 5.68 (t, J = 9.8 Hz, 1H), 5.57 (t, J = 9.7 Hz, 1H), 4.92 (d, J = 10.1 Hz, 1H), 4.66 (dd, J = 12.2, 3.0 Hz, 1H), 4.52–4.46 (m, 2H), 4.44–4.35 (m, 1H), 4.19 (ddd, J = 9.0, 5.5, 2.9 Hz, 1H), 3.10 (ddd, J = 14.1, 8.0, 6.2 Hz, 1H), 2.94 (ddd, J = 14.0, 7.9, 6.1 Hz, 1H). 13C NMR (101 MHz, CDCl3) δ 166.16, 165.84, 165.31, 165.29, 133.62, 133.51, 133.40, 133.22, 130.09, 130.03, 129.96, 129.84, 129.81, 129.79, 129.60, 129.07, 129.02, 128.81, 128.77, 128.55, 128.52, 128.46, 128.42, 126.05, 125.36, 124.16, 84.27, 76.56, 73.99, 70.57, 69.50, 64.01, 63.22, 29.23. HRMS (ESI): calc. for C44H36N2NaO11S (M + Na): 823.1938; found: 823.1956.
- 2,3,4,6-tetra-O-benzyl-α/β-d-mannopyranoside EDPA thioglycoside donor (2d): This was prepared according to general procedure A: a solution of 2,3,4,6-tetra-O-benzyl-d-mannopyranosyl thiol 1d [28] (0.25 g, 0.449 mmol, 1.0 equiv.) was coupled with diazo linker-1 [23] (0.139 g, 0.52 mmol, 1.15 equiv.) to afford EDPA thioglycoside donor 2d (0.177 g, 53%, α:β=10:1). Rf = 0.4 (hexane:ethyl acetate = 80:20). 1H NMR (400 MHz, CDCl3) δ 7.47 (dd, J = 8.6, 1.2 Hz, 2H), 7.41–7.36 (m, 4H), 7.34 (t, J = 2.0 Hz, 1H), 7.31 (dd, J = 6.9, 2.3 Hz, 7H), 7.29–7.27 (m, 7H), 7.21–7.15 (m, 3H), 5.44 (s, 1H), 4.88 (d, J = 10.7 Hz, 1H), 4.73 (d, J = 12.3 Hz, 1H), 4.67 (s, 1H), 4.64 (s, 1H), 4.55 (d, J = 4.3 Hz, 2H), 4.51 (dd, J = 12.1, 7.8 Hz, 4H), 4.35–4.28 (m, 1H), 4.14–4.09 (m, 1H), 4.05–3.99 (m, 1H), 3.84–3.79 (m, 3H), 3.72 (dd, J = 10.8, 2.0 Hz, 1H), 2.99–2.91 (m, 1H), 2.82 (dt, J = 13.7, 6.8 Hz, 1H). 13C NMR (101 MHz, CDCl3) δ 164.91, 138.46, 138.35, 138.23, 138.01, 129.08, 128.49, 128.43, 128.38, 128.07, 127.92, 127.84, 127.83, 127.79, 127.72, 127.59, 126.00, 125.46, 124.09, 82.42, 80.18, 76.07, 75.26, 74.98, 73.43, 72.31, 72.19, 72.04, 69.12, 63.23, 30.10. HRMS (ESI): calc. for C44H44N2NaO7S (M + Na): 767.2767; found: 767.2784.
- 2-O-acetyl-3,4,6-tri-O-benzyl-α/β-d-mannopyranoside 4-OMe-EDPA thioglycoside donor (2e): This was prepared according to general procedure A: a solution of 2-O-acetyl-3,4,6-tri-O-benzyl-d-mannopyranosyl thiol 1a [28] (0.1 g, 0.197 mmol, 1.0 equiv.) was coupled with diazo linker-2 [25] (0.068 g, 0.226 mmol, 1.15 equiv.) to afford EDPA thioglycoside donor 2e (0.087 g, 61%, α:β = 10:1). Rf = 0.3 (hexane:ethyl acetate = 80:20). 1H NMR (400 MHz, CDCl3) δ 7.38–7.35 (m, 3H), 7.33–7.27 (m, 17H), 7.16 (dd, J = 7.2, 2.3 Hz, 3H), 6.95–6.92 (m, 2H), 5.45 (dd, J = 3.0, 1.7 Hz, 1H), 5.35 (d, J = 1.6 Hz, 1H), 4.85 (d, J = 10.7 Hz, 1H), 4.68 (dd, J = 11.6, 6.2 Hz, 3H), 4.52 (d, J = 11.1 Hz, 1H), 4.50–4.45 (m, 4H), 4.35 (dt, J = 11.3, 7.0 Hz, 1H), 3.93–3.89 (m, 2H), 3.80 (s, 3H), 3.70 (dd, J = 10.7, 2.1 Hz, 1H), 2.97 (dt, J = 13.8, 7.0 Hz, 1H), 2.90–2.82 (m, 1H), 2.16 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 170.46, 158.21, 138.32, 138.19, 137.65, 128.57, 128.45, 128.42, 128.30, 128.28, 128.05, 128.01, 127.99, 127.96, 127.91, 127.88, 127.80, 127.72, 126.14, 116.78, 114.72, 114.37, 114.22, 114.16, 83.28, 78.49, 77.34, 75.34, 74.49, 73.57, 73.51, 72.14, 72.04, 70.27, 68.77, 63.48, 55.45, 55.41, 49.57, 30.35, 29.81, 21.23, 21.17, 14.30, 14.24.
- 2-O-acetyl-3,4,6-tri-O-benzyl-α/β-d-mannopyranoside 4-NO2-EDPA thioglycoside donor (2f): This was prepared according to general procedure A: a solution of 2-O-acetyl-3,4,6-tri-O-benzyl-d-mannopyranosyl thiol 1a [28] (0.1 g, 0.196 mmol, 1.0 equiv.) was coupled with diazo linker-3 (0.071 g, 0.226 mmol, 1.15 equiv.) to afford EDPA thioglycoside donor 2f (0.063 g, 43%, α:β = 10:1). Rf = 0.2 (hexane:ethyl acetate = 80:20). 1H NMR (400 MHz, CDCl3) δ 8.22 (d, J = 9.1 Hz, 2H), 7.64 (d, J = 9.1 Hz, 2H), 7.31 (q, J = 7.9 Hz, 13H), 7.19–7.13 (m, 2H), 5.43 (s, 1H), 5.35 (s, 1H), 4.85 (d, J = 10.7 Hz, 1H), 4.70–4.62 (m, 2H), 4.53 (d, J = 11.1 Hz, 2H), 4.49 (d, J = 4.7 Hz, 1H), 4.46 (d, J = 3.2 Hz, 1H), 4.41 (dt, J = 11.3, 6.8 Hz, 1H), 4.15 (dd, J = 7.6, 4.5 Hz, 1H), 3.94–3.86 (m, 2H), 3.81 (dd, J = 10.7, 4.6 Hz, 1H), 3.72–3.68 (m, 1H), 2.99 (dt, J = 13.7, 6.8 Hz, 1H), 2.88 (dt, J = 13.6, 6.4 Hz, 1H), 2.17 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 170.28, 163.25, 145.09, 138.13, 138.05, 137.49, 133.64, 128.43, 128.33, 128.27, 128.10, 127.88, 127.86, 127.70, 127.64, 127.57, 124.27, 123.19, 83.15, 78.32, 75.23, 74.43, 73.38, 72.14, 71.93, 70.17, 68.79, 63.82, 30.17, 21.05. HRMS (ESI): calc. for C39H39N3NaO10S (M + Na): 764.2254; found: 764.2269.
3.3. General Procedure B for the Activation of EDPA Thioglycosides for the Synthesis of Disaccharides (4–27)
In an oven-dried vial equipped with a stir bar, accepter 3 (a–l) (0.050 mmol) was combined with EDPA thioglycoside donor 2 (a–f) (0.075 mmol, 1.5 equiv.), 4 Å molecular sieves (50 mg or 100 wt.%), and [Cu(OTf)]2·tol (0.002 mmol, 0.1 equiv.) dissolved in CH2Cl2 (0.05 M). Upon complete addition, the reaction mixture was stirred at the ambient temperature, and the reaction progress was monitored using TLC and quenched with saturated aqueous NaHCO3 upon completion. The layers were separated, and the aqueous layer was washed with ethyl acetate. The organic layers were combined, dried over Na2SO4, filtered, and evaporated under reduced pressure. The crude product was dissolved in ethyl acetate and passed through a short silica pad to remove traces of iron (to check the crude material using 1H-NMR). The filtrate was evaporated, and the resulting crude material was purified using column chromatography.
Note: The donor was added in three portions initially; 0.8 equiv. was charged and TLC monitored during the reaction progress, and upon complete consumption of the donor, 0.35 equiv. was added into the reaction mixture twice.
- Methyl 2-O-acetyl-3,4,6-tri-O-benzyl-α-d-mannopyranosyl-(1→6)-2,3,4-tri-O-benzyl-α-d-glucopyranoside (4): Following general procedure B, glycosyl acceptor 3a (24.0 mg, 50.0 μmol) was coupled with EDPAT donor 2a (54.0 mg, 75.0 μmol) to afford 4 (43.0 mg, 45.8 μmol, 88% yield) as a colorless viscous oil. Rf = 0.3 (hexane:EtOAc = 70:30). 1H NMR (400 MHz, CDCl3) δ 7.38–7.35 (m, 2H), 7.34–7.30 (m, 10H), 7.29–7.26 (m, 10H), 7.25–7.23 (m, 3H), 7.20 (dd, J = 5.5, 2.1 Hz, 2H), 7.15 (dd, J = 7.5, 2.0 Hz, 1H), 7.13 (dd, J = 7.2, 2.1 Hz, 2H), 5.39 (td, J = 3.8, 1.9 Hz, 1H), 4.99 (d, J = 10.8 Hz, 1H), 4.90 (d, J = 1.9 Hz, 1H), 4.88–4.86 (m, 1H), 4.86–4.84 (m, 1H), 4.79 (d, J = 4.1 Hz, 1H), 4.78 (d, J = 5.3 Hz, 1H), 4.70–4.69 (m, 1H), 4.67 (s, 1H), 4.64–4.62 (m, 1H), 4.58 (d, J = 3.5 Hz, 1H), 4.53 (d, J = 6.0 Hz, 1H), 4.52–4.49 (m, 1H), 4.48 (s, 1H), 4.46 (d, J = 3.5 Hz, 1H), 4.43 (d, J = 8.6 Hz, 1H), 3.98 (t, J = 9.3 Hz, 1H), 3.91 (dd, J = 9.5, 3.2 Hz, 1H), 3.86 (t, J = 9.5 Hz, 1H), 3.81–3.78 (m, 1H), 3.73–3.70 (m, 3H), 3.66 (dd, J = 10.8, 4.2 Hz, 1H), 3.63 (dd, J = 11.4, 1.9 Hz, 1H), 3.54 (ddd, J = 13.3, 10.2, 2.8 Hz, 2H), 3.45–3.41 (m, 1H), 3.32 (s, 3H), 2.13 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 170.31, 138.67, 138.50, 138.27, 138.20, 138.16, 138.09, 137.76, 128.45, 128.38, 128.34, 128.31, 128.24, 128.21, 128.15, 128.09, 128.07, 128.00, 127.92, 127.89, 127.86, 127.75, 127.72, 127.66, 127.63, 127.61, 127.59, 127.52, 127.48, 97.99, 97.78, 82.07, 80.07, 77.61, 77.58, 75.75, 75.12, 75.03, 74.90, 74.48, 74.17, 73.48, 73.32, 71.79, 71.57, 71.45, 71.38, 69.62, 68.90, 68.67, 68.48, 66.07, 55.08, 21.12. The data are identical to the literature report [29].
- Methyl 2-O-acetyl-3,4,6-tri-O-benzyl-α-d-mannopyranosyl-(1→6)-2,3,4-tri-O-benzyl-α-d-mannopyranoside (5): Following general procedure B, glycosyl acceptor 3b (24.0 mg, 50.0 μmol) was coupled with EDPAT donor 2a (54.0 mg, 75.0 μmol) to afford 5 (39.0 mg, 41.5 μmol, 81% yield) as a colorless viscous oil. Rf = 0.3 (hexane:EtOAc = 70:30). 1H NMR (400 MHz, CDCl3) δ 7.39–7.36 (m, 2H), 7.34–7.27 (m, 21H), 7.24–7.22 (m, 4H), 7.16–7.11 (m, 3H), 5.48–5.45 (m, 1H), 4.96 (d, J = 2.1 Hz, 1H), 4.91 (d, J = 11.2 Hz, 1H), 4.86 (dd, J = 10.9, 3.4 Hz, 1H), 4.73–4.70 (m, 3H), 4.67–4.65 (m, 1H), 4.64 (d, J = 3.9 Hz, 1H), 4.59 (d, J = 1.2 Hz, 2H), 4.55 (d, J = 6.9 Hz, 1H), 4.50 (d, J = 3.6 Hz, 1H), 4.48–4.46 (m, 2H), 4.44 (d, J = 4.3 Hz, 1H), 3.96 (dd, J = 9.2, 3.2 Hz, 1H), 3.91 (d, J = 9.4 Hz, 1H), 3.87 (t, J = 2.3 Hz, 2H), 3.80–3.77 (m, 2H), 3.72 (d, J = 5.2 Hz, 1H), 3.70 (t, J = 3.3 Hz, 1H), 3.67 (s, 1H), 3.59 (dd, J = 10.8, 1.9 Hz, 1H), 3.25 (s, 3H), 2.14 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 170.28, 138.59, 138.45, 138.42, 138.30, 138.28, 137.82, 128.38, 128.35, 128.32, 128.30, 128.28, 128.23, 128.19, 128.06, 127.92, 127.86, 127.81, 127.76, 127.72, 127.62, 127.60, 127.53, 127.48, 127.43, 98.69, 97.94, 80.25, 77.56, 75.12, 74.99, 74.94, 74.59, 74.53, 74.47, 74.18, 73.48, 73.32, 72.58, 71.97, 71.79, 71.39, 71.35, 71.31, 70.90, 69.18, 68.90, 68.72, 68.52, 66.70, 54.64, 21.12. The data are identical to the literature report [30].
- Methyl 2-O-acetyl-3,4,6-tri-O-benzyl-α-d-mannopyranosyl-(1→6)-2,3,4-tri-O-benzoyl-α-d-glucopyranoside (6): Following general procedure B, glycosyl acceptor 3c (25.0 mg, 50.0 μmol) was coupled with EDPAT donor 2a (52.0 mg, 75.0 μmol) to afford 6 (34.0 mg, 34.7 μmol, 70% yield) as a colorless viscous oil. Rf = 0.2 (hexane:EtOAc = 70:30). 1H NMR (400 MHz, CDCl3) δ 7.98 (dd, J = 8.5, 1.3 Hz, 2H), 7.94 (dd, J = 8.5, 1.3 Hz, 2H), 7.87 (dd, J = 8.4, 1.4 Hz, 2H), 7.50 (ddt, J = 14.1, 7.1, 1.3 Hz, 2H), 7.46–7.41 (m, 2H), 7.41–7.35 (m, 3H), 7.33–7.27 (m, 15H), 7.16 (dd, J = 7.4, 2.1 Hz, 2H), 6.13 (t, J = 9.9 Hz, 1H), 5.56 (t, J = 9.9 Hz, 1H), 5.33 (dd, J = 3.3, 1.8 Hz, 1H), 5.26 (dd, J = 10.2, 3.7 Hz, 1H), 5.19 (d, J = 3.7 Hz, 1H), 4.87–4.83 (m, 2H), 4.60 (d, J = 2.2 Hz, 1H), 4.57 (s, 1H), 4.46 (d, J = 7.3 Hz, 1H), 4.43 (d, J = 7.7 Hz, 1H), 4.38 (d, J = 12.0 Hz, 1H), 4.20 (ddd, J = 10.2, 5.4, 3.2 Hz, 1H), 3.94 (dd, J = 9.3, 3.4 Hz, 1H), 3.91–3.86 (m, 1H), 3.84 (d, J = 9.5 Hz, 1H), 3.74–3.69 (m, 1H), 3.69–3.65 (m, 1H), 3.63 (dd, J = 11.6, 3.7 Hz, 1H), 3.53 (dd, J = 10.7, 1.9 Hz, 1H), 3.39 (s, 3H), 2.13 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 170.30, 165.79, 165.15, 138.56, 138.18, 137.97, 133.31, 133.03, 129.92, 129.67, 129.24, 129.07, 128.94, 128.40, 128.38, 128.32, 128.25, 128.22, 128.12, 127.79, 127.71, 127.66, 127.50, 127.48, 97.88, 77.84, 77.21, 75.01, 74.15, 73.30, 72.09, 71.73, 71.45, 70.48, 69.64, 68.65, 68.60, 68.05, 66.30, 55.49, 21.09. The data are identical to the literature report [30].
- Methyl 2-O-acetyl-3,4,6-tri-O-benzyl-α-d-mannopyranosyl-(1→6)-2,3,4-tri-O-benzoyl-α-d-mannopyranoside (7): Following general procedure B, glycosyl acceptor 3d (25.0 mg, 50.0 μmol) was coupled with EDPAT donor 2a (52.0 mg, 75.0 μmol) to afford 7 (36.0 mg, 36.7 μmol, 73% yield) as a colorless viscous oil. Rf = 0.2 (hexane:EtOAc = 70:30). 1H NMR (400 MHz, CDCl3) δ 8.08 (d, J = 7.7 Hz, 2H), 7.96 (d, J = 7.9 Hz, 2H), 7.82 (d, J = 7.6 Hz, 2H), 7.62–7.51 (m, 1H), 7.44 (dt, J = 15.1, 7.8 Hz, 4H), 7.30 (dd, J = 19.6, 5.3 Hz, 17H), 7.19–7.09 (m, 2H), 5.97–5.76 (m, 2H), 5.65 (s, 1H), 5.34 (s, 1H), 4.91 (d, J = 16.7 Hz, 2H), 4.83 (d, J = 11.1 Hz, 1H), 4.65–4.29 (m, 6H), 4.27–4.17 (m, 1H), 4.00–3.81 (m, 3H), 3.71 (dd, J = 23.6, 11.8 Hz, 3H), 3.54 (d, J = 11.2 Hz, 1H), 3.45 (s, 3H), 2.12 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 170.47, 165.66, 165.60, 165.53, 138.64, 138.24, 138.03, 133.64, 133.50, 133.25, 130.02, 129.99, 129.87, 129.48, 129.23, 129.14, 128.75, 128.59, 128.55, 128.48, 128.40, 128.38, 128.24, 128.18, 128.11, 128.03, 127.96, 127.92, 127.89, 127.82, 127.67, 98.63, 98.02, 78.25, 75.21, 74.21, 73.44, 71.76, 71.58, 70.63, 70.13, 69.18, 68.65, 68.60, 67.60, 66.83, 55.54, 21.27. The data are identical to the literature report [31].
- Methyl 2-O-acetyl-3,4,6-tri-O-benzyl-α-d-mannopyranosyl-(1→4)-2,3,6-tri-O-benzyl-α-d-glucopyranoside (8): Following general procedure B, glycosyl acceptor 3e (24.0 mg, 50.0 μmol) was coupled with EDPAT donor 2a (54.0 mg, 75.0 μmol) to afford 8 (33.0 mg, 35.1 μmol, 67% yield) as a colorless viscous oil. Rf = 0.3 (hexane:EtOAc = 70:30). 1H NMR (400 MHz, CDCl3) δ 7.38–7.34 (m, 1H), 7.33 (d, J = 1.9 Hz, 3H), 7.31–7.26 (m, 21H), 7.25–7.19 (m, 3H), 7.16–7.13 (m, 2H), 5.48–5.44 (m, 1H), 5.42 (d, J = 2.2 Hz, 1H), 5.04 (d, J = 11.2 Hz, 1H), 4.81 (d, J = 10.8 Hz, 1H), 4.76–4.72 (m, 1H), 4.67 (d, J = 18.7 Hz, 1H), 4.62 (d, J = 2.8 Hz, 1H), 4.59 (d, J = 2.3 Hz, 2H), 4.54 (d, J = 11.8 Hz, 2H), 4.48–4.41 (m, 3H), 4.41–4.34 (m, 2H), 3.93 (t, J = 9.1 Hz, 1H), 3.88–3.85 (m, 1H), 3.85–3.80 (m, 2H), 3.72 (s, 1H), 3.69 (t, J = 4.2 Hz, 2H), 3.67–3.63 (m, 1H), 3.54 (dd, J = 9.5, 3.5 Hz, 1H), 3.50 (dd, J = 10.8, 1.9 Hz, 1H), 3.38 (s, 3H), 1.98 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 169.85, 138.52, 138.45, 138.20, 137.97, 137.92, 128.44, 128.30, 128.24, 128.20, 128.14, 128.05, 127.93, 127.88, 127.82, 127.61, 127.56, 127.53, 127.40, 127.27, 127.25, 99.30, 97.76, 81.74, 80.05, 78.20, 77.22, 75.71, 75.13, 75.09, 74.02, 73.44, 73.27, 72.41, 71.67, 69.57, 69.20, 68.72, 55.28, 20.95. The data are identical to the literature report [30].
- p-Methoxyphenyl 2-O-acetyl-3,4,6-tri-O-benzyl-α-d-mannopyranoside (9): Following general procedure B, glycosyl acceptor 3f (6.0 mg, 50.0 μmol) was coupled with EDPAT donor 2a (54.0 mg, 75.0 μmol) to afford 9 (22.0 mg, 36.8 μmol, 76% yield) as a colorless viscous oil. Rf = 0.5 (hexane:EtOAc = 70:30). 1H NMR (400 MHz, CDCl3) δ 7.38–7.27 (m, 13H), 7.18 (dd, J = 7.8, 1.7 Hz, 2H), 7.01–6.97 (m, 2H), 6.81–6.78 (m, 2H), 5.55 (dd, J = 3.4, 2.0 Hz, 1H), 5.46 (d, J = 2.0 Hz, 1H), 4.89 (d, J = 10.7 Hz, 1H), 4.77 (d, J = 11.1 Hz, 1H), 4.66 (d, J = 12.0 Hz, 1H), 4.62 (d, J = 11.1 Hz, 1H), 4.52 (d, J = 10.7 Hz, 1H), 4.46 (d, J = 12.0 Hz, 1H), 4.19 (dd, J = 9.0, 3.4 Hz, 1H), 4.00 (t, J = 9.5 Hz, 1H), 3.96 (ddd, J = 9.8, 4.1, 1.8 Hz, 1H), 3.82 (dd, J = 10.8, 4.1 Hz, 1H), 3.76 (s, 3H), 3.68 (dd, J = 10.8, 1.9 Hz, 1H), 2.18 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 170.44, 155.10, 149.95, 138.31, 138.13, 137.87, 128.43, 128.32, 128.27, 128.09, 127.84, 127.80, 127.78, 127.65, 127.57, 117.81, 114.57, 96.86, 78.02, 75.22, 74.16, 73.35, 71.97, 71.86, 68.68, 68.65, 55.60, 21.12. The data are identical to the literature report [32].
- l-Menthyl 2-O-acetyl-3,4,6-tri-O-benzyl-α-d-mannopyranoside (10): Following general procedure B, glycosyl acceptor 3g (8.0 mg, 50.0 μmol) was coupled with EDPAT donor 2a (54.0 mg, 75.0 μmol) to afford 10 (22.0 mg, 36.8 μmol, 74% yield) as a colorless viscous oil. Rf = 0.6 (hexane:EtOAc = 70:30). 1H NMR (400 MHz, CDCl3) δ 7.39–7.26 (m, 13H), 7.18 (dd, J = 7.4, 2.1 Hz, 2H), 5.26 (dd, J = 3.3, 1.9 Hz, 1H), 4.94–4.86 (m, 2H), 4.71 (dd, J = 11.7, 6.6 Hz, 2H), 4.58 (d, J = 11.3 Hz, 1H), 4.50 (dd, J = 11.4, 10.0 Hz, 2H), 4.02–3.95 (m, 2H), 3.91–3.80 (m, 2H), 3.71 (dd, J = 10.6, 1.9 Hz, 1H), 3.35 (td, J = 10.6, 4.3 Hz, 1H), 2.16 (s, 3H), 2.05 (pd, J = 7.0, 2.5 Hz, 1H), 1.66–1.57 (m, 2H), 1.41–1.31 (m, 1H), 1.24–1.15 (m, 1H), 1.03–0.90 (m, 5H), 0.85 (d, J = 6.5 Hz, 3H), 0.78 (d, J = 7.0 Hz, 4H). 13C NMR (101 MHz, CDCl3) δ 170.58, 138.40, 138.31, 137.98, 128.37, 128.32, 128.25, 128.08, 127.92, 127.72, 127.70, 127.62, 127.50, 99.27, 81.63, 77.97, 75.19, 74.62, 73.36, 71.76, 71.41, 69.34, 69.05, 48.55, 42.71, 34.26, 31.58, 25.84, 23.30, 22.20, 21.20, 21.02, 16.31. The data are identical to the literature report [33].
- Adamantanyl 2-O-acetyl-3,4,6-tri-O-benzyl-α-d-mannopyranoside (11): Following general procedure B, glycosyl acceptor 3h (8.0 mg, 50.0 μmol) was coupled with EDPAT donor 2a (54.0 mg, 75.0 μmol) to afford 11 (28.0 mg, 44.8 μmol, 84% yield) as a colorless viscous oil. Rf = 0.7 (hexane:EtOAc = 70:30). 1H NMR (400 MHz, CDCl3) δ 7.37–7.26 (m, 12H), 7.17 (dd, J = 7.4, 2.1 Hz, 2H), 5.27 (d, J = 2.1 Hz, 1H), 5.18 (dd, J = 3.3, 2.0 Hz, 1H), 4.86 (d, J = 10.6 Hz, 1H), 4.73–4.68 (m, 2H), 4.55 (d, J = 11.1 Hz, 1H), 4.48 (dd, J = 11.3, 4.4 Hz, 2H), 4.08–3.99 (m, 2H), 3.90 (t, J = 9.6 Hz, 1H), 3.83 (dd, J = 10.6, 4.2 Hz, 1H), 3.68 (dd, J = 10.6, 2.0 Hz, 1H), 2.15 (s, 3H), 2.14–2.10 (m, 3H), 1.79 (d, J = 2.0 Hz, 6H), 1.64–1.55 (m, 6H). 13C NMR (101 MHz, CDCl3) δ 170.75, 138.46, 138.35, 138.11, 128.35, 128.30, 128.22, 128.06, 127.94, 127.69, 127.65, 127.59, 127.46, 90.95, 78.24, 75.20, 75.03, 74.68, 73.36, 71.71, 70.91, 70.44, 69.07, 42.28, 36.20, 30.59, 21.24. The data are identical to the literature report [33].
- Cholesteryl 2-O-acetyl-3,4,6-tri-O-benzyl-α-d-mannopyranoside (12): Following general procedure B, glycosyl acceptor 3i (19.0 mg, 50.0 μmol) was coupled with EDPAT donor 2a (54.0 mg, 75.0 μmol) to afford 12 (34.0 mg, 40.8 μmol, 83% yield) as a colorless viscous oil. Rf = 0.7 (hexane:EtOAc = 70:30). 1H NMR (400 MHz, CDCl3) δ 7.38–7.27 (m, 12H), 7.19–7.15 (m, 2H), 5.34 (dd, J = 3.4, 1.8 Hz, 1H), 5.29 (dd, J = 4.8, 2.7 Hz, 1H), 5.01 (d, J = 1.8 Hz, 1H), 4.86 (d, J = 10.6 Hz, 1H), 4.71 (dd, J = 11.5, 9.0 Hz, 2H), 4.55 (d, J = 11.1 Hz, 1H), 4.50 (d, J = 9.3 Hz, 1H), 4.48 (d, J = 8.0 Hz, 1H), 4.03 (dt, J = 6.1, 3.3 Hz, 1H), 3.92–3.89 (m, 2H), 3.85–3.81 (m, 1H), 3.72 (dd, J = 10.6, 1.3 Hz, 1H), 3.49 (dq, J = 10.9, 5.6 Hz, 1H), 2.35–2.27 (m, 2H), 2.15 (s, 3H), 2.05–1.97 (m, 2H), 1.95–1.81 (m, 4H), 1.61–1.42 (m, 8H), 1.41–1.30 (m, 4H), 1.29–1.24 (m, 2H), 1.13 (ddt, J = 15.7, 9.9, 5.6 Hz, 6H), 1.00 (s, 4H), 0.92 (d, J = 6.5 Hz, 3H), 0.88 (dd, J = 6.6, 1.8 Hz, 6H), 0.68 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 170.58, 140.54, 138.37, 138.25, 138.06, 128.36, 128.32, 128.26, 128.03, 127.95, 127.78, 127.67, 127.63, 127.52, 121.92, 109.99, 95.81, 78.35, 77.22, 75.24, 74.49, 73.38, 71.76, 71.32, 69.31, 68.93, 56.75, 56.14, 50.07, 42.31, 39.84, 39.75, 39.52, 36.96, 36.67, 36.19, 35.78, 31.93, 31.87, 28.23, 28.01, 27.68, 24.29, 23.82, 22.82, 22.57, 21.18, 21.04, 19.33, 18.72, 11.86. The data are identical to the literature report [33].
- Methyl 2-O-acetyl-3,4,6-tri-O-benzyl-β-d-glucopyranosyl-(1→6)-2,3,4-tri-O-benzyl-α-d-glucopyranoside (13): Following general procedure B, glycosyl acceptor 3a (24.0 mg, 50.0 μmol) was coupled with EDPAT donor 2b (54.0 mg, 75.0 μmol) to afford 13 (33.0 mg, 35.2 μmol, 68% yield) as a colorless viscous oil. Rf = 0.3 (hexane:EtOAc = 70:30). 1H NMR (400 MHz, CDCl3) δ 7.35 (dd, J = 8.2, 1.6 Hz, 2H), 7.33–7.29 (m, 16H), 7.29–7.25 (m, 9H), 7.24 (s, 1H), 7.18 (dd, J = 7.4, 2.1 Hz, 2H), 5.05 (t, J = 8.6 Hz, 1H), 4.97 (d, J = 11.1 Hz, 1H), 4.83 (d, J = 10.8 Hz, 1H), 4.80–4.76 (m, 4H), 4.65 (dd, J = 11.8, 5.1 Hz, 2H), 4.59–4.55 (m, 3H), 4.55–4.51 (m, 2H), 4.39 (d, J = 8.1 Hz, 1H), 4.09 (dd, J = 10.8, 2.0 Hz, 1H), 3.97 (t, J = 9.3 Hz, 1H), 3.78–3.74 (m, 1H), 3.73 (dd, J = 11.1, 2.1 Hz, 1H), 3.69–3.61 (m, 4H), 3.52 (dd, J = 9.6, 3.6 Hz, 1H), 3.50–3.47 (m, 1H), 3.44 (t, J = 9.5 Hz, 1H), 3.35 (s, 3H), 1.87 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 169.17, 138.83, 138.25, 138.14, 138.09, 137.81, 128.44, 128.42, 128.33, 128.15, 128.01, 127.89, 127.86, 127.83, 127.73, 127.72, 127.65, 127.56, 127.51, 100.95, 98.00, 79.82, 78.03, 77.74, 75.66, 75.36, 75.04, 75.00, 74.86, 73.43, 73.41, 72.93, 69.70, 68.81, 67.90, 55.06, 20.93. The data are identical to the literature report [33].
- Methyl 2-O-acetyl-3,4,6-tri-O-benzyl-β-d-glucopyranosyl-(1→6)-2,3,4-tri-O-benzyl-α-d-mannopyranoside (14): Following general procedure B, glycosyl acceptor 3b (24.0 mg, 50.0 μmol) was coupled with EDPAT donor 2b (54.0 mg, 75.0 μmol) to afford 14 (39.0 mg, 41.6 μmol, 81% yield) as a colorless viscous oil. Rf = 0.3 (hexane:EtOAc = 70:30). 1H NMR (400 MHz, CDCl3) δ 7.36 (dd, J = 8.3, 1.9 Hz, 2H), 7.34–7.27 (m, 26H), 7.18 (dd, J = 7.3, 2.2 Hz, 2H), 5.04 (dd, J = 9.5, 7.9 Hz, 1H), 4.91 (d, J = 11.2 Hz, 1H), 4.78 (d, J = 11.7 Hz, 2H), 4.72 (s, 2H), 4.68 (s, 2H), 4.65–4.63 (m, 1H), 4.61 (s, 1H), 4.58 (s, 3H), 4.55 (s, 1H), 4.51 (d, J = 7.3 Hz, 1H), 4.44 (t, J = 8.6 Hz, 2H), 4.18–4.14 (m, 1H), 3.88–3.85 (m, 1H), 3.77 (dd, J = 3.1, 1.9 Hz, 1H), 3.75–3.71 (m, 3H), 3.68 (d, J = 3.8 Hz, 1H), 3.66 (t, J = 2.2 Hz, 1H), 3.63 (s, 1H), 3.29 (s, 3H), 1.92 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 169.31, 138.43, 138.25, 138.17, 137.97, 128.39, 128.38, 128.34, 128.32, 127.97, 127.89, 127.82, 127.79, 127.75, 127.69, 127.67, 127.64, 127.57, 127.53, 101.31, 98.83, 82.95, 80.29, 78.00, 77.21, 75.21, 75.04, 74.98, 74.94, 74.81, 74.45, 73.49, 73.10, 72.72, 72.07, 71.51, 68.91, 68.69, 54.58, 20.91. The data are identical to the literature report [34].
- Methyl 2-O-acetyl-3,4,6-tri-O-benzyl-β-d-glucopyranosyl-(1→6)-2,3,4-tri-O-benzoyl-α-d-glucopyranoside (15): Following general procedure B, glycosyl acceptor 3c (25.0 mg, 50.0 μmol) was coupled with EDPAT donor 2b (52.0 mg, 75.0 μmol) to afford 15 (36.0 mg, 36.7 μmol, 74% yield) as a colorless viscous oil. Rf = 0.2 (hexane:EtOAc = 70:30). 1H NMR (400 MHz, CDCl3) δ 7.99–7.97 (m, 2H), 7.94–7.92 (m, 2H), 7.85 (dd, J = 8.5, 1.3 Hz, 2H), 7.51 (td, J = 7.3, 1.4 Hz, 2H), 7.42 (tt, J = 7.2, 1.4 Hz, 1H), 7.38 (d, J = 8.5 Hz, 2H), 7.37–7.35 (m, 2H), 7.33 (dt, J = 8.5, 1.4 Hz, 1H), 7.30 (dd, J = 10.7, 1.2 Hz, 3H), 7.29–7.26 (m, 10H), 7.24 (dd, J = 4.9, 3.7 Hz, 1H), 7.18–7.16 (m, 2H), 6.14 (t, J = 9.7 Hz, 1H), 5.42 (t, J = 9.9 Hz, 1H), 5.23 (dd, J = 10.1, 3.7 Hz, 1H), 5.20 (d, J = 3.7 Hz, 1H), 5.06–5.03 (m, 1H), 4.79 (dd, J = 11.1, 7.2 Hz, 2H), 4.69 (d, J = 11.4 Hz, 1H), 4.55–4.53 (m, 2H), 4.47–4.42 (m, 2H), 4.27 (ddd, J = 9.2, 7.2, 1.9 Hz, 1H), 4.07 (dd, J = 10.9, 2.0 Hz, 1H), 3.70–3.67 (m, 4H), 3.66–3.64 (m, 1H), 3.45 (s, 3H), 2.02 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 169.50, 165.81, 165.69, 165.37, 138.17, 138.01, 137.87, 133.40, 133.31, 133.01, 129.91, 129.85, 129.63, 129.26, 129.08, 128.87, 128.41, 128.38, 128.31, 128.22, 127.98, 127.83, 127.80, 127.71, 127.69, 127.55, 101.35, 96.64, 82.86, 77.81, 75.20, 75.03, 75.01, 73.41, 73.07, 72.14, 70.56, 69.53, 68.70, 68.39, 55.33, 20.95. The data are identical to the literature report [33].
- Methyl 2-O-acetyl-3,4,6-tri-O-benzyl-β-d-glucopyranosyl-(1→6)-1,2:3,4-di-O-isopropylidene-α-d-galactopyranoside (16): Following general procedure B, glycosyl acceptor 3j (13.0 mg, 50.0 μmol) was coupled with EDPAT donor 2b (53.0 mg, 75.0 μmol) to afford 16 (29.0 mg, 39.5 μmol, 79% yield) as a colorless viscous oil. Rf = 0.4 (hexane:EtOAc = 70:30). 1H NMR (400 MHz, CDCl3) (600 MHz, cdcl3) δ 7.35–7.31 (m, 4H), 7.31 (d, J = 2.3 Hz, 1H), 7.29 (d, J = 2.8 Hz, 2H), 7.27 (d, J = 12.1 Hz, 6H), 7.18 (dd, J = 7.7, 2.0 Hz, 2H), 5.49 (d, J = 5.0 Hz, 1H), 5.02–4.98 (m, 1H), 4.78 (dd, J = 11.1, 8.1 Hz, 2H), 4.68 (d, J = 11.4 Hz, 1H), 4.63 (d, J = 12.1 Hz, 1H), 4.58–4.55 (m, 2H), 4.54 (d, J = 6.4 Hz, 1H), 4.44 (d, J = 8.1 Hz, 1H), 4.27 (dd, J = 4.9, 2.4 Hz, 1H), 4.18 (dd, J = 8.1, 1.9 Hz, 1H), 4.06 (dd, J = 11.3, 3.5 Hz, 1H), 3.92 (ddt, J = 5.4, 3.5, 1.9 Hz, 1H), 3.73 (d, J = 2.9 Hz, 2H), 3.71–3.65 (m, 2H), 3.62 (dd, J = 11.3, 7.4 Hz, 1H), 3.47 (dt, J = 9.4, 3.2 Hz, 1H), 2.01 (s, 3H), 1.50 (s, 3H), 1.42 (s, 3H), 1.31 (s, 3H), 1.30 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 169.75, 138.23, 138.16, 137.96, 128.39, 128.38, 128.33, 127.99, 127.83, 127.79, 127.76, 127.67, 127.55, 109.29, 108.65, 101.83, 96.20, 82.79, 77.94, 75.17, 75.03, 74.96, 73.51, 73.05, 71.30, 70.62, 70.53, 69.48, 68.60, 67.84, 26.06, 25.94, 25.10, 24.30, 20.95. The data are identical to the literature report [35].
- l-Menthyl 2-O-acetyl-3,4,6-tri-O-benzyl-β-d-glucopyranoside (17): Following general procedure B, glycosyl acceptor 3g (8.0 mg, 50.0 μmol) was coupled with EDPAT donor 2b (54.0 mg, 75.0 μmol) to afford 17 (30.0 mg, 47.6 μmol, 94% yield) as a colorless viscous oil. Rf = 0.6 (hexane:EtOAc = 70:30). 1H NMR (400 MHz, CDCl3) δ 7.34–7.30 (m, 7H), 7.30–7.26 (m, 6H), 7.23 (dd, J = 7.8, 1.8 Hz, 2H), 4.94 (dd, J = 9.0, 8.0 Hz, 1H), 4.80 (dd, J = 11.2, 4.3 Hz, 2H), 4.67 (d, J = 11.5 Hz, 1H), 4.64–4.60 (m, 2H), 4.55 (d, J = 12.1 Hz, 1H), 4.40 (d, J = 8.0 Hz, 1H), 3.73–3.64 (m, 4H), 3.44 (ddd, J = 9.3, 4.5, 2.1 Hz, 1H), 3.39 (td, J = 10.7, 4.3 Hz, 1H), 2.31 (td, J = 7.0, 2.7 Hz, 1H), 1.96 (s, 3H), 1.93 (d, J = 3.2 Hz, 1H), 1.63 (ddt, J = 13.0, 6.3, 3.3 Hz, 2H), 1.33 (dtd, J = 12.0, 6.0, 3.2 Hz, 1H), 1.23–1.18 (m, 1H), 0.95–0.93 (m, 1H), 0.90 (d, J = 6.5 Hz, 3H), 0.88 (d, J = 7.1 Hz, 3H), 0.83–0.80 (m, 1H), 0.78 (d, J = 6.9 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 169.41, 138.33, 138.30, 137.98, 128.42, 128.37, 128.33, 128.11, 127.83, 127.81, 127.63, 127.60, 127.51, 98.96, 83.14, 78.33, 78.10, 75.10, 75.02, 74.83, 73.71, 73.42, 69.14, 47.50, 40.89, 34.30, 31.38, 24.99, 23.00, 22.29, 21.00, 20.97, 15.76. The data are identical to the literature report [33].
- Adamantanyl 2-O-acetyl-3,4,6-tri-O-benzyl-β-d-glucopyranoside (18): Following general procedure B, glycosyl acceptor 3h (8.0 mg, 50.0 μmol) was coupled with EDPAT donor 2b (54.0 mg, 75.0 μmol) to afford 18 (29.0 mg, 46.3 μmol, 87% yield) as a colorless viscous oil. Rf = 0.7 (hexane:EtOAc = 70:30). 1H NMR (400 MHz, CDCl3) δ 7.34 (d, J = 7.3 Hz, 2H), 7.32 (d, J = 6.0 Hz, 6H), 7.28 (d, J = 8.2 Hz, 5H), 7.16 (dd, J = 7.5, 1.9 Hz, 2H), 5.46 (d, J = 3.8 Hz, 1H), 4.81 (d, J = 2.5 Hz, 1H), 4.80 (s, 1H), 4.78–4.74 (m, 1H), 4.66 (d, J = 12.1 Hz, 1H), 4.52–4.48 (m, 2H), 4.04 (t, J = 9.5 Hz, 2H), 3.78 (dd, J = 10.8, 3.9 Hz, 1H), 3.70 (t, J = 9.4 Hz, 1H), 3.65 (dd, J = 10.7, 2.2 Hz, 1H), 2.13–2.09 (m, 3H), 2.02 (s, 3H), 1.78 (dd, J = 10.8, 1.8 Hz, 3H), 1.73–1.69 (m, 3H), 1.62 (d, J = 11.9 Hz, 3H), 1.59–1.55 (m, 3H). 13C NMR (101 MHz, CDCl3) δ 170.42, 138.86, 138.19, 138.14, 128.37, 128.34, 128.30, 127.99, 127.78, 127.72, 127.56, 127.49, 127.44, 88.80, 80.41, 78.04, 75.28, 75.14, 74.43, 74.17, 73.43, 69.90, 68.68, 42.30, 36.20, 30.54, 21.02. The data are identical to the literature report [33].
- Cholesteryl 2-O-acetyl-3,4,6-tri-O-benzyl-β-d-glucopyranoside (19): Following general procedure B, glycosyl acceptor 3i (19.0 mg, 50.0 μmol) was coupled with EDPAT donor 2b (54.0 mg, 75.0 μmol) to afford 19 (32.0 mg, 38.4 μmol, 78% yield) as a colorless viscous oil. Rf = 0.7 (hexane:EtOAc = 70:30). 1H NMR (400 MHz, CDCl3) δ 7.34–7.29 (m, 7H), 7.28 (ddd, J = 8.8, 3.6, 1.4 Hz, 6H), 7.21–7.19 (m, 2H), 5.34–5.32 (m, 1H), 4.96 (dd, J = 9.3, 8.0 Hz, 1H), 4.79 (dd, J = 11.1, 4.3 Hz, 2H), 4.67 (d, J = 11.4 Hz, 1H), 4.62 (d, J = 12.2 Hz, 1H), 4.56 (dd, J = 11.5, 3.9 Hz, 2H), 4.43 (d, J = 8.0 Hz, 1H), 3.76–3.73 (m, 1H), 3.70–3.64 (m, 3H), 3.50–3.45 (m, 2H), 2.24–2.18 (m, 1H), 1.97 (s, 6H), 1.83 (dt, J = 13.4, 3.0 Hz, 2H), 1.62–1.44 (m, 8H), 1.36–1.33 (m, 2H), 1.26 (td, J = 6.3, 3.3 Hz, 2H), 1.17–1.02 (m, 7H), 0.99 (s, 5H), 0.92 (d, J = 6.5 Hz, 4H), 0.87 (dd, J = 6.6, 2.6 Hz, 6H), 0.68 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 169.42, 140.63, 138.21, 137.89, 128.41, 128.39, 128.31, 128.03, 127.86, 127.83, 127.68, 127.67, 127.53, 121.83, 99.95, 83.02, 79.54, 78.13, 77.20, 75.11, 75.02, 74.94, 73.42, 73.36, 68.90, 56.75, 56.14, 50.18, 42.31, 39.76, 39.51, 39.05, 37.26, 36.71, 36.18, 35.77, 31.94, 31.86, 29.61, 28.22, 28.01, 24.28, 23.80, 22.81, 22.55, 21.04, 20.96, 19.37, 18.71, 11.84. The data are identical to the literature report [33].
- Methyl (2,3,4,6-tetra-O-benzoyl-β-d-glucopyranosyl)-(1→6)-2,3,4-tri-O-benzyl-α-d-glucopyranoside (20): Following general procedure B, glycosyl acceptor 3a (24.0 mg, 50.0 μmol) was coupled with EDPAT donor 2c (62.0 mg, 75.0 μmol) to afford 20 (47.0 mg, 45.2 μmol, 88% yield) as a colorless viscous oil. Rf = 0.3 (hexane:EtOAc = 70:30). 1H NMR (400 MHz, CDCl3) δ 7.98–7.96 (m, 2H), 7.87 (ddd, J = 8.3, 3.2, 1.3 Hz, 5H), 7.80 (dd, J = 8.4, 1.3 Hz, 2H), 7.53–7.45 (m, 3H), 7.38 (dd, J = 15.1, 7.8 Hz, 6H), 7.33–7.29 (m, 6H), 7.29–7.25 (m, 7H), 7.24–7.17 (m, 6H), 7.04–7.01 (m, 2H), 5.87 (t, J = 9.7 Hz, 1H), 5.65 (t, J = 9.7 Hz, 1H), 5.58 (dd, J = 9.8, 7.8 Hz, 1H), 4.87 (d, J = 10.9 Hz, 1H), 4.80 (d, J = 7.8 Hz, 1H), 4.72 (d, J = 12.1 Hz, 1H), 4.66 (d, J = 11.0 Hz, 1H), 4.61–4.56 (m, 2H), 4.52–4.45 (m, 3H), 4.26 (d, J = 11.0 Hz, 1H), 4.13 (d, J = 8.7 Hz, 1H), 4.11–4.05 (m, 1H), 3.86 (t, J = 9.3 Hz, 1H), 3.75–3.68 (m, 2H), 3.41 (dd, J = 9.7, 3.6 Hz, 1H), 3.36 (t, J = 9.3 Hz, 1H), 3.19 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 166.23, 165.95, 165.27, 165.03, 138.87, 138.26, 138.22, 133.55, 133.37, 133.25, 133.23, 129.92, 129.86, 129.85, 129.82, 129.80, 129.62, 129.23, 128.85, 128.80, 128.54, 128.52, 128.46, 128.44, 128.41, 128.38, 128.24, 128.01, 127.99, 127.71, 127.59, 127.57, 101.41, 98.04, 81.97, 79.79, 75.65, 74.81, 73.49, 72.91, 72.27, 71.86, 69.84, 69.52, 68.38, 63.32, 55.11. The data are identical to the literature report [36].
- Methyl (2,3,4,6-tetra-O-benzoyl-β-d-glucopyranosyl)-(1→6)-2,3,4-tri-O-benzyl-α-d-mannopyranoside (21): Following general procedure B, glycosyl acceptor 3d (25.0 mg, 50.0 μmol) was coupled with EDPAT donor 2c (59.0 mg, 75.0 μmol) to afford 21 (49.0 mg, 45.2 μmol, 91% yield) as a colorless viscous oil. Rf = 0.2 (hexane:EtOAc = 70:30). 1H NMR (400 MHz, CDCl3) δ 8.08–8.04 (m, 2H), 8.00–7.97 (m, 2H), 7.93 (dd, J = 8.3, 1.4 Hz, 2H), 7.89–7.85 (m, 4H), 7.82 (dd, J = 8.5, 1.4 Hz, 2H), 7.76 (dd, J = 8.3, 1.3 Hz, 2H), 7.64–7.59 (m, 1H), 7.56–7.45 (m, 6H), 7.44–7.37 (m, 5H), 7.33 (td, J = 8.1, 3.9 Hz, 6H), 7.30–7.27 (m, 2H), 7.22 (d, J = 7.5 Hz, 2H), 5.90 (t, J = 9.7 Hz, 1H), 5.78 (dd, J = 9.9, 3.4 Hz, 1H), 5.69–5.62 (m, 2H), 5.60–5.56 (m, 1H), 5.54 (dd, J = 3.5, 1.8 Hz, 1H), 4.97 (d, J = 7.9 Hz, 1H), 4.64–4.59 (m, 2H), 4.43 (dd, J = 12.1, 5.1 Hz, 1H), 4.25 (t, J = 8.5 Hz, 1H), 4.15 (d, J = 14.9 Hz, 2H), 3.85 (dd, J = 11.1, 8.4 Hz, 1H), 3.11 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 166.21, 165.90, 165.77, 165.58, 165.39, 165.26, 165.24, 133.57, 133.54, 133.35, 133.29, 133.24, 130.02, 129.92, 129.90, 129.86, 129.83, 129.77, 129.42, 128.90, 128.85, 128.83, 128.70, 128.54, 128.49, 128.40, 128.33, 102.02, 98.05, 72.90, 72.29, 71.91, 70.51, 69.91, 69.80, 69.71, 67.41, 63.08, 54.96, 29.81. The data are identical to the literature report [37].
- Methyl (2,3,4,6-tetra-O-benzyl-α/β-d-mannopyranosyl)-(1→6)-2,3,4-tri-O-benzyl-α-d-glucopyranoside (22): Following general procedure B, glycosyl acceptor 3a (24.0 mg, 50.0 μmol) was coupled with EDPAT donor 2d (58.0 mg, 77.5 μmol) to afford 22 (27.0 mg, 27.4 μmol, 53% yield) as a colorless viscous oil. Rf = 0.3 (hexane:EtOAc = 70:30). 1H NMR (400 MHz, CDCl3) δ 7.39–7.20 (m, 37H), 7.17–7.13 (m, 2H), 4.99 (d, J = 10.9 Hz, 2H), 4.87 (dd, J = 10.9, 7.8 Hz, 2H), 4.82–4.75 (m, 3H), 4.73–4.65 (m, 4H), 4.64–4.59 (m, 3H), 4.57 (d, J = 3.5 Hz, 1H), 4.52–4.42 (m, 4H), 3.99 (q, J = 9.4 Hz, 3H), 3.88–3.81 (m, 2H), 3.79 (s, 1H), 3.73–3.57 (m, 6H), 3.46 (dd, J = 9.7, 3.4 Hz, 1H), 3.43–3.37 (m, 1H), 3.31 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 138.66, 138.63, 138.46, 138.42, 138.36, 138.18, 138.13, 128.48, 128.40, 128.35, 128.26, 128.21, 128.01, 127.99, 127.93, 127.84, 127.75, 127.70, 127.64, 127.60, 127.59, 127.55, 127.48, 127.44, 127.37, 98.24, 97.81, 82.13, 79.98, 79.56, 77.62, 75.80, 75.01, 74.92, 74.86, 73.25, 72.42, 71.98, 71.93, 69.80, 69.11, 65.79, 55.07. The data are identical to the literature report [38].
- Methyl (2,3,4,6-tetra-O-benzyl-d-mannopyranosyl)-(1→6) 2,3,4-tri-O-benzoyl-α-d-glucopyranoside (23): Following general procedure B, glycosyl acceptor 3b (25.0 mg, 50.0 μmol) was coupled with EDPAT donor 2d (55.0 mg, 77.5 μmol) to afford 23 (36.0 mg, 35.0 μmol, 71% yield) as a colorless viscous oil. Rf = 0.2 (hexane:EtOAc = 70:30). 1H NMR (400 MHz, CDCl3) δ 8.01–7.97 (m, 2H), 7.93–7.86 (m, 4H), 7.52 (t, J = 7.4 Hz, 1H), 7.47–7.33 (m, 10H), 7.29 (dt, J = 9.8, 5.1 Hz, 16H), 7.20–7.16 (m, 2H), 6.13 (t, J = 9.9 Hz, 1H), 5.57 (t, J = 9.9 Hz, 1H), 5.24 (dd, J = 10.1, 3.6 Hz, 1H), 5.18 (d, J = 3.6 Hz, 1H), 4.93–4.91 (m, 1H), 4.88 (d, J = 10.9 Hz, 1H), 4.75–4.66 (m, 2H), 4.59–4.52 (m, 1H), 4.47 (dd, J = 11.2, 7.8 Hz, 3H), 4.40 (d, J = 12.1 Hz, 1H), 3.99–3.85 (m, 3H), 3.70 (s, 1H), 3.68–3.60 (m, 3H), 3.55 (d, J = 9.0 Hz, 1H), 3.39 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 165.83, 165.80, 165.06, 138.69, 138.59, 138.47, 138.40, 133.34, 133.23, 133.04, 129.92, 129.89, 129.65, 129.10, 129.08, 128.40, 128.37, 128.29, 128.26, 128.21, 127.91, 127.75, 127.66, 127.65, 127.49, 127.43, 127.35, 98.19, 96.91, 79.87, 74.99, 74.75, 73.20, 72.55, 72.10, 72.05, 71.92, 70.51, 69.83, 69.03, 68.07, 66.16, 55.53. The data are identical to the literature report [30].
- l-Menthyl 2,3,4,6-tetra-O-benzyl-d-mannopyranoside (24): Following general procedure B, glycosyl acceptor 3g (8.0 mg, 50.0 μmol) was coupled with EDPAT donor 2d (57.0 mg, 75.0 μmol) to afford 24 (22.0 mg, 32.4 μmol, 64% yield) as a colorless viscous oil. Rf = 0.6 (hexane:EtOAc = 70:30). α-anomer: 1H NMR (400 MHz, CDCl3) δ 7.37–7.27 (m, 18H), 7.15 (dd, J = 7.5, 2.0 Hz, 2H), 5.04 (d, J = 3.7 Hz, 1H), 4.99 (d, J = 11.0 Hz, 1H), 4.87–4.82 (m, 2H), 4.74–4.68 (m, 2H), 4.65 (d, J = 12.1 Hz, 1H), 4.48 (dd, J = 11.4, 5.1 Hz, 2H), 4.03 (t, J = 9.3 Hz, 1H), 3.99 (ddd, J = 10.1, 4.0, 2.2 Hz, 1H), 3.77 (dd, J = 10.5, 4.0 Hz, 1H), 3.67–3.63 (m, 2H), 3.56 (dd, J = 9.8, 3.7 Hz, 1H), 3.37 (dd, J = 10.6, 4.5 Hz, 1H), 2.43 (pd, J = 7.1, 2.6 Hz, 1H), 2.17–2.13 (m, 1H), 1.62 (dp, J = 13.2, 3.3 Hz, 2H), 1.41–1.30 (m, 2H), 1.08–1.02 (m, 1H), 0.96–0.92 (m, 1H), 0.86 (dd, J = 6.7, 3.2 Hz, 7H), 0.72 (d, J = 6.9 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 138.91, 138.38, 138.30, 138.05, 128.34, 128.32, 128.30, 128.23, 127.89, 127.87, 127.63, 127.62, 127.61, 127.48, 127.46, 98.62, 81.97, 80.99, 80.54, 75.47, 75.03, 73.44, 73.18, 70.29, 68.66, 48.75, 43.04, 34.26, 31.73, 24.57, 22.95, 22.28, 21.10, 16.05. β-anomer: 1H NMR (400 MHz, CDCl3) δ 7.36–7.32 (m, 5H), 7.31–7.26 (m, 9H), 7.20 (dd, J = 7.8, 1.8 Hz, 1H), 4.94 (dd, J = 15.9, 11.1 Hz, 2H), 4.80 (dd, J = 16.7, 11.0 Hz, 2H), 4.69 (d, J = 10.8 Hz, 1H), 4.63–4.53 (m, 3H), 4.48 (d, J = 7.9 Hz, 1H), 3.70 (d, J = 3.4 Hz, 2H), 3.65–3.58 (m, 2H), 3.51 (td, J = 10.8, 4.3 Hz, 1H), 3.43–3.39 (m, 2H), 2.35 (pd, J = 6.8, 2.5 Hz, 1H), 2.16–2.12 (m, 1H), 1.69–1.64 (m, 2H), 1.39–1.32 (m, 1H), 1.30–1.27 (m, 1H), 1.04–0.94 (m, 2H), 0.92 (dd, J = 12.2, 6.8 Hz, 6H), 0.89–0.84 (m, 1H), 0.83 (d, J = 6.9 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 138.82, 138.56, 138.38, 138.22, 128.36, 128.30, 128.27, 128.06, 127.76, 127.70, 127.61, 127.57, 127.48, 127.46, 100.75, 84.96, 82.21, 77.96, 77.74, 75.57, 74.97, 74.80, 73.66, 69.33, 48.12, 40.96, 34.45, 31.46, 25.27, 23.21, 22.23, 21.07, 15.95. The data are identical to the literature report [39].
- Propargyl 2-O-acetyl-3,4,6-tri-O-benzyl-α-d-mannopyranosyl-(1→6) 2,3,4-tri-O-benzyl-α-d-mannopyranoside (25): Following general procedure B, glycosyl acceptor 3k (25.0 mg, 51.0 μmol) was coupled with EDPAT donor 2a (54.0 mg, 77.5 μmol) to afford 25 (38.0 mg, 39.5 μmol, 77% yield) as a colorless viscous oil. Rf = 0.3 (hexane:EtOAc = 70:30). 1H NMR (400 MHz, CDCl3) δ 7.42–7.18 (m, 28H), 7.17–7.09 (m, 2H), 5.46 (dd, J = 3.2, 1.9 Hz, 1H), 4.99 (dd, J = 27.0, 1.8 Hz, 2H), 4.88 (dd, J = 21.7, 11.0 Hz, 2H), 4.73 (s, 2H), 4.69–4.62 (m, 2H), 4.59 (d, J = 1.5 Hz, 2H), 4.45 (ddd, J = 13.8, 11.6, 4.9 Hz, 4H), 4.13 (dd, J = 4.0, 2.4 Hz, 2H), 3.98–3.81 (m, 6H), 3.80–3.76 (m, 1H), 3.69 (td, J = 10.9, 10.1, 2.6 Hz, 3H), 3.59 (dd, J = 10.9, 1.9 Hz, 1H), 2.38 (t, J = 2.4 Hz, 1H), 2.15 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 170.49, 138.69, 138.47, 138.35, 138.25, 137.91, 128.56, 128.52, 128.50, 128.46, 128.40, 128.34, 128.05, 127.95, 127.89, 127.85, 127.80, 127.77, 127.73, 127.67, 127.59, 98.10, 96.38, 80.14, 78.93, 75.16, 74.94, 74.56, 74.43, 74.24, 73.46, 72.80, 72.11, 71.64, 71.53, 71.47, 68.78, 68.61, 66.66, 54.30, 29.86, 21.34. The data are identical to the literature report [31].
- Methyl 6-O-(6-O-(2-O-Acetyl-3,4,6-tri-O-benzyl-α-d-mannopyranosyl)-2,3,4-tri-O-benzyl-α-d-mannopyranosyl)-2,3,4-tri-O-benzyl-α-d-mannopyranoside (26): Procedure 1: This was prepared according to the literature procedure [40] using donor 25 (7 μmol), acceptor 3b (1.2 equiv.), and AuCl3 (1 mol%) in acetonitrile at 60 °C in 3 h (76%, α:β = 2:1). Procedure 2: This was prepared according to the literature procedure [41] using donor 27 (20 μmol), acceptor 3b (1.1 equiv.), and NIS (1.5 equiv.)/TMSOTf (10 mol%) in CH2Cl2 at 0 °C in 3 h (52%, α:β = 2:1). 1H NMR (400 MHz, CDCl3) δ 7.47–7.44 (m, 1H), 7.40–7.37 (m, 2H), 7.34 (dt, J = 4.3, 2.4 Hz, 9H), 7.32–7.26 (m, 20H), 7.26–7.15 (m, 39H), 7.09 (dd, J = 6.6, 3.0 Hz, 4H), 5.49 (q, J = 3.2 Hz, 2H), 5.09 (d, J = 1.7 Hz, 1H), 5.00–4.87 (m, 7H), 4.85–4.80 (m, 3H), 4.73–4.52 (m, 19H), 4.50–4.35 (m, 12H), 4.20 (d, J = 16.7 Hz, 2H), 3.98–3.82 (m, 15H), 3.82–3.74 (m, 5H), 3.72–3.59 (m, 9H), 3.53 (ddd, J = 21.6, 10.8, 1.9 Hz, 4H), 3.40 (dd, J = 9.4, 3.0 Hz, 1H), 3.22 (s, 3Hα), 3.18 (s, 1.5Hβ), 2.13 (s, 3Hα), 2.11 (s, 1.5Hβ). 13C NMR (101 MHz, CDCl3) δ 170.22, 138.71, 138.69, 138.64, 138.62, 138.60, 138.46, 138.41, 138.24, 138.21, 138.17, 138.06, 137.84, 137.80, 128.61, 128.36, 128.33, 128.31, 128.26, 128.23, 128.17, 128.16, 128.14, 128.09, 127.92, 127.89, 127.86, 127.79, 127.78, 127.73, 127.71, 127.67, 127.64, 127.62, 127.60, 127.58, 127.53, 127.48, 127.44, 127.41, 127.39, 127.32, 127.27, 102.22, 98.85, 98.74, 98.16, 98.06, 97.81, 82.15, 80.27, 79.25, 77.80, 77.67, 77.22, 74.98, 74.85, 74.67, 74.56, 74.44, 74.27, 74.04, 73.52, 73.30, 72.88, 72.77, 72.62, 72.30, 72.05, 71.89, 71.41, 71.29, 71.23, 70.99, 68.55, 68.34, 66.40, 65.93, 54.67, 54.58, 21.18, 21.16. The data are identical to the literature report [31].
- Phenyl 2-O-acetyl-3,4,6-tri-O-benzyl-α-d-mannopyranosyl-(1→6) 2,3,4-tri-O-benzyl-1-deoxy-1-thio-α-d-mannopyranoside (27): Following general procedure B, glycosyl acceptor 3m (27.0 mg, 50.0 μmol) was coupled with EDPAT donor 2a (54.0 mg, 77.5 μmol) to afford 27 (43.0 mg, 42.5 μmol, 84% yield) as a colorless viscous oil. Rf = 0.3 (hexane:EtOAc = 70:30). 1H NMR (400 MHz, CDCl3) δ 7.32 (qd, J = 24.1, 4.6 Hz, 32H), 7.18–7.09 (m, 3H), 5.57 (s, 1H), 5.45 (s, 1H), 4.94 (d, J = 7.8 Hz, 2H), 4.86 (d, J = 10.8 Hz, 1H), 4.74 (d, J = 13.3 Hz, 1H), 4.71–4.57 (m, 6H), 4.50 (d, J = 10.9 Hz, 1H), 4.47–4.36 (m, 2H), 4.23 (dd, J = 10.7, 6.2 Hz, 1H), 4.01 (s, 1H), 3.97–3.89 (m, 3H), 3.89–3.76 (m, 3H), 3.70 (dd, J = 17.7, 12.6 Hz, 2H), 3.59 (d, J = 10.9 Hz, 1H), 2.14 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 170.45, 138.70, 138.42, 138.32, 138.16, 137.97, 137.94, 134.79, 131.97, 131.01, 129.27, 128.60, 128.57, 128.54, 128.50, 128.41, 128.33, 128.09, 128.00, 127.91, 127.86, 127.82, 127.74, 127.68, 127.61, 127.34, 98.20, 86.11, 85.52, 80.38, 77.95, 76.19, 75.25, 74.71, 74.20, 73.44, 72.24, 72.08, 71.96, 71.62, 71.43, 68.71, 68.56, 66.87, 21.34. The data are identical to the literature report [31].
4. Conclusions
In conclusion, we have developed a sustainable and efficient glycosylation approach by utilizing copper, an earth-abundant catalyst, to activate ethyl 2-diazo-2-phenylacetate (EDPA)-based thioglycoside donors. This method provides an alternative to traditional glycosylation strategies that often require harsh conditions or precious metals. The versatility of this approach allows it to accommodate a broad range of nucleophiles, glycosyl donors, and protecting groups, demonstrating its wide applicability. Additionally, the orthogonality of this method to other thioglycoside and alkyne donors further enhances its utility. The successful application of this methodology to the iterative synthesis of trisaccharides, along with the mechanistic insights gained from investigating the electronic effects of different donor groups, highlights the potential of this approach for advancing glycosylation chemistry.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29225367/s1, Figure S1: List of synthesized sugar anomeric thiols; Figure S2: List of synthesized diazo linkers; Figure S3: List of literature-known glycosyl acceptors; references for the synthesis of glycosyl thiols and diazo linkers; copy of the NMR spectra.
Author Contributions
S.P.S. developed this work along with I.S. S.P.S. elaborated on the scope and performed mechanistic studies. U.C. synthesized donors (3d and 3e) and performed Cu(II)-based experiments. In the end, S.P.S. completed the manuscript writing with I.S.; and funding acquisition was carried out by I.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the NSF CHE-1753187.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
We thank Novruz G. Akhmedov and Steven Foster from the Research Support Services, University of Oklahoma, for NMR and mass spectral analyses, respectively.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ling, J.; Bennett, C.S. Recent developments in stereoselective chemical glycosylation. Asian J. Org. Chem. 2019, 8, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, X.; Yu, B. O-Glycosylation methods in the total synthesis of complex natural glycosides. Nat. Prod. Rep. 2015, 32, 1331–1355. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.M.; Pedersen, C.M. Catalytic Glycosylations in Oligosaccharide Synthesis. Chem. Rev. 2018, 118, 8285–8358. [Google Scholar] [CrossRef] [PubMed]
- Garegg, P.J. Thioglycosides as Glycosyl Donors in Oligosaccharide Synthesis. In Advances in Carbohydrate Chemistry and Biochemistry; Horton, D., Ed.; Academic Press: Cambridge, MA, USA, 1997; Volume 52, pp. 179–205. [Google Scholar]
- Cai, L.; Meng, L.; Zeng, J.; Wan, Q. Sequential activation of thioglycosides enables one-pot glycosylation. Org. Chem. Front. 2021, 8, 3150–3165. [Google Scholar] [CrossRef]
- Konradsson, P.; Udodong, U.E.; Fraser-Reid, B. Iodonium promoted reactions of disarmed thioglycosides. Tetrahedron Lett. 1990, 31, 4313–4316. [Google Scholar] [CrossRef]
- Smith, R.; Müller-Bunz, H.; Zhu, X. Investigation of α-Thioglycoside Donors: Reactivity Studies toward Configuration-Controlled Orthogonal Activation in One-Pot Systems. Org. Lett. 2016, 18, 3578–3581. [Google Scholar] [CrossRef]
- Basu, N.; Kumar Maity, S.; Ghosh, R. Trichloroisocyanuric acid (TCCA)–TMSOTf: An efficient activator system for glycosylation reactions based on thioglycosides. RSC Adv. 2012, 2, 12661–12664. [Google Scholar] [CrossRef]
- Xiong, D.-C.; Zhang, L.-H.; Ye, X.-S. Bromodimethylsulfonium Bromide-Silver Triflate: A New Powerful Promoter System for the Activation of Thioglycosides. Adv. Synth. Catal. 2008, 350, 1696–1700. [Google Scholar] [CrossRef]
- Dasgupta, F.; Garegg, P.J. Alkyl sulfenyl triflate as activator in the thioglycoside-mediated formation of β-glycosidic linkages during oligosaccharide synthesis. Carbohydr. Res. 1988, 177, c13–c17. [Google Scholar] [CrossRef]
- He, H.; Zhu, X. Thioperoxide-Mediated Activation of Thioglycoside Donors. Org. Lett. 2014, 16, 3102–3105. [Google Scholar] [CrossRef]
- Duong, T.; Valenzuela, E.A.; Ragains, J.R. Benzyne-Promoted, 1,2-cis-Selective O-Glycosylation with Benzylchalcogenoglycoside Donors. Org. Lett. 2023, 25, 8526–8529. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Wang, Q.; Yu, B. Ortho-Alkynylphenyl thioglycosides as a new type of glycosylation donors under the catalysis of Au(I) complexes. Tetrahedron Lett. 2012, 53, 5231–5234. [Google Scholar] [CrossRef]
- Adhikari, S.; Baryal, K.N.; Zhu, D.; Li, X.; Zhu, J. Gold-Catalyzed Synthesis of 2-Deoxy Glycosides Using S-But-3-ynyl Thioglycoside Donors. ACS Catal. 2013, 3, 57–60. [Google Scholar] [CrossRef]
- Liu, H.; Liang, Z.-F.; Liu, H.-J.; Liao, J.-X.; Zhong, L.-J.; Tu, Y.-H.; Zhang, Q.-J.; Xiong, B.; Sun, J.-S. Ortho-Methoxycarbonylethynylphenyl Thioglycosides (MCEPTs): Versatile Glycosyl Donors Enabled by Electron-Withdrawing Substituents and Catalyzed by Gold(I) or Cu(II) Complexes. J. Am. Chem. Soc. 2023, 145, 3682–3695. [Google Scholar] [CrossRef]
- Ding, H.; Lv, J.; Zhang, X.-L.; Xu, Y.; Zhang, Y.-H.; Liu, X.-W. Efficient O- and S-glycosylation with ortho-2,2-dimethoxycarbonylcyclopropylbenzyl thioglycoside donors by catalytic strain-release. Chem. Sci. 2024, 15, 3711–3720. [Google Scholar] [CrossRef]
- Xiao, X.; Ding, H.; Peng, L.-C.; Fang, X.-Y.; Qin, Y.-Y.; Mu, Q.-Q.; Liu, X.-W. Sweet Strain Release: Donor–Acceptor Cyclopropane Mediated Glycosylation. CCS Chem. 2023, 5, 2910–2921. [Google Scholar] [CrossRef]
- Du, S.; Ragains, J.R. MPTGs: Thioglycoside Donors for Acid-Catalyzed O-Glycosylation and Latent-Active Synthetic Strategies. Org. Lett. 2019, 21, 980–983. [Google Scholar] [CrossRef]
- Xiao, X.; Zhao, Y.; Shu, P.; Zhao, X.; Liu, Y.; Sun, J.; Zhang, Q.; Zeng, J.; Wan, Q. Remote Activation of Disarmed Thioglycosides in Latent-Active Glycosylation via Interrupted Pummerer Reaction. J. Am. Chem. Soc. 2016, 138, 13402–13407. [Google Scholar] [CrossRef]
- Njeri, D.K.; Pertuit, C.J.; Ragains, J.R. 1,2-cis-Selective glucosylation enabled by halogenated benzyl protecting groups. Org. Biomol. Chem. 2020, 18, 2405–2409. [Google Scholar] [CrossRef]
- Meng, L.; Wu, P.; Fang, J.; Xiao, Y.; Xiao, X.; Tu, G.; Ma, X.; Teng, S.; Zeng, J.; Wan, Q. Glycosylation Enabled by Successive Rhodium(II) and Brønsted Acid Catalysis. J. Am. Chem. Soc. 2019, 141, 11775–11780. [Google Scholar] [CrossRef]
- Doyle, L.M.; O’Sullivan, S.; Di Salvo, C.; McKinney, M.; McArdle, P.; Murphy, P.V. Stereoselective Epimerizations of Glycosyl Thiols. Org. Lett. 2017, 19, 5802–5805. [Google Scholar] [CrossRef] [PubMed]
- Ba, D.; Wen, S.; Tian, Q.; Chen, Y.; Lv, W.; Cheng, G. Rhodium(II)-catalyzed multicomponent assembly of α,α,α-trisubstituted esters via formal insertion of O–C(sp3)–C(sp2) into C–C bonds. Nat. Commun. 2020, 11, 4219. [Google Scholar] [CrossRef] [PubMed]
- Crich, D. Mechanism of a Chemical Glycosylation Reaction. Acc. Chem. Res. 2010, 43, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xia, Y.; Feng, S.; Zhang, Y.; Wang, J. Rh(I)-Catalyzed coupling of 2-bromoethyl aryldiazoacetates with tertiary propargyl alcohols through carbene migratory insertion. Org. Chem. Front. 2016, 3, 1691–1698. [Google Scholar] [CrossRef]
- Kafle, P.; Ghosh, B.; Hunter, A.C.; Mukherjee, R.; Nicholas, K.M.; Sharma, I. Iron-Carbene Initiated O–H Insertion/Aldol Cascade for the Stereoselective Synthesis of Functionalized Tetrahydrofurans. ACS Catal. 2024, 14, 1292–1299. [Google Scholar] [CrossRef]
- Ghosh, B.; Alber, A.; Lander, C.W.; Shao, Y.; Nicholas, K.M.; Sharma, I. Catalytic Activation of Thioglycosides with Copper-Carbenes for Stereoselective 1,2-Cis-Furanosylations. Org. Lett. 2024, 26, 9436–9441. [Google Scholar] [CrossRef]
- Johnston, B.D.; Pinto, B.M. Synthesis of Thio-Linked Disaccharides by 1→2 Intramolecular Thioglycosyl Migration: Oxacarbenium versus Episulfonium Ion Intermediates. J. Org. Chem. 2000, 65, 4607–4617. [Google Scholar] [CrossRef]
- Manmode, S.; Sato, T.; Sasaki, N.; Notsu, I.; Hayase, S.; Nokami, T.; Itoh, T. Rational optimization of the mannoside building block for automated electrochemical assembly of the core trisaccharide of GPI anchor oligosaccharides. Carbohydr. Res. 2017, 450, 44–48. [Google Scholar] [CrossRef]
- Kim, K.S.; Lee, Y.J.; Kim, H.Y.; Kang, S.S.; Kwon, S.Y. Glycosylation with glycosyl benzyl phthalates as a new type of glycosyl donor. Org. Biomol. Chem. 2004, 2, 2408–2410. [Google Scholar] [CrossRef]
- Pratap Singh, S.; Ghosh, B.; Sharma, I. Catalytic Orthogonal Glycosylation Enabled by Enynal-Derived Copper Carbenes. Adv. Synth. Catal. 2024, 366, 1847–1856. [Google Scholar] [CrossRef]
- Nitta, K.; Kuribara, T.; Totani, K. Synthetic trisaccharides reveal discrimination of endo-glycosidic linkages by exo-acting α-1,2-mannosidases in the endoplasmic reticulum. Org. Biomol. Chem. 2021, 19, 4137–4145. [Google Scholar] [CrossRef] [PubMed]
- Mallick, A.; Mallikharjunarao, Y.; Rajasekaran, P.; Roy, R.; Vankar, Y.D. AuIII-Halide/Phenylacetylene-Catalysed Glycosylations Using 1-O-Acetyl-furanoses and Pyranose 1,2-Ortho-esters as Glycosyl Donors. Eur. J. Org. Chem. 2016, 2016, 579–588. [Google Scholar] [CrossRef]
- MacCoss, R.N.; Brennan, P.E.; Ley, S.V. Synthesis of carbohydrate derivatives using solid-phase work-up and scavenging techniques. Org. Biomol. Chem. 2003, 1, 2029–2031. [Google Scholar] [CrossRef] [PubMed]
- Molla, M.R.; Das, P.; Guleria, K.; Subramanian, R.; Kumar, A.; Thakur, R. Cyanomethyl Ether as an Orthogonal Participating Group for Stereoselective Synthesis of 1,2-trans-β-O-Glycosides. J. Org. Chem. 2020, 85, 9955–9968. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, Z.-X.; Wu, X.; Gao, C.-F.; Wang, P.-Y.; Chai, J.-Z.; Liu, M.; Ye, X.-S.; Xiong, D.-C. Photosensitizer-free visible-light-promoted glycosylation enabled by 2-glycosyloxy tropone donors. Nat. Commun. 2023, 14, 8025. [Google Scholar] [CrossRef]
- Mishra, B.; Neralkar, M.; Hotha, S. Stable alkynyl glycosyl carbonates: Catalytic anomeric activation and synthesis of a tridecasaccharide reminiscent of Mycobacterium tuberculosis cell wall lipoarabinomannan. Angew. Chem. Int. Ed. 2016, 128, 7917–7922. [Google Scholar] [CrossRef]
- Toshima, K.; Nagai, H.; Kasumi, K.-I.; Kawahara, K.; Matsumura, S. Stereocontrolled glycosidations using a heterogeneous solid acid, sulfated zirconia, for the direct syntheses of α- and β-manno- and 2-deoxyglucopyranosides. Tetrahedron 2004, 60, 5331–5339. [Google Scholar] [CrossRef]
- Palanivel, A.; Chennaiah, A.; Dubbu, S.; Mallick, A.; Vankar, Y.D. AuCl3-AgOTf promoted O-glycosylation using anomeric sulfoxides as glycosyl donors at room temperature. Carbohydr. Res. 2017, 437, 43–49. [Google Scholar] [CrossRef]
- Hotha, S.; Kashyap, S. Propargyl Glycosides as Stable Glycosyl Donors: Anomeric Activation and Glycoside Syntheses. J. Am. Chem. Soc. 2006, 128, 9620–9621. [Google Scholar] [CrossRef]
- Hevey, R.; Ling, C.-C. Studies on the 6-homologation of β-D-idopyranosides. Carbohydr. Res. 2017, 445, 65–74. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).