Structure Characterization of Four New Sesquiterpene Pyridine Alkaloids from Tripterygium wilfordii Hook. f. and Anti-Inflammatory Activity Evaluations
Abstract
1. Introduction
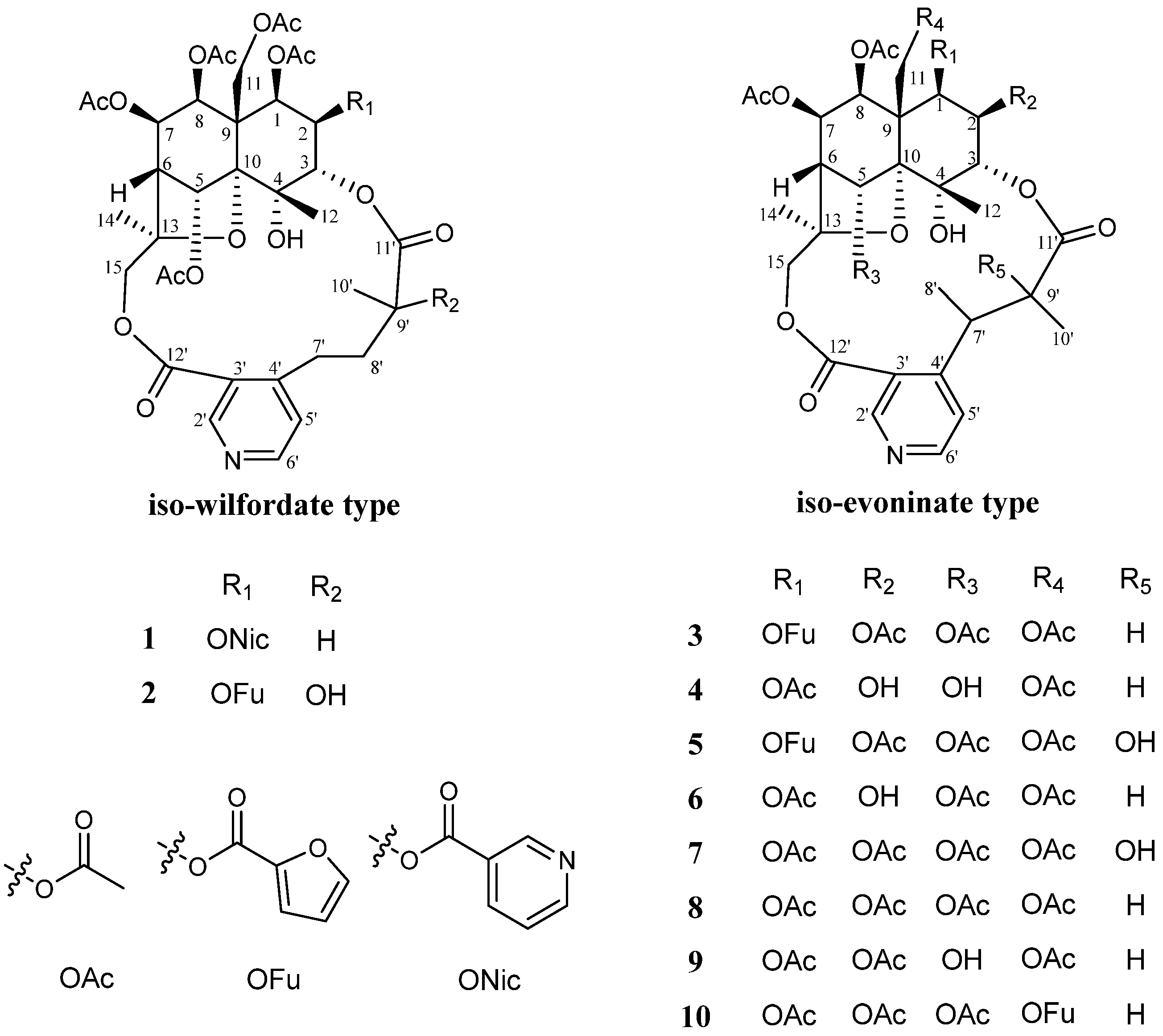
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Characterization of New Compounds
3.5. Bioassays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lv, H.W.; Jiang, L.P.; Zhu, M.D.; Li, Y.X.; Luo, M.; Jiang, P.H.; Tong, S.Q.; Zhang, H.J.; Yan, J.Z. The genus Tripterygium: A phytochemistry and pharmacological review. Fitoterapia 2019, 137, 104190. [Google Scholar] [CrossRef] [PubMed]
- Law, S.K.Y.; Simmons, M.P.; Techen, N.; Khan, I.A.; He, M.F.; Shaw, P.C.; But, P.P.H. Molecular analyses of the Chinese herb Leigongteng (Tripterygium wilfordii Hook.f.). Phytochemistry 2011, 72, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Brinker, A.M.; Ma, J.; Lipsky, P.E.; Raskin, I. Medicinal chemistry and pharmacology of genus Tripterygium (Celastraceae). Phytochemistry 2007, 68, 732–766. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Dai, S.M. A Chinese herb Tripterygium wilfordii Hook F in the treatment of rheumatoid arthritis: Mechanism, efficacy, and safety. Rheumatol. Int. 2011, 31, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.L.; Lipsky, P.E. The Chinese anti-inflammatory and immunosuppressive herbal remedy Tripterygium wilfordii Hook F. Rheum. Dis. Clin. N. Am. 2000, 26, 29–50. [Google Scholar] [CrossRef]
- Song, C.Y.; Xu, Y.G.; Lu, Y.Q. Use of Tripterygium wilfordii Hook F for immune-mediated inflammatory diseases: Progress and future prospects. J. Zhejiang Univ.-Sci. B 2020, 21, 280–290. [Google Scholar] [CrossRef]
- Shan, Y.; Zhao, J.; Wei, K.; Jiang, P.; Xu, L.; Chang, C.; Xu, L.; Shi, Y.; Zheng, Y.; Bian, Y.; et al. A comprehensive review of Tripterygium wilfordii hook. f. in the treatment of rheumatic and autoimmune diseases: Bioactive compounds, mechanisms of action, and future directions. Front. Pharmacol. 2023, 14, 1282610. [Google Scholar] [CrossRef]
- Sun, X.; Shen, B.; Yu, H.; Wu, W.; Sheng, R.; Fang, Y.; Guo, R. Therapeutic potential of demethylzeylasteral, a triterpenoid of the genus Tripterygium wilfordii. Fitoterapia 2022, 163, 105333. [Google Scholar] [CrossRef]
- Chen, Y.W.; Wang, Y.D.; Yan, J.G.; Xu, Y.W.; Liu, Y.Y.; Ma, S.C.; Wu, X.F. Quantification of sesquiterpene pyridine alkaloids from genus Tripterygium by band-selective HSQC NMR. Anal. Chim. Acta 2023, 1274, 341568. [Google Scholar] [CrossRef]
- Kuo, Y.H.; Chen, C.H.; Kuo, L.M.Y.; King, M.L.; Wu, T.S.; Lu, S.T.; Chen, I.S.; McPhail, D.R.; McPhail, A.T.; Lee, K.H. Structure and stereochemistry of Emarginatine-A, a novel cytotoxic sesquiterpene pyridine alkaloid from Maytenus emarginata: X-ray crystal structure of Emarginatine-A monohydrate. Heterocycles 1989, 29, 1465–1468. [Google Scholar] [CrossRef]
- Yan, J.G.; Wu, X.F.; Chen, M.H.; Dai, Z.; Wang, Y.D.; Ma, S.C. Spectral characteristics of sesquiterpene pyridine alkaloids from Tripterygium plants. Zhongguo Zhong Yao Za Zhi 2022, 47, 4292–4304. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.X.; Guan, P.P.; Li, M.L.; Liu, B.; Xu, Y.Y.; Zhang, Z.G.; Han, L.; Huang, X.S. New dihydro-β-agarofuran sesquiterpenoids from Tripterygium wilfordii and their anti-inflammatory activity. Bioorgan. Chem. 2021, 114, 105140. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.S.; Zhu, G.Y.; Li, T.; Jiang, Z.H.; Bai, L.P. Dimacrolide Sesquiterpene Pyridine Alkaloids from the Stems of Tripterygium regelii. Molecules 2016, 21, 1146. [Google Scholar] [CrossRef] [PubMed]
- Ning, R.N.; Zhao, J.; Chen, L.; Mu, H.Y.; Chen, Z.X.; Yang, K.; Xu, X.; Litifu, D.; Zuo, J.P.; He, S.J.; et al. Macrolide sesquiterpene pyridine alkaloids from Celastrus monospermus and evaluation of their immunosuppressive and anti-osteoclastogenesis activities. Bioorgan. Chem. 2024, 145, 107246. [Google Scholar] [CrossRef]
- Kuo, Y.H.; Chen, C.H.; Kuo, L.M.Y.; King, M.L.; Wu, T.S.; Haruna, M.; Lee, K.H. Antitumor Agents, 112. Emarginatine B, a Novel Potent Cytotoxic Sesquiterpene Pyridine Alkaloid from Maytenus emarginata. J. Nat. Prod. 1990, 53, 422–428. [Google Scholar] [CrossRef]
- Kuo, Y.H.; Huang, H.C.; Chiou, W.F.; Shi, L.S.; Wu, T.S.; Wu, Y.C. A Novel NO-Production-Inhibiting Triterpene and Cytotoxicity of Known Alkaloids from Euonymus laxiflorus. J. Nat. Prod. 2003, 66, 554–557. [Google Scholar] [CrossRef]
- Duan, H.; Takaishi, Y.; Bando, M.; Kido, M.; Imakura, Y.; Lee, K. Novel sesquiterpene esters with alkaloid and monoterpene and related compounds from Tripterygium hypoglaucum: A new class of potent anti-HIV agents. Tetrahedron Lett. 1999, 40, 2969–2972. [Google Scholar] [CrossRef]
- Duan, H.Q.; Takaishi, Y.; Imakura, Y.; Jia, Y.F.; Li, D.; Cosentino, L.M.; Lee, K.H. Sesquiterpene Alkaloids from Tripterygium hypoglaucum and Tripterygium wilfordii: A New Class of Potent Anti-HIV Agents. J. Nat. Prod. 2000, 63, 357–361. [Google Scholar] [CrossRef]
- Horiuch, M.; Murakami, C.; Fukamiya, N.; Yu, D.L.; Chen, T.H.; Bastow, K.F.; Zhang, D.C.; Takaishi, Y.; Imakura, Y.; Lee, K.H. Tripfordines A−C, Sesquiterpene Pyridine Alkaloids from Tripterygium wilfordii, and Structure Anti-HIV Activity Relationships of Tripterygium Alkaloids. J. Nat. Prod. 2006, 69, 1271–1274. [Google Scholar] [CrossRef]
- Furukawa, M.; Makino, M.; Uchiyama, T.; Ishimi, K.; Ichinohe, Y.; Fujimoto, Y. Sesquiterpene pyridine alkaloids from Hippocratea excelsa. Phytochemistry 2002, 59, 767–777. [Google Scholar] [CrossRef]
- Núñez, M.J.; Guadaño, A.; Jiménez, I.A.; Ravelo, A.G.; González Coloma, A.; Bazzocchi, I.L. Insecticidal Sesquiterpene Pyridine Alkaloids from Maytenus chiapensis. J. Nat. Prod. 2004, 67, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.Q.; Zhang, Q.Q.; Shi, B.J.; Wei, S.P.; Wang, M.G.; Wu, W.J. Three new insecticidal sesquiterpene pyridine alkaloids from Celastrus angulatus. Nat. Prod. Res. 2009, 23, 470–478. [Google Scholar] [CrossRef]
- Wang, C.; Li, C.-J.; Yang, J.-Z.; Ma, J.; Chen, X.-G.; Hou, Q.; Zhang, D.-M. Anti-inflammatory Sesquiterpene Derivatives from the Leaves of Tripterygium wilfordii. J. Nat. Prod. 2013, 76, 85–90. [Google Scholar] [CrossRef]
- Gao, C.; Huang, X.X.; Bai, M.; Wu, J.; Li, J.Y.; Liu, Q.B.; Li, L.Z.; Song, S.J. Anti-inflammatory sesquiterpene pyridine alkaloids from Tripterygium wilfordii. Fitoterapia 2015, 105, 49–54. [Google Scholar] [CrossRef]
- Duan, H.Q.; Takaishi, Y.; Momota, H.; Ohmoto, Y.; Taki, T.; Jia, Y.F.; Li, D. Immunosuppressive Sesquiterpene Alkaloids from Tripterygium wilfordii. J. Nat. Prod. 2001, 64, 582–587. [Google Scholar] [CrossRef]
- Wang, Y.D.; Yan, J.G.; Zhang, Z.M.; Chen, M.H.; Wu, X.F.; Ma, S.C. Immunosuppressive Sesquiterpene Pyridine Alkaloids from Tripterygium wilfordii Hook. f. Molecules 2022, 27, 7274. [Google Scholar] [CrossRef]
- Duan, H.Q.; Takaishi, Y. Structures of sesquiterpene polyol alkaloids from Tripterygium hypoglaucum. Phytochemistry 1998, 49, 2185–2189. [Google Scholar] [CrossRef]
- Ye, H.Y.; Ignatova, S.; Luo, H.D.; Li, Y.F.; Peng, A.; Chen, L.J.; Sutherland, I. Preparative separation of a terpenoid and alkaloids from Tripterygium wilfordii Hook. f. using high-performance counter-current chromatography: Comparison of various elution and operating strategies. J. Chromatogr. A 2008, 1213, 145–153. [Google Scholar] [CrossRef]
- Duan, H.Q.; Takaishi, Y. Sesquiterpene evoninate alkaloids from Tripterygium hypoglaucum. Phytochemistry 1999, 52, 1735–1738. [Google Scholar] [CrossRef]
- You, C.X.; Yang, K.; Wang, C.F.; Zhang, W.J.; Wang, Y.; Han, J.; Fan, L.; Du, S.S.; Geng, Z.F.; Deng, Z.W. Cytotoxic Compounds Isolated from Murraya tetramera Huang. Molecules 2014, 19, 13225–13234. [Google Scholar] [CrossRef]
- Cai, L.; Qin, X.J.; Xu, Z.H.; Song, Y.Y.; Jiang, H.J.; Wu, Y.; Ruan, H.J.; Chen, J. Comparison of Cytotoxicity Evaluation of Anticancer Drugs between Real-Time Cell Analysis and CCK-8 Method. ACS Omega 2019, 4, 12036–12042. [Google Scholar] [CrossRef] [PubMed]
- Fakhrudin, N.; Waltenberger, B.; Cabaravdic, M.; Atanasov, A.G.; Malainer, C.; Schachner, D.; Heiss, E.H.; Liu, R.; Noha, S.M.; Grzywacz, A.M.; et al. Identification of plumericin as a potent new inhibitor of the NF-κB pathway with anti-inflammatory activity in vitro and in vivo. Br. J. Pharmacol. 2013, 171, 1676–1686. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Huang, J.; Li, X.T.; Lin, R.J.; Wang, X.Y.; Xiao, G.; Zeng, J.N.; Wang, Z.Q. Periplaneta americana extract promotes infectious diabetic ulcers wound healing by downregulation of LINC01133/SLAMF9. Chin. J. Nat. Med. 2024, 22, 608–618. [Google Scholar] [CrossRef] [PubMed]


| Position | δH (J in Hz) a | |||
|---|---|---|---|---|
| 1 | 3 | 4 | 5 | |
| 1 | 5.75 (1H, d, 3.6) | 5.79 (1H, d, 4.2) | 5.46 (1H, d, 3.6) | 5.84 (1H, d, 3.6) |
| 2 | 5.47 (1H, t, 3.0) | 5.28 (1H, dd, 3.6, 2.4) | 4.10 (1H, t, 3.0) | 5.39 (1H, dd, 3.6, 2.4) |
| 3 | 5.06 (1H, d, 3.0) | 4.78 (1H, d, 2.4) | 4.80 (1H, d, 2.4) | 4.75 (1H, d, 2.4) |
| 5 | 6.98 (1H, s) | 7.07 (1H, s) | 5.45 (1H, s) | 7.07 (1H, s) |
| 6 | 2.37 (1H, d, 3.6) | 2.37 (1H, d, 4.2) | 2.44 (1H, d, 3.0) | 2.42 (1H, d, 3.6) |
| 7 | 5.55 (1H, t, 4.2) | 5.54 (1H, dd, 6.0, 4.2) | 5.51 (1H, dd, 6.0, 4.2) | 5.54 (1H, dd, 6.0, 4.2) |
| 8 | 5.42 (1H, d, 6.0) | 5.43 (1H, d, 6.0) | 5.34 (1H, d, 6.0) | 5.41 (1H, d, 6.0) |
| 11 | 5.47 (1H, d, 13.2) 4.37 (1H, d, 13.2) | 5.25 (1H, d, 13.2) 4.59 (1H, d, 13.2) | 5.24 (1H, d, 13.2) 4.64 (1H, d, 13.2) | 5.25 (1H, d, 13.2) 4.59 (1H, d, 13.2) |
| 12 | 1.64 (3H, s) | 1.57 (3H, s) | 1.86 (3H, d, 1.2) | 1.56 (3H, s) |
| 14 | 1.71 (3H, s) | 1.75 (3H, s) | 1.67 (3H, s) | 1.65 (3H, s) |
| 15 | 5.84 (1H, d, 12.0) 3.79 (1H, d, 12.0) | 6.05 (1H, d, 11.4) 3.70 (1H, d, 11.4) | 6.06 (1H, d, 12.0) 3.72 (1H, d, 12.0) | 5.11 (1H, d, 11.4) 4.29 (1H, d, 11.4) |
| 2′ | 9.23 (1H, s) | 9.00 (1H, s) | 9.04 (1H, br, s) | 9.00 (1H, s) |
| 5′ | 7.27 (1H, overlapped) | 7.36 (1H, d, 5.4) | 7.37 (1H, br, s) | 7.83 (1H, d, 5.4) |
| 6′ | 8.69 (1H, d, 5.4) | 8.72 (1H, d, 5.4) | 8.72 (1H, br, s) | 8.69 (1H, d, 5.4) |
| 7′ | 3.90 (1H, m) 2.69 (1H, m) | 4.71 (1H, q, 7.2) | 4.79 (1H, q, 7.2) | 4.25 (1H, q, 7.2) |
| 8′ | 2.36 (1H, m) 1.65 (1H, m) | 1.37(3H, d, 7.2) | 1.34 (3H, d, 7.2) | 1.19 (3H, d, 7.2) |
| 9′ | 2.36 (1H, m) | 2.46 (1H, q, 7.2) | 2.43 (1H, q, 7.2) | |
| 10′ | 1.20 (3H, d, 6.6) | 1.09 (3H, d, 7.2) | 1.03 (3H, d, 7.2) | 1.39 (3H, s) |
| Position | δC a | |||
|---|---|---|---|---|
| 1 | 3 | 4 | 5 | |
| 1 | 73.4 | 73.0 | 75.4 | 72.5 |
| 2 | 70.3 | 69.1 | 69.4 | 68.3 |
| 3 | 75.9 | 75.8 | 77.3 | 77.4 |
| 4 | 69.8 | 70.8 | 72.5 | 70.4 |
| 5 | 73.6 | 73.7 | 74.3 | 74.2 |
| 6 | 51.3 | 50.5 | 51.8 | 50.5 |
| 7 | 69.0 | 68.8 | 69.1 | 68.8 |
| 8 | 70.7 | 71.3 | 71.0 | 71.2 |
| 9 | 52.1 | 52.3 | 51.4 | 52.5 |
| 10 | 93.8 | 94.3 | 93.2 | 93.2 |
| 11 | 60.3 | 59.8 | 60.7 | 59.7 |
| 12 | 23.0 | 22.7 | 23.4 | 22.2 |
| 13 | 84.7 | 84.5 | 84.5 | 83.4 |
| 14 | 17.9 | 18.4 | 18.6 | 18.5 |
| 15 | 70.4 | 70.2 | 71.1 | 69.8 |
| 2′ | 152.0 | 151.0 | 151.2 | 151.5 |
| 3′ | 124.4 | 125.2 | 123.6 | 127.4 |
| 4′ | 155.0 | 156.5 | 156.4 | 151.6 |
| 5′ | 126.4 | 121.5 | 122.0 | 123.4 |
| 6′ | 153.4 | 153.0 | 152.9 | 152.6 |
| 7′ | 31.1 | 33.3 | 33.0 | 41.8 |
| 8′ | 38.1 | 11.3 | 11.2 | 17.2 |
| 9′ | 81.8 | 45.7 | 45.8 | 76.7 |
| 10′ | 22.1 | 10.1 | 9.4 | 23.9 |
| 11′ | 174.6 | 173.6 | 173.7 | 175.0 |
| 12′ | 166.7 | 168.1 | 168.2 | 167.6 |
| Compounds | Cell Viability (%) (n = 3) | NF-κB Inhibitory Rates (%) (n = 3) | IC50 (μM) |
|---|---|---|---|
| 2 | 94.25 ± 5.75 | 33.87 ± 2.06 | |
| 4 | 100.00 ± 2.55 | 56.41 ± 2.17 | 1.64 |
| 6 | 92.38 ± 7.62 | 48.28 ± 2.84 | 9.05 |
| 7 | 95.01 ± 36.23 | 32.12 ± 5.70 | |
| 8 | 93.90 ± 6.10 | 19.11 ± 3.20 | |
| Blank control | 100.00 ± 3.33 | ||
| JSH23 a | 74.56 ± 1.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-J.; Yan, J.-G.; Zhang, Z.-M.; Fang, Q.-F.; Wang, Y.-D.; Ma, S.-C. Structure Characterization of Four New Sesquiterpene Pyridine Alkaloids from Tripterygium wilfordii Hook. f. and Anti-Inflammatory Activity Evaluations. Molecules 2024, 29, 5284. https://doi.org/10.3390/molecules29225284
Wang Y-J, Yan J-G, Zhang Z-M, Fang Q-F, Wang Y-D, Ma S-C. Structure Characterization of Four New Sesquiterpene Pyridine Alkaloids from Tripterygium wilfordii Hook. f. and Anti-Inflammatory Activity Evaluations. Molecules. 2024; 29(22):5284. https://doi.org/10.3390/molecules29225284
Chicago/Turabian StyleWang, Yong-Jian, Jian-Gong Yan, Zhong-Mou Zhang, Qiu-Fang Fang, Ya-Dan Wang, and Shuang-Cheng Ma. 2024. "Structure Characterization of Four New Sesquiterpene Pyridine Alkaloids from Tripterygium wilfordii Hook. f. and Anti-Inflammatory Activity Evaluations" Molecules 29, no. 22: 5284. https://doi.org/10.3390/molecules29225284
APA StyleWang, Y.-J., Yan, J.-G., Zhang, Z.-M., Fang, Q.-F., Wang, Y.-D., & Ma, S.-C. (2024). Structure Characterization of Four New Sesquiterpene Pyridine Alkaloids from Tripterygium wilfordii Hook. f. and Anti-Inflammatory Activity Evaluations. Molecules, 29(22), 5284. https://doi.org/10.3390/molecules29225284






