2.2. Mechanical Properties
In order to assess the stability of SrAl
2O
4 and gain deeper insights into its pressure-induced anisotropic structural characteristics, we conducted elasticity calculations for this compound. In particular, calculations were performed to determine the elastic constants
Cij. For crystals with an orthorhombic structure, the elastic constants must meet certain criteria to ensure mechanical stability [
17].
The obtained effective elastic constants are provided in
Table 2, along with the theoretical data at a pressure of 0 GPa. The obtained data align with the findings reported in Ref. [
16]. In the pressure range of 0 to 50 GPa, it can be observed that the mechanical stability of the orthorhombic phase of SrAl
2O
4 is maintained, as evidenced by meeting the criteria for stability. In the subsequent stage, we employed the Reuss–Voigt–Hill approximation techniques to determine the bulk modulus
B, shear modulus
G, and Young’s modulus
E of SrAl
2O
4 [
18,
19,
20]. In
Table 3, we have included the pressure-dependent values of
B,
G, and
E for SrAl
2O
4. The values of
B,
G, and
E at 0 K and 0 GPa are slightly higher than the theoretical predictions of 163.1 GPa, 101.09 GPa, and 256.28 GPa, respectively [
16], with measured values of approximately 180.52 GPa, 108.31 GPa, and 270.77 GPa. Poisson’s ratio
v can be judged as a criterion of brittleness/ductility. Poisson’s ratio
v of SrAl
2O
4 remains consistently below 0.26 throughout a pressure range of 0–50 GPa, indicating its inherent brittleness. The ratio
B/
G has also been used to judge the brittleness/ductility of a solid [
21]. The
B/
G ratio of SrAl
2O
4 is less than 1.75, indicating that SrAl
2O
4 is a brittle structure.
Table 3 displays the values of Poisson’s ratio (
v) and
B/
G for SrAl
2O
4 under zero pressure conditions. These values, 0.25 and 1.67, respectively, exhibit a remarkable agreement with the results obtained from other simulation methods, as shown in
Table 3, where the corresponding values were 0.2381 and 1.58. The pressure-dependent variations in
v and
B/
G for SrAl
2O
4 are presented in
Table 3, indicating an upward trend with increasing pressure. The findings indicate that SrAl
2O
4 demonstrates a tendency towards brittleness within the pressure intervals of 0–50 GPa.
The anisotropic factors for shear on various crystallographic planes (
A1,
A2, and
A3), the universal anisotropic index (
AU), the percentage of anisotropy in shear and bulk moduli (
AG and
AB), as well as the directional bulk moduli (
Ba,
Bb, and
Bc) were computed and are summarized in
Table 4. It is possible to assess the mechanical anisotropy indexes through the following methods [
22,
23,
24]
The orthorhombic crystal’s elastic anisotropy can be characterized by the bulk moduli
Ba,
Bb, and
Bc along the
a,
b, and
c axes, respectively. These values are interrelated.
where
The anisotropy factor percentage for the orthorhombic crystal, derived from the bulk modulus (
AB) and shear modulus (
AG), can be determined using the equation proposed by Chung and Buessem [
22,
23,
24].
Table 4 presents the calculated values of
A1,
A2, and
A3, indicating a deviation from unity. This suggests that SrAl
2O
4 demonstrates a significant elastic anisotropy, reaching up to 50 GPa. Meanwhile, the
a-axis exhibits the highest directional bulk modulus for
Ba compared to
Bb and
Bc. The observed trend indicates that the compressibility of SrAl
2O
4 is comparatively higher along the
a and
c axes, while it exhibits relatively lower compressibility along the
b axis. Additionally, it can be noted that compression along the
c axis is relatively more favorable. This observation is consistent with our previous findings in
Figure S1, indicating that the lattice parameter
b exhibits a more rapid decrease under pressure than parameters
a and
c. If the value of
AU is equal to zero, it indicates that the material exhibits isotropic properties. Otherwise, a greater departure from zero results in increased anisotropic elasticity. The range of
AB and
AG values spans from zero to one, denoting the presence of elastic anisotropy in terms of compression and shear characteristics, respectively. In
Table 4, we additionally present the pressure-dependent anisotropic indexes. The anisotropy of SrAl
2O
4 is more pronounced within the pressure range of 0–50 GPa, leading to higher
AU values. The
AU of SrAl
2O
4 exhibits a continuous increase until reaching 50 GPa.
The Debye temperature (
θ) has a close correlation with both the specific heat and melting temperature. Acoustic vibrations solely contribute to the crystal vibrations in conditions of low temperatures. Hence, by utilizing the elastic constants as input parameters, it is possible to determine the Debye temperature at low temperatures through established relationships [
25]
The given formula involves the utilization of various constants such as
ħ,
k, and
NA, and variables including
n,
M,
ρ, and
vm. These elements are employed to calculate the average sound velocity in a manner that accounts for Planck’s constant divided by 2π, Boltzmann’s constant, Avogadro’s number, the number of atoms per formula unit, the molecular mass per formula unit, density, and the average sound velocity [
26]
The calculation of longitudinal and transverse elastic wave velocities involves utilizing the following relationship, where
vt represents the longitudinal velocity and
vl denotes the transverse velocity [
27,
28]
The values for the longitudinal, transverse, and average sound velocities and Debye temperature of SrAl
2O
4 under different pressures are presented in
Table 3, alongside the findings reported in ref. [
16]. After conducting a thorough comparison, it can be concluded that our calculated outcomes align well with existing research findings, affirming the accuracy of our calculations. According to the data provided in
Table 3, it can be deduced that an upward trend exists between pressure and the longitudinal, transverse, and average sound velocities, as well as the Debye temperature.
In addition, the hardness
HV, melting point
Tm, and thermal conductivity
k of the material are calculated utilizing the subsequent formulas [
29,
30]
Here,
kB is Boltzmann’s constant,
ρ is the density, and
Ma is the average mass per atom, respectively. At zero pressure, the material exhibits a hardness value of 14.2 GPa, a melting point temperature of 1646.10 K, and a thermal conductivity rate of 1.92 W·m
−1·K
−1. It is evident from the data presented in
Table 4 that under increased pressure,
Hv,
Tm, and
k exhibit noticeable increments.
To provide a comprehensive analysis of the mechanical anisotropy, a study was conducted on the spatial variation in linear compressibility (
β), shear modulus (
G), and Young’s modulus (
E) in SrAl
2O
4 at varying pressure levels [
31].
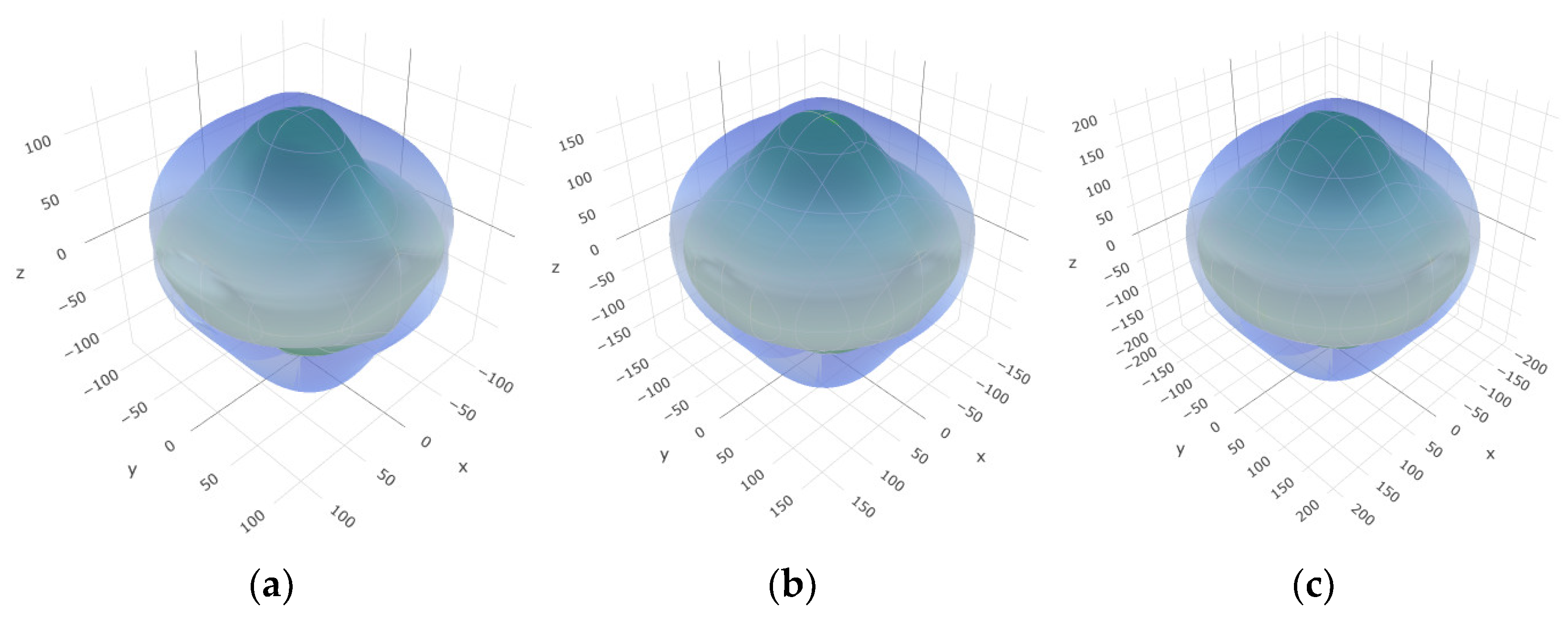
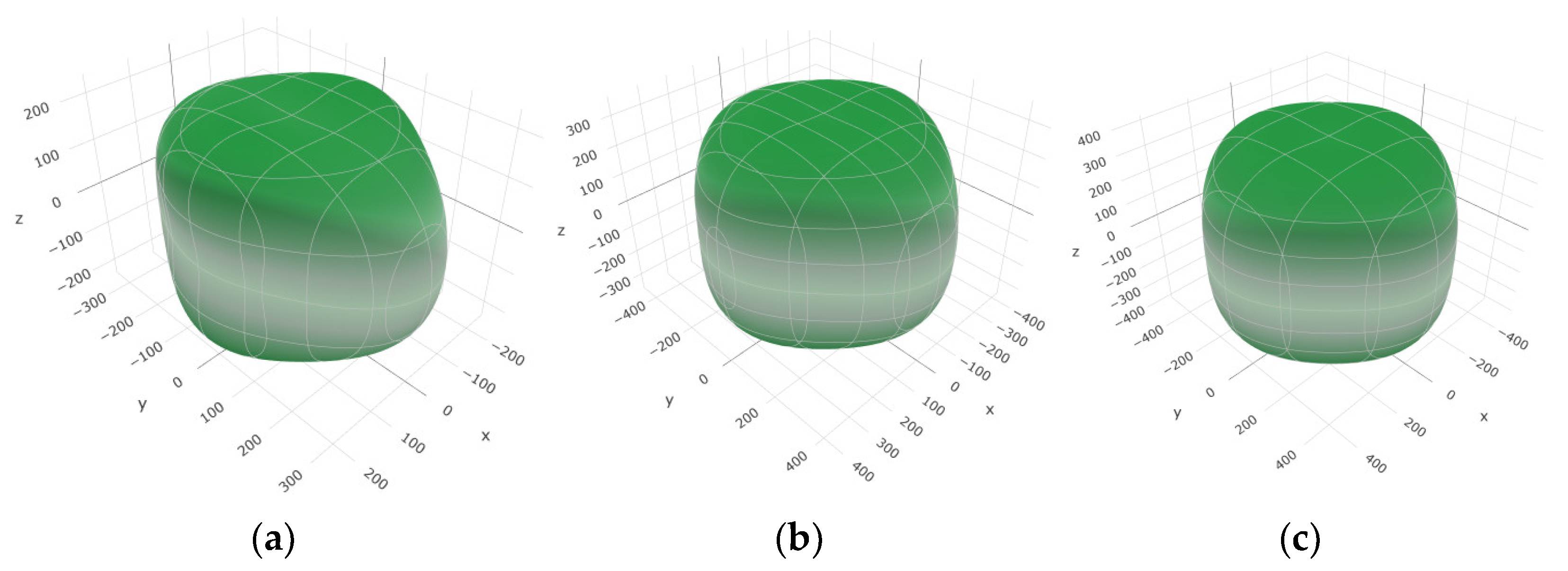
Figure 2,
Figure 3 and
Figure 4 illustrate the spatial correlation between linear compressibility (
β), shear modulus (
G), and Young’s modulus (
E) in SrAl
2O
4. In the event of a structure exhibiting isotropy, the spatial correlation will be evident in a spherical configuration, whereby any deviation from this particular shape can be utilized as an indicator for identifying anisotropy. The non-spherical spatial distribution of linear compressibility (
β), shear modulus (
G), and Young’s modulus (
E) at varying pressures in SrAl
2O
4 suggests a significant level of material anisotropy. For a more extensive view of the directional responsiveness of SrAl
2O
4’s linear compressibility (
β), shear modulus (
G), and Young’s modulus (
E) under varying pressure conditions,
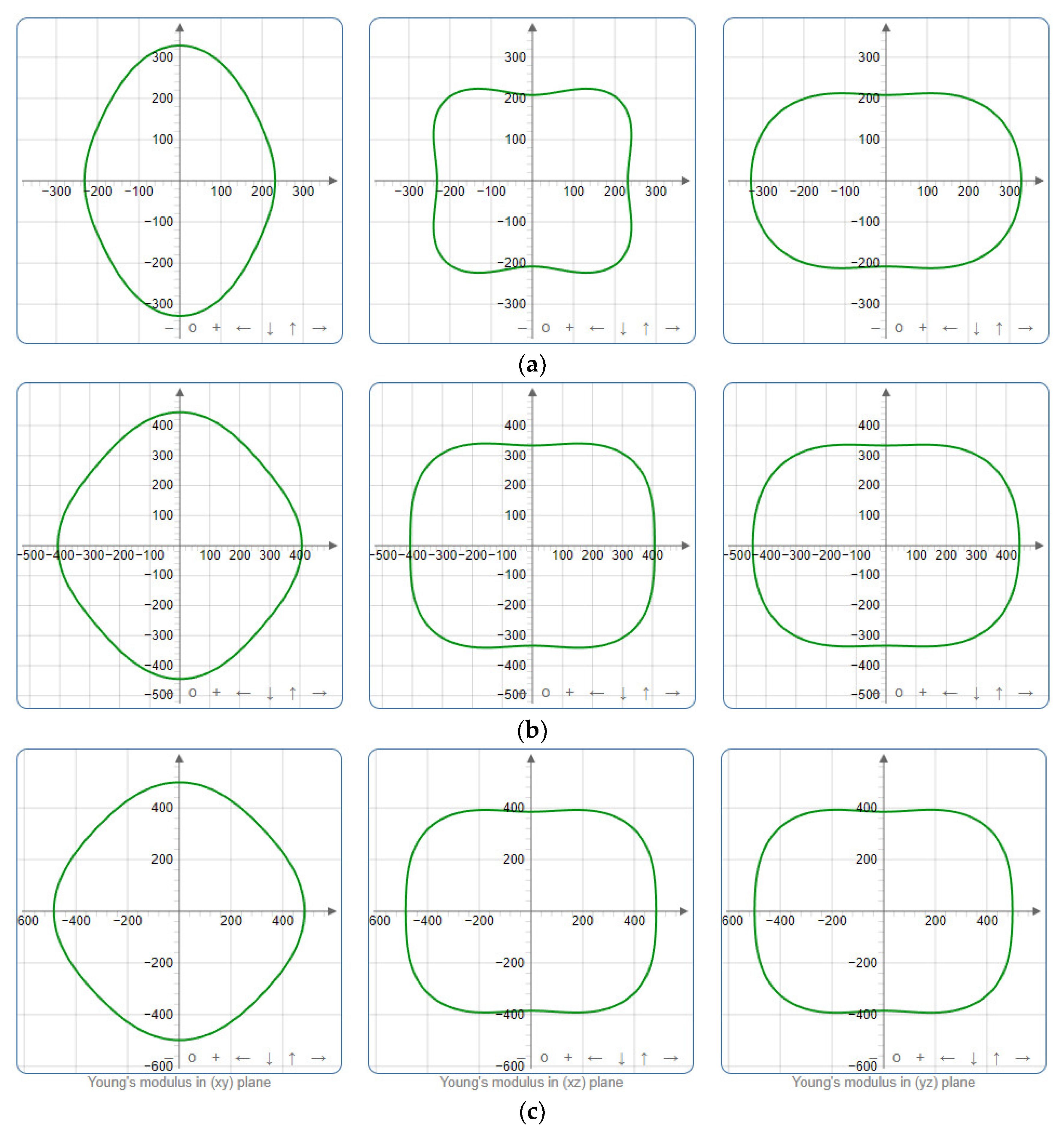
Figure 5,
Figure 6 and
Figure 7 present the planar projections of linear compressibility (
β), shear modulus (
G), and Young’s modulus (
E) in the xy, yz, and xz planes. For materials with properties that are the same in all directions, the curve of projection displays a circular form. For the xy and xz planes, there is a noticeable increase in deviation for the linear compressibility (
β), shear modulus (
G), and Young’s modulus (
E), as shown in
Figure 5,
Figure 6 and
Figure 7. This indicates a significant variation in elastic properties between the xy and xz planes for these solids. It suggests a notable disparity in the elastic characteristics between the xy and xz orientations within these materials. Simultaneously, the elastic anisotropy of SrAl
2O
4 was thoroughly examined under different pressure conditions by employing
βmax/
βmin,
Gmax/
Gmin, and
Emax/
Emin ratios. Higher linear compression anisotropy can be inferred from a greater
βmax/
βmin and the corresponding
Gmax/
Gmin and
Emax/
Emin ratios. The
βmax/
βmin values are 1.50, 1.18, 1.08, 1.03, 1.02, and 1.04 at 0 GPa, 10 GPa, 20 GPa, 30 GPa, 40 GPa, and 50 GPa, respectively; the
Gmax/
Gmin values are 1.59, 1.48, 1.44, 1.43, 1.41, and 1.41 at 0 GPa, 10 GPa, 20 GPa, 30 GPa, 40 GPa, and 50 GPa, respectively; and the
Emax/
Emin values are 1.58, 1.43, 1.37, 1.35, 1.35, and 1.35 at 0 GPa, 10 GPa, 20 GPa, 30 GPa, 40 GPa, and 50 GPa, respectively. According to the data provided, anisotropy decreases with increasing pressure within the range of 0–50 GPa.
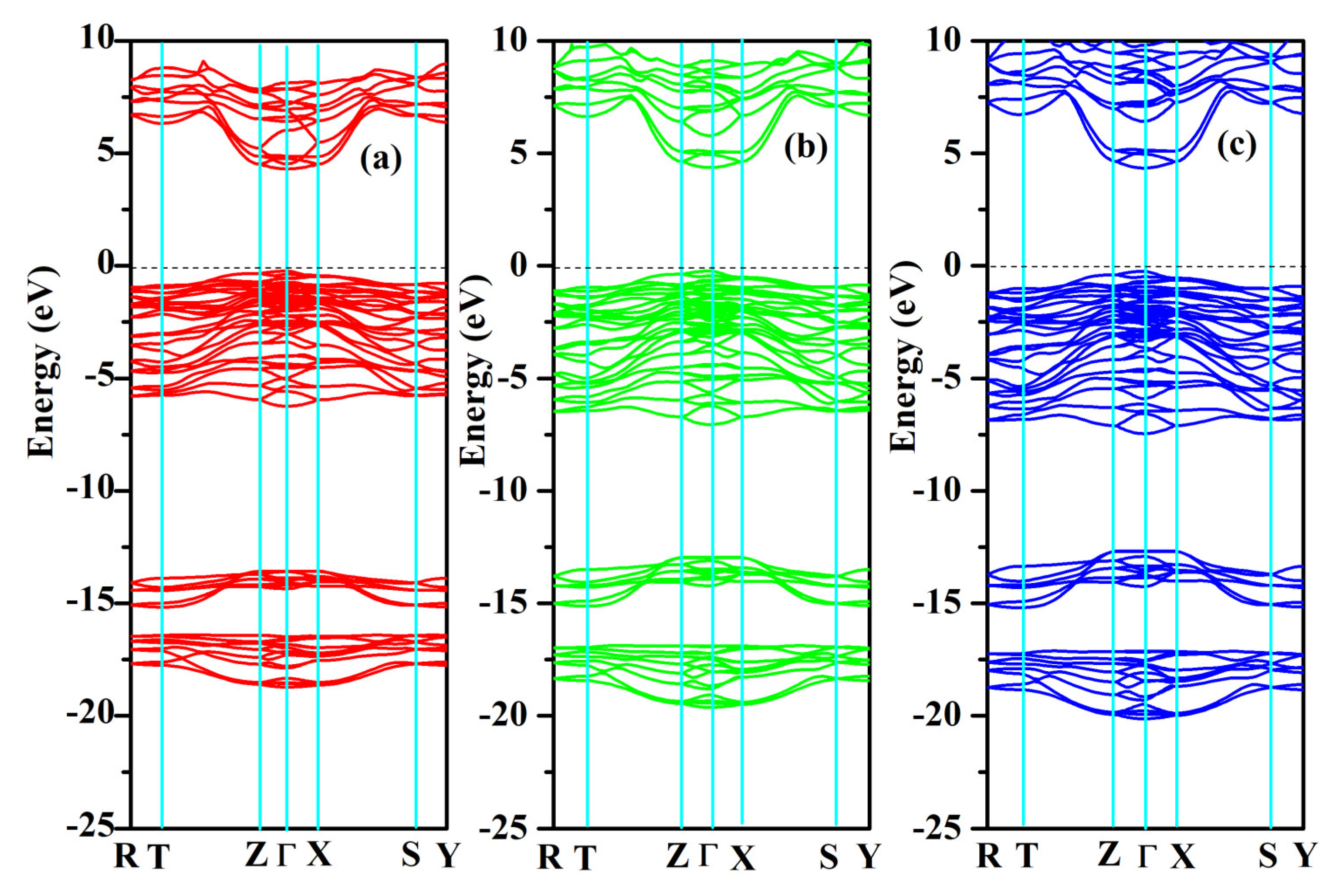
2.4. Phonon Properties
The phonon dispersion relation and the total and partial phonon density of states for SrAl
2O
4 in its orthorhombic structure are depicted in
Figure 10 and
Figure 11, respectively. The dispersion relation curves indicate the absence of any gap between the overall spectra, and no negative phonon frequencies can be observed. Hence, our findings suggest that the investigated SrAl
2O
4 exhibits favorable dynamic stability. According to the results depicted in
Figure 10, the phonon dispersion curves of SrAl
2O
4 exhibit similarities to those observed at 0 GPa, even under increased pressures of 30 GPa and 50 GPa. As the pressure rises, there is a shift in the phonon frequency towards higher energy levels. It is evident that the low energy region depicted in
Figure 11a primarily exhibits vibrations within the frequency range of 0–7.5 THz, predominantly originating from Sr atoms with a minor contribution from Al and O atoms. As the frequency rises, the influence of Sr atom vibrations diminishes while the impact of O and Al atom vibrations amplifies. In the mid-frequency range of 7.5–15.0 THz, the dominant vibrations are attributed to O and Al atoms, while the involvement of Sr atom vibrations diminishes. In the higher frequency range of 15.0–22.5 THz, the dominant vibrations are primarily attributed to the vibrational contributions from O and Al atoms, while the involvement of Sr atom vibrations is minimal in this high energy region. When examining
Figure 11b,c, it becomes apparent that the vibration frequencies of Sr atoms, O atoms, and Al atoms progressively shift towards higher values as the applied pressure increases. In the low frequency range, there is a reduction in the vibrational contribution of Sr atoms as pressure levels increase.
The Pnma space group is associated with the orthorhombic crystal system of SrAl
2O
4. The irreducible representation of crystals with the Pnma structure in the center of the Brillouin zone (
k = 0) is as follows:
The Raman activity is observed in the 13A
g, 8B
1g, 13B
2g, and 8B
3g modes, while the infrared activity is detected in the 12B
1u, 7B
2u, and 12B
3u modes. One B
1u, one B
2u, and one B
3u mode exhibit acoustic characteristics, whereas the 8A
u mode does not display Raman or infrared activity. Consequently, the Au mode can be classified as an acoustic mode.
Table 5,
Table 6 and
Table 7 present the phonon frequencies at the
Γ point and other computed values for SrAl
2O
4 under varying pressure conditions. In contrast, the computed values obtained under zero pressure concur with alternative theoretical findings [
16].
Finally, a graphical representation of the temperature-dependent thermodynamic properties, including specific heat
CV and entropy
S, for SrAl
2O
4 can be observed in
Figure 12a,b. As the temperature increases, there is a rapid increase in heat capacity until it reaches approximately 400 K. Beyond this temperature, the rate of increase becomes smaller. The heat capacity reaches a stable value called the Dulong–Petit limit when temperatures are elevated. In addition, the increase in temperature shown in
Figure 12b is accompanied by an increase in entropy. Regrettably, there is a lack of empirical and theoretical information available for the comparison of thermodynamic properties.