A Novel Benzothiazole-Based Fluorescent AIE Probe for the Detection of Hydrogen Peroxide in Living Cells
Abstract
1. Introduction
2. Results and Discussion
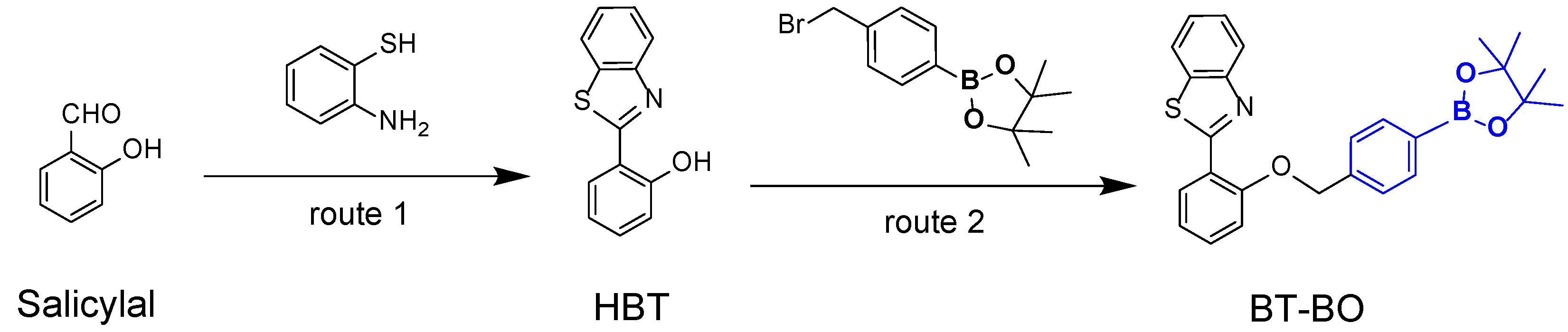
2.1. Synthesis and Structural Analysis of HBT and Probe BT-BO
2.2. AIE Property of HBT
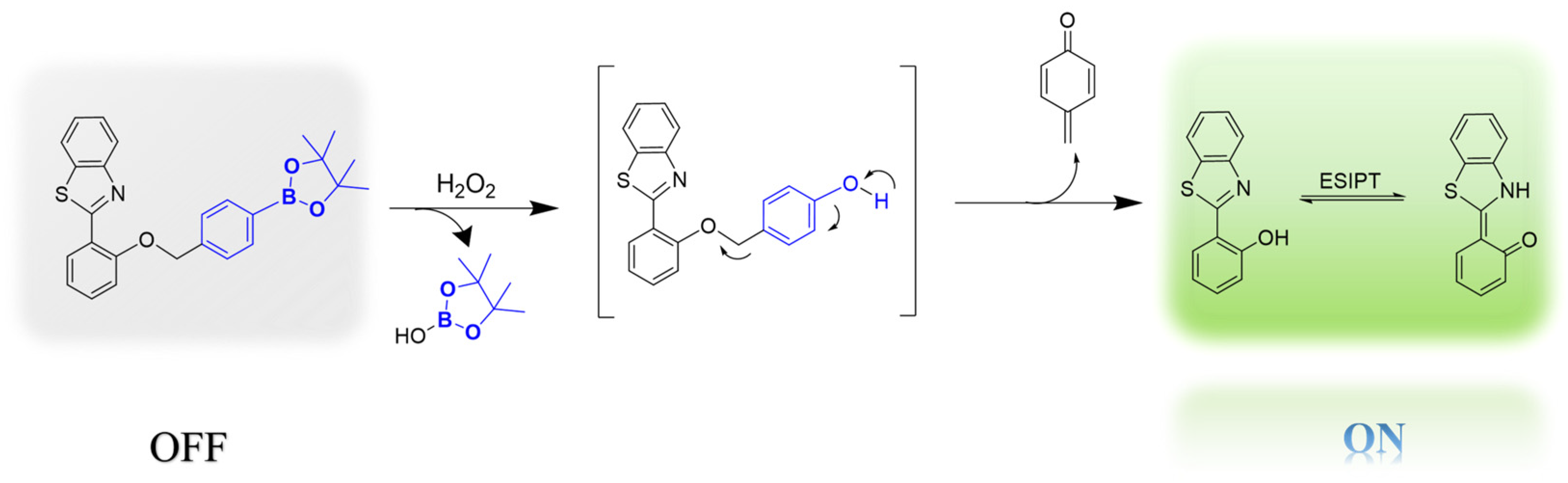
2.3. Spectral Properties of Probe BT-BO to H2O2
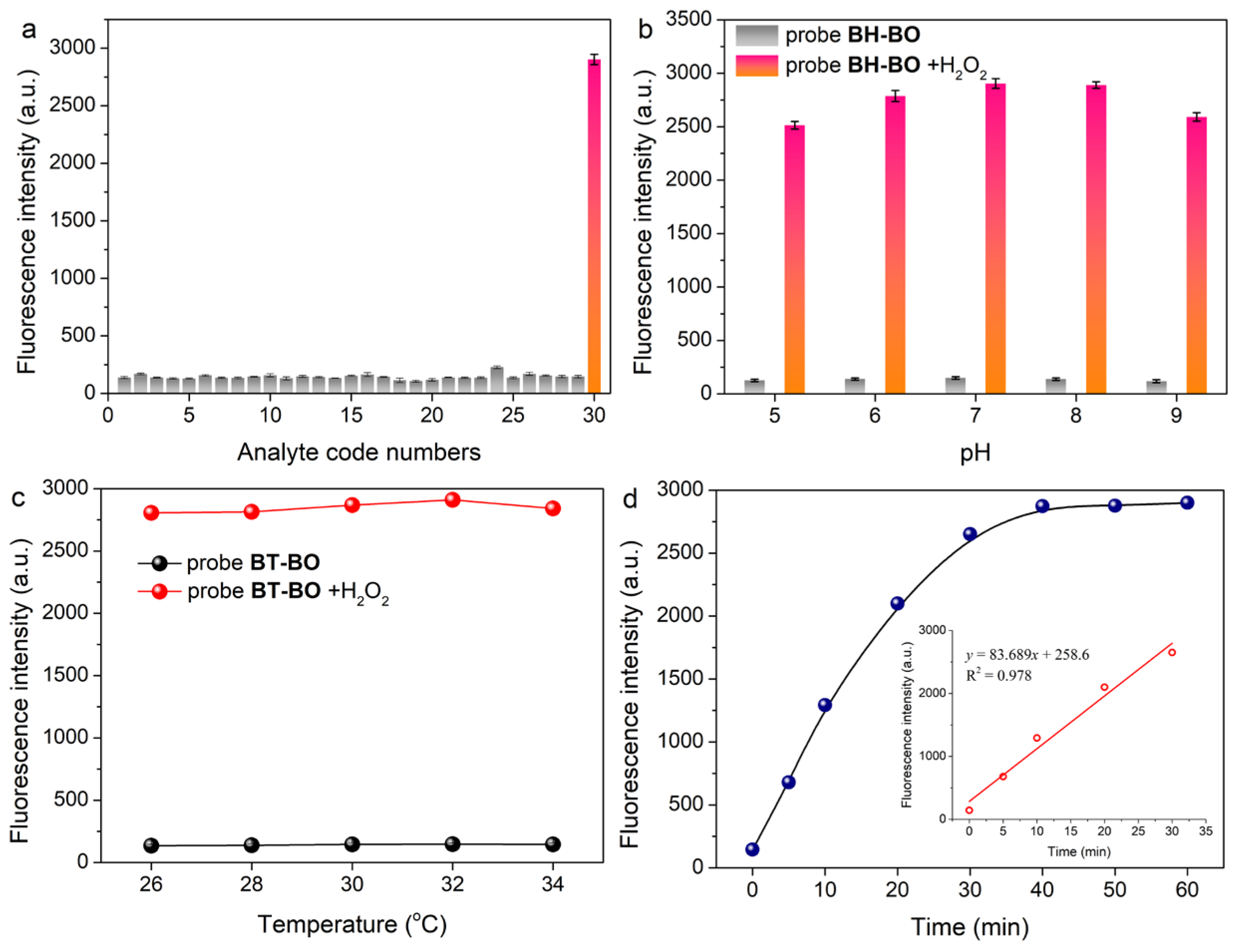
2.4. Selectivity, Effect of pH, Temperature and Response Time of Probe BT-BO
2.5. Cytotoxicity and Cellular Imaging
3. Materials and Methods
3.1. Materials and Measurements
3.2. Synthesis of the Fluorescence Probe BT-BO
3.2.1. Synthesis of 2-(2’-Hydroxyphenyl)Benzothiazole (HBT)
3.2.2. Synthesis of 2-(2-((4-(4,4,5,5-Tetramethyl-1,3,2-Dioxaborolan-2-Yl)Benzyl)Oxy)Phenyl)Benzo[d]Thiazole (Probe BT-BO)
3.3. Liquid Chromatography–Mass Spectrometry
3.4. Cytotoxic Assay
3.5. Cell Image Experiments
3.6. DFT Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shashkovskaya, V.S.; Vetosheva, P.I.; Shokhina, A.G.; Aparin, I.O.; Prikazchikova, T.A.; Mikaelyan, A.S.; Kotelevtsev, Y.V.; Belousov, V.V.; Zatsepin, T.S.; Zatsepin, T.O. Delivery of Lipid Nanoparticles with ROS Probes for Improved Visualization of Hepatocellular Carcinoma. Biomedicines 2023, 11, 1783. [Google Scholar] [CrossRef] [PubMed]
- Murrant, C.L.; Reid, M.B. Detection of Reactive Oxygen and Reactive Nitrogen Species in Skeletal Muscle. Microsc. Res. Techniq. 2001, 55, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Cui, M.; Chu, J.; Chen, J.; Wang, P. A novel AIE fluorescent probe for the detection and imaging of hydrogen peroxide in living tumor cells and in vivo. Bioorg. Chem. 2024, 150, 107592. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Chang, H.; Li, H.; Wang, S. Induction of reactive oxygen species: An emerging approach for cancer therapy. Apoptosis 2017, 22, 1321–1335. [Google Scholar] [CrossRef]
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA damage response in cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef]
- Galle, P.R.; Forner, A.; Llovet, J.M.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.-L.; Schirmacher, P.; Vilgrain, V. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Arnandis, T.; Monteiro, P.; Adams, S.D.; Bridgeman, V.L.; Rajeeve, V.; Gadaleta, E.; Marzec, J.; Chelala, C.; Malanchi, I.; Cutillas, P.R.; et al. Oxidative Stress in Cells with Extra Centrosomes Drives Non-Cell-Autonomous Invasion. Dev. Cell 2018, 47, 409–424. [Google Scholar] [CrossRef]
- Jiang, G.; Li, C.; Liu, X.; Chen, Q.; Li, X.; Gu, X.; Zhang, P.; Lai, Q.; Wang, J. Lipid Droplet-Targetable Fluorescence Guided Photodynamic Therapy of Cancer Cells with an Activatable AIE-Active Fluorescent Probe for Hydrogen Peroxide. Adv. Opt. Mater. 2020, 8, 2001119. [Google Scholar] [CrossRef]
- Jiang, X.; Li, Y.; Fu, D.; You, T.; Wu, S.; Xin, J.; Wen, J.; Huang, Y.; Hu, C. Caveolin-1 ameliorates acetaminophen-aggravated inflammatory damage and lipid deposition in non-alcoholic fatty liver disease via the ROS/TXNIP/NLRP3 pathway. Int. Immunopharmacol. 2023, 114, 109558. [Google Scholar] [CrossRef]
- Masch, W.R.; Kampalath, R.; Parikh, N.; Shampain, K.A.; Aslam, A.; Chernyak, V. Imaging of treatment response during systemic therapy for hepatocellular carcinoma. Abdom. Radiol. 2021, 46, 3625–3633. [Google Scholar] [CrossRef]
- Li, Z.; He, Y.; Li, Y.; Li, J.; Zhao, H.; Song, G.; Miyagishi, M.; Wu, S.; Kasim, V. NeuroD1 promotes tumor cell proliferation and tumorigenesis by directly activating the pentose phosphate pathway in colorectal carcinoma. Oncogene 2021, 40, 6736–6747. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Huang, Y.; Li, Y.; Chen, Y.; Jin, Q.; Ji, J. A heparan sulfate proteoglycan-mimicking AIE fluorescent probe for SARS-CoV-2 detection. New J. Chem. 2024, 48, 4208–4212. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Baumann, S.; Paul, W.; Choi, T.; Lutz, C.P.; Ardavan, A.; Heinrich, A.J. Electron paramagnetic resonance of individual atoms on a surface. Science 2015, 350, 417–420. [Google Scholar] [CrossRef]
- Guo, S.; Deng, Q.; Xiao, J.; Xie, J.; Sun, Z. Evaluation of antioxidant activity and preventing DNA damage effect of pomegranate extracts by chemiluminescence method. J. Agric. Food Chem. 2007, 55, 3134–3140. [Google Scholar] [CrossRef]
- Gill, T.M.; Zheng, X. Comparing Methods for Quantifying Electrochemically Accumulated H2O2. Chem. Mater. 2020, 32, 6285–6294. [Google Scholar] [CrossRef]
- Płotka, J.; Tobiszewski, M.; Sulej, A.M.; Kupska, M.; Górecki, T.; Namieśnik, J. Green chromatography. J. Chromatogr. A 2013, 1307, 1–20. [Google Scholar] [CrossRef]
- Tang, J.; Li, F.; Liu, C.; Shu, J.; Yue, J.; Xu, B.; Liu, X.; Zhang, K.; Jiang, W. Attractive benzothiazole-based fluorescence probe for the highly efficient detection of hydrogen peroxide. Anal. Chim. Acta 2022, 1214, 339939. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chang, Y.T. Fluorescent probe strategy for live cell distinction. Chem. Soc. Rev. 2022, 51, 1573–1591. [Google Scholar] [CrossRef]
- Terai, T.; Nagano, T. Fluorescent probes for bioimaging applications. Curr. Opin. Chem. Biol. 2008, 12, 515–521. [Google Scholar] [CrossRef]
- Tian, M.; Ma, Y.; Lin, W. Fluorescent probes for the visualization of cell viability. Acc. Chem. Res. 2019, 52, 2147–2157. [Google Scholar] [CrossRef] [PubMed]
- Marvin, J.S.; Scholl, B.; Wilson, D.E.; Podgorski, K.; Kazemipour, A.; Müller, J.A.; Schoch, S.; Quiroz, F.J.U.; Rebola, N.; Bao, H.; et al. Stability, affinity, and chromatic variants of the glutamate sensor iGluSnFR. Nat. Methods 2018, 15, 936–939. [Google Scholar] [CrossRef]
- Willett, R.M.; Marcia, R.F.; Nichols, J.M. Compressed sensing for practical optical imaging systems: A tutorial. Opt. Eng. 2011, 50, 072601. [Google Scholar]
- Liao, Y.X.; Li, K.; Wu, M.Y.; Wu, T.; Yu, X.Q. A selenium-contained aggregation-induced “turn on” fluorescent probe for hydrogen peroxide. Org. Biomol. Chem. 2014, 12, 3004–3008. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Huang, S.; Feng, B.; Lou, T.; Chu, F.; Zheng, F.; Zhu, Y.; Chen, F.; Zeng, W. An ESIPT-based AIE fluorescent probe to visualize mitochondrial hydrogen peroxide and its application in living cells and rheumatoid arthritis. Org. Biomol. Chem. 2023, 21, 5063–5071. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Nie, J.; Niu, J.; Meng, F.; Lin, W. Ratiometric fluorescent probe with AIE property for monitoring endogenous hydrogen peroxide in macrophages and cancer cells. Sci. Rep. 2017, 7, 7293. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Topics in Fluorescence Spectroscopy Nonlinear and Two Photon Induced Fluorescence; Springer: Berlin/Heidelberg, Germany, 2002; pp. 103–121. [Google Scholar]
- Lou, J.; Xie, Z.; Lam, J.W.Y.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; Zhu, D.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 18, 1740–1741. [Google Scholar]
- Tang, Y.X.; Cao, Y.; Shi, W.J.; Li, J.C.; Lu, W.L.; Fan, T.; Zheng, L.; Yan, J.; Han, D.; Niu, L. Construction of cationic meso-thiazolium-BODIPY AIE fluorescent probes for viscosity imaging in dual organelles. Chem. Commun. 2024, 60, 8864–8867. [Google Scholar] [CrossRef] [PubMed]
- Sagara, Y.; Yamane, S.; Mitani, M.; Weder, C.; Kato, T. Mechanoresponsive Luminescent Molecular Assemblies: An Emerging Class of Materials. Adv. Mater. 2016, 28, 1073–1095. [Google Scholar] [CrossRef]
- Ding, D.; Li, K.; Tang, B.Z. Bioprobes Based on AIE Fluorogens. Acc. Chem. Res. 2013, 46, 2441–2453. [Google Scholar] [CrossRef]
- Jiang, H.; Lin, Q.; Yu, Z.; Wang, C.; Zhang, R. Nanotechnologies for Reactive Oxygen Species “Turn-On” Detection. Front. Bioeng. Biotechnol. 2021, 9, 780032. [Google Scholar] [CrossRef] [PubMed]
- Sedgwick, A.C.; Wu, L.; Han, H.H.; Bull, S.D.; He, X.P.; James, T.D.; Sessler, J.L.; Tang, B.Z.; Tian, H.; Yoon, J. Excited-state intramolecular proton-transfer (ESIPT) based fluorescence sensors and imaging agents. Chem. Soc. Rev. 2018, 47, 8842. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Huang, H.; Jing, F.; Wang, Y.; Chen, S.; Wang, L.; Li, Y.; Hou, S. A fluorescent probe based on the ESIPT (excited state intramolecular proton transfer) mechanism for rapid detection of endogenous and exogenous H2O2 (hydrogen peroxide) in cells. Spectrochim. Acta A 2024, 304, 123394. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Xu, J.; Ma, Q.; Mao, G.; Zhang, J.; Li, L.; Liu, S. A new lysosome-targeted fluorescent probe for hydrogen peroxide based on a benzothiazole derivative. Methods 2023, 215, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; He, X.; Wu, M.; Huang, Y.; Zhao, L.; Xu, L.; Ma, P.; Sun, Y.; Song, D.; Wang, X. A novel colorimetric and near-infrared fluorescence probe for detecting and imaging exogenous and endogenous hydrogen peroxide in living cells. Talanta 2020, 217, 121000. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ji, L.; Yu, Y. Rational design of a selective and sensitive “turn-on” fluorescent probe for monitoring and imaging hydrogen peroxide in living cells. RSC Adv. 2021, 11, 35093–35098. [Google Scholar] [CrossRef]
- Ren, M.; Deng, B.; Wang, J.Y.; Kong, X.; Liu, Z.R.; Zhou, K.; He, L.; Lin, W. A fast responsive two-photon fluorescent probe for imaging H2O2 in lysosomes with a large turn-on fluorescence signal. Biosens. Bioelectron. 2016, 79, 237–243. [Google Scholar] [CrossRef]
- Li, X.; Yu, W.; Zhao, H.; Fan, Z.; Xiao, M.; Xi, R.; Xu, Y.; Meng, M. Fluorogenic Biosensors Constructed via Aggregation-induced Emission Based on Enzyme-catalyzed Coupling Reactions for Detection of Hydrogen Peroxide. Anal. Sci. 2021, 37, 1275–1279. [Google Scholar] [CrossRef]
- Song, D.; Lim, J.M.; Cho, S.; Park, S.J.; Cho, J.; Kang, D.; Rhee, S.G.; You, Y.; Nam, W. A fluorescence turn-on H2O2 probe exhibits lysosome-localized fluorescence signals. Chem. Commun. 2012, 48, 5449–5451. [Google Scholar] [CrossRef]
- Ma, T.; Zhang, Y.; Fu, K.; Li, Z.; Yuan, C.; Ma, W. Design, synthesis and properties of hydrogen peroxide fluorescent probe based on benzothiazole. Bioorg. Chem. 2022, 123, 105798. [Google Scholar] [CrossRef]
- Wu, Q.; Li, Y.; Li, Y.; Wang, D.; Tang, B.Z. Hydrogen peroxide-responsive AIE probe for imaging-guided organelle targeting and photodynamic cancer cell ablation. Mater. Chem. Front. 2021, 5, 3489–3496. [Google Scholar] [CrossRef]
- Abo, M.; Minakami, R.; Miyano, K.; Kamiya, M.; Nagano, T.; Urano, Y.; Sumimoto, H. Visualization of Phagosomal Hydrogen Peroxide Production by a Novel Fluorescent Probe That Is Localized via SNAP-tag Labeling. Anal. Chem. 2014, 86, 5983–5990. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Song, X.; Kong, X.; Wang, C.; Tang, Y.; Liu, Y.; Lin, W. Simultaneous Near-Infrared and Two-Photon In Vivo Imaging of H2O2 Using a Ratiometric Fluorescent Probe based on the Unique Oxidative Rearrangement of Oxonium. Adv. Mater. 2016, 28, 8755–8759. [Google Scholar] [CrossRef] [PubMed]
- Masanta, G.; Heo, C.H.; Lim, C.S.; Bae, S.K.; Cho, B.R.; Kim, H.M. A mitochondria-localized two-photon fluorescent probe for ratiometric imaging of hydrogen peroxide in live tissue. Chem. Commun. 2012, 48, 3518–3520. [Google Scholar] [CrossRef]
- He, Y.; Miao, L.; Yu, L.; Chen, Q.; Qiao, Y.; Zhang, J.F.; Zhou, Y. A near-infrared fluorescent probe for detection of exogenous and endogenous hydrogen peroxide in vivo. Dyes Pigments 2019, 168, 160–165. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, J.; Tao, B. Cloning of cyp57A1 gene from Fusarium verticillioides for degradation of herbicide fomesafen. Environ. Technol. Innov. 2024, 36, 103822. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, D.; Yang, Y.; Tong, L.; Zhang, L.; Yang, F.; Tao, J.; Zhao, M. A Novel Benzothiazole-Based Fluorescent AIE Probe for the Detection of Hydrogen Peroxide in Living Cells. Molecules 2024, 29, 5181. https://doi.org/10.3390/molecules29215181
Shi D, Yang Y, Tong L, Zhang L, Yang F, Tao J, Zhao M. A Novel Benzothiazole-Based Fluorescent AIE Probe for the Detection of Hydrogen Peroxide in Living Cells. Molecules. 2024; 29(21):5181. https://doi.org/10.3390/molecules29215181
Chicago/Turabian StyleShi, Dezhi, Yulong Yang, Luan Tong, Likang Zhang, Fengqing Yang, Jiali Tao, and Mingxia Zhao. 2024. "A Novel Benzothiazole-Based Fluorescent AIE Probe for the Detection of Hydrogen Peroxide in Living Cells" Molecules 29, no. 21: 5181. https://doi.org/10.3390/molecules29215181
APA StyleShi, D., Yang, Y., Tong, L., Zhang, L., Yang, F., Tao, J., & Zhao, M. (2024). A Novel Benzothiazole-Based Fluorescent AIE Probe for the Detection of Hydrogen Peroxide in Living Cells. Molecules, 29(21), 5181. https://doi.org/10.3390/molecules29215181




