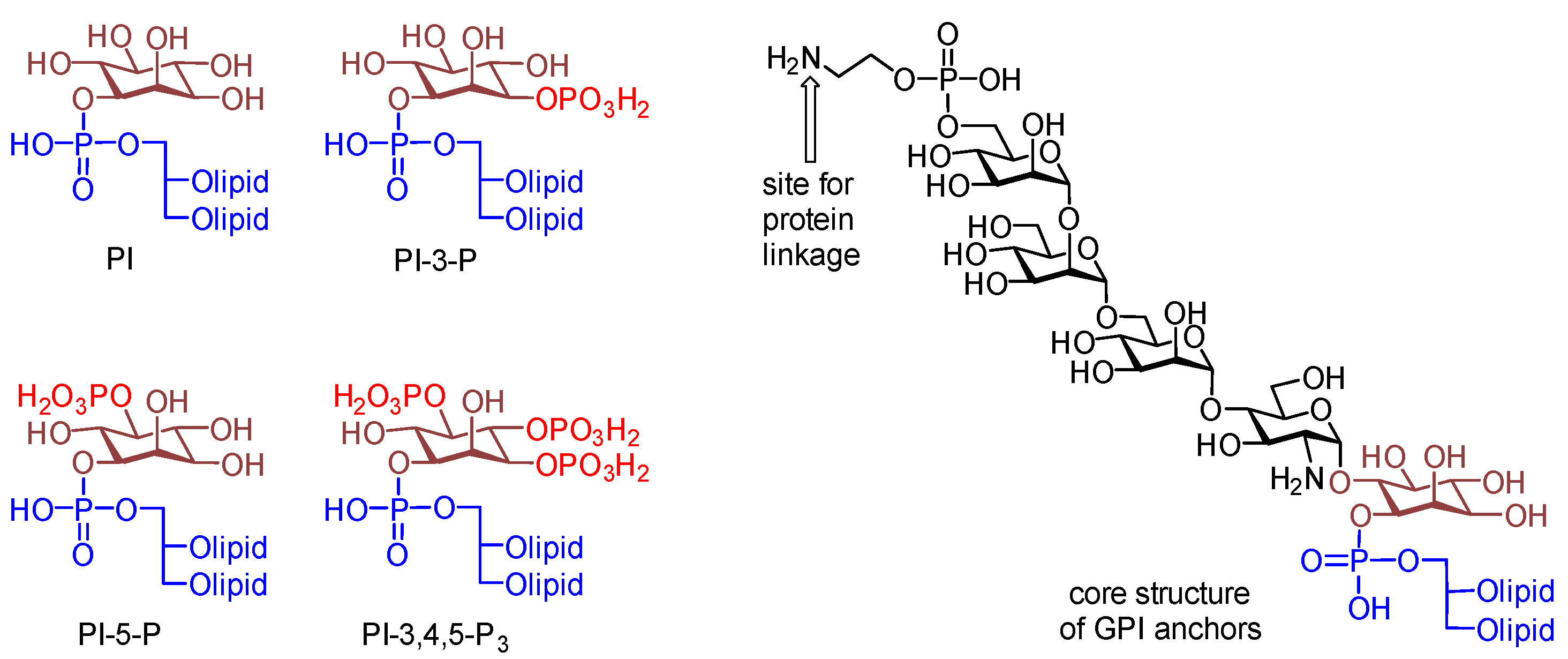
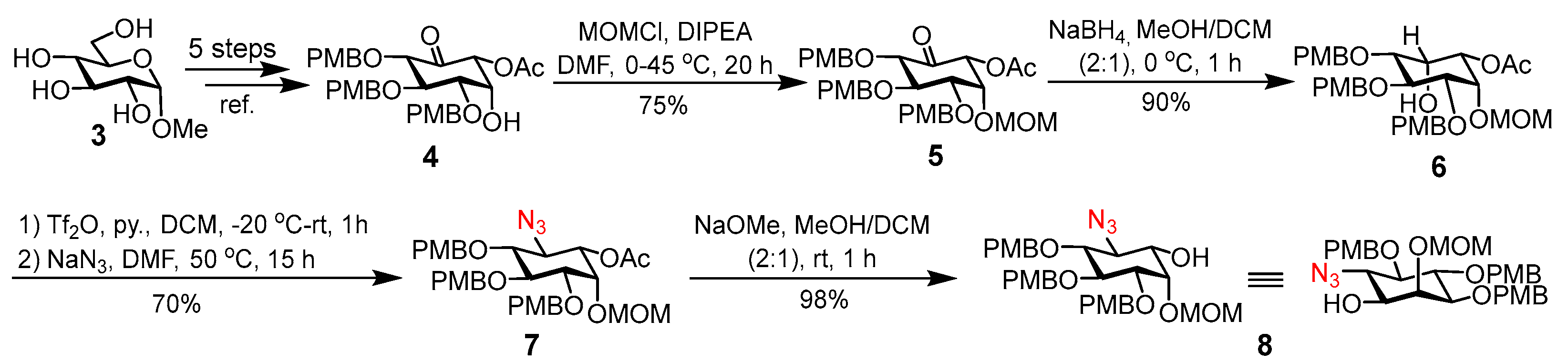
3. Materials and Methods
General methods: Commercial chemicals and materials were used without further purification unless specified otherwise. Flash chromatography used silica gel 60 (230–400 mesh). Thin-layer chromatography (TLC) was performed on silica gel 60G F254 25 glass plates detected with a UV lamp, with charring using 10% (v/v) H2SO4 in ethanol, and staining using p-anisaldehyde. 1H, 13C, COSY, and HSQC NMR spectra were obtained with Bruker (Billerica, MA, USA) 400 and 600 MHz NMR spectrometers. Coupling constants (J) were reported in hertz (Hz). Chemical shifts (δ) were reported in ppm referenced to CDCl3 (1H NMR: δ 7.26 ppm; 13C NMR: δ 77.00 ppm) or CD3OD (1H NMR: δ 3.31 ppm; 13C NMR: δ 49.0 ppm). The chemical shifts of 31P NMR signals were calculated using a lock signal in reference to 85% phosphoric acid in water. Abbreviations that describe peak splitting patterns are as follows: s, singlet; d, doublet; t, triplet; dd, double doublet; m, multiplet; br, broad. High-resolution electrospray ionization mass spectra (HR ESI MS) were obtained with an Agilent (Santa Clara, CA, USA) 6230 time-of-flight (TOF) machine. Aluminum heating blocks were used to heat the reaction mixtures.
(2R,3R,4S,5R,6S)-2-Acetoxy-4,5,6-tris[(4-methoxybenzyl)oxy]-3-methoxymethoxy-cyclohexan-1-one (5). To a solution of
4 [
16] (100 mg, 0.172 mmol) in anhydrous DMF (0.6 mL), DIPEA (207 µL, 1.19 mmol) was added at 0 °C, which was followed by the addition of MOMCl (65 µL, 0.861 mmol). The reaction mixture was heated to 45 °C and stirred at this temperature for 20 h. The reaction mixture was diluted with water and extracted with DCM (10 mL × 3). The organic layers were combined, dried with Na
2SO
4, filtered, and condensed under reduced pressure. The crude product was purified by silica gel column chromatography to afford
5 (81 mg, 75%) as a colorless syrup. R
f = 0.40 (40% EtOAc in hexane);
1H NMR (600 MHz, CDCl
3): δ 7.32 (d,
J = 8.6 Hz, 2H), 7.27 (d,
J = 9.3 Hz, 2H), 7.24 (d,
J = 8.6 Hz, 2H), 6.89–6.83 (m, 6H), 5.15 (d,
J = 2.4 Hz, 1H), 4.85 (d,
J = 10.9 Hz, 1H), 4.80–4.77 (m, 2H), 4.73 (d,
J = 10.3 Hz, 1H), 4.72–4.68 (m, 2H), 4.64 (d,
J = 11.2 Hz, 1H), 4.48 (d,
J = 10.9 Hz, 1H), 4.34 (t,
J = 2.3 Hz, 1H), 4.08–4.04 (m, 2H), 3.82–3.79 (m, 10H), 3.39 (s, 3H), 2.21 (s, 3H);
13C NMR (151 MHz, CDCl
3): δ 197.8, 169.8, 159.4, 159.4, 159.2, 130.6, 129.9, 129.7, 129.6, 129.5, 113.9, 113.8, 113.7, 96.8, 83.5, 81.9, 79.0, 75.7, 75.2, 73.4, 72.8, 72.6, 55.7, 55.3, 55.24, 55.23, 20.6; HR-ESI-TOF MS
m/z: Calcd for C
34H
40NaO
11 [M + Na]
+ 647.2468; Found 647.2464.
3-O-Acetyl-1,5,6-tri-O-(4-methoxybenzyl)-2-O-methoxymethyl-epi-inositol (6). A mixture of 5 (310 mg, 0.496 mmol) and NaBH4 (28 mg, 0.744 mmol) in MeOH and DCM (2:1, 6 mL) was stirred at 0 °C for 1 h, and then saturated aqueous NH4Cl solution was added. The reaction mixture was diluted with DCM, dried over Na2SO4, filtered, and then concentrated in vacuo. The residue was purified by silica gel column chromatography (EtOAc/hexane, 2:1) to provide 6 (280 mg, 90%) as a white solid. Rf = 0.30 (50% EtOAc in hexane); 1H NMR (600 MHz, CDCl3): δ 7.34–7.24 (m, 7H), 6.90–6.85 (m, 6H), 4.88–4.83 (m, 2H), 4.79 (d, J = 10.2 Hz, 1H), 4.74 (d, J = 6.9 Hz, 1H), 4.71 (d, J = 11.4 Hz, 1H), 4.67–4.62 (m, 3H), 4.61 (t, J = 2.9 Hz, 1H), 4.33 (d, J = 1.8 Hz, 1H), 4.21 (d, J = 9.4 Hz, 1H), 4.11 (t, J = 9.8 Hz, 1H), 3.83 (s, 3H), 3.82 (s, 3H), 3.82 (s, 3H), 3.46 (s, 3H), 3.42 (dd, J = 9.8, 2.7 Hz, 1H), 3.38 (dd, J = 9.8, 3.3 Hz, 1H), 2.18 (s, 3H); 13C NMR (151 MHz, CDCl3): δ 170.2, 159.3, 159.2, 159.15, 131.1, 130.1, 130.0, 129.7, 129.5, 129.4, 113.8, 113.79, 113.7, 97.8, 79.9, 79.5, 78.4, 76.1, 75.8, 72.9, 71.9, 70.2, 68.9, 56.0, 55.3, 55.2, 21.0; HR-ESI-TOF MS m/z: Calcd for C34H46NO11 [M + NH4]+ 644.3065; Found 644.3063.
1-O-Acetyl-6-azido-6-deoxy-3,4,5-tri-O-(p-methoxybenzyl)-2-O-methoxymethyl-myo-inositol (7). To a solution of 6 (330 mg, 0.526 mmol) and pyridine (85 µL, 1.05 mmol) in DCM (5 mL), Tf2O (104 µL, 0.631 mmol) was added at −20 °C. The solution was allowed to warm at room temperature (rt) and was stirred for 1 h. After the disappearance of the starting material as shown by TLC, the reaction was quenched with saturated aqueous NaHCO3. The mixture was extracted with DCM, and the organic layers were combined, washed with brine, and dried over Na2SO4. The solvent was evaporated to provide crude triflate, which was dissolved in DMF (2 mL) and then treated with NaN3 (135 mg, 2.08 mmol, 4 equiv) at 40 °C overnight. The reaction mixture was diluted with ethyl acetate and washed with water and brine. The organic layer was dried over Na2SO4, filtered, and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography to afford 7 as a colorless syrup (240 mg, 70%). Rf = 0.50 (40% EtOAc in Hexane); 1H NMR (600 MHz, CDCl3): δ 7.30–7.21 (m, 6H), 6.89–6.83 (m, 6H), 4.86–4.77 (m, 3H), 4.76–4.71 (m, 2H), 4.66 (d, J = 6.8 Hz, 1H), 4.62 (d, J = 11.0 Hz, 1H), 4.56 (dd, J = 10.9, 2.4 Hz, 1H), 4.54 (d, J = 11.1 Hz, 1H), 4.23 (t, J = 2.2 Hz, 1H), 3.96 (d, J = 10.6 Hz, 1H), 3.93 (d, J = 9.8 Hz, 1H), 3.81 (s, 3H), 3.80 (s, 6H), 3.41 (dd, J = 9.9, 2.2 Hz, 1H), 3.39 (s, 3H), 3.29 (t, J = 9.5 Hz, 1H), 2.16 (s, 3H); 13C NMR (151 MHz, CDCl3): δ 170.1, 159.3, 159.3, 159.2, 130.8, 130.1, 129.8, 129.8, 129.5, 129.4, 113.84, 113.8, 113.77, 97.5, 81.2, 81.1, 79.6, 75.5, 75.49, 72.5, 72.4, 71.7, 63.4, 55.7, 55.3, 20.9; HR-ESI-TOF MS m/z: Calcd for C34H45N4O10 [M + NH4]+ 669.3130; Found 669.3134.
6-Azido-6-deoxy-3,4,5-tri-O-(p-methoxybenzyl)-2-O-(methoxymethyl)-myo-inositol (8). To a solution of 7 (26 mg, 0.040 mmol) in MeOH and DCM (2:1, 1 mL), NaOMe (5 M in MeOH, 10 µL) was added at rt. After stirring the solution at rt for 2 h, Amberlite IR 120 H+ resin was added, followed by filtration and condensation under reduced pressure. The crude product was purified by silica gel column chromatography to afford 8 (25 mg, 98%) as a white solid. Rf = 0.35 (40% EtOAc in hexane); 1H NMR (600 MHz, CDCl3): δ 7.31–7.22 (m, 6H), 6.91–6.82 (m, 6H), 4.84 (d, J = 10.4 Hz, 1H), 4.83 (d, J = 6.8 Hz, 1H), 4.80 (d, J = 10.1 Hz, 1H), 4.74 (d, J = 10.2 Hz, 2H), 4.66 (d, J = 6.7 Hz, 1H), 4.64 (d, J = 11.4 Hz, 1H), 4.59 (d, J = 11.4 Hz, 1H), 3.92 (t, J = 2.4 Hz, 1H), 3.90 (t, J = 9.5 Hz, 1H), 3.81 (s, 6H), 3.80 (s, 3H), 3.62 (t, J = 10.1 Hz, 1H), 3.55 (d, J = 8.5 Hz, 1H), 3.45 (s, 3H), 3.37 (dd, J = 9.9, 2.6 Hz, 1H), 3.29–3.20 (m, 2H); 13C NMR (151 MHz, CDCl3): δ 159.4, 159.3, 159.2, 130.8, 130.2, 129.9, 129.8, 129.5, 129.47, 113.9, 113.8, 113.79, 98.6, 81.4, 81.3, 79.4, 79.2, 75.5, 75.4, 72.7, 70.3, 66.9, 56.1, 55.3; HR-ESI-TOF MS m/z: Calcd for C32H43N4O9 [M + NH4]+ 627.3025; Found 627.3032.
(R)-1-[(tert-butyldimethylsilyl)oxy]-3-(stearoyloxy)propan-2-yl (E)-octadec-9-enoate (12). To a solution of
11 [
18,
21] (0.20 g, 0.42 mmol) and oleic acid (0.3 mL, 0.97 mmol) in DCM (5.0 mL), EDC (0.17 g, 1.14 mmol) and DMAP (15.5 mg, 0.13 mmol) were added under a N
2 atmosphere. The solution was stirred in the dark at rt for 16 h. The reaction mixture was diluted with water and extracted with DCM three times. The combined organic layers were washed with brine, dried over Na
2SO
4, and concentrated under reduced pressure. The crude product was purified by column chromatography with 4% EtOAc in hexane as the eluent to give
12 (0.25 g, 82%) as colorless syrup. R
f = 0.45 (8% EtOAc in Hex);
1H NMR (400 MHz, CDCl
3): δ 5.39–5.30 (m, 2H), 5.10–5.05 (m, 1H), 4.34 (dd,
J = 11.8, 3.7 Hz, 1H), 4.16 (dd,
J = 11.9, 6.3 Hz, 1H), 3.75–3.67 (m, 2H), 2.32–2.28 (m, 4H), 2.03–1.98 (m, 4H), 1.63–1.59 (m, 4H), 1.35−1.25 (m, 48H), 0.90–0.86 (m, 15H), 0.05 (s, 6H, SiMe
2). The
1H NMR data match with those reported in the literature [
22].
(S)-3-Hydroxy-1-(stearoyloxy)propan-2-yl (E)-octadec-9-enoate (13). Et
3N·3HF (0.68 mL, 4.17 mmol) was slowly added to a solution of
12 (0.26 g, 0.35 mmol) in CH
3CN and THF (1:1, 6.0 mL). After the solution was stirred at rt for 16 h, the reaction was quenched by dropwise addition of saturated aqueous NaHCO
3 at 0 °C. The aqueous layer was extracted with DCM three times. The combined organic layers were washed with brine, dried over Na
2SO
4, and concentrated under vacuum. The crude product was purified by column chromatography with 12% EtOAc in hexane as the eluent to afford
13 (0.20 g, 90%) as colorless oil. R
f = 0.38 (20% EtOAc in Hex);
1H NMR (400 MHz, CDCl
3): δ 5.35−5.31 (m, 2H), 5.08 (p,
J = 5.0 Hz, 1H), 4.32 (dd,
J = 11.9, 4.6 Hz, 1H), 4.24 (dd,
J = 11.9, 5.6 Hz, 1H), 3.73 (t,
J = 5.3 Hz, 2H), 2.37–2.31 (m, 4H), 2.06–1.98 (m, 4H), 1.62–1.60 (m, 4H), 1.34−1.25 (m, 48H), 0.87 (t,
J = 8.0 Hz, 6H, CH
3). The
1H NMR data match with those reported in the literature [
23].
(2-Cyanoethoxyl)-(diisopropylamino)-[(R)-2-O-oleoyl-3-O-stearoyl-sn-glycerol)]-phosphine (10). To a solution of
13 (0.20 g, 0.31 mmol) and commercial
16 (0.30 mL, 0.94 mmol) in dry DCM and CH
3CN (2:1, 3.0 mL), diisopropylammonium tetrazolide (161 mg, 0.94 mmol) was added at rt under a nitrogen atmosphere. After stirring for 2 h, the reaction mixture was diluted with DCM and poured into a saturated aqueous NaHCO
3 solution. The mixture was extracted with DCM three times, and the combined organic layers were dried with Na
2SO
4 and concentrated under reduced pressure. The crude product was purified by column chromatography using triethylamine-neutralized silica gel with 10% EtOAc in hexane containing ~1% triethylamine as the eluent to provide
10 (0.24 g, 92%) as a colorless syrup. R
f = 0.33 (20% EtOAc in Hex);
1H NMR (400 MHz, CDCl
3): 5.32–5.20 (m, 2H), 5.15–5.09 (m, 1H), 4.31–4.22 (m, 1H), 4.13–4.05 (m, 1H), 3.82–3.68 (m, 3H), 3.65–3.59 (m, 1H), 3.54–3.48 (m, 2H), 2.57 (t,
J = 6.4 Hz, 2H), 2.28–2.22 (m, 4H), 1.96–1.91 (m, 4H), 1.57–1.52 (m, 4H), 1.26−1.18 (m, 54H), 1.11 (dd,
J = 6.9, 5.0 Hz, 12H), 0.81 (t,
J = 6.7 Hz, 7H);
31P NMR (CDCl
3, 162 MHz): δ 149.51, 149.36. The
1H NMR data match with those reported in the literature [
23].
(2-Cyanoethoxyl)-(diisopropylamino)-[(R)-2,3-di-O-stearoyl-sn-glycerol)]-phosphine (9). It was synthesized from commercial
14 in a 95% yield by the same method utilized to synthesize
10, and its
1H NMR data match those reported in the literature [
11].
6-Azido-1-O-{(2-cyanoethoxy)-[(R)-2,3-di-O-stearoyl-sn-glycerol]-phosphono}-6-deoxy-3,4,5-tri-O-(4-methoxybenzyl)-2-O-(methoxymethyl)-myo-inositol (16). To a solution of 8 (15 mg, 0.024 mmol) in DCM and CH3CN (4:1, 1.5 mL), activated MS 4Å (50 mg) and 1H-tetrazole (0.45 M in acetonitrile, 0.53 mL, 0.236 mmol) were added at rt. This was followed by dropwise addition of 9 (72 mg, 0.086 mmol) dissolved in DCM (0.5 mL). After 30 min of stirring, the mixture was cooled to 0 °C, and then t-BuO2H (105 µL, 0.577 mmol) was added. After 1 h of stirring, the mixture was diluted with DCM and washed with saturated aqueous NaHCO3. The aqueous layer was extracted with DCM three times. The organic layers were combined, washed with brine, dried with Na2SO4, filtered, and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography to afford 16 (15 mg, 55%) as a white solid. Rf = 0.30 (40% EtOAc in hexane); 1H NMR (400 MHz, CDCl3): δ 7.31–7.20 (m, 8H), 6.90–6.82 (m, 4H), 5.35–5.25 (m, 1H), 4.90–4.80 (m, 3H), 4.78–4.62 (m, 4H), 4.54 (dd, J = 10.9, 1.8 Hz, 1H), 4.41–4.12 (m, 6H), 4.11–4.00 (m, 1H), 3.93 (td, J = 9.9, 2.6 Hz, 2H), 3.81 (m, 9H), 3.41–3.34 (m, 3H), 3.29 (td, J = 9.4, 2.9 Hz, 1H), 2.87–2.73 (m, 2H), 2.46–2.21 (m, 4H), 1.67–1.53 (m, 8H), 1.45–1.10 (m, 56H), 0.91–0.83 (m, 6H); 13C NMR (151 MHz, CDCl3): δ 173.2, 172.9, 159.3, 159.3, 159.2, 130.7, 129.9, 129.9, 129.8, 129.5, 116.3, 116.25, 113.8, 113.78, 113.77, 97.6, 97.5, 81.0, 80.9, 79.33, 79.3, 75.6, 75.5, 73.5, 73.3, 72.5, 72.4, 69.2, 64.3, 61.5, 55.94, 55.9, 55.3, 34.1, 34.0, 31.9, 29.7, 29.65, 29.5, 29.49, 29.4, 29.3, 29.29, 29.13, 29.12, 24.8, 22.7, 14.1; 31P NMR (162 MHz, CDCl3): δ −2.06, −2.12; HR-ESI-TOF MS m/z: Calcd for C74H121N5O16P [M + NH4]+ 1366.8541; Found 1366.8567.
6-Azido-1-O-[(2-cyanoethoxyl)-[(R)-2-O-oleoyl-3-O-stearoyl-sn-glycerol)]-phosphono-6-deoxy-3,4,5-tri-O-4-methoxybenzyl-2-O-(methoxymethyl)-myo-inositol (17). To a solution of 8 (35 mg, 0.057 mmol) in dry DCM and CH3CN (3:1, 6.4 mL), MS 4Å and 1H-tetrazole (0.45 M in acetonitrile, 1.26 mL, 0.57 mmol) were added, followed by dropwise addition of a fresh solution of 10 (0.24 g, 0.28 mmol) in DCM under nitrogen at rt. After the mixture was stirred at rt for 30 min, it was cooled to −40 °C and treated with t-BuO2H (5.5 M in decane, 0.21 mL, 1.14 mmol). After the solution was stirred at −40 °C for 1 h, Me2S (0.17 mL, 2.28 mmol) was added, with stirring for 1 h. While the reaction mixture was slowly warmed to rt, it was diluted with DCM and then filtered through a Celite pad. The mixture was poured into saturated aqueous NaHCO3 solution and extracted with DCM three times. The organic layers were combined, dried with Na2SO4, filtered, and condensed under reduced pressure. The crude product was purified by silica gel column chromatography with 12% EtOAc in toluene as the eluent to give 17 (33 mg, 42%) as a colorless syrup. Rf = 0.28 (40% EtOAc in Hex); 1H NMR (600 MHz, CDCl3): δ 7.29–7.24 (m, 6H), 6.89–6.86 (m, 6H), 5.39–5.34 (m, 2H), 5.33–5.31 (m, 1H), 4.87–4.83 (m, 3H), 4.78–4.66 (m, 4H), 4.56 (dd, J = 10.9, 2.6 Hz, 1H), 4.40–4.25 (m, 7H), 4.24–4.17 (m, 2H), 4.11–4.07 (m, 1H), 3.83 (d, J = 1.7 Hz, 9H), 3.41–3.39 (m, 4H), 3.32 (dd, J = 11.2, 7.9 Hz, 1H), 2.83–2.80 (m, 2H), 2.39–2.32 (m, 4H), 2.04–2.01 (m, 4H), 1.65–1.61 (m, 4H), 1.36–1.25 (m, 48H), 0.90 (t, J = 6.9 Hz, 6H); 13C NMR (151 MHz, CDCl3): δ 173.3, 172.9, 172.8, 159.4, 159.3, 159.2, 130.7, 130.0, 129.97, 129.92, 129.8 (2C), 129.72, 129.7, 129.5 (3C), 113.9 (3C), 113.82, 113.8 (3C), 97.6, 97.57, 97.55, 81.0, 80.9, 79.4, 79.3, 76.0, 75.6, 75.5, 73.5, 73.4, 73.35, 72.5, 72.48, 72.42, 72.4, 69.3, 69.2, 66.2, 65.9, 64.4, 64.3, 62.4, 62.3, 62.2, 62.1, 61.6, 61.5, 61.49, 56.0, 55.9, 55.3 (3C), 55.27, 34.2, 34.1 (2C), 34.0 (2C), 33.98, 31.94 (2C), 31.9 (2C), 29.8 (2C), 29.74, 29.72 (multiple C), 29.70, 29.67 (3C), 29.66 (2C), 29.54 (2C), 29.5 (2C), 29.4 (2C), 29.34 (2C), 29.3 (2C), 29.24, 29.2, 29.16, 29.14 (2C), 29.1, 27.24 (2C), 27.2 (2C), 24.9, 24.8, 22.7 (2C), 19.7, 19.65, 19.6, 14.1 (2C). 31P NMR (CDCl3, 243 MHz) δ −1.93, −2.06, −2.12, −2.16. HR-ESI-TOF MS m/z: Calcd for C74H119N4O16P [M + NH4]+ 1364.8383; Found 1364.8367.
6-Azido-6-deoxy-1-O-{[(R)-2,3-di-O-stearoyl-sn-glycerol]-phosphono}-myo-inositol (1). To a solution of 16 (15 mg, 0.011 mmol) in anhydrous DCM (3 mL), DBU (2 µL, 0.013 mmol) was added. After stirring at rt for 1 h, the solvent was evaporated under reduced pressure, and the product was briefly purified by silica gel column chromatography and then dissolved in DCM (3 mL). After the solution was cooled to 0 °C, a 20% TFA solution in DCM (3 mL) was added. The solution was warmed to rt, stirred for 2 h, diluted with toluene, and condensed under reduced pressure. The residue was co-evaporated with toluene five times, and the product was washed with diethyl ether three times to afford 1 as a white solid (7.5 mg, 77%). Rf = 0.1 (15% methanol in DCM); 1H NMR (600 MHz, MeOD:CDCl3 = 3:2): δ 5.26 (s, 1H), 4.41 (d, J = 11.9 Hz, 1H), 4.21–3.98 (m, 3H), 3.86 (br, 1H), 3.77–3.54 (m, 3H), 3.40–3.34 (m, 1H), 3.23–3.13 (m, 1H), 2.43–2.17 (m, 4H), 1.67–1.54 (m, 4H), 1.42–1.06 (m, 56H), 0.87 (t, J = 6.9 Hz, 6H); 13C NMR (151 MHz, MeOD:CDCl3 3:2): δ 174.0, 73.7, 73.3, 72.8, 71.1, 71.0, 70.3, 64.9, 63.9, 62.5, 54.5, 34.1, 34.0, 31.8, 29.6, 29.5, 29.4, 29.24, 29.2, 29.04, 29.0, 24.82, 24.8, 22.5, 13.8; 31P NMR (162 MHz, MeOD:CDCl3 = 3:2): δ −2.44; HR-ESI-TOF MS m/z: Calcd for C45H90N4O12P [M + NH4]+ 909.6287; Found 909.6282.
6-Azido-6-deoxy-1-O-{[(R)-2-O-oleoyl-3-O-stearoyl-sn-glycerol]-phosphono}-myo-inositol (2). DBU (7.2 µL, 0.048 mmol) was added to a solution of 17 (33 mg, 0.024 mmol) in dry DCM (5 mL) at rt. After stirring the solution for 1 h, glacial acetic acid (14 µL, 0.24 mmol) was added. The mixture was stirred for another 5 min and condensed under reduced pressure. The product was briefly purified by silica gel chromatography and then dissolved in dry DCM (6 mL), followed by dropwise addition of HCl in dioxane (4 M, 2.5 mL) at 0 °C. After the mixture was stirred for 10 min, it was warmed to rt with stirring for 1.5 h, diluted with toluene, and condensed under reduced pressure. The residue was co-evaporated with toluene six times, and the product was purified by silica gel column chromatography using 12% MeOH in DCM as the eluent to afford 2 as a white solid (14.5 mg, 67%). Rf = 0.18 (30% MeOH in CHCl3); 1H NMR (600 MHz, MeOD:CDCl3 = 3:2): δ 5.29–5.22 (m, 2H), 5.19–5.18 (m, 1H), 4.36–4.32 (m, 1H), 4.19 (br, 1H), 4.12–4.08 (m, 1H), 4.02–3.97 (m, 2H), 3.79 (br, 1H), 3.63–3.56 (m, 2H), 3.27–3.26 (m, 1H), 3.14–3.08 (m, 1H), 2.27–2.22 (m, 4H), 1.95–1.91 (m, 4H), 1.56–1.49 (m, 4H), 1.26–1.17 (m, 48H), 0.81–0.78 (m, 6H); 13C NMR: (151 MHz, MeOD:CDCl3 = 3:2): δ 173.9, 173.5, 129.8, 129.5, 73.7, 72.7, 71.1 (2C), 70.3, 65.1, 63.5, 62.4, 52.6, 34.0, 33.9, 31.8 (2C), 29.6 (2C), 29.5 (multiple C), 29.47 (3C), 29.3, 29.2, 29.15 (2C), 29.1, 29.0, 28.94, 28.9, 27.0, 26.96, 24.8, 24.7, 22.5 (3), 13.5 (2C). 31P NMR (243 MHz, MeOD:CDCl3 = 3:2): δ −4.74. HR-ESI-TOF MS m/z: Calcd for C45H88N3O12P [M + NH4]+ 907.6130; Found 907.6117.