Abstract
To achieve artificial photosynthesis, it is crucial to develop a catalytic system for CO2 reduction using water as the electron source. However, photochemical CO2 reduction by homogeneous molecular catalysts has predominantly been conducted in organic solvents. This study investigates the impact of water content on catalytic activity in photochemical CO2 reduction in N,N-dimethylacetamide (DMA), using [Ru(bpy)3]2+ (bpy: 2,2′-bipyridine) as a photosensitizer, 1-benzyl-1,4-dihydronicotinamide (BNAH) as an electron donor, and two ruthenium diimine carbonyl complexes, [Ru(bpy)2(CO)2]2+ and trans(Cl)-[Ru(Ac-5Bpy-NHMe)(CO)2Cl2] (5Bpy: 5′-amino-2,2′-bipyridine-5-carboxylic acid), as catalysts. Increasing water content significantly decreased CO and formic acid production. The similar rates of decrease for both catalysts suggest that water primarily affects the formation efficiency of free one-electron-reduced [Ru(bpy)3]2+, rather than the intrinsic catalytic activity. The reduction in cage-escape efficiency with higher water content underscores the challenges in replacing organic solvents with water in photochemical CO2 reduction.
1. Introduction
In recent years, technology for CO2 reduction has emerged as an effective solution for addressing the global warming problem and the storage of fossil fuels. Metal complexes have become a promising candidate for catalysts in CO2 reduction because of their multiple accessible redox states, high activation energies against proton reduction, and various molecular design possibilities through the combination of appropriate metal ions and ligands [1,2,3,4,5,6,7,8,9,10]. Among them, metal diimine carbonyl complexes have been identified as efficient catalysts for selectively yielding CO and/or formic acid without accompanying hydrogen evolution, even though proton reduction is a more thermodynamically favorable process than the CO2 reduction. In photochemical CO2 reduction, a rhenium diimine carbonyl complex, fac-Re(bpy)(CO)3Cl (bpy = 2,2′-bipyridine), acts as a highly efficient CO2 reduction photocatalyst to selectively produce CO in N,N-dimethylformamide (DMF) solution containing triethanolamine (TEOA) as an electron donor [11,12]. Here, fac-Re(bpy)(CO)3Cl functions not only as the catalyst but also as the photosensitizer. In the case of ruthenium diimine carbonyl and manganese diimine carbonyl complexes, direct irradiation causes the dissociation of the CO ligands [13,14,15]. Therefore, the use of additional photosensitizer is essential. Tris(diimine)ruthenium complexes are often used as the photosensitizers due to their intense absorption band in the visible region, their long-lived excited triplet state, and the stability of their one-electron-reduced species after electron transfer from an electron donor. In particular, [Ru(bpy)3]2+ has been widely used not only in CO2 reduction but also in hydrogen evolution [16] and photoredox reactions for chemical bond formations [17]. In the photochemical CO2 reductions using ruthenium diimine carbonyl complexes, such as [Ru(bpy)2(CO)(X)]+ (X =Cl, H), [Ru(bpy)2(CO)2]2+, and cis-Ru(bpy)(CO)2(Cl)2, formic acid is selectively formed using [Ru(bpy)3]2+ as the photosensitizer in DMF containing TEOA [18,19,20]. When 1-benzyl-1,4-dihydronicotinamide (BNAH) was used as the electron source instead of TEOA and water was used as a proton donor in DMF (DMF:water = 9:1 v/v), the photochemical CO2 reduction using [Ru(bpy)2(CO)2]2+ in the presence of [Ru(bpy)3]2+ produces CO as well as formic acid [19,20,21]. The covalently linked systems of a tris(diimine)ruthenium complex and a ruthenium diimine carbonyl complex were used in the photochemical CO2 reduction with BNAH as the electron donor in a mixed solvent of DMA and TEOA, yielding formic acid as the reduction product with high selectivity [22]. The photochemical CO2 reduction using [Ru(bpy)2(CO)H]+ in the presence of BNAH and [Ru(bpy)3]2+ in a biphasic liquid-condensed CO2 gas system using a mixed solvent of DMF and water produces a mixture of CO and formic acid [23].
Homogeneous photochemical reactions catalyzed by many molecular catalysts have typically been carried out in organic solvents. In the photochemical CO2 reduction, since Lehn et al. discovered that fac-Re(bpy)(CO)3Cl serves as a highly efficient CO2 reduction photocatalyst, DMF has been widely used as the solvent. However, DMF has a disadvantage of generating formic acid by hydrolysis, which complicates the distinction between this blank formic acid and the formic acid produced by the CO2 reduction [24]. Therefore, we proposed using N,N-dimethylacetamide (DMA) as an alternative solvent to DMF [25]. DMA is stable against hydrolysis and does not produce formic acid even if hydrolysis occurs. Currently, various researchers are using DMA as a solvent instead of DMF in different CO2 reduction systems [26,27,28,29,30,31].
On the other hand, water is an abundant and environmentally friendly solvent, making the replacement of organic solvents with water an important issue. While heterogeneous systems have achieved the photochemical CO2 reduction in water, where water also acts as the electron donor [32,33], attempts to replace organic solvents with water in homogeneous catalytic reactions have often been unsuccessful. We have previously reported that the photocatalytic CO2 reduction using [Ru(bpy)2(CO)2]2+, [Ru(bpy)3]2+, and BNAH produces CO and formic acid in a mixed solvent of DMA and water (DMA:water = 9:1 v/v) with high efficiency, but increasing the water ratio decreases the catalytic activity [25]. This study demonstrates that the changes in the water ratio significantly affect the initial stages of the photocatalytic reaction. Here, the concentration of the catalyst, [Ru(bpy)2(CO)2]2+, is 1.0 × 10−4 M, which is comparable to that of the photosensitizer, [Ru(bpy)3]2+ (5.0 × 10−4 M). Under such conditions, it is considered that the rate-determining step in the photocatalytic reaction is not the reaction on the catalyst (“catalytic cycle”) but rather the process in which the photosensitizer is reduced and the electron is transferred from the reduced photosensitizer to the catalyst (“electron relay cycle”) (vide infra) [8]. In other words, the decrease in the catalytic activity with increasing ratio of water is related to the decreased efficiency of the “electron relay cycle” process. To support this, we investigate the water dependence in a different ruthenium diimine carbonyl complex, trans(Cl)-[Ru(Ac-5Bpy-NHMe)(CO)2Cl2] (Figure 1) [34]. This complex differs in neutral initial charge, reduction potential, and catalytic activity for CO2 reduction compared to the divalent cationic complex, [Ru(bpy)2(CO)2]2+ but exhibits a similar dependence on water content. This indicates that increasing the ratio of water does not affect the catalytic reaction on the catalyst but rather the generation of the one-electron-reduced free species of [Ru(bpy)3]2+ in the “electron relay cycle”. The significant decreases in the catalytic activity cannot be explained by the decrease in the quenching efficiency of the excited state of [Ru(bpy)3]2+ by BNAH. It is further suggested that the decrease in the solvent cage-escape efficiency from the solvent cage after the electron transfer between the excited [Ru(bpy)3]2+ and BNAH is also involved. The importance of the cage-escape process from the solvent cage has recently gained attention in various photocatalytic reactions [35,36,37,38,39,40,41]. In this study, we demonstrate that the solvent composition affects the solvent cage-escape efficiency and has a substantial impact on the photocatalytic CO2 reduction reaction.
Figure 1.
Chemical structures of [Ru(bpy)3]2+, BNAH, and ruthenium diimine carbonyl complexes.
2. Results and Discussion
2.1. Effect of Water Content on Catalytic Activity
Photocatalytic CO2 reductions were conducted in DMA or DMA/water solutions using two types of the ruthenium diimine carbonyl complexes as catalysts: one was the cationic complex [Ru(bpy)2(CO)2]2+ [25], and the other was the neutral complex trans(Cl)-[Ru(Ac-5Bpy-NHMe)(CO)2Cl2] [34]. [Ru(bpy)3]2+ and BNAH were used as a photosensitizer and an electron donor, respectively. Figure 2 shows the relationships between the water content and the amount of the reduction products. Both plots, using [Ru(bpy)2(CO)2]2+ and trans(Cl)-[Ru(Ac-5Bpy-NHMe)(CO)2Cl2], show similar trends. The main reaction products are CO and formic acid resulting from the CO2 reduction, with negligible amounts of hydrogen regardless of the water content in the DMA. The total amount of CO and formic acid is the highest at 10 vol% water content in DMA and significantly decreases with the addition of water above 10 vol%. Moreover, the amounts in DMA without the addition of water show lower values, possibly due to a shortage of the proton source in the catalytic reaction on the ruthenium complex catalysts [42,43,44,45,46,47,48,49]. The catalytic systems described in this study are thought to consist of two main cycles: the “electron relay cycle” and the “catalytic cycle” (Figure 3) [8]. Here, relatively high catalyst concentrations of 0.10 mM are used, where the rate-determining step is thought to be the process in the “electron relay cycle” including electron transfer from BNAH to the excited photosensitizer, followed by electron transfer from the one-electron-reduced species of the photosensitizer to the catalyst (Figure 3) [50,51]. Thus, the decreases in the catalytic activities above 10 vol% water is explainable by decreases in the efficiency of the process leading to electron transfer to the ruthenium complex catalysts.
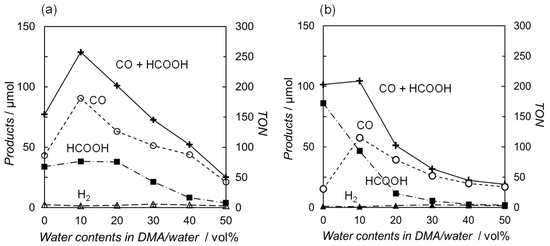
Figure 2.
Effects of water content on the reduction products after 1h of irradiation (λ > 400 nm) using (a) [Ru(bpy)2(CO)2](PF6)2 (1.0 × 10−4 M) [25] and (b) trans(Cl)-[Ru(Ac-5Bpy-NHMe)(CO)2Cl2] (1.0 × 10−4 M) [34] in DMA containing [Ru(bpy)3](PF6)2 (5.0 × 10−4 M) and BNAH (0.10 M) under CO2 atmosphere: CO (○), HCOOH (■), H2 (Δ), and CO+HCOOH (+).
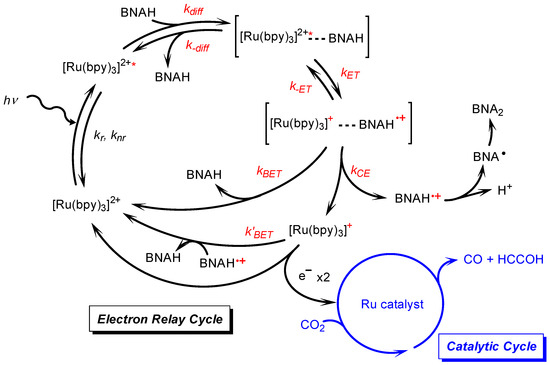
Figure 3.
Reaction mechanism for the photocatalytic CO2 reduction, consisting of the electron–relay cycle and the catalytic cycle [8,51].
The electron–relay process begins with the formation of an encounter complex resulting from the diffusional encounter between BNAH and the excited [Ru(bpy)3]2+ (Figure 3). Within the encounter complex in a solvent cage, an electron transfer occurs to form the charge-separated encounter complex. The resulting species may diffuse out of the solvent cage to give the one-electron-reduced photosensitizer ([Ru(bpy)3]+) and BNAH+ or recombine to produce the ground state of [Ru(bpy)3]2+ and BNAH [8,35,51,52,53,54,55,56,57,58,59,60]. The free [Ru(bpy)3]+ that escapes from the solvent cage can provide the electron to the catalyst. We have observed the quantitative formation of BNA dimers from BNAH in the photocatalytic CO2 reduction [25], indicating that the BNA• does not function as an electron donor and that both two electrons for the catalyst are supplied by the free [Ru(bpy)3]+. The BNA dimers are known to cause an undesired quenching process during the catalytic reaction. We previously investigated the dependence of the quenching efficiency of the excited state of [Ru(bpy)3]2+ on the water ratio in the presence of the BNA dimer [25]. The results indicated that the undesired quenching by the BNA dimer was suppressed with increasing water content, which is the opposite trend to the decrease in activity observed with a higher water ratio, as shown in Figure 2. Furthermore, since we are discussing the initial reaction rate, the contribution of the BNA dimer is considered negligible. Thus, the decline in catalytic activity observed at high water contents in Figure 2 would be attributed to a decrease in the formation efficiency of the free [Ru(bpy)3]+ by BNAH.
2.2. Diffusion Rate Constants of Excited [Ru(bpy)3]2+ and BNAH
The initial step in the electron relay process involves diffusional collision between the excited [Ru(bpy)3]2+ and BNAH. As shown in Figure 4, the viscosity of the binary solvent mixture comprising DMA and water increases as the water content increases from 0 to 40 vol.% [61,62]. The diffusion rate constants (kdiff) between the excited [Ru(bpy)3]2+ and BNAH are calculated by Equation (1) [52], and the value of kdiff is significantly affected by the diffusion coefficient, i.e., the viscosity of the solvent.
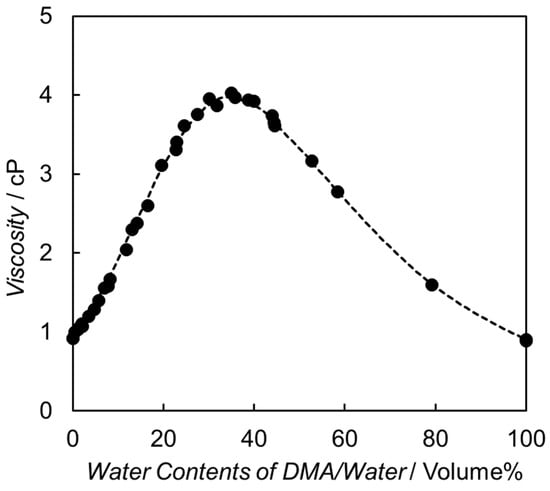
Figure 4.
Viscosity as a function of water content in the DMA and water-mixed solvent. These plots are reconstructed using the values reported in reference [61,62].
NA is Avogadro’s number. The diffusion coefficient (DRu and DBNAH) for the excited [Ru(bpy)3]2+ and BNAH are calculated by the Stokes–Einstein equation (D = kBT/6πηr, where D is the diffusion coefficient, kB is Boltzmann’s constant, T is the absolute temperature, and r is the effective radius) using the effective radii of the excited [Ru(bpy)3]2+ (r Ru = 7.1 Å, assuming that the molecular size does not change by the excitation) [55,56,57] and BNAH (r BNAH ~ 6.7 Å) estimated from r = (dx dy dz)1/3 [63,64,65,66]. The diffusion constants and the quenching rate constants are summarized in Table 1. The quenching rate constants (kq) have been determined by the Stern–Volmer plots and the emission lifetimes of [Ru(bpy)3]2+ [25]. While the value of kdiff decreases as the water content increases, the values of kdiff are one order of magnitude larger than those of kq. Thus, it is thought that the rate-determining step for kq is not the diffusional process (kdiff) but the electron transfer process from BNAH to the excited [Ru(bpy)3]2+ (kET in Figure 3).

Table 1.
Solvent effect on diffusion and quenching rate constants for excited [Ru(bpy)3]2+ and BNAH in DMA/water at 298 K.
2.3. Electron Transfer on the Encounter Complex
The values of kq are correlated with the diffusional effect using Equation (2) [52]
In Equation (2), kq’ is the rate constant for activated quenching and kdiff is the diffusion rate constant. A kinetic analysis in Figure 3 gives the following relationships (Equation (3a,b)) [54,55,56,57].
where Fet = (kBET + kCE)/(k−ET + kBET + kCE), ν is the frequency factor of kET, ∆GET is the free energy change of the electron transfer step, and λ is the sum of the inner- (λi) and outer-sphere (λo) reorganization energies. Keq is the equilibrium constant for the formation of the encounter complex (=kdiff/k-diff). The value of Keq can be calculated from the Fuoss equation to be 6.62 M−1 ([Ru(bpy)3]2+ and the neutral quencher system, d = r Ru + r BNAH = 13.8 Å) [55,56,57]. The values of ∆GET are determined from the Rehm–Weller equation, which is based on the half-wave reduction potential of [Ru(bpy)3]2+, the half-wave oxidation potential of BNAH, the excited state energy of [Ru(bpy)3]2+ (E00 = 2.12 eV), and a coulombic attraction term (Equation (4)) [52]. Judging from the emission peaks (Table 2), E00 is expected not to be changed by the water content.
wp is the electrostatic work necessary to bring two product ions to the close-contact distance: wp = (ZD+ZA−) e2/(d εs), where ZD+ and ZA− are the ion charges, and d is the distance between the ion centers. The values of E1/2(Ru2+/Ru+) and E1/2(BNAH+/BNAH) are determined by the cyclic voltammograms (CV) or the differential pulse voltammetry (DPV). The E1/2(BNAH+/BNAH) is estimated to be +0.14 V vs. Ag/Ag+, whose value does not change by increasing the water content in the solvent. On the other hand, the E1/2(Ru2+/Ru+) shows a shift from − 1.65 V vs. Ag/Ag+ in DMA to −1.76 V vs. Ag/Ag+ in DMA/water (6:4. v/v). As a result, the value of −∆GET decreases as the water content increases (Table 2).

Table 2.
Emission peak of [Ru(bpy)3]2+, oxidation potentials of BNAH, and reduction potentials of [Ru(bpy)3]2+ in DMA and water.
Meyer and coworkers reported that there are two limiting forms for kq’ in Equation (3b), where Case I is defined as k−ET << (kCE + kBET) and Fet = 1 and Case II as k−ET >> (kCE + kBET) and Fet = (kBET + kCE)/k−ET. They also reported that the reductive quenching proceeded via case I [54,55,56,57,67]. Then, Equation (3b) gives Equation (5).
The logarithmic form becomes
When |∆GET| << 2λ, Equation (6) is simplified to Equation (7).
Since λ =λi + λo, Equation (8) then becomes
Equation (8) indicates that a plot of (RT ln kq’ + λo/4) vs. ∆GET has a linear region of slope = 1/2 when λi and ν are constant. λo was calculated by Equation (9).
where n and εs are the refractive index and the static dielectric constant, respectively. The values of wp, λo/4, −∆GET, and kq’ for the quenching of the excited [Ru(bpy)3]2+ by BNAH in DMA/water are summarized in Table 3.

Table 3.
Gibbs free energy changes in electron transfer step and activation-controlled quenching rate constants in DMA and water.
We have observed a decrease in the quenching rate constant (kq) with increasing water content of the solvent, as observed in Table 1. Figure 5 displays a linear relationship with a slope of 1/2 in the plots of (RT ln kq’ + λo/4) vs. ∆GET, indicating that the quenching process obeys Equation (8) and that the back-electron transfer process (k−ET) from the encounter complex to return the excited [Ru(bpy)3]2+ is negligible. The quenching rate decreases with increasing water content, resulting from a decrease in −∆GET caused by the large negative shift of E1/2(Ru2+/Ru+) with increasing water content. Thus, the decrease in the quenching rate is one reason why the efficiency of the free [Ru(bpy)3]+ formation decreases at high water content.
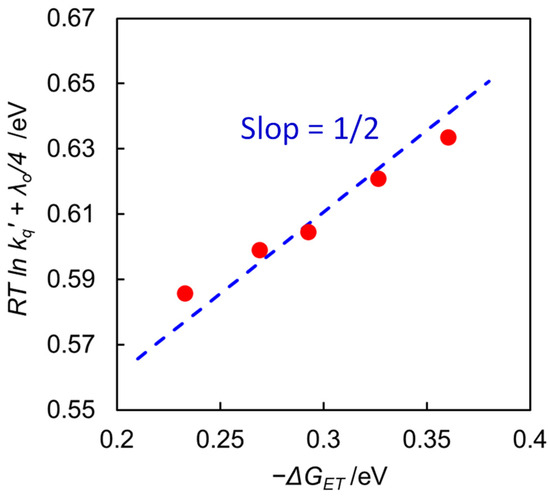
Figure 5.
Plots of (RT ln kq’ + λo/4) vs. ∆GET in DMA/water at 298 K. The line is drawn with slope = 1/2.
2.4. Competition Between Cage-Escape and Back-Electron Transfer After Charge Separation
In Figure 2, the concentration of BNAH is 0.10 M. The quenching efficiency (ηq) of the excited [Ru(bpy)3]2+, which is calculated from the Stern–Volmer plot [8], decreases from 98% in DMA to 81% in DMA containing 40 vol.% water (Table 4). This decrease does not fully reflect the decrease in catalytic activity observed in Figure 2, suggesting that there is another factor contributing to the decreasing in efficiency of free [Ru(bpy)3]+ formation. To obtain the free [Ru(bpy)3]+ that can provide an electron to the catalyst, the cage-escape process is required. This process competes with the back-electron transfer that occurs after charge separation in the solvent cage (Figure 3). In some cases, the cage-escape rate constant (kCE) can be theoretically determined from the Eigen equation (Equation (10)) [52,55,56,57,58,59,60].
where kB, R, T, and η are the Boltzmann constant, gas constant, temperature, and solvent viscosity, respectively. wp is the electrostatic work required to bring two product ions to the close-contact distance (Table 2). The electron transfer from BNAH to the excited state of [Ru(bpy)3]2+ results in two cationic molecules which promote the cage-escape process by their electric repulsion. Increasing the water content in water/DMA solutions causes both an increase in the solvent viscosity and a decrease in the electrostatic work (wp). In particular, the increase in solvent viscosity leads to a significant decrease in the kCE value (Figure 6a).

Table 4.
Effect of water content on quenching efficiency, cage-escape rate constant, and TON in Figure 2.
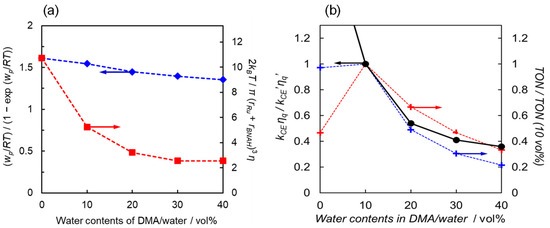
Figure 6.
(a) Values of the electrostatic work term (blue diamonds) and viscosity term (red squares) in Equation (10) as a function of water content. (b) Relationship between the relative ratio of the cage-escape rate constants corrected by the quenching constants (black circles) and the relative TONs in the photochemical CO2 reduction catalysed by [Ru(bpy)2(CO)2]2+ (red points) and trans(Cl)-[Ru(Ac-5Bpy-NHMe)(CO)2Cl2] (blue points).
Table 4 summarizes the kCE values for different water ratios, the kCE values corrected by the quenching efficiency, and the relative TON values of the photocatalytic CO2 reduction against the TON at 10 vol% water. At 0 vol% water, the reaction on the catalyst is slow due to proton deficiency, and the rate-determining step is thought to occur in the “catalytic cycle” shown in Figure 3. In the region with more than 10 vol% water, the catalytic reaction proceeds sufficiently, and the rate-determining process is expected to occur within the “electron transfer cycle”. If the back-electron transfer rate constant (kBET) in the solvent cage remains unchanged as the water content varies, the formation efficiency of free [Ru(bpy)3]+ should be proportional to the kCE value corrected with the quenching efficiency (ηq). Figure 6b shows the relationship between the relative kCE values corrected by the quenching efficiency and the relative TON values for two Ru catalysts, both of which exhibit a similar decreasing trend with increasing water content. The consistent decreasing trend observed for the two different Ru complex catalysts strongly supports that the origin of this phenomenon lies in the electron transfer cycle rather than the catalytic cycle in the region with more than 10 vol% water content. Thus, the decrease in catalytic activity with increasing water content would be significantly influenced by the efficiency of cage-escape, i.e., the efficiency of free [Ru(bpy)3]+ formation.
In the electrochemical experiments, the Faradic efficiencies of the CO2 reduction were estimated to be nearly 100% for the ruthenium diimine carbonyl complexes [42,43,44,45,46,47,48,49]. Considering that the electrons acquired by the catalyst are almost exclusively used for CO2 reduction, the relatively low photochemical quantum yield for CO and formic acid production (Φ = 15%) [25] would mainly originate from the efficiency of the electron–relay process for free [Ru(bpy)3]+ formation. Since the quenching efficiency (ηq) of the excited [Ru(bpy)3]2+ is almost unity (96%) in DMA/water (9:1, v/v), the efficiency of the cage-escape, ηCE = kCE/(kCE + kBET), could dominantly affect the reaction quantum yield for CO2 reduction. Mataga et al. reported a back-electron transfer rate constant of an order of ~1010 s−1 in the electron transfer between [Ru(bpy)3]2+ and quenchers.[70] Assuming this rate is constant, the cage-escape yield in DMA/water (9:1, v/v) is roughly estimated to be 17%, which seems to be comparable to the values of reaction quantum yield, Φ.
3. Materials and Methods
3.1. General Procedure
The ruthenium complexes, trans(Cl)-[Ru(Ac-5Bpy-NHMe)(CO)2Cl2], [Ru(bpy)2(CO)2](PF6)2, and [Ru(bpy)3](PF6)2, were prepared according to the literature [34,71,72]. BNAH was prepared according to the literature [73] and stored in a refrigerator. DMA (Wako, dehydrate) was used as supplied. High-purity water (resistivity: 18.2 MΩ cm) was obtained by an ultra-pure water system (RFU424TA, Advantec, Tokyo, Japan). Cyclic voltammograms (CV) and differential pulse voltmmograms (DPV) were obtained by a Bio-Logic VSP Potentiostat with a glassy-carbon working electrode (ϕ 3 mm), a Pt counter electrode, using tetrabutylammonium perchlorate (nBu4NClO4) as a supporting electrolyte and a Ag/AgNO3 (10 mM) reference electrode in DMA and water. The quenching experiments were carried out on a F-4500 spectrometer (Hitachi, Tokyo, Japan) (λex = 453 nm) by recording the emissions of [Ru(bpy)3](PF6)2 (5.8 × 10−6 M) in the Ar-saturated DMA/water solutions in the absence and presence of BNAH. The emission lifetimes (τ) were measured at 298 K with a FluoroCube fluorescence lifetime spectrometer (Horiba Jobin Yvon, Kyoto, Japan) using a 455 nm laser diode (NanoLED, Horiba, Kyoto, Japan).
3.2. Photochemical CO2 Reduction
Ar-saturated DMA/water solutions (5 mL) of [Ru(bpy)2(CO)2](PF6)2 (1.0 × 10−4 M) or trans(Cl)-[Ru(Ac-5Bpy-NHMe)(CO)2Cl2] (1.0 × 10−4 M), [Ru(bpy)3](PF6)2 (5.0 × 10−4 M), and BNAH (0.10 M) were placed in Quartz tubes (23 mL volume, i.d. = 14 mm). Each solution was bubbled through a septum cap with CO2 gas (1 atm) for 20 min. Ten tubes were set in a merry-go-round irradiation apparatus (Riko Kagaku, RH400-10W) and then were irradiated using a 400 W high-pressure Hg lamp at λ > 400 nm (L-39 cutoff filter, Riko Kagaku, Tokyo, Japan) at room temperature. The reaction temperature was maintained at 298 ± 3 K by using a water bath. After irradiated for a definite time, CO and H2 were analyzed on a system gas chromatograph (GC) based on Shimadzu GC-2014 (Shimadzu, Kyoto, Japan). The product gases (0.10 mL) were injected with a gastight syringe into the GC equipped with successive Porapak-N (GL Science, Tokyo, Japan), Molecular Sieve 13X (GL Science, Tokyo, Japan), and Shimalite-Q (Shinwa Chemical, Kyoto, Japan) columns (stainless steel columns). N2 (>99.9995%) was used as the carrier gas. CO was methanized through a Shimadzu MTN-1 methanizer (Shimadzu, Kyoto, Japan), followed by detection with FID (Shimadzu, Kyoto, Japan). H2 was detected with TCD (Shimadzu, Kyoto, Japan). Formate was extracted as formic acid with ethyl acetate prior to the GC analyses, according to the previous procedure [25]. The sample was injected into a Shimadzu GC-2014 equipped with DB-WAX (Agilent, Santa Clara, CA, USA) columns (i.d. 0.53 mm, 15 m × 2). Formic acid was detected with FID after methanization by a Shimadzu MTN-1 methanizer. The turnover numbers (TON) were calculated based on the amount of [Ru(bpy)2(CO)2](PF6)2 or trans(Cl)-[Ru(Ac-5Bpy-NHMe)(CO)2Cl2].
4. Conclusions
In this study, we systematically investigated the relationship between the water content of DMA solutions and catalytic activity for the photochemical CO2 reduction using [Ru(bpy)3]2+ as the photosensitizer, two types of ruthenium diimine carbonyl complexes as the catalyst, and BNAH as the electron donor. Increasing the water content led to a significant decrease in catalytic activity, which was attributed to the reduced efficiency of the electron relay cycle in the overall photocatalytic cycle (Figure 3). The water content raised the solvent viscosity, resulting in a lower diffusion rate. However, the diffusion rate constants (kdiff) were an order of magnitude larger than the quenching rate constants (kq), indicating that the rate-determining step in the initial quenching process was governed by the electron transfer process in the solvent cage. The water content also affected the driving force (−∆GET) for the electron transfer from BNAH to the excited [Ru(bpy)3]2+, where the slope of the logarithmic quenching rate constants versus the driving forces was 1/2, indicating that the back-electron transfer to form the excited [Ru(bpy)3]2+ (k−ET) was considered negligible. However, the decrease in the initial quenching process did not fully account for the significant decrease in catalytic activity. The increase in solvent viscosity also dramatically decreased the cage-escape rate (kCE), suggesting that the cage-escape efficiency was the main reason for the decrease in the efficiency of free [Ru(bpy)3]+ formation, resulting in lower catalytic activity. While the efficiency of the cage-escape process has been reported to affect catalytic activity in binary Ru(II)-Re(I) catalyst systems in water and in Ru(II) and Os(II) photosensitizers using 1,3-dimethyl-2-phenyl-2,3-dihydro-1H-benzo[d]imidazole (BIH) [36,37], systematic studies on the effects of solvents are still scarce. The present systematic investigation provides important insights not only for the reduction process of [Ru(bpy)3]2+ in photochemical CO2 reduction but also for replacing water in a wide range of photoredox catalytic systems involving C–C bond formation typically performed in organic solvents. In the future, the insights gained from this study on the effect of water are expected to be valuable for applications such as CO2 reduction reactions using water as an electron source by combining mediator molecules and water oxidation catalysts.
Author Contributions
Y.K.; Conceptualization, methodology, data curation, writing—original draft preparation, funding acquisition. M.K.; investigation. H.I.; writing—review and editing, supervision, project administration, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by JSPS KAKENHI (grant number: JP22H02186, JP23K04784). This is a product of research which was financially supported (in part) by the Kansai University Fund for Supporting formation of strategic Research Centers (University initiative type), 2024.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Further inquiries can be directed to the corresponding author/s.
Acknowledgments
We thank Makoto Yoshida for experimental supports.
Conflicts of Interest
There are no conflicts of interest to declare.
References
- Bizzarri, C. Homogeneous Systems Containing Earth-Abundant Metal Complexes for Photoactivated CO2 Reduction: Recent Advances. Eur. J. Org. Chem. 2022, 24, e202200185. [Google Scholar] [CrossRef]
- Fujita, E.; Grills, D.C.; Manbeck, G.F.; Polyansky, D.E. Understanding the Role of Inter- and Intramolecular Promoters in Electro and Photochemical CO2 Reduction Using Mn, Re, and Ru Catalysts. Acc. Chem. Res. 2022, 55, 616–628. [Google Scholar] [CrossRef] [PubMed]
- Pirzada, B.M.; Dar, A.H.; Shaikh, M.N.; Qurashi, A. Reticular-Chemistry-Inspired Supramolecule Design as a Tool to Achieve Efficient Photocatalysts for CO2 Reduction. ACS Omega 2021, 6, 29291–29324. [Google Scholar] [CrossRef] [PubMed]
- Nakada, A.; Kumagai, H.; Robert, M.; Ishitani, O.; Maeda, K. Molecule/Semiconductor Hybrid Materials for Visible-Light CO2 Reduction: Design Principles and Interfacial Engineering. Acc. Mater. Res. 2021, 2, 458–470. [Google Scholar] [CrossRef]
- Son, H.-J.; Pac, C.; Kang, S.O. Inorganometallic Photocatalyst for CO2 Reduction. Acc. Chem. Res. 2021, 54, 4530–4544. [Google Scholar] [CrossRef]
- Maeda, K. Metal-Complex/Semiconductor Hybrid Photocatalysts and Photoelectrodes for CO2 Reduction Driven by Visible Light. Adv. Mater. 2019, 31, 1808205. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.-H.; Dong, L.-Z.; Liu, J.; Li, S.-L.; Lan, Y.Q. From Molecular Metal Complex to Metal-Organic Framework: The CO2 Reduction Photocatalysts with Clear and Tunable Structure. Coord. Chem. Rev. 2019, 390, 86–126. [Google Scholar] [CrossRef]
- Kuramochi, Y.; Ishitani, O.; Ishida, H. Reaction Mechanisms of Catalytic Photochemical CO2 Reduction Using Re(I) and Ru(II) Complexes. Coord. Chem. Rev. 2018, 373, 333–356. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Takeda, H.; Ishitani, O. Photocatalytic Reduction of CO2 Using Metal Complexes. J. Photochem. Photobiol. C 2015, 25, 106–137. [Google Scholar] [CrossRef]
- Elgrishi, N.; Chambers, M.B.; Wang, X.; Fontecave, M. Molecular Polypyridine-Based Metal Complexes as Catalysts for the Reduction of CO2. Chem. Soc. Rev. 2017, 46, 761–796. [Google Scholar] [CrossRef]
- Hawecker, J.; Lehn, J.-M.; Ziessel, R. Photochemical and Electrochemical Reduction of Carbon Dioxide to Carbon Monoxide Mediated by (2,2′-Bipyridine)tricarbonylchlororhenium(I) and Related Complexes as Homogeneous Catalysts. Helv. Chim. Acta 1986, 69, 1990–2012. [Google Scholar] [CrossRef]
- Hawecker, J.; Lehn, J.-M.; Ziessel, R. Efficient Photochemical Reduction of CO2 to CO by Visible Light Irradiation of Systems Containing Re(bipy)(CO)3X or Ru(bipy)32+–Co2+ Combinations as Homogeneous Catalysts. J. Chem. Soc. Chem. Commun. 1983, 9, 536–538. [Google Scholar] [CrossRef]
- Gabrielsson, A.; Záliš, S.; Matousek, P.; Towrie, M.; Vlček, A. Ultrafast Photochemical Dissociation of an Equatorial CO Ligand from trans(X,X)-[Ru(X)2(CO)2(bpy)] (X = Cl, Br, I): A Picosecond Time-Resolved Infrared Spectroscopic and DFT Computational Study. Inorg. Chem. 2004, 43, 7380–7388. [Google Scholar] [CrossRef] [PubMed]
- Pinnick, D.V.; Durham, B. Photosubstitution Reactions of Ru(bpy)2XYn+ Complexes. Inorg. Chem. 1984, 23, 1440–1445. [Google Scholar] [CrossRef]
- Stor, G.J.; Morrison, S.L.; Stufkens, D.L.; Oskam, A. The Remarkable Photochemistry of fac-XMn(CO)3(α-diimine) (X = Halide): Formation of Mn2(CO)6(α-diimine)2 via the mer Isomer and Photocatalytic Substitution of X− in the Presence of PR3. Organometallics 1994, 13, 2641–2650. [Google Scholar] [CrossRef]
- Li, G.; Zhu, D.; Wang, X.; Su, Z.; Bryce, M.R. Dinuclear Metal Complexes: Multifunctional Properties and Applications. Chem. Soc. Rev. 2020, 49, 765–838. [Google Scholar] [CrossRef]
- Prier, C.K.; Rankic, D.A.; MacMillan, D.W.C. Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem. Rev. 2013, 113, 5322–5363. [Google Scholar] [CrossRef]
- Lehn, J.-M.; Ziessel, R. Photochemical Reduction of Carbon Dioxide to Formate Catalyzed by 2,2′-Bipyridine- or 1,10-Phenanthroline-Ruthenium(II) Complexes. J. Organomet. Chem. 1990, 382, 157–173. [Google Scholar] [CrossRef]
- Ishida, H.; Terada, T.; Tanaka, K.; Tanaka, T. Photochemical Carbon Dioxide Reduction Catalyzed by Bis(2,2′-bipyridine)dicarbonylruthenium(2+) Using Triethanolamine and 1-Benzyl-1,4-dihydronicotinamide as an Electron Donor. Inorg. Chem. 1990, 29, 905–911. [Google Scholar] [CrossRef]
- Ishida, H.; Tanaka, K.; Tanaka, T. Photoreduction of CO2 in the [Ru(bpy)2(CO)2]2+/[Ru(bpy)3]2+ or [Ru(phen)3]2+/Triethanolamine/N,N-Dimethylformamide System. Chem. Lett. 1987, 16, 1035–1036. [Google Scholar] [CrossRef]
- Ishida, H.; Tanaka, K.; Tanaka, T. Photochemical CO2 Reduction by an NADH Model Compound in the Presence of [Ru(bpy)3]2+ and [Ru(bpy)2(CO)2]2+ (bpy = 2,2′-Bipyridine) in H2O/DMF. Chem. Lett. 1988, 17, 339–342. [Google Scholar] [CrossRef]
- Tamaki, Y.; Morimoto, T.; Koike, K.; Ishitani, O. Photocatalytic Reduction of CO2 with a Ru(II)-Re(I) Supramolecular Complex under Visible Light: Detailed Mechanistic Study. Proc. Natl. Acad. Sci. USA. 2012, 109, 15673–15678. [Google Scholar] [CrossRef] [PubMed]
- Voyame, P.; Toghill, K.E.; Méndez, M.A.; Girault, H.H. Photoreduction of CO2 Using [Ru(bpy)2(CO)L]n+ Catalysts in Biphasic Solution/Supercritical CO2 Systems. Inorg. Chem. 2013, 52, 10949–10957. [Google Scholar] [CrossRef][Green Version]
- Paul, A.; Connolly, D.; Schulz, M.; Pryce, M.T.; Vos, J.G. Effect of Water during the Quantitation of Formate in Photocatalytic Studies on CO2 Reduction in Dimethylformamide. Inorg. Chem. 2012, 51, 1977–1979. [Google Scholar] [CrossRef]
- Kuramochi, Y.; Kamiya, M.; Ishida, H. Photocatalytic CO2 Reduction in N,N-Dimethylacetamide/Water as an Alternative Solvent System. Inorg. Chem. 2014, 53, 3326–3332. [Google Scholar] [CrossRef]
- Kuramochi, Y.; Hashimoto, M.; Satake, A. Methane Formation Induced via Face-to-Face Orientation of Cyclic Fe Porphyrin Dimer in Photocatalytic CO2 Reduction. Molecules 2024, 29, 2453. [Google Scholar] [CrossRef]
- An, D.; Nishioka, S.; Yasuda, S.; Kanazawa, T.; Kamakura, Y.; Yokoi, T.; Nozawa, S.; Maeda, K. Alumina-Supported Alpha-Iron(III) Oxyhydroxide as a Recyclable Solid Catalyst for CO2 Photoreduction under Visible Light. Angew. Chem. Int. Ed. 2022, 61, e202204948. [Google Scholar] [CrossRef]
- Rotundo, L.; Grills, D.C.; Gobetto, R.; Priola, E.; Nervi, C.; Polyansky, D.E.; Fujita, E. Photochemical CO2 Reduction Using Rhenium(I) Tricarbonyl Complexes with Bipyridyl-Type Ligands with and without Second Coordination Sphere Effects. ChemPhotoChem 2021, 5, 526–537. [Google Scholar] [CrossRef]
- Hong, D.; Kawanishi, T.; Tsukakoshi, Y.; Kotani, H.; Ishizuka, T.; Kojima, T. Efficient Photocatalytic CO2 Reduction by a Ni(II) Complex Having Pyridine Pendants through Capturing a Mg2+ Ion as a Lewis-Acid Cocatalyst. J. Am. Chem. Soc. 2019, 141, 20309–20317. [Google Scholar] [CrossRef]
- Lee, S.K.; Kondo, M.; Okamura, M.; Enomoto, T.; Nakamura, G.; Masaoka, S. Function-Integrated Ru Catalyst for Photochemical CO2 Reduction. J. Am. Chem. Soc. 2018, 140, 16899–16903. [Google Scholar] [CrossRef]
- Kuriki, R.; Sekizawa, K.; Ishitani, O.; Maeda, K. Visible-Light-Driven CO2 Reduction with Carbon Nitride: Enhancing the Activity of Ruthenium Catalysts. Angew. Chem. Int. Ed. 2015, 54, 2406. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Arai, T.; Morikawa, T.; Uemura, K.; Suzuki, T.M.; Tanaka, H.; Kajino, T. Selective CO2 Conversion to Formate Conjugated with H2O Oxidation Utilizing Semiconductor/Complex Hybrid Photocatalysts. J. Am. Chem. Soc. 2011, 133, 15240–15243. [Google Scholar] [CrossRef]
- Suzuki, T.M.; Yoshino, S.; Takayama, T.; Iwase, A.; Kudo, A.; Morikawa, T. Z-Schematic and Visible-Light-Driven CO2 Reduction Using H2O as an Electron Donor by a Particulate Mixture of a Ru-Complex/(CuGa)1−xZn2xS2 Hybrid Catalyst, BiVO4, and an Electron Mediator. Chem. Commun. 2018, 54, 10199–10202. [Google Scholar] [CrossRef]
- Kuramochi, Y.; Fukaya, K.; Yoshida, M.; Ishida, H. Trans-(Cl)-[Ru(5,5′-Diamide-2,2′-Bipyridine)(CO)2Cl2]: Synthesis, Structure, and Photocatalytic CO2 Reduction Activity. Chem. Eur. J. 2015, 21, 10049–10060. [Google Scholar] [CrossRef]
- Goodwin, M.J.; Dickenson, J.J.; Ripak, A.; Deetz, A.M.; McCarthy, J.S.; Meyer, G.J.; Troian-Gautier, L. Factors that Impact Photochemical Cage Escape Yields. Chem. Rev. 2024, 124, 7379–7464. [Google Scholar] [CrossRef] [PubMed]
- Nakada, A.; Koike, K.; Nakashima, T.; Morimoto, T.; Ishitani, O. Photocatalytic CO2 Reduction to Formic Acid Using a Ru(II)–Re(I) Supramolecular Complex in an Aqueous Solution. Inorg. Chem. 2015, 54, 1800–1807. [Google Scholar] [CrossRef]
- Ozawa, K.; Tamaki, Y.; Kamogawa, K.; Koike, K.; Ishitani, O. Ishitani, Factors determining formation efficiencies of one-electron-reduced species of redox photosensitizers. J. Chem. Phys. 2020, 153, 154302. [Google Scholar] [CrossRef] [PubMed]
- Draper, F.; DiLuzio, S.; Sayre, H.J.; Pham, L.N.; Coote, M.L.; Doeven, E.H.; Francis, P.S.; Connell, T.U. Maximizing Photon-to-Electron Conversion for Atom Efficient Photoredox Catalysis. J. Am. Chem. Soc. 2024, 146, 26830–26843. [Google Scholar] [CrossRef]
- Ripak, A.; Vega Salgado, A.K.; Valverde, D.; Cristofaro, S.; de Gary, A.; Olivier, Y.; Elias, B.; Troian-Gautier, L. Factors Controlling Cage Escape Yields of Closed—and Open-Shell Metal Complexes in Bimolecular Photoinduced Electron Transfer. J. Am. Chem. Soc. 2024, 146, 22818–22828. [Google Scholar] [CrossRef]
- De Kreijger, S.; Ripak, A.; Elias, B.; Troian-Gautier, L. Investigation of the Excited-State Electron Transfer and Cage Escape Yields Between Halides and a Fe(III) Photosensitizer. J. Am. Chem. Soc. 2024, 146, 10286–10292. [Google Scholar] [CrossRef]
- Wang, C.; Li, H.; Bürgin, T.H.; Wenger, O.S. Cage Escape Governs Photoredox Reaction Rates and Quantum Yields. Nat. Chem. 2024, 16, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Ishida, H.; Fujiki, K.; Ohba, T.; Ohkubo, K.; Tanaka, K.; Terada, T.; Tanaka, T. Ligand Effects of Ruthenium 2,2′-Bipyridine and 1,10-Phenanthroline Complexes on the Electrochemical Reduction of CO2. J. Chem. Soc., Dalton Trans. 1990, 10, 2155–2160. [Google Scholar] [CrossRef]
- Ishida, H.; Tanaka, K.; Tanaka, T. Electrochemical CO2 Reduction Catalyzed by [Ru(bpy)2(CO)2]2+ and [Ru(bpy)2(CO)CI]+. The Effect of pH on the Formation of CO and HCOO−. Organometallics 1987, 6, 181–186. [Google Scholar] [CrossRef]
- Chardon-Noblat, S.; Deronzier, A.; Ziessel, R.; Zsoldos, D. Electroreduction of CO2 Catalyzed by Polymeric [Ru(bpy)(CO)2]n Films in Aqueous Media: Parameters Influencing the Reaction Selectivity. J. Electroanal. Chem. 1998, 444, 253–260. [Google Scholar] [CrossRef]
- Chardon-Noblat, S.; Deronzier, A.; Ziessel, R.; Zsoldos, D. Selective Synthesis and Electrochemical Behavior of trans(Cl)- and cis(Cl)-[Ru(bpy)(CO)2Cl2] Complexes (bpy = 2,2′-Bipyridine). Comparative Studies of Their Electrocatalytic Activity toward the Reduction of Carbon Dioxide. Inorg. Chem. 1997, 36, 5384–5389. [Google Scholar] [CrossRef]
- Chardon-Noblat, S.; Collomb-Dunand-Sauthier, M.N.; Deronzier, A.; Ziessel, R.; Zsoldos, D. Formation of Polymeric [{Ru0(bpy)(CO)2}n] Films by Electrochemical Reduction of [Ru(bpy)2(CO)2](PF6)2: Its Implication in CO2 Electrocatalytic Reduction. Inorg. Chem. 1994, 33, 4410–4412. [Google Scholar] [CrossRef]
- Collomb-Dunand-Sauthier, M.N.; Deronzier, A.; Ziessel, R. Electrocatalytic Reduction of Carbon Dioxide with Mono(bipyridine)carbonylruthenium Complexes in Solution or as Polymeric Thin Films. Inorg. Chem. 1994, 33, 2961–2967. [Google Scholar] [CrossRef]
- Collomb-Dunand-Sauthier, M.N.; Deronzier, A.; Ziessel, R. Electrocatalytic Reduction of CO2 in Water on a Polymeric [{Ru0(bpy)(CO)2}n] (bpy = 2,2′-Bipyridine) Complex Immobilized on Carbon Electrodes. J. Chem. Soc. Chem. Commun. 1994, 189–191. [Google Scholar] [CrossRef]
- Machan, C.W.; Sampson, M.D.; Kubiak, C.P. A Molecular Ruthenium Electrocatalyst for the Reduction of Carbon Dioxide to CO and Formate. J. Am. Chem. Soc. 2015, 137, 8564–8571. [Google Scholar]
- Kuramochi, Y.; Itabashi, J.; Toyama, M.; Ishida, H. Photochemical CO2 Reduction Catalyzed by trans(Cl)-[Ru(2,2′-Bipyridine)(CO)2Cl2] Bearing Two Methyl Groups at 4,4′-, 5,5′- or 6,6′-Positions in the Ligand. ChemPhotoChem 2018, 2, 314–322. [Google Scholar] [CrossRef]
- Kuramochi, Y.; Itabashi, J.; Fukaya, K.; Enomoto, A.; Yoshida, M.; Ishida, H. Unexpected Effect of Catalyst Concentration on Photochemical CO2 Reduction by trans(Cl)–Ru(bpy)(CO)2Cl2: New Mechanistic Insight into the CO/HCOO− Selectivity. Chem. Sci. 2015, 6, 3063–3074. [Google Scholar] [CrossRef] [PubMed]
- Kavarnos, G.J. Fundamentals of Photoinduced Electron Transfer; VCH Publishers: New York, NY, USA, 1993. [Google Scholar]
- Thompson, D.W.; Ito, A.; Meyer, T.J. [Ru(bpy)3]2+* and other remarkable metal-to-ligand charge transfer (MLCT) excited states. Pure. Appl. Chem. 2013, 85, 1257–1305. [Google Scholar] [CrossRef]
- Bock, C.R.; Connor, J.A.; Gutierrez, A.R.; Meyer, T.J.; Whitten, D.G.; Sullivan, B.P.; Nagle, J.K. Estimation of Excited-State Redox Potentials by Electron-Transfer Quenching. Application of Electron-Transfer Theory to Excited-State Redox Processes. J. Am. Chem. Soc. 1979, 101, 4815–4824. [Google Scholar] [CrossRef]
- Kim, H.-B.; Kitamura, N.; Kawanishi, Y.; Tazuke, S. Photoinduced Electron-Transfer Reactions of Ruthenium(II) Complexes. 2. Oxidative Quenching of Excited Ru(bpy)32+ by Neutral Organic Electron Acceptors. J. Phys. Chem. 1989, 93, 5757–5764. [Google Scholar] [CrossRef]
- Kitamura, N.; Kim, H.-B.; Okano, S.; Tazuke, S. Photoinduced Electron-Transfer Reactions of Ruthenium(II) Complexes. 1. Reductive Quenching of Excited Ru(bpy)32+ by Aromatic Amines. J. Phys. Chem. 1989, 93, 5750–5756. [Google Scholar] [CrossRef]
- Kim, H.-B.; Kitamura, N.; Kawanishi, Y.; Tazuke, S. Bell-Shaped Temperature Dependence in Quenching of Excited Ru(bpy)32+ by Organic Acceptor. J. Am. Chem. Soc. 1987, 109, 2506–2508. [Google Scholar] [CrossRef]
- Sun, H.; Hoffman, M.Z. Reductive Quenching of the Excited States of Ruthenium(II) Complexes Containing 2,2′-Bipyridine, 2,2′-Bipyrazine, and 2,2′-Bipyrimidine Ligands. J. Phys. Chem. 1994, 98, 11719–11726. [Google Scholar] [CrossRef]
- Hoffman, M.Z. Cage Escape Yields from the Quenching of *Ru(bpy)32+ by Methylviologen in Aqueous Solution. J. Phys. Chem. 1988, 92, 3458–3464. [Google Scholar] [CrossRef]
- Prasad, D.R.; Hessler, D.; Hoffman, M.Z. Quantum yield of formation of methylviologen radical cation from the photolysis of the Ru(bpz)32+/methylviologen/EDTA system. Chem. Phys. Lett. 1985, 121, 61–64. [Google Scholar] [CrossRef]
- Petersen, R.C. Petersen, Interactions in the binary liquid system N,N-Dimethylacetamide—Water: Viscosity and density. J. Phys. Chem. 1960, 64, 184–185. [Google Scholar] [CrossRef]
- Assarsson, P.; Eirich, F.R. Properties of Amides in Aqueous Solution. I. A. Viscosity and Density Changes of Amide-Water Systems. B. An Analysis of Volume Deficiencies of Mixtures Based on Molecular Size Differences (Mixing of Hard Spheres). J. Phys. Chem. 1968, 72, 2710–2719. [Google Scholar] [CrossRef]
- Hamada, T.; Tsukamoto, M.; Ohtsuka, H.; Sakaki, S. Charge Effects in Photoinduced Electron-Transfer Reactions between [Ru(bpy)3]2+ and Viologen Derivatives. Bull. Chem. Soc. Jpn. 1998, 71, 2281–2291. [Google Scholar] [CrossRef]
- Clark, C.D.; Hoffman, M.Z. Ion-Pairing Control of Excited-State Electron-Transfer Reactions. Quenching, Charge Recombination, and Back Electron Transfer. J. Phys. Chem. 1996, 100, 7526–7532. [Google Scholar] [CrossRef]
- Pac, C.; Miyauchi, Y.; Ishitani, O.; Ihama, M.; Yasuda, M.; Sakurai, H. Redox-Photosensitized Reactions. II. Ru(bpy)32+-Photosensitized Reactions of 1-Benzyl-1,4-dihydronicotinamide with Aryl-Substituted Enones, Derivatives of Methyl Cinnamate, and Substituted Cinnamonitriles: Electron-Transfer Mechanism and Structure-Reactivity Relationships. J. Org. Chem. 1984, 49, 26–34. [Google Scholar]
- Fukuzumi, S.; Koumitsu, S.; Hironaka, K.; Tanaka, T. Energetic Comparison between Photoinduced Electron-Transfer Reactions from NADH Model Compounds to Organic and Inorganic Oxidants and Hydride-Transfer Reactions from NADH Model Compounds to p-Benzoquinone Derivatives. J. Am. Chem. Soc. 1987, 109, 305–316. [Google Scholar] [CrossRef]
- Sreenath, K.; Suneesh, C.V.; Gopidas, K.R.; Flowers, R.A., II. Generation of Triarylamine Radical Cations through Reaction of Triarylamines with Cu(II) in Acetonitrile. A Kinetic Investigation of the Electron-Transfer Reaction. J. Phys. Chem. A 2009, 113, 6477–6483. [Google Scholar] [CrossRef] [PubMed]
- Rohdewald, P.; Moldner, M. Dielectric Constants of Amide-Water Systems. J. Phys. Chem. 1973, 77, 373–377. [Google Scholar] [CrossRef]
- Aminabhavi, T.M.; Gopalakrishna, B. Density, Viscosity, Refractive Index, and Speed of Sound in Aqueous Mixtures of N,N-Dimethylformamide, Dimethyl Sulfoxide, N,N-Dimethylacetamide, Acetonitrile, Ethylene Glycol, Diethylene Glycol, 1,4-Dioxane, Tetrahydrofuran, 2-Methoxyethanol, and 2-Ethoxyethanol at 298.15 K. J. Chem. Eng. Data 1995, 40, 856–861. [Google Scholar]
- Ohno, T.; Yoshimura, A.; Mataga, N. Bell-Shaped Energy Gap Dependence of Backward Electron-Transfer Rate of Geminate Radical Pairs Produced by Electron-Transfer Quenching of Ru(II) Complexes by Aromatic Amines. J. Phys. Chem. 1986, 90, 3295–3297. [Google Scholar] [CrossRef]
- Kelly, J.M.; O’Connell, C.M.; Vos, J.G. Preparation, Spectroscopic Characterisation, Electrochemical and Photochemical Properties of cis-Bis(2,2’-Bipyridyl)Carbonylruthenium(II) Complexes. J. Chem. Soc. Dalton Trans. 1986, 253–258. [Google Scholar]
- Suzuki, K.; Kobayashi, A.; Kaneko, S.; Takehira, K.; Yoshihara, T.; Ishida, H.; Shiina, Y.; Oishi, S.; Tobita, S. Reevaluation of Absolute Luminescence Quantum Yields of Standard Solutions Using a Spectrometer with an Integrating Sphere and a Back-Thinned CCD Detector. Phys. Chem. Chem. Phys. 2009, 11, 9850–9860. [Google Scholar] [CrossRef]
- Mauzerall, D.; Westheimer, F.H. 1-Benzyldihydronicotinamide—A Model for Reduced DPN. J. Am. Chem. Soc. 1955, 77, 2261–2264. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).