Preparation and Properties of Flexible Phenolic Silicone Hybrid Aerogels for Thermal Insulation
Abstract
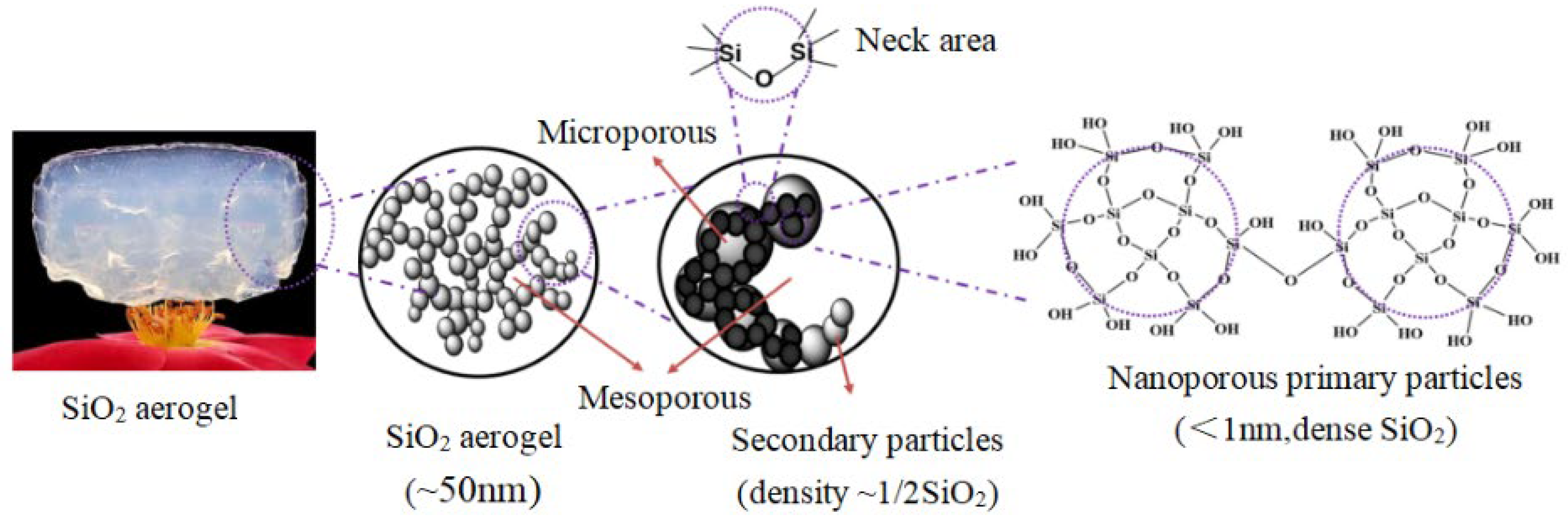
1. Introduction
2. Results and Discussion
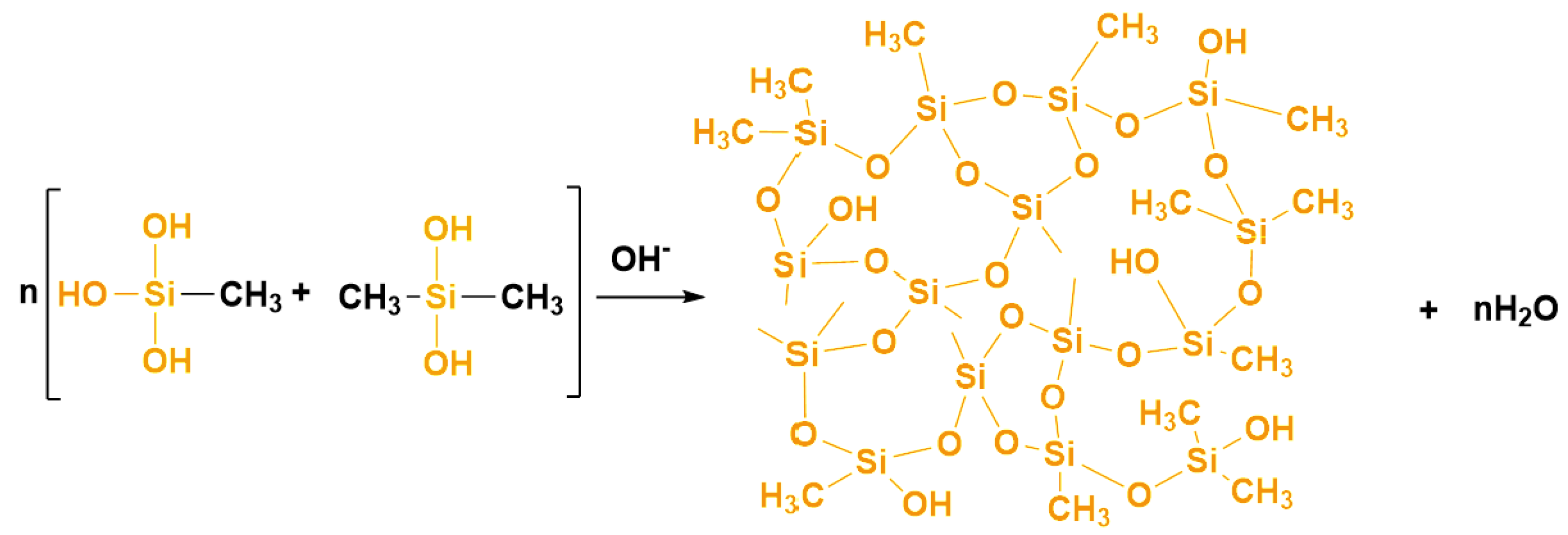
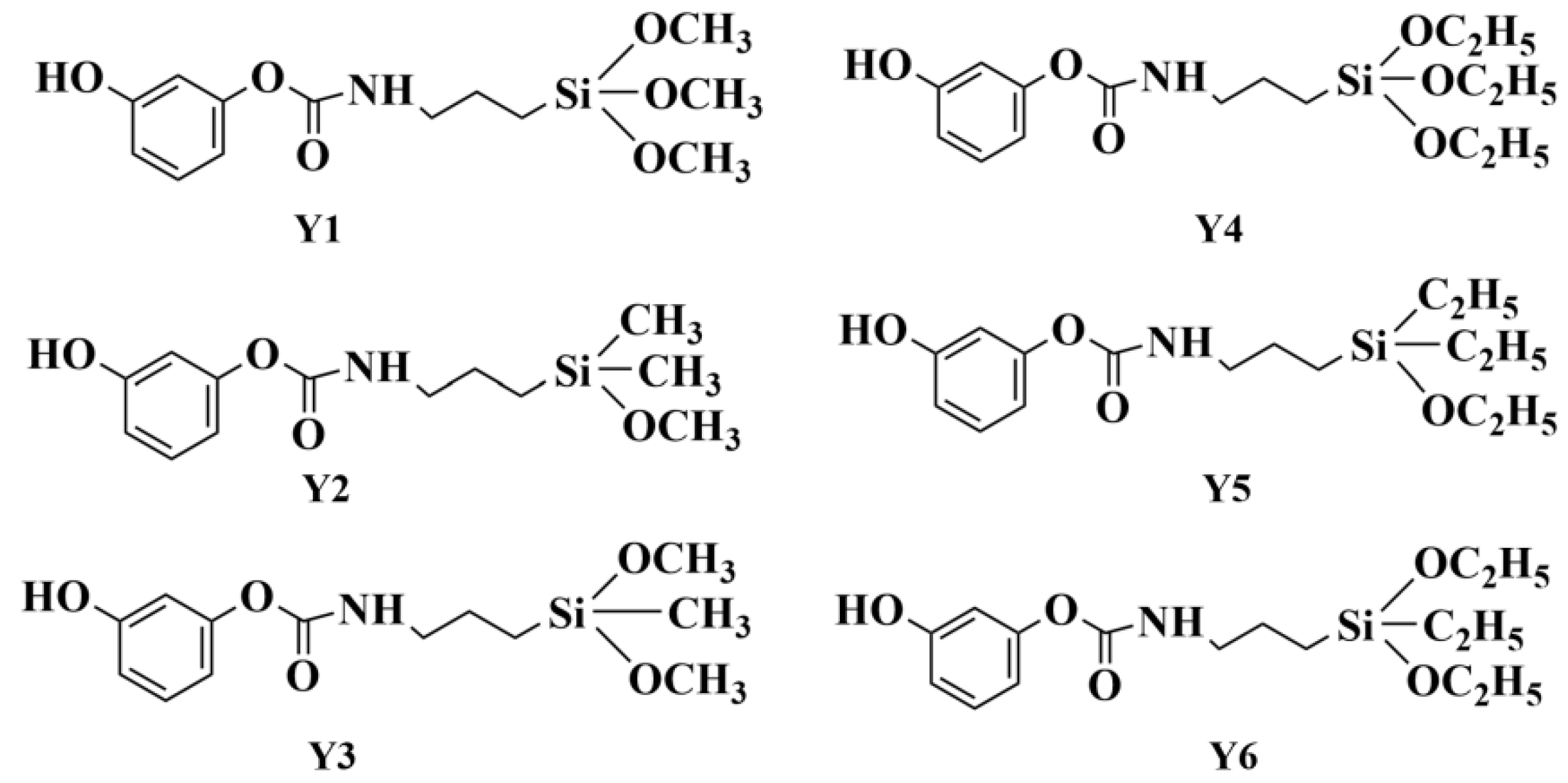
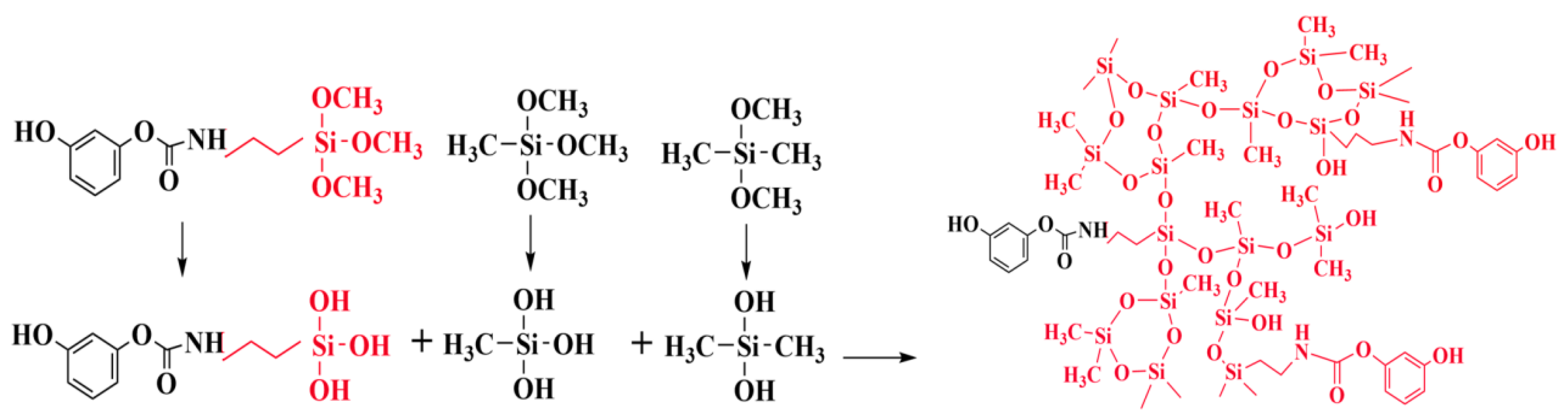
2.1. Reaction Mechanism of the DM Aerogel
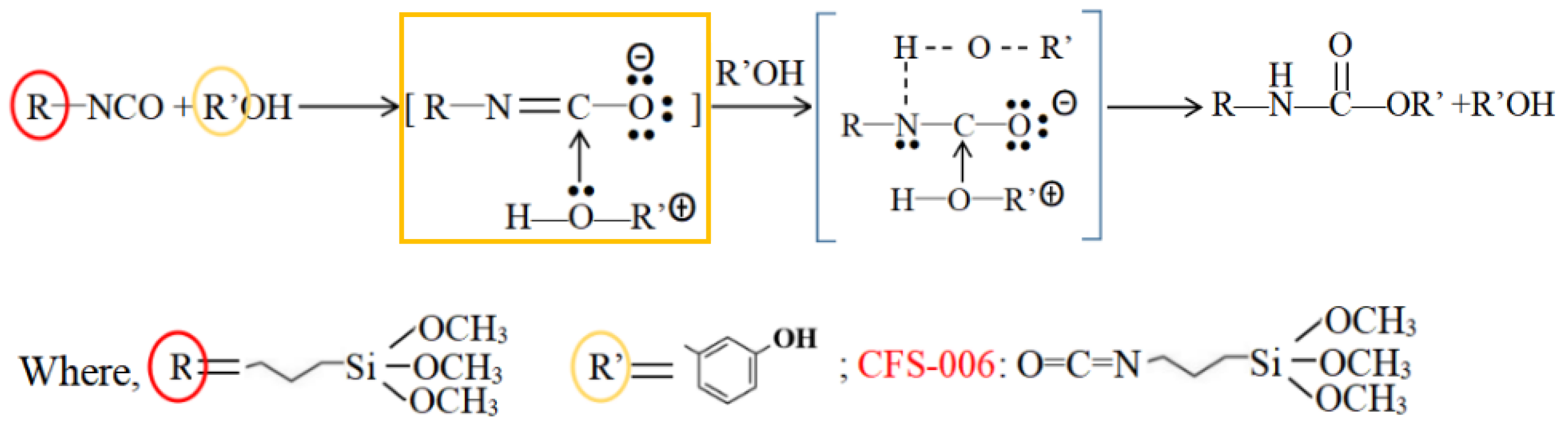
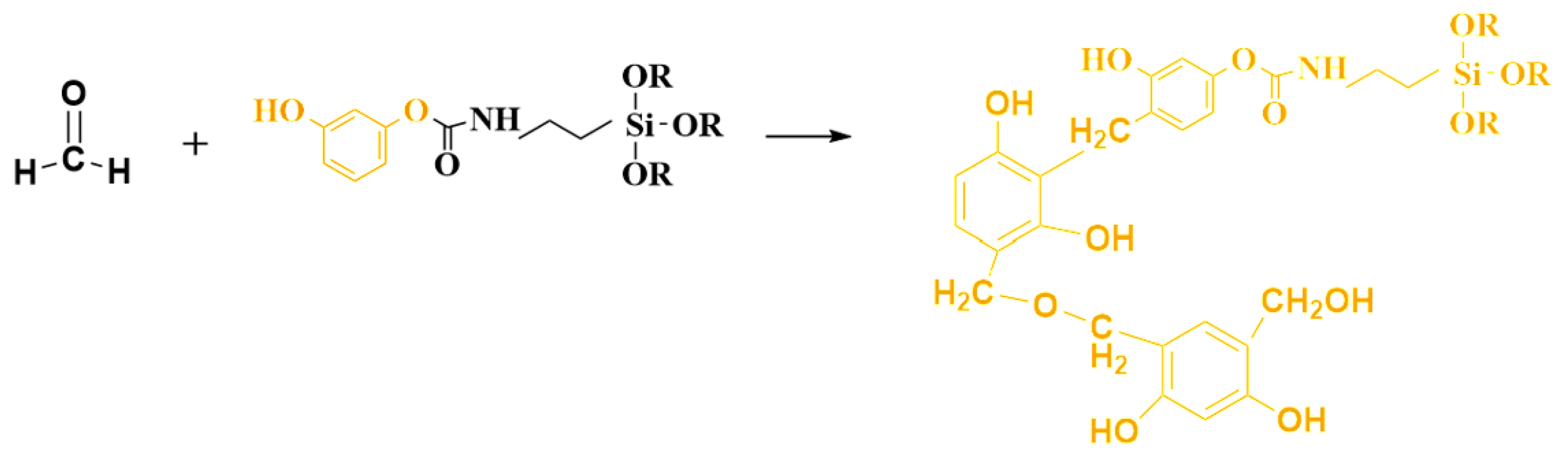
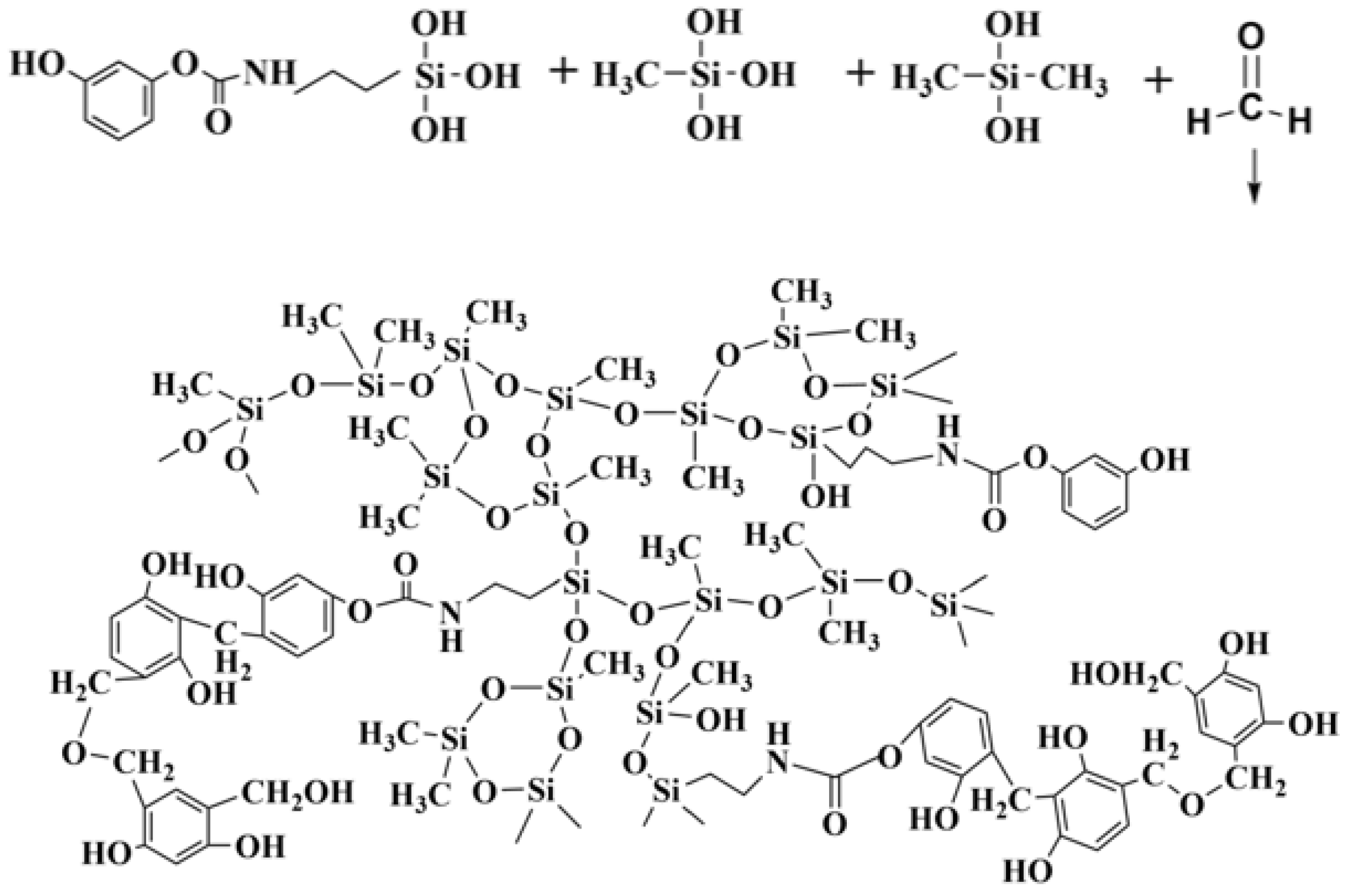
2.2. Hybrid Reaction of the Phenolic Silicone Aerogel
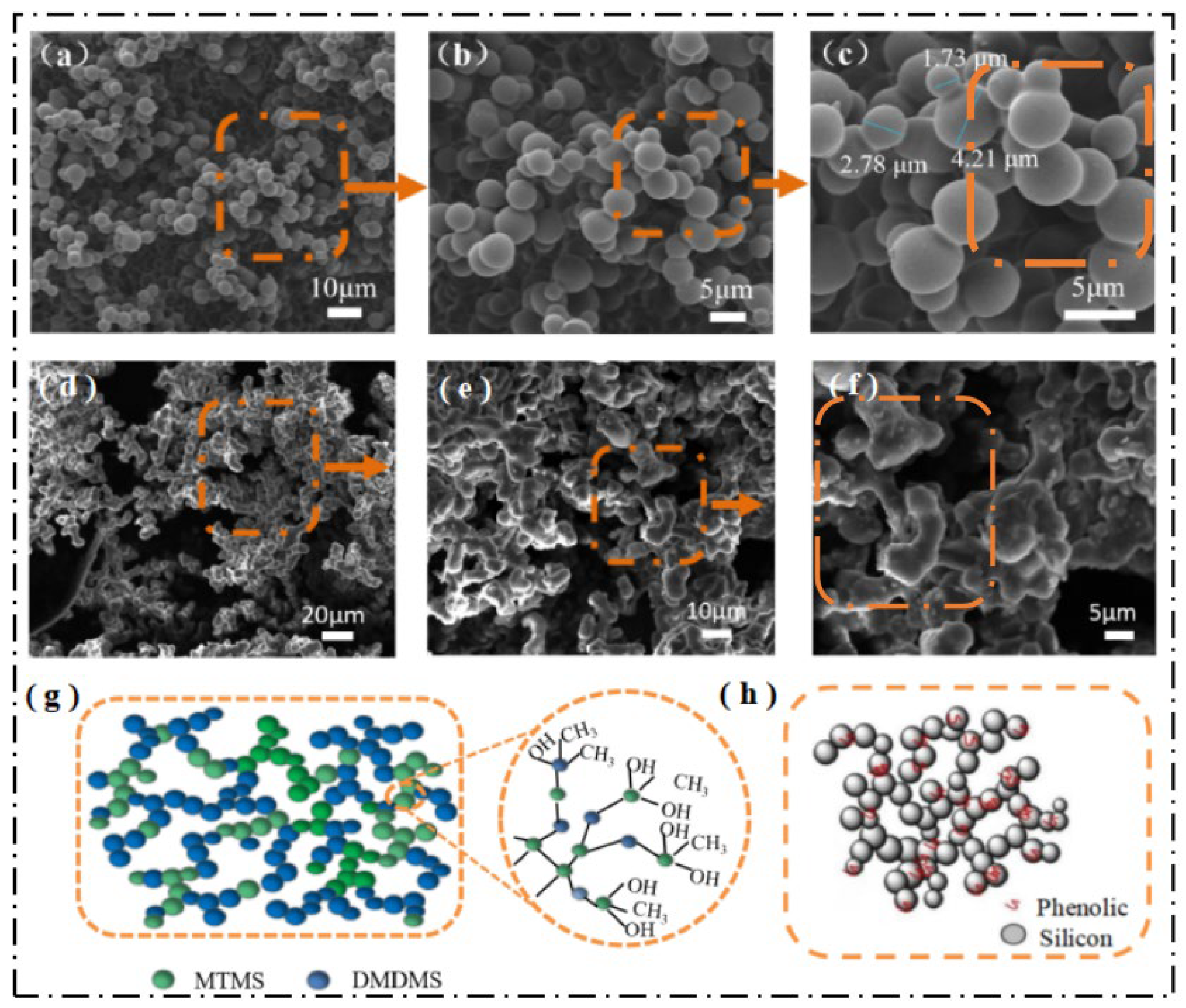
2.3. Surface Morphology
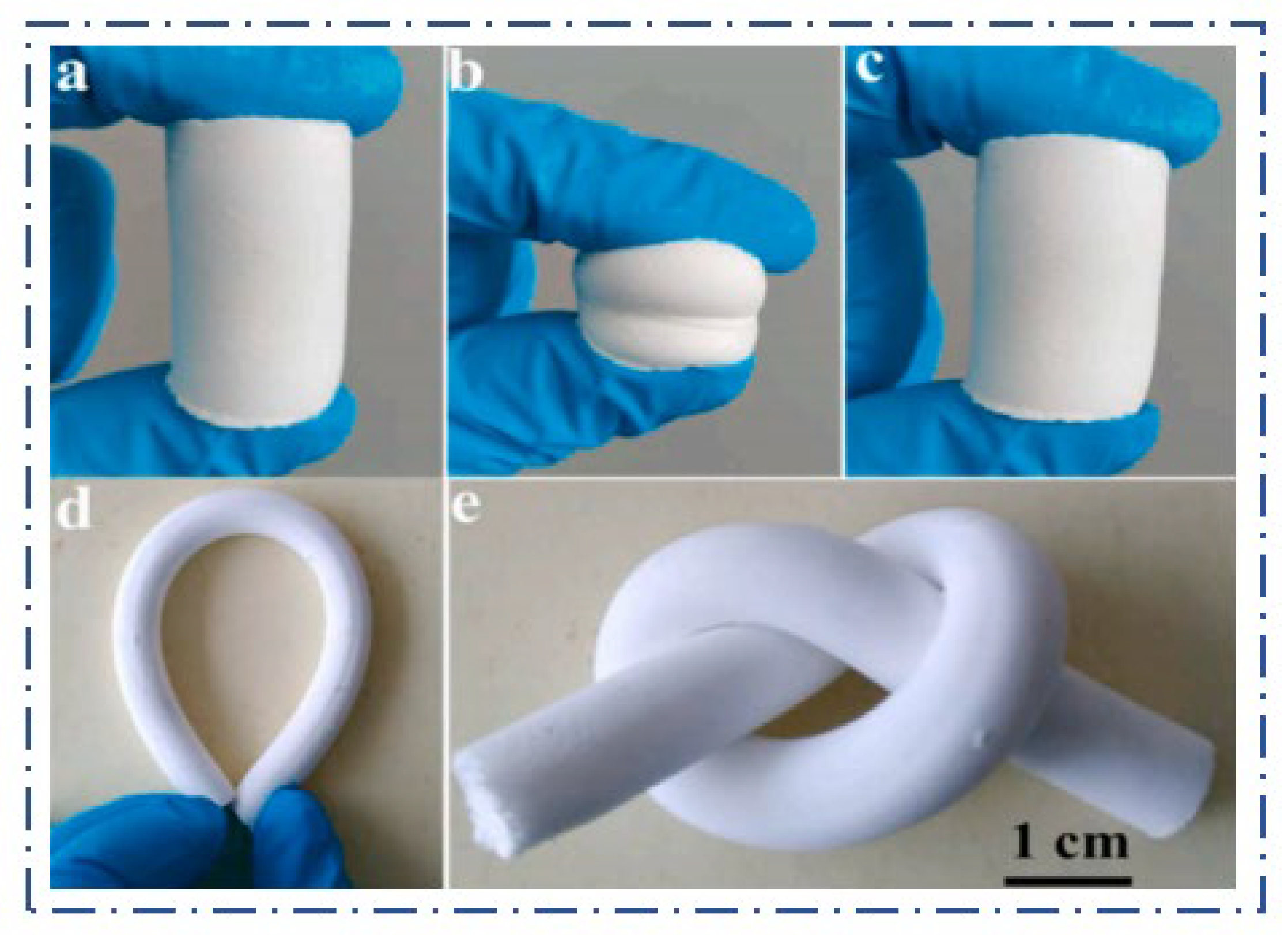
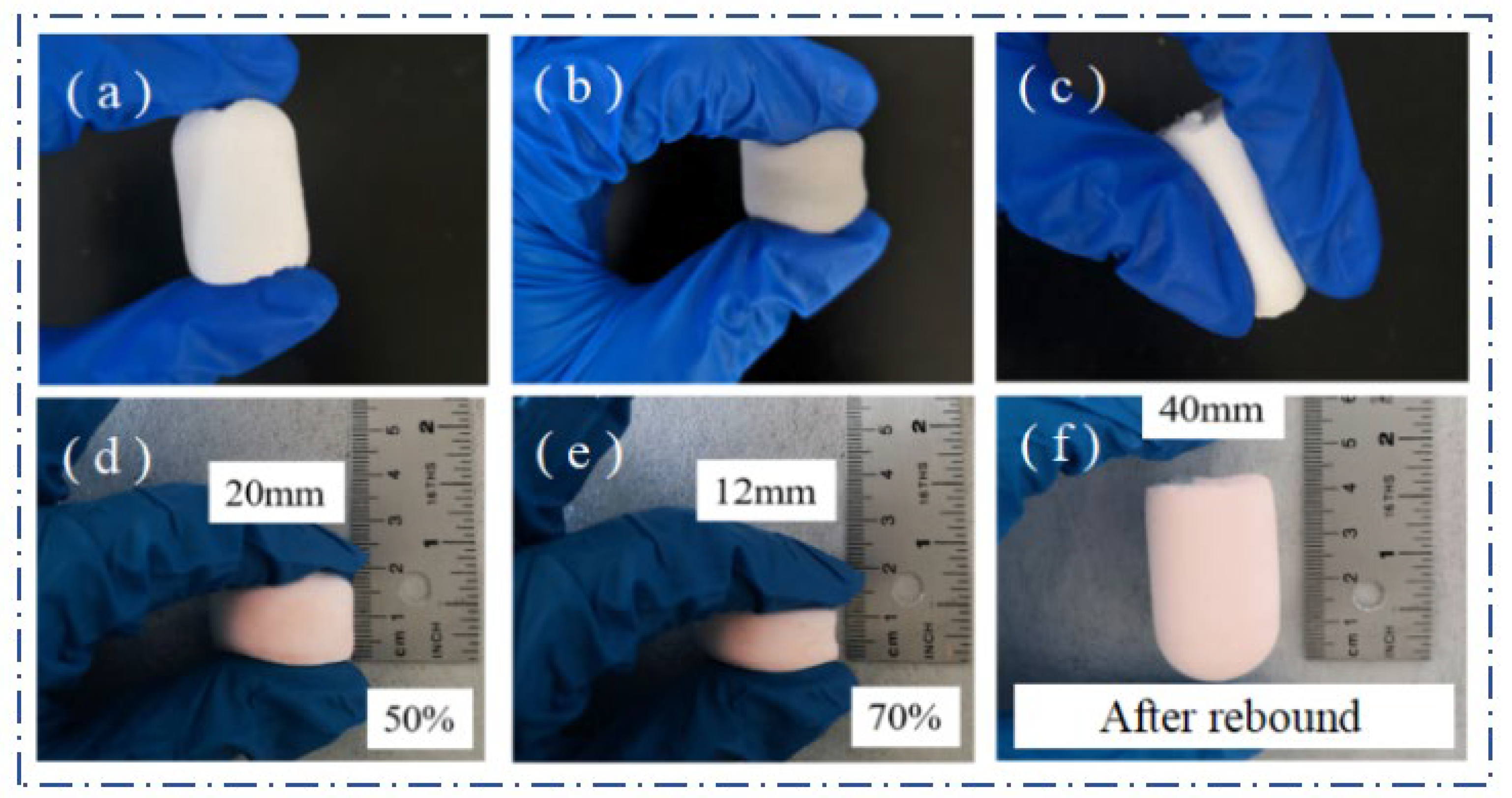
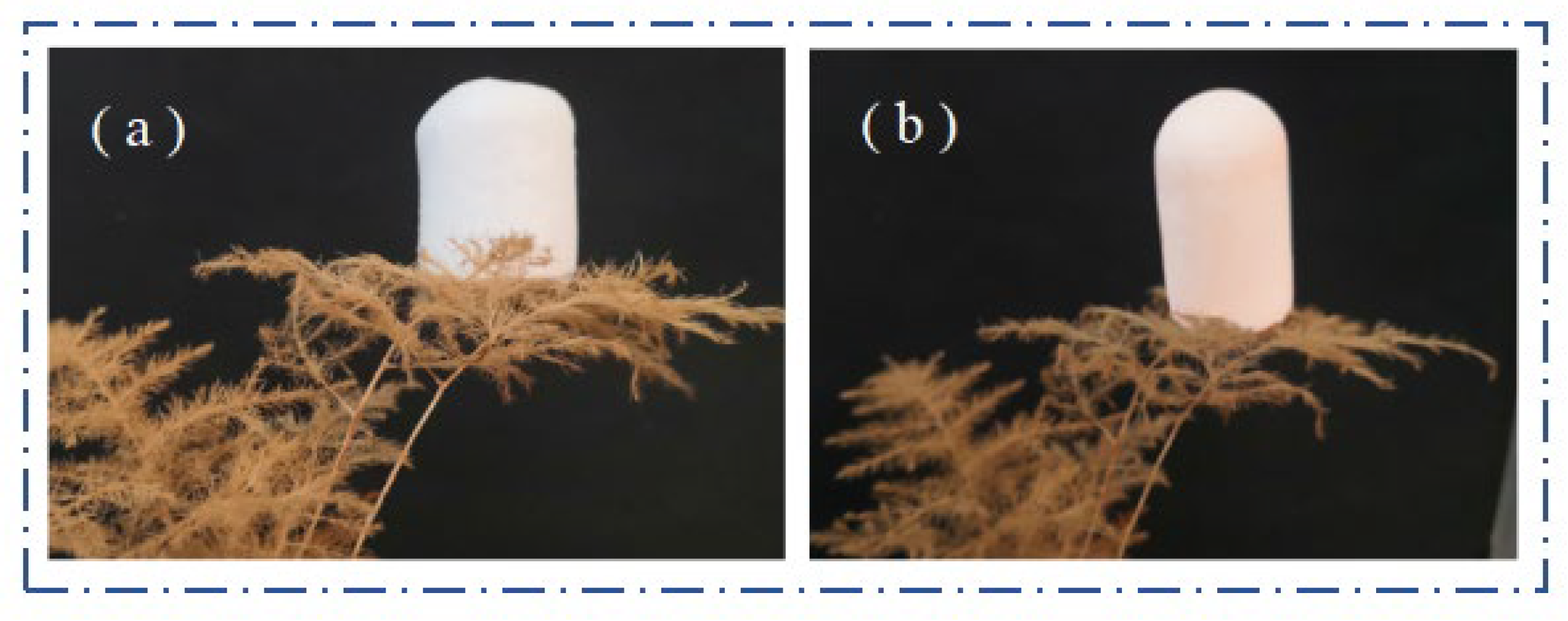
2.4. Mechanical Properties
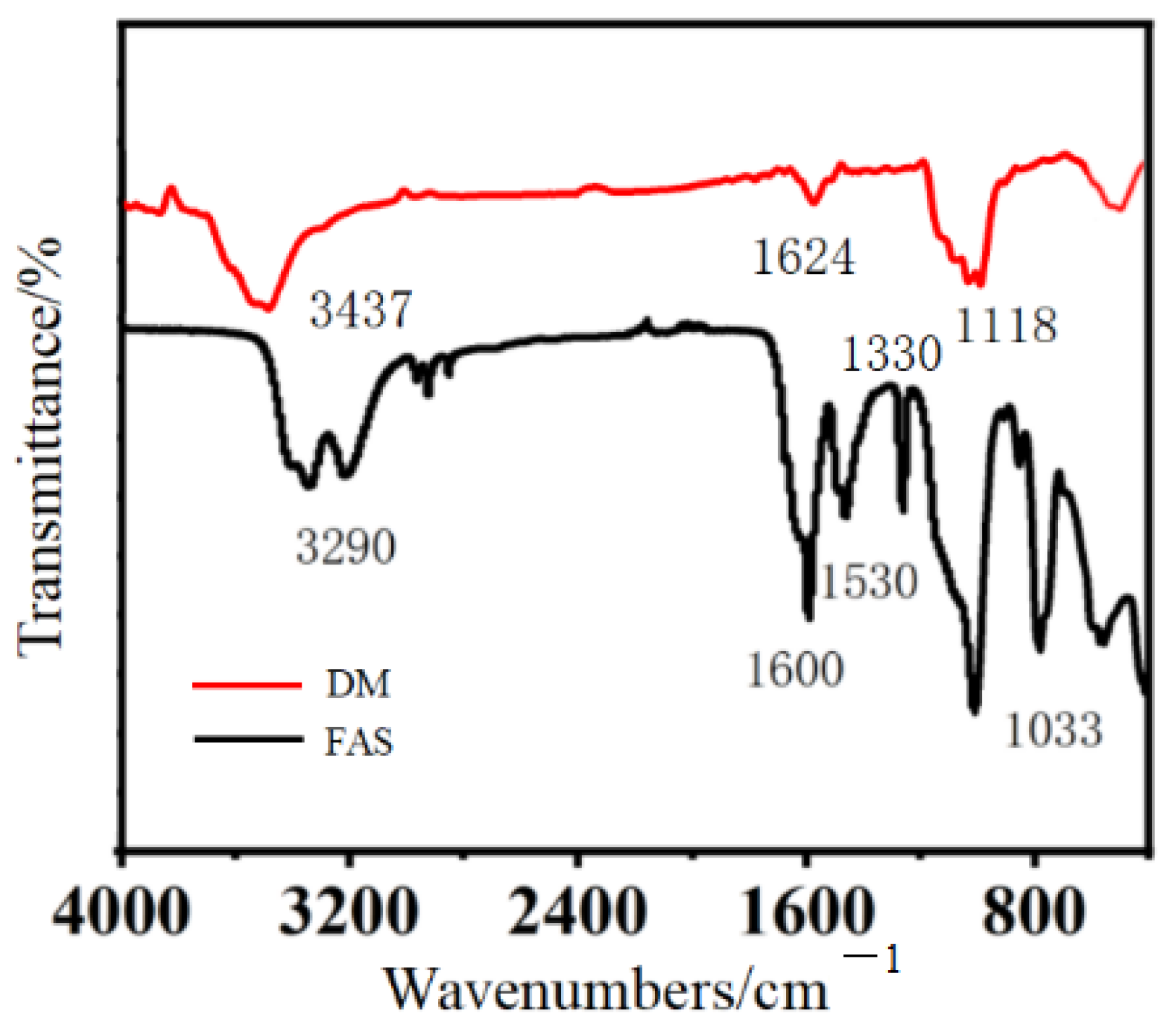
2.5. Infrared Spectroscopic Analysis
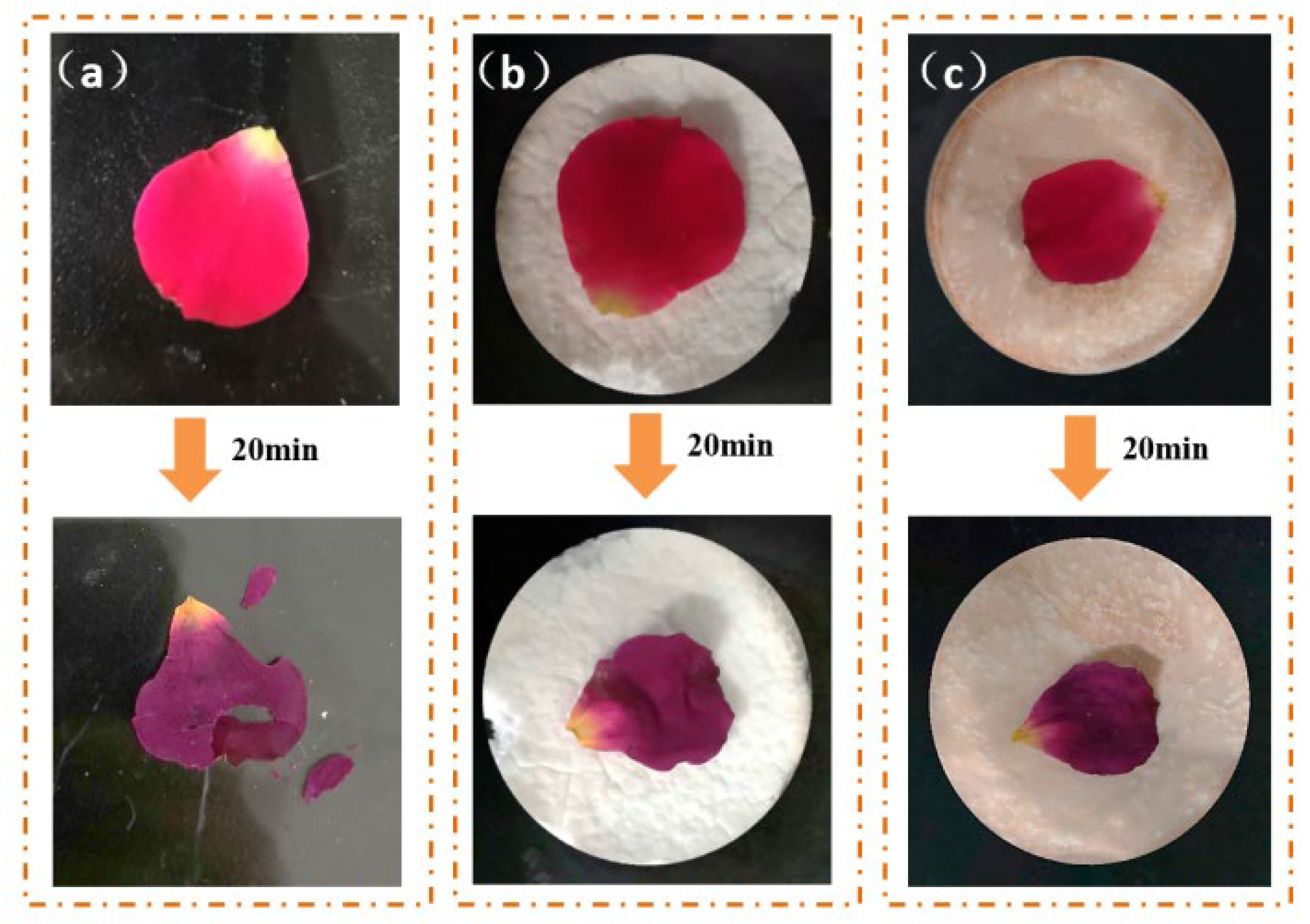
2.6. Thermal Insulation Properties
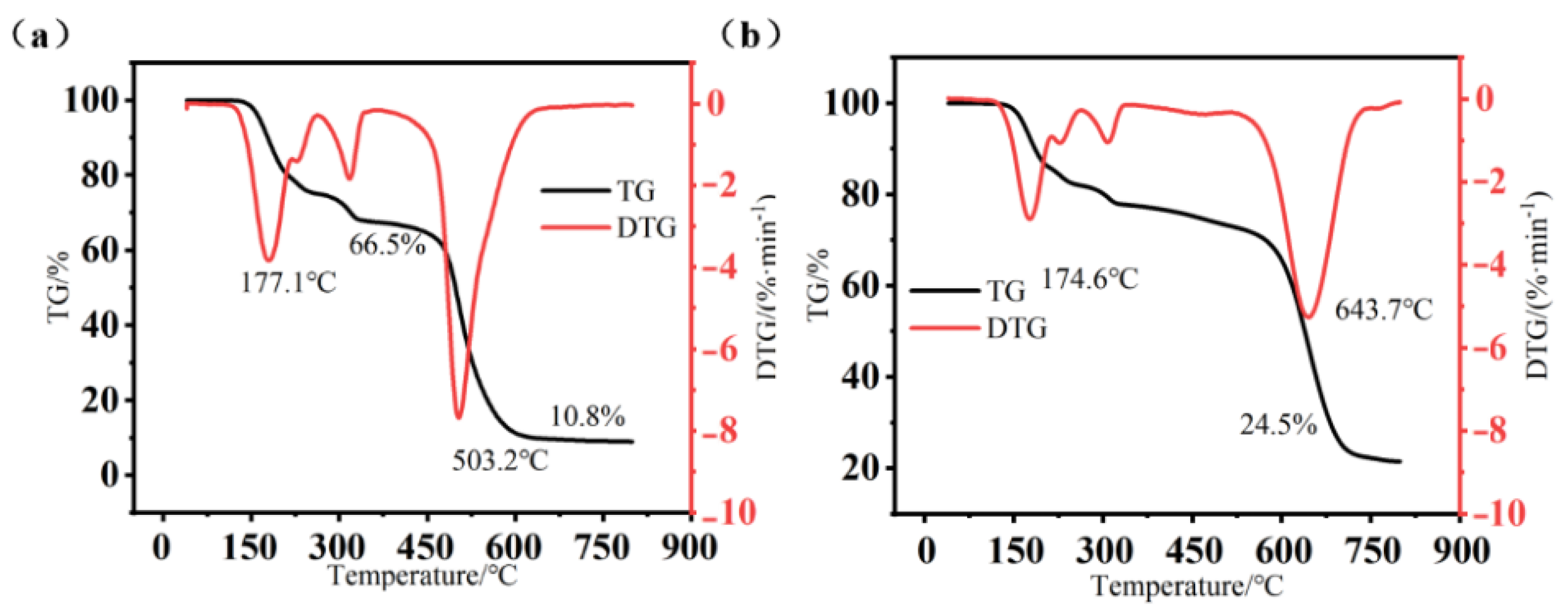
2.7. Thermal Stability Analysis
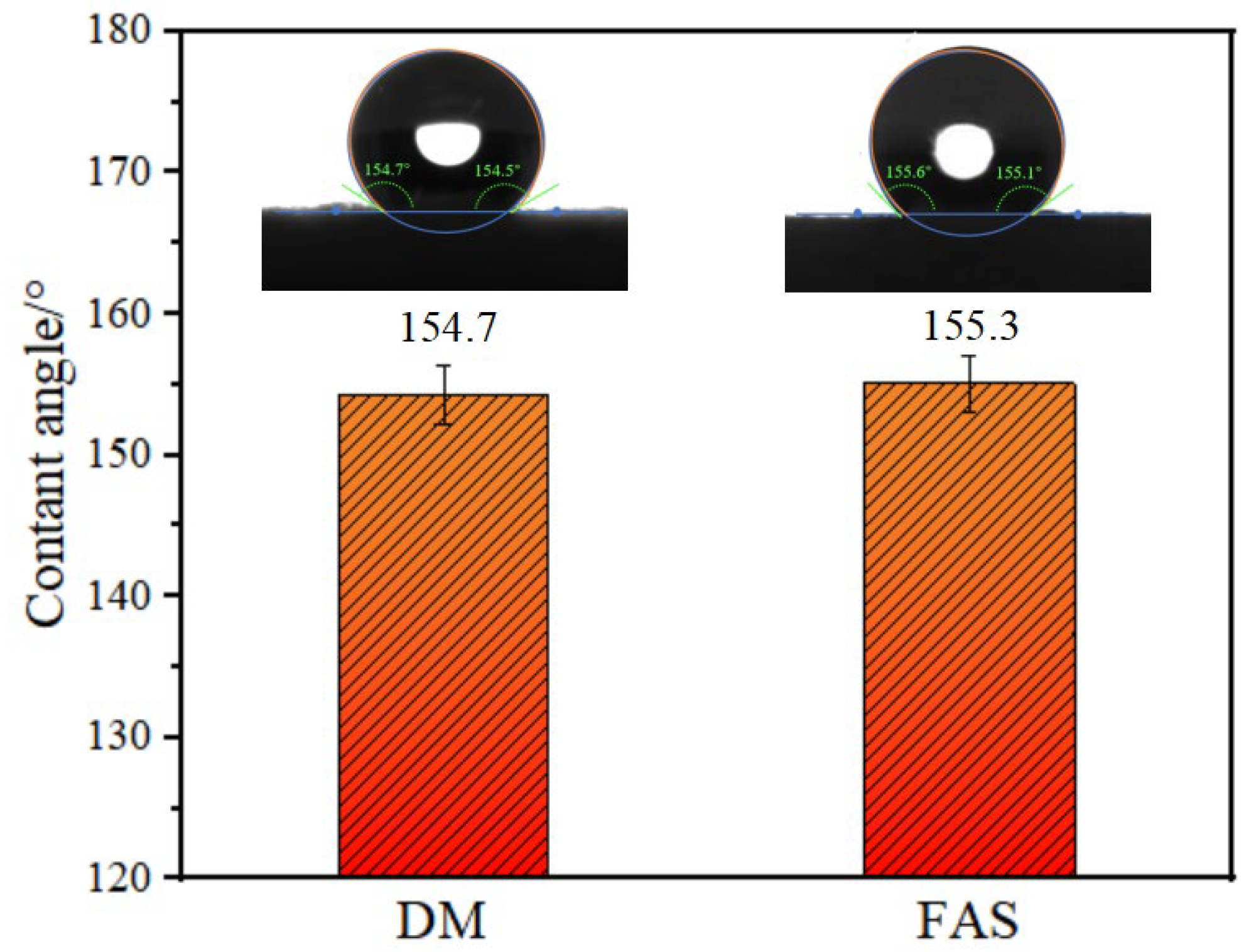
2.8. Hydrophobic Property Analysis
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Preparation of the Phenolic Silicone Hybrid FAS Aerogel
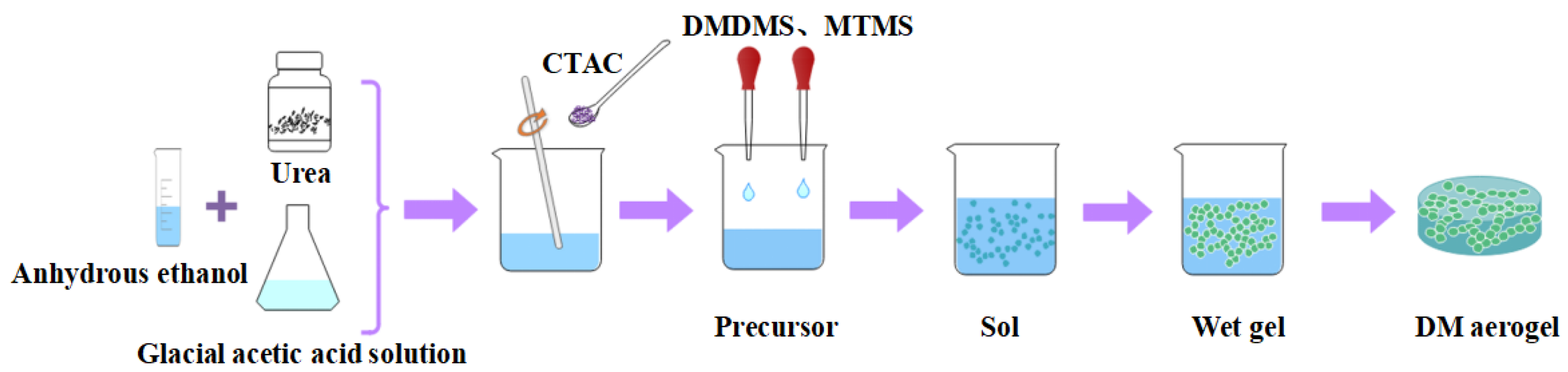
3.2.2. Preparation of the Flexible Silicone DM Aerogel
3.3. Characterization
3.3.1. Shrinkage Test
3.3.2. Microtopography Measurement
3.3.3. Infrared Spectrum Test
3.3.4. Thermogravimetric Test
3.3.5. Hydrophobic Performance Test
3.3.6. Thermal Conductivity Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jamshaid, J.M.; Kotresh, T.M.; Mishra, R.; Venkataraman, M. Aerogels for thermal insulation in high-performance textiles. Text. Prog. 2016, 48, 55–118. [Google Scholar]
- Deng, M.; Jiang, L.L.; Li, J.; Wang, Y.Y. Progress in the application of aerogel materials in firefighting clothing. Fangzhi Xuebao 2011, 42, 187–194. [Google Scholar]
- Meng, Y.; Pan, H.; Shen, Y.; Sheng, Y.; Wang, L.M.; Xu, L.H. Preparation of superhydrophobic photocatalytic UV-protective fabrics with SiO2/TiO2 composite aerogels. Fangzhi Xuebao 2019, 40, 90–96. [Google Scholar]
- Fabian, I.; Gyori, E.; Lazar, I.; Varga, A. Supercritical CO2 extraction and selective adsorption of aroma materials of selected spice plants in functionalized silica aerogels. J. Supercrit. Fluids 2019, 148, 16–23. [Google Scholar]
- Jia, L.C.; Li, M.Z.; Li, Z.M.; Yan, D.X.; Zhang, Q.C.; Zhang, X.P. Robust carbon nanotube foam for efficient electromagnetic interference shielding and microwave absorption. J. Colloid Interface Sci. 2018, 530, 113–119. [Google Scholar]
- Chen, Z.F.; Dong, W.F.; Li, C.D.; Lin, L.L.; Zhu, X.M.; Zhang, Y. A review of silicon-based aerogel thermal insulation materials: Performance optimization through composition and microstructure. J. Non-Cryst. Solids. 2021, 553, 120517. [Google Scholar]
- Licciulli, A.; Padmanabhan, S.K.; Ul Haq, E. Synthesis of silica cryogel-glass fiber blanket by vacuum drying. Ceram. Int. 2016, 42, 7216–7222. [Google Scholar]
- Duraes, L.; Lamy-Mendes, A.; Torres, R.B.; Vareda, J.P. Effect of different silylation agents on the properties of ambient pressure dried and supercritically dried vinyl-modified silica aerogels. J. Supercrit. Fluids 2019, 147, 81–89. [Google Scholar]
- Huang, R.; Huang, S.Q.; Huber, L.; Li, Z.; Liu, Q.; Wang, Y.F.; Wu, X.X.; Zhang, Y. Reducing the thermal hazard of hydrophobic silica aerogels by using dimethyldichlorosilane as modifier. J. Sol Gel Sci. Technol. 2020, 93, 111–122. [Google Scholar]
- Karamikamkar, S.; Naguib, H.E.; PARK, C.B. Advances in precursor system for silica-based aerogel production toward improved mechanical properties, customized morphology, and multifunctionality: A review. Adv. Colloid Interface Sci. 2020, 276, 102101. [Google Scholar] [CrossRef]
- Feng, J.; Feng, J.Z.; Jiang, Y.G.; Wang, L.K. Polyvinylmethyldimethoxysilane reinforced methyltrime-thoxysilane based silica aerogels for thermal insulation with super-high specific surface area. Mater. Lett. 2019, 256, 126644. [Google Scholar]
- Sohu Technology. Available online: https://m.sohu.com (accessed on 24 October 2023).
- Liu, C.; Wu, S.J.; Yang, Z.F.; Sun, H.X.; Zhu, Z.Q.; Liang, W.D.; Li, A. Mechanically robust and flame-retardant silicon aerogel elastomers for thermal insulation and efficient solar steam generation. ACS Omega 2020, 5, 8638–8646. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.J.; Cheng, Y.Y.; Ma, Q.L.; Wang, G.L.; Hu, P.Y.; Wang, J. Amphiphilic silica monomers induced superhydrophilic and flexible silica aerogels for radiative cooling and atmospheric water harvesting. Chem. Eng. J. 2024, 497, 154948. [Google Scholar] [CrossRef]
- Li, K.W.; He, S.; Du, C.H.; Guo, S.P.; Huang, Y.J. Ultra flexible silica aerogel with excellent mechanical properties for durable oil-water separation. J. Environ. Chem. Eng. 2024, 12, 113752. [Google Scholar] [CrossRef]
- Li, Y.C.; Song, Z.W.; Hao, L.; Sun, A.; Li, X.L. Study of light transmission-hydrophobic thermal insulation-mechanical properties of TMOS-DMDMS composite SiO2 aerogels. Huazhong Keji Daxue Xuebao Ziran Kexueban 2024, 52, 139–145. [Google Scholar]
- Guo, T.; Yun, S.; Li, Y.X.; Chen, Z.; Cao, C.X.; Gao, Y.F. Facile synthesis of highly flexible polymethylsilsesquioxane aerogel monoliths with low density, low thermal conductivity and superhydrophobicity. Vacuum 2021, 183, 109825. [Google Scholar] [CrossRef]
- Ji, J.R.; Jiang, X.; Jing, X.L.; Lei, Z.X.; Liu, Y.H.; Lv, Y. Time-temperature-transformation diagram of modified resol phenolic resin and the thermomechanical performance of resol phenolic resin/glass fabric composite. Polym. Adv. Technol. 2018, 29, 2827–2837. [Google Scholar]
- Guo, D.H.; Jia, X.L.; Jin, R.Z.; Liu, J.; Shi, B.L.; Wang, X.Q.; Xu, B.S.; Zhou, N.; Zhou, Z.H. Aerogels for Thermal Protection and Their Application in Aerospace. Gels 2023, 9, 606. [Google Scholar] [CrossRef]
- Alves, P.; Duraes, L.; Lamy-Mendes, A.; Pontinha, A.D.R.; Santos, P. Progress in silica aerogel-containing materials for buildings’ thermal insulation. Constr. Build. Mater. 2021, 286, 122815. [Google Scholar]
- Bhuiyan, M.A.R.; Shaid, A.; Wang, L.J. Aerogel incorporated flexible nonwoven fabric for thermal protective clothing. Fire Saf. J. 2021, 125, 103444. [Google Scholar]
- Feng, J.; Feng, J.Z.; Jiang, Y.K.; Li, L.J.; Liu, F.Q.; Liu, L.F.; Men, J.; Peng, F.; Wang, L.K. Researc-h progress in preparation of aerogel thermal insulation materials and application of aerospace thermal protection. Yuhang Cailiao Gongyi 2022, 52, 26–47. [Google Scholar]
- Pan, Y.L.; He, S.; Gong, L.L.; Cheng, X.D.; Li, C.C.; Li, Z.; Liu, Z.; Zhang, H.P. Low thermal-conductivity and high thermal stable silica aerogel based on MTMS/Water-glass co-precursor prepared by freeze drying. Mater. Des. 2017, 113, 246–253. [Google Scholar] [CrossRef]
- Wu, G.Y.; Yu, Y.X.; Cheng, X.; Zhang, Y. Preparation and surface modification mechanism of silica aerogels via ambient pressure drying. Mater. Chem. Phys. 2011, 129, 308–314. [Google Scholar] [CrossRef]
- Yang, Z.C.; Yu, H.J.; Li, X.L. Hyperelastic and hydrophobic silica aerogels with enhanced compressive strength by using VTES/MTMS as precursors. J. Non-Cryst. Solids. 2019, 525, 119677. [Google Scholar] [CrossRef]
- Zong, S.K.; Wei, W.; Jiang, Z.F.; Yan, Z.X.; Zhu, J.J.; Xie, J.M. Characterization and comparison of uniform hydrophilic/hydrophobic transparent silica aerogel beads: Skeleton strength and surface modification. Rsc Adv. 2015, 5, 55579–55587. [Google Scholar] [CrossRef]
- Lv, H.L. Research on the Preparation and Performance of Aerogel-Based Thermal Insulation Layer Materials for Firefighting. Master’s Thesis, Tiangong University, Tianjin, China, March 2023. [Google Scholar]
- Ge, D.T.; Li, Y.; Yang, L.L.; Zhao, J.P. Hydrophobic and thermal insulation properties of silica aerogel/epoxy composite. J. Non-Cryst. Solids 2009, 355, 2610–2615. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, D.; Lv, H.; Zheng, Z.; Luo, L. Preparation and Properties of Flexible Phenolic Silicone Hybrid Aerogels for Thermal Insulation. Molecules 2024, 29, 4942. https://doi.org/10.3390/molecules29204942
Ye D, Lv H, Zheng Z, Luo L. Preparation and Properties of Flexible Phenolic Silicone Hybrid Aerogels for Thermal Insulation. Molecules. 2024; 29(20):4942. https://doi.org/10.3390/molecules29204942
Chicago/Turabian StyleYe, Danni, Hongli Lv, Zhenrong Zheng, and Lijuan Luo. 2024. "Preparation and Properties of Flexible Phenolic Silicone Hybrid Aerogels for Thermal Insulation" Molecules 29, no. 20: 4942. https://doi.org/10.3390/molecules29204942
APA StyleYe, D., Lv, H., Zheng, Z., & Luo, L. (2024). Preparation and Properties of Flexible Phenolic Silicone Hybrid Aerogels for Thermal Insulation. Molecules, 29(20), 4942. https://doi.org/10.3390/molecules29204942





