Liquid Organic Hydrogen Carrier Concepts and Catalysts for Hydrogenation and Dehydrogenation Reactions
Abstract
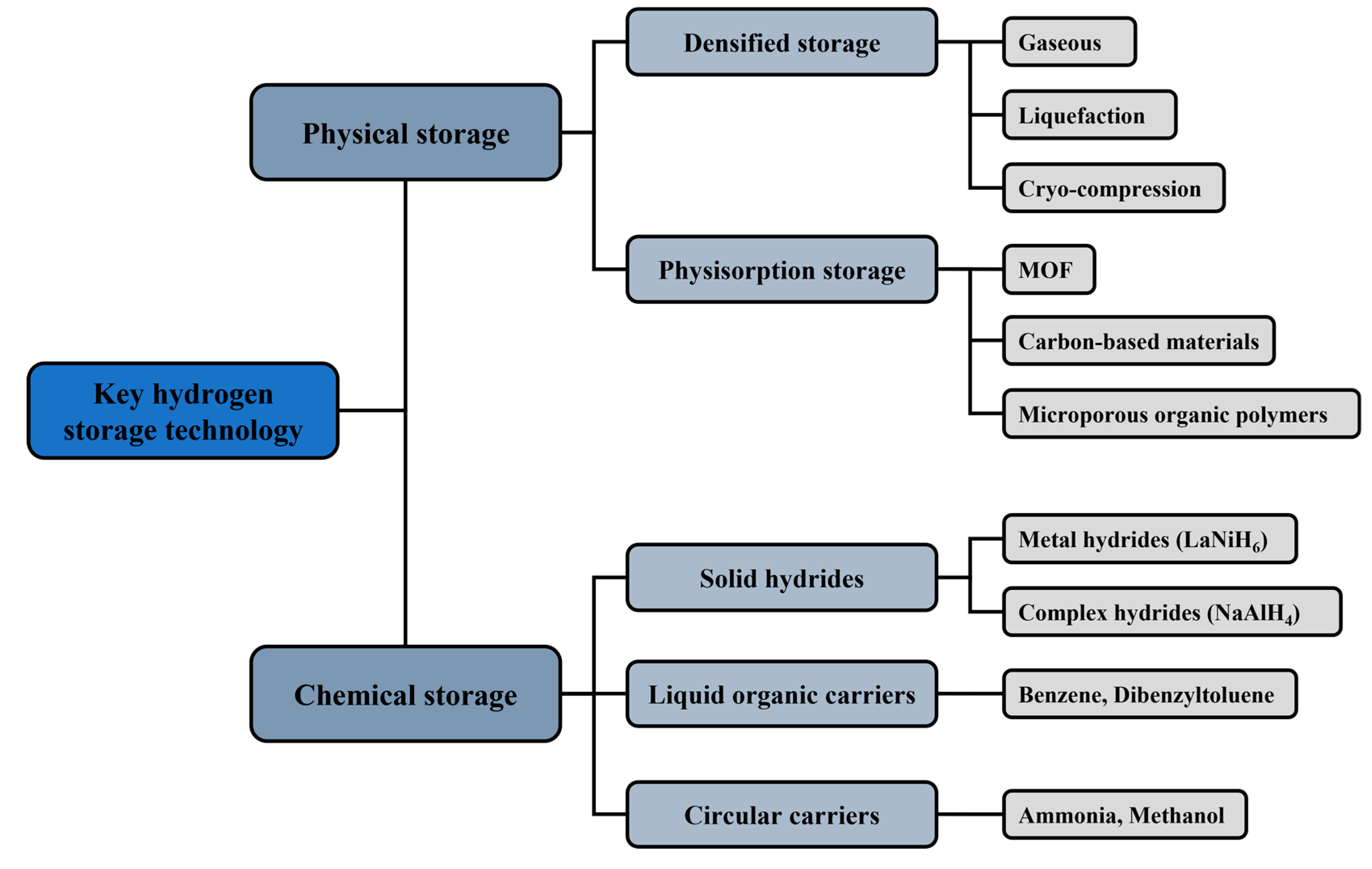
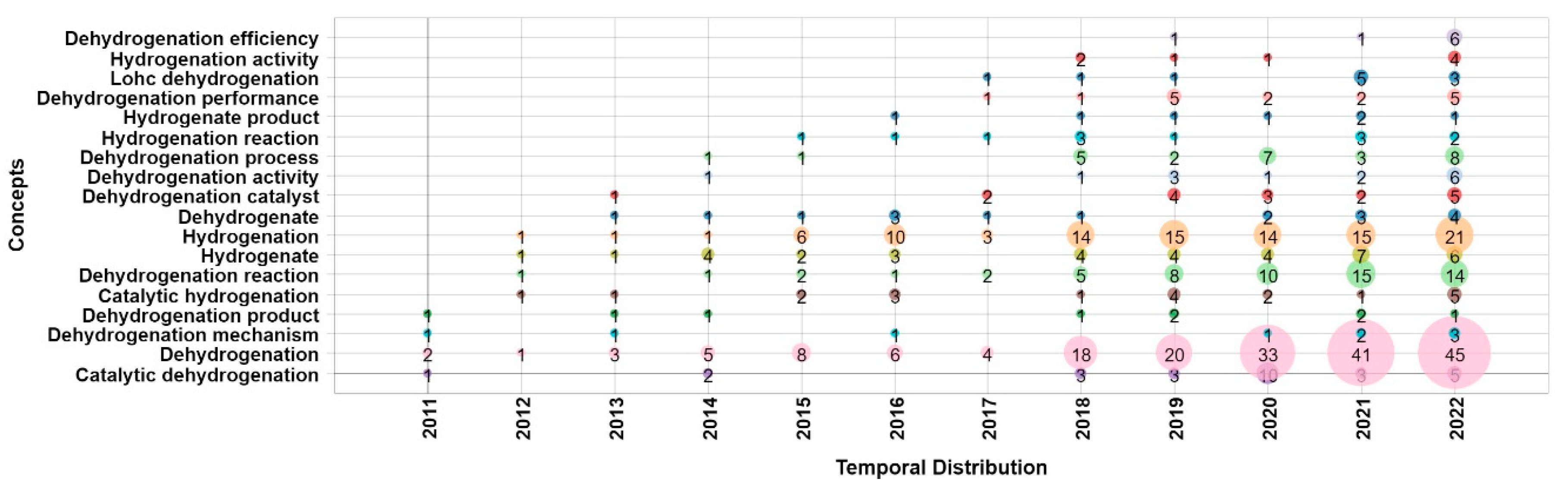
1. Introduction
2. Results and Discussion
2.1. Organic Hydrogen Carrier Liquids
2.1.1. Aromatic N-Heterocyclic Compounds
2.1.2. Homocyclic Aromatic Compounds
2.1.3. Compounds Containing Oxygen
2.2. Catalysts for Hydrogenation and Dehydrogenation Reactions
3. Methodology
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Renewables 2020 Global Status Report. 2020. Available online: https://www.ren21.net/wp-content/uploads/2019/05/gsr_2020_full_report_en.pdf (accessed on 5 April 2023).
- Liu, C.; Li, F.; Ma, L.-P.; Cheng, H.-M. Advanced Materials for Energy Storage. Adv. Mater. 2010, 22, E28–E62. [Google Scholar] [CrossRef] [PubMed]
- Jorschick, H.; Bulgarin, A.; Alletsee, L.; Preuster, P.; Bösmann, A.; Wasserscheid, P. Charging a Liquid Organic Hydrogen Carrier with Wet Hydrogen from Electrolysis. ACS Sustain. Chem. Eng. 2019, 7, 4186–4194. [Google Scholar] [CrossRef]
- Niermann, M.; Drünert, S.; Kaltschmitt, M.; Bonhoff, K. Liquid organic hydrogen carriers (LOHCs)—Techno-economic analysis of LOHCs in a defined process chain. Energy Environ. Sci. 2019, 12, 290–307. [Google Scholar] [CrossRef]
- Hank, C.; Sternberg, A.; Köppel, N.; Holst, M.; Smolinka, T.; Schaadt, A.; Hebling, C.; Henning, H.M. Energy efficiency and economic assessment of imported energy carriers based on renewable electricity. Sustain. Energy Fuels 2020, 4, 2256–2273. [Google Scholar] [CrossRef]
- Kojima, H.; Nagasawa, K.; Todoroki, N.; Ito, Y.; Matsui, T.; Nakajima, R. Influence of renewable energy power fluctuations on water electrolysis for green hydrogen production. Int. J. Hydrogen Energy. 2023, 48, 4572–4593. [Google Scholar] [CrossRef]
- Chang, P.-L.; Hsu, C.-W.; Lin, C.-Y. Assessment of hydrogen fuel cell applications using fuzzy multiple-criteria decision making method. Appl. Energy 2012, 100, 93–99. [Google Scholar] [CrossRef]
- Hosseini, S.E.; Wahid, M.A. Hydrogen production from renewable and sustainable energy resources: Promising green energy carrier for clean development. Renew. Sustain. Energy Rev. 2016, 57, 850–866. [Google Scholar] [CrossRef]
- Biniwale, R.B.; Rayalu, S.; Devotta, S.; Ichikawa, M. Chemical hydrides: A solution to high capacity hydrogen storage and supply. Int. J. Hydrogen Energy 2008, 33, 360–365. [Google Scholar] [CrossRef]
- Pasquini, L. Design of Nanomaterials for Hydrogen Storage. Energies 2020, 13, 3503. [Google Scholar] [CrossRef]
- Aziz, M.; Wijayanta, A.T.; Nandiyanto, A.B. Ammonia as Effective Hydrogen Storage: A Review on Production, Storage and Utilization. Energies 2020, 13, 3062. [Google Scholar] [CrossRef]
- Chamoun, R.; Demirci, U.B.; Miele, P. Cyclic Dehydrogenation–(Re)Hydrogenation with Hydrogen-Storage Materials: An Overview. Energy Technol. 2015, 3, 100–117. [Google Scholar] [CrossRef]
- Niaz, S.; Manzoor, T.; Pandith, A.H. Hydrogen storage: Materials, methods and perspectives. Renew. Sustain. Energy Rev. 2015, 50, 457–469. [Google Scholar] [CrossRef]
- Moradi, R.; Groth, K.M. Hydrogen storage and delivery: Review of the state of the art technologies and risk and reliability analysis. Int. J. Hydrogen Energy 2019, 44, 12254–12269. [Google Scholar] [CrossRef]
- Goeppert, A.; Czaun, M.; Jones, J.-P.; Prakash, G.S.; Olah, G.A. Recycling of carbon dioxide to methanol and derived products—Closing the loop. Chem. Soc. Rev. 2014, 43, 7995–8048. [Google Scholar] [CrossRef]
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef]
- Niermann, M.; Timmerberg, S.; Drünert, S.; Kaltschmitt, M. Liquid Organic Hydrogen Carriers and alternatives for international transport of renewable hydrogen. Renew. Sustain. Energy Rev. 2021, 135, 110171. [Google Scholar] [CrossRef]
- Zhang, C.; Song, P.; Zhang, Y.; Xiao, L.; Hou, J.; Wang, X. Technical and cost analysis of imported hydrogen based on MCH-TOL hydrogen storage technology. Int. J. Hydrogen Energy 2022, 47, 27717–27732. [Google Scholar] [CrossRef]
- Abdin, Z.; Tang, C.; Liu, Y.; Catchpole, K. Large-scale stationary hydrogen storage via liquid organic hydrogen carriers. iScience 2021, 24, 102966. [Google Scholar] [CrossRef]
- Teichmann, D.; Arlt, W.; Wasserscheid, P. Liquid Organic Hydrogen Carriers as an efficient vector for the transport and storage of renewable energy. Int. J. Hydrogen Energy 2012, 37, 18118–18132. [Google Scholar] [CrossRef]
- Teichmann, D.; Arlt, W.; Wasserscheid, P.; Freymann, R. A future energy supply based on Liquid Organic Hydrogen Carriers (LOHC). Energy Environ. Sci. 2011, 4, 2767–2773. [Google Scholar] [CrossRef]
- Sultan, O.; Shaw, H. Study of Automotive Storage of Hydrogen Using Recyclable Liquid Chemical Carriers. [Catalytic Dehydrogenation of Naphthenes]. 1975. Available online: https://www.osti.gov/biblio/5000657 (accessed on 5 April 2023).
- Choi, I.Y.; Shin, B.S.; Kwak, S.K.; Kang, K.S.; Yoon, C.W.; Kang, J.W. Thermodynamic efficiencies of hydrogen storage processes using carbazole-based compounds. Int. J. Hydrogen Energy 2016, 41, 9367–9373. [Google Scholar] [CrossRef]
- Stepanenko, S.A.; Shivtsov, D.M.; Koskin, A.P.; Koskin, I.P.; Kukushkin, R.G.; Yeletsky, P.M.; Yakovlev, V.A. N-Heterocyclic Molecules as Potential Liquid Organic Hydrogen Carriers: Reaction Routes and Dehydrogenation Efficacy. Catalysts 2022, 12, 1260. [Google Scholar] [CrossRef]
- Perreault, P.; Van Hoecke, L.; Pourfallah, H.; Kummamuru, N.B.; Boruntea, C.R.; Preuster, P. Critical challenges towards the commercial rollouts of a LOHC-based H2 economy. Curr. Opin. Green. Sustain. Chem. 2023, 41, 100836. [Google Scholar] [CrossRef]
- Kim, T.W.; Jeong, H.; Baik, J.H.; Suh, Y.-W. State-of-the-art Catalysts for Hydrogen Storage in Liquid Organic Hydrogen Carriers. Chem. Lett. 2022, 51, 239–255. [Google Scholar] [CrossRef]
- Cho, J.-Y.; Kim, H.; Oh, J.-E.; Park, B.Y. Recent Advances in Homogeneous/Heterogeneous Catalytic Hydrogenation and Dehydrogenation for Potential Liquid Organic Hydrogen Carrier (LOHC) Systems. Catalysts 2021, 11, 1497. [Google Scholar] [CrossRef]
- Niermann, M.; Beckendorff, A.; Kaltschmitt, M.; Bonhoff, K. Liquid Organic Hydrogen Carrier (LOHC)—Assessment based on chemical and economic properties. Int. J. Hydrogen Energy 2019, 44, 6631–6654. [Google Scholar] [CrossRef]
- Aakko-Saksa, P.T.; Cook, C.; Kiviaho, J.; Repo, T. Liquid organic hydrogen carriers for transportation and storing of renewable energy—Review and discussion. J. Power Sources 2018, 396, 803–823. [Google Scholar] [CrossRef]
- Jo, Y.; Oh, J.; Kim, D.; Park, J.H.; Baik, J.H.; Suh, Y.W. Recent progress in dehydrogenation catalysts for heterocyclic and homocyclic liquid organic hydrogen carriers. Korean J. Chem. Eng. 2022, 39, 20–37. [Google Scholar] [CrossRef]
- Koutsonikolas, D.; Kaldis, S.; Zaspalis, V.T.; Sakellaropoulos, G.P. Potential application of a microporous silica membrane reactor for cyclohexane dehydrogenation. Int. J. Hydrogen Energy 2012, 37, 16302–16307. [Google Scholar] [CrossRef]
- Kou, Z.; Zhi, Z.; Xu, G.; An, Y.; He, C. Investigation of the performance and deactivation behavior of Raney-Ni catalyst in continuous dehydrogenation of cyclohexane under multiphase reaction conditions. Appl. Catal. A Gen. 2013, 467, 96–201. [Google Scholar] [CrossRef]
- Ali, J.K.; Rippin, D.W.T. Comparing Mono- and Bimetallic Noble-Metal Catalysts in a Catalytic Membrane Reactor for Methylcyclohexane Dehydrogenation. Ind. Eng. Chem. Res. 1995, 34, 722–729. [Google Scholar] [CrossRef]
- Ferreira-Aparicio, P.; Rodriguez-Ramos, I.; Guerrero-Ruiz, A. On the Performance of Porous Vycor Membranes for Conversion Enhancement in the Dehydrogenation of Methylcyclohexane to Toluene. J. Catal. 2002, 212, 182–192. [Google Scholar] [CrossRef]
- Oda, K.; Akamatsu, K.; Sugawara, T.; Sikuchi, R.; Segawa, A.; Nakao, S.I. Dehydrogenation of Methylcyclohexane to Produce High-Purity Hydrogen Using Membrane Reactors with Amorphous Silica Membranes. Ind. Eng. Chem. Res. 2010, 49, 11287–11293. [Google Scholar] [CrossRef]
- Hodoshima, S.; Arai, H.; Saito, Y. Liquid-film-type catalytic decalin dehydrogeno-aromatization for long-term storage and long-distance transportation of hydrogen. Int. J. Hydrogen Energy 2003, 28, 197–204. [Google Scholar] [CrossRef]
- Jiang, N.; Rao, K.S.R.; Jin, M.-J.; Park, S.-E. Effect of hydrogen spillover in decalin dehydrogenation over supported Pt catalysts. Appl. Catal. A Gen. 2012, 425–426, 62–67. [Google Scholar] [CrossRef]
- Morawa Eblagon, K.; Tam, K.; Yu, K.M.K.; Zhao, S.L.; Gong, X.Q.; He, H.; Ye, L.; Wang, L.C.; Ramirez-Cuesta, A.J.; Tsang, S.C. Study of Catalytic Sites on Ruthenium for Hydrogenation of N-ethylcarbazole: Implications of Hydrogen Storage via Reversible Catalytic Hydrogenation. J. Phys. Chem. C 2010, 114, 9720–9730. [Google Scholar] [CrossRef]
- Sotoodeh, F.; Smith, K.J. Kinetics of Hydrogen Uptake and Release from Heteroaromatic Compounds for Hydrogen Storage. Ind. Eng. Chem. Res. 2010, 49, 1018–1026. [Google Scholar] [CrossRef]
- Modisha, P.M.; Jordaan, J.H.L.; Bösmann, A.; Wasserscheid, P.; Bessarabov, D. Analysis of reaction mixtures of perhydro-dibenzyltoluene using two-dimensional gas chromatography and single quadrupole gas chromatography. Int. J. Hydrogen Energy 2018, 43, 5620–5636. [Google Scholar] [CrossRef]
- Geburtig, D.; Preuster, P.; Bösmann, A.; Müller, K.; Wasserscheid, P. Chemical utilization of hydrogen from fluctuating energy sources—Catalytic transfer hydrogenation from charged Liquid Organic Hydrogen Carrier systems. Int. J. Hydrogen Energy. 2016, 41, 1010–1017. [Google Scholar] [CrossRef]
- Byun, M.; Lee, A.; Cheon, S.; Kim, H.; Lim, H. Preliminary feasibility study for hydrogen storage using several promising liquid organic hydrogen carriers: Technical, economic, and environmental perspectives. Energy Convers. Manag. 2022, 268, 116001. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, F.; Wang, B.; Li, D.; Wei, M.; Fang, T.; Zhang, Z. Heterogeneous Catalysts in N-Heterocycles and Aromatics as Liquid Organic Hydrogen Carriers (LOHCs): History, Present Status and Future. Materials 2023, 16, 3735. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yang, M.; Dong, Y.; Mei, P.; Cheng, H. Hydrogen storage and release from a new promising liquid Organic Hydrogen storage carrier (LOHC): 2-methylindole. Int. J. Hydrogen Energy 2016, 41, 16129–16134. [Google Scholar] [CrossRef]
- Dobereiner, G.E.; Crabtree, R.H. Dehydrogenation as a Substrate-Activating Strategy in Homogeneous Transition-Metal Catalysis. Chem. Rev. 2010, 110, 681–703. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, R.H. Nitrogen-Containing Liquid Organic Hydrogen Carriers: Progress and Prospects. ACS Sustain. Chem. Eng. 2017, 5, 4491–4498. [Google Scholar] [CrossRef]
- Ouimet, R.J.; Glenn, J.R.; De Porcellinis, D.; Motz, A.R.; Carmo, M.; Ayers, K.E. The Role of Electrocatalysts in the Development of Gigawatt-Scale PEM Electrolyzers. ACS Catal. 2022, 12, 6159–6171. [Google Scholar] [CrossRef]
- Kim, T.W.; Kim, M.; Kim, S.K.; Oh, H.; Suh, Y.-W. Remarkably fast low-temperature hydrogen storage into aromatic benzyltoluenes over MgO-supported Ru nanoparticles with homolytic and heterolytic H2 adsorption. Appl. Catal. B Environ. 2021, 286, 119889. [Google Scholar] [CrossRef]
- Kim, T.W.; Park, S.; Oh, J.; Shin, C.-H.; Suh, Y.-W. Hydrogenation of the LOHC Compound Monobenzyl Toluene over ZrO2-supported Ru Nanoparticles: A Consequence of Zirconium Hydroxide’s Surface Hydroxyl Group and Surface Area. ChemCatChem 2018, 10, 3406–3410. [Google Scholar] [CrossRef]
- Leinweber, A.; Müller, K. Hydrogenation of the Liquid Organic Hydrogen Carrier Compound Monobenzyl Toluene: Reaction Pathway and Kinetic Effects. Energy Technol. 2018, 6, 513–520. [Google Scholar] [CrossRef]
- Kim, T.W.; Jeong, H.; Kim, D.; Park, J.H.; Suh, Y.-W. Feasible coupling of CH4/H2 mixtures to H2 storage in liquid organic hydrogen carrier systems. J. Power Sources 2022, 541, 231721. [Google Scholar] [CrossRef]
- Ali, A.; Rohini, A.K.; Noh, Y.S.; Moon, D.J.; Lee, H.J. Hydrogenation of dibenzyltoluene and the catalytic performance of Pt/Al2O3 with various Pt loadings for hydrogen production from perhydro-dibenzyltoluene. Int. J. Energy Res. 2022, 46, 6672–6688. [Google Scholar] [CrossRef]
- Ali, A.; Kumar, G.U.; Lee, H.J. Investigation of hydrogenation of Dibenzyltoluene as liquid organic hydrogen carrier. Mater. Today Proc. 2021, 45, 1123–1127. [Google Scholar] [CrossRef]
- Modisha, P.; Bessarabov, D. Stress tolerance assessment of dibenzyltoluene-based liquid organic hydrogen carriers. Sustain. Energy Fuels 2020, 4, 4662–4670. [Google Scholar] [CrossRef]
- Ali, A.; Kumar, G.U.; Lee, H.J. Parametric study of the hydrogenation of dibenzyltoluene and its dehydrogenation performance as a liquid organic hydrogen carrier. J. Mech. Sci. Technol. 2020, 34, 3069–3077. [Google Scholar] [CrossRef]
- Heller, A.; Rausch, M.H.; Schulz, P.S.; Wasserscheid, P.; Fröba, A.P. Binary Diffusion Coefficients of the Liquid Organic Hydrogen Carrier System Dibenzyltoluene/Perhydrodibenzyltoluene. J. Chem. Eng. Data. 2016, 61, 504–511. [Google Scholar] [CrossRef]
- Jo, Y.; Wan Kim, T.; Oh, J.; Kim, D.; Suh, Y.-W. Mesoporous sulfur-decorated Pt–Al2O3 for dehydrogenation of perhydro benzyltoluenes: Activity-favorable adsorption of reaction species onto electron-deficient Pt atoms. J. Catal. 2022, 413, 127–137. [Google Scholar] [CrossRef]
- Kim, C.H.; Lee, M.-W.; Jang, J.S.; Lee, S.H.; Lee, K.-Y. Enhanced activity of a WOx-incorporated Pt/Al2O3 catalyst for the dehydrogenation of homocyclic LOHCs: Effects of impregnation sequence on Pt–WOx interactions. Fuel 2022, 313, 122654. [Google Scholar] [CrossRef]
- Auer, F.; Blaumeiser, D.; Bauer, T.; Libuda, J.; Wasserscheid, P. Boosting the activity of hydrogen release from liquid organic hydrogen carrier systems by sulfur-additives to Pt on alumina catalysts. Catal. Sci. Technol. 2019, 9, 3537–3547. [Google Scholar] [CrossRef]
- Modisha, P.; Garidzirai, R.; Güneş, H.; Erkey, C.; Bessarabov, D. A Promising Catalyst for the Dehydrogenation of Perhydro-Dibenzyltoluene: Pt/Al2O3 Prepared by Supercritical CO2 Deposition. Catalysts 2022, 12, 489. [Google Scholar] [CrossRef]
- Naseem, M.; Usman, M.; Lee, S. A parametric study of dehydrogenation of various Liquid Organic Hydrogen Carrier (LOHC) materials and its application to methanation process. Int. J. Hydrogen Energy 2021, 46, 4100–4115. [Google Scholar] [CrossRef]
- Rao, N.; Lele, A.K.; Patwardhan, A.W. Optimization of Liquid Organic Hydrogen Carrier (LOHC) dehydrogenation system. Int. J. Hydrogen Energy 2022, 47, 28530–28547. [Google Scholar] [CrossRef]
- Shi, L.; Qi, S.; Smith, K.J.; Alamoudi, M.; Zhou, Y. Dehydrogenation of the liquid organic hydrogen carrier perhydrodibenzyltoluene—Reaction pathway over Pt/ Al2O3. React. Chem. Eng. 2023, 8, 96–103. [Google Scholar] [CrossRef]
- Mrusek, S.; Preuster, P.; Müller, K.; Bösmann, A.; Wasserscheid, P. Pressurized hydrogen from charged liquid organic hydrogen carrier systems by electrochemical hydrogen compression. Int. J. Hydrogen Energy 2021, 46, 15624–15634. [Google Scholar] [CrossRef]
- Feng, X.; Jiang, L.; Li, Z.; Wu, Y.; Yuan, B. Boosting the hydrogenation activity of dibenzyltoluene catalyzed by Mg-based metal hydrides. Int. J. Hydrogen Energy 2022, 47, 23994–24003. [Google Scholar] [CrossRef]
- Jorschick, H.; Geißelbrecht, M.; Eßl, M.; Bösmann, A.; Wasserscheid, P. Benzyltoluene/dibenzyltoluene-based mixtures as suitable liquid organic hydrogen carrier systems for low temperature applications. Int. J. Hydrogen Energy 2020, 45, 14897–14906. [Google Scholar] [CrossRef]
- Modisha, P.; Gqogqa, P.; Garidzirai, R.; Ouma, C.N.M.; Bessarabov, D. Evaluation of catalyst activity for release of hydrogen from liquid organic hydrogen carriers. Int. J. Hydrogen Energy 2019, 44, 21926–21935. [Google Scholar] [CrossRef]
- Wunsch, A.; Mohr, M.; Pfeifer, P. Intensified LOHC-dehydrogenation using multi-stage microstructures and Pd-based membranes. Membranes 2018, 8, 112. [Google Scholar] [CrossRef]
- Cabrera Papamija, G.; Diosa Astaiza, J.E.; Mosquera Vargas, E.E. Nanomateriales Carbonosos en Electrodos de Celdas PEM Utilizadas en la Electrolisis del Agua Para la Producción de Hidrógeno; Ocronos—Editorial Científico-Técnica, 2024. Available online: https://ocronos.com/libros-publicados-isbn/doi-nanomateriales-carbonosos-electrodos-celdas-pem-electrolisis-agua-produccion-hidrogeno/ (accessed on 10 March 2023).
- Shi, L.; Qi, S.; Qu, J.; Che, T.; Yi, C.; Yang, B. Integration of hydrogenation and dehydrogenation based on dibenzyltoluene as liquid organic hydrogen energy carrier. Int. J. Hydrogen Energy 2019, 44, 5345–5354. [Google Scholar] [CrossRef]
- Shi, L.; Zhou, Y.; Tan, X.; Qi, S.; Smith, K.J.; Yi, C.; Yang, B. The effects of alumina morphology and Pt electron property on reversible hydrogenation and dehydrogenation of dibenzyltoluene as a liquid organic hydrogen carrier. Int. J. Hydrogen Energy 2022, 47, 4704–4715. [Google Scholar] [CrossRef]
- Shi, L.; Zhou, Y.; Qi, S.; Smith, K.J.; Tan, X.; Yan, J.; Yi, C. Pt catalysts supported on H2 and O2 plasma-treated Al2O3 for hydrogenation and dehydrogenation of the liquid organic hydrogen carrier pair dibenzyltoluene and perhydrodibenzyltoluene. ACS Catal. 2020, 10, 10661–10671. [Google Scholar] [CrossRef]
- Elsevier-Scopus. Available online: https://www.scopus.com (accessed on 10 March 2023).
- VOSviewer. Available online: https://www.vosviewer.com (accessed on 5 April 2023).
- Orbit Intellixir. Available online: https://carlac.intellixir.fr/cenm (accessed on 10 March 2023).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]


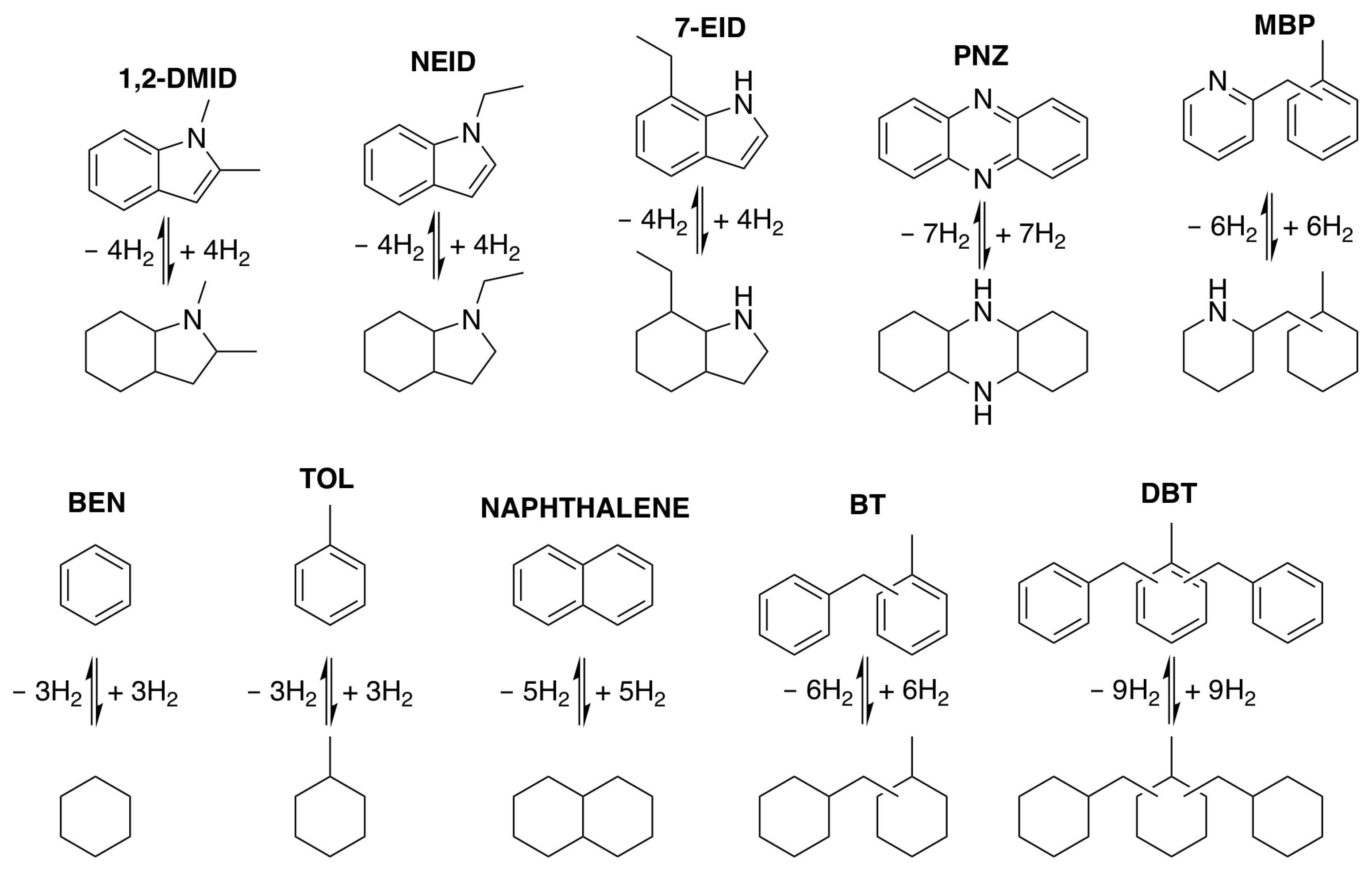
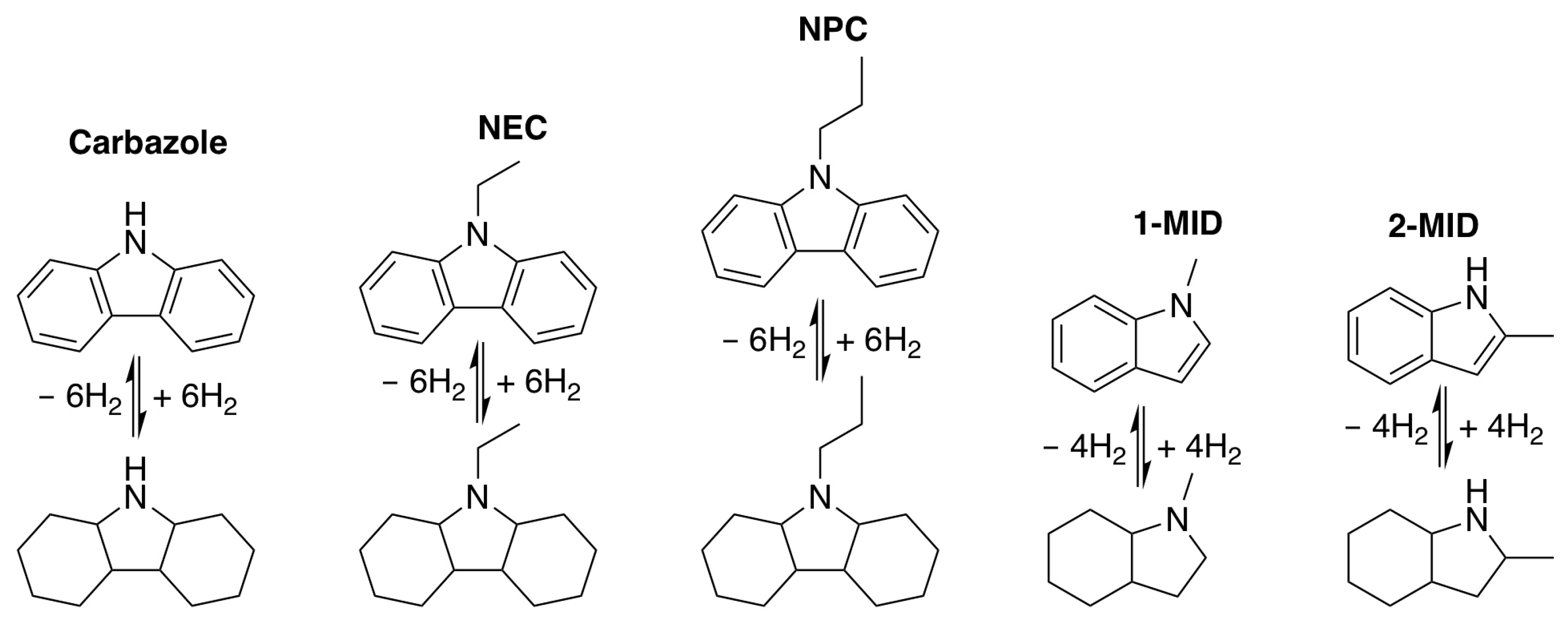

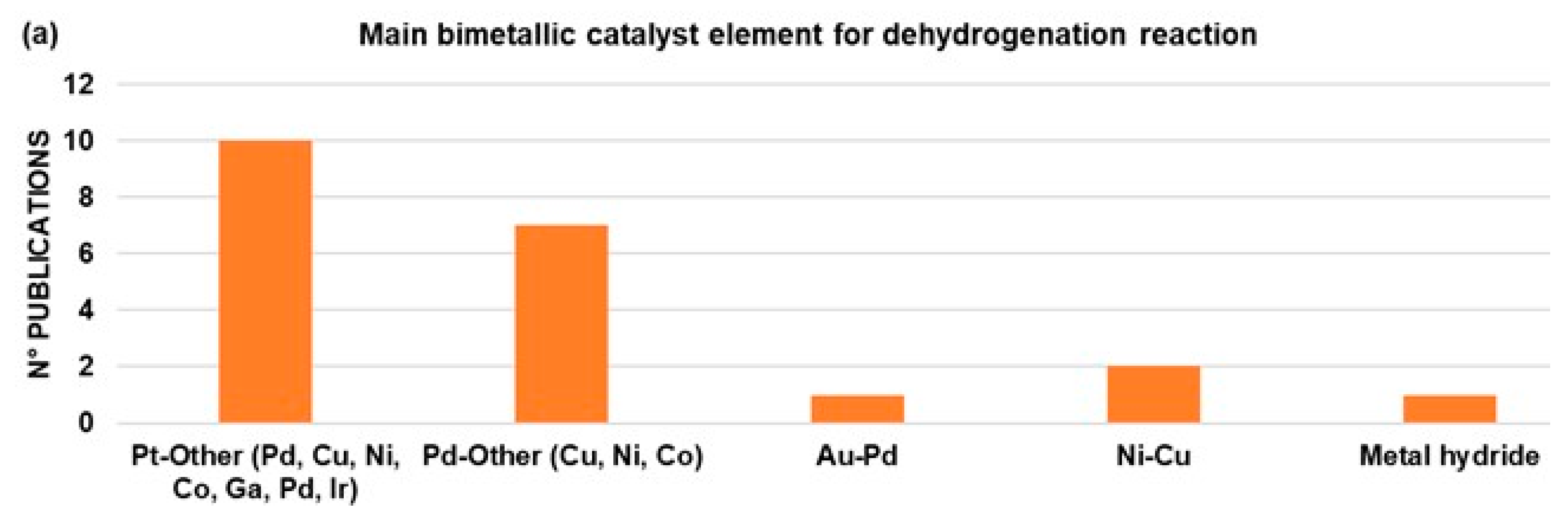
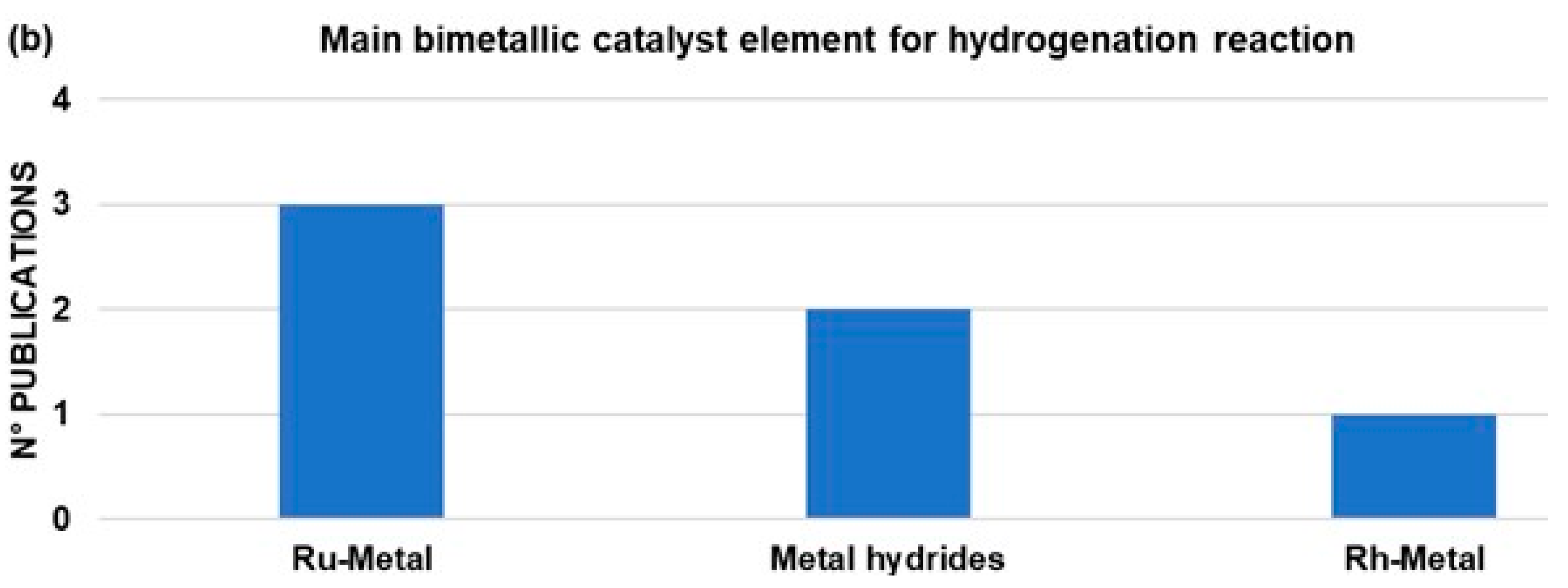
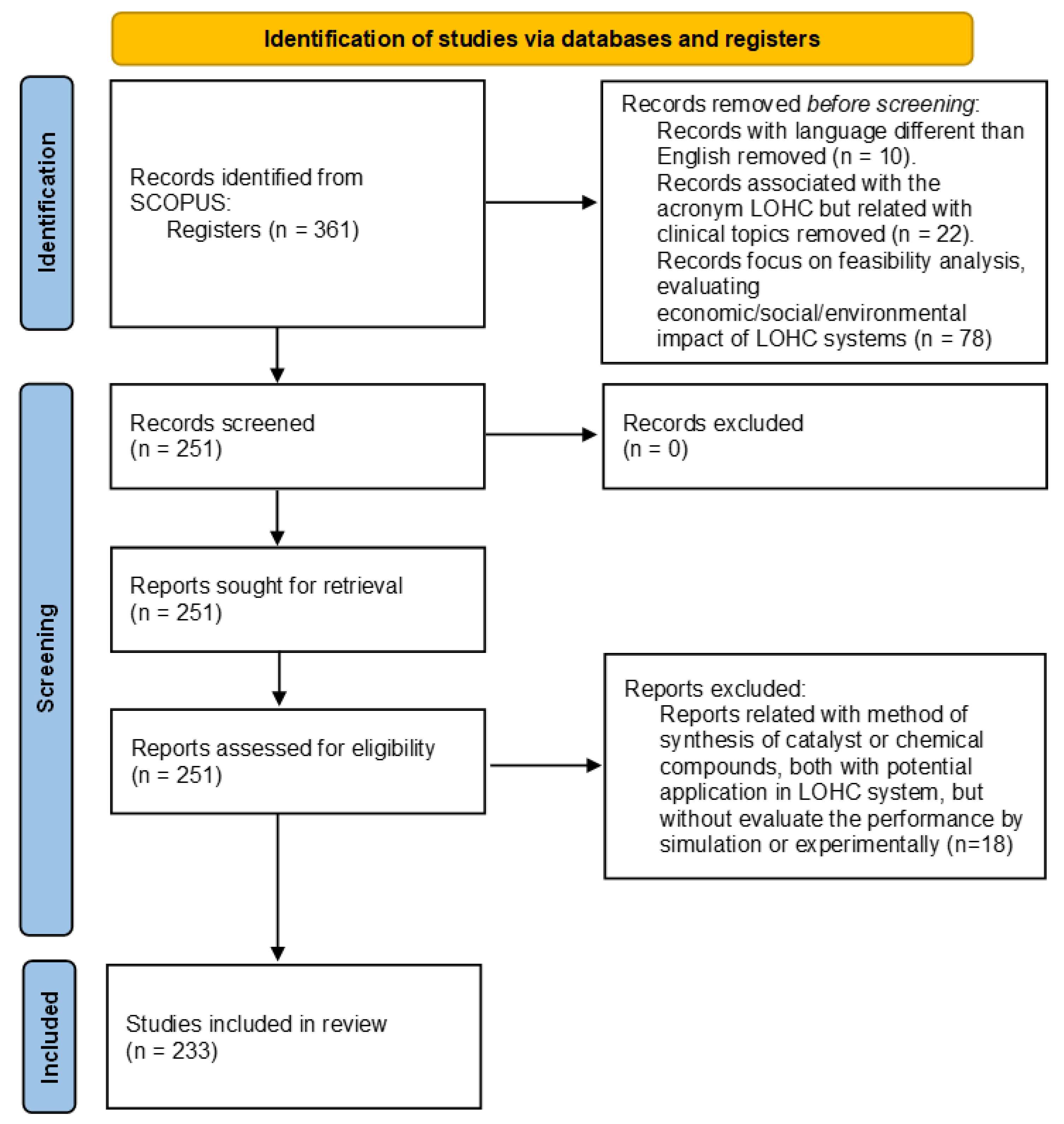
| LOHCs | Melting Point (°C) | Boiling Point (°C) | H2 Storage Capacity wt% |
|---|---|---|---|
| Benzene (BEN) | 5.5 | 80 | 7.2 |
| Toluene (TOL) | −95 | 111 | 6.2 |
| Naphthalene | 80 | 218 | 7.3 |
| Carbazole | 245 | 355 | 6.7 |
| N-ethylcarbazole (NEC) | 69 | 378 | 5.8 |
| N-propylcarbazole (NPC) | 48 | 336 | 5.43 |
| Benzyltoluene (BT) | −55 | 280 | 6.2 |
| Dibenzyltoluene (DBT) | −39~−34 | 390 | 6.2 |
| 1-methylindole (1-MID) | −20 | 239 | 5.76 |
| 2-methylindole (2-MID) | 57 | 273 | 5.76 |
| 1,2-dimethylindole (1,2-DMID) | 55 | 260 | 5.23 |
| N-ethylindole (NEID) | −17.8 | 253.5 | 5.23 |
| 7-ethylindole (7-EID) | 14 | 230 | 5.23 |
| 2-(N-methylbenzyl)-pyridine (MBP) | −50.1~−40.2 | 291~293 | 6.15 |
| Phenazine (PNZ) | 174–177 | 357.2 | 7.2 |
| LOHCs | Catalysts | T (°C) | PH2 (MPa) | Time (h) | Conv a (%) | Yield b (%) | TOF (h−1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| BEN | Ru/SBA-15 | 20 | 1 | - | 100 | 100 | 85.3 | [43] |
| BEN | Ru/MOF | 60 | 6 | 1.5 | 100 | 100 | 3200 | [43] |
| BEN | 4.2 wt% Ru/C-silica | 110 | 8 | 0.53 | 100 | 99.8 | 37.7 | [43] |
| BEN | Ru(0)-Zeolite-Y | 22 | 0.28 | 1 | 100 | 100 | 1040 | [43] |
| BEN | Ru/CNTs | 80 | 4 | 0.5 | 100 | 99.97 | 6983 | [43] |
| BEN | Pd/SiO2 (co-SEA) | 150 | 7 | 6 | 84.1 | 84.1 | - | [43] |
| TOL | Ni nanoflowers | 140 | 5 | 0.5 | 100 | 100 | - | [43] |
| TOL | Pd/SiO2 (co-SEA) | 150 | 7 | 6 | 85.4 | 85.4 | - | [43] |
| TOL | Pt (MP)/CeO2-A-400 | 100 | 0.5 | 3 | 90.8 | 90.8 | - | [43] |
| NAP | Pt/WO3-500 | 70 | 3 | 1 | 100 | 100 | - | [43] |
| NAP | Pd/HY-9.5 | 200 | 4 | 1 | 100 | 73.15 | - | [43] |
| TEN | 1 wt% Ni/Al2O3–YH3 | 150 | 10 | 5 | - | 95 | - | [43] |
| MBT | 0.5 wt% Ru/MgO | 150 | 5 | 1.87 | - | 100 | 7680 | [47] |
| MBT | 5 wt% Ru/ZrO2 | 150 | 5 | - | - | 100 | - | [48] |
| MBT | 0.5 wt% Ru/Al2O3 | 175 | 10 | 0.104 | - | 53 | - | [49] |
| DBT | 0.5 wt% Ru/MgO | 150 | 5 | 3.33 | - | 100 | 2880 | [47] |
| DBT | Ni70/AlSiO-1/1 | 150 | 7 | 1.5 | 100 | 100 | - | [43] |
| DBT | 0.3 wt% Pt/Al2O3 | 270 | 3 | 1.42 | - | 100 | - | [43] |
| DBT | 5 wt% Pd/Al2O3 | 260 | 3 | 6 | - | 100 | - | [43] |
| DBT | 5 wt% Ru/Al2O3 | 170 | 5 | 0.772 | - | 100 | - | [50] |
| DBT | Raney-Ni | 170 | 7 | 30 | - | 100 | - | [51] |
| DBT | Raney-Ni | 170 | 0.8 | 18 | - | - | - | [52] |
| DBT | Ni-based (NISAT 310) | 170 | 2 | 3 | - | 100 | - | [53] |
| DBT | Raney-Ni | 170 | 0.8 | 10 | - | 100 | - | [54] |
| DBT | Ru/Al2O3 | 130 | 3 | 16 | - | 100 | - | [55] |
| NEC | Ru/pg-BC | 130 | 6 | 1.17 | 100 | 99.41 | - | [43] |
| NEC | Raney-Ni | 180 | 5 | 1.3 | - | 86.2 | [43] | |
| NEC | Ni70/AlSiO-1/1 | 150 | 7 | 1.5 | 100 | 100 | -- | [43] |
| NEC | 1.3 wt% Ru/YH3 | 130 | 7 | 2.5 | 100 | 100 | - | [43] |
| NEC | 5 wt% Ru/TiO2 | 130 | 7 | - | - | 95 | - | [43] |
| NEC | Ru black | 130 | 7 | - | - | 85 | - | [43] |
| NEC | 1.5 wt% Ru-Ni1Al2-LDO | 150 | 8 | 1 | 100 | 100 | - | [43] |
| NEC | 1 wt%Ni/Al2O3–YH3 | 180 | 10 | 1.5 | 100 | 100 | - | [43] |
| NEC | 5 wt% Ru/LDH-3.9CNT | 120 | 6 | 0.4 | 100 | 98.31 | - | [43] |
| NEC | Ru/P25 | 150 | 7 | 24 | 100 | 92.4 | - | [43] |
| NEC | Ru/anatase | 150 | 7 | 24 | 100 | 95.7 | - | [43] |
| NEC | Ru/Ni-Fe LDH | 110 | 6 | 1.33 | - | 98.88 | - | [43] |
| NPC | 5 wt% Ru/Al2O3 | 150 | 7 | 0.5 | - | 100 | - | [43] |
| NPC | Ni70/AlSiO-1/1 | 150 | 7 | 1 | 100 | 100 | [43] |
| LOHCs | Catalysts | T (°C) | P (MPa) | Time (h) | Conv a (%) | Yield b (%) | H2 Release (wt%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 12H-MBT | 3 wt% Pt/Al2O3 (decorated with S) | 250 | - | 4 | - | 86.8 | - | [56] |
| 12H-MBT | 5 wt% Pt/Al2O3 (doped with WOx) | 250 | - | 3.5 | - | 75.9 | - | [57] |
| 18H-DBT | 0.3 wt% Pt/Al2O3 | 310 | 0.1 | 2 | - | 95 | - | [58] |
| 18H-DBT | 1 wt% Pd/C | 290 | - | - | - | 97.6 | - | [54] |
| 18H-DBT | 0.5 wt% Pt/Al2O3 | 300 | - | 1.67 | - | 60 | - | [59] |
| 18H-DBT | Pt/Al2O3 | 315 | 0.1 | - | - | 100 | - | [60] |
| 18H-DBT | 0.3 wt% Pt/Al2O3 | 320 | 0.122 | - | - | 90 | - | [61] |
| 18H-DBT | 1 wt% Pt/Al2O3 | 290 | 0.1 | 14 | - | 91.0 | - | [62] |
| 18H-DBT | 5 wt% Pt/Al2O3 | 290 | - | 7 | - | 90.2 | - | [52] |
| 18H-DBT | 0.3 wt% Pt/Al2O3 | 240 | 0.1 | 4 | - | 58.2 | - | [63] |
| 18H-DBT | 5 wt% Pt/Al2O3 (doped with WOx) | 270 | - | 3.5 | - | 64.8 | - | [57] |
| 18H-DBT | 3 wt% Pt/Al2O3 (decorated with S) | 250 | - | 4 | - | 86.8 | - | [56] |
| 12H-NEC | 5 wt% Pd/NGC | 180 | 0.1 | 10 | 100 | 98.72 | 5.76 | [43] |
| 12H-NEC | 2.5 wt% Pt/SiO2-TiO(OH)2 | 180 | 0.1 | 7 | 100 | 97.9 | 5.75 | [43] |
| 12H-NEC | 2.5 wt% Pd/LDHs-us | 180 | 0.1 | 6 | 100 | - | 5.72 | [43] |
| 12H-NEC | 1 wt% Pd-EU/KIT-6 | 190 | 0.1 | 6 | 100 | 100 | - | [43] |
| 12H-NEC | 5 wt% Pd/Al2O3 | 180 | 0.1 | 4 | 100 | 100 | - | [43] |
| 12H-NEC | 5 wt% Pt/Al2O3 | 180 | 0.1 | 5 | 100 | 100 | - | [43] |
| 12H-NEC | 4 wt% Pd/SiO2 | 170 | 1.6 | 100 | 100 | 5.8 | [43] | |
| 12H-NEC | 2.5 wt% Pd/rGO-EG | 170 | 0.1 | 12 | 100 | 84.61 | 5.49 | [43] |
| 12H-NEC | 5 wt% Pt/TiO2 | 180 | 0.1 | 6 | 100 | 79 | 5.38 | [43] |
| 12H-NEC | 2.32 wt% Pd/rGO | 180 | 0.1 | - | 100 | 97.65 | 5.74 | [43] |
| 12H-NPC | 1 wt% Pd/Al2O3-120 | 180 | 7 | 6 | 100 | 100 | 5.43 | [43] |
| 12H-NPC | 3 wt% Pd@MIL-101 | 190 | 0.1 | 4 | 100 | 100 | 5.43 | [43] |
| LOHCs | Catalysts | T (°C) | P (MPa) | Time (h) | Conv a (%) | Yield b (%) | Ref. |
|---|---|---|---|---|---|---|---|
| BEN | Pd-Ni/SiO2 (co-SEA) | 150 | 7 | 6 | 99.9 | 99.9 | [43] |
| BEN | Pd-Pt/SiO2 (co-SEA) | 150 | 7 | 6 | 90.8 | 90.8 | [43] |
| BEN | 0.024 wt% Ru–1.00 wt% Ni/C | 60 | 700 psi | 2 | 100 | 100 | [43] |
| BEN | Ru0.56Ni0.44/C | 60 | 5.3 | 0.5 | - | 99.8 | [43] |
| BEN | 1 wt% Ru2Pt1 MIL-101 | 60 | 1 | 6 | 100 | 100 | [43] |
| TOL | Pd-Ni/SiO2 (co-SEA) | 150 | 7 | 6 | 99.9 | 99.9 | [43] |
| TOL | Pd-Pt/SiO2 (co-SEA) | 150 | 7 | 6 | 91.4 | 91.4 | [43] |
| TOL | 6 wt% Pt1Pd1/HBEA | 150 | 7–12 | 2 | 100 | 100 | [43] |
| TOL | Pt–Rh/MWNTs | 20 | 1 | 3 | 100 | 100 | [43] |
| DBT | Mg2NiH4 | 280 | 6 | 20 | - | - | [64] |
| NAP | Ru/Ni/Ni(OH)2/C | 100 | 4.48 | 1 | - | >99 | [43] |
| NAP | Ru/Ni/NiO/C | 100 | 4.5 | 1 | 100 | 100 | [43] |
| NAP | Ru/Co/Co3O4/C | 100 | 4.5 | 0.8 | 100 | 100 | [43] |
| NEC | 5.0 wt% Ni0.5Ru4.5/pg-BC | 130 | 6 | 1.17 | 100 | 99.06 | [43] |
| NEC | 5.0 wt% Co@Ru/NGC | 130 | 6 | 1 | 100 | 99.1 | [43] |
| NEC | Ru-Ni/P25 | 150 | 7 | 24 | 100 | 93 | [43] |
| NEC | Ru-Ni/anatase | 150 | 7 | 24 | 100 | 94.8 | [43] |
| NEC | Ru0.7Ni0.3/SBA15 | 100 | 5 | 1.33 | 100 | 99.82 | [43] |
| NPC | Ru2.5Ni2.5/Al2O3 | 150 | 4 | 0.5 | 100 | 100 | [43] |
| LOHCs | Catalysts | T (°C) | P (MPa) | Time (h) | Conv a (%) | Yield b (%) | H2 Release (wt%) | H2 Evolution Rate mmol/gmet/min | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| CYH | 10 wt% Ni0.8Cu0.2/ACC | 350 | - | 10 | 25.78 | - | - | 39.45 | [43] |
| CYH | 10 wt% Ag-1 wt% Pd/ACC | 300 | - | 7 | - | - | - | 7.5 | [43] |
| CYH | 10 wt% Ag-1 wt% Rh/ACC | 300 | - | 6 | - | - | - | 12.34 | [43] |
| CYH | 10 wt% Ag-1 wt% Pt/ACC | 300 | - | 6 | - | - | - | 13.36 | [43] |
| CYH | 5 wt% 1:4 Ag-Rh/Y2O3 | 300 | 0.1 | 4 | - | - | - | 400 | [43] |
| CYH | 5 wt%1:4 Ag-Rh/ACC | 300 | 0.1 | 5 | - | - | - | 178.7 | [43] |
| MCH | 2.5wt%Pt0.8Ir0.2/Mg-Al-O | 350 | - | 1.6 | 91.1 | 99.9 | - | 263.9 | [43] |
| MCH | 2.0 wt% Pt-0.5 wt% Sn/MgAleO-350 | 300 | - | 12 | 90.5 | - | - | 262.1 | [43] |
| MCH | Pt-Cu/S-1 | 400 | 0.1 | 6 | 92.26 | - | - | 445.3 | [43] |
| 12H-MBT | Pt-Pd/Al2O3 | 250 | 0.1 | 6 | - | 99 | - | - | [65] |
| 18H-DBT | 1 wt% Pt-1 wt% Pd/Al2O3 | 320 | - | 1.33 | - | 10 | - | - | [66] |
| 18H-DBT | Membranas basadas en Pd-Ag | 350 | 0.4 | - | - | - | - | - | [67] |
| 12H-NEC | 5 wt% PdCo/NGC | 180 | 0.1 | 6 | 100 | 97.87 | 5.71 | - | [43] |
| 12H-NEC | Pd3 (3.75 wt%)-Ni1/SiO2 | 180 | 0.1 | 8 | 100 | 91.1 | 5.63 | - | [43] |
| 12H-NEC | Pd3 (3.75wt%)-Cu1/SiO2 | 180 | 0.1 | 8 | 100 | 83.11 | 5.47 | - | [43] |
| 12H-NEC | Pd3 (3.75wt%)-Au1/SiO2 | 180 | 0.1 | 8 | 100 | 94.9 | 5.7 | - | [43] |
| 12H-NEC | 0.65 mol%Pd1.3–0.52 mol% Au1/rGO | 180 | 0.1 | 4 | 100 | 100 | 5.79 | - | [43] |
| 12H-NEC | 0.58 mol%Pd1.3–0.42 mol% Ru1/rGO | 180 | 0.1 | 4 | 100 | 84.11 | 5.48 | - | [43] |
| 12H-NEC | Pd1 (2.5 wt%)-Co1/Al2O3 | 180 | 0.1 | 8 | 100 | 85.4 | 5.52 | - | [43] |
| 12H-NEC | Pd4Ni1/KIT-6 | 180 | 0.1 | 6 | - | - | 5.74 | - | [43] |
| 12H-NEC | Pd1.2Cu/rGO | 180 | 0.1 | 7 | 100 | 100 | 5.79 | - | [43] |
| 12H-NPC | 5 wt%Pd1-Ni1/Al2O3 | 180 | 6 | 7 | 100 | 100 | 5.43 | - | [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabrera, G.; Mora, M.; Gil-Burgos, J.P.; Visbal, R.; Machuca-Martínez, F.; Mosquera-Vargas, E. Liquid Organic Hydrogen Carrier Concepts and Catalysts for Hydrogenation and Dehydrogenation Reactions. Molecules 2024, 29, 4938. https://doi.org/10.3390/molecules29204938
Cabrera G, Mora M, Gil-Burgos JP, Visbal R, Machuca-Martínez F, Mosquera-Vargas E. Liquid Organic Hydrogen Carrier Concepts and Catalysts for Hydrogenation and Dehydrogenation Reactions. Molecules. 2024; 29(20):4938. https://doi.org/10.3390/molecules29204938
Chicago/Turabian StyleCabrera, Gerardo, Malka Mora, Juan P. Gil-Burgos, Renso Visbal, Fiderman Machuca-Martínez, and Edgar Mosquera-Vargas. 2024. "Liquid Organic Hydrogen Carrier Concepts and Catalysts for Hydrogenation and Dehydrogenation Reactions" Molecules 29, no. 20: 4938. https://doi.org/10.3390/molecules29204938
APA StyleCabrera, G., Mora, M., Gil-Burgos, J. P., Visbal, R., Machuca-Martínez, F., & Mosquera-Vargas, E. (2024). Liquid Organic Hydrogen Carrier Concepts and Catalysts for Hydrogenation and Dehydrogenation Reactions. Molecules, 29(20), 4938. https://doi.org/10.3390/molecules29204938









