Efficient Toluene Decontamination and Resource Utilization through Ni/Al2O3 Catalytic Cracking
Abstract
1. Introduction
2. Results and Discussion
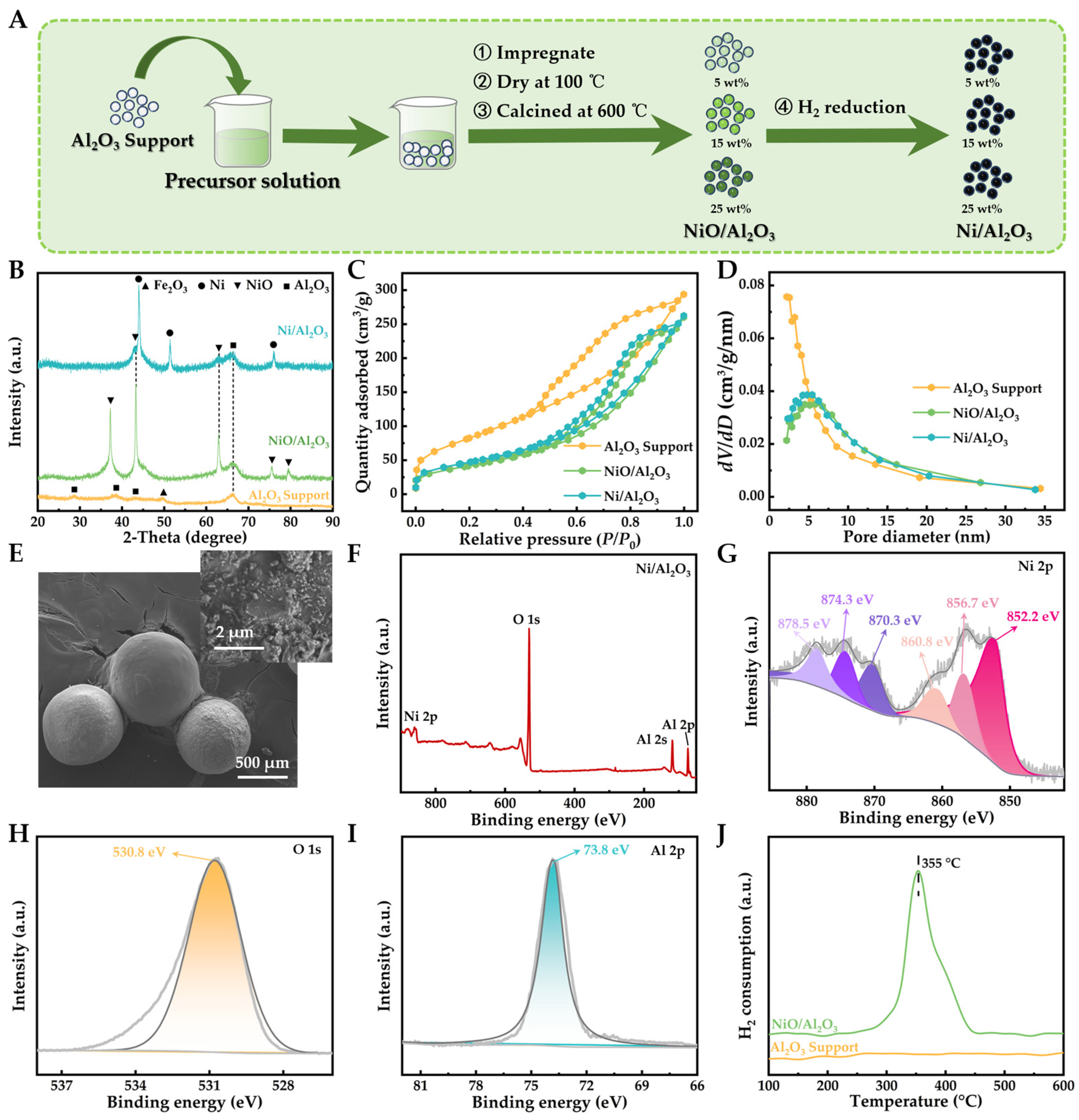
2.1. Comprehensive Characterization and Preparation of Ni/Al2O3 Catalyst for Toluene Pyrolysis
2.2. Effect of Temperature on Pyrolytic Conversion of Toluene
2.3. Effect of Process Conditions on Pyrolysis Conversion of Toluene
2.4. Re-Usability of a Ni/Al2O3 Catalyst
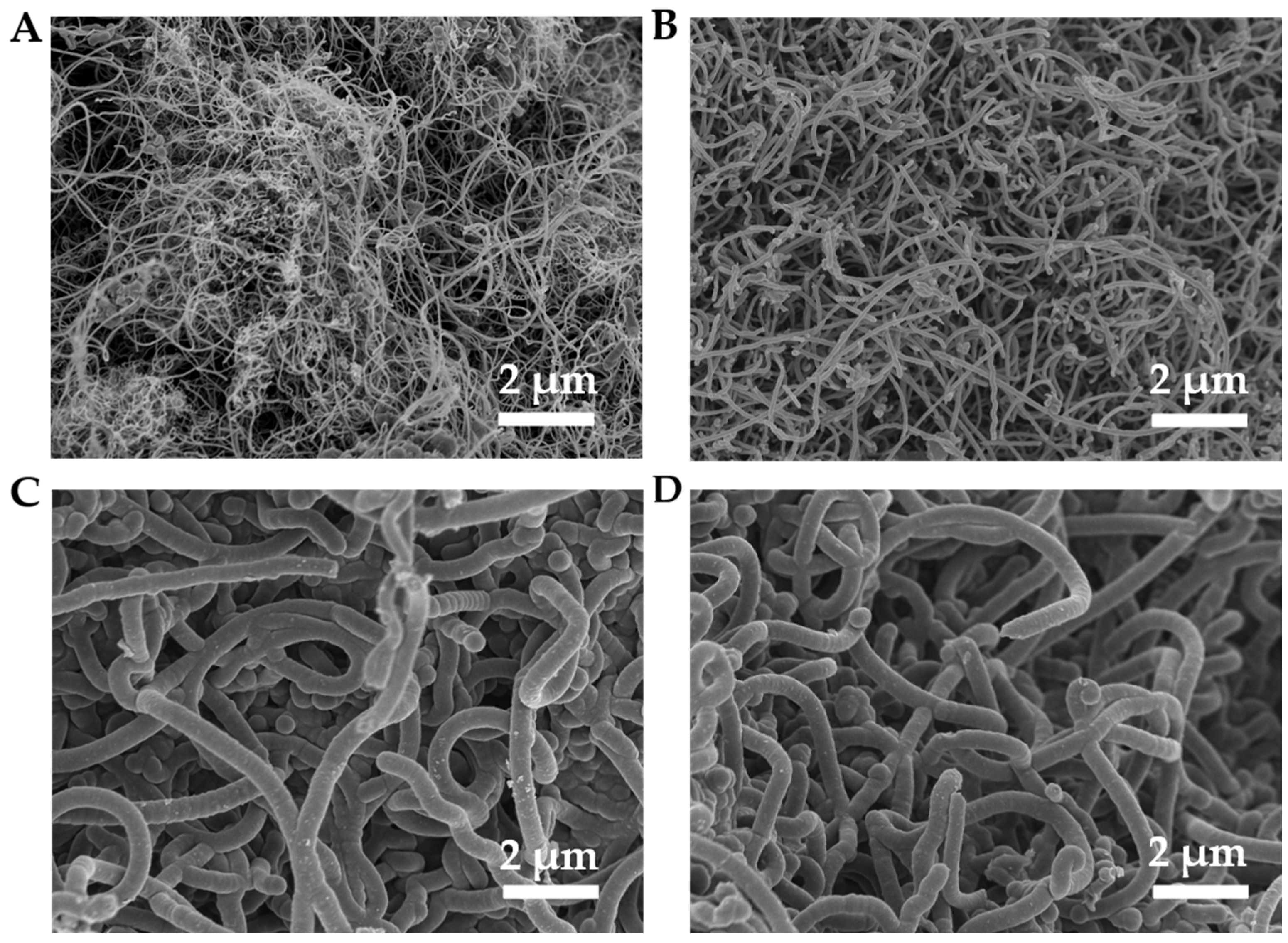
2.5. Structure of Deposited Carbon on a Ni/Al2O3 Catalyst
3. Materials and Methods
3.1. Regents and Material
3.2. Preparation of the NiO/Al2O3 Precursor
3.3. Catalytic Pyrolysis of Toluene in a Ni/Al2O3 Filled Bed
3.4. Reuse Experiment of a Ni/Al2O3 Catalyst
3.5. Materials Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, C.; Cheng, J.; Zhang, X.; Douthwaite, M.; Pattisson, S.; Hao, Z. Recent advances in the catalytic oxidation of volatile organic compounds: A review based on pollutant sorts and sources. Chem. Rev. 2019, 119, 4471–4568. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Z.; Wei, L.; Liu, Y.; Dai, H.; Deng, J. Recent progress on VOC pollution control via the catalytic method. Chin. J. Catal. 2024, 61, 71–96. [Google Scholar] [CrossRef]
- Wu, P.; Jin, X.; Qiu, Y.; Ye, D. Recent progress of thermocatalytic and photo/thermocatalytic oxidation for VOCs purification over manganese-based oxide catalysts. Environ. Sci. Technol. 2021, 55, 4268–4286. [Google Scholar] [CrossRef]
- Liu, B.; Ji, J.; Zhang, B.; Huang, W.; Gan, Y.; Leung, D.Y.C.; Huang, H. Catalytic ozonation of VOCs at low temperature: A comprehensive review. J. Hazard. Mater. 2022, 422, 126847. [Google Scholar] [CrossRef]
- Ma, X.; Zhao, D.; Qian, J.; Ma, Z.; Cui, J. Utilization of hematite particles for economical removal of o-xylene in a high-temperature gas-solid reactor. Molecules 2022, 27, 1502. [Google Scholar] [CrossRef]
- Shi, Y.; Li, Z.; Wang, J.; Zhou, R. Synergistic effect of Pt/Ce and USY zeolite in Pt-based catalysts with high activity for VOCs degradation. Appl. Catal. B Environ. 2021, 286, 119936. [Google Scholar] [CrossRef]
- He, F.; Muliane, U.; Weon, S.; Choi, W. Substrate-specific mineralization and deactivation behaviors of TiO2 as an air-cleaning photocatalyst. Appl. Catal. B Environ. 2020, 275, 119145. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Z.; Wang, Q.; Wang, T.; Zhong, L.; Pan, W.-P. Insights into the removal of volatile organic compounds by three-dimensional ordered microporous zeolite-templated carbon: A combined experimental study and density functional theory calculation. Chem. Eng. J. 2024, 494, 152965. [Google Scholar] [CrossRef]
- Ma, M.; Gao, K.; Ma, Z.; Ding, J. Influence of preparation method on the adsorptive performance of silica sulfuric acid for the removal of gaseous o-xylene. Sep. Purif. Technol. 2021, 265, 118484. [Google Scholar] [CrossRef]
- Gao, K.; Ma, M.; Liu, Y.; Ma, Z. A comparative study of the removal of o-xylene from gas streams using mesoporous silicas and their silica supported sulfuric acids. J. Hazard. Mater. 2021, 409, 124965. [Google Scholar] [CrossRef]
- Deng, H.; Li, K.; Li, H.; Li, X.; Zhang, L.; Cao, W. Densification behavior and microstructure of carbon/carbon composites prepared by chemical vapor infiltration from xylene at temperatures between 900 and 1250 °C. Carbon 2011, 49, 2561–2570. [Google Scholar] [CrossRef]
- Guo, Y.; Wen, M.; Song, S.; Liu, Q.; Li, G.; An, T. Enhanced catalytic elimination of typical VOCs over ZnCoOx catalyst derived from in situ pyrolysis of ZnCo bimetallic zeolitic imidazolate frameworks. Appl. Catal. B Environ. 2022, 308, 121212. [Google Scholar] [CrossRef]
- Xiang, W.; Zhang, X.; Chen, K.; Fang, J.; He, F.; Hu, X.; Tsang, D.C.W.; Ok, Y.S.; Gao, B. Enhanced adsorption performance and governing mechanisms of ball-milled biochar for the removal of volatile organic compounds (VOCs). Chem. Eng. J. 2020, 385, 123842. [Google Scholar] [CrossRef]
- Sorcar, S.; Rosen, B.A. Methane pyrolysis using a multiphase molten metal reactor. ACS Catal. 2023, 13, 10161–10166. [Google Scholar] [CrossRef]
- Kang, D.; Palmer, C.; Mannini, D.; Rahimi, N.; Gordon, M.J.; Metiu, H.; McFarland, E.W. Catalytic methane pyrolysis in molten alkali chloride salts containing iron. ACS Catal. 2020, 10, 7032–7042. [Google Scholar] [CrossRef]
- Prabowo, J.; Lai, L.; Chivers, B.; Burke, D.; Dinh, A.H.; Ye, L.; Wang, Y.; Wang, Y.; Wei, L.; Chen, Y. Solid carbon co-products from hydrogen production by methane pyrolysis: Current understandings and recent progress. Carbon 2024, 216, 118507. [Google Scholar] [CrossRef]
- Patlolla, S.R.; Katsu, K.; Sharafian, A.; Wei, K.; Herrera, O.E.; Mérida, W. A review of methane pyrolysis technologies for hydrogen production. Renew. Sustain. Energy Rev. 2023, 181, 113323. [Google Scholar] [CrossRef]
- Patlolla, S.R.; Sharafian, A.; Mérida, W. Characterization of solid carbon from hydrocarbon pyrolysis in molten aluminum. Carbon 2024, 224, 119054. [Google Scholar] [CrossRef]
- Ji, Y.; Palmer, C.; Foley, E.E.; Giovine, R.; Yoshida, E.; Sebti, E.; Patterson, A.R.; McFarland, E.; Clément, R.J. Valorizing the carbon byproduct of methane pyrolysis in batteries. Carbon 2023, 204, 26–35. [Google Scholar] [CrossRef]
- Gadd, G.E.; Blackford, M.; Moricca, S.; Webb, N.; Evans, P.J.; Smith, A.M.; Jacobsen, G.; Leung, S.; Day, A.; Hua, Q. The world’s smallest gas cylinders? Science 1997, 277, 933–936. [Google Scholar] [CrossRef]
- Chen, P.; Wu, X.; Lin, J.; Tan, K.L. High H2 uptake by alkalidoped carbon nanotubes under ambient pressure and moderate temperatures. Science 1999, 285, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, N.M.; Kim, M.-S.; Baker, R.T.K. Carbon nanofibers: A unique catalyst support medium. J. Phys. Chem. 1994, 98, 13108–13111. [Google Scholar] [CrossRef]
- Kovalenko, G.A.; Rudina, N.A.; Chuenko, T.V.; Ermakov, D.Y.; Perminova, L.V. Synthesis of catalytic filamentous carbon by the pyrolysis of alkanes on alumina–silica foam supporting nickel nanoparticles. Carbon 2009, 47, 428–435. [Google Scholar] [CrossRef]
- Zhang, K.; Huang, Z.; Yang, M.; Liu, M.; Zhou, Y.; Zhan, J.; Zhou, Y. Recent progress in melt pyrolysis: Fabrication and applications of high-value carbon materials from abundant sources. SusMat 2023, 3, 558–580. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhong, J.; Feng, F.; Ye, D.; Hu, Y. Synergistic photothermal catalytic oxidation of methanol and toluene mixture over Co-MOFs-derived catalyst: Interfacial and promotion effects. Chem. Eng. J. 2024, 485, 149720. [Google Scholar] [CrossRef]
- Hoecker, C.; Smail, F.; Pick, M.; Boies, A. The influence of carbon source and catalyst nanoparticles on CVD synthesis of CNT aerogel. Chem. Eng. J. 2017, 314, 388–395. [Google Scholar] [CrossRef]
- Shandakov, S.D.; Kosobutsky, A.V.; Rybakov, M.S.; Sevostyanov, O.G.; Russakov, D.M.; Lomakin, M.V.; Vershinina, A.I.; Chirkova, I.M. Effect of gaseous and condensate products of ethanol decomposition on aerosol CVD synthesis of single-walled carbon nanotubes. Carbon 2018, 126, 522–531. [Google Scholar] [CrossRef]
- Baker, K.; Harris, P.; Thomas, R.; Waite, R. Formation of filamentous carbon from iron, cobalt and chromium catalyzed decomposition of acetylene. J. Catal. 1973, 30, 86–95. [Google Scholar] [CrossRef]
- Serp, P.; Castillejos, E. Catalysis in carbon nanotubes. ChemCatChem 2010, 2, 41–47. [Google Scholar] [CrossRef]
- Bae, K.; Kim, D.; Dung, P.A.; Lee, D.; Hwang, B.; Go, K.S.; Kim, W.; Lee, J.K.; Im, J.S.; Kang, S.C.; et al. Simultaneous and continuous production of carbon nanotubes and hydrogen by catalytic CH4 decomposition in a pressurized fluidized-bed reactor. Ind. Eng. Chem. Res. 2024, 63, 930–941. [Google Scholar] [CrossRef]
- Hernadi, K.; Fonseca, A.; Nagy, J.B.; Siska, A.; Kiricsi, I. Production of nanotubes by the catalytic decomposition of different carbon-containing compounds. Appl. Catal. A Gen. 2000, 199, 245–255. [Google Scholar] [CrossRef]
- Ermakova, M.A.; Ermakov, D.Y.; Kuvshinov, G.G. Effective catalysts for direct cracking of methane to produce hydrogen and filamentous carbon. Appl. Catal. A 2000, 201, 61–70. [Google Scholar] [CrossRef]
- Zhang, Y.; Tao, Y.; Huang, J.; Williams, P. Influence of silica–alumina support ratio on H2 production and catalyst carbon deposition from the Ni-catalytic pyrolysis/reforming of waste tyres. Waste Manag. Res. 2017, 35, 1045–1054. [Google Scholar] [CrossRef]
- Kim, S.; Kim, K.; Ahn, H.; Cho, K. Characterization of graphitic nanofibers synthesized by the CVD method using nickel–copper as a catalyst. J. Alloys Compd. 2008, 449, 274–278. [Google Scholar] [CrossRef]
- Schwarz, H. Chemistry with methane: Concepts rather than recipes. Angew. Chem. Int. Ed. Engl. 2011, 50, 10096–10115. [Google Scholar] [CrossRef]
- Szwarc, M. The C–H bond energy in toluene and xylenes. J. Chem. Phys. 1948, 16, 128–136. [Google Scholar] [CrossRef]
- Lv, Y.; Li, J.; Feng, S.; Liu, P.; Hao, F.; Xiong, W.; Luo, H.A. Multi-walled carbon nanotubes supported nickel nanoparticles doped with magnesia and copper for adiponitrile hydrogenation with high activity and chemoselectivity under mild conditions. Chem. Eng. J. 2018, 346, 203–216. [Google Scholar] [CrossRef]
- Kang, Y.; Wang, W.; Pu, Y.; Li, J.; Chai, D.; Lei, Z. An effective Pd-NiOx -P composite catalyst for glycerol electrooxidation: Co-existed phosphorus and nickel oxide to enhance performance of Pd. Chem. Eng. J. 2017, 308, 419–427. [Google Scholar] [CrossRef]
- Damyanovaa, S.; Shterevaa, I.; Pawelecb, B.; Mihaylovc, L.; Fierro, J. Characterization of none and yttrium-modified Ni-based catalysts for dry reforming of methane. Appl. Catal. B 2020, 278, 119335. [Google Scholar] [CrossRef]
- Sun, M.; Xia, J.; Wang, H.; Liu, X.; Xia, Q.; Wang, Y. An efficient NixZryO catalyst for hydrogenation of bio-derived methyl levulinate to γ-valerolactone in water under low hydrogen pressure. Appl. Catal. B Environ. 2018, 227, 488–498. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, X.; Wang, H.; Zhao, D.; Liu, Y.; Ma, Z. Enhancement of gaseous o-xylene elimination by chlorosulfonic acid-modified H-zeolite socony mobil-5. Molecules 2024, 29, 3507. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Liu, Y.; Ma, X.; Qian, J.; Ma, Z. Reactive adsorption performance and behavior of gaseous cumene on MCM-41 supported sulfuric acid. Molecules 2022, 27, 5129. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Deng, L.; Kinloch, I.; Young, R. Raman spectroscopy of carbon materials and their composites: Graphene, nanotubes and fibres. Prog. Mater. Sci. 2023, 135, 101089. [Google Scholar] [CrossRef]

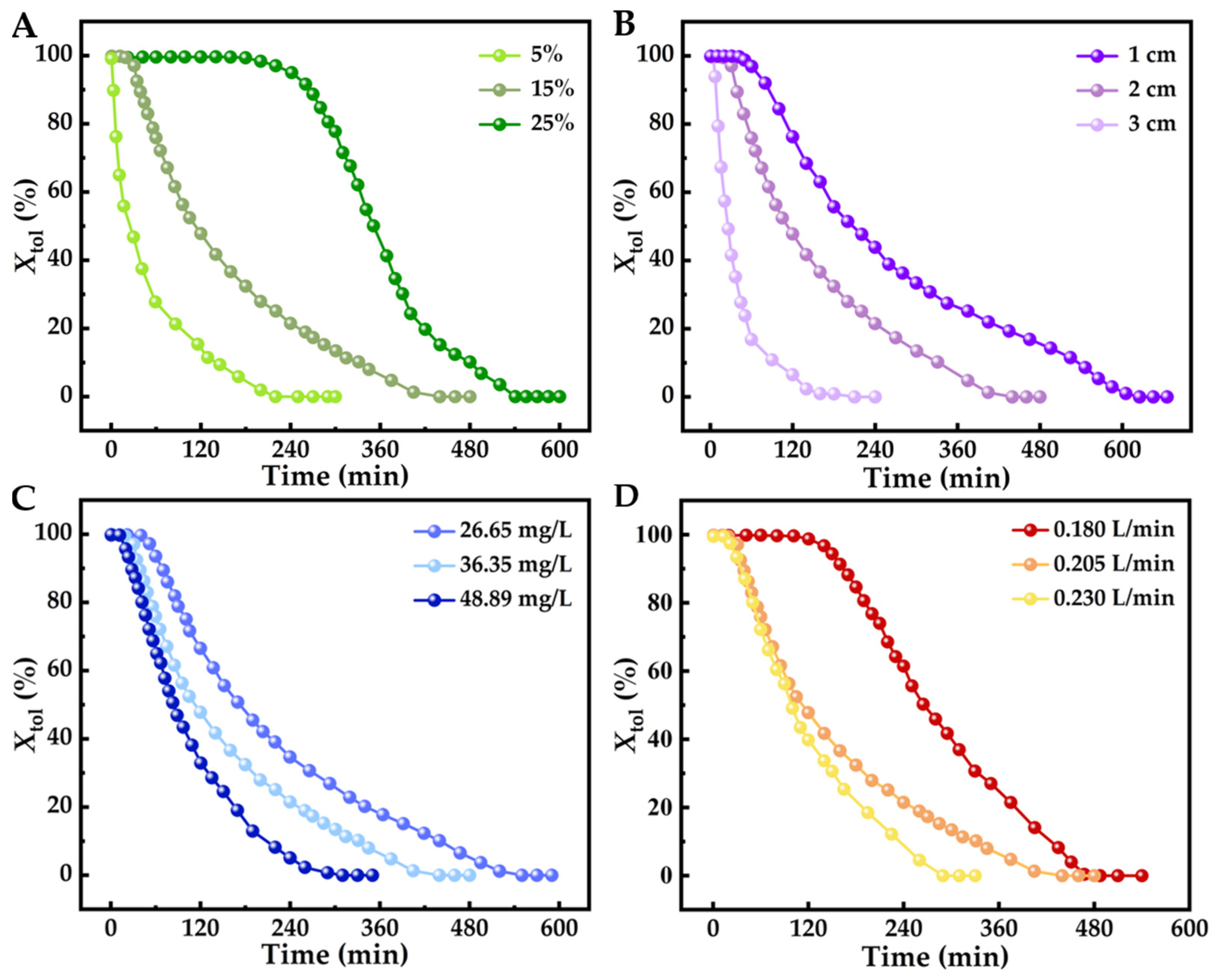

| Reaction Condition | RC (mg/g) | RH (mL/g) | Qt (mg/g) | |
|---|---|---|---|---|
| Metal loading (%) | 5 | 191 | 169 | 180 |
| 15 | 453 | 551 | 554 | |
| 25 | 915 | 1234 | 1328 | |
| Bed height (cm) | 1 | 280 | 254 | 247 |
| 2 | 453 | 551 | 554 | |
| 3 | 510 | 723 | 716 | |
| Inlet Concentration (mg/L) | 26.65 | 475 | 556 | 579 |
| 36.35 | 453 | 551 | 554 | |
| 48.89 | 450 | 481 | 512 | |
| Flow Rate (L/min) | 0.180 | 683 | 753 | 918 |
| 0.205 | 453 | 551 | 554 | |
| 0.230 | 346 | 455 | 486 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, Y.; Ma, X.; Lu, G.; Zhao, D.; Ma, Z. Efficient Toluene Decontamination and Resource Utilization through Ni/Al2O3 Catalytic Cracking. Molecules 2024, 29, 4868. https://doi.org/10.3390/molecules29204868
Niu Y, Ma X, Lu G, Zhao D, Ma Z. Efficient Toluene Decontamination and Resource Utilization through Ni/Al2O3 Catalytic Cracking. Molecules. 2024; 29(20):4868. https://doi.org/10.3390/molecules29204868
Chicago/Turabian StyleNiu, Yifei, Xiaolong Ma, Guangyi Lu, Dandan Zhao, and Zichuan Ma. 2024. "Efficient Toluene Decontamination and Resource Utilization through Ni/Al2O3 Catalytic Cracking" Molecules 29, no. 20: 4868. https://doi.org/10.3390/molecules29204868
APA StyleNiu, Y., Ma, X., Lu, G., Zhao, D., & Ma, Z. (2024). Efficient Toluene Decontamination and Resource Utilization through Ni/Al2O3 Catalytic Cracking. Molecules, 29(20), 4868. https://doi.org/10.3390/molecules29204868










