Recent Progress in Photocatalytic Applications of Electrospun Nanofibers: A Review
Abstract
1. Introduction
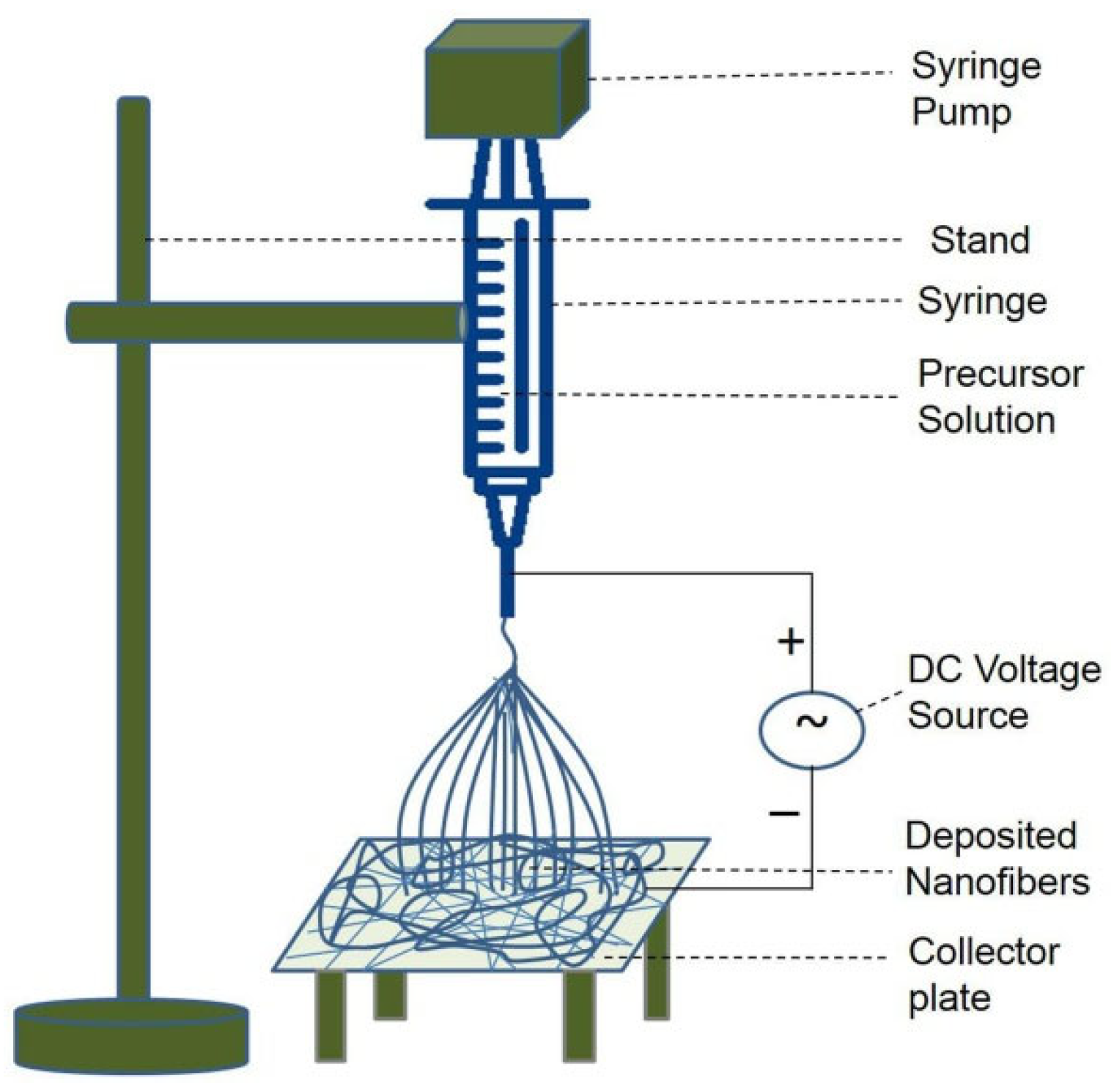
2. Principle of Electrospinning Method
- -
- Stretching of the jet due to the increased electric field voltage, leading to electrical bending instability.
- -
- Solidification of the jet in the form of solid fiber(s) on a grounded collector.
3. Application of Electrospun One-Dimensional Photocatalysts
3.1. Development of Various Electrospun Composite Nanofibers for Photocatalytic Applications
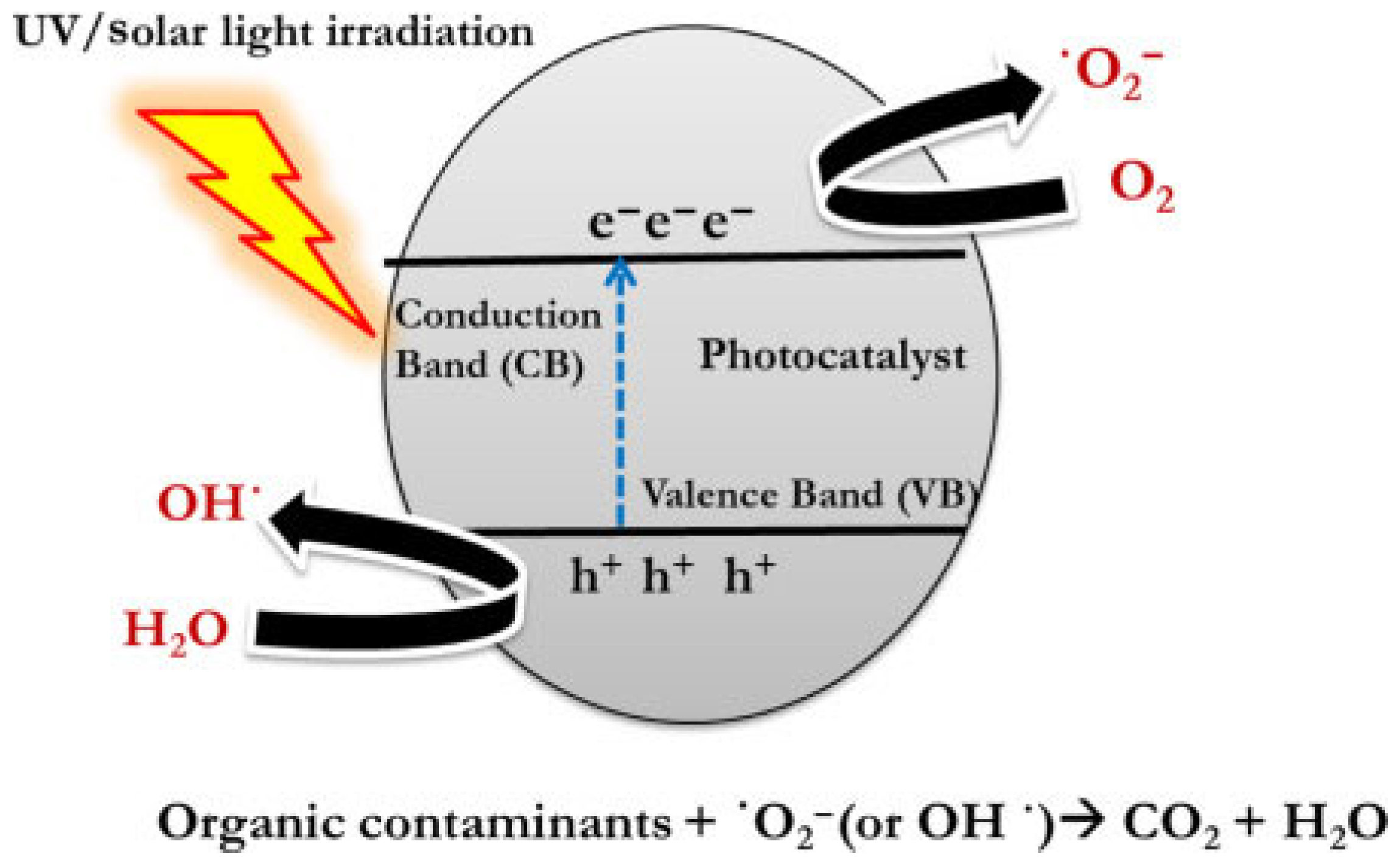
3.2. Application of Electrospun Nanofibers for Hydrogen Production
3.3. Application of Electrospun Nanofibers for Water Treatment
| Year | Photocatalyst | Light Source | Pollutant | Time | Efficiency | Ref. |
|---|---|---|---|---|---|---|
| 2021 | ZnFe2O4/Ag/AgBr | UV light | Rhodamine B | 100 min | 86% | [121] |
| 2020 | Bimetal-PANNM | UV-visible | Reactive blue | 60 min | 99.99% | [122] |
| 2021 | TiO2@Ag@Cu2O | Visible light | Methylene Blue | 90 min | 99% | [123] |
| 2020 | ZnIn2S4/SnO2 | Visible light | Cr(VI) | 80 min | 100% | [124] |
| 2020 | Co-CdSe@ECNFs | Visible light | Methylene Blue | 90 min | 87% | [125] |
| 2021 | ZnO | UV light | Methylene Blue | 85 min | 90% | [87] |
| 2021 | Bi2O3/g-C3N4 | Visible light | Tetracycline | 180 min | ~60% | [126] |
| 2020 | Mn2+/ZnO | Visible light | Rhodamine B | 260 min | ~80% | [127] |
| 2021 | Ag3PO4-TiO2CNFs | Visible light | Methylene Blue | 10 min | 100% | [128] |
| 2021 | Ag/BiVO4 | Visible light | Rhodamine B | 20 min | ~100% | [129] |
| 2020 | ZnO-TiO2CNFs | Visible light | Methylene Blue | 120 min | ~95% | [130] |
| 2023 | g-C3N4 (TiO2/g-C3N4@LCNFs | UV light | Rhodamine B | 90 min | 83.8% | [131] |
| 2022 | Chitin-modified and graphene oxide (GO) bridged TiO2/carbon fibers (CGTC) | Visible light | Rhodamine B | 60 min | 86.8% | [132] |
| 2022 | PAN/Bi2MoO6/Ti3C2 (PAN/BT) | UV-visible | Tetracycline | 180 min | 90.3% | [133] |
| 2023 | CuBi2O4@WO3 | Visible light | Tetracycline hydrochloride (TCH) | 120 min | 70.42% | [134] |
3.4. Application of Electrospun Nanofibers for CO2 Reduction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Letcher, T.M. 9.01—Introduction to Environmental and Social Issues. In Comprehensive Renewable Energy, 2nd ed.; Letcher, T.M., Ed.; Elsevier: Oxford, UK, 2022; pp. 1–8. ISBN 978-0-12-819734-9. [Google Scholar]
- Sharma, S.; Basu, S.; Shetti, N.P.; Kamali, M.; Walvekar, P.; Aminabhavi, T.M. Waste-to-Energy Nexus: A Sustainable Development. Environ. Pollut. 2020, 267, 115501. [Google Scholar] [CrossRef] [PubMed]
- Yergaziyeva, G.; Kuspanov, Z.; Mambetova, M.; Khudaibergenov, N.; Makayeva, N.; Daulbayev, C. Advancements in Catalytic, Photocatalytic, and Electrocatalytic CO2 Conversion Processes: Current Trends and Future Outlook. J. CO2 Util. 2024, 80, 102682. [Google Scholar] [CrossRef]
- Nations, U. Renewable Energy—Powering a Safer Future. Available online: https://www.un.org/en/climatechange/raising-ambition/renewable-energy (accessed on 5 April 2023).
- Nations, U. The Paris Agreement. Available online: https://www.un.org/en/climatechange/paris-agreement (accessed on 5 April 2023).
- Directorate-General for Communication (European Commission). REPowerEU with Clean Energy; Publications Office of the European Union: Luxembourg, 2022; ISBN 978-92-76-52458-8. [Google Scholar]
- Environment, U.N. Emissions Gap Report. 2022. Available online: http://www.unep.org/resources/emissions-gap-report-2022 (accessed on 5 April 2023).
- Hermesmann, M.; Müller, T.E. Green, Turquoise, Blue, or Grey? Environmentally Friendly Hydrogen Production in Transforming Energy Systems. Prog. Energy Combust. Sci. 2022, 90, 100996. [Google Scholar] [CrossRef]
- Daulbayev, C.; Lesbayev, B.; Bakbolat, B.; Kaidar, B.; Sultanov, F.; Yeleuov, M.; Ustayeva, G.; Rakhymzhan, N. A Mini-Review on Recent Trends in Prospective Use of Porous 1D Nanomaterials for Hydrogen Storage. S. Afr. J. Chem. Eng. 2022, 39, 52–61. [Google Scholar] [CrossRef]
- Ratnakar, R.R.; Gupta, N.; Zhang, K.; van Doorne, C.; Fesmire, J.; Dindoruk, B.; Balakotaiah, V. Hydrogen Supply Chain and Challenges in Large-Scale LH2 Storage and Transportation. Int. J. Hydrogen Energy 2021, 46, 24149–24168. [Google Scholar] [CrossRef]
- Qureshi, F.; Yusuf, M.; Arham Khan, M.; Ibrahim, H.; Ekeoma, B.C.; Kamyab, H.; Rahman, M.M.; Nadda, A.K.; Chelliapan, S. A State-of-The-Art Review on the Latest Trends in Hydrogen Production, Storage, and Transportation Techniques. Fuel 2023, 340, 127574. [Google Scholar] [CrossRef]
- Insights into Renewable Hydrogen Energy: Recent Advances and Prospects. Mater. Sci. Energy Technol. 2020, 3, 319–327. [CrossRef]
- Razzhivin, I.A.; Andreev, M.V.; Kievec, A.V. Increasing the Stability of Hydrogen Production in the Wind Energy-Hydrogen System through the Use of Synthetic Inertia of the Wind Turbine. Int. J. Hydrogen Energy 2022, 47, 38495–38505. [Google Scholar] [CrossRef]
- Partho, A.T.; Tahir, M.; Tahir, B. Recent Advances in Covalent Organic Framework (COF) Nanotextures with Band Engineering for Stimulating Solar Hydrogen Production: A Comprehensive Review. Int. J. Hydrogen Energy 2022, 47, 34323–34375. [Google Scholar] [CrossRef]
- Steingrímsson, B.; Gunnlaugsson, E.; Saemundsson, K.; Axelsson, G. 7.02—Nature and Classification of Geothermal Resources. In Comprehensive Renewable Energy, 2nd ed.; Letcher, T.M., Ed.; Elsevier: Oxford, UK, 2022; pp. 3–17. ISBN 978-0-12-819734-9. [Google Scholar]
- Mahmoud, M.; Ramadan, M.; Naher, S.; Pullen, K.; Ali Abdelkareem, M.; Olabi, A.-G. A Review of Geothermal Energy-Driven Hydrogen Production Systems. Therm. Sci. Eng. Prog. 2021, 22, 100854. [Google Scholar] [CrossRef]
- Muritala, I.K.; Guban, D.; Roeb, M.; Sattler, C. High Temperature Production of Hydrogen: Assessment of Non-Renewable Resources Technologies and Emerging Trends. Int. J. Hydrogen Energy 2020, 45, 26022–26035. [Google Scholar] [CrossRef]
- Monga, D.; Shetti, N.P.; Basu, S.; Kakarla, R.R. Recent Advances in Various Processes for Clean and Sustainable Hydrogen Production. Nano-Struct. Nano-Objects 2023, 33, 100948. [Google Scholar] [CrossRef]
- Mandade, P. Chapter 5—Introduction, Basic Principles, Mechanism, and Challenges of Photocatalysis. In Handbook of Nanomaterials for Wastewater Treatment; Bhanvase, B., Sonawane, S., Pawade, V., Pandit, A., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2021; pp. 137–154. ISBN 978-0-12-821496-1. [Google Scholar]
- Kuspanov, Z.; Bakbolat, B.; Baimenov, A.; Issadykov, A.; Yeleuov, M.; Daulbayev, C. Photocatalysts for a Sustainable Future: Innovations in Large-Scale Environmental and Energy Applications. Sci. Total Environ. 2023, 885, 163914. [Google Scholar] [CrossRef]
- Ahmed, S.N.; Haider, W. Heterogeneous Photocatalysis and Its Potential Applications in Water and Wastewater Treatment: A Review. Nanotechnology 2018, 29, 342001. [Google Scholar] [CrossRef]
- Nishiyama, H.; Yamada, T.; Nakabayashi, M.; Maehara, Y.; Yamaguchi, M.; Kuromiya, Y.; Nagatsuma, Y.; Tokudome, H.; Akiyama, S.; Watanabe, T.; et al. Photocatalytic Solar Hydrogen Production from Water on a 100-M2 Scale. Nature 2021, 598, 304–307. [Google Scholar] [CrossRef]
- Goto, Y.; Hisatomi, T.; Wang, Q.; Higashi, T.; Ishikiriyama, K.; Maeda, T.; Sakata, Y.; Okunaka, S.; Tokudome, H.; Katayama, M.; et al. A Particulate Photocatalyst Water-Splitting Panel for Large-Scale Solar Hydrogen Generation. Joule 2018, 2, 509–520. [Google Scholar] [CrossRef]
- Tai, L.N.; Long, P.D.; Hong Le, N.T.; Van Hong, L.; Linh, P.T.; Khuyen, B.X.; Tung, B.S.; Lam, V.D. Photocatalytic and Water-Splitting Properties of TiO2 and Ag–TiO2 Films in the Visible Light Region. AIP Adv. 2021, 11, 075118. [Google Scholar] [CrossRef]
- Peng, R.; Zhang, S.; Yao, Y.; Wang, J.; Zhu, X.; Jiang, R.; Zhang, J.; Zhang, W.; Wang, C. MOFs Meet Electrospinning: New Opportunities for Water Treatment. Chem. Eng. J. 2023, 453, 139669. [Google Scholar] [CrossRef]
- Liverani, L.; Guarino, V.; La Carrubba, V.; Boccaccini, A.R. Porous Biomaterials and Scaffolds for Tissue Engineering. In Encyclopedia of Biomedical Engineering; Narayan, R., Ed.; Elsevier: Oxford, UK, 2019; pp. 188–202. ISBN 978-0-12-805144-3. [Google Scholar]
- Mohammadalizadeh, Z.; Bahremandi-Toloue, E.; Karbasi, S. Recent Advances in Modification Strategies of Pre- and Post-Electrospinning of Nanofiber Scaffolds in Tissue Engineering. React. Funct. Polym. 2022, 172, 105202. [Google Scholar] [CrossRef]
- Ghosal, K.; Augustine, R.; Zaszczynska, A.; Barman, M.; Jain, A.; Hasan, A.; Kalarikkal, N.; Sajkiewicz, P.; Thomas, S. Novel Drug Delivery Systems Based on Triaxial Electrospinning Based Nanofibers. React. Funct. Polym. 2021, 163, 104895. [Google Scholar] [CrossRef]
- Tan, S.M.; Teoh, X.Y.; Le Hwang, J.; Khong, Z.P.; Sejare, R.; Almashhadani, A.Q.; Assi, R.A.; Chan, S.Y. Electrospinning and Its Potential in Fabricating Pharmaceutical Dosage Form. J. Drug Deliv. Sci. Technol. 2022, 76, 103761. [Google Scholar] [CrossRef]
- Asgari, S.; Mohammadi Ziarani, G.; Badiei, A.; Ajalloueian, F.; Vasseghian, Y. Electrospun Composite Nanofibers as Novel High-Performance and Visible-Light Photocatalysts for Removal of Environmental Pollutants: A Review. Environ. Res. 2022, 215, 114296. [Google Scholar] [CrossRef] [PubMed]
- Tebyetekerwa, M.; Ramakrishna, S. What Is Next for Electrospinning? Matter 2020, 2, 279–283. [Google Scholar] [CrossRef]
- SalehHudin, H.S.; Mohamad, E.N.; Mahadi, W.N.L.; Muhammad Afifi, A. Multiple-Jet Electrospinning Methods for Nanofiber Processing: A Review. Mater. Manuf. Process. 2018, 33, 479–498. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Ghosal, K.; Agatemor, C.; Tucker, N.; Kny, E.; Thomas, S. Electrical Spinning to Electrospinning: A Brief History; The Royal Society of Chemistry: Cambridge, UK, 2018. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Kang, I.-K. A Comprehensive Review Summarizing the Effect of Electrospinning Parameters and Potential Applications of Nanofibers in Biomedical and Biotechnology. Arab. J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]
- Shepa, I.; Mudra, E.; Dusza, J. Electrospinning through the Prism of Time. Mater. Today Chem. 2021, 21, 100543. [Google Scholar] [CrossRef]
- Panda, P.K.; Sahoo, B.; Ramakrishna, S. Electrospun Nanofibers for Photocatalytic Water Treatment and Hydrogen Generation Application: A Review. Int. J. Hydrogen Energy 2023, 48, 37193–37208. [Google Scholar] [CrossRef]
- Joshi, B.; Samuel, E.; Kim, Y.; Yarin, A.L.; Swihart, M.T.; Yoon, S.S. Review of Recent Progress in Electrospinning-Derived Freestanding and Binder-Free Electrodes for Supercapacitors. Coord. Chem. Rev. 2022, 460, 214466. [Google Scholar] [CrossRef]
- Can-Herrera, L.A.; Oliva, A.I.; Dzul-Cervantes, M.a.A.; Pacheco-Salazar, O.F.; Cervantes-Uc, J.M. Morphological and Mechanical Properties of Electrospun Polycaprolactone Scaffolds: Effect of Applied Voltage. Polymers 2021, 13, 662. [Google Scholar] [CrossRef]
- Tlili, I.; Alkanhal, T.A. Nanotechnology for Water Purification: Electrospun Nanofibrous Membrane in Water and Wastewater Treatment. J. Water Reuse Desalination 2019, 9, 232–248. [Google Scholar] [CrossRef]
- Wei, L.; Sun, R.; Liu, C.; Xiong, J.; Qin, X. Mass Production of Nanofibers from Needleless Electrospinning by a Novel Annular Spinneret. Mater. Des. 2019, 179, 107885. [Google Scholar] [CrossRef]
- Zhao, K.; Wang, W.; Yang, Y.; Wang, K.; Yu, D.-G. From Taylor Cone to Solid Nanofiber in Tri-Axial Electrospinning: Size Relationships. Results Phys. 2019, 15, 102770. [Google Scholar] [CrossRef]
- Ngadiman, N.H.A.; Noordin, M.Y.; Idris, A.; Kurniawan, D. Effect of Electrospinning Parameters Setting towards Fiber Diameter. Adv. Mater. Res. 2014, 845, 985–988. [Google Scholar] [CrossRef]
- Sharma, G.K.; James, N.R.; Sharma, G.K.; James, N.R. Electrospinning: The Technique and Applications. In Recent Developments in Nanofibers Research; IntechOpen: London, UK, 2022; ISBN 978-1-80356-387-9. [Google Scholar]
- Abdul Hameed, M.M.; Mohamed Khan, S.A.P.; Thamer, B.M.; Rajkumar, N.; El-Hamshary, H.; El-Newehy, M. Electrospun Nanofibers for Drug Delivery Applications: Methods and Mechanism. Polym. Adv. Technol. 2023, 34, 6–23. [Google Scholar] [CrossRef]
- Shi, X.; Zhou, W.; Ma, D.; Ma, Q.; Bridges, D.; Ma, Y.; Hu, A. Electrospinning of Nanofibers and Their Applications for Energy Devices. J. Nanomater. 2015, 2015, e140716. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Tan, Z. A Review of Electrospun Nanofiber-Based Separators for Rechargeable Lithium-Ion Batteries. J. Power Sources 2019, 443, 227262. [Google Scholar] [CrossRef]
- Mwiiri, F.K.; Daniels, R. Influence of PVA Molecular Weight and Concentration on Electrospinnability of Birch Bark Extract-Loaded Nanofibrous Scaffolds Intended for Enhanced Wound Healing. Molecules 2020, 25, 4799. [Google Scholar] [CrossRef]
- Lin, H.-N.; Peng, T.-Y.; Kung, Y.-R.; Chiou, Y.-J.; Chang, W.-M.; Wu, S.-H.; Mine, Y.; Chen, C.-Y.; Lin, C.-K. Effects of the Methyl Methacrylate Addition, Polymerization Temperature and Time on the MBG@PMMA Core-Shell Structure and Its Application as Addition in Electrospun Composite Fiber Bioscaffold. Ceram. Int. 2023, 49, 7630–7639. [Google Scholar] [CrossRef]
- Ki, C.S.; Baek, D.H.; Gang, K.D.; Lee, K.H.; Um, I.C.; Park, Y.H. Characterization of Gelatin Nanofiber Prepared from Gelatin–Formic Acid Solution. Polymer 2005, 46, 5094–5102. [Google Scholar] [CrossRef]
- Suresh, S.; Becker, A.; Glasmacher, B. Impact of Apparatus Orientation and Gravity in Electrospinning-A Review of Empirical Evidence. Polymers 2020, 12, 2448. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, S. An Introduction to Electrospinning and Nanofibers; World Scientific: Hackensack, NJ, USA, 2005; ISBN 978-981-256-761-1. [Google Scholar]
- Yang, G.-Z.; Li, H.-P.; Yang, J.-H.; Wan, J.; Yu, D.-G. Influence of Working Temperature on The Formation of Electrospun Polymer Nanofibers. Nanoscale Res. Lett. 2017, 12, 55. [Google Scholar] [CrossRef] [PubMed]
- Ramazani, S.; Karimi, M. Investigating the Influence of Temperature on Electrospinning of Polycaprolactone Solutions. e-Polymers 2014, 14, 323–333. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Ni, J.; Li, L. Electrospinning for Flexible Sodium-Ion Batteries. Energy Storage Mater. 2022, 45, 704–719. [Google Scholar] [CrossRef]
- Joly, D.; Jung, J.-W.; Kim, I.-D.; Demadrille, R. Electrospun Materials for Solar Energy Conversion: Innovations and Trends. J. Mater. Chem. C 2016, 4, 10173–10197. [Google Scholar] [CrossRef]
- Lu, X.; Wang, C.; Wei, Y. One-Dimensional Composite Nanomaterials: Synthesis by Electrospinning and Their Applications. Small 2009, 5, 2349–2370. [Google Scholar] [CrossRef]
- Hariganesh, S.; Vadivel, S.; Maruthamani, D.; Rangabhashiyam, S. Chapter 12—Disinfection by-Products in Drinking Water: Detection and Treatment Methods. In Disinfection By-Products in Drinking Water; Prasad, M.N.V., Ed.; Butterworth-Heinemann: Oxford, UK, 2020; pp. 279–304. ISBN 978-0-08-102977-0. [Google Scholar]
- Mayorov, A.S.; Gorbachev, R.V.; Morozov, S.V.; Britnell, L.; Jalil, R.; Ponomarenko, L.A.; Blake, P.; Novoselov, K.S.; Watanabe, K.; Taniguchi, T.; et al. Micrometer-Scale Ballistic Transport in Encapsulated Graphene at Room Temperature. Nano Lett. 2011, 11, 2396–2399. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Stoller, M.D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-Based Ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef]
- Nasr, M.; Balme, S.; Eid, C.; Habchi, R.; Miele, P.; Bechelany, M. Enhanced Visible-Light Photocatalytic Performance of Electrospun rGO/TiO2 Composite Nanofibers. J. Phys. Chem. C 2017, 121, 261–269. [Google Scholar] [CrossRef]
- Song, M.; Cao, H.; Zhu, Y.; Wang, Y.; Zhao, S.; Huang, C.; Zhang, C.; He, X. Electrochemical and Photocatalytic Properties of Electrospun C/TiO2 Nanofibers. Chem. Phys. Lett. 2020, 747, 137355. [Google Scholar] [CrossRef]
- Yao, Y. Visible-Light Photocatalysis of Carbon-Based Materials; IntechOpen Limited: London, UK, 2018; ISBN 978-1-78923-029-1. [Google Scholar]
- Latorre-Sánchez, M.; Lavorato, C.; Puche, M.; Fornés, V.; Molinari, R.; Garcia, H. Visible-Light Photocatalytic Hydrogen Generation by Using Dye-Sensitized Graphene Oxide as a Photocatalyst. Chem.—A Eur. J. 2012, 18, 16774–16783. [Google Scholar] [CrossRef] [PubMed]
- Zhurenok, A.V.; Vasilchenko, D.B.; Kozlova, E.A. Comprehensive Review on G-C3N4-Based Photocatalysts for the Photocatalytic Hydrogen Production under Visible Light. Int. J. Mol. Sci. 2023, 24, 346. [Google Scholar] [CrossRef] [PubMed]
- Singla, S.; Sharma, S.; Basu, S.; Shetti, N.P.; Reddy, K.R. Graphene/Graphitic Carbon Nitride-Based Ternary Nanohybrids: Synthesis Methods, Properties, and Applications for Photocatalytic Hydrogen Production. FlatChem 2020, 24, 100200. [Google Scholar] [CrossRef]
- Mishra, A.; Mehta, A.; Kainth, S.; Basu, S. A Comparative Study on the Effect of Different Precursors for Synthesis and Efficient Photocatalytic Activity of G-C3N4/TiO2/Bentonite Nanocomposites. J. Mater. Sci. 2018, 53, 13126–13142. [Google Scholar] [CrossRef]
- Kim, Y.K.; Kim, M.; Hwang, S.-H.; Lim, S.K.; Park, H.; Kim, S. CdS-Loaded Flexible Carbon Nanofiber Mats as a Platform for Solar Hydrogen Production. Int. J. Hydrogen Energy 2015, 40, 136–145. [Google Scholar] [CrossRef]
- Dai, Z.; Ren, P.; Cao, Q.; Gao, X.; He, W.; Xiao, Y.; Jin, Y.; Ren, F. Synthesis of TiO2@lignin Based Carbon Nanofibers Composite Materials with Highly Efficient Photocatalytic to Methylene Blue Dye. J. Polym. Res. 2020, 27, 108. [Google Scholar] [CrossRef]
- Abbasi, S.; Hasanpour, M.; Ekrami-Kakhki, M.-S. Removal Efficiency Optimization of Organic Pollutant (Methylene Blue) with Modified Multi-Walled Carbon Nanotubes Using Design of Experiments (DOE). J. Mater. Sci. Mater. Electron. 2017, 28, 9900–9910. [Google Scholar] [CrossRef]
- Roozban, N.; Abbasi, S.; Ghazizadeh, M. The Experimental and Statistical Investigation of the Photo Degradation of Methyl Orange Using Modified MWCNTs with Different Amount of ZnO Nanoparticles. J. Mater. Sci. Mater. Electron. 2017, 28, 7343–7352. [Google Scholar] [CrossRef]
- Sultanov, F.; Daulbayev, C.; Bakbolat, B.; Daulbayev, O.; Bigaj, M.; Mansurov, Z.; Kuterbekov, K.; Bekmyrza, K. Aligned Composite SrTiO3/PAN Fibers as 1D Photocatalyst Obtained by Electrospinning Method. Chem. Phys. Lett. 2019, 737, 136821. [Google Scholar] [CrossRef]
- Daulbayev, C.; Sultanov, F.; Korobeinyk, A.V.; Yeleuov, M.; Azat, S.; Bakbolat, B.; Umirzakov, A.; Mansurov, Z. Bio-Waste-Derived Few-Layered Graphene/SrTiO3/PAN as Efficient Photocatalytic System for Water Splitting. Appl. Surf. Sci. 2021, 549, 149176. [Google Scholar] [CrossRef]
- Serik, A.; Kuspanov, Z.; Bissenova, M.; Idrissov, N.; Yeleuov, M.; Umirzakov, A.; Daulbayev, C. Effective Photocatalytic Degradation of Sulfamethoxazole Using PAN/SrTiO3 Nanofibers. J. Water Process Eng. 2024, 66, 106052. [Google Scholar] [CrossRef]
- Pereira, M.F.G.; Nascimento, M.M.; Cardoso, P.H.N.; Oliveira, C.Y.B.; Tavares, G.F.; Araújo, E.S. Preparation, Microstructural Characterization and Photocatalysis Tests of V5+-Doped TiO2/WO3 Nanocomposites Supported on Electrospun Membranes. Inorganics 2022, 10, 143. [Google Scholar] [CrossRef]
- Guerrero-Pérez, M.O. Research Progress on the Applications of Electrospun Nanofibers in Catalysis. Catalysts 2022, 12, 9. [Google Scholar] [CrossRef]
- Hilal Elhousseini, M.; Isık, T.; Kap, Ö.; Verpoort, F.; Horzum, N. Dual Remediation of Waste Waters from Methylene Blue and Chromium (VI) Using Thermally Induced ZnO Nanofibers. Appl. Surf. Sci. 2020, 514, 145939. [Google Scholar] [CrossRef]
- Navidpour, A.H.; Salehi, M.; Salimijazi, H.R.; Kalantari, Y.; Azarpour Siahkali, M. Photocatalytic Activity of Flame-Sprayed Coating of Zinc Ferrite Powder. J. Therm. Spray Technol. 2017, 26, 2030–2039. [Google Scholar] [CrossRef]
- Karim, A.V.; Shriwastav, A. Degradation of Amoxicillin with Sono, Photo, and Sonophotocatalytic Oxidation under Low-Frequency Ultrasound and Visible Light. Environ. Res. 2021, 200, 111515. [Google Scholar] [CrossRef]
- Bharathi, P.; Harish, S.; Archana, J.; Navaneethan, M.; Ponnusamy, S.; Muthamizhchelvan, C.; Shimomura, M.; Hayakawa, Y. Enhanced Charge Transfer and Separation of Hierarchical CuO/ZnO Composites: The Synergistic Effect of Photocatalysis for the Mineralization of Organic Pollutant in Water. Appl. Surf. Sci. 2019, 484, 884–891. [Google Scholar] [CrossRef]
- Ullah, H.; Haneef, Z.; Ahmad, A.; Butler, I.S.; Nasir Dara, R.; Rehman, Z. MoS2 and CdS Photocatalysts for Water Decontamination: A Review. Inorg. Chem. Commun. 2023, 153, 110775. [Google Scholar] [CrossRef]
- Paraskevopoulou, A.; Pandis, P.; Argirusis, C.; Sourkouni, G. Sonochemical Synthesis of Indium Nitride Nanoparticles and Photocatalytic Composites with Titania. Ceramics 2024, 7, 478–490. [Google Scholar] [CrossRef]
- Alahmadi, N. Recent Progress in Photocatalytic Removal of Environmental Pollution Hazards in Water Using Nanostructured Materials. Separations 2022, 9, 264. [Google Scholar] [CrossRef]
- Samadi, M.; Moshfegh, A.Z. Recent Developments of Electrospinning-Based Photocatalysts in Degradation of Organic Pollutants: Principles and Strategies. ACS Omega 2022, 7, 45867–45881. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Huang, Y.; Zhang, D.; Chen, S. Electrospinning Fabricating Au/TiO2 Network-like Nanofibers as Visible Light Activated Photocatalyst. Sci. Rep. 2019, 9, 8008. [Google Scholar] [CrossRef] [PubMed]
- Pantò, F.; Dahrouch, Z.; Saha, A.; Patanè, S.; Santangelo, S.; Triolo, C. Photocatalytic Degradation of Methylene Blue Dye by Porous Zinc Oxide Nanofibers Prepared via Electrospinning: When Defects Become Merits. Appl. Surf. Sci. 2021, 557, 149830. [Google Scholar] [CrossRef]
- Ruiz-Ramírez, L.R.; Torres-Pérez, J.; Medellín-Castillo, N.; Reyes-López, S.Y. Photocatalytic Degradation of Oxytetracycline by SiO2–TiO2–Ag Electrospun Fibers. Solid State Sci. 2023, 140, 107188. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Xie, S.; Huang, H.; Guo, G.; Dai, H.; Deng, J. Supported Ceria-Modified Silver Catalysts with High Activity and Stability for Toluene Removal. Environ. Int. 2019, 128, 335–342. [Google Scholar] [CrossRef]
- Lontio Fomekong, R.; Saruhan, B. Synthesis of Co3+ Doped TiO2 by Co-Precipitation Route and Its Gas Sensing Properties. Front. Mater. 2019, 6, 252. [Google Scholar] [CrossRef]
- Na, K.-H.; Kim, B.-S.; Yoon, H.-S.; Song, T.-H.; Kim, S.-W.; Cho, C.-H.; Choi, W.-Y. Fabrication and Photocatalytic Properties of Electrospun Fe-Doped TiO2 Nanofibers Using Polyvinyl Pyrrolidone Precursors. Polymers 2021, 13, 2634. [Google Scholar] [CrossRef]
- Ghoreishian, S.M.; Ranjith, K.S.; Lee, H.; Park, B.; Norouzi, M.; Nikoo, S.Z.; Kim, W.-S.; Han, Y.-K.; Huh, Y.S. Tuning the Phase Composition of 1D TiO2 by Fe/Sn Co-Doping Strategy for Enhanced Visible-Light-Driven Photocatalytic and Photoelectrochemical Performances. J. Alloys Compd. 2021, 851, 156826. [Google Scholar] [CrossRef]
- Wang, L.; Sun, M.; Zhou, B.; Rao, Y.; Yan, T.; Du, B.; Shao, Y. Electrospinning Mediated Solvothermal Preparation of Hierarchical MGa2O4/ZnIn2S4 (M = Ni, Co) Hollow Nanofibers with p-n Heterojunction for Boosting Photocatalytic H2 Evolution. Fuel 2024, 374, 132448. [Google Scholar] [CrossRef]
- Kuspanov, Z.; Umirzakov, A.; Serik, A.; Baimenov, A.; Yeleuov, M.; Daulbayev, C. Multifunctional Strontium Titanate Perovskite-Based Composite Photocatalysts for Energy Conversion and Other Applications. Int. J. Hydrogen Energy 2023, 48, 38634–38654. [Google Scholar] [CrossRef]
- Kudaibergen, A.D.; Kuspanov, Z.B.; Issadykov, A.N.; Beisenov, R.E.; Mansurov, Z.A.; Yeleuov, M.A.; Daulbayev, C.B. Synthesis, Structure, and Energetic Characteristics of Perovskite Photocatalyst SrTiO3: An Experimental and DFT Study. Eurasian Chem.-Technol. J. 2023, 25, 139–146. [Google Scholar] [CrossRef]
- Bissenova, M.; Umirzakov, A.; Mit, K.; Mereke, A.; Yerubayev, Y.; Serik, A.; Kuspanov, Z. Synthesis and Study of SrTiO3/TiO2 Hybrid Perovskite Nanotubes by Electrochemical Anodization. Molecules 2024, 29, 1101. [Google Scholar] [CrossRef] [PubMed]
- Matus, E.V.; Ismagilov, I.Z.; Mikhaylova, E.S.; Ismagilov, Z.R. Hydrogen Production from Coal Industry Methane. Eurasian Chem.-Technol. J. 2022, 24, 69–91. [Google Scholar] [CrossRef]
- Sun, F.; Qi, H.; Xie, Y.; Ma, Q.; He, W.; Xu, D.; Wang, G.; Yu, W.; Wang, T.; Dong, X. Flexible Self-Supporting Bifunctional [TiO2/C]//[Bi2WO6/C] Carbon-Based Janus Nanofiber Heterojunction Photocatalysts for Efficient Hydrogen Evolution and Degradation of Organic Pollutant. J. Alloys Compd. 2020, 830, 154673. [Google Scholar] [CrossRef]
- Zhang, Y.; Niu, L.; Li, Z.; Yang, T.; Liu, Y.; Kang, Z. A Recyclable ZnIn2S4/PAN Photocatalytic Nanofiber Membrane for Boosting Visible Light Hydrogen Evolution in Seawater without Cocatalyst. Appl. Catal. B Environ. Energy 2024, 357, 124300. [Google Scholar] [CrossRef]
- Ismagilov, I.Z.; Matus, E.V.; Kuznetsov, V.V.; Yashnik, S.A.; Kerzhentsev, M.A.; Gerritsen, G.; Abbenhuis, H.C.; Ismagilov, Z.R. Application of POSS Nanotechnology for Preparation of Efficient Ni Catalysts for Hydrogen Production. Eurasian Chem.-Technol. J. 2017, 19, 3–16. [Google Scholar] [CrossRef]
- Sultanov, F.; Daulbayev, C.; Azat, S.; Kuterbekov, K.; Bekmyrza, K.; Bakbolat, B.; Bigaj, M.; Mansurov, Z. Influence of Metal Oxide Particles on Bandgap of 1D Photocatalysts Based on SrTiO3/PAN Fibers. Nanomaterials 2020, 10, 1734. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Zeng, C.-J.; Chen, C.-Y.; Tsay, C.-Y.; Lee, G.-J.; Wu, J.J. NiS/Pt Loaded on Electrospun TiO2 Nanofiber with Enhanced Visible-Light-Driven Photocatalytic Hydrogen Production. Mater. Res. Bull. 2023, 157, 112041. [Google Scholar] [CrossRef]
- Peng, H.; Yong, J.; Wang, H.; Gou, Y.; Wang, F.; Zheng, X. Dual CdS–CoS/S,N-Doped TiO2 Nanofibers for Efficient Visible-Light Induced H2 Evolution. Int. J. Hydrogen Energy 2022, 47, 31269–31278. [Google Scholar] [CrossRef]
- Li, R.; Luan, J.; Zhang, Y.; Jiang, L.; Yan, H.; Chi, Q.; Yan, Z. A Review of Efficient Photocatalytic Water Splitting for Hydrogen Production. Renew. Sustain. Energy Rev. 2024, 206, 114863. [Google Scholar] [CrossRef]
- Abu-Sari, S.M.; Ang, B.C.; Wan Daud, W.M.A.; Patah, M.F.A. Visible-Light-Driven Photocatalytic Hydrogen Production on Defective, Sulfur Self-Doped g-C3N4 Nanofiber Fabricate via Electrospinning Method. J. Environ. Chem. Eng. 2023, 11, 109318. [Google Scholar] [CrossRef]
- Shang, Q.; Wang, J.; Yang, J.; Wang, Z.; Wang, G.; Wang, K.; Wu, X.; Li, J. Photocatalytic Hydrogen Evolution Coupled with Tetracycline Photodegradation over S-Scheme BaTiO3/Ag2S Dual-Function Nanofibers: Performance and Mechanism. Appl. Surf. Sci. 2023, 635, 157760. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Syu, S.-Y.; Hsu, P.-C. Construction of In2S3–In(OH)3–ZnS Nanofibers for Boosting Photocatalytic Hydrogen Evolution. Int. J. Hydrogen Energy 2024, 86, 24–35. [Google Scholar] [CrossRef]
- Peng, H.; Luo, M.; Zheng, X. Boosting Solar-Light Photocatalytic Activity of Ni2P-Decorated ZnCr2O4 Nanofibers for H2 Evolution and Tetracycline Elimination. Ceram. Int. 2024, 50, 26474–26481. [Google Scholar] [CrossRef]
- Al-Enizi, A.M.; Karim, A.; Yousef, A. A Novel Method for Fabrication of Electrospun Cadmium Sulfide Nanoparticles- Decorated Zinc Oxide Nanofibers as Effective Photocatalyst for Water Photosplitting. Alex. Eng. J. 2023, 65, 825–835. [Google Scholar] [CrossRef]
- Aravind, M.; Amalanathan, M.; Aslam, S.; Noor, A.E.; Jini, D.; Majeed, S.; Velusamy, P.; Alothman, A.A.; Alshgari, R.A.; Saleh Mushab, M.S.; et al. Hydrothermally Synthesized Ag-TiO2 Nanofibers (NFs) for Photocatalytic Dye Degradation and Antibacterial Activity. Chemosphere 2023, 321, 138077. [Google Scholar] [CrossRef]
- Mousa, H.M.; Alenezi, J.F.; Mohamed, I.M.A.; Yasin, A.S.; Hashem, A.-F.M.; Abdal-hay, A. Synthesis of TiO2@ZnO Heterojunction for Dye Photodegradation and Wastewater Treatment. J. Alloys Compd. 2021, 886, 161169. [Google Scholar] [CrossRef]
- Villa, K.; Galán-Mascarós, J.R.; López, N.; Palomares, E. Photocatalytic Water Splitting: Advantages and Challenges. Sustain. Energy Fuels 2021, 5, 4560–4569. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Mohamed, A.A. Recent Progress in Semiconductor/Graphene Photocatalysts: Synthesis, Photocatalytic Applications, and Challenges. RSC Adv. 2022, 13, 421–439. [Google Scholar] [CrossRef]
- Chen, Y.; Li, A.; Fu, X.; Peng, Z. Electrospinning-Based (N,F)-Co-Doped TiO2-δ Nanofibers: An Excellent Photocatalyst for Degrading Organic Dyes and Heavy Metal Ions under Visible Light. Mater. Chem. Phys. 2022, 291, 126672. [Google Scholar] [CrossRef]
- Yasin, A.S.; Mohamed, I.M.A.; Mousa, H.M.; Park, C.H.; Kim, C.S. Facile Synthesis of TiO2/ZrO2 Nanofibers/Nitrogen Co-Doped Activated Carbon to Enhance the Desalination and Bacterial Inactivation via Capacitive Deionization. Sci. Rep. 2018, 8, 541. [Google Scholar] [CrossRef] [PubMed]
- Le, P.H.; Hieu, L.T.; Lam, T.-N.; Hang, N.T.N.; Truong, N.V.; Tuyen, L.T.C.; Phong, P.T.; Leu, J. Enhanced Photocatalytic Performance of Nitrogen-Doped TiO2 Nanotube Arrays Using a Simple Annealing Process. Micromachines 2018, 9, 618. [Google Scholar] [CrossRef] [PubMed]
- Enculescu, M.; Costas, A.; Evanghelidis, A.; Enculescu, I. Fabrication of ZnO and TiO2 Nanotubes via Flexible Electro-Spun Nanofibers for Photocatalytic Applications. Nanomaterials 2021, 11, 1305. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wang, Y.; Shen, Q.; Xiong, X.; Ren, S.; Dai, G.; Wu, C. Fabrication of TiO2 Nanofibers Assembled by Bi2WO6 Nanosheets with Enhanced Visible Light Photocatalytic Activity. Ceram. Int. 2020, 46, 21304–21310. [Google Scholar] [CrossRef]
- Lou, C.-W.; Xie, M.-M.; Yang, Y.-D.; Wang, H.-Y.; Wang, Z.-K.; Zhang, L.; Hsieh, C.-T.; Liu, L.-Y.; Lin, M.-C.; Li, T.-T. Carbon Nanofiber Membranes Loaded with MXene@g-C3N4: Preparation and Photocatalytic Property. Nanomaterials 2024, 14, 896. [Google Scholar] [CrossRef]
- Liu, Y.; Hao, M.; Zhou, C.; Yang, B.; Jiang, S.; Huang, J.; Chen, Z.; Liu, Y.; Ramakrishna, S. Chapter 9—Scale-up Strategies for Electrospun Nanofiber Production. In Electrospun and Nanofibrous Membranes; Kargari, A., Matsuura, T., Shirazi, M.M.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 205–266. ISBN 978-0-12-823032-9. [Google Scholar]
- Sabzehmeidani, M.M.; Karimi, H.; Ghaedi, M.; Avargani, V.M. Construction of Efficient and Stable Ternary ZnFe2O4/Ag/AgBr Z-Scheme Photocatalyst Based on ZnFe2O4 Nanofibers under LED Visible Light. Mater. Res. Bull. 2021, 143, 111449. [Google Scholar] [CrossRef]
- Yi, S.; Sun, S.; Zhang, Y.; Zou, Y.; Dai, F.; Si, Y. Scalable Fabrication of Bimetal Modified Polyacrylonitrile (PAN) Nanofibrous Membranes for Photocatalytic Degradation of Dyes. J. Colloid Interface Sci. 2020, 559, 134–142. [Google Scholar] [CrossRef]
- Li, X.; Raza, S.; Liu, C. Directly Electrospinning Synthesized Z-Scheme Heterojunction TiO2@Ag@Cu2O Nanofibers with Enhanced Photocatalytic Degradation Activity under Solar Light Irradiation. J. Environ. Chem. Eng. 2021, 9, 106133. [Google Scholar] [CrossRef]
- Li, X.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Hollow SnO2 Nanotubes Decorated with ZnIn2S4 Nanosheets for Enhanced Visible-Light Photocatalytic Activity. J. Alloys Compd. 2020, 843, 155772. [Google Scholar] [CrossRef]
- Panthi, G.; Kwon, O.H.; Kuk, Y.-S.; Gyawali, K.R.; Park, Y.W.; Park, M. Ternary Composite of Co-Doped CdSe@electrospun Carbon Nanofibers: A Novel Reusable Visible Light-Driven Photocatalyst with Enhanced Performance. Catalysts 2020, 10, 348. [Google Scholar] [CrossRef]
- Zhang, L.; Shen, Q.; Zheng, S.; Yu, L.; Huang, F.; Zhang, C.; Sheng, J.; Yang, H. Direct Electrospinning Preparation of Z-Scheme Mixed-Crystal Bi2O3/g-C3N4 Composite Photocatalysts with Enhanced Visible-Light Photocatalytic Activity. New J. Chem. 2021, 45, 14522–14531. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, X.; Wang, Z.; Dong, M.; Cui, L. Facile Fabrication of Mn2+-Doped ZnO Photocatalysts by Electrospinning. R. Soc. Open Sci. 2020, 7, 191050. [Google Scholar] [CrossRef] [PubMed]
- Pant, B.; Prasad Ojha, G.; Acharya, J.; Park, M. Ag3PO4-TiO2-Carbon Nanofiber Composite: An Efficient Visible-Light Photocatalyst Obtained from Electrospinning and Hydrothermal Methods. Sep. Purif. Technol. 2021, 276, 119400. [Google Scholar] [CrossRef]
- Xing, Y.; Cheng, J.; Li, H.; Lin, D.; Wang, Y.; Wu, H.; Pan, W. Electrospun Ceramic Nanofibers for Photocatalysis. Nanomaterials 2021, 11, 3221. [Google Scholar] [CrossRef]
- Pant, B.; Ojha, G.P.; Kuk, Y.-S.; Kwon, O.H.; Park, Y.W.; Park, M. Synthesis and Characterization of ZnO-TiO2/Carbon Fiber Composite with Enhanced Photocatalytic Properties. Nanomaterials 2020, 10, 1960. [Google Scholar] [CrossRef]
- Zhai, G.; Zhou, J.; Xie, M.; Jia, C.; Hu, Z.; Xiang, H.; Zhu, M. Improved Photocatalytic Property of Lignin-Derived Carbon Nanofibers through Catalyst Synergy. Int. J. Biol. Macromol. 2023, 233, 123588. [Google Scholar] [CrossRef]
- Yu, W.; Zheng, B.; Mao, K.; Jiang, J.; Luo, B.; Wu, X.; Tao, T.; Min, X.; Mi, R.; Huang, Z.; et al. Interfacial Structure and Photocatalytic Degradation Performance of Graphene Oxide Bridged Chitin-Modified TiO2/Carbon Fiber Composites. J. Clean. Prod. 2022, 361, 132261. [Google Scholar] [CrossRef]
- Zhang, J.-J.; Kai, C.-M.; Zhang, F.-J.; Wang, Y.-R. Novel PAN/Bi2MoO6/Ti3C2 Ternary Composite Membrane via Electrospinning with Enhanced Photocatalytic Degradation of Tetracycline. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129255. [Google Scholar] [CrossRef]
- Zhang, L.; Shen, Q.; Huang, F.; Jiang, L.; Liu, J.; Sheng, J.; Li, Y.; Yang, H. Electrospinning Directly Synthesis of 0D/1D CuBi2O4@WO3 Nanofiber Photocatalyst with S-Scheme Heterojunction. Appl. Surf. Sci. 2023, 608, 155064. [Google Scholar] [CrossRef]
- Fu, J.; Jiang, K.; Qiu, X.; Yu, J.; Liu, M. Product Selectivity of Photocatalytic CO2 Reduction Reactions. Mater. Today 2020, 32, 222–243. [Google Scholar] [CrossRef]
- Adekoya, D.; Tahir, M.; Amin, N.A.S. Recent Trends in Photocatalytic Materials for Reduction of Carbon Dioxide to Methanol. Renew. Sustain. Energy Rev. 2019, 116, 109389. [Google Scholar] [CrossRef]
- Kang, S.; Hwang, J. rGO-Wrapped Ag-Doped TiO2 Nanofibers for Photocatalytic CO2 Reduction under Visible Light. J. Clean. Prod. 2022, 374, 134022. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Huang, Z.; Dong, P.; Nie, X.; Jin, Z. Electrospun PVDF Nanofibers Decorated with Graphene and Titania for Improved Visible Light Photocatalytic Methanation of CO2. Plasmonics 2020, 15, 717–725. [Google Scholar] [CrossRef]
- Lu, N.; Zhang, M.; Jing, X.; Zhang, P.; Zhu, Y.; Zhang, Z. Electrospun Semiconductor-Based Nano-Heterostructures for Photocatalytic Energy Conversion and Environmental Remediation: Opportunities and Challenges. Energy Environ. Mater. 2023, 6, e12338. [Google Scholar] [CrossRef]
- Xu, F.; Tan, H.; Fan, J.; Cheng, B.; Yu, J.; Xu, J. Electrospun TiO2-Based Photocatalysts. Sol. RRL 2021, 5, 2000571. [Google Scholar] [CrossRef]
- Kang, S.; Im, T.; Koh, M.; Lee, C.S. Facile Fabrication of Electrospun Black Titania Nanofibers Decorated with Graphitic Carbon Nitride for the Application of Photocatalytic CO2 Reduction. J. CO2 Util. 2020, 41, 101230. [Google Scholar] [CrossRef]
- Song, J.; Guan, R.; Xie, M.; Dong, P.; Yang, X.; Zhang, J. Advances in Electrospun TiO2 Nanofibers: Design, Construction, and Applications. Chem. Eng. J. 2022, 431, 134343. [Google Scholar] [CrossRef]
- Ma, H.; Wu, X.; Li, X.; Liu, J.; Dong, H.; Liu, Y.; Niu, L.; Zhang, F.; Wang, W.; Shao, C.; et al. Photocatalytic CO2 Reduction to Ethanol by ZnCO2O4/ZnO Janus Hollow Nanofibers. Inorg. Chem. 2024, 63, 15735–15751. [Google Scholar] [CrossRef]
- Wei, Z.; Ji, T.; Zhou, X.; Guo, J.; Yu, X.; Liu, H.; Wang, J. Synergistic Enhancement of Photocatalytic CO2 Reduction by Built-in Electric Field/Piezoelectric Effect and Surface Plasmon Resonance via PVDF/CdS/Ag Heterostructure. Small 2023, 19, 2304202. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, J.; Zhu, C.; Zhang, L.; Yan, J. MXene-Intercalation Induced Ordered Brick-Mortar Structures of Allomorph Junctions for Enhanced Flexibility in TiO2 Nanofibers and Photocatalytic Efficiency. Chem. Eng. J. 2023, 465, 142798. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, X.; Zhang, M.; Ge, M.; Wang, J.; Tang, Y.; Zhang, Y.; Mi, J.; Cai, W.; Lai, Y.; et al. Rational Design of Electrospun Nanofibers for Gas Purification: Principles, Opportunities, and Challenges. Chem. Eng. J. 2022, 446, 137099. [Google Scholar] [CrossRef]
- Wang, S.; Wang, L.; Wang, D.; Li, Y. Recent Advances of Single-Atom Catalysts in CO2 Conversion. Energy Environ. Sci. 2023, 16, 2759–2803. [Google Scholar] [CrossRef]
- Li, L.; ul Hasan, I.M.; Farwa1; He, R.; Peng, L.; Xu, N.; Niazi, N.K.; Zhang, J.-N.; Qiao, J. Copper as a Single Metal Atom Based Photo-, Electro-, and Photoelectrochemical Catalyst Decorated on Carbon Nitride Surface for Efficient CO2 Reduction: A Review. Nano Res. Energy 2022, 1, e9120015. [Google Scholar] [CrossRef]
- Zhu, L.; Qin, C.; Wang, Y.; Cao, J. Single-Atom Pt Supported on Non-Metal Doped WS2 for Photocatalytic CO2 Reduction: A First-Principles Study. Appl. Surf. Sci. 2023, 626, 157252. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Z.; Cao, S.-W.; Xue, C. Au/Pt Nanoparticle-Decorated TiO2 Nanofibers with Plasmon-Enhanced Photocatalytic Activities for Solar-to-Fuel Conversion. J. Phys. Chem. C 2013, 117, 25939–25947. [Google Scholar] [CrossRef]
- Gong, S.; Hou, M.; Niu, Y.; Teng, X.; Liu, X.; Xu, M.; Xu, C.; Ka-Man Au, V.; Chen, Z. Molybdenum Phosphide Coupled with Highly Dispersed Nickel Confined in Porous Carbon Nanofibers for Enhanced Photocatalytic CO2 Reduction. Chem. Eng. J. 2022, 427, 131717. [Google Scholar] [CrossRef]
- Photocatalytic CO2 Reduction of C/ZnO Nanofibers Enhanced by an Ni-NiS Cocatalyst—Nanoscale (RSC Publishing). Available online: https://pubs.rsc.org/en/content/articlelanding/2020/nr/c9nr10451h (accessed on 11 September 2024).
- Shao, X.; Li, K.; Li, J.; Cheng, Q.; Wang, G.; Wang, K. Investigating S-Scheme Charge Transfer Pathways in NiS@Ta2O5 Hybrid Nanofibers for Photocatalytic CO2 Conversion. Chin. J. Catal. 2023, 51, 193–203. [Google Scholar] [CrossRef]
- Kang, S.; Khan, H.; Lee, C.; Kwon, K.; Sunyong Lee, C. Investigation of Hydrophobic MoSe2 Grown at Edge Sites on TiO2 Nanofibers for Photocatalytic CO2 Reduction. Chem. Eng. J. 2021, 420, 130496. [Google Scholar] [CrossRef]
- Prado, A.C.F.; Malafatti, J.O.D.; Oliveira, J.A.; Ribeiro, C.; Joya, M.R.; Luz, A.P.; Paris, E.C. Preparation and Application of Nb2O5 Nanofibers in CO2 Photoconversion. Nanomaterials 2021, 11, 3268. [Google Scholar] [CrossRef]
- Li, Y.; Ren, Z.; Gu, M.; Duan, Y.; Zhang, W.; Lv, K. Synergistic Effect of Interstitial C Doping and Oxygen Vacancies on the Photoreactivity of TiO2 Nanofibers towards CO2 Reduction. Appl. Catal. B Environ. 2022, 317, 121773. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, R.; Wang, L.; Chen, X.; Ding, J.; Zhang, J.; Wan, H.; Guan, G. Synergistic Effect of Cu2+ and Cu+ in SrTiO3 Nanofibers Promotes the Photocatalytic Reduction of CO2 to Methanol. Appl. Surf. Sci. 2023, 609, 155297. [Google Scholar] [CrossRef]
- Kang, S.; Khan, H.; Lee, C.S. CO2 Selectivity of Flower-like MoS2 Grown on TiO2 Nanofibers Coated with Acetic Acid-Treated Graphitic Carbon Nitride. Sol. Energy Mater. Sol. Cells 2021, 221, 110890. [Google Scholar] [CrossRef]

| Year | Photocatalyst | Light Source | Sacrificial Agent | H2 Evolution Rate (mmol h−1g−1) and AQY | Ref. |
|---|---|---|---|---|---|
| 2023 | Sg-C3N4 nanofiber | Metal halide 400 W, full spectrum | 20 vol.% methanol | 0.632 | [105] |
| 2023 | TiO2/NiS/Pt nanofiber | 5 W blue LED light, λmax = 420 nm | 50 vol.% methanol | 4.411 | [102] |
| 2024 | NiGa2O4/ZnIn2S4 nanofiber | 300 W Xe-lamp, AM 1.5 filter, 41.7 mW/cm2 | 10 vol.% TEOA | 9.292 | [93] |
| 2024 | CoGa2O4/ZnIn2S4 nanofiber | 300 W Xe-lamp, AM 1.5 filter, 41.7 mW/cm2 | 10 vol.% TEOA | 6.283 | [93] |
| 2022 | Cd0.5Co0.5S/SN-TiO2 nanofiber | 300 W Xe-lamp | 2.4 g Na2S, and 1.26 g Na2SO3 into 100 mL deionized H2O | 4.55 and AQY of 19.01% at 410 nm | [103] |
| 2024 | ZnIn2S4/PAN nanofiber membrane | Visible light (420 nm ≤ λ ≤ 700 nm) | 10 vol.% TEOA | 1.836 and AQY of 1.77% at 365 nm | [99] |
| 2023 | S-scheme BaTiO3/Ag2S nanofiber | 300 W Xe-lamp | Na2S (0.35 mol/L) and Na2SO3 (0.25 mol/L) | 0.597 | [106] |
| 2024 | In2S3–In(OH)3–ZnS nanofibers | 5 W blue LED light (λmax = 420 nm, 41.7 mW cm−2) | 0.1 M Na2S solution | 0.2236 | [107] |
| 2024 | C–Ni2P/ZnCr2O4 nanofibers | Xe lamp intensity of 350 mW cm−2 | 0.2 g Na2S, and 0.2 g Na2SO3 into 100 mL deionized H2O | 0.5759 and AQY of 15.25% at 420 nm | [108] |
| 2023 | CdS NPs-decorated ZnO nanofibers | 500 W Xe lamp with 425 nm band pass filter | 0.35 M Na2S and 0.25 M Na2SO3 | 0.820 | [109] |
| Photocatalytic Nanofibers | Light Source | Reagent | Products | Reaction Rate | Ref. |
|---|---|---|---|---|---|
| g-C3N4/black titania | 300 W Xe-arc lamp | CO2 + H2O + TEOA | CO and CH4 | 5.19 and 1.65 μmol/g | [141] |
| (rGO)-wrapped Ag/TiO2 | 500 W Xe lamp with a 400-nm long pass filter | CO2 + H2O vapor | CH4 | 4.301 μmol g−1 | [137] |
| Ni-NiS/C/ZnO | 350 W simulated solar Xe arc lamp, 10,117 μW cm−2 | CO2 + H2O + NaHCO3 | CO and CH4 | 5.86 and 1.14 μmol g−1 h−1 | [152] |
| NiS@Ta2O5 | Xe lamp, 920 mW cm−2 | CO2 + H2O | CO and CH4 | 43.27 and 6.56 μmol g−1 h−1 | [153] |
| TiO2/MoSe2 | 300 W Xe-arc lamp, 12 mW/cm2 | CO2 + H2O + TEOA | CH4 and CO | 174.02 and 478.46 μmol/g | [154] |
| Nb2O5 | 18 W mercury lamp, 254 nm | CO2 + H2O vapor | CO and CH4 | 8.5 and 0.55 μmol g−1 | [155] |
| Ni-MoP@NCPF | 300 W Xe lamp with a UVCUT 420-nm filter | CO2 + acetonitrile/H2O + TEOA | CO | 953.33 μmol g−1h−1 | [151] |
| C doped TiO2 | 300 W Xe lamp, AM 1.5 filter | CO2 + H2O + NaHCO3 + H2SO4 | CH4 | 55.17 μmol g−1 h−1 | [156] |
| SrTi1-xCuxO3-H2 | 300 W Xe lamp, (400 nm < λ < 780 nm) | CO2 + H2O | CH3OH | 5.38 μmol g−1 h−1 | [157] |
| Graphene@PVDF@TiO2 | Two 300 W visible light sources (UV < 5%) | CO2 + H2O | CH4 | 363 μmol g−1 h−1 | [138] |
| TiO2/MoS2/g-C3N | 300 W Xe-arc lamp, 12 mW/cm2 | CO2 + H2O + TEOA | CH4 | 21.78 μmol g−1 | [158] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serik, A.; Idrissov, N.; Baratov, A.; Dikov, A.; Kislitsin, S.; Daulbayev, C.; Kuspanov, Z. Recent Progress in Photocatalytic Applications of Electrospun Nanofibers: A Review. Molecules 2024, 29, 4824. https://doi.org/10.3390/molecules29204824
Serik A, Idrissov N, Baratov A, Dikov A, Kislitsin S, Daulbayev C, Kuspanov Z. Recent Progress in Photocatalytic Applications of Electrospun Nanofibers: A Review. Molecules. 2024; 29(20):4824. https://doi.org/10.3390/molecules29204824
Chicago/Turabian StyleSerik, Aigerim, Nurlan Idrissov, Aibol Baratov, Alexey Dikov, Sergey Kislitsin, Chingis Daulbayev, and Zhengisbek Kuspanov. 2024. "Recent Progress in Photocatalytic Applications of Electrospun Nanofibers: A Review" Molecules 29, no. 20: 4824. https://doi.org/10.3390/molecules29204824
APA StyleSerik, A., Idrissov, N., Baratov, A., Dikov, A., Kislitsin, S., Daulbayev, C., & Kuspanov, Z. (2024). Recent Progress in Photocatalytic Applications of Electrospun Nanofibers: A Review. Molecules, 29(20), 4824. https://doi.org/10.3390/molecules29204824





