The Potential of Glucosinolates and Their Hydrolysis Products as Inhibitors of Cytokine Storms
Abstract
1. Introduction
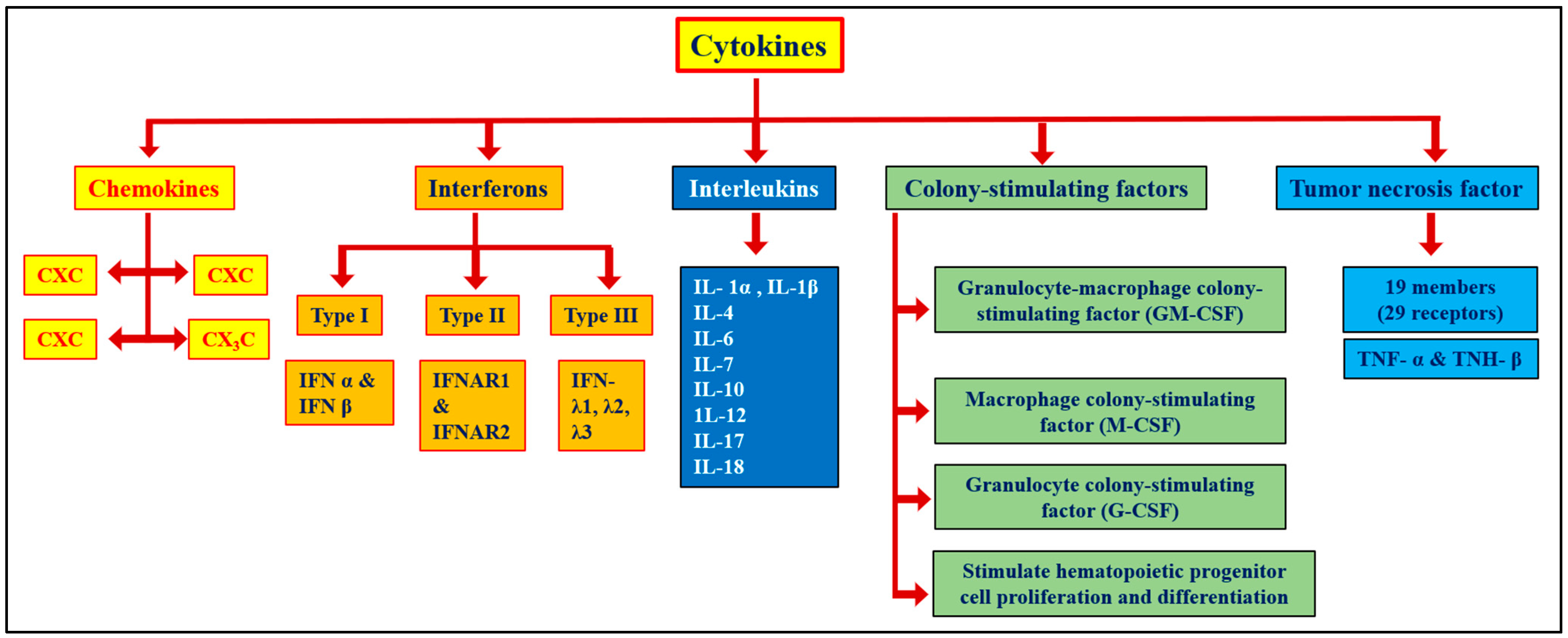
2. Cytokines and Cytokine Storms
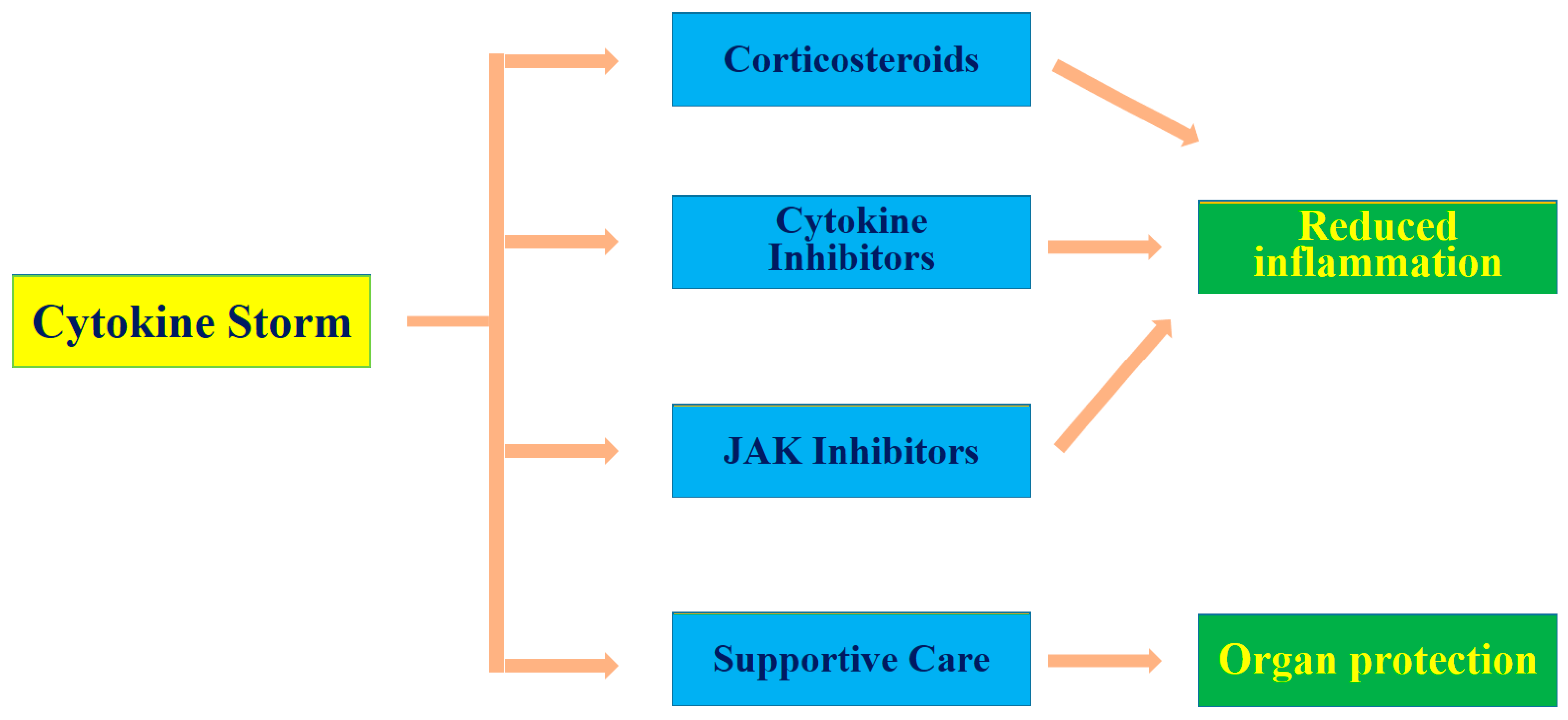
3. Pathophysiology and Management of Cytokine Storms in COVID-19
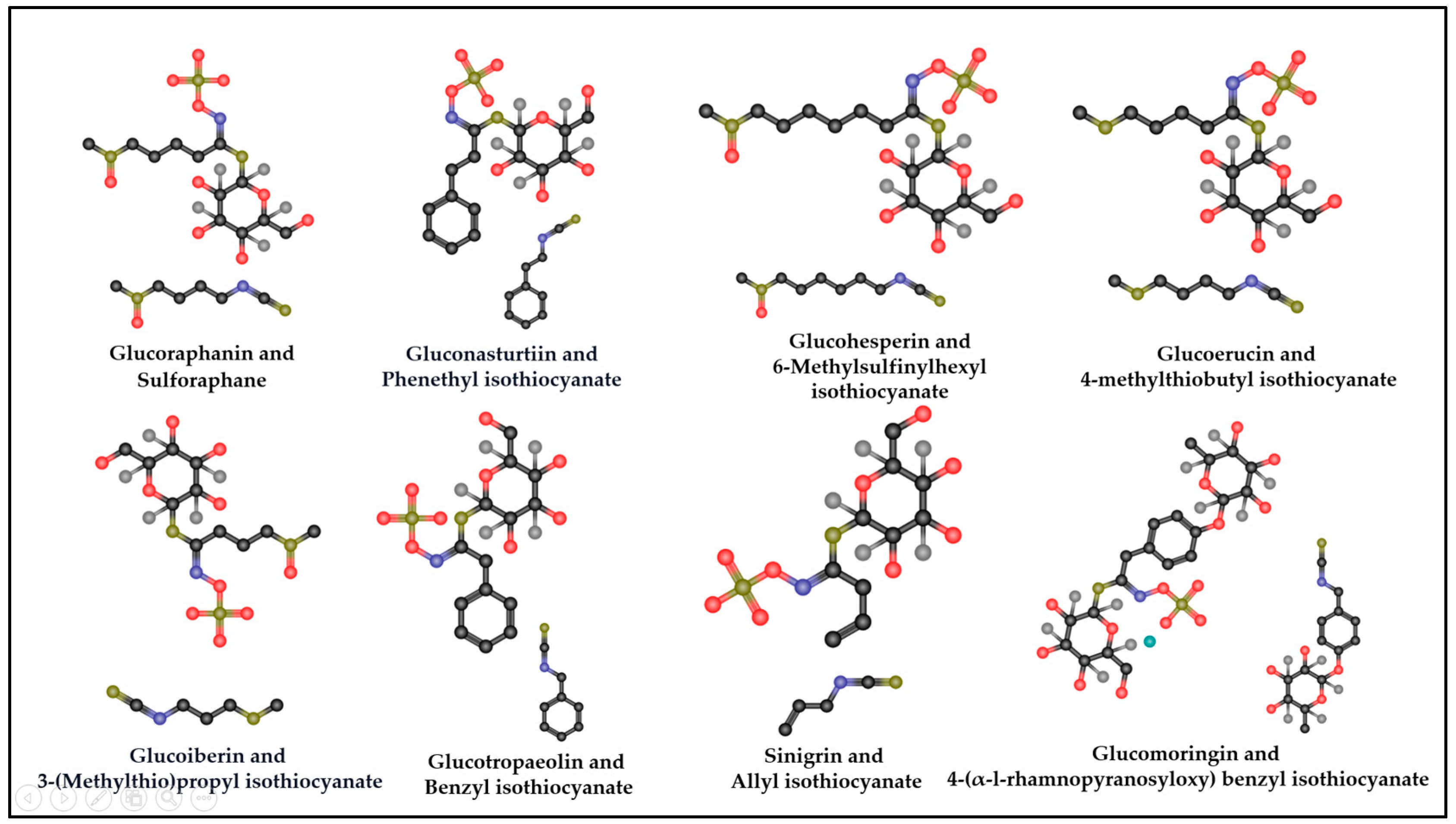
4. GSL Hydrolysis Products as Potential Modulators of Cytokine Storms
4.1. Glucoraphanin and Its Hydrolysis Product, SFN
4.2. Gluconasturtiin and Its Hydrolysis Product, PEITC
4.3. Glucohesperin and Its Hydrolysis Product, 6-MSITC
4.4. Glucoerucin and Its Hydrolysis Product, Erucin
4.5. Glucoiberin and Glucoiberverin and Their Hydrolysis Products, Iberin and Iberverin, Respectively
4.6. Glucotropaeolin and Their Hydrolysis Product, Benzyl ITC (BITC)
4.7. Sinigrin and Its Hydrolysis Product, Allyl ITC (AITC)
4.8. Glucomoringin and Its Hydrolysis Product, Moringin
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in inflammatory disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef] [PubMed]
- Tisoncik, J.R.; Korth, M.J.; Simmons, C.P.; Farrar, J.; Martin, T.R.; Katze, M.G. Into the eye of the cytokine storm. Microbiol. Mol. Biol. Rev. 2012, 76, 16–32. [Google Scholar] [CrossRef]
- Jiang, Y.; Rubin, L.; Peng, T.; Liu, L.; Xing, X.; Lazarovici, P.; Zheng, W. Cytokine storm in COVID-19: From viral infection to immune responses, diagnosis and therapy. Int. J. Biol. Sci. 2022, 18, 459. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Ad’hiah, A.H. Interleukin-37 is down-regulated in serum of patients with severe coronavirus disease 2019 (COVID-19). Cytokine 2021, 148, 155702. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Castejon, G.; Brough, D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 2011, 22, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Montazersaheb, S.; Hosseiniyan Khatibi, S.M.; Hejazi, M.S.; Tarhriz, V.; Farjami, A.; Ghasemian Sorbeni, F.; Farahzadi, R.; Ghasemnejad, T. COVID-19 infection: An overview on cytokine storm and related interventions. Virol. J. 2022, 19, 92. [Google Scholar] [CrossRef]
- Vinayagam, S.; Sattu, K. SARS-CoV-2 and coagulation disorders in different organs. Life Sci. 2020, 260, 118431. [Google Scholar] [CrossRef]
- Zhou, Z.; Ren, L.; Zhang, L.; Zhong, J.; Xiao, Y.; Jia, Z.; Guo, L.; Yang, J.; Wang, C.; Jiang, S. Heightened innate immune responses in the respiratory tract of COVID-19 patients. Cell Host Microbe 2020, 27, 883–890. [Google Scholar] [CrossRef]
- Rudd, J.M.; Tamil Selvan, M.; Cowan, S.; Kao, Y.-F.; Midkiff, C.C.; Narayanan, S.; Ramachandran, A.; Ritchey, J.W.; Miller, C.A. Clinical and histopathologic features of a feline SARS-CoV-2 infection model are analogous to acute COVID-19 in humans. Viruses 2021, 13, 1550. [Google Scholar] [CrossRef]
- Rathi, M.; Singh, P.; Bi, H.P.; Shivanna, A.; Kavadichanda, C.; Tripathy, S.R.; Parthasarathy, J.; Tota, S.; Maurya, S.; Vijayalekshmi, V. Impact of the COVID-19 pandemic on patients with systemic lupus erythematosus: Observations from an Indian inception cohort. Lupus 2021, 30, 158–164. [Google Scholar] [CrossRef]
- Que, Y.; Hu, C.; Wan, K.; Hu, P.; Wang, R.; Luo, J.; Li, T.; Ping, R.; Hu, Q.; Sun, Y. Cytokine release syndrome in COVID-19: A major mechanism of morbidity and mortality. Int. Rev. Immunol. 2022, 41, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Bergamaschi, C.; Terpos, E.; Rosati, M.; Angel, M.; Bear, J.; Stellas, D.; Karaliota, S.; Apostolakou, F.; Bagratuni, T.; Patseas, D. Systemic IL-15, IFN-γ, and IP-10/CXCL10 signature associated with effective immune response to SARS-CoV-2 in BNT162b2 mRNA vaccine recipients. Cell Rep. 2021, 36, 109504. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Huang, S.; Yin, L. The cytokine storm and COVID-19. J. Med. Virol. 2021, 93, 250–256. [Google Scholar] [CrossRef]
- Kolodnitsky, A.; Ionov, N.; Gravel, I.; Poroikov, V. Natural compounds from medicinal plants against COVID-19. Explor. Drug Sci. 2023, 1, 253–275. [Google Scholar] [CrossRef]
- Raghav, A.; Khan, Z.A.; Upadhayay, V.K.; Tripathi, P.; Gautam, K.A.; Mishra, B.K.; Ahmad, J.; Jeong, G.-B. Mesenchymal stem cell-derived exosomes exhibit promising potential for treating SARS-CoV-2-infected patients. Cells 2021, 10, 587. [Google Scholar] [CrossRef] [PubMed]
- Tzvetkov, N.T.; Kirilov, K.; Matin, M.; Atanasov, A.G. Natural product drug discovery and drug design: Two approaches shaping new pharmaceutical development. Nephrol. Dial. Transplant. 2024, 39, 375–378. [Google Scholar] [CrossRef]
- Salam, U.; Ullah, S.; Tang, Z.-H.; Elateeq, A.A.; Khan, Y.; Khan, J.; Khan, A.; Ali, S. Plant metabolomics: An overview of the role of primary and secondary metabolites against different environmental stress factors. Life 2023, 13, 706. [Google Scholar] [CrossRef]
- Reshi, Z.A.; Ahmad, W.; Lukatkin, A.S.; Javed, S.B. From Nature to lab: A review of secondary metabolite biosynthetic pathways, environmental influences, and in vitro approaches. Metabolites 2023, 13, 895. [Google Scholar] [CrossRef] [PubMed]
- Tariq, H.; Asif, S.; Andleeb, A.; Hano, C.; Abbasi, B.H. Flavonoid production: Current trends in plant metabolic engineering and de novo microbial production. Metabolites 2023, 13, 124. [Google Scholar] [CrossRef]
- Chen, S.; Cai, R.; Liu, Z.; Cui, H.; She, Z. Secondary metabolites from mangrove-associated fungi: Source, chemistry and bioactivities. Nat. Prod. Rep. 2022, 39, 560–595. [Google Scholar] [CrossRef]
- Hacker, K. The burden of chronic disease. Mayo Clin. Proc. Innov. Qual. Outcomes 2024, 8, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Elshafie, H.S.; Camele, I.; Mohamed, A.A. A comprehensive review on the biological, agricultural and pharmaceutical properties of secondary metabolites based-plant origin. Int. J. Mol. Sci. 2023, 24, 3266. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.; Martini, D.; Venturi, S.; Tucci, M.; Porrini, M.; Riso, P.; Del Bo’, C. An overview of registered clinical trials on glucosinolates and human health: The current situation. Front. Nutr. 2021, 8, 730906. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.T.; Stewart, J.; Lopez, M.; Ioannou, I.; Allais, F. Glucosinolates: Natural occurrence, biosynthesis, accessibility, isolation, structures, and biological activities. Molecules 2020, 25, 4537. [Google Scholar] [CrossRef] [PubMed]
- Prieto, M.; López, C.J.; Simal-Gandara, J. Glucosinolates: Molecular structure, breakdown, genetic, bioavailability, properties and healthy and adverse effects. Adv. Food Nutr. Res. 2019, 90, 305–350. [Google Scholar]
- Ruhee, R.T.; Suzuki, K. The Immunomodulatory Effects of Sulforaphane in Exercise-Induced Inflammation and Oxidative Stress: A Prospective Nutraceutical. Int. J. Mol. Sci. 2024, 25, 1790. [Google Scholar] [CrossRef]
- Miękus, N.; Marszałek, K.; Podlacha, M.; Iqbal, A.; Puchalski, C.; Świergiel, A.H. Health benefits of plant-derived sulfur compounds, glucosinolates, and organosulfur compounds. Molecules 2020, 25, 3804. [Google Scholar] [CrossRef]
- Ruhee, R.T.; Roberts, L.A.; Ma, S.; Suzuki, K. Organosulfur compounds: A review of their anti-inflammatory effects in human health. Front. Nutr. 2020, 7, 64. [Google Scholar] [CrossRef]
- Bahoosh, S.R.; Shokoohinia, Y.; Eftekhari, M. Glucosinolates and their hydrolysis products as potential nutraceuticals to combat cytokine storm in SARS-CoV-2. DARU J. Pharm. Sci. 2022, 30, 245–252. [Google Scholar] [CrossRef]
- Bousquet, J.; Anto, J.; Czarlewski, W.; Haahtela, T.; Fonseca, S.; Iaccarino, G.; Blain, H.; Vidal, A. Cabbage and fermented vegetables. Allergy 2020, 76, 735–750. [Google Scholar] [CrossRef]
- Kim, H.J.; Kwon, M.S.; Hwang, H.; Choi, H.-S.; Lee, W.; Choi, S.-P.; Jo, H.; Hong, S.W. A review of the health benefits of kimchi functional compounds and metabolites. Microbiol. Biotechnol. Lett. 2023, 51, 353–373. [Google Scholar] [CrossRef]
- Park, K.-Y.; Ju, J. Kimchi and its health benefits. In Korean Functional Foods; CRC Press: Boca Raton, FL, USA, 2018; pp. 43–78. [Google Scholar]
- Lavefve, L.; Marasini, D.; Carbonero, F. Microbial ecology of fermented vegetables and non-alcoholic drinks and current knowledge on their impact on human health. Adv. Food Nutr. Res. 2019, 87, 147–185. [Google Scholar] [PubMed]
- Kim, S.-A.; Joung, H.; Shin, S. Dietary pattern, dietary total antioxidant capacity, and dyslipidemia in Korean adults. Nutr. J. 2019, 18, 37. [Google Scholar] [CrossRef]
- Das, G.; Paramithiotis, S.; Sivamaruthi, B.S.; Wijaya, C.H.; Suharta, S.; Sanlier, N.; Shin, H.-S.; Patra, J.K. Traditional fermented foods with anti-aging effect: A concentric review. Food Res. Int. 2020, 134, 109269. [Google Scholar] [CrossRef] [PubMed]
- An, S.-Y.; Lee, M.S.; Jeon, J.Y.; Ha, E.S.; Kim, T.H.; Yoon, J.Y.; Ok, C.-O.; Lee, H.-K.; Hwang, W.-S.; Choe, S.J. Beneficial effects of fresh and fermented kimchi in prediabetic individuals. Ann. Nutr. Metab. 2013, 63, 111–119. [Google Scholar] [CrossRef]
- Soundararajan, P.; Kim, J.S. Anti-carcinogenic glucosinolates in cruciferous vegetables and their antagonistic effects on prevention of cancers. Molecules 2018, 23, 2983. [Google Scholar] [CrossRef]
- Maina, A.; Mureithi, M.; Kiiru, J.; Revathi, G. Systemic and Mucosal Concentrations of Nine Cytokines Among Individuals with Neisseria gonorrhoeae infection in Nairobi Kenya. AAS Open Res. 2022, 5, 12. [Google Scholar]
- Qudus, M.S.; Tian, M.; Sirajuddin, S.; Liu, S.; Afaq, U.; Wali, M.; Liu, J.; Pan, P.; Luo, Z.; Zhang, Q. The roles of critical pro-inflammatory cytokines in the drive of cytokine storm during SARS-CoV-2 infection. J. Med. Virol. 2023, 95, e28751. [Google Scholar] [CrossRef]
- Chams, N.; Chams, S.; Badran, R.; Shams, A.; Araji, A.; Raad, M.; Mukhopadhyay, S.; Stroberg, E.; Duval, E.J.; Barton, L.M. COVID-19: A multidisciplinary review. Front. Public Health 2020, 8, 383. [Google Scholar] [CrossRef]
- Panda, S.S.; Panda, D.S.; Dixit, R. Revolutionary Solutions for Comprehensive Assessment of COVID-19 Pandemic. In Proceedings of International Conference on Computational Intelligence: ICCI 2021, Online, 27–28 December 2021; Springer: Singapore, 2022; pp. 183–195. [Google Scholar]
- Jarczak, D.; Nierhaus, A. Cytokine storm—Definition, causes, and implications. Int. J. Mol. Sci. 2022, 23, 11740. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, B.; Mao, J. The pathogenesis and treatment of theCytokine Storm’in COVID-19. J. Infect. 2020, 80, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Chatenoud, L.; Ferran, C.; Bach, J.-F. The anti-CD3-induced syndrome: A consequence of massive in vivo cell activation. In Superantigens; Springer: Heidelberg, Germany, 1991; pp. 121–134. [Google Scholar]
- Ferrara, J.L. Cytokine dysregulation as a mechanism of graft versus host disease. Curr. Opin. Immunol. 1993, 5, 794–799. [Google Scholar] [CrossRef]
- Fara, A.; Mitrev, Z.; Rosalia, R.A.; Assas, B.M. Cytokine storm and COVID-19: A chronicle of pro-inflammatory cytokines. Open Biol. 2020, 10, 200160. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, G.; Colafrancesco, S.; Emmi, G.; Imazio, M.; Lopalco, G.; Maggio, M.C.; Sota, J.; Dinarello, C.A. Interleukin 1α: A comprehensive review on the role of IL-1α in the pathogenesis and treatment of autoimmune and inflammatory diseases. Autoimmun. Rev. 2021, 20, 102763. [Google Scholar] [CrossRef]
- Di Paolo, N.C.; Shayakhmetov, D.M. Interleukin 1α and the inflammatory process. Nat. Immunol. 2016, 17, 906–913. [Google Scholar] [CrossRef]
- Kim, B.; Lee, Y.; Kim, E.; Kwak, A.; Ryoo, S.; Bae, S.H.; Azam, T.; Kim, S.; Dinarello, C.A. The interleukin-1α precursor is biologically active and is likely a key alarmin in the IL-1 family of cytokines. Front. Immunol. 2013, 4, 391. [Google Scholar] [CrossRef]
- Al-Qahtani, A.A.; Alhamlan, F.S.; Al-Qahtani, A.A. Pro-inflammatory and anti-inflammatory interleukins in infectious diseases: A comprehensive review. Trop. Med. Infect. Dis. 2024, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Renu, K.; Subramaniam, M.D.; Chakraborty, R.; Myakala, H.; Iyer, M.; Bharathi, G.; Siva, K.; Vellingiri, B.; Gopalakrishnan, A.V. The role of Interleukin-4 in COVID-19 associated male infertility–A hypothesis. J. Reprod. Immunol. 2020, 142, 103213. [Google Scholar] [CrossRef]
- Liu, X.-G.; Li, J.; Zheng, L.-J.; Han, B.; Huang, F. Interleukin-36 receptor antagonist alleviates airway inflammation in asthma via inhibiting the activation of interleukin-36 pathway. Int. Immunopharmacol. 2020, 81, 106200. [Google Scholar] [CrossRef]
- Clinchy, B.; Gunnerås, M.; Håkansson, A.; Håkansson, L. Production of IL-1Ra by human mononuclear blood cells in vitro: Influence of serum factors. Cytokine 2006, 34, 320–330. [Google Scholar] [CrossRef]
- Ihim, S.A.; Abubakar, S.D.; Zian, Z.; Sasaki, T.; Saffarioun, M.; Maleknia, S.; Azizi, G. Interleukin-18 cytokine in immunity, inflammation, and autoimmunity: Biological role in induction, regulation, and treatment. Front. Immunol. 2022, 13, 919973. [Google Scholar] [CrossRef]
- Kaplanski, G. Interleukin-18: Biological properties and role in disease pathogenesis. Immunol. Rev. 2018, 281, 138–153. [Google Scholar] [CrossRef]
- Dinarello, C.A. Overview of the interleukin-1 family of ligands and receptors. Semin. Immunol. 2013, 25, 389–393. [Google Scholar] [CrossRef]
- Makaremi, S.; Asgarzadeh, A.; Kianfar, H.; Mohammadnia, A.; Asghariazar, V.; Safarzadeh, E. The role of IL-1 family of cytokines and receptors in pathogenesis of COVID-19. Inflamm. Res. 2022, 71, 923–947. [Google Scholar] [CrossRef]
- Queen, D.; Ediriweera, C.; Liu, L. Function and regulation of IL-36 signaling in inflammatory diseases and cancer development. Front. Cell Dev. Biol. 2019, 7, 317. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Ad’hiah, A.H. Interleukin-37 gene polymorphism and susceptibility to coronavirus disease 19 among Iraqi patients. Meta Gene 2022, 31, 100989. [Google Scholar] [CrossRef]
- Rudloff, I.; Ung, H.K.; Dowling, J.K.; Mansell, A.; D’Andrea, L.; Ellisdon, A.M.; Whisstock, J.C.; Berger, P.J.; Nold-Petry, C.A.; Nold, M.F. Parsing the IL-37-mediated suppression of inflammasome function. Cells 2020, 9, 178. [Google Scholar] [CrossRef]
- Su, Z.; Tao, X. Current understanding of IL-37 in human health and disease. Front. Immunol. 2021, 12, 696605. [Google Scholar] [CrossRef]
- Vaz de Paula, C.B.; de Azevedo, M.L.V.; Nagashima, S.; Martins, A.P.C.; Malaquias, M.A.S.; Miggiolaro, A.F.R.d.S.; da Silva Motta Júnior, J.; Avelino, G.; do Carmo, L.A.P.; Carstens, L.B. IL-4/IL-13 remodeling pathway of COVID-19 lung injury. Sci. Rep. 2020, 10, 18689. [Google Scholar] [CrossRef] [PubMed]
- Cesta, M.C.; Zippoli, M.; Marsiglia, C.; Gavioli, E.M.; Mantelli, F.; Allegretti, M.; Balk, R.A. The role of interleukin-8 in lung inflammation and injury: Implications for the management of COVID-19 and hyperinflammatory acute respiratory distress syndrome. Front. Pharmacol. 2022, 12, 808797. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liu, Y.; Xiang, J. Synergistic effect of adoptive T-cell therapy and intratumoral interferon γ-inducible protein-10 transgene expression in treatment of established tumors. Cell. Immunol. 2002, 217, 12–22. [Google Scholar] [CrossRef]
- Yadav, V.; Sharma, S.; Kumar, A.; Singh, S.; Ravichandiran, V. Serratiopeptidase Attenuates Lipopolysaccharide-Induced Vascular Inflammation by Inhibiting the Expression of Monocyte Chemoattractant Protein-1. Curr. Issues Mol. Biol. 2023, 45, 2201–2212. [Google Scholar] [CrossRef]
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-γ in tumor progression and regression: A review. Biomark. Res. 2020, 8, 49. [Google Scholar] [CrossRef]
- He, Z.; Tian, H.; Xing, J.; Tang, X.; Sheng, X.; Chi, H.; Zhan, W. Full-length transcriptome sequencing of lymphocytes respond to IFN-γ reveals a Th1-skewed immune response in flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 2023, 134, 108636. [Google Scholar] [CrossRef]
- Kandikattu, H.K.; Venkateshaiah, S.U.; Kumar, S.; Mishra, A. IL-15 immunotherapy is a viable strategy for COVID-19. Cytokine Growth Factor Rev. 2020, 54, 24–31. [Google Scholar] [CrossRef]
- Wilz, S.W. A clinical trial of IL-15 and IL-21 combination therapy for COVID-19 is warranted. Cytokine Growth Factor Rev. 2021, 58, 49–50. [Google Scholar] [CrossRef]
- Malahe, S.R.K.; den Hartog, Y.; Rietdijk, W.J.; van Baarle, D.; de Kuiper, R.; Reijerkerk, D.; Ras, A.M.; Geers, D.; Diavatopoulos, D.A.; Messchendorp, A.L. The role of interleukin-21 in COVID-19 vaccine–induced B cell–mediated immune responses in patients with kidney disease and kidney transplant recipients. Am. J. Transplant. 2023, 23, 1411–1424. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, J. Interleukin-2 family cytokines: An overview of genes, expression, signaling and functional roles in teleost. Dev. Comp. Immunol. 2023, 141, 104645. [Google Scholar] [CrossRef]
- Yazan, A. Interleukin-2 level for normal people and COVID-19 infection: Is it our concern is COVID-19 infection or interleukin-2 level before the infection. Eurasian J. Med. Oncol. 2021, 5, 309. [Google Scholar] [CrossRef]
- Chen, D.; Tang, T.-X.; Deng, H.; Yang, X.-P.; Tang, Z.-H. Interleukin-7 biology and its effects on immune cells: Mediator of generation, differentiation, survival, and homeostasis. Front. Immunol. 2021, 12, 747324. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; McGoogan, J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
- Gupta, R.; Kumar, S. Ten Weeks of COVID-19 Infection in India-Description and Analysis. SSRN 3576320. 2020. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3576320 (accessed on 7 October 2024).
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Rothan, H.A.; Byrareddy, S.N. The potential threat of multisystem inflammatory syndrome in children during the COVID-19 pandemic. Pediatr. Allergy Immunol. 2021, 32, 17–22. [Google Scholar] [CrossRef]
- Chen, R.-X.; Gong, H.-Y.; Wang, X.; Sun, M.-H.; Ji, Y.-F.; Tan, S.-M.; Chen, J.-M.; Shao, J.-W.; Liao, M. Zoonotic Hantaviridae with global public health significance. Viruses 2023, 15, 1705. [Google Scholar] [CrossRef]
- Jarczak, D.; Nierhaus, A. What Is Cytokine Storm? In Management of Dysregulated Immune Response in the Critically Ill; Springer: Berlin/Heidelberg, Germany, 2023; pp. 35–54. [Google Scholar]
- Vassiliou, A.G.; Kotanidou, A.; Dimopoulou, I.; Orfanos, S.E. Endothelial damage in acute respiratory distress syndrome. Int. J. Mol. Sci. 2020, 21, 8793. [Google Scholar] [CrossRef]
- Van Paassen, J.; Vos, J.S.; Hoekstra, E.M.; Neumann, K.M.; Boot, P.C.; Arbous, S.M. Corticosteroid use in COVID-19 patients: A systematic review and meta-analysis on clinical outcomes. Crit. Care 2020, 24, 696. [Google Scholar] [CrossRef]
- Belletti, A.; Campochiaro, C.; Marmiere, M.; Likhvantsev, V.; Yavorovskiy, A.; Dagna, L.; Landoni, G.; Zangrillo, A.; Hajjar, L.A. Efficacy and safety of IL-6 inhibitors in patients with COVID-19 pneumonia: A systematic review and meta-analysis of multicentre, randomized trials. Ann. Intensive Care 2021, 11, 152. [Google Scholar] [CrossRef]
- Zhang, X.; Shang, L.; Fan, G.; Gu, X.; Xu, J.; Wang, Y.; Huang, L.; Cao, B. The efficacy and safety of janus kinase inhibitors for patients with COVID-19: A living systematic review and meta-analysis. Front. Med. 2022, 8, 800492. [Google Scholar] [CrossRef]
- Bajwah, S.; Wilcock, A.; Towers, R.; Costantini, M.; Bausewein, C.; Simon, S.T.; Bendstrup, E.; Prentice, W.; Johnson, M.J.; Currow, D.C. Managing the supportive care needs of those affected by COVID-19. Eur. Respir. J. 2020, 55, 2000815. [Google Scholar] [CrossRef] [PubMed]
- Alipour, Z.; Zarezadeh, S.; Ghotbi-Ravandi, A.A. The potential of anti-coronavirus plant secondary metabolites in COVID-19 drug discovery as an alternative to repurposed drugs: A review. Planta Medica 2023, 90, 172–203. [Google Scholar] [CrossRef]
- López-Chillón, M.T.; Carazo-Díaz, C.; Prieto-Merino, D.; Zafrilla, P.; Moreno, D.A.; Villaño, D. Effects of long-term consumption of broccoli sprouts on inflammatory markers in overweight subjects. Clin. Nutr. 2019, 38, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wang, X.-J.; Zhang, Y.-Y.; Xing, Y.; Yang, L.; Ni, H.; Li, H.-H. Simultaneous extraction and separation of oil, proteins, and glucosinolates from Moringa oleifera seeds. Food Chem. 2019, 300, 125162. [Google Scholar] [CrossRef] [PubMed]
- Tetteh, O.N.A.; Ulrichs, C.; Huyskens-Keil, S.; Mewis, I.; Amaglo, N.K.; Oduro, I.N.; Adarkwah, C.; Obeng-Ofori, D.; Förster, N. Effects of harvest techniques and drying methods on the stability of glucosinolates in Moringa oleifera leaves during post-harvest. Sci. Hortic. 2019, 246, 998–1004. [Google Scholar] [CrossRef]
- Brunelli, D.; Tavecchio, M.; Falcioni, C.; Frapolli, R.; Erba, E.; Iori, R.; Rollin, P.; Barillari, J.; Manzotti, C.; Morazzoni, P. The isothiocyanate produced from glucomoringin inhibits NF-kB and reduces myeloma growth in nude mice in vivo. Biochem. Pharmacol. 2010, 79, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Hiraga, Y.; Ara, T.; Sato, N.; Akimoto, N.; Sugiyama, K.; Suzuki, H.; Kera, K. Metabolic analysis of unripe papaya (Carica papaya L.) to promote its utilization as a functional food. Biosci. Biotechnol. Biochem. 2021, 85, 1194–1204. [Google Scholar] [CrossRef]
- Santana, L.F.; Inada, A.C.; Espirito Santo, B.L.S.d.; Filiú, W.F.; Pott, A.; Alves, F.M.; Guimarães, R.d.C.A.; Freitas, K.d.C.; Hiane, P.A. Nutraceutical potential of Carica papaya in metabolic syndrome. Nutrients 2019, 11, 1608. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.J.; Pun, S.; Chaliha, M.; Scheelings, P.; O’Hare, T. An unusual combination in papaya (Carica papaya): The good (glucosinolates) and the bad (cyanogenic glycosides). J. Food Compos. Anal. 2013, 29, 82–86. [Google Scholar] [CrossRef]
- Kim, S.-H.; Ochar, K.; Iwar, K.; Lee, Y.-J.; Kang, H.J.; Na, Y.-W. Variations of Major Glucosinolates in Diverse Chinese Cabbage (Brassica rapa ssp. pekinensis) Germplasm as Analyzed by UPLC-ESI-MS/MS. Int. J. Mol. Sci. 2024, 25, 4829. [Google Scholar] [CrossRef]
- Kim, S.-H.; Lee, G.-A.; Subramanian, P.; Hahn, B.-S. Quantification and Diversity Analyses of Major Glucosinolates in Conserved Chinese Cabbage (Brassica rapa L. ssp. pekinensis) Germplasms. Foods 2023, 12, 1243. [Google Scholar] [CrossRef]
- Connolly, E.L.; Sim, M.; Travica, N.; Marx, W.; Beasy, G.; Lynch, G.S.; Bondonno, C.P.; Lewis, J.R.; Hodgson, J.M.; Blekkenhorst, L.C. Glucosinolates from cruciferous vegetables and their potential role in chronic disease: Investigating the preclinical and clinical evidence. Front. Pharmacol. 2021, 12, 767975. [Google Scholar] [CrossRef]
- Burčul, F.; Ivana, G.M.; Mila, R.; Patrick, R.; Ivica, B. Isothiocyanates: Cholinesterase inhibiting, antioxidant, and anti-inflammatory activity. Enzyme Inhib. Med. Chem. 2018, 33, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Kamal, R.M.; Abdull Razis, A.F.; Mohd Sukri, N.S.; Perimal, E.K.; Ahmad, H.; Patrick, R.; Djedaini-Pilard, F.; Mazzon, E.; Rigaud, S. Beneficial health effects of glucosinolates-derived isothiocyanates on cardiovascular and neurodegenerative diseases. Molecules 2022, 27, 624. [Google Scholar] [CrossRef]
- Ghaffari, M. Herbal Remedies for Management of COVID-19 Induced Myocarditis. Suntext Rev. Cardiovasc. Sci. 2021, 1, 104. [Google Scholar]
- Sapra, L.; Bhardwaj, A.; Azam, Z.; Madhry, D.; Verma, B.; Rathore, S.; Srivastava, R.K. Phytotherapy for treatment of cytokine storm in COVID-19. Front. Biosci.-Landmark 2021, 26, 51–75. [Google Scholar]
- Savant, S.; Srinivasan, S.; Kruthiventi, A.K. Potential nutraceuticals for COVID-19. Nutr. Diet. Suppl. 2021, 13, 25–51. [Google Scholar] [CrossRef]
- Bousquet, J.; Le Moing, V.; Blain, H.; Czarlewski, W.; Zuberbier, T.; De La Torre, R.; Lozano, N.P.; Reynes, J.; Bedbrook, A.; Cristol, J.-P. Efficacy of broccoli and glucoraphanin in COVID-19: From hypothesis to proof-of-concept with three experimental clinical cases. World Allergy Organ. J. 2021, 14, 100498. [Google Scholar] [CrossRef] [PubMed]
- Giacoppo, S.; Galuppo, M.; Iori, R.; De Nicola, G.R.; Cassata, G.; Bramanti, P.; Mazzon, E. Protective role of (RS)-glucoraphanin bioactivated with myrosinase in an experimental model of multiple sclerosis. CNS Neurosci. Ther. 2013, 19, 577–584. [Google Scholar] [CrossRef]
- Esteve, M. Mechanisms underlying biological effects of cruciferous glucosinolate-derived isothiocyanates/indoles: A focus on metabolic syndrome. Front. Nutr. 2020, 7, 111. [Google Scholar] [CrossRef]
- Caglayan, B.; Kilic, E.; Dalay, A.; Altunay, S.; Tuzcu, M.; Erten, F.; Orhan, C.; Gunal, M.Y.; Yulug, B.; Juturu, V. Allyl isothiocyanate attenuates oxidative stress and inflammation by modulating Nrf2/HO-1 and NF-κB pathways in traumatic brain injury in mice. Mol. Biol. Rep. 2019, 46, 241–250. [Google Scholar] [CrossRef]
- Subedi, L.; Venkatesan, R.; Kim, S.Y. Neuroprotective and anti-inflammatory activities of allyl isothiocyanate through attenuation of JNK/NF-κB/TNF-α signaling. Int. J. Mol. Sci. 2017, 18, 1423. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.L.; Kong, A.-N. Molecular targets of dietary phenethyl isothiocyanate and sulforaphane for cancer chemoprevention. AAPS J. 2010, 12, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Ernst, I.M.; Palani, K.; Esatbeyoglu, T.; Schwarz, K.; Rimbach, G. Synthesis and Nrf2-inducing activity of the isothiocyanates iberverin, iberin and cheirolin. Pharmacol. Res. 2013, 70, 155–162. [Google Scholar] [CrossRef]
- Gasparello, J.; D’Aversa, E.; Papi, C.; Gambari, L.; Grigolo, B.; Borgatti, M.; Finotti, A.; Gambari, R. Sulforaphane inhibits the expression of interleukin-6 and interleukin-8 induced in bronchial epithelial IB3-1 cells by exposure to the SARS-CoV-2 Spike protein. Phytomedicine 2021, 87, 153583. [Google Scholar] [CrossRef] [PubMed]
- Jaafaru, M.S.; Razis, A.F.A. Sulfur compounds. In Bioactive Food Components Activity in Mechanistic Approach; Elsevier: Amsterdam, The Netherlands, 2022; pp. 211–222. [Google Scholar]
- Tran, H.T.; Herz, C.; Ruf, P.; Stetter, R.; Lamy, E. Human T2R38 bitter taste receptor expression in resting and activated lymphocytes. Front. Immunol. 2018, 9, 2949. [Google Scholar] [CrossRef]
- Jaafaru, M.S.; Abd Karim, N.A.; Enas, M.E.; Rollin, P.; Mazzon, E.; Abdull Razis, A.F. Protective effect of glucosinolates hydrolytic products in neurodegenerative diseases (NDDs). Nutrients 2018, 10, 580. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhou, H.-J.; Sheng, J.; Su, L.-Y.; Tian, Y. Extraction, structural properties, and bioactivities of Moringa (Moringa oleifera Lam.) isothiocyanates: A review. Food Biosci. 2023, 57, 103447. [Google Scholar] [CrossRef]
- Su, C.-M.; Wang, L.; Yoo, D. Activation of NF-κB and induction of proinflammatory cytokine expressions mediated by ORF7a protein of SARS-CoV-2. Sci. Rep. 2021, 11, 13464. [Google Scholar] [CrossRef] [PubMed]
- Negi, G.; Kumar, A.; S Sharma, S. Nrf2 and NF-κB modulation by sulforaphane counteracts multiple manifestations of diabetic neuropathy in rats and high glucose-induced changes. Curr. Neurovasc. Res. 2011, 8, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Shen, G.; Chen, C.; Gélinas, C.; Kong, A.-N.T. Suppression of NF-κB and NF-κB-regulated gene expression by sulforaphane and PEITC through IκBα, IKK pathway in human prostate cancer PC-3 cells. Oncogene 2005, 24, 4486–4495. [Google Scholar] [CrossRef]
- Pant, T.; Uche, N.; Juric, M.; Zielonka, J.; Bai, X. Regulation of immunomodulatory networks by Nrf2-activation in immune cells: Redox control and therapeutic potential in inflammatory diseases. Redox Biol. 2024, 70, 103077. [Google Scholar] [CrossRef]
- Deramaudt, T.B.; Ali, M.; Vinit, S.; Bonay, M. Sulforaphane reduces intracellular survival of Staphylococcus aureus in macrophages through inhibition of JNK and p38 MAPK-induced inflammation. Int. J. Mol. Med. 2020, 45, 1927–1941. [Google Scholar] [CrossRef] [PubMed]
- Tarozzi, A.; Angeloni, C.; Malaguti, M.; Morroni, F.; Hrelia, S.; Hrelia, P. Sulforaphane as a potential protective phytochemical against neurodegenerative diseases. Oxidative Med. Cell. Longev. 2013, 2013, 415078. [Google Scholar] [CrossRef] [PubMed]
- Olagnier, D.; Farahani, E.; Thyrsted, J.; Blay-Cadanet, J.; Herengt, A.; Idorn, M.; Hait, A.; Hernaez, B.; Knudsen, A.; Iversen, M.B. SARS-CoV2-mediated suppression of NRF2-signaling reveals potent antiviral and anti-inflammatory activity of 4-octyl-itaconate and dimethyl fumarate. Nat. Commun. 2020, 11, 4938. [Google Scholar] [CrossRef]
- Kiser, C.; Gonul, C.P.; Olcum, M.; Genc, S. Inhibitory effects of sulforaphane on NLRP3 inflammasome activation. Mol. Immunol. 2021, 140, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Gasparello, J.; Marzaro, G.; Papi, C.; Gentili, V.; Rizzo, R.; Zurlo, M.; Scapoli, C.; Finotti, A.; Gambari, R. Effects of Sulforaphane on SARS-CoV-2 infection and NF-κB dependent expression of genes involved in the COVID-19 ‘cytokine storm’. Int. J. Mol. Med. 2023, 52, 1–14. [Google Scholar] [CrossRef]
- Ordonez, A.A.; Bullen, C.K.; Villabona-Rueda, A.F.; Thompson, E.A.; Turner, M.L.; Merino, V.F.; Yan, Y.; Kim, J.; Davis, S.L.; Komm, O. Sulforaphane exhibits antiviral activity against pandemic SARS-CoV-2 and seasonal HCoV-OC43 coronaviruses in vitro and in mice. Commun. Biol. 2022, 5, 242. [Google Scholar] [CrossRef]
- Kow, C.S.; Ramachandram, D.S.; Hasan, S.S. Use of sulforaphane in COVID-19: Clinical trials are needed. Mol. Immunol. 2022, 145, 78–79. [Google Scholar] [CrossRef]
- Pan, P.; Shen, M.; Yu, Z.; Ge, W.; Chen, K.; Tian, M.; Xiao, F.; Wang, Z.; Wang, J.; Jia, Y. SARS-CoV-2 N protein promotes NLRP3 inflammasome activation to induce hyperinflammation. Nat. Commun. 2021, 12, 4664. [Google Scholar] [CrossRef]
- Tufekci, K.U.; Ercan, I.; Isci, K.B.; Olcum, M.; Tastan, B.; Gonul, C.P.; Genc, K.; Genc, S. Sulforaphane inhibits NLRP3 inflammasome activation in microglia through Nrf2-mediated miRNA alteration. Immunol. Lett. 2021, 233, 20–30. [Google Scholar] [CrossRef]
- Chen, Z.; Du, R.; Cooper, L.; Achi, J.G.; Dong, M.; Ran, Y.; Zhang, J.; Zhan, P.; Rong, L.; Cui, Q. Sulforaphane is a reversible covalent inhibitor of 3-chymotrypsin-like protease of SARS-CoV-2. J. Med. Virol. 2023, 95, e28609. [Google Scholar] [CrossRef]
- Hoch, C.C.; Shoykhet, M.; Weiser, T.; Griesbaum, L.; Petry, J.; Hachani, K.; Multhoff, G.; Dezfouli, A.B.; Wollenberg, B. Isothiocyanates in medicine: A comprehensive review on phenylethyl-, allyl-, and benzyl-isothiocyanates. Pharmacol. Res. 2024, 201, 107107. [Google Scholar] [CrossRef] [PubMed]
- Castro, V.; Carpena, M.; Fraga-Corral, M.; Lopez-Soria, A.; Garcia-Perez, P.; Barral-Martinez, M.; Perez-Gregorio, R.; Cao, H.; Simal-Gandara, J.; Prieto, M. Sulfur-containing compounds from plants. In Natural Secondary Metabolites: From Nature, through Science, to Industry; Springer: Berlin/Heidelberg, Germany, 2023; pp. 363–402. [Google Scholar]
- Sita, G.; Hrelia, P.; Tarozzi, A.; Morroni, F. Isothiocyanates are promising compounds against oxidative stress, neuroinflammation and cell death that may benefit neurodegeneration in Parkinson’s disease. Int. J. Mol. Sci. 2016, 17, 1454. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, F.; Paredes-Gonzalez, X.; Kong, A.-N.T. Dietary glucosinolates sulforaphane, phenethyl isothiocyanate, indole-3-carbinol/3, 3′-diindolylmethane: Antioxidative stress/inflammation, Nrf2, epigenetics/epigenomics and in vivo cancer chemopreventive efficacy. Curr. Pharmacol. Rep. 2015, 1, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, C.N.; Li, W.; Zhang, C.; Wu, R.; Su, S.; Wang, C.; Gao, L.; Yin, R.; Kong, A.-N. In vitro-in vivo dose response of ursolic acid, sulforaphane, PEITC, and curcumin in cancer prevention. AAPS J. 2018, 20, 19. [Google Scholar] [CrossRef] [PubMed]
- Baskar, V.; Krishnan, M.; Rajakumar, G.; Hariram Nile, S.; Thiruvengadam, M. Modulation of Expression Levels of Various Cytokines and Inflammatory Responses by Glucosinolate Derivatives. Curr. Top. Med. Chem. 2023, 23, 1575–1578. [Google Scholar] [CrossRef]
- Aslam, M.S.; Ahmad, M.A. Multidisciplinary Applications of Natural Science for Drug Discovery and Integrative Medicine; IGI Global: Pennsylvania, PA, USA, 2023. [Google Scholar]
- Okamoto, T.; Akita, N.; Nagai, M.; Hayashi, T.; Suzuki, K. 6-Methylsulfinylhexyl isothiocyanate modulates endothelial cell function and suppresses leukocyte adhesion. J. Nat. Med. 2014, 68, 144–153. [Google Scholar] [CrossRef]
- Chen, J.; Uto, T.; Tanigawa, S.; Yamada-Kato, T.; Fujii, M.; Hou, D.-X. Microarray-based determination of anti-inflammatory genes targeted by 6-(methylsulfinyl) hexyl isothiocyanate in macrophages. Exp. Ther. Med. 2010, 1, 33–40. [Google Scholar] [CrossRef]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016, 7, 11624. [Google Scholar] [CrossRef]
- Yang, L.; Xie, X.; Tu, Z.; Fu, J.; Xu, D.; Zhou, Y. The signal pathways and treatment of cytokine storm in COVID-19. Signal Transduct. Target. Ther. 2021, 6, 255. [Google Scholar] [CrossRef]
- Pagnotta, E.; Ugolini, L.; Matteo, R.; Righetti, L. Bioactive compounds from Eruca sativa seeds. Encyclopedia 2022, 2, 1866–1879. [Google Scholar] [CrossRef]
- Melchini, A.; Traka, M.H. Biological profile of erucin: A new promising anticancer agent from cruciferous vegetables. Toxins 2010, 2, 593–612. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.E.; Sturm, C.; Piegholdt, S.; Wolf, I.M.; Esatbeyoglu, T.; De Nicola, G.R.; Iori, R.; Rimbach, G. Myrosinase-treated glucoerucin is a potent inducer of the Nrf2 target gene heme oxygenase 1—Studies in cultured HT-29 cells and mice. J. Nutr. Biochem. 2015, 26, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Maresca, D.C.; Conte, L.; Romano, B.; Ianaro, A.; Ercolano, G. Antiproliferative and Proapoptotic Effects of Erucin, a Diet-Derived H2S Donor, on Human Melanoma Cells. Antioxidants 2022, 12, 41. [Google Scholar] [CrossRef]
- Hanlon, N. The Chemopreventive Potential of Sulforaphane and Erucin; University of Surrey: Guildford, UK, 2008. [Google Scholar]
- Genah, S.; Ciccone, V.; Filippelli, A.; Simonis, V.; Martelli, A.; Piragine, E.; Pagnotta, E.; Pecchioni, N.; Calderone, V.; Morbidelli, L. Erucin, a natural isothiocyanate, exerts pro-angiogenic properties in cultured endothelial cells and reverts angiogenic impairment induced by high glucose. Phytother. Res. 2024, 38, 2641–2655. [Google Scholar] [CrossRef]
- Singh, S.; Singh, G.; Attri, S.; Kaur, P.; Rashid, F.; Bedi, N.; Haque, S.; Janahi, E.M.; Arora, S. Development and optimization of nanoparticles loaded with erucin, a dietary isothiocyanate isolated from Eruca sativa: Antioxidant and antiproliferative activities in ehrlich-ascites carcinoma cell line. Front. Pharmacol. 2023, 13, 1080977. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Lee, K.W.; Park, J.H.Y. Erucin exerts anti-inflammatory properties in murine macrophages and mouse skin: Possible mediation through the inhibition of NFκB signaling. Int. J. Mol. Sci. 2013, 14, 20564–20577. [Google Scholar] [CrossRef]
- Yehuda, H.; Soroka, Y.; Zlotkin-Frušić, M.; Gilhar, A.; Milner, Y.; Tamir, S. Isothiocyanates inhibit psoriasis-related proinflammatory factors in human skin. Inflamm. Res. 2012, 61, 735–742. [Google Scholar] [CrossRef]
- Hosokawa, Y.; Hosokawa, I.; Shimoyama, M.; Fujii, A.; Sato, J.; Kadena, K.; Ozaki, K.; Hosaka, K. The anti-inflammatory effects of iberin on TNF-α-stimulated human oral epithelial cells: In vitro research. Biomedicines 2022, 10, 3155. [Google Scholar] [CrossRef]
- Mazumder, A.; Dwivedi, A.; Du Plessis, J. Sinigrin and its therapeutic benefits. Molecules 2016, 21, 416. [Google Scholar] [CrossRef]
- Subedi, L.; Lee, J.H.; Yumnam, S.; Ji, E.; Kim, S.Y. Anti-inflammatory effect of sulforaphane on LPS-activated microglia potentially through JNK/AP-1/NF-κB inhibition and Nrf2/HO-1 activation. Cells 2019, 8, 194. [Google Scholar] [CrossRef]
- Liu, Y.; Chin, F.W.L.; Huang, D.; Liu, S.-Q.; Lu, Y. The thermal degradation of glucomoringin and changes of phenolic compounds in moringa seed kernels during different degrees of roasting. Food Chem. 2024, 454, 139782. [Google Scholar] [CrossRef] [PubMed]
- Manjunath, S.H.; Nataraj, P.; Swamy, V.H.; Sugur, K.; Dey, S.K.; Ranganathan, V.; Daniel, S.; Leihang, Z.; Sharon, V.; Chandrashekharappa, S. Development of Moringa oleifera as functional food targeting NRF2 signaling: Antioxidant and anti-inflammatory activity in experimental model systems. Food Funct. 2023, 14, 4734–4751. [Google Scholar] [CrossRef] [PubMed]
- Chiricosta, L.; Gugliandolo, A.; Diomede, F.; Pizzicannella, J.; Trubiani, O.; Iori, R.; Tardiolo, G.; Guarnieri, S.; Bramanti, P.; Mazzon, E. Moringin pretreatment inhibits the expression of genes involved in mitophagy in the stem cell of the human periodontal ligament. Molecules 2019, 24, 3217. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhao, L.; Xu, M.; Jiang, P.; Zhang, K. Moringin alleviates DSS-induced ulcerative colitis in mice by regulating Nrf2/NF-κB pathway and PI3K/AKT/mTOR pathway. Int. Immunopharmacol. 2024, 134, 112241. [Google Scholar] [CrossRef]
- Giacoppo, S.; Rajan, T.S.; De Nicola, G.R.; Iori, R.; Rollin, P.; Bramanti, P.; Mazzon, E. The isothiocyanate isolated from Moringa oleifera shows potent anti-inflammatory activity in the treatment of murine subacute Parkinson’s disease. Rejuvenation Res. 2017, 20, 50–63. [Google Scholar] [CrossRef]
| Cytokine/Chemokine | Outcome | Cell Source | Function | Reference |
|---|---|---|---|---|
| Interleukin-1α (IL-1α) or pro-IL-1α | Proinflammatory cytokine | Activated macrophages, epithelial cells, endothelial cells, neutrophils, monocytes, fibroblasts, mesenchymal cells, necrotic cells, and dendritic cells | 1. Initiates and triggers the secretion of other inflammatory cytokines, such as IL-6 and TNF-α. 2. Contributes to tumor development in humans. 3. Activates T lymphocytes (causes fever), producing INF and IL-2 required for immune response. 4. Plays a role in tissue repair and remodeling in chronic obstructive pulmonary disease. | [39,47,48,49] |
| Interleukin-1β (IL-1β) or pro-IL-1β | Proinflammatory cytokine | Macrophages, monocytes, fibroblasts, neutrophils, epithelial cells, endothelial cells, dendritic cells, activated T cells, and T-helper 1 (Th1) cells | 1. Activates immune response and promotes fever. 2. Activates Th1 cells. 3. Induces IL-6 production. 4. Mediates inflammatory response, which is crucial for host response and resistance to pathogens. 5. Aggravates tissue damage under chronic and acute disease conditions. 6. Acts as a major cytokine in many rheumatic disorders. | [5,39,50] |
| Interleukin-1 receptor antagonist (IL-1Ra) | Anti-inflammatory cytokine | Hepatic cells, monocytes, macrophages, neutrophils, keratinocytes, epithelial cells, and fibroblasts | Limits IL-1-mediated inflammation. | [50,51,52,53] |
| Interleukin-18 (IL-18) or IFN-γ-inducing factor (IGIF) | Proinflammatory cytokine | Monocytes, macrophages, keratinocytes, mesenchymal cells, endothelial cells, osteoblasts, epithelial cells, and dendritic cells | 1. Stimulates IL-6 and IFN-γ expression. 2. Controls neutrophil function. 3. Mediates adaptive immune responses by regulating the activities of several cytokines. 4. Together with IL-2, IL-18 enhances the production of Th2 cytokines, such as IL-3, IL-4, IL-9, and IL-13. 5. Together with IL-3, IL-18 stimulates the production of IL-4, IL-13, and histamine by various cells, such as mast cells and basophils. 6. Stimulates the production of other cytokines, such as IL-1β, IL-6, IL-17, and TNF-α; C-reactive protein (CRP); and chemokines that are essential for host innate defense and cytokine storms. 7. Overexpression enhances the accumulation of inflammatory cells, such as neutrophils, eosinophils, cytotoxic T lymphocytes (CD8 + T cells), and macrophages, in lung injury and fibrosis. 8. Aberrant expression can be induced by constant NLRP3 inflammasome activity, resulting in the increased production of downstream inflammatory mediators, such as IFN-γ, IL-6, and TNF-α, involved in cytokine storms. 9. Abundant presence in the lungs relative to other tissues increases the activity of IL-18 and the likelihood of a cytokine storm. 10. Induces inflammatory and cytotoxic immune cell activities, leading to autoimmunity. 11. Together with IL-12, IL-18 enhances natural killer (NK) cell activities in response to cancers and infections. | [5,39,54,55,56] |
| Interleukin-33 (IL-33) | Proinflammatory cytokine | Myofibroblasts, endothelial cells, epithelial cells, and active fibroblasts | 1. Activates various immune subgroups. 2. Promotes type 2 immune response. 3. Promotes pulmonary fibrosis. 4. Reduces IFN-γ expression and increases IL-4, IL-5, and IL-13 expression. 5. Elevated levels can increase joint inflammation through the increased activation of neutrophils and mast cells, in addition to the increased expression of inflammatory cytokines, such as IL-6 and IL-1β. 6. Increases the production of type 2 cytokines, such as IL-4, IL-9, IL-10, IL-13, and tumor growth factor-beta (TGF-β). 7. Regulates ARDS-induced inflammation by mediating the secretion of IL-13 by T-reg cells. 8. Participates in the mechanism of fibrosis. | [39,57] |
| Interleukin-36α (IL-36α) | Proinflammatory cytokine | Bronchial epithelium, monocytes, macrophages, keratinocytes, dendritic cells, brain tissue, gut, and skin | 1. Induces inflammatory responses by stimulating NF-κB and mitogen-activated protein kinases (MAPKs). 2. Abnormally activates IL-36 signaling to enhance inflammatory conditions. | [58] |
| Interleukin-36β (IL-36β) | Proinflammatory cytokine | Bronchial epithelium, monocytes, macrophages, keratinocytes, dendritic cells, brain tissue, gut, and skin | 1. Induces inflammatory responses by activating NF-κB and MAPK via the intracellular signaling cascade. 2. Abnormal activation promotes inflammatory conditions detrimental to various organs, such as the kidneys, lungs, and intestines. | [58] |
| Interleukin-36γ (IL-36γ) | Proinflammatory cytokine | Bronchial epithelium, monocytes, macrophages, keratinocytes, dendritic cells, brain tissue, gut, and skin | 1. Induces inflammatory responses by activating NF-κB and MAPK via the intracellular signaling cascade. 2. Abnormal activation increases inflammatory conditions that may be detrimental to various organs, such as the lungs, kidneys, and intestines. 3. Exhibits both proinflammatory and anti-inflammatory properties. | [58] |
| Interleukin-37 (IL-37) | Anti-inflammatory cytokine | Monocytes, dendritic cells, macrophages, leukocytes, tonsil B cells, plasma cells, natural killer cells, stimulated B lymphocytes, and epithelial cells | 1. Powerful anti-inflammatory cytokine capable of countering a broad spectrum of proinflammatory cytokines, such as IL-1β, TNF, and IFN-γ, as well as TLRs. 2. Transduces anti-inflammatory signals by activating Mer–PTEN–DOK pathways, while suppressing NF-κB and MAPK pathways. 3. Promotes innate and acquired immunity and increases aging-related immunosenescence. 4. Represses tumor angiogenesis and metastasis. | [58,59,60,61] |
| Interleukin-4 (IL-4) | Anti-inflammatory cytokine | Mast cells, T-helper (Th1 and Th2) cells, and basophils | 1. Mediates Th2 cell-mediated synthesis of various cytokines (IL-4, IL-5, IL-6, and IL-13) and Th2 immune response. 2. IL-4 and IL-13 are largely associated with the remodeling of fibrogenic inflammation. 3. Involved in the activation of the JAK–STAT pathway. 4. Inhibits proinflammatory monocyte-related cytokines, such as IL-8, IL-6, IL-1, and TNF-α. 5. Suppresses the cytotoxic activity of macrophages. 6. Promotes antibody production. | [50,51,62] |
| Interleukin-6 (IL-6) | Proinflammatory cytokine | Macrophages, T cells, and fibroblasts | 1. Facilitates immune response. 2. Induces and releases other proinflammatory cytokines (promotes inflammation). 3. Acts as an antibody and pyrogen potentiator (enhances the sensitization of antibodies). 4. Plays a crucial role in cytokine storms. 5. Increases vascular permeability. 6. Causes lymphocyte exhaustion and necrosis. | [39,50,51] |
| Interleukin-12 (IL-12) | Proinflammatory cytokine | Dendritic cells and macrophages | Enhances cellular immune response. | [50] |
| Interleukin 13 (IL-13) | Anti-inflammatory cytokine | T cells, mast cells, basophils, and activated Th2 cells | 1. Downregulates inflammation and supports tissue repair. 2. Associated with the remodeling of fibrogenic inflammation. 3. Similar to IL-4, IL-13 is a key participant in the T2 pathway. 4. Coordinates with IL-4 to activate M2 macrophages. | [50,51,62] |
| Interleukin-17 (IL-17) | Proinflammatory cytokine | Macrophages, neutrophils, Th17 cells, and iNKT cells | 1. Acts as a proinflammatory cytokine. 2. Promotes neutrophilic inflammation, stimulating cytokine production. | [39] |
| Interleukin-10 (IL-10) | Anti-inflammatory cytokine | Macrophages, regulatory T cells, monocytes, Th1 cells, and Th2 cells | 1. Downregulates immune response and suppresses inflammation. 2. Suppresses Th1 cell-mediated cytokine release. 3. Negatively regulates T cells by increasing cytotoxic CD8+ T cells. | [39,50] |
| Tumor necrosis factor-alpha (TNF-α) | Proinflammatory cytokine | Macrophages, monocytes, epithelial cells, dendritic cells, Th1 cells, Th17 cells, CD8+ T cells, and endothelial cells | 1. Promotes vascular permeability, enhances autoimmune response, and induces a proinflammatory state. 2. Improves IL-6 production and functions as a pyrogenic cytokine. 3. Acts as a principal participant in cytokine storm interactions. | [39,51] |
| Interleukin 8 (IL-8) or C-X-C motif chemokine ligand 8 (CXCL8) | Proinflammatory chemokine | Macrophages, monocytes, endothelial cells, fibroblasts, activated T cells, Th1 cells, neutrophils, epithelial cells, and hepatocytes | 1. Acts as a proinflammatory cytokine. 2. Controls Th1 and Th2 responses. 3. Elevated concentrations enhance ARDS and increase the severity of pneumonia or COVID-19. 4. Acts as a neutrophil chemotactic agent that recruits neutrophils to infected tissues, increasing the severity of COVID-19. 5. Associated with many disorders, such as severe lung damage in SARS-CoV and MERS-CoV infections. | [39,63] |
| C-X-C motif chemokine 10 ligand (CXCL10)/IP-10 | Proinflammatory chemokine | Dendritic cells, Th2 cells, and macrophages | 1. Acts as a chemoattractant for immune cells. 2. Activates Th1 cell function. 3. Binds to the CXCR3 receptor and recruits various cells, such as macrophages, Th1 cells, and NK cells. 4. Regulates cellular angiostasis, proliferation, apoptosis, and chemotaxis. | [39] |
| C-X-C motif chemokine ligand 1 (CX3CL1), also known as fractalkine (FKN) | Inflammatory chemokine | Vascular endothelial cells | 1. Recruits mononuclear phagocytes. 2. Causes neurological vascular damage and thrombosis. 3. Induces endothelial dysfunction, causing atherosclerosis, cardiovascular diseases, and other complications. 4. Acts as an invasion signal, attracting mononuclear phagocytes to the lungs. 5. Facilitates thrombosis and vascular damage. | [39] |
| Chemokine ligand 2 (CCL2)/Monocytechemoattractant protein-1 (MCP-1) | Inflammatory chemokine | Monocytes, endothelial cells, epithelial cells, fibroblasts, astrocytes, microglial cells, and mesangial cells | 1. Facilitates communication between lymphocytes and myeloid cells. 2. Activates Th1 cell function. 3. Regulates the movement, infiltration, and differentiation of monocytes to macrophages, thereby determining the body’s response to inflammation. 4. High levels upregulate the expression of IL-1, IL-6, TNF-α, matrix metalloproteinase-8, and intercellular adhesion molecule-1, causing inflammation. 5. Increased levels aggravate neurological conditions, such as stroke, Alzheimer’s disease, and dementia. 6. Amplified expression increases the chances of developing kidney failure and worsens prognosis in patients with severe COVID-19. | [39] |
| Granulocyte-macrophage colony-stimulating factor (GM-CSF) | Inflammatory chemokine | Epithelial cells, monocytes, activated T cells, and macrophages | 1. Serves as a chemoattractant for immune cells. 2. Activates Th1 cell function. 3. Enhances proinflammatory cytokines and chemokines, influencing Th17 responses. 4. Significantly enhances GM-CSF-activated immune cells. | [39] |
| Interferon-γ-inducible protein (IP10) | Inflammatory chemokine | Monocytes, neutrophils, endothelial cells, keratinocytes, fibroblasts, mesenchymal cells, dendritic cells, astrocytes, hepatocytes, and leukocytic eosinophils | 1. Secreted in response to IFN-γ and plays a key role in the inflammatory response. 2. Both amplifies and suppresses the proliferation of different cells and receptors. 3. Promotes cancer cell proliferation. 4. Elevated levels cause uncontrolled inflammatory responses, induce tissue damage, and facilitate the development of ARDS, leading to a cytokine storm. | [51,64] |
| Monocyte chemoattractant protein-1 (MCP-1), also known as chemokine (CC-motif) ligand 2 (CCL2) | Inflammatory cytokine | Macrophages, monocytes, fibroblasts, epithelial cells, endothelial cells, smooth muscle cells, astrocytes, and microglial cells | 1. Plays a vital role in the inflammation process by attracting other inflammatory cells, such as monocytes, T cells, macrophages, and dendritic cells, to the site of inflammation or infection and enhancing their expression. 2. Elevated levels are associated with chronic inflammatory and respiratory failure in patients with COVID-19. | [65] |
| Interferon-gamma (IFN-γ) | Proinflammatory cytokine | Macrophages, T cells, NK cells, and cytotoxic T lymphocytes | 1. Exhibits both anti- and protumorigenic effects. 2. Stimulates the activation of immune response and elimination of pathogens. 3. Prevents the overactivation of the immune system and tissue damage. 4. Promotes the activation of macrophages, thereby enhancing their ability to kill intracellular pathogens. 5. Promotes Th1/Th2 cell differentiation, which is important for cell-mediated immunity. | [51,66,67] |
| Interleukin-15 (IL-15) | Proinflammatory cytokine | Macrophages, monocytes, epithelial cells, dendritic cells, keratinocytes, epidermal skin cells, fibroblasts, bone marrow stromal cells, and nerve cell lymphocytes | 1. Promotes the survival, proliferation, and activities of NK cells. 2. Acts as a potent autocrine regulator of proinflammatory cytokine production. 3. High concentrations are suitable for TNF-α, IL-1, and IL-6 production, while very low concentrations favor IL-10 production. 4. Stimulates neutrophil movement and function, e.g., the secretion of IL-8, via NF-κB activation. 5. Induces the proliferation of mast cells in the absence of IL-15Rα and the β chain. 6. Maintains the balance of induced inflammatory cytokines and the homeostatic responses of natural killer and CD8+ T cells. 7. Stimulates cytotoxic CD8+ T cell and NK cell responses. 8. Supports lymphocyte homeostasis by contributing to the overall maintenance of lymphoid cell populations in the body. | [68,69] |
| Interleukin-21 (IL-21) | Anti- and proinflammatory cytokine | T cells, natural killer cells, and stromal cells | 1. Lowers the expression of IL-6 and TNF-α, thereby reducing inflammatory proteins involved in cytokine storms. 2. Promotes the development of B cells and plasma cells and upregulates IgG1 antibody formation. 3. Stimulates the proliferation of CD8 effector cells and costimulates the proliferation and function of T and NK cells. | [69,70] |
| Interleukin-2 (IL-2) | Proinflammatory cytokine | Activated T cells, Th1 cells, dendritic cells, and lymphocytes | 1. Promotes the development of T cells, B cells, natural killer (NK) cells, and innate lymphoid cells (ILCs) associated with the survival or death of immune cell populations. 2. Regulates white blood cells, especially lymphocytes. 3. Participates in the activation of T cells to produce TNF-α and IFN-γ, particularly in response to antigen recognition. 4. Influences the survival and differentiation of cells and negatively regulates immune activation. | [51,71,72] |
| Interleukin-7 (IL-7) | Proinflammatory cytokine | Keratinocytes, dendritic cells, hepatocytes, neurons, epithelial cells, bone marrow stromal cells, and fibroblasts | 1. Promotes the growth, survival, proliferation, and differentiation of B and T cells. 2. Maintains the survival and homeostasis of naive and memory T cells in the peripheral immune system. | [73] |
| Glucosinolate | Hydrolysis Product | Mechanism of Action | Effect on Cytokine Storm | Reference |
|---|---|---|---|---|
| Glucoraphanin | Sulforaphane and sulforaphane nitrile | Downregulates NF-kB translocation and Bax and caspase 3 expression. | Reduces the production of proinflammatory cytokines (e.g., IL-1β) and apoptosis. | [101,102] |
| Sinigrin | Allyl isothiocyanate (AITC) | 1. Regulates the NF-κB-mediated inflammatory cascade. 2. Inhibits the production of IL-6 and TNF-α by downregulating NF-κB-mediated transcription. 3. Upregulates the Nrf2 pathway via the suppression of NF-κB. | 1. Reduces the expression levels of proinflammatory markers, such as IL-1β and IL-6. 2. Increases antioxidative mechanisms. 3. Demonstrates other therapeutic effects, such as anticancer, antimicrobial, and wound-healing effects. | [103,104,105] |
| Gluconasturtiin | Phenethyl isothiocyanate (PEITC) | 1. Influences Nrf2 expression and nuclear translocation. 2. Inhibits the transcription of NF-κB. | Reduces the secretion of proinflammatory mediators, exhibiting anti-inflammatory properties. | [106] |
| Glucoiberin | Iberin and iberverin | Induces the Nrf2-dependent gene expression signaling pathway. | Stimulates immune cell activity. | [29,107] |
| Glucoiberverin | Iberin and iberverin | A homolog of sulforaphane that can induce Nrf2-dependent gene expression. | Stimulates immune cell activity. | [29,107] |
| Glucotropaeolin | Benzyl isothiocyanate (BITC) | Downregulates NF-κB signaling. | Possesses anti-inflammatory properties. | [37,103] |
| Glucoerucin | Erucin | Directly inhibits the NF-κB signaling pathway by activating Nrf2 to inhibit IL-6, IL-8, and IL-12 expression. | Possesses anti-inflammatory properties, reducing the transcription of proinflammatory molecules. | [108,109] |
| Progoitrin | Goitrin | Reduces TNF-α secretion. | Induces an anti-inflammatory response. | [29,110] |
| Epiprogoitrin | Goitrin | Reduces TNF-α secretion. | Induces an anti-inflammatory response. | [29,110] |
| Glucohesperin | 6-(Methylsulfinyl) hexyl isothiocyanate (6-MSITC) | 1. Regulates COX-2, iNOS, TNF-α, IL-1β, and IL-6 gene transcription. 2. Modulates Nrf2 activity within the nucleus. | Possesses anti-inflammatory, antimicrobial, and anticancer properties. | [111] |
| Glucomoringin | Moringin | 1. Interacts with Nrf2 to inhibit oxidative stress 2. Exhibits better inhibitory effects against NF-kB activity than SFN. | Exhibits a broad range of biological effects by counteracting inflammation, antioxidative stress, tumorigenesis, and microbial infections. | [29,112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ochar, K.; Iwar, K.; Nair, V.D.; Chung, Y.-J.; Ha, B.-K.; Kim, S.-H. The Potential of Glucosinolates and Their Hydrolysis Products as Inhibitors of Cytokine Storms. Molecules 2024, 29, 4826. https://doi.org/10.3390/molecules29204826
Ochar K, Iwar K, Nair VD, Chung Y-J, Ha B-K, Kim S-H. The Potential of Glucosinolates and Their Hydrolysis Products as Inhibitors of Cytokine Storms. Molecules. 2024; 29(20):4826. https://doi.org/10.3390/molecules29204826
Chicago/Turabian StyleOchar, Kingsley, Kanivalan Iwar, Vadakkemuriyil Divya Nair, Yun-Jo Chung, Bo-Keun Ha, and Seong-Hoon Kim. 2024. "The Potential of Glucosinolates and Their Hydrolysis Products as Inhibitors of Cytokine Storms" Molecules 29, no. 20: 4826. https://doi.org/10.3390/molecules29204826
APA StyleOchar, K., Iwar, K., Nair, V. D., Chung, Y.-J., Ha, B.-K., & Kim, S.-H. (2024). The Potential of Glucosinolates and Their Hydrolysis Products as Inhibitors of Cytokine Storms. Molecules, 29(20), 4826. https://doi.org/10.3390/molecules29204826







