Impact of Protein Coronas on Lipid Nanoparticle Uptake and Endocytic Pathways in Cells
Abstract
1. Introduction
2. Results
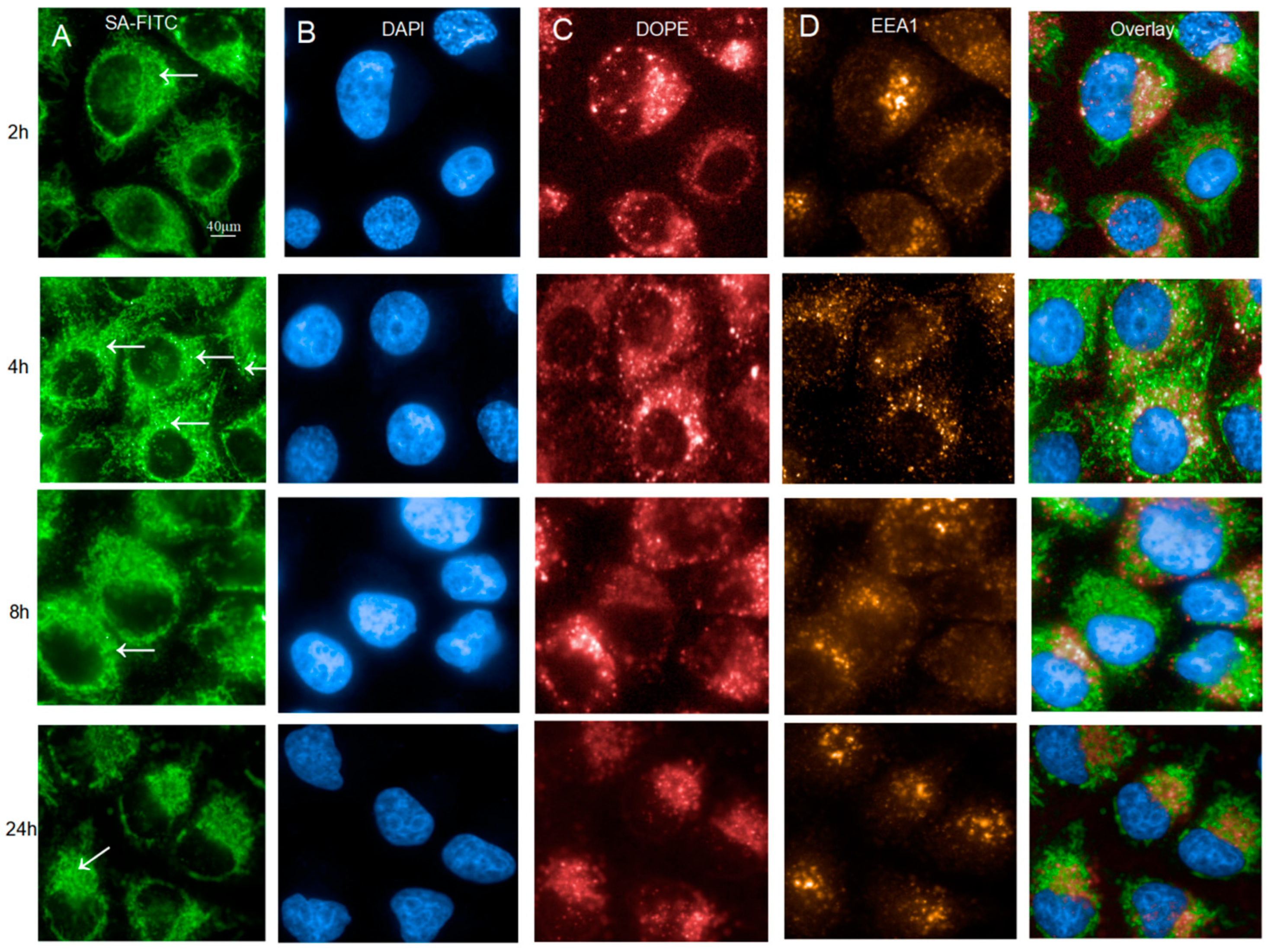
2.1. Characterization of Intracellular Uptake Activity of DNA-LNPs at Different Time Points
2.2. Characterization of Endocytic Activity in Cells Undergoing Nutrient Depletion
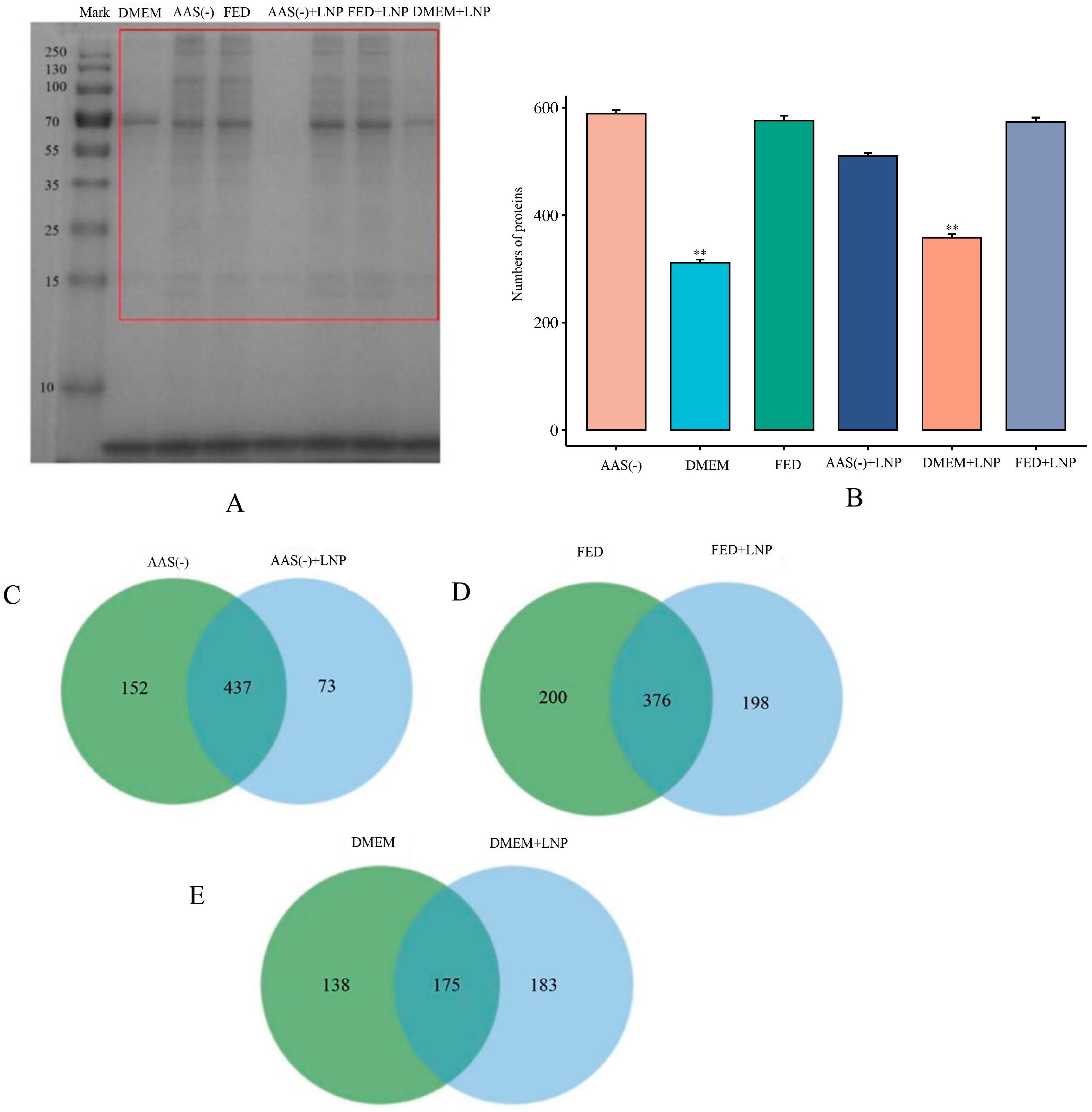
2.3. LNP-PC-Based Proteomic Analysis Strategy
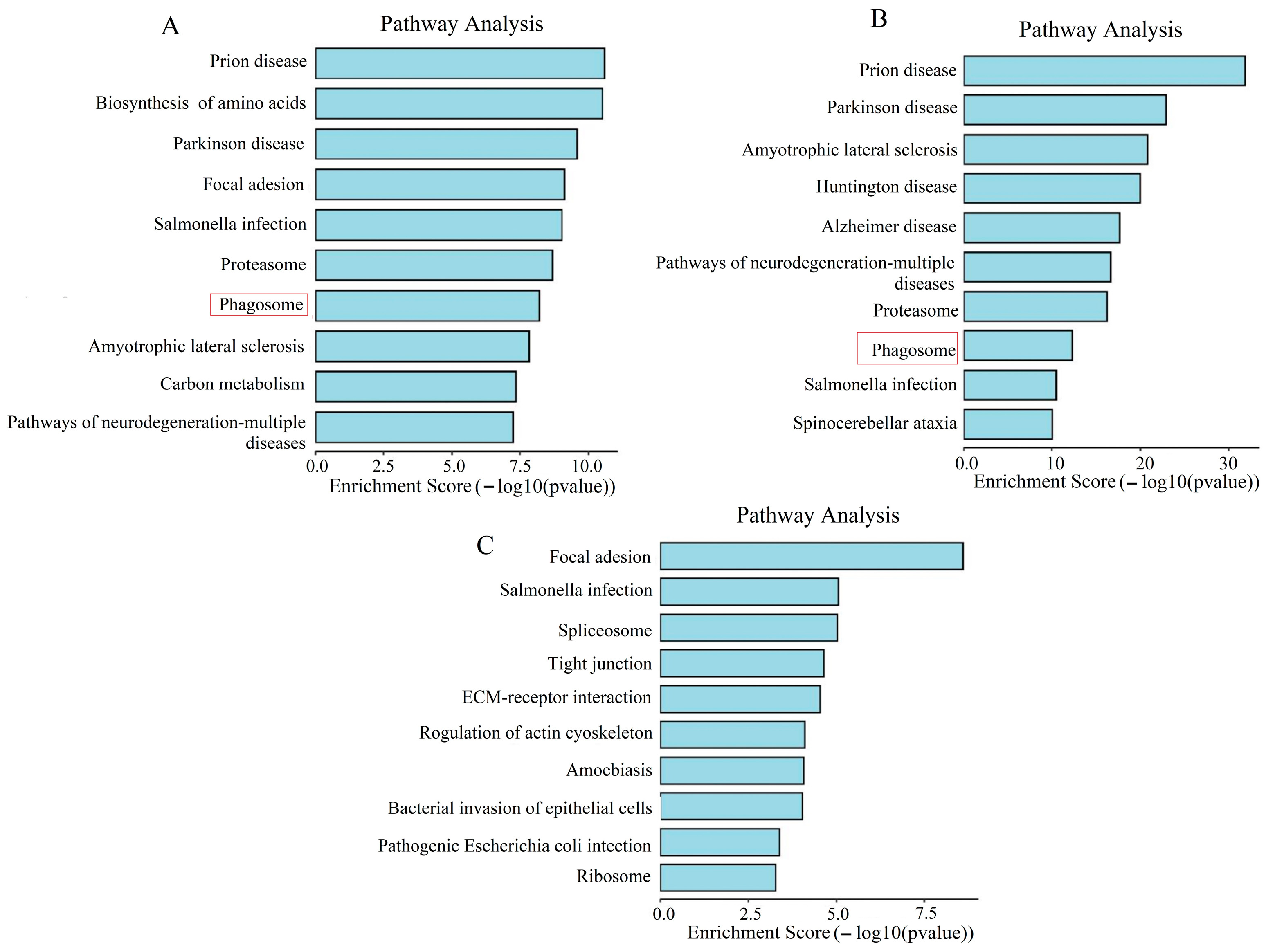
2.4. KEGG Enrichment Analysis
2.5. Phagosome and Differential Analysis
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Antibodies and Reagents
5.2. Linear DNA Amplification
5.3. Nucleic Acid Formulation into Lipid Nanoparticles
5.4. Stains
5.5. Cell Culture
5.6. Immunofluorescence and Uptake Assay
5.7. Coomassie Brilliant Blue
5.8. LC-MS/MS Analysis
5.9. Data Processing
5.10. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vroman, L. Effect of Adsorbed Proteins on the Wettability of Hydrophilic and Hydrophobic Solids. Nature 1962, 196, 476–477. [Google Scholar] [CrossRef] [PubMed]
- Nienhaus, K.; Nienhaus, G.U. Mechanistic Understanding of Protein Corona Formation around Nanoparticles: Old Puzzles and New Insights. Small 2023, 19, e2301663. [Google Scholar] [CrossRef] [PubMed]
- Abid, N.; Khan, A.; Shujait, S.; Chaudhary, K.; Ikram, M.; Imran, M.; Haider, J.; Khan, M.; Khan, Q.; Maqbool, M. Synthesis of nanomaterials using various top-down and bottom-up approaches, influencing factors, advantages, and disadvantages: A review. Adv. Colloid Interface Sci. 2022, 300, 102597. [Google Scholar] [PubMed]
- Lee, H. Differences in protein distribution, conformation, and dynamics in hard and soft coronas: Dependence on protein and particle electrostatics. Phys. Chem. Chem. Phys. 2023, 25, 7496–7507. [Google Scholar] [CrossRef]
- Bashiri, G.; Padilla, M.; Swingle, K.; Shepherd, S.; Mitchell, M.; Wang, K. Nanoparticle protein corona: From structure and function to therapeutic targeting. Lab A Chip 2023, 23, 1432–1466. [Google Scholar] [CrossRef]
- Piltti, K.M.; Cummings, B.J.; Carta, K.; Manughian-Peter, A.; Worne, C.L.; Singh, K.; Anderson, A.J. Live-cell time-lapse imaging and single-cell tracking of in vitro cultured neural stem cells Tools for analyzing dynamics of cell cycle, migration, and lineage selection. Methods 2018, 133, 81–90. [Google Scholar] [CrossRef]
- Jaccard, N.; Szita, N.; Griffin, L.D. Segmentation of phase contrast microscopy images based on multi-scale local Basic Image Features histograms. Comput. Methods Biomech. Biomed. Eng. Imaging Vis. 2017, 5, 359–367. [Google Scholar] [CrossRef]
- Booij, T.H.; Price, L.S.; Danen, E.H.J. 3D Cell -Based Assays for Drug Screens: Challenges in Imaging, Image Analysis, and High-Content Analysis. SLAS Discov. 2019, 24, 615–627. [Google Scholar] [CrossRef]
- Gunn, A.L.; Yashchenko, A.I.; Dubrulle, J.J.; Hatch Emily, M. A high-content screen reveals new regulators of nuclear membrane stability. Sci. Rep. 2024, 14, 6013. [Google Scholar] [CrossRef]
- Chen Cherry, C.; Borden Mark, A. Ligand conjugation to bimodal poly(ethylene glycol) brush layers on microbubbles. Langmuir 2010, 26, 13183–13194. [Google Scholar] [CrossRef]
- Wienke, H.S.; Pinilla, B.M.C.; Contri, V.R.; Brandelli, A. Development of Polyelectrolyte-Coated Liposomes as Nanostructured Systems for Nisin Delivery: Antimicrobial Activity and Long-Term Stability. Food Biophys. 2024, 1–13. [Google Scholar] [CrossRef]
- Palchetti, S.; Colapicchioni, V.; Digiacomo, L.; Caracciolo, G.; Pozzi, D.; Capriotti, A.L.; Barbera, G.A. The protein corona of circulating PEGylated liposomes. Biochim. Biophys. Acta-Biomembr. 2016, 1858, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Benne, N.; Van Duijn, J.; Lozano Vigario, F.; Leboux, R.J.; Van Veelen, T.P.; Kuiper, J.; Jiskoot, W.; Slütter, B. Anionic 1,2-distearoyl-sn-glycero-3-phosphoglycerol (DSPG) liposomes induce antigen-specific regulatory T cells and prevent atherosclerosis in mice. J. Control Release 2018, 291, 135–146. [Google Scholar] [CrossRef]
- Palchetti, A.S.; Pozzi, D.; Capriotti, A.L.; Barbera, G.L.; Chiozzi, R.Z.; Peruzzi, G.; Caracciolo, G.; Palchetti, S.; Pozzi, D.; Capriotti, A.L.; et al. Influence of dynamic flow environment on nanoparticle-protein corona: From protein patterns to uptake in cancer cells. Colloids Surf. B Biointerfaces 2017, 153, 263–271. [Google Scholar] [CrossRef]
- Yasmin, A.; Ezeddine, H.; François, D. The interplay between lysosome, protein corona and biological effects of cationic carbon dots: Role of surface charge titratability. Int. J. Pharm. 2023, 645, 123388. [Google Scholar]
- Francesca, L.; Ilaria, T.; Alessandro, M.D.; Dominici, F.; Argentati, C.; Morena, F.; Torre, L.; Puglia, D.; Martino, S. Novel Nanocomposite PLA Films with Lignin/Zinc Oxide Hybrids: Design, Characterization, Interaction with Mesenchymal Stem Cells. Nanomaterials 2020, 10, 2176. [Google Scholar] [CrossRef] [PubMed]
- De, L.M.; Ferraro, M.M.; Hartmann, R.; Rivera, P.; Klingl, A.; Nazarenus, M.; Ramirez, A.; Parak, W.J.; Bucci, C.; Rinaldi, R. Advances in Use of Capsule-Based Fluorescent Sensors for Measuring Acidification of Endocytic Compartments in Cells with Altered Expression of V-ATPase Subunit V1G1. ACS Appl. Mater. Interfaces 2015, 7, 15052–15060. [Google Scholar]
- Ispanixtlahuatl-Meráz, O.; Delgado-Buenrostro, N.L.; Déciga-Alcaraz, A.; Del Pilar Ramos-Godinez, M.; Oliva-Rico, D.; López-Villegas, E.O.; Chirino, Y.I. Differential response of immobile (pneumocytes) and mobile (monocytes) barriers against 2 types of metal oxide nanoparticles. Chem.-Biol. Interact. 2021, 347, 109596. [Google Scholar] [CrossRef]
- Zhu, X.; Sun, J.; Zhang, Y.; Sun, X.L. Immunization with functionalized carbon nanotubes enhances the antibody response against mode antigen ovalbumin. Immunol. Lett. 2016, 178, 77–84. [Google Scholar] [CrossRef]
- Takuma, I.; Safaa, N.; Megan, V. Therapeutic strategies to target connective tissue growth factor in fibrotic lung diseases. Pharmacol. Ther. 2024, 253, 108578. [Google Scholar]
- Chen, D.; Ganesh, S.; Wang, W.; Amiji, M. The role of surface chemistry in serum protein corona-mediated cellular delivery and gene silencing with lipid nanoparticles. Nanoscale 2019, 11, 8760–8775. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Parayath, N.; Ganesh, S.; Wang, W.; Amiji, M. The role of apolipoprotein- and vitronectin-enriched protein corona on lipid nanoparticles for in vivo targeted delivery and transfection of oligonucleotides in murine tumor models. Nanoscale 2019, 11, 18806–18824. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Kon, E.; Sharma, P.; Dan, P. Endosomal escape: A bottleneck for LNP-mediated therapeutics. Proc. Natl. Acad. Sci. USA 2024, 121, e2307800120. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, C.; Carril, M.; Parak, W.J. Techniques for the experimental investigation of the protein corona. Curr. Opin. Biotechnol. 2017, 46, 106–113. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]

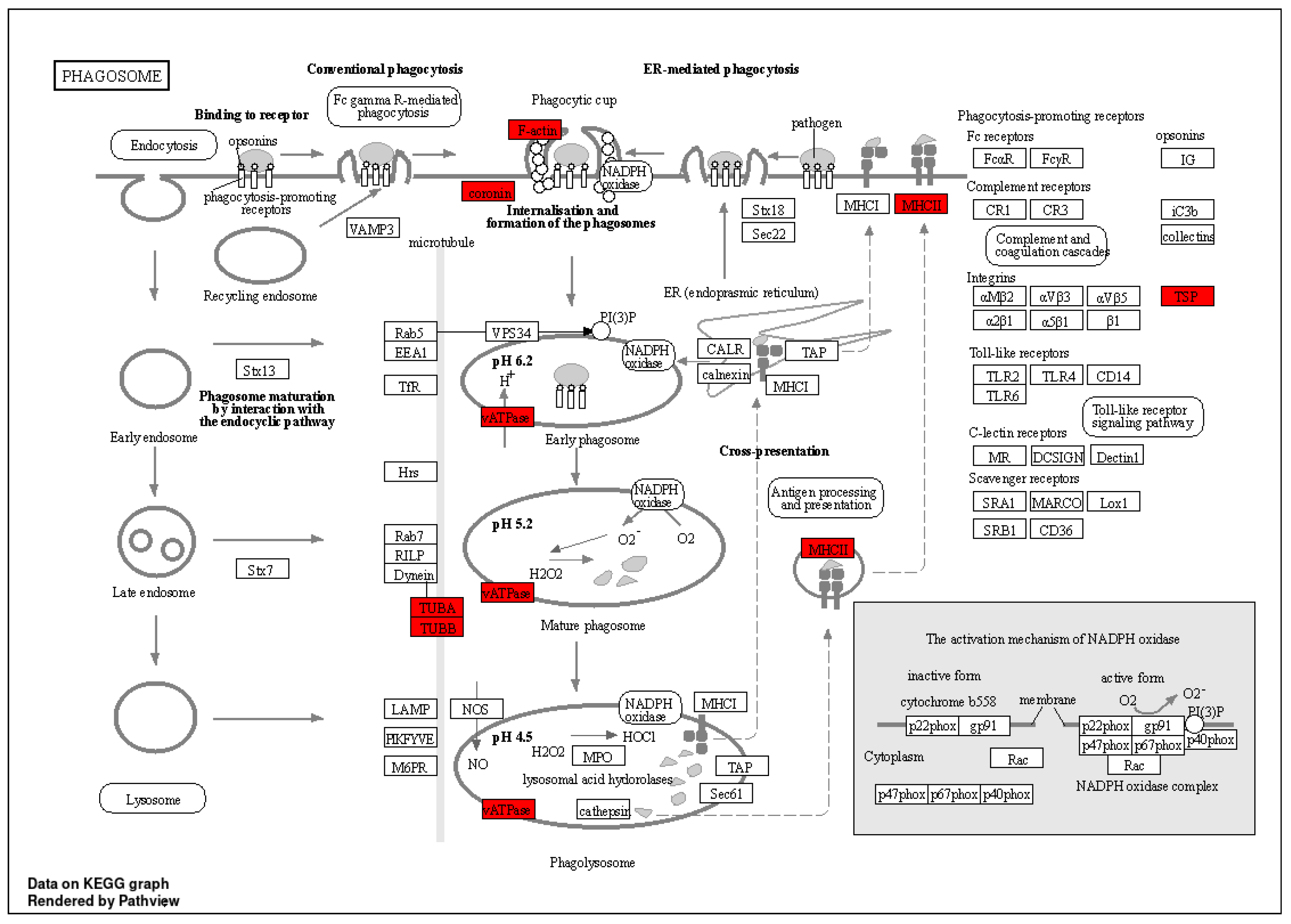

| Name | Protein Description | Gene Name |
|---|---|---|
| F-actin | F-actin-capping protein subunit alpha-2 | CAPZA2 |
| F-actin-capping protein subunit alpha-1 | CAPZA1 | |
| F-actin-capping protein subunit beta | CAPZB | |
| Coronin | Coronin-1A | CORO1A |
| Coronin-1B | CORO1B | |
| CORO-2B | CORO2B | |
| Coronin-6 | CORO6 | |
| Coronin-1C | CORO1C | |
| vATPase | V-type proton ATPase catalytic subunit A | ATP6V1A |
| V-type proton ATPase subunit B, brain isoform | ATP6V1B2 | |
| V-type proton ATPase subunit H | ATP6V1H | |
| TUBA | Tubulin alpha-4A chain | TUBA4A |
| Tubulin alpha-1C chain | TUBA1C | |
| Tubulin alpha-8 chain | TUBA8 | |
| Tubulin alpha chain-like 3 | TUBAL3 | |
| TUBB | Tubulin beta chain | TUBB |
| Tubulin beta-4B chain | TUBB4B | |
| Tubulin beta-2A chain | TUBB2A | |
| Tubulin beta-4A chain | TUBB4A | |
| Tubulin beta-3 chain | TUBB3 | |
| Tubulin beta-6 chain | TUBB6 | |
| Tubulin beta-8 chain | TUBB8 | |
| MHCII | HLA class I histocompatibility antigen, alpha chain G | HLA-G |
| HLA class I histocompatibility antigen, A alpha chain | HLA-A | |
| TSP | Thrombospondin-1 | THBS1 |
| Thrombospondin-2 | THBS2 | |
| Thrombospondin-3 | THBS3 | |
| Thrombospondin-4 | THBS4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; He, J.; Xu, Y.; Peng, B. Impact of Protein Coronas on Lipid Nanoparticle Uptake and Endocytic Pathways in Cells. Molecules 2024, 29, 4818. https://doi.org/10.3390/molecules29204818
Wang R, He J, Xu Y, Peng B. Impact of Protein Coronas on Lipid Nanoparticle Uptake and Endocytic Pathways in Cells. Molecules. 2024; 29(20):4818. https://doi.org/10.3390/molecules29204818
Chicago/Turabian StyleWang, Rui, Jing He, Yuhong Xu, and Baowei Peng. 2024. "Impact of Protein Coronas on Lipid Nanoparticle Uptake and Endocytic Pathways in Cells" Molecules 29, no. 20: 4818. https://doi.org/10.3390/molecules29204818
APA StyleWang, R., He, J., Xu, Y., & Peng, B. (2024). Impact of Protein Coronas on Lipid Nanoparticle Uptake and Endocytic Pathways in Cells. Molecules, 29(20), 4818. https://doi.org/10.3390/molecules29204818






