Comprehensive Evaluation of Quality and Differences in Silene viscidula Franch from Different Origins Based on UPLC-ZENO-Q-TOF-MS/MS Compounds Analysis and Antioxidant Capacity
Abstract
1. Introduction
2. Results
2.1. Optimization of Extraction Conditions
2.2. Optimization of UPLC-Q-ZENO-TOF-MS/MS Conditions
2.3. Identification of the Compounds of SF
2.3.1. Identification of Phytosterols and Their Glycosides
2.3.2. Identification of the Steroidal Saponins
2.3.3. Identification of Other Compounds
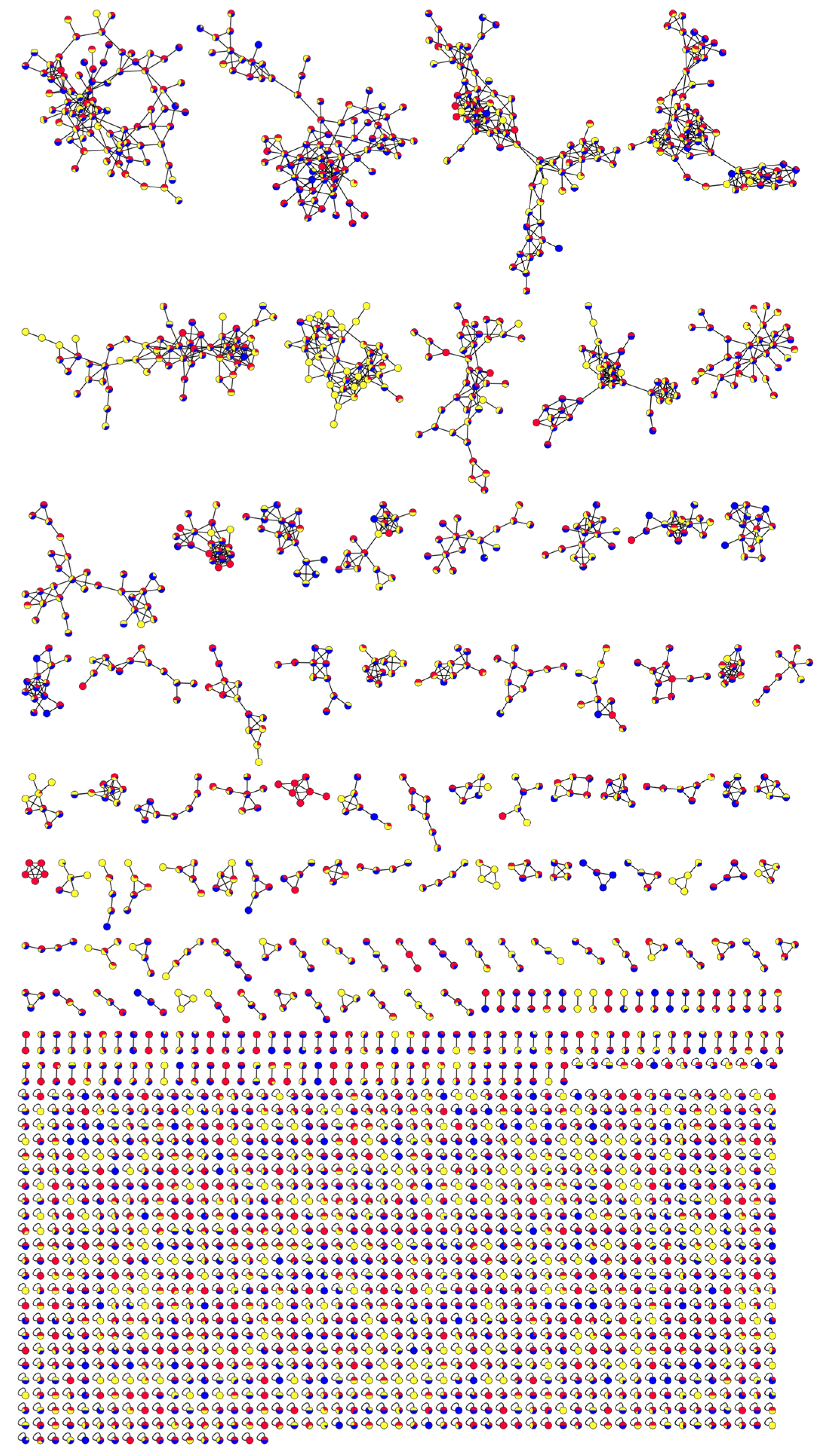
2.4. Molecular Network Visualization and Analysis of SF Mass Spectrometry Data
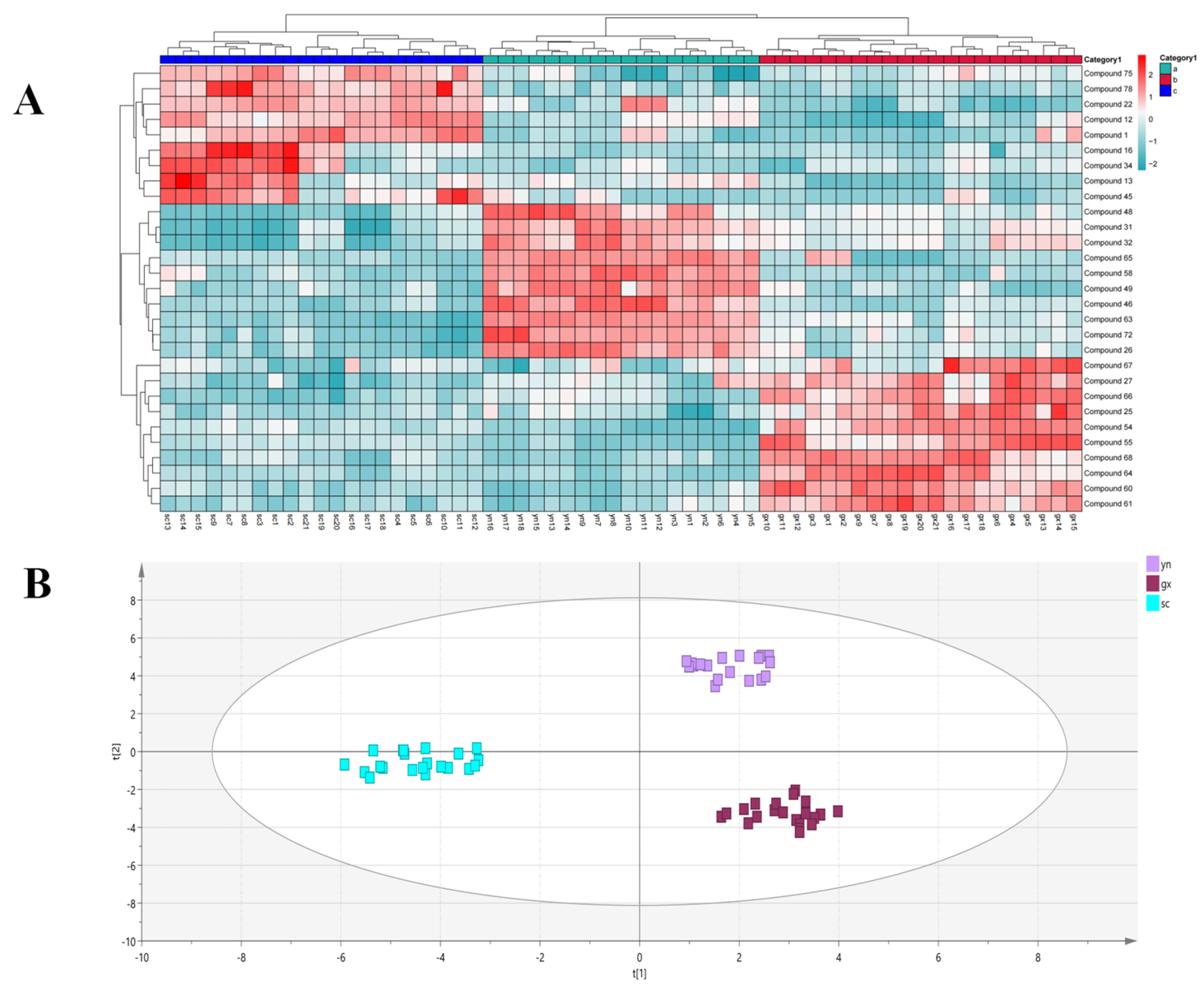
2.5. Differential Analysis of the Chemical Compounds of SF from Different Origins
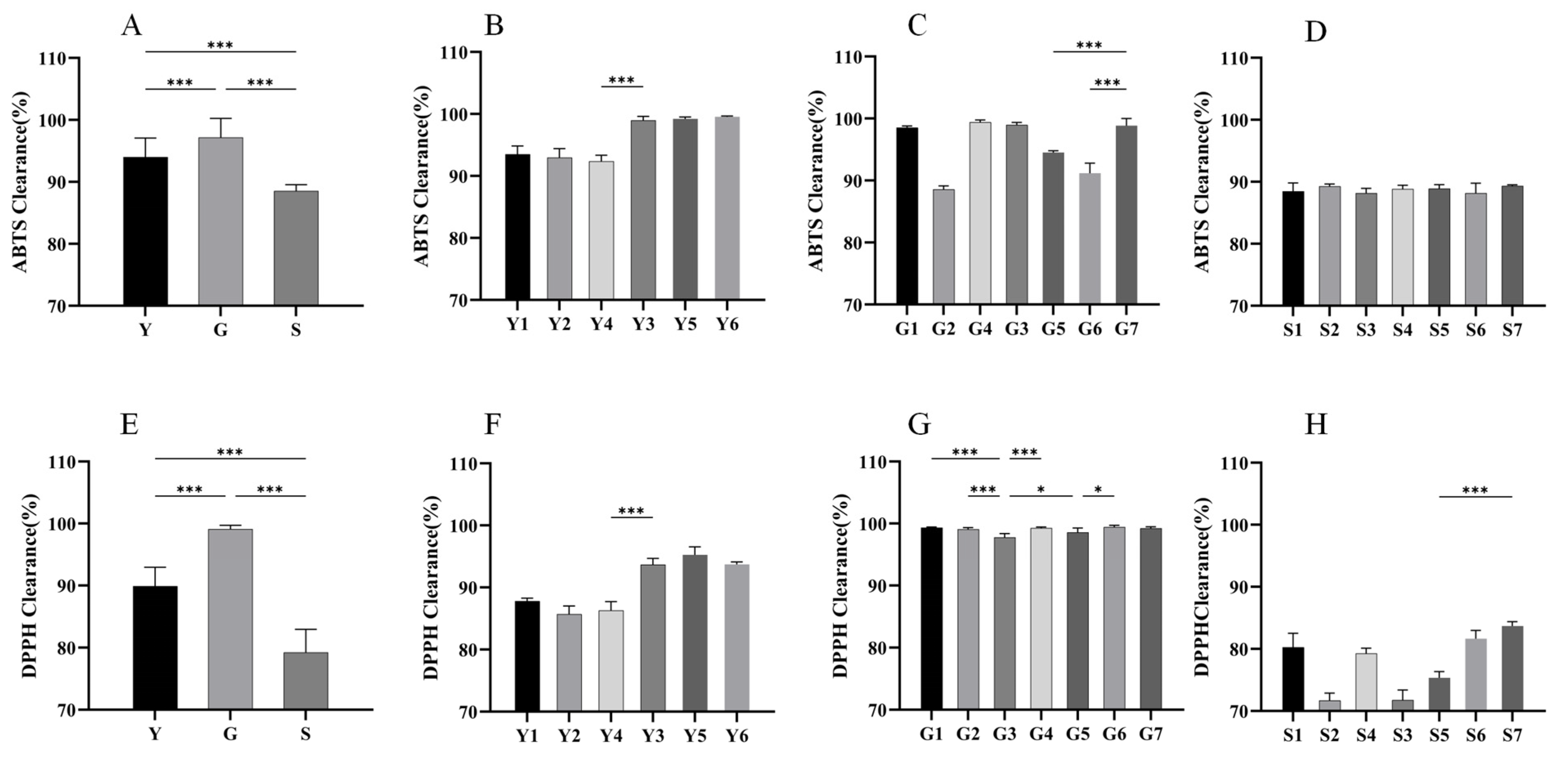
2.6. Results of SF Samples of Different Origins in DPPH and ABTS Radical Scavenging Assays
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Collection and Preparation of Medicinal Herbs
4.3. UPLC-MS/MS Conditions Analysis
4.4. Molecular Network and Compound Identification Analysis
4.5. Comparative Analysis of the Chemical Compounds and Antioxidant Activity of SF from Different Origins
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mao, L.; Guoyou, S. Yunnan Materia Medica, 1st ed.; Yunnan People’s Publishing House: Kunming, China, 2017; p. 534. [Google Scholar]
- Liao, C.; Zhao, B.; Wang, X.; Liu, C.; Xu, G.; Liu, S.; Zhang, X.; Wang, X. Isolation and Characterization of Chemical Constituents from the Roots of Silene viscidula Franch. Asian J. Chem. 2013, 25, 10311–10314. [Google Scholar] [CrossRef]
- Zhang, R.P.; Zou, C.; He, Y.N.; Tan, N.H.; Zhou, J. Three new cyclopeptides from silene szechuensis. Acta Bot. Yunnanica 1997, 19, 304–310. [Google Scholar]
- Zhang, C.; Qiao, S.; Wu, J.; Xu, W.; Ma, s.; Zhao, B.; Wang, X. A new insulin-sensitive enhancer from Silene viscidula, WPTS, treats type 2 diabetes by ameliorating insulin resistance, reducing dyslipidemia, and promoting proliferation of islet β cells. Pharmacol. Res. 2021, 165, 105416. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ma, Q.; Xu, W.; Li, W.-M.; Yuan, D.-Z.; Wu, J.-M.; Li, Y.-S.; Fang, J. Anticancer Effects of Sinocrassulosides VI/VII from Silene viscidula on HeLa Cells. Evid.-Based Complement. Altern. Med. 2017, 2017, 8240820. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.Q. Studies on the Anti-Inflammatory and Analgesic Activity of Silene viscidula Franch Saponins. Master’s Thesis, Minzu University of China, Beijing, China, 2015. [Google Scholar]
- Lv, J.; Wang, Y.; Xu, J.; Zhou, T.; Chen, Z.; Yang, H.; Di, T.; Li, P. Protective effect of Yangxue Jiedu Soup against psoriasis-like lesions by regulating TLR4/NF-κB signaling pathway mediated by secretion of exosome HSP70. Biomed. Pharmacother. 2022, 147, 112604. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, H.; Xue, J.; Yuan, J.; Cai, Z.; Wu, N.; Zou, L.; Yin, S.; Yang, W.; Liu, X.; et al. Qualitative Analysis and Componential Differences of Chemical Constituents in Lysimachiae Herba from Different Habitats (Sichuan Basin) by UFLC-Triple TOF-MS/MS. Molecules 2022, 27, 4600. [Google Scholar] [CrossRef]
- Shi, D.; Liao, N.; Liu, H.; Gao, W.; Zhong, S.; Zheng, C.; Chen, H.; Xiao, L.; Zhu, Y.; Huang, S.; et al. Rapid Analysis of Compounds from Piperis Herba and Piperis Kadsurae Caulis and Their Differences Using High-Resolution Liquid–Mass Spectrometry and Molecular Network Binding Antioxidant Activity. Molecules 2024, 29, 439. [Google Scholar] [CrossRef]
- GNPS. Available online: https://gnps.ucsd.edu/ (accessed on 12 July 2024).
- Rumpf, J.; Burger, R.; Schulze, M. Statistical evaluation of DPPH, ABTS, FRAP, and Folin-Ciocalteu assays to assess the antioxidant capacity of lignins. Int. J. Biol. Macromol. Struct. Funct. Interact. 2023, 233, 123470. [Google Scholar] [CrossRef]
- Yao, C.-L.; Qian, Z.-M.; Tian, W.-S.; Xu, X.-Q.; Yan, Y.; Shen, Y.; Lu, S.-M.; Li, W.-J.; Guo, D.-A. Profiling and identification of aqueous extract of Cordyceps sinensis by ultra-high performance liquid chromatography tandem quadrupole-orbitrap mass spectrometry. Chin. J. Nat. Med. 2019, 17, 631–640. [Google Scholar] [CrossRef]
- Hou, S.X. Comprehensive Quality Evaluation of Psammosilene Tunicoidesand Silene viscidula and Basic Study on Anti-Inflammatorypharmacodynamic Substances of Silene viscidula. Master’s Thesis, Tianjin University of Traditional Chinese Medicine, Tianjin, China, 2023. [Google Scholar]
- Seo, C.; Shin, H.S.; Lee, J.E.; Jung, Y.W.; Kim, J.K.; Kwon, J.G.; Jeong, W.; Choi, C.W.; Oh, J.S.; Hong, S.S. Isolation and structure elucidation of siliendines A–D, new β-carboline alkaloids from Silene seoulensis. Phytochem. Lett. 2020, 36, 58–62. [Google Scholar] [CrossRef]
- Saatov, Z.; Gorovits, M.B.; Abubakirov, N.K. Phytoecdysteroids of plants of the genus Silene. XV. 2-Deoxy-α-ecdysone 22-O-benzoate from Silene wallichiana. Chem. Nat. Compd. 1987, 23, 708–711. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Shukla, S.; Kang, S.C. Chemical composition and antifungal activity of essential oil and various extract of Silene armeria L. Bioresour. Technol. 2008, 99, 8903–8908. [Google Scholar] [CrossRef] [PubMed]
- Al Kateeb, A.I.M.; Tüfekci, E.F.; Altunoglu, Y.C.; Baloglu, M.C.; Nilofar, N.; Yıldıztugay, E.; Jekő, J.; Cziáky, Z.; Zengin, G. Multidirectional research for the therapeutic potential of Phlomoides molucelloides (Bunge) Salmaki: LC-MS/MS, antioxidant, enzyme inhibition, and antiproliferative characteristics. Process Biochem. 2024, 143, 302–314. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, W.; Xie, S.; Liu, Y.; Xu, Y. Multi-omics analysis of brain tissue metabolome and proteome reveals the protective effect of gross saponins of Tribulus terrestris L. fruit against ischemic stroke in rat. J. Ethnopharmacol. 2021, 278, 114280. [Google Scholar] [CrossRef]
- Cui, Y.; Yang, H.; Jing, J.; Liu, T.; Wang, R.; Di, F.; Han, F.; Zhao, Y.; Yu, Z. Rapid characterization of chemical constituents of Gansuibanxia decoction by UHPLC-FT-ICR-MS analysis. J. Pharm. Biomed. Anal. 2020, 179, 113029. [Google Scholar] [CrossRef]
- Duan, S.; Li, X.; Yao, Z.; Zhang, X.; Yao, X.; Yang, J.; Qin, Z. Visual authentication of steroidal saponins in Allium macrostemon Bge. and Allium chinense G. Don using MALDI-TOF imaging mass spectrometry and their structure activity relationship. Arab. J. Chem. 2022, 15, 104138. [Google Scholar] [CrossRef]
- Zemtsova, G.N.; Glyzin, V.Y.; Dzhumyrko, S.F. Flavones and their C-glycosides from Silene saxatilis. Chem. Nat. Compd. 1975, 11, 538. [Google Scholar] [CrossRef]
- Li, Y.-Z.; Song, B.; Zheng, X.-D.; Huang, W.-L.; Zhang, H.-W. Five new polyhydroxylated furostanol saponins from the rhizomes of Tupistra chinensis. Chin. J. Nat. Med. 2019, 17, 624–630. [Google Scholar] [CrossRef]
- Báthori, M.; Girault, J.P.; Kalász, H.; Máthé, I.; Lafont, R. New minor ecdysteroids from Silene otites (L.) Wib. J. Pharm. Biomed. Anal. 1997, 16, 327–336. [Google Scholar] [CrossRef]
- Liu, C.-H.; Qi, J.; Zhou, D.-Z.; Ju, A.-C.; Yu, B.-Y. Influence of ultrafiltration membrane on ophiopogonins and homoisoflavonoids in Ophiopogon japonicus as measured by ultra-fast liquid chromatography coupled with ion trap time-of-flight mass spectrometry. Chin. J. Nat. Med. 2017, 15, 121–141. [Google Scholar] [CrossRef]
- Yahyazadeh, R.; Rahimi, V.B.; Mohajeri, S.A.; Iranshahy, M.; Hasanpour, M.; Askari, V.R. Intra-peritoneal lavage of Zingiber officinale rhizome and its active constituent gingerol impede inflammation, angiogenesis, and fibrosis following post-operative peritoneal adhesion in male rats. Saudi Pharm. J. 2024, 32, 102092. [Google Scholar] [CrossRef] [PubMed]
- Mamadalieva, N.Z.; Zibareva, L.N.; Lafont, R.; Dainan, L.; Saatov, Z. Phytoecdysteroids from the Silene genus. Chem. Nat. Compd. 2004, 40, 574–578. [Google Scholar] [CrossRef]
- Takahashi, N.; Li, W.; Koike, K. Oleanane-type triterpenoid saponins from Silene armeria. Phytochemistry 2016, 129, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Saatov, Z.; Gorovits, M.B.; Abubakirov, N.K. Phytoecdysteroids of plants of the genusSileneXIII. Ecdysterone 20,22-monoacetonide fromSilene scabrifolia. Chem. Nat. Compd. 1987, 23, 643. [Google Scholar] [CrossRef]
- Mamadalieva, N.Z.; Ramazanov, N.S.; Dinan, L.N.; Saatov, Z. Phytoecdysteroids of Plants of the Silene Genus. 2-Dehydroxyecdysterone-3-O-benzoate from Silene wallichiana. Chem. Nat. Compd. 2000, 36, 513–515. [Google Scholar] [CrossRef]
- Chang, Y.; Wang, S.; Xu, J.; Zhu, T.; Ma, S.; Zhou, A.; Song, Y.; Liu, M.; Tian, C. Optimization of extraction process of Dioscorea nipponica Makino saponins and their UPLC-QTOF-MS profiling, antioxidant, antibacterial and anti- inflammatory activities. Arab. J. Chem. 2023, 16, 104630. [Google Scholar] [CrossRef]
- Chen, W.; Yu, J.W.; Deng, Y.Y. Identification of sedative-hypnotic compounds shared by five medicinal Polyporales mushrooms using UPLC-Q-TOF-MS/MS-based untargeted metabolomics. Phytomedicine Int. J. Phytother. Phytopharm. 2024, 128, 155355. [Google Scholar] [CrossRef]
- Jayasinghe, L.; Kumarihamy, B.M.M.; Arundathie, B.G.S.; Dissanayake, L.; Fujimoto, Y. A new ecdysteroid, 2-deoxy-5β,20-dihydroxyecdysone from the fruits of Diploclisia glaucescens. Steroids 2003, 68, 447–450. [Google Scholar] [CrossRef]
- Bechkri, S.; Magid, A.A.; Sayagh, C.; Berrehal, D.; Kabouche, A. Triterpene saponins from Silene gallica collected in North-Eastern Algeria. Phytochemistry 2020, 172, 112274. [Google Scholar] [CrossRef]
- Saatov, Z.; Gorovits, M.B.; Abdullaev, N.D. Phytoecdysteroids of plants of the genus Silene. V. Sileneoside B-An ecdysterone digalactoside from Silene brahuica. Chem. Nat. Compd. 1982, 578–582. [Google Scholar] [CrossRef]
- Saatov, Z.; Abdullaev, N.D.; Gorovits, M.B.; Abubakirov, N.K. Phytoecdysteroids of plants of the genus Silene. VI. α-Ecdysone 22-sulfate—A new ecdysteroid from Silene brahuica. Chem. Nat. Compd. 1984, 20, 441–444. [Google Scholar] [CrossRef]
- Zheng, Q.A.; Yang, C.R. Pregnane glycosides from Dracaena cochinchinensis. J. Asian Nat. Prod. Res. 2003, 5, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Saatov, A.; Abdullaev, N.D.; Gorovits, M.B.; Abubakirov, N.K. Phytoecdysteroids of plants of the genus Silene. XVII. Ecdysterone 22,25-di-O-benzoate from Silene scabrifolia. Chem. Nat. Compd. 1990, 26, 301–303. [Google Scholar] [CrossRef]
- Xu, W.; Fang, J.; Zhu, Z.; Wu, J.; Li, Y. A new triterpenoid saponin from the roots of Silene viscidula. Nat. Prod. Res. 2011, 26, 2002–2007. [Google Scholar] [CrossRef]
- Ramazanov, N.S.; Maksimov, E.S.; Saatov, Z.; Abdullaev, N.D. Phytoecdysteroids of plants of the Silene genus. XVIII. Tomentesterone from Silene tomentella. Chem. Nat. Compd. 1996, 32, 47–49. [Google Scholar] [CrossRef]
- Yu, H.S.; Zhang, J.; Kang, L.P.; Han, L.F.; Zou, P.; Zhao, Y.; Xiong, C.Q.; Tan, D.W.; Song, X.B.; Yu, K. Three new saponins from the fresh rhizomes of Polygonatum kingianum. Chem. Pharm. Bull. 2009, 57, 1. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-C.; Chen, C.-R.; Chen, C.-Y.; Liang, P.-H. Synthesis of Quillaic Acid through Sustainable C-H Bond Activations. J. Org. Chem. 2024, 89, 5491–5497. [Google Scholar] [CrossRef]
- Yamasaki, K.K. Iridoid and phenolic diglycosides from Canthium berberidifolium. Phytochemistry 2002, 61, 461–464. [Google Scholar]
- Chen, Y.; Yan, T.; Zhang, Y.; Wang, Q.; Li, G. Characterization of the incense ingredients of cultivated grafting Kynam by TG-FTIR and HS-GC–MS. Fitoterapia 2020, 142, 104493. [Google Scholar] [CrossRef]
- Liu, M.S.; Li, X. Spectral rules of phytoecdysteroids. Nat. Prod. Res. Dev. 1998, 4, 5–11. [Google Scholar]
- Jiang, C.L.; Wu, J.H.; Zhao, L.C.; Wang, X.Y. The antitumor effect of Sinocrassulosides VI/VII and its effect on apoptosis and cell cycle of BGC-823 cells. Glob. Tradit. Chin. Med. 2024, 17, 1050–1054. [Google Scholar]
- Yuan, L.; Ma, Y.H.; Yin, Z.H.; Gu, X.Z.; Kang, W.Y. Antioxidant Activity of Psammosilene Tunicoides in vitro. Chin. J. Exp. Tradit. Med. Formulae 2012, 18, 109–112. [Google Scholar]

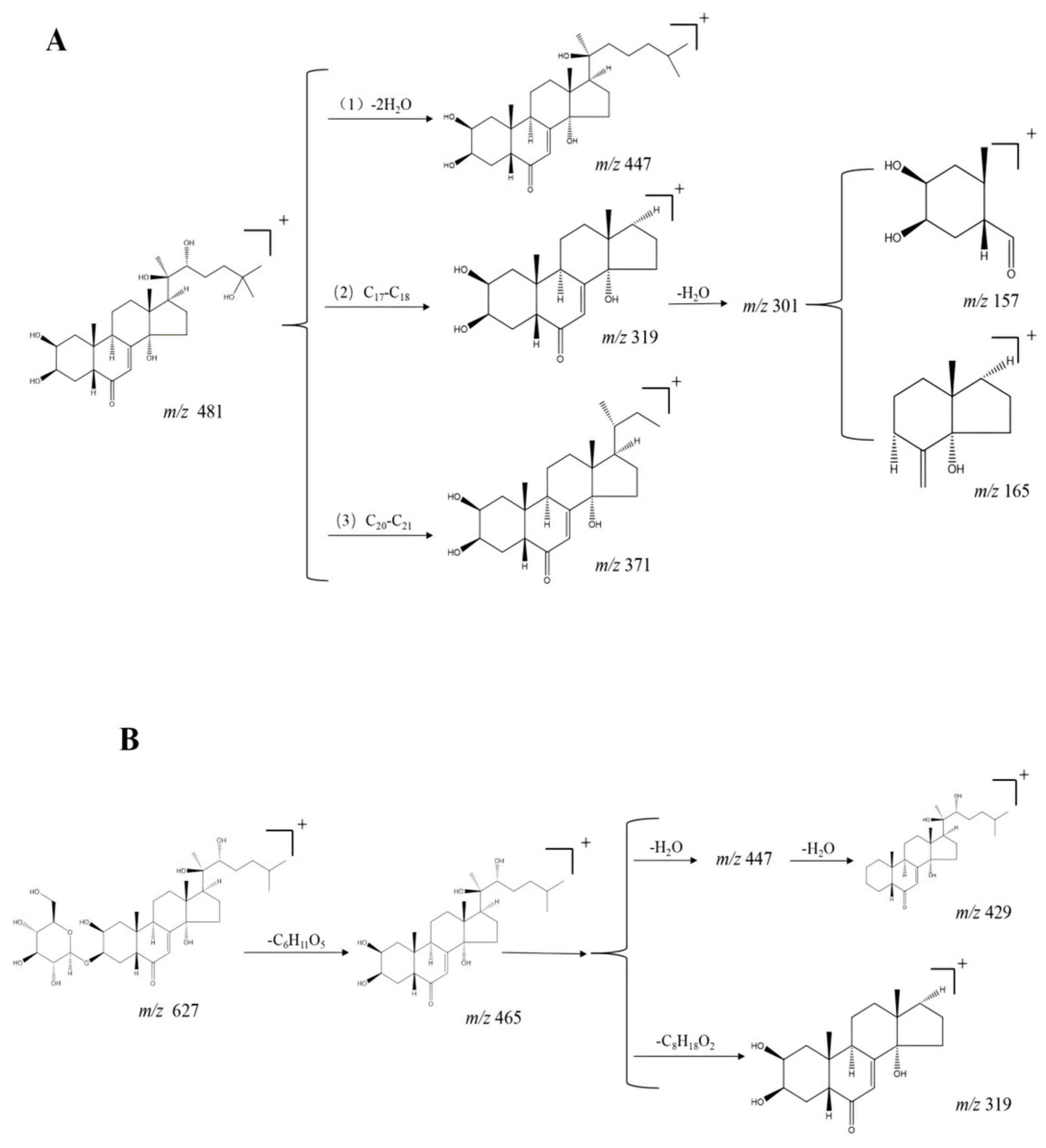
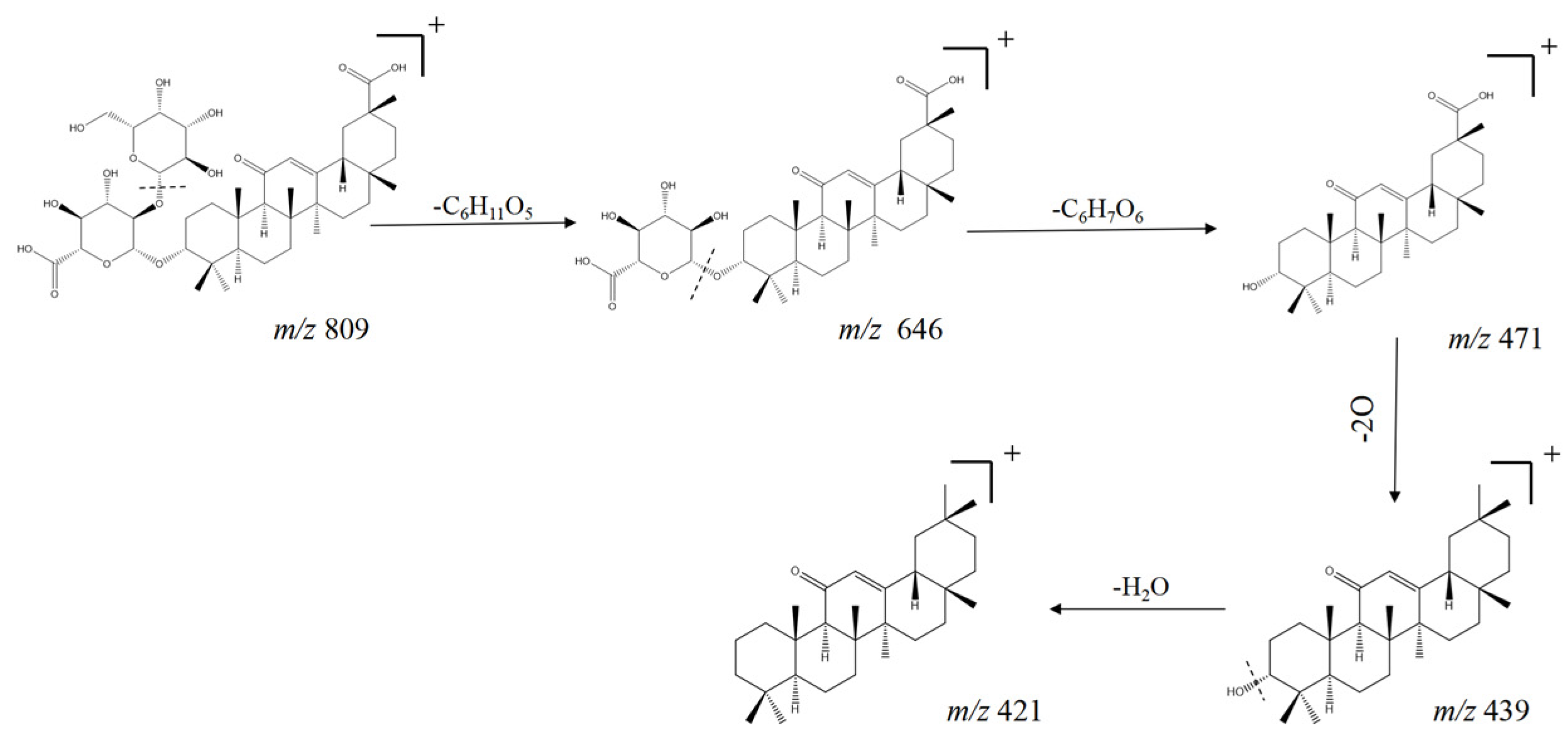
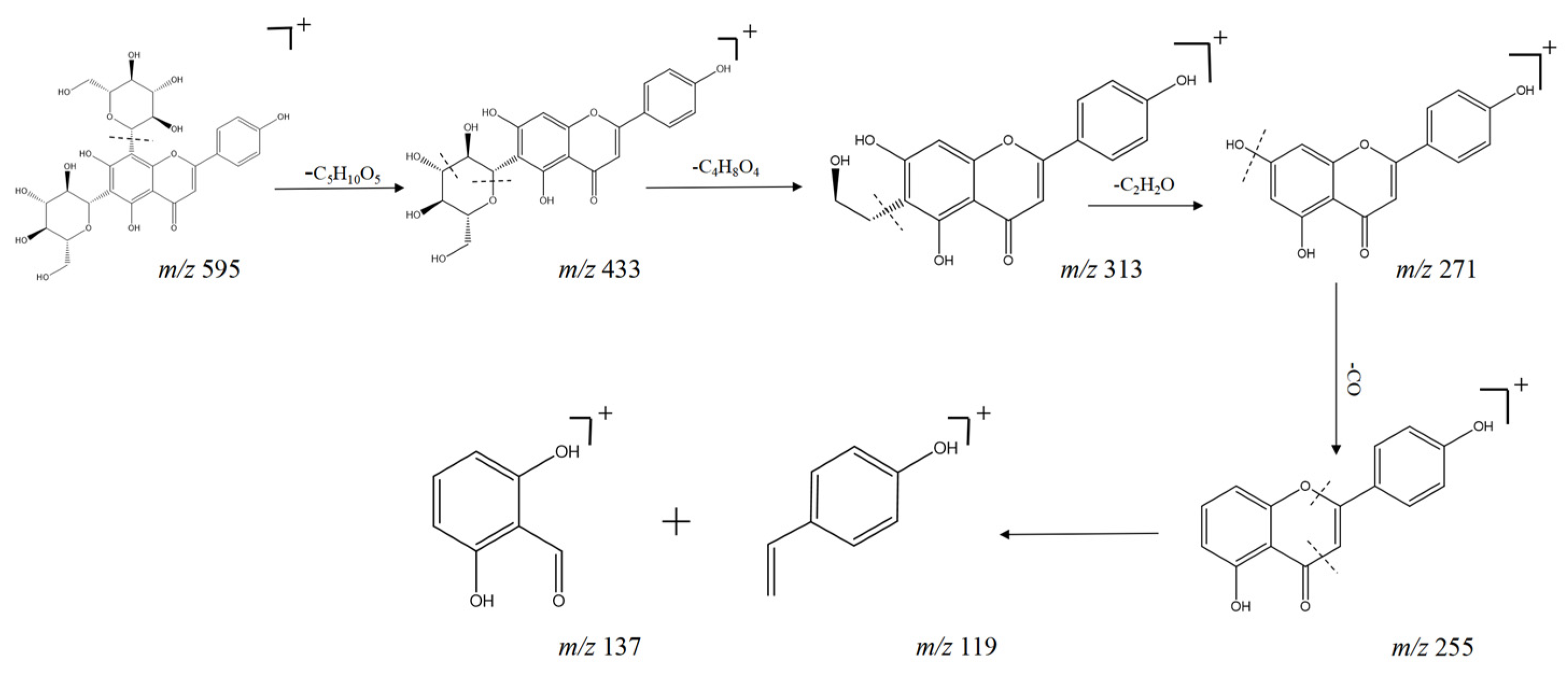
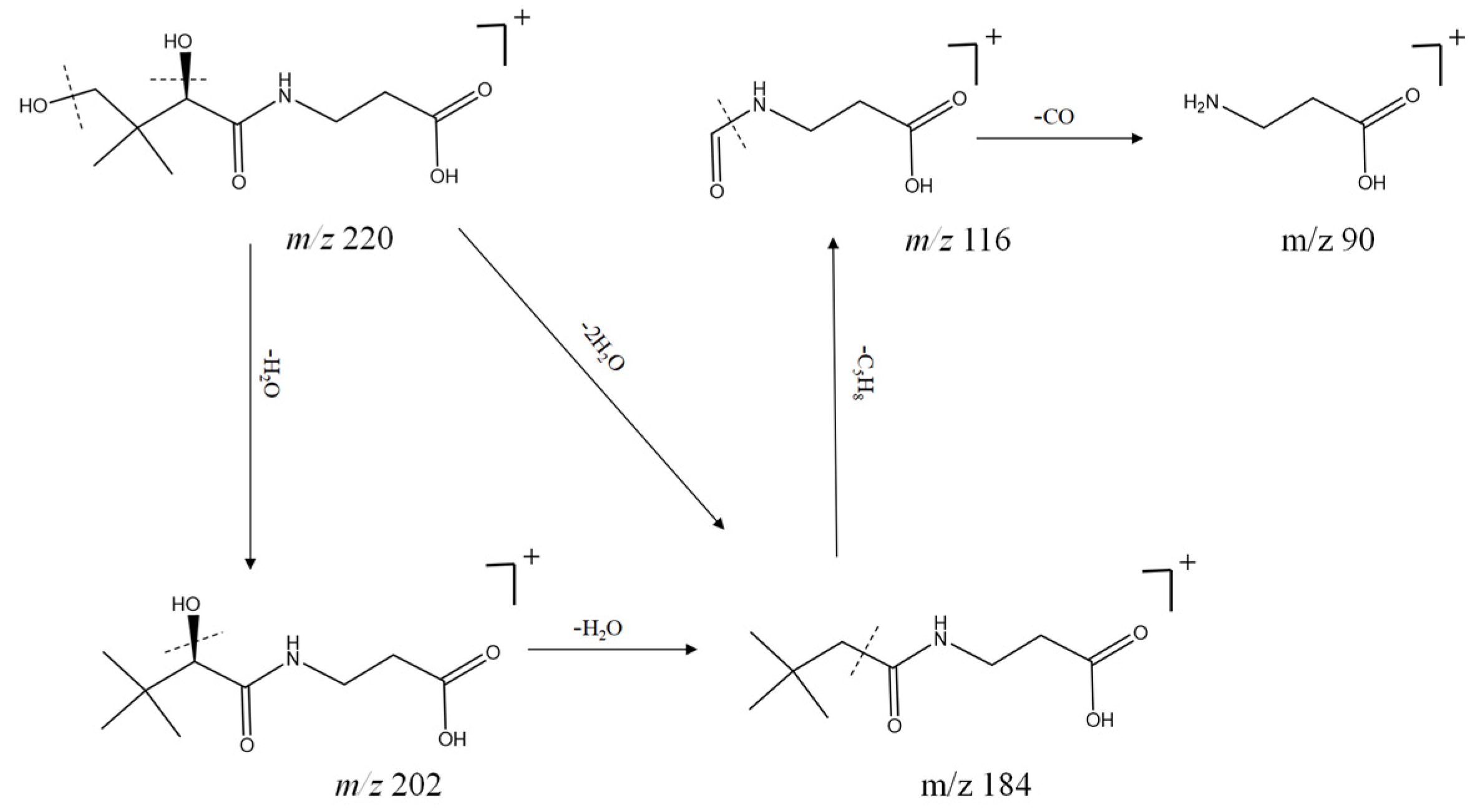
| No. | tR min | Molecular Formula | [M + H] + | Error (ppm) | MS2 | Compound | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | 0.78 | C6H14N4O2 | 175.1191 | 0.6 | 116.0728, 72.0807, 70.0722, 60.0600 | Arginine a | [12] |
| 2 | 0.87 | C11H12N2O2 | 205.0964 | −3.6 | 205.0964, 159.0712, 118.0545, 74.0251 | L-5-Hydroxytryptophan a | [13] |
| 3 | 0.89 | C14H10N2O4 | 271.0804 | 3.5 | 271.0806, 109.0291 | Siliendine A a | [14] |
| 4 | 0.89 | C5H9NO2 | 116.0705 | −0.8 | 116.0709, 70.0663 | Proline a | [12] |
| 5 | 0.89 | C29H44O5 | 473.3252 | −2 | 445.3173, 427.3191, 161.0903 | 3β-Acetoxy-(25R)-5α-spirostan-12-one a | [15] |
| 6 | 0.92 | C9H11NO3 | 182.0813 | 0.5 | 182.0817, 119.0497, 91.0569 | Tyrosine a | [12] |
| 7 | 1.15 | C9H6O2 | 147.0438 | −1.6 | 147.0438 | Coumarin a | [16] |
| 8 | 1.16 | C6H13NO2 | 132.1019 | −0.3 | 86.0959, 69.0699, 56.0490 | Isoleucine a | [12] |
| 9 | 1.32 | C12H12N2O2 | 217.0971 | −0.5 | 144.0826, 127.0541, 171.0928 | 1,2,3,4-Tetrahydro-1H-pyrido[3,4-b] indole-3-carboxylic Acid a | [13] |
| 10 | 1.43 | C9H17NO5 | 220.1179 | −0.4 | 205.1581, 90.0546, 72.0443 | Pantothenic acid b | [17] |
| 11 | 1.82 | C17H22N2O7 | 367.1502 | 0.5 | 332.1155, 229.0973, 188.0705, 146.0600 | N-(1-Deoxy-1-fructosyl) Tryptophan b | [17] |
| 12 | 2.01 | C11H9NO2 | 188.0706 | 0.1 | 146.0604, 118.0653, 91.0539 | Indoleacrylic acid b | [18] |
| 13 | 2.46 | C18H24O12 | 433.1337 | −0.8 | 145.0493, 127.0399, 85.0283 | Licoagroside B b | [19] |
| 14 | 3.06 | C45H72O20 | 933.4657 | −3.5 | 933.4667 | Macrostemonoside I b | [20] |
| 15 | 3.13 | C27H30O16 | 611.1591 | −2.6 | 611.0689, 383.0768, 329.0637, 299.0539 | Vincetoxicoside A a,b | [21] |
| 16 | 3.24 | C26H28O14 | 565.1547 | −0.8 | 427.1007, 271.0812, 349.0703, 433.0598 | Apiin b | [21] |
| 17 | 3.24 | C39H62O16 | 787.4101 | −1.2 | 625.3588, 463.3044, 427.2846, 301.1797 | Pregn-5-en-3β,20(S)-diol-3-O-bis-β-d-glucopyranosyl-(l-2,1-6)-β-D-glucopyranoside a, b | [13] |
| 18 | 3.27 | C33H54O13 | 659.3623 | −2.2 | 659.3643, 443.2792, 425.2693, 407.2540 | Tupistroside K b | [22] |
| 19 | 3.29 | C37H50O8 | 623.3534 | 2.6 | 623.3372, 301.3236 | Ecdysterone 2,3-Monoacetonide 22-O-Benzoate a, b | [15] |
| 20 | 3.59 | C18H18N3O5 | 357.1172 | −0.9 | 357.1169 | Siliendine C a | [14] |
| 21 | 3.61 | C39H64O17 | 805.4046 | −1.7 | 465.3208, 301.3105, 426.2977, 363.2268 | Sileneoside G a | [23] |
| 22 | 3.62 | C27H30O15 | 595.1654 | −0.5 | 433.1140, 283.0929, 313.0713, 271.0604 | Oroxin B b | [21] |
| 23 | 3.68 | C39H62O15 | 771.4153 | −1.2 | 593.3193, 411.2659, 162.2843 | Ophiopogonin R b | [24] |
| 24 | 3.68 | C39H64O16 | 789.4241 | −3.4 | 609.3670, 447.3123, 429.2993, 355.2274 | Tupistroside L a | [22] |
| 25 | 3.69 | C33H52O11 | 625.3575 | −1.2 | 463.1238, 367.0820, 343.0815, 313.0715, 301.1798 | Hydroxyecdysone-3-O-α-d-mannose—H2O a, b | [13] |
| 26 | 3.7 | C33H54O12 | 643.3683 | −0.8 | 481.3165, 445.2971, 371.2220, 165.1276, 127.2848 | Tupistroside J a, b | [13] |
| 27 | 3.7 | C33H50O10 | 607.3472 | −0.8 | 607.3473, 427.2853, 283.1798 | Caucasicoside A b | [10] |
| 28 | 3.71 | C19H26O5 | 335.1847 | −1.8 | 335.1847 | Dehydro-8-gingerdione b | [25] |
| 29 | 3.73 | C28H44O7 | 493.3152 | −1.4 | 457.2950, 173.1342, 311.2003 | Polyporoid B b | [13] |
| 30 | 3.75 | C27H44O9 | 513.3045 | −2.7 | 513.2899, 423.2436, 369.2074, 211.1110 | 26-Hydroxypolipodine B a | [26] |
| 31 | 4.01 | C27H44O7 | 481.3157 | −0.6 | 463.3076, 371.2248, 301.1801, 165.1292 | Hydroxyecdysone a, b | [13] |
| 32 | 4.01 | C27H42O6 | 463.3050 | −0.8 | 445.2979, 301.1840, 283.1702, 81.071 | 3-Dehydroecdysone a, b | [13] |
| 33 | 4.01 | C42H64O17 | 841.5618 | −1.4 | 841.3145, 501.1545, 163.0599 | Armeroside B a | [27] |
| 34 | 4.04 | C21H20O10 | 433.1131 | 0.4 | 431.0699, 313.0908, 271.0695, 283.0608 | Apigenin-7-O glucoside b | [21] |
| 35 | 4.05 | C27H44O8 | 497.3107 | −0.4 | 497.3115, 443.2790, 425.2714, 81.0699 | Abutasteronea | [13] |
| 36 | 4.05 | C34H52O9 | 605.3545 | −5.8 | 447.3715, 383.2166 | Ecdysteroid a | [28] |
| 37 | 4.06 | C34H48O7 | 569.3501 | 4.9 | 514.3484, 311.2843, 303.1743 | 2-Dehydroxyecdysterone-3-O-benzoate a | [29] |
| 38 | 4.11 | C22H22O11 | 463.1233 | −0.5 | 367.0800, 343.0796, 283.0243, 301.1794 | Chrysoeriol-7-O-glucoside b | [17] |
| 39 | 4.11 | C33H52O10 | 609.3626 | −1.2 | 447.3109, 429.3000, 411.2892 | 20,22-Trihydroxy-6-Oxocholesta-7,14-dien-3-yl beta-d-glucopyranoside a | [13] |
| 40 | 4.66 | C35H58O12 | 671.3982 | −2.9 | 671.3982, 507.3372, 313.1837 | Hydroxyecdysone-3-O-α-d-mannose + CH2CH3 a | [13] |
| 41 | 4.69 | C28H46O7 | 495.3306 | −2.2 | 459.3092, 357.2051, 237.1633, 131.0859 | Makisterone A a | [13] |
| 42 | 4.83 | C33H54O11 | 627.3732 | −1.1 | 465.3192, 445.3121, 429.3010, 411.2906 | Hydroxyecdysone-3-O-α-d-mannose—O a, b | [13] |
| 43 | 4.97 | C24H32O6 | 417.2267 | −1.1 | 417.2312, 335.2024, 187.1114, 163.0717 | Sidisterone a | [26] |
| 44 | 5.01 | C33H54O10 | 611.3670 | −2.3 | 413.3051, 593.2316, 287.2004 | 2-Deoxyecdysone 22β-d-glycoside a | [26] |
| 45 | 5.13 | C27H42O3 | 415.3202 | −1.1 | 415.3201, 397.3096, 271.2064, 285.1843 | Diosgenin b | [30] |
| 46 | 5.19 | C29H46O8 | 523.3262 | −0.7 | 445.2928, 427.2849, 165.1266, 110.7474 | Viticosterone E a | [26] |
| 47 | 5.2 | C27H40O7 | 477.2842 | −0.9 | 477.2765, 423.2492, 459.2448, 223.0979 | Lucidenic acid C b | [31] |
| 48 | 5.26 | C27H44O6 | 465.3201 | −2 | 429.3006, 411.2875, 233.1544, 159.0815 | Ponasterone A a | [13] |
| 49 | 5.57 | C29H48O7 | 509.3467 | −1.1 | 473.3248, 371.2201, 223.1479, 455.1563 | Makisterone C a, b | [32] |
| 50 | 5.74 | C56H88O26 | 1178.5041 | −1.9 | 805.3985, 499.3032, 453.3121, 163.0604, | Silenegallisaponin B a | [33] |
| 51 | 5.78 | C45H72O17 | 884.4549 | −3.8 | 749.4031, 426.3206, 327.3086, 145.1844 | Sileneoside B-diacetonide a, b | [34] |
| 52 | 5.83 | C33H49O9 | 590.3378 | 4.8 | 479.3005, 383.2894, 172.1786 | a-Ecdysone 2,3,25-Triacetate a, b | [35] |
| 53 | 5.99 | C41H62O16 | 811.4085 | −3.1 | 473.3268, 325.1130, 163.0600, 145.0495 | QUDA-GlcA-Ara/Xyl a, b | [13] |
| 54 | 6.04 | C54H86O26 | 1147.5151 | −1.4 | 517.3145, 469.1545, 163.0599 | Armeroside D a, b | [27] |
| 55 | 6.04 | C48H72O21 | 985.4619 | −2 | 679.3699, 517.3156, 215.1379 | Licorice saponin A3 a, b | [19] |
| 56 | 6.24 | C30H48O7 | 521.3457 | −3 | 457.3277, 207.0323, 187.1468, 189.1354, 143.0806 | Ecdysterone 20,22-monoacetonide a | [28] |
| 57 | 6.29 | C39H60O16 | 785.3945 | −1.2 | 447.3103, 429.2993, 411.2878, 393.2778 | Dracaenoside C b | [36] |
| 58 | 6.56 | C30H46O6 | 503.3360 | −1.5 | 485.3254, 467.3153, 301.1629, 187.1473 | 3β-16β-Dihydroxy-olean-12-ene-23,28-dioic acid a | [13] |
| 59 | 6.66 | C41H52O9 | 793.3977 | −3.6 | 426.3977, 311.3153, 301.1629, 187.1473 | Ecdysterone 22,25-Di-O-benzoate a, b | [37] |
| 60 | 7.48 | C48H76O21 | 989.4934 | −1.8 | 503.3363, 485.3266, 457.3309, 439.3201 163.0595 | Sinocrassuloside I a, b | [13] |
| 61 | 7.48 | C42H66O16 | 827.4408 | −1.9 | 629.3245, 503.3298, 439.3204, 163.1430 | Dianchinenoside D b | [38] |
| 62 | 7.48 | C15H24 | 205.1947 | −2.1 | 205.085 | α-Humulene a | [21] |
| 63 | 7.49 | C34H48O6 | 553.3253 | −1.8 | 445.3001, 427.2864, 313.2248, 165.1292 | Tomentesterone B a | [39] |
| 64 | 8.03 | C27H42O7 | 479.1813 | 0.5 | 479.3003, 317.1755, 443.2789 | 4-Dehydroecdysterone a | [13] |
| 65 | 8.19 | C39H60O14 | 753.4034 | −2.9 | 426.3202, 591.3079, 162.1846 | Kingianoside A b | [40] |
| 66 | 8.23 | C54H84O25 | 1133.5372 | −0.2 | 307.1028, 289.0912, 163.0595, 145.0486 | Tunicosaponin J a, b | [13] |
| 67 | 8.25 | C48H74O20 | 971.4828 | −1.8 | 439.3204, 307.1017, 163.0603, 145.0495 | QUDA-(Glc)-(Glc-Glc) a, b | [13] |
| 68 | 8.25 | C42H64O15 | 809.4302 | −2 | 457.3298, 439.3200, 163.0596, 145.0493 | QUDA-Glc-Glc a, b | [13] |
| 69 | 8.48 | C51H58O10N8 | 943.4332 | −1.8 | 943.4354, 332.1593, 302.1485, 261.1226 | Silenin C a, b | [3] |
| 70 | 9.14 | C36H50O9 | 627.3514 | −2.1 | 426.4773, 301.3539, 363.2021, 162.2333 | Viticosterone E 22-O-benzoate a, b | [35] |
| 71 | 9.68 | C69H100O30 | 1409.6319 | −3.8 | 485.3254, 955.4653, 775.2523 | Sinocrassuloside X a, b | [13] |
| 72 | 11.39 | C30H46O5 | 487.3408 | −2.1 | 451.3190, 201.1622, 187.1464 | Quillaic acid b | [41] |
| 73 | 11.94 | C48H74O21 | 987.5050 | −1.1 | 987.4961, 163.4233 | Ameroside F a, b | [27] |
| 74 | 12.28 | C17H15N3O5 | 342.0969 | −3.6 | 342.1383 | Siliendine D a | [14] |
| 75 | 12.66 | C18H26O12 | 435.1488 | −2.1 | 435.0381, 127.0282, 92.0333 | Canthoside C b | [42] |
| 76 | 15.91 | C48H74O22 | 1003.4725 | −1.9 | 1003.4725 | Armeroside C a | [27] |
| 77 | 16.44 | C48H66O8N8 | 883.5061 | −1.7 | 883.5079, 211.1427, 70.0646 | Silenin B a | [3] |
| 78 | 18.11 | C10H8O2 | 161.0596 | −0.7 | 133.0644, 120.0406, 92.0383 | 2-Methylchromone b | [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, S.; Shi, D.; Fei, Y.; Wu, C.; Zha, J.; Lu, F.; Zhang, Y.; Ji, J.; Liu, T.; Cheng, J. Comprehensive Evaluation of Quality and Differences in Silene viscidula Franch from Different Origins Based on UPLC-ZENO-Q-TOF-MS/MS Compounds Analysis and Antioxidant Capacity. Molecules 2024, 29, 4817. https://doi.org/10.3390/molecules29204817
Zhong S, Shi D, Fei Y, Wu C, Zha J, Lu F, Zhang Y, Ji J, Liu T, Cheng J. Comprehensive Evaluation of Quality and Differences in Silene viscidula Franch from Different Origins Based on UPLC-ZENO-Q-TOF-MS/MS Compounds Analysis and Antioxidant Capacity. Molecules. 2024; 29(20):4817. https://doi.org/10.3390/molecules29204817
Chicago/Turabian StyleZhong, Shaohui, Dezhi Shi, Yingxue Fei, Chengchao Wu, Jinyao Zha, Fangqi Lu, Yunyu Zhang, Jing Ji, Taoshi Liu, and Jianming Cheng. 2024. "Comprehensive Evaluation of Quality and Differences in Silene viscidula Franch from Different Origins Based on UPLC-ZENO-Q-TOF-MS/MS Compounds Analysis and Antioxidant Capacity" Molecules 29, no. 20: 4817. https://doi.org/10.3390/molecules29204817
APA StyleZhong, S., Shi, D., Fei, Y., Wu, C., Zha, J., Lu, F., Zhang, Y., Ji, J., Liu, T., & Cheng, J. (2024). Comprehensive Evaluation of Quality and Differences in Silene viscidula Franch from Different Origins Based on UPLC-ZENO-Q-TOF-MS/MS Compounds Analysis and Antioxidant Capacity. Molecules, 29(20), 4817. https://doi.org/10.3390/molecules29204817






