Synthesis of Alkyl/Aryloxymethyl Derivatives of 1,2,4-Triazole-3-Carboxamides and Their Biological Activities
Abstract
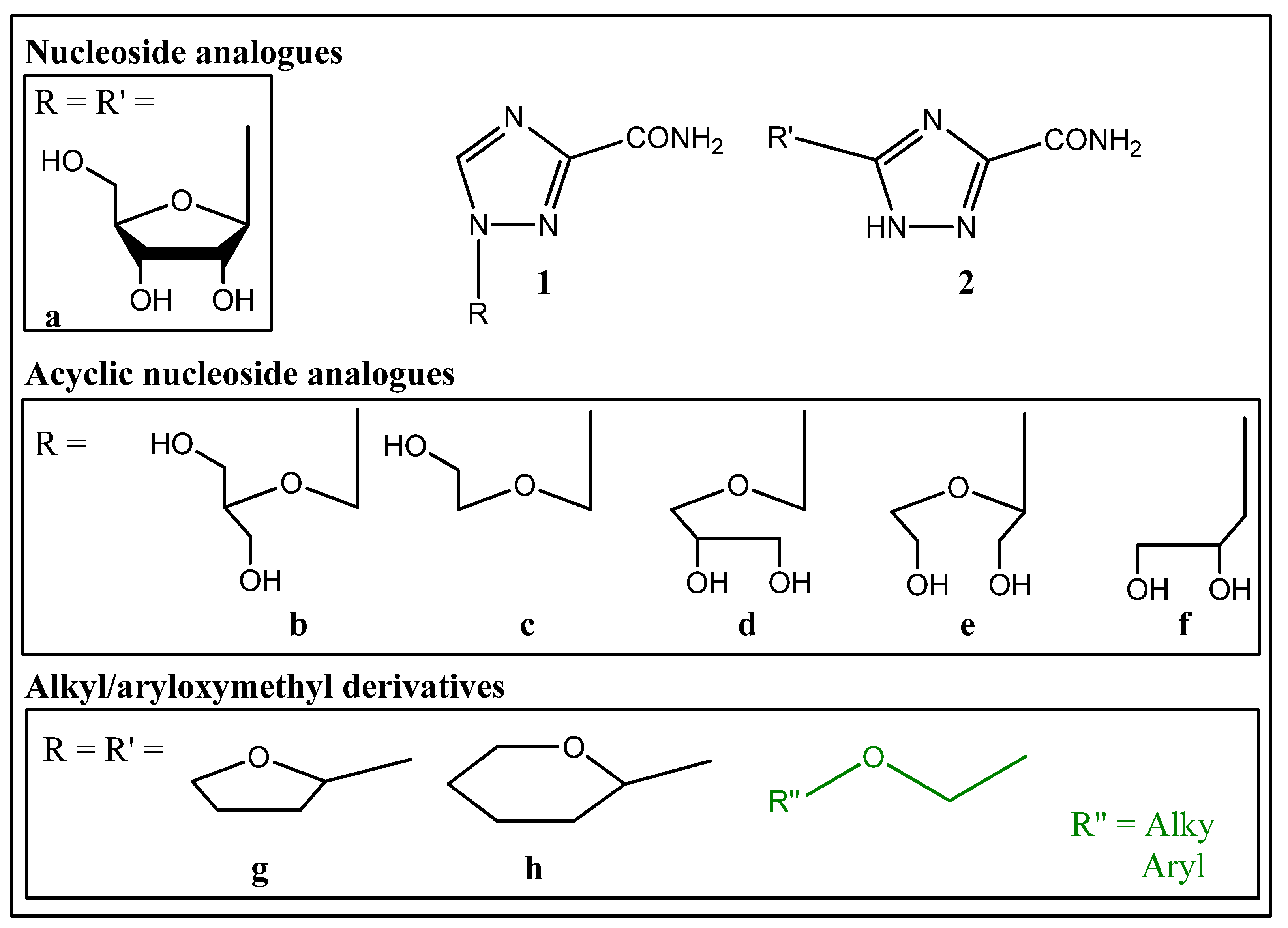
1. Introduction
2. Results and Discussions
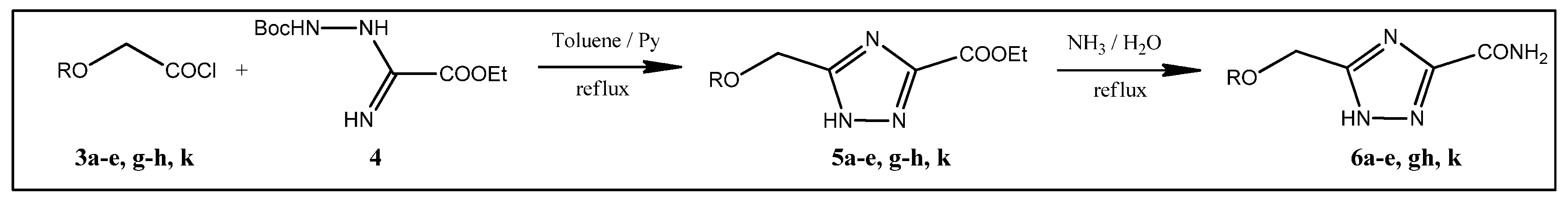
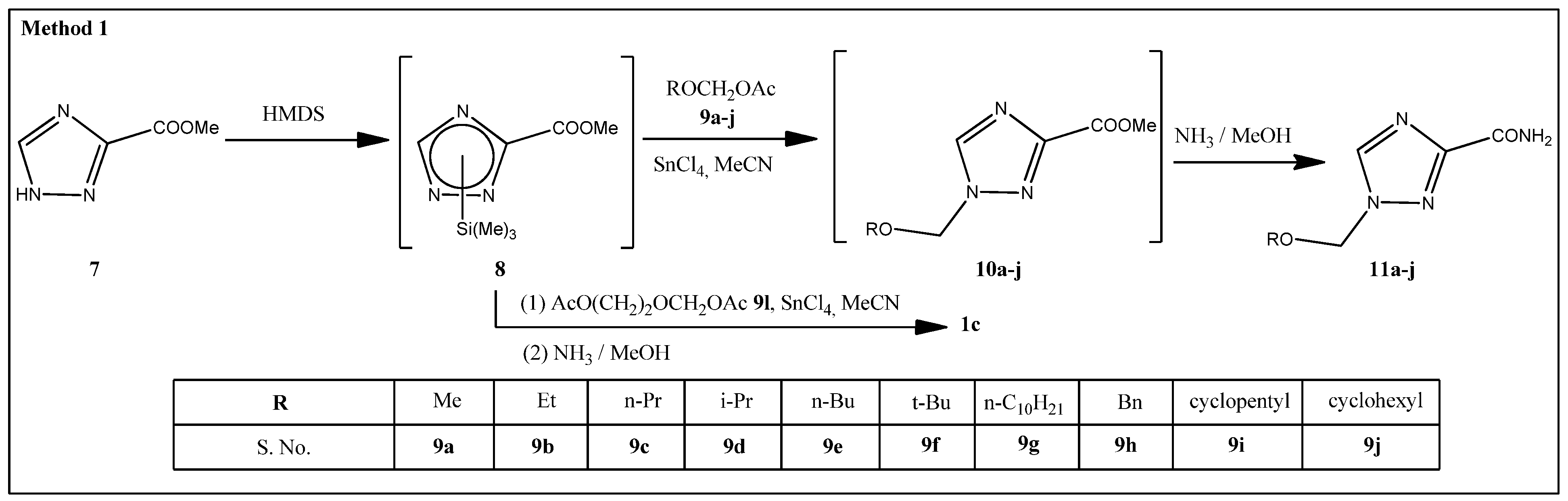
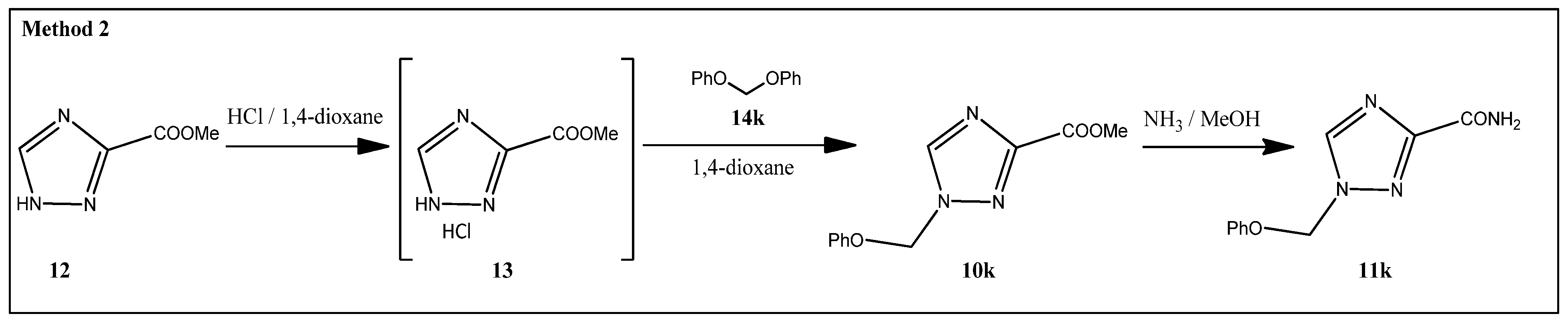
2.1. Synthesis
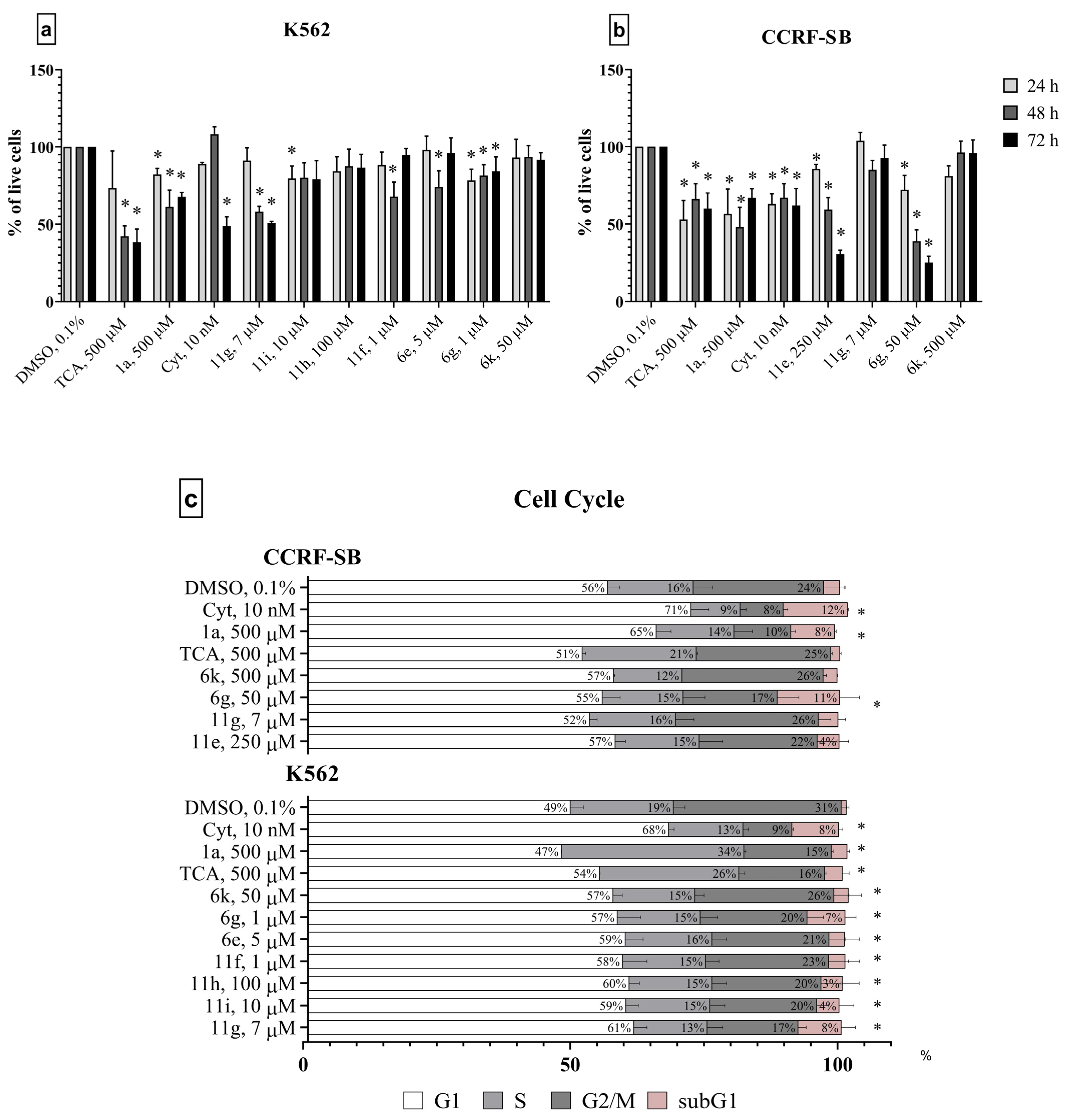
2.2. In Vitro Studies
2.2.1. Anti-Cancer Activity In Vitro
2.2.2. Antimicrobial Effect Studies
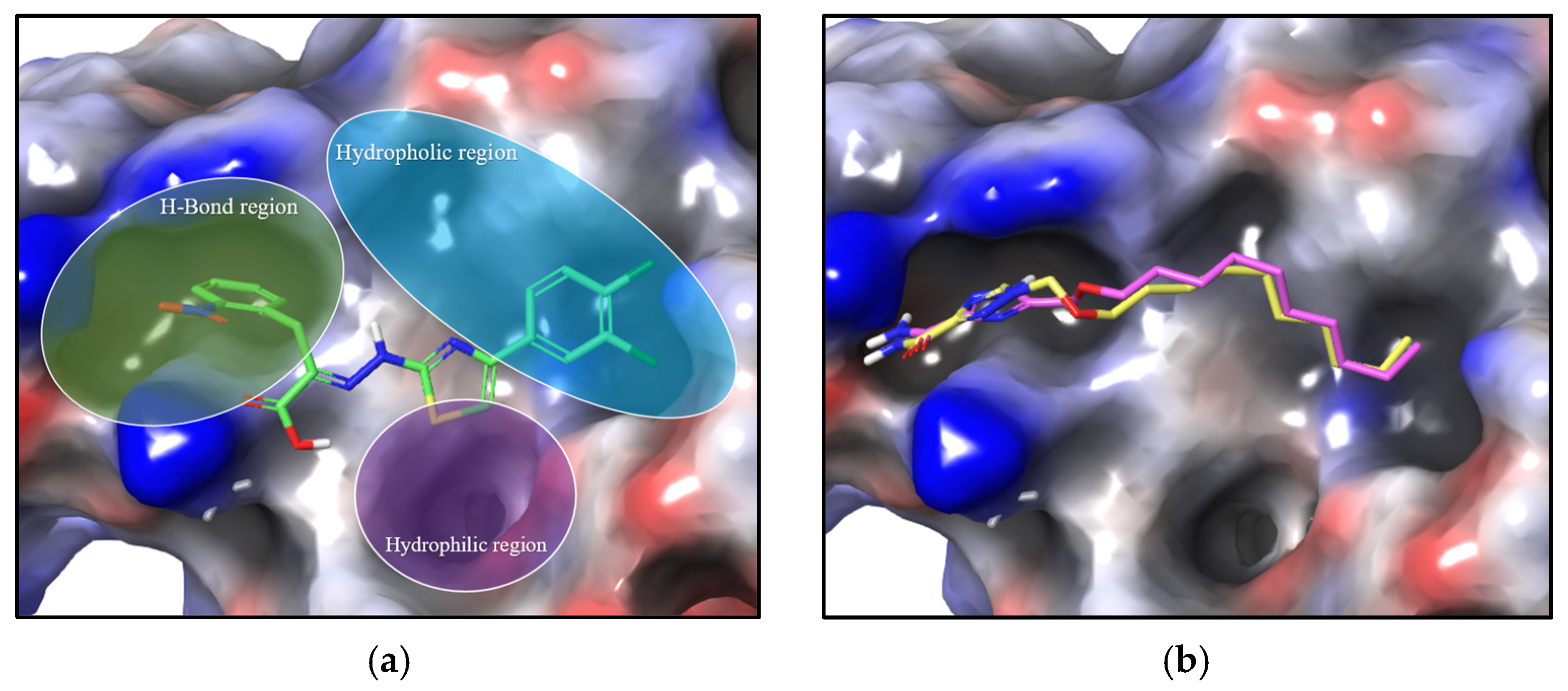
2.3. Molecular Docking
3. Materials and Methods
3.1. Synthetic Section
3.1.1. 5-(n-Propoxymethyl)-1,2,4-triazole-3-carboxamide 6c
3.1.2. Methyl 1-(Methoxymethyl)-1,2,4-triazole-3-carboxylate (10a)
3.1.3. 1-(Methoxymethyl)-1,2,4-triazole-3-carboxamide (11a)
3.1.4. General Procedure for the Preparation of 1-Substituted of 1,2,4-Triazole-3-carboxamides 11b–j, 1c
3.1.5. Methyl 1-(Phenoxymethyl)-1,2,4-triazole-3-carboxylate (10k)
3.1.6. 1-(Phenoxymethyl)-1,2,4-triazole-3-carboxamide (11k)
3.2. Antiproliferative Assays
3.2.1. Cell Cultures
3.2.2. MTT Assay
3.2.3. Cell Proliferation
3.2.4. Cell Cycle
3.2.5. Human Peripheral Blood Mononuclear Cell (PBMC) Isolation and Culture
3.3. Antimicrobial Assays
3.4. Statistical Analysis
3.5. In Silico Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chudinov, M.V. Ribavirin and its analogues: Can you teach an old dog new tricks? Fine Chem. Technol. 2019, 14, 7–23. [Google Scholar] [CrossRef]
- Gonzalez, S. Anti-HCV and Zika activities of ribavirin C-nucleosides analogues. Bioorg. Med. Chem. 2022, 68, 116850–116858. [Google Scholar] [CrossRef] [PubMed]
- Sardushkin, M.V.; Shiryaeva, Y.K.; Donskaya, L.; Vifor, R.; Donina, M.V. Colloid-Chemical and Antimicrobial Properties of Ribavirin Aqueous Solutions. Sys. Rev. Pharm. 2020, 11, 2050–2053. [Google Scholar]
- Assouline, S.; Culjkovic-Kraljacic, B.; Bergeron, J.; Caplan, S.; Cocolakis, E.; Lambert, C.; Borden, K.L.B. A phase I trial of ribavirin and low-dose cytarabine for the treatment of relapsed and refractory acute myeloid leukemia with elevated eIF4E. Haematologica 2014, 100, e7–e9. [Google Scholar] [CrossRef]
- Way, H.; Roh, J.; Venteicher, B.; Chandra, S.; Thomas, A.A. Synthesis of ribavirin 1,2,3-and 1,2,4-triazolyl analogs with changes at the amide and cytotoxicity in breast cancer cell lines. Nucleosides Nucleotides Nucleic Acids 2020, 42, 38–64. [Google Scholar] [CrossRef]
- Bózsity, N.; Minorics, R.; Szabó, J.; Mernyák, E.; Schneider, G.; Wölfling, J.; Wang, H.C.; Wuc, C.C.; Ocsovszki, I.; Zupkó, I. Mechanism of antiproliferative action of a new d-secoestrone-triazole derivative in cervical cancer cells and its effect on cancer cell motility. J. Steroid Biochem. Mol. Biol. 2017, 165, 247–257. [Google Scholar] [CrossRef]
- Szabó, J.; Bacsa, I.; Wölfling, J.; Schneider, G.; Zupkó, I.; Varga, M.; Hermab, B.E.; Kalmar, L.; Szecsi, M.; Mernyák, E. Synthesis and in vitro pharmacological evaluation of N-[(1-benzyl-1,2,3-triazol-4-yl)methyl]-carboxamides on d-secoestrone scaffolds. J. Enzym. Inhib. 2015, 31, 574–579. [Google Scholar] [CrossRef]
- Ilovaisky, A.I.; Scherbakov, A.M.; Chernoburova, E.A.; Povarov, A.A.; Shchetinina, M.A.; Merkulova, V.M.; Salnikova, D.I.; Sorokin, D.V.; Bozhenko, E.I.; Zavarzin, I.V.; et al. Secosteroid thiosemicarbazides and secosteroid–1,2,4-triazoles as antiproliferative agents targeting breast cancer cells: Synthesis and biological evaluation. J. Steroid Biochem. Mol. Biol. 2023, 234, e1–e12. [Google Scholar] [CrossRef]
- Zhidkova, E.; Stepanycheva, D.; Grebenkina, L.; Mikhina, E.; Maksimova, V.; Grigoreva, D.; Matveev, A.; Lesovaya, E. Synthetic 1,2,4-triazole-3-carboxamides Induce Cell Cycle Arrest and Apoptosis in Leukemia Cells. Curr. Pharm. Des. 2023, 29, 3478–3487. [Google Scholar]
- Wittine, K.; Stipković, B.M.; Makuc, D.; Plavec, J.; Kraljević, P.S.; Sedić, M.; Pavelić, K.; Leyssen, P.; Neyts, J.; Balzarini, J.; et al. Novel 1,2,4-triazole and imidazole derivatives of L-ascorbic and imino-ascorbic acid: Synthesis, anti-HCV and antitumor activity evaluations. Bioorg. Med. Chem. 2012, 20, 3675–3785. [Google Scholar] [CrossRef]
- Shen, G.Y.; Robins, R.K.; Revankar, G.R. Synthetic Studies on the Isomeric N-Methyl Derivatives of C-Ribavirin. Nucleosides Nucleotides 1991, 10, 1707–1717. [Google Scholar] [CrossRef]
- Wan, J.Q.; Xia, Y.; Liu, Y.; Wang, M.H.; Rocchi, P.; Yao, J.H.; Qu, F.Q.; Neyts, J.; Iovanna, J.L.; Peng, L. Discovery of novel arylethynyltriazole ribonucleosides with selective and effective antiviral and antiproliferative activity. J. Med. Chem. 2009, 52, e1144–e1155. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.Z.; Wang, M.H.; Xia, Y.; Qu, F.Q.; Neytsc, J.; Peng, L. Arylethynyltriazole acyclonucleosides inhibit hepatitis C virus replication. Bioorg. Med. Chem. Lett. 2008, 18, e3321–e3327. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Chunxian, L.; Lianjia, Z. Advance of structural modification of 13 nucleosides scaffold. Eur. J. Med. Chem. 2021, 214, e1–e22. [Google Scholar]
- Grebenkina, L.E.; Prutkov, A.N.; Matveev, A.V.; Chudinov, M.V. Synthesis of 5-oxymethyl-1,2,4-triazole-3-carboxamides. Fine Chem. Technol. 2022, 17, 311–322. [Google Scholar] [CrossRef]
- Tsilevich, T.L.; Shchaveleva, I.L.; Nosach, L.N.; Zhovnovataia, V.L.; Smirnov, I.P. Acyclic analogues of ribavirine. Synthesis and antiviral activity. Bioorg. Chem. 1988, 14, 689–693. [Google Scholar]
- Tsilevich, T.L.; Zavgorodniy, S.G.; Marks, U.; Ionova, L.V.; Florentev, V.L. Synthesis of ribavirin acyclic analogues. Bioorg. Chem. 1986, 12, 819–827. [Google Scholar]
- Hughes, W.B.; Kleene, R.D. Reaction of methylal with some acid anhydrides. Serv. Res. Dev. Co. 1954, 31, 5159–5161. [Google Scholar] [CrossRef]
- Thenet, K.; Beydoun, K.; Wiesenthal, J.; Leitner, W.; Klankermayer, J. Ruthenium-catalyzed synthesis of dialkoxymethane ethers utilizing carbon dioxide and molecular hydrogen. Angew. Chem. Int. Ed. 2016, 55, 12266–12269. [Google Scholar] [CrossRef]
- Matsumoto, H.; Kaneko, C.; Yamada, K.; Takeuchi, T.; Mori, T.; Mizuno, Y. A Convenient Synthesis of 9-(2-Hydroxyethoxymethyl)guanine (Acyclovir) and Related Compounds. Chem. Pharm. Bull. 1988, 36, 1153–1157. [Google Scholar] [CrossRef]
- Wenming, L.; Szewczy, J.; Liladhar, W. Practical synthesis of diaryloxymethanes. J. Synth. Org. Chem. Jpn. 2003, 33, 2719–2723. [Google Scholar]
- Cruz-Hernandez, E.; Medina-Franco, J.; Trujillo, J.; Chavez-Blanco, A.; Dominguez-Gomez, G.; Perez-Cardenas, E.; Gonzalez-Fierro, A.; Taja-Chayeb, L.; Dueïas-Gonzalez, A. Ribavirin as a tri-targeted antitumor repositioned drug. Oncol. Rep. 2015, 33, 2384–2392. [Google Scholar] [CrossRef] [PubMed]
- Hedstrom, L. IMP Dehydrogenase: Structure, mechanism, and inhibition. Chem. Rev. 2009, 109, 2903–2928. [Google Scholar] [CrossRef] [PubMed]
- Konno, Y.; Natsumeda, Y.; Nagai, M.; Yamaji, Y.; Ohno, S.; Suzuki, K.; Weber, G. Expression of human IMP dehydrogenase types I and II in Escherichia coli and distribution in human normal lymphocytes and leukemic cell lines. J. Biol. Chem. 1991, 266, 506–509. [Google Scholar] [CrossRef]
- Hongxia, T.; Li, H.; Zhenshun, C. Inhibition of eIF4E signaling by ribavirin selectively targets lung cancer and angiogenesis. Biochem. Biophys. Res. Commun. 2020, 529, 519–525. [Google Scholar]
- Nagai, M.; Natsumeda, Y.; Konno, Y.; Hoffman, R.; Irino, S.; Weber, G. Selective up-regulation of type II inosine 5′-monophosphate dehydrogenase messenger RNA ex-pression in human leukemias. Cancer Res. 1991, 51, 3886–3890. [Google Scholar]
- Urtishak, K.A.; Wang, L.S.; Culjkovic-Kraljacic, B.; Davenport, J.W.; Porazzi, P.; Vincent, T.L.; Teachey, D.T.; Tasian, S.K.; Moore, J.S.; Seif, A.E.; et al. Targeting EIF4E signaling with ribavirin in infant acute lymphoblastic leukemia. Oncogene 2019, 38, 2241–2262. [Google Scholar] [CrossRef]
- Volpin, F.; Casaos, J.; Sesen, J.; Mangraviti, A.; Choi, J.; Gorelick, N.; Frikeche, J.; Lott, T.; Felder, R.; Scotland, S.J.; et al. Use of an anti-viral drug, Ribavirin, as an anti-glioblastoma therapeutic. Oncogene 2017, 36, 3037–3047. [Google Scholar] [CrossRef]
- Kraljaci, B.C.; Arguello, M.; Amri, A.; Cormack, G.; Borden, K. Inhibition of eIF4E with ribavirin cooperates with common chemotherapies in primary acute myeloid leukemia specimens. Leukemia 2011, 25, 1197–1200. [Google Scholar] [CrossRef]
- Kentsis, A.; Volpon, L.; Topisirovic, I.; Soll, C.E.; Culjkovic, B.; Shao, L.; Borden, K.L. Further evidence that ribavirin interacts with eIF4E. RNA 2005, 11, 1762–1766. [Google Scholar] [CrossRef]
- Silvera, D.; Formenti, S.C.; Schneider, R.J. Translational control in cancer. Nat. Rev. Cancer. 2010, 10, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, E.; Jenni, S.; Kabha, E.; Wagner, G. Structure of the eukaryotic translation initiation factor eIF4E in complex with 4EGI-1 reveals an allosteric mechanism for dissociating eIF4G. Proc. Natl. Acad. Sci. USA 2014, 111, E3187–E3195. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Li, Y.; Yue, P.; Khuri, F.R.; Sun, S.Y. The eIF4E/eIF4G interaction inhibitor 4EGI-1 augments TRAIL-mediated apoptosis through c-FLIP down-regulation and DR5 induction independent of inhibition of cap-dependent protein translation. Neoplasia 2010, 12, 346–356. [Google Scholar] [CrossRef]
- Moerke, N.J.; Aktas, H.; Chen, H.; Cantel, S.; Reibarkh, M.Y.; Fahmy, A.; Gross, J.D.; Degterev, A.; Yuan, J.; Chorev, M.; et al. Small-molecule inhibition of the interaction between the translation initiation factors eIF4E and eIF4G. Cell 2007, 128, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Aktas, B.H.; Wang, Y.; He, X.; Sahoo, R.; Zhang, N.; Denoyelle, S.; Kabha, E.; Yang, H.; Freedman, R.Y.; et al. Tumor suppression by small molecule inhibitors of translation initiation. Oncotarget 2012, 3, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Tamburini, J.; Green, A.S.; Bardet, V.; Chapuis, N.; Park, S.; Willems, L.; Uzunov, M.; Ifrah, N.; Dreyfus, F.; Lacombe, C.; et al. Protein synthesis is resistant to rapamycin and constitutes a promising therapeutic target in acute myeloid leukemia. Blood 2009, 114, 1618–1627. [Google Scholar] [CrossRef]
- Descamps, G.; Gomez-Bougie, P.; Tamburini, J.; Green, A.; Bouscary, D.; Maiga, S.; Moreau, P.; Gouill, S.L.; Pellat-Deceunynck, C.; Amiot, M. The cap-translation inhibitor 4EGI-1 induces apoptosis in multiple myeloma through Noxa induction. Br. J. Cancer. 2012, 106, 1660–1667. [Google Scholar] [CrossRef]
- Fieser, L.; Fieser, M. Reagents for Organic Synthesis; John and Wiley and Sons: Hoboken, NJ, USA, 1967. [Google Scholar]
- Gottlieb, H.E.; Kotlyar, V.; Nudelman, A. NMR Chemical Shifts of Common Laboratory Solvents as Trace Impurities. J. Org. Chem. 1997, 62, 7512–7515. [Google Scholar] [CrossRef]


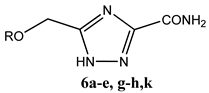 | ||
|---|---|---|
| S. No. | R | Yield |
| 6a | Me | 60% |
| 6b | Et | 53% |
| 6c | n-Pr | 62% |
| 6d | i-Pr | 24% |
| 6e | n-Bu | 33% |
| 6g | n-C10H21 | 43% |
| 6h | Bn | 68% |
| 6k | Ph | 76% |
 | ||
|---|---|---|
| S. No. | R | Yield |
| 11a | Me | 87% |
| 11b | Et | 78% |
| 11c | n-Pr | 78% |
| 11d | i-Pr | 91% |
| 11e | n-Bu | 81% |
| 11f | t-Bu | 49% |
| 11g | n-C10H21 | 78% |
| 11h | Bn | 89% |
| 11i | cyclopentyl | 82% |
| 11j | cyclohexyl | 53% |
| 11k | Ph | 52% |
| 1c | HO(CH2)2 | 83% |
| 5-Alkyl/aryloxymethyl-1,2,4-triazole-3-carboxamides | 1-Alkyl/aryloxymethyl-1,2,4-triazole-3-carboxamides | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CC50, µM | CC50, µM | ||||||||
| S. No. | K562 | CCRF-SB | S. No. | K562 | CCRF-SB | ||||
| 24 h | 72 h | 24 h | 72 h | 24 h | 72 h | 24 h | 72 h | ||
| 1a (ribavirin) | 270 ± 11 | 10 ± 1 | - | 188 ± 31 | |||||
| 2g | 260 ± 12 * | 240 ± 16 * | 1g | 240 ± 22 * | 230 ± 18 * | ||||
| 2h | 240 ± 21 * | 250 ± 13 * | 1h | 230 ± 13 * | 270 ± 25 * | ||||
| 6a | - | - | - | - | 11a | - | - | - | - |
| 6b | - | - | - | - | 11b | - | - | - | - |
| 6c | - | - | - | - | 11c | - | - | - | - |
| 6d | - | - | - | - | 11d | - | - | - | - |
| 6e | - | - | - | - | 11e | - | - | - | - |
| 11f | - | - | - | - | |||||
| 6g | 391 ± 15 | 43 ± 7 | 500 ± 100 | - | 11g | 14 ± 0 | 13 ± 3 | 112 ± 19 | 62 ± 2 |
| 6h | - | - | - | - | 11h | - | - | - | - |
| 11i | - | - | - | - | |||||
| 11j | - | - | - | - | |||||
| 6k | - | - | - | - | 11k | - | - | - | - |
| 1c | - | - | - | - | |||||
| Cyt | 59.4 ± 14.0 | 58.1 ± 16.9 | 15.8 ± 4.1 | 0.1 ± 0.1 | |||||
| S. No. | Zone of Growth Inhibition, mm | |||
|---|---|---|---|---|
| S. aureus | M. luteus | P. aeruginosa | C. albicans | |
| 1-Alkyl/aryloxymethyl-1,2,4-triazole-3-carboxamides | ||||
| 1a | - | - | 25 ± 1 | 30 ± 1 |
| 11a | - | - | - | - |
| 11b | - | - | - | - |
| 11c | - | - | 12 ± 1 | - |
| 11d | - | - | - | - |
| 11e | - | - | - | - |
| 11f | - | - | - | - |
| 11g | - | - | - | - |
| 11h | - | - | - | - |
| 11i | - | 12 ± 1 | - | - |
| 11j | - | 12 ± 1 | - | - |
| 11k | - | - | - | - |
| 1c | - | 12 ± 1 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikhina, E.A.; Stepanycheva, D.V.; Maksimova, V.P.; Sineva, O.N.; Markelova, N.N.; Grebenkina, L.E.; Lesovaya, E.A.; Yakubovskaya, M.G.; Matveev, A.V.; Zhidkova, E.M. Synthesis of Alkyl/Aryloxymethyl Derivatives of 1,2,4-Triazole-3-Carboxamides and Their Biological Activities. Molecules 2024, 29, 4808. https://doi.org/10.3390/molecules29204808
Mikhina EA, Stepanycheva DV, Maksimova VP, Sineva ON, Markelova NN, Grebenkina LE, Lesovaya EA, Yakubovskaya MG, Matveev AV, Zhidkova EM. Synthesis of Alkyl/Aryloxymethyl Derivatives of 1,2,4-Triazole-3-Carboxamides and Their Biological Activities. Molecules. 2024; 29(20):4808. https://doi.org/10.3390/molecules29204808
Chicago/Turabian StyleMikhina, Ekaterina A., Daria V. Stepanycheva, Varvara P. Maksimova, Olga N. Sineva, Natalia N. Markelova, Lyubov E. Grebenkina, Ekaterina A. Lesovaya, Marianna G. Yakubovskaya, Andrey V. Matveev, and Ekaterina M. Zhidkova. 2024. "Synthesis of Alkyl/Aryloxymethyl Derivatives of 1,2,4-Triazole-3-Carboxamides and Their Biological Activities" Molecules 29, no. 20: 4808. https://doi.org/10.3390/molecules29204808
APA StyleMikhina, E. A., Stepanycheva, D. V., Maksimova, V. P., Sineva, O. N., Markelova, N. N., Grebenkina, L. E., Lesovaya, E. A., Yakubovskaya, M. G., Matveev, A. V., & Zhidkova, E. M. (2024). Synthesis of Alkyl/Aryloxymethyl Derivatives of 1,2,4-Triazole-3-Carboxamides and Their Biological Activities. Molecules, 29(20), 4808. https://doi.org/10.3390/molecules29204808






