Rapid and Ultrasensitive Detection of H. aduncum via the RPA-CRISPR/Cas12a Platform
Abstract
1. Introduction
2. Results and Discussion
2.1. Nematode DNA Quality and RPA Primer Selection
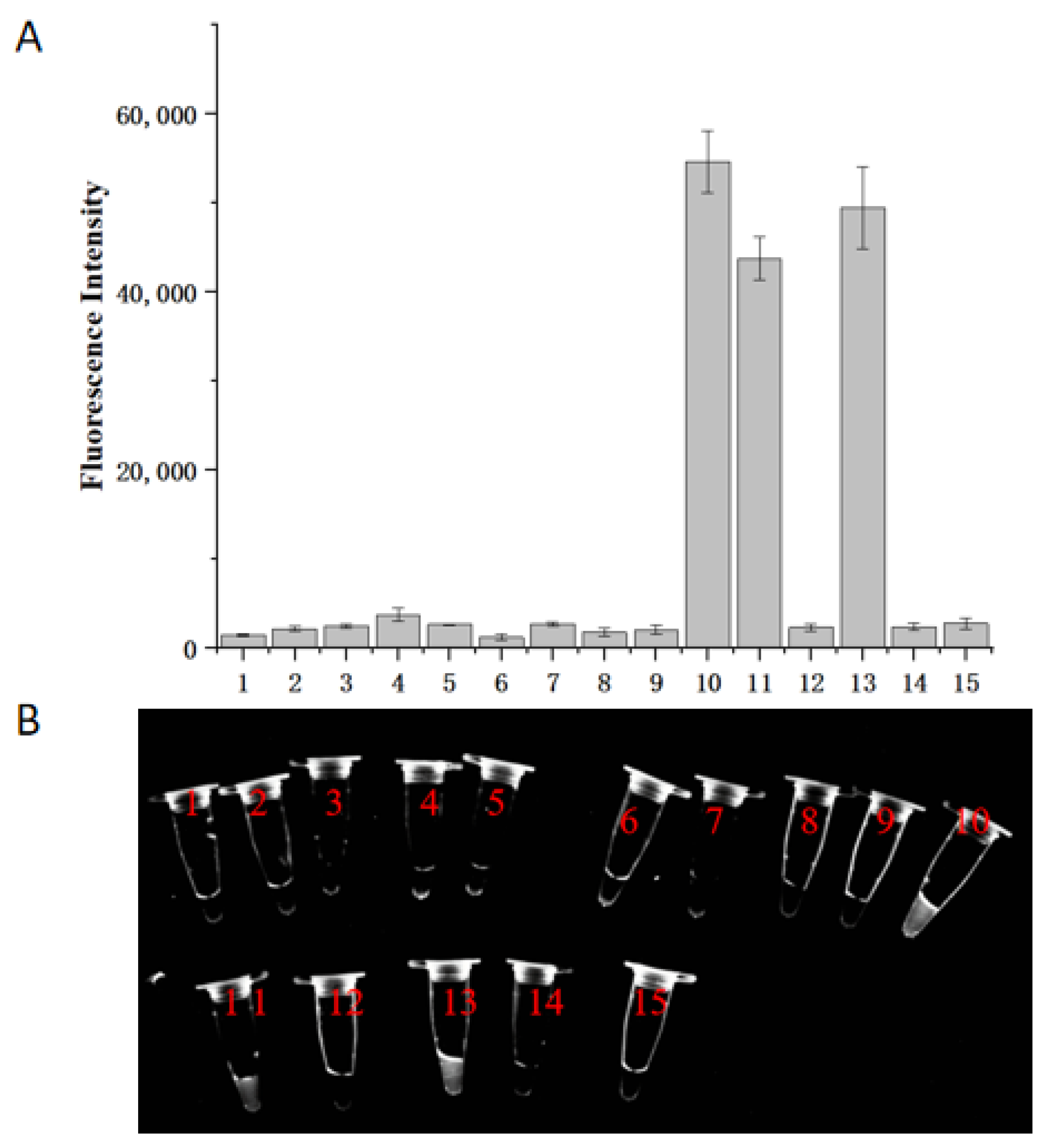
2.2. Specificity of the RPA-CRISPR/Cas12a Assay
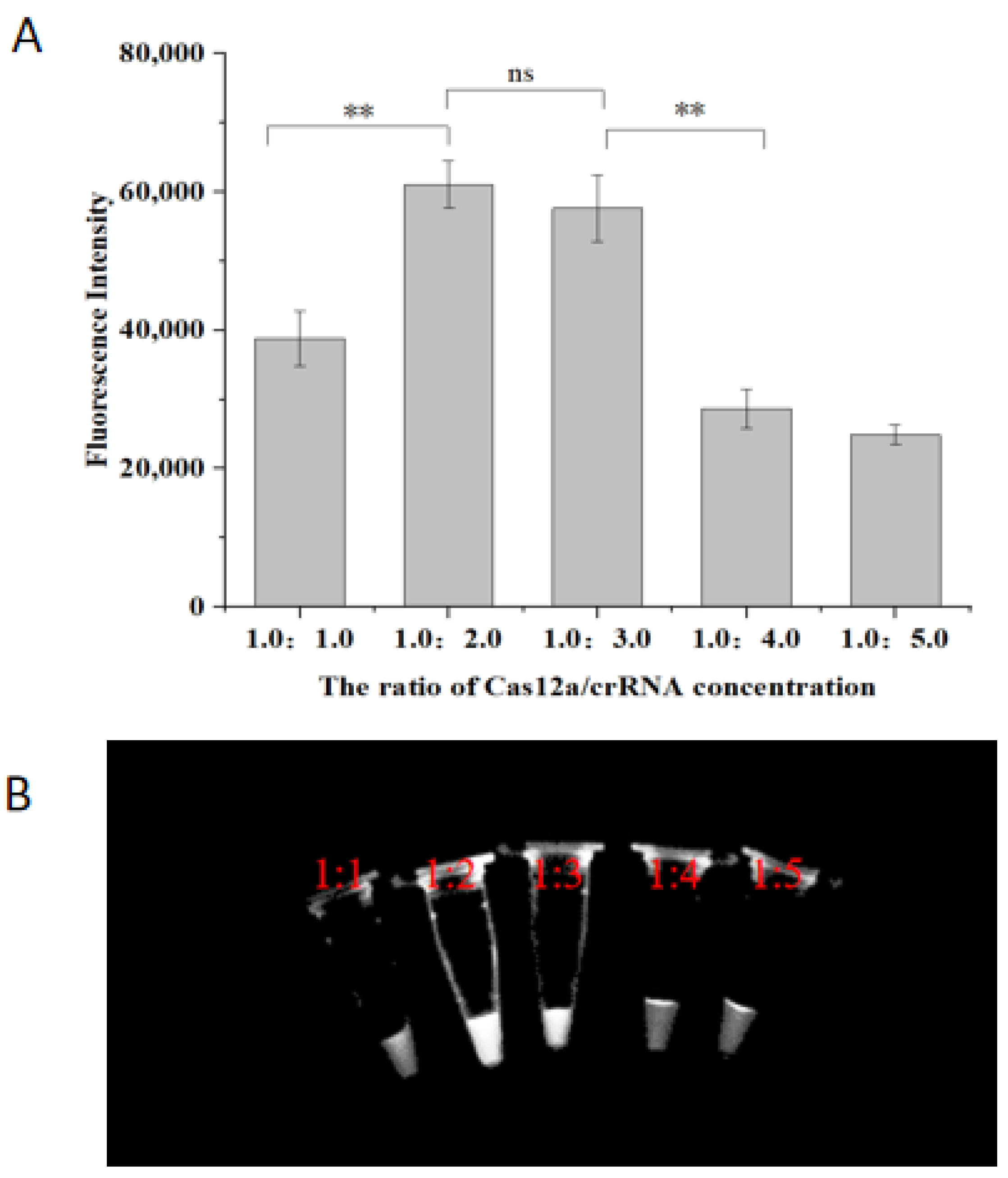
2.3. Optimization of Cas12a/crRNA Concentration Ratio
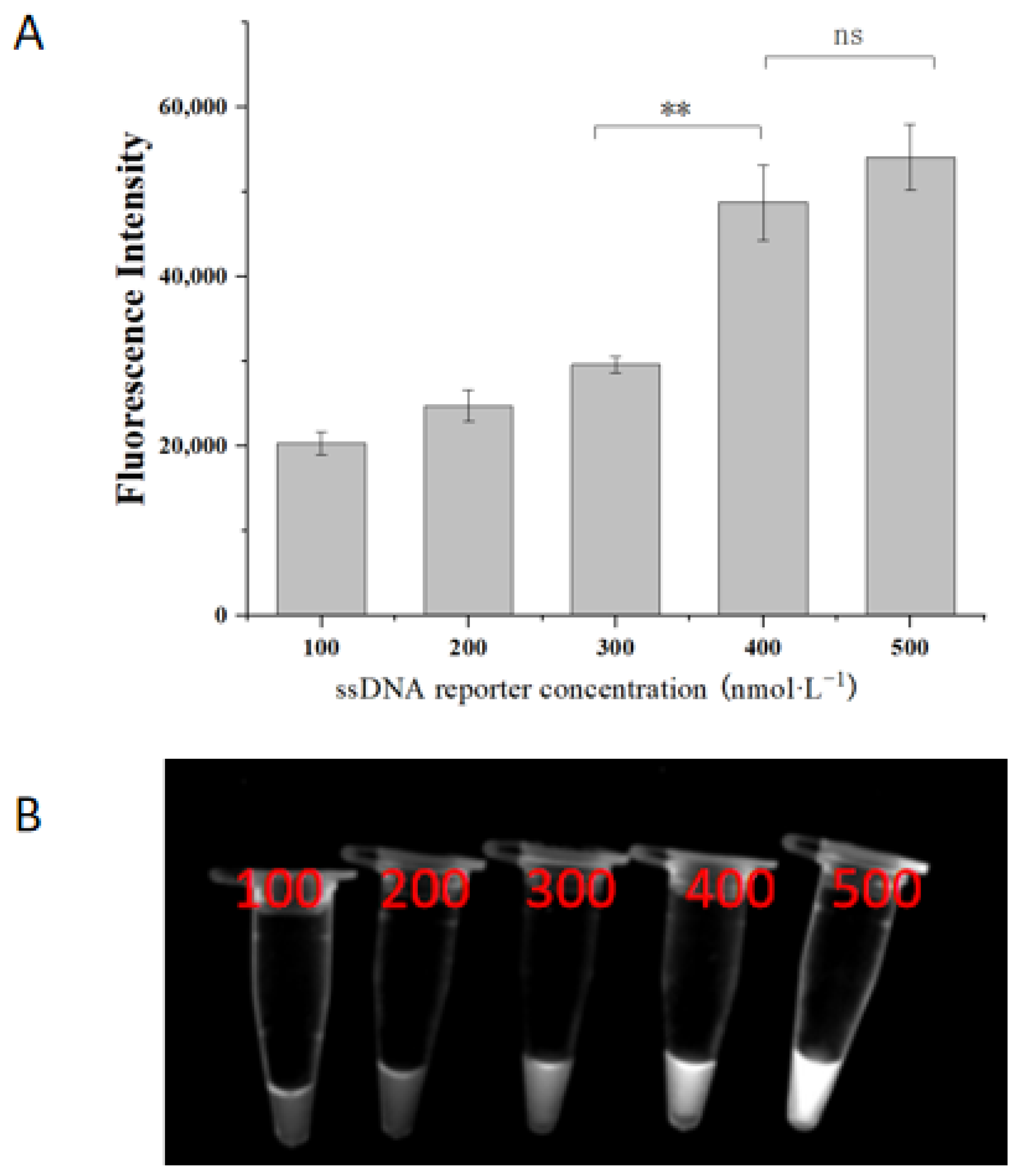
2.4. Optimization of ssDNA Concentration
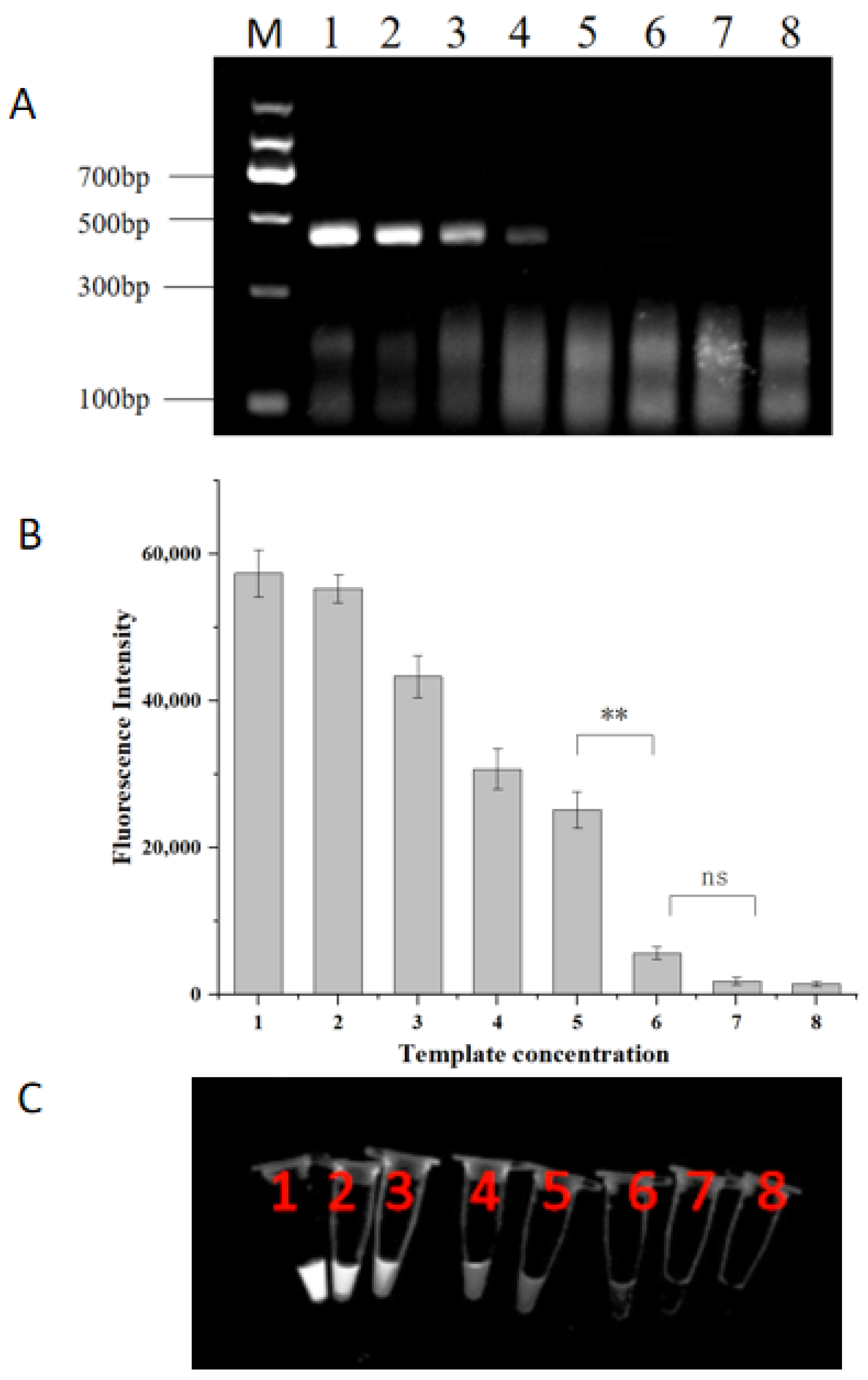
2.5. Sensitivity of the RPA-CRISPR/Cas12a
2.6. Commercial Sample Testing Using RPA-CRISPR/Cas12a
3. Materials and Methods
3.1. Sources of Experimental Samples
3.2. Main Reagents and Instruments
3.3. RPA Primer Design and Amplification Test
3.4. RPA-CRISPR/Cas12 Assay and Its Specificity
3.5. Optimization for the Concentration Ratio between Cas12a and CrRNA
3.6. Optimization of the ssDNA Concentration
3.7. Sensitivity for the RPA-CRISPR/Cas12a Assay
3.8. Detection of Commercial Samples Using the RPA-CRISPR/Cas12a Assay
3.9. Experimental Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, L.; Gibson, D.I.; Zhang, L. An annotated catalogue of the ascaridoid nematode parasites of Chinese vertebrates. Syst. Parasitol. 2016, 93, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xu, Z.; Zhang, L. Investigation on the Nematode of Hysterothylacium aduncum (Anisakidae) from Bohai Sea andYellow Sea in China. Chin. J. Parasitol. Parasit. Dis. 2007, 25, 364–367. [Google Scholar]
- Guo, Y.-N.; Xu, Z.; Zhang, L.-P.; Hu, Y.-H.; Li, L. Occurrence of Hysterothylacium and Anisakis nematodes (Ascaridida: Ascaridoidea) in the tanaka’s snailfish Liparis tanakae (Gilbert & Burke) (Scorpaeniformes: Liparidae). Parasitol. Res. 2014, 113, 1289–1300. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhao, J.-Y.; Chen, H.-X.; Ju, H.-D.; An, M.; Xu, Z.; Zhang, L.-P. Survey for the presence of ascaridoid larvae in the cinnamon flounder Pseudorhombus cinnamoneus (Temminck & Schlegel) (Pleuronectiformes: Paralichthyidae). Int. J. Food Microbiol. 2017, 241, 108–116. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, H.; Chen, X.; Wang, L.; Abulaizi, W.; Yang, Y.; Li, B.; Wang, C.; Bai, X. Agarose Hydrogel-Boosted One-Tube RPA-CRISPR/Cas12a Assay for Robust Point-of-Care Detection of Zoonotic Nematode. Anisakis J. Agric. Food Chem. 2024, 72, 8257–8268. [Google Scholar] [CrossRef]
- Sánchez-Alonso, I.; Rodríguez, S.; Tejada, M.; Navas, A.; González-Muñoz, M.; Careche, M. The artificial digestion method underestimates the viability of Anisakis simplex (s.l.) L3 present in processed fish products. Food Waterborne Parasitol. 2021, 23, e00121. [Google Scholar] [CrossRef]
- D’amelio, S.; Bellini, I.; Chiovoloni, C.; Magliocco, C.; Pronio, A.; Di Rocco, A.; Pentassuglio, I.; Rosati, M.; Russo, G.; Cavallero, S. A Case of Gastroallergic and Intestinal Anisakiasis in Italy: Diagnosis Based on Double Endoscopy and Molecular Identification. Pathogens 2023, 12, 1172. [Google Scholar] [CrossRef]
- Abattouy, N.; López, A.V.; Maldonado, J.L.; Benajiba, M.; Martín-Sánchez, J. Epidemiology and molecular identification of Anisakis pegreffii (Nematoda: Anisakidae) in the horse mackerel Trachurus trachurus from northern Morocco. J. Helminthol. 2014, 88, 257–263. [Google Scholar] [CrossRef]
- Wang, X.; Xu, T.; Ding, S.; Xu, Y.; Jin, X.; Guan, F. Recombinase polymerase amplification combined with lateral flow dipstick assay for rapid visual detection of A. simplex (s. s.) and A. pegreffii in sea foods. Heliyon 2024, 10, e28943. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.X.; Zhao, Y.G.; Li, L.; Wu, X.D.; Wang, Z.L. Research Progress of Recombinant Enzyme Polymerase Amplification (RPA) in Rapid Detection of Diseases. China Anim. Health Insp. 2016, 33, 72–77. Available online: http://en.cnki.com.cn/Article_en/CJFDTOTAL-ZGDW201608023.htm (accessed on 6 August 2024).
- Ren, Y.; Du, B.; Cai, N.; Xiu, M.; Liu, W. Investigation and molecular identification of Anisakis infection in marine fish sold in Fuxin, Liaoning Province. China Trop. Med. 2023, 23, 489–494. [Google Scholar] [CrossRef]
- Huang, S. Rapid Detection of Anisakid Nematodes in Seafood by Multiplex Real-time Fluorescence PCR. J. Guangdong Ocean. Univ. 2022, 42, 122–129. [Google Scholar]
- Qiao, Y.; Zhou, Q.; Li, X. Rapid detection of Hysterothylacium aduncum by loop-mediated isothermal amplificationcombined with lateral flow dipstick. Chin. J. Prev. Vet. Med. 2017, 39, 6. Available online: https://xueshu.baidu.com/usercenter/paper/show?paperid=1w0608g08d0y0af0pr05082006784105&site=xueshu_se&hitarticle=1 (accessed on 6 August 2024).
- Castellanos-Gonzalez, A.; White, A.; Melby, P.; Travi, B. Molecular diagnosis of protozoan parasites by Recombinase Polymerase Amplification. Acta Trop. 2018, 182, 4–11. [Google Scholar] [CrossRef]
- Hoff, M. DNA amplification and detection made simple (relatively). PLoS Biol. 2006, 4, e222. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, A.; Fu, M.; Guo, J.; Wang, L.; Zuo, X.; Ma, F. Establishment and Clinical Application of a RPA-LFS Assay for Detection of Capsulated and Non-Capsulated Haemophilus influenzae. Front. Cell Infect. Microbiol. 2022, 12, 878813. [Google Scholar] [CrossRef]
- Wang, F.; Ge, D.; Wang, L.; Li, N.; Chen, H.; Zhang, Z.; Zhu, W.; Wang, S.; Liang, W. Rapid and sensitive recombinase polymerase amplification combined with lateral flow strips for detecting Candida albicans. Anal. Biochem. 2021, 633, 114428. [Google Scholar] [CrossRef]
- Gong, L.; Wang, X.; Li, Z.; Huang, G.; Zhang, W.; Nie, J.; Wu, C.; Liu, D. Integrated Trinity Test with RPA-CRISPR/Cas12a-Fluorescence for Real-Time Detection of Respiratory Syncytial Virus A or B. Front. Microbiol. 2022, 13, 819931. [Google Scholar] [CrossRef] [PubMed]
- Onchan, W.; Ritbamrung, O.; Changtor, P.; Pradit, W.; Chomdej, S.; Nganvongpanit, K.; Siengdee, P.; Suyasunanont, U.; Buddhachat, K. Sensitive and rapid detection of Babesia species in dogs by recombinase polymerase amplification with lateral flow dipstick (RPA-LFD). Sci. Rep. 2022, 12, 20560. [Google Scholar] [CrossRef]
- Wang, P.; Guo, B.; Zhang, X.; Wang, Y.; Yang, G.; Shen, H.; Gao, S.; Zhang, L. One-Pot Molecular Diagnosis of Acute Hepatopancreatic Necrosis Disease by Recombinase Polymerase Amplification and CRISPR/Cas12a with Specially Designed crRNA. J. Agric. Food Chem. 2023, 71, 6490–6498. [Google Scholar] [CrossRef]
- Dai, Y.; Sun, J.; Zhang, M.; Liu, Y.; Chen, H.; Lu, Y. Principles and applications of trans-cleavage CRlSPR/Cas technology for the detection of foodborne pathogenic microorganisms. Food Sci. 2024, 1–16. Available online: https://link.cnki.net/urlid/11.2206.TS.20240226.1741.002 (accessed on 6 August 2024).
- Hu, J.; Liu, D.; Cai, M.; Zhou, Y.; Yin, W.; Luo, C. One-Pot Assay for Rapid Detection of Benzimidazole Resistance in Venturia carpophila by Combining RPA and CRISPR/Cas12a. J. Agric. Food Chem. 2023, 71, 1381–1390. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, L.; Chen, X.; Yang, Y.; Wu, F.; Liu, X.; Wang, Y.; Bai, X. RPA-CRISPR/Cas12a-based visual and rapid detection of Anisakis. Chin. Vet. Sci. 2024, 56, 735–741. [Google Scholar] [CrossRef]
- Vermeil, C.; Petter, A.; Morin, O.; Le Bodic, M.F.; Daniel, C.; Guegan, J.; Kerneis, J.P. Do the eosinophilic granulomas observed in Brittany represent a form of anisakiasis? The larvae of Thynnascaris aduncum do not produce these granulomas experimentally. Bull. Soc. Pathol. Exot. Fil. 1975, 68, 79–83. [Google Scholar]
- González-Amores, Y.; Clavijo-Frutos, E.; Salas-Casanova, C.; Alcain-Martínez, G. Direct parasitologial diagnosis of infection with Hysterothylacium aduncum in a patient with epigastralgia. Rev. Esp. Enferm. Dig. 2015, 107, 699–700. [Google Scholar]
- Shamsi, S.; Barton, D. A critical review of anisakidosis cases occurring globally. Parasitol. Res. 2023, 122, 1733–1745. [Google Scholar] [CrossRef]
- Cammilleri, G.; Ferrantelli, V.; Pulvirenti, A.; Drago, C.; Stampone, G.; Macias, G.D.R.Q.; Drago, S.; Arcoleo, G.; Costa, A.; Geraci, F.; et al. Validation of a Commercial Loop-Mediated Isothermal Amplification (LAMP) Assay for the Rapid Detection of Anisakis spp. DNA in Processed Fish Products. Foods 2020, 9, 92. [Google Scholar] [CrossRef]
- Herrero, B.; Vieites, J.M.; Espiñeira, M. Detection of anisakids in fish and seafood products by real-time PCR. Food Control 2011, 22, 933–939. [Google Scholar] [CrossRef]
- Cho, J.; Lim, H.; Jung, B.-K.; Shin, E.-H.; Chai, J.-Y. Anisakis pegreffii Larvae in Sea Eels (Astroconger myriaster) from the South Sea, Republic of Korea. Korean J. Parasitol. 2015, 53, 349–353. [Google Scholar] [CrossRef]
- Lakemeyer, J.; Siebert, U.; Abdulmawjood, A.; Ryeng, K.A.; Ijsseldijk, L.L.; Lehnert, K. Anisakid nematode species identification in harbour porpoises (Phocoena phocoena) from the North Sea, Baltic Sea and North Atlantic using RFLP analysis. Int. J. Parasitol. Parasites Wildl. 2020, 12, 93–98. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, H.; Liu, J.; Ni, F.; Xu, S.; Luo, D. Detection of anisakid nematodes by an SYBR green I real-time PCR. Chin. J. Parasitol. Parasit. Dis. 2010, 28, 194–199. [Google Scholar]
- Yao, K.; Peng, D.; Jiang, C.; Zhao, W.; Li, G.; Huang, W.; Kong, L.; Gao, H.; Zheng, J.; Peng, H. Rapid and Visual Detection of Heterodera schachtii Using Recombinase Polymerase Amplification Combined with Cas12a-Mediated Technology. Int. J. Mol. Sci. 2021, 22, 12577. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jin, W.; Yang, Y.; Ma, H.; Liu, H.; Lei, J.; Wu, Y.; Zhang, L. CRISPR/Cas12a-mediated Enzymatic recombinase amplification for rapid visual quantitative authentication of halal food. Anal. Chim. Acta 2023, 1255, 341144. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhao, L.; Wang, J.; Wang, H.; Qiu, Y.; Dong, Z.; Zhang, C.; Liu, M.; Wang, X.; Bai, X. Rapid visual detection of anisakid nematodes using recombinase polymerase amplification and SYBR Green I. Front. Microbiol. 2022, 13, 1026129. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, M.; Mattiucci, S.; Colantoni, A.; Levsen, A.; Gay, M.; Nascetti, G. Species-specific Real Time-PCR primers/probe systems to identify fish parasites of the genera Anisakis, Pseudoterranova and Hysterothylacium (Nematoda: Ascaridoidea). Fish. Res. 2018, 202, 38–48. [Google Scholar] [CrossRef]
- Godínez-González, C.; Roca-Geronès, X.; Cancino-Faure, B.; Montoliu, I.; Fisa, R. Quantitative SYBR Green qPCR technique for the detection of the nematode parasite Anisakis in commercial fish-derived food Int. J. Food Microbiol. 2017, 261, 89–94. [Google Scholar] [CrossRef]



| Primer/Probe | Sequence (5′-3′) | Product Size (bp) | |
|---|---|---|---|
| ADU 1 | ADU-F1 | GTTGTGAAGGTACTTCCTCGTGTTGTGCCTTTG | 470 bp |
| ADU-R1 | CACGTGCTCGGAGCCGTATCTATGGTTAAG | ||
| ADU 2 | ADU-F1 | GTTGTGAAGGTACTTCCTCGTGTTGTGCCTTTG | 420 bp |
| ADU-R2 | GACACTTGACGGCCATCATCGCCTCGCATGGAG | ||
| ADU 3 | ADU-F1 | GTTGTGAAGGTACTTCCTCGTGTTGTGCCTTTG | 500 bp |
| ADU-R3 | CTAGCATCACCCTCTCTTCCATTTCCAGCA | ||
| crRNA | UAAUUUCUAAGUGUAGAUAAGGAGAGGUCA CUUCAUGUGCUC | ||
| ssDNA | (FAM)-TTATT-(BHQ-1) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Chen, X.; Xu, T.; Jin, X.; Jiang, J.; Guan, F. Rapid and Ultrasensitive Detection of H. aduncum via the RPA-CRISPR/Cas12a Platform. Molecules 2024, 29, 4789. https://doi.org/10.3390/molecules29204789
Wang X, Chen X, Xu T, Jin X, Jiang J, Guan F. Rapid and Ultrasensitive Detection of H. aduncum via the RPA-CRISPR/Cas12a Platform. Molecules. 2024; 29(20):4789. https://doi.org/10.3390/molecules29204789
Chicago/Turabian StyleWang, Xiaoming, Xiang Chen, Ting Xu, Xingsheng Jin, Junfang Jiang, and Feng Guan. 2024. "Rapid and Ultrasensitive Detection of H. aduncum via the RPA-CRISPR/Cas12a Platform" Molecules 29, no. 20: 4789. https://doi.org/10.3390/molecules29204789
APA StyleWang, X., Chen, X., Xu, T., Jin, X., Jiang, J., & Guan, F. (2024). Rapid and Ultrasensitive Detection of H. aduncum via the RPA-CRISPR/Cas12a Platform. Molecules, 29(20), 4789. https://doi.org/10.3390/molecules29204789






