Hydrodynamic Cavitation as a Method of Removing Surfactants from Real Carwash Wastewater
Abstract
1. Introduction
2. Results and Discussion
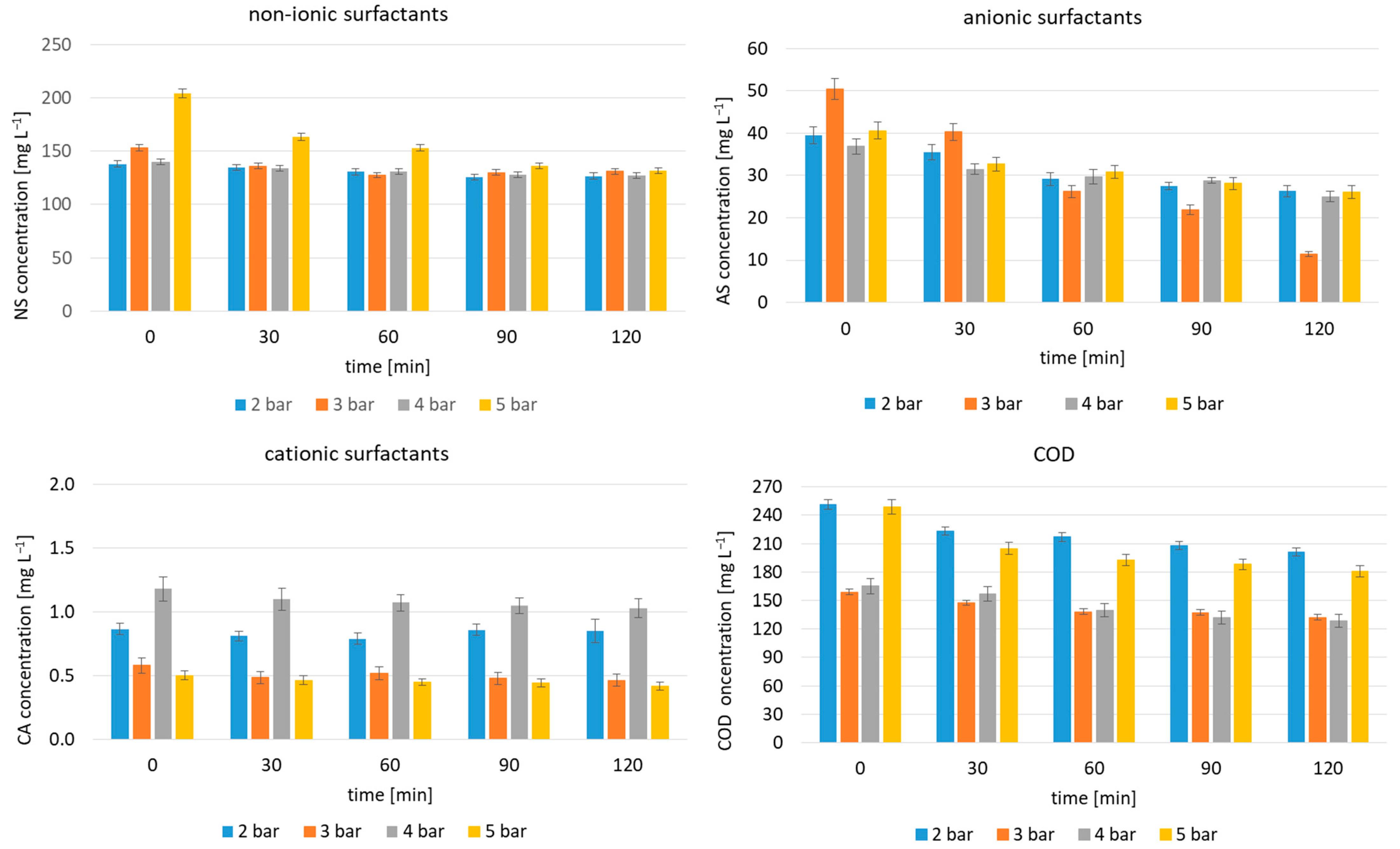
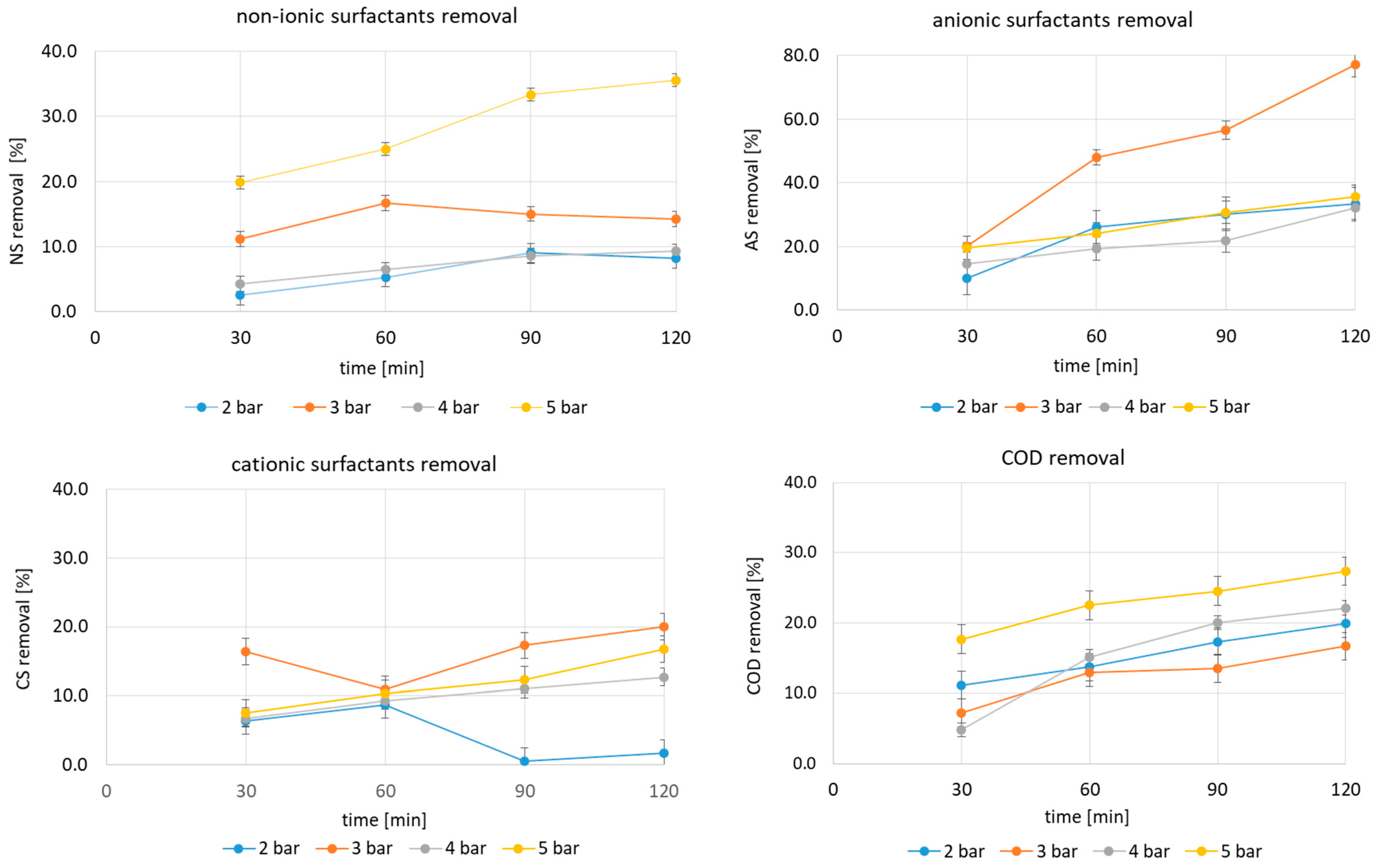
2.1. HC for Surfactant Removal from Carwash Wastewater
2.2. Multi-Criteria Decision Support of HC Process
2.3. Physicochemical Analysis of the Wastewater under Optimal HC Conditions
2.4. FT-IR/ATR Analysis under Optimal HC Conditions
3. Materials and Methods
3.1. Materials
3.2. Operational Set Up and Experimental Installation
3.3. Physicochemical Measurements of the Wastewater
3.4. Methodology of Conducting Multi-Criteria Decision Support
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alazaiza, M.Y.D.; Alzghoul, T.M.; Amr, S.A.; Bangalore Ramu, M.; Nassani, D.E. Bibliometric Insights into Car Wash Wastewater Treatment Research: Trends and Perspectives. Water 2024, 16, 2034. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Vieira dos Santos, E.; Tossi de Araújo Costa, E.C.; Martínez-Huitle, C.A. Electrochemical advanced oxidation processes (EAOPs) as alternative treatment techniques for carwash wastewater reclamation. Chemosphere 2018, 211, 998–1006. [Google Scholar] [CrossRef]
- Torkashvand, J.; Pasalari, H.; Gholami, M.; Younesi, G.S.; Oskoei, V.; Farzadkia, M. On-site carwash wastewater treatment and reuse: A systematic review. Int. J. Environ. Anal. Chem. 2020, 102, 3613–3627. [Google Scholar] [CrossRef]
- Espinoza-Montero, P.J.; Martínez-Huitle, C.A.; Loor-Urgilés, L.D. Technologies employed for carwash wastewater recovery. J. Clean. Prod. 2023, 401, 136722. [Google Scholar] [CrossRef]
- Kuan, W.-H.; Hu, C.-Y.; Ke, L.-W.; Wu, J.-M. A review of on-site carwash wastewater treatment. Sustainability 2022, 14, 5764. [Google Scholar] [CrossRef]
- Dadebo, D.; Ibrahim, M.G.; Fujii, M.; Nasr, M. Transition towards sustainable carwash wastewater management: Trends and enabling technologies at global scale. Sustainability 2022, 14, 5652. [Google Scholar] [CrossRef]
- Terechova, E.L.; Zhang, G.; Chen, J.; Sosnina, N.A.; Yang, F. Combined chemical coagulation–flocculation/ultraviolet photolysis treatment for anionic surfactants in laundry wastewater. J. Environ. Chem. Eng. 2014, 2, 2111–2119. [Google Scholar] [CrossRef]
- Tamjidi, S.; Moghadas, B.K.; Esmaeili, H.; Khoo, F.S.; Gholami, G.; Ghasemi, M. Improving the surface properties of adsorbents by surfactants and their role in the removal of toxic metals from wastewater: A review study. Process Saf. Environ. Prot. 2021, 148, 775–795. [Google Scholar] [CrossRef]
- Park, B.H.; Kim, S.; Seo, A.Y.; Lee, T.G. Evaluation of optimal conditions for anionic surfactant removal in wastewater. Chemosphere 2021, 263, 128174. [Google Scholar] [CrossRef]
- Jena, G.; Dutto, K.; Daverey, A. Surfactants in water and wastewater (greywater): Environmental toxicity and treatment options. Chemosphere 2023, 341, 140082. [Google Scholar] [CrossRef]
- Rodriguez Boluarte, I.A.; Andersen, M.; Pramanik, B.; Kumar, B.; Chang, C.; Bagshaw, S.; Farago, L.; Jegatheesan, V.; Shu, L. Reuse of car wash wastewater by chemical coagulation and membrane bioreactor treatment processes. Int. Biodeterior. Biodegrad. 2016, 113, 44–48. [Google Scholar] [CrossRef]
- Tajuddin, M.F.; Al-Gheethi, A.; Mohamed, R.; Noman, E.; Talip, B.A.; Bakar, A. Optimizing of heavy metals removal from car wash wastewater by chitosan-ceramic beads using response surface methodology. Mater. Today Proc. 2020, 31, 43–47. [Google Scholar] [CrossRef]
- Nagamani, V.; Sunder, R.S.; Lakshman, V. A cost effective membrane integrated process for the treatment of vehicle wash wastewater. Int. J. Eng. Res. 2020, 8, 833–837. [Google Scholar] [CrossRef]
- Kazembeigi, F.; Bayad, S.; Nasab, A.Y.; Doraghi, M.; Parseh, I. Techno-environmental study on the consequences of carwash wastewater and its management methods. Heliyon 2023, 9, e19764. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Zhou, M.; Martínez-Huitle, C.A. Heterogeneous electro-Fenton and photoelectro-Fenton processes: A critical review of fundamental principles and application for water/wastewater treatment. Appl. Catal. B 2018, 235, 103–125. [Google Scholar] [CrossRef]
- Gönder, Z.B.; Balcioğlu, G.; Vergili, I.; Kaya, Y. Electrochemical treatment of carwash wastewater using Fe and Al electrode: Techno-economic analysis and sludge characterization. J. Environ. Manag. 2017, 200, 380–390. [Google Scholar] [CrossRef]
- Veit, M.T.; Novais, I.G.V.; Juchen, P.T.; Palácio, S.M.; da Cunha Gonçalves, G.; Zanette, J.C. Automotive wash effluent treatment using combined process of coagulation/flocculation/sedimentation–adsorption. Water Air Soil Pollut. 2020, 231, 494. [Google Scholar] [CrossRef]
- Moazzem, S.; Wills, J.; Fan, L.; Roddick, F.; Jegatheesan, V. Performance of ceramic ultrafiltration and reverse osmosis membranes in treating car wash wastewater for reuse. Environ. Sci. Pollut. Res. 2018, 25, 8654–8668. [Google Scholar] [CrossRef]
- Gönder, Z.B.; Balcioğlu, G.; Vergili, I.; Kaya, Y. An integrated electrocoagulation-nanofiltration process for carwash wastewater reuse. Chemosphere 2020, 253, 126713. [Google Scholar] [CrossRef]
- Mkilima, T.; Zharkenov, Y.; Utepbergenova, L.; Smagulova, E.; Fazylov, K.; Zhumadilov, I.; Kirgizbayeva, K.; Baketova, A.; Abdukalikova, G. Carwash wastewater treatment through the synergistic efficiency of microbial fuel cells and metal-organic frameworks with graphene oxide integration. Case Stud. Chem. Environ. Eng. 2024, 9, 100582. [Google Scholar] [CrossRef]
- Thanekar, P.; Gogate, P.R. Combined hydrodynamic cavitation based processes as an efficient treatment option for real industrial effluent. Ultrason. Sonochem. 2019, 53, 202–213. [Google Scholar] [CrossRef]
- Li, C.; Yang, F.; Huang, Y.; Huang, C.; Zhang, K.; Yan, L. Comparison of hydrodynamic and ultrasonic cavitation effects on soy protein isolate functionality. J. Food Eng. 2020, 265, 109697. [Google Scholar]
- Raut-Jadhav, S.; Badve, M.P.; Pinjari, D.V.; Saini, D.R.; Sonawane, S.H.; Pandit, A.B. Treatment of the pesticide industry effluent using hydrodynamic cavitation and its combination with process intensifying additives (H2O2 and ozone). Chem. Eng. J. 2016, 295, 326–335. [Google Scholar] [CrossRef]
- Nakashima, K.; Ebi, Y.; Shibasaki-Kitakawa, N.; Soyama, H.; Yonemoto, T. Hydrodynamic cavitation reactor for efficient pretreatment of lignocellulosic biomass. Ind. Eng. Chem. Res. 2016, 55, 1866–1871. [Google Scholar] [CrossRef]
- Burzio, E.; Bersani, F.; Caridi, G.C.A.; Vesipa, R.; Ridolfi, L.; Manes, C. Water disinfection by orifice-induced hydrodynamic cavitation. Ultrason. Sonochem. 2020, 60, 104740. [Google Scholar] [CrossRef]
- Wang, B.; Su, H.; Zhang, B. Hydrodynamic cavitation as a promising route for wastewater treatment—A review. Chem. Eng. J. 2021, 412, 128685. [Google Scholar] [CrossRef]
- Song, Y.; Hou, R.; Zhang, W.; Liu, J. Hydrodynamic cavitation as an efficient water treatment method for various sewage—A review. Water Sci. Technol. 2022, 86, 302–320. [Google Scholar] [CrossRef]
- Patil, Y.; Sonawane, S.H.; Shyam, P.; Sun, X.; Manickam, S. Hybrid hydrodynamic cavitation (HC) technique for the treatment and disinfection of lake water. Ultrason. Sonochem. 2023, 97, 106454. [Google Scholar] [CrossRef]
- Mevada, J.; Devi, S.; Pandit, A. Large scale microbial cell disruption using hydrodynamic cavitation: Energy saving options. Biochem. Eng. J. 2019, 143, 151–160. [Google Scholar] [CrossRef]
- Sun, X.; Liu, S.; Zhang, X.; Tao, Y.; Boczkaj, G.; Yoon, J.Y.; Xuan, X. Recent advances in hydrodynamic cavitation-based pretreatments of lignocellulosic biomass for valorization. Bioresour. Technol. 2022, 345, 126251. [Google Scholar] [CrossRef]
- Patil, A.; Baral, S.; Dhanke, P. Hydrodynamic cavitation for process intensification of biodiesel synthesis—A review. Curr. Opin. Green Sustain. Chem. 2021, 4, 100144. [Google Scholar] [CrossRef]
- Ghayal, D.; Pandit, A.B.; Rathod, V.K. Optimization of biodiesel production in a hydrodynamic cavitation reactor using used frying oil. Ultrason. Sonochem. 2013, 20, 322–328. [Google Scholar] [CrossRef]
- Panda, D.; Saharan, V.K.; Manickam, S. Controlled Hydrodynamic Cavitation: A Review of Recent Advances and Perspectives for Greener Processing. Processes 2020, 8, 220. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, Y.; Lu, L.; Shi, C.; Huang, Y.; Mao, Z.; Duan, C.; Ren, X.; Guo, Y.; Huang, C. Hydrodynamic cavitation: A feasible approach to intensify the emulsion cross-linking process for chitosan nanoparticle synthesis. Ultrason. Sonochem. 2021, 74, 105551. [Google Scholar] [CrossRef]
- Zupanc, M.; Kosjek, T.; Petkovšek, M.; Dular, M.; Kompare, B.; Širok, B.; Blažeka, Ž.; Heath, E. Removal of pharmaceuticals from wastewater by biological processes, hydrodynamic cavitation and UV treatment. Ultrason. Sonochem. 2013, 20, 1104–1112. [Google Scholar] [CrossRef]
- Panda, D.; Manickam, S. Hydrodynamic cavitation assisted degradation of persistent endocrine-disrupting organochlorine pesticide Dicofol: Optimization of operating parameters and investigations on the mechanism of intensification. Ultrason. Sonochem. 2019, 51, 526–532. [Google Scholar] [CrossRef]
- Das, S.; Bhat, A.P.; Gogate, P.R. Degradation of dyes using hydrodynamic cavitation: Process overview and cost estimation. J. Water Process Eng. 2021, 42, 102126. [Google Scholar] [CrossRef]
- Wang, B.; Jiao, H.; Su, H.; Wang, T. Degradation of pefloxacin by hybrid hydrodynamic cavitation with H2O2 and O3. Chemosphere 2022, 303, 135299. [Google Scholar] [CrossRef]
- Mukherjee, A.; Mullick, A.; Vadthya, P.; Moulik, S.; Roy, A. Surfactant degradation using hydrodynamic cavitation based hybrid advanced oxidation technology: A techno economic feasibility study. Chem. Eng. J. 2020, 398, 125599. [Google Scholar] [CrossRef]
- Pereira, J.C.R.; Mateus, M.V.; Malpass, G.R.P.; Ferreira, D.C.; da Luz, M.S.; de Souza Inácio Gonçalves, J.C. Hybrid technology combining hydrodynamic cavitation and oxidative processes to degrade surfactants from a real effluent. Braz. J. Chem. Eng. 2023, 40, 723–732. [Google Scholar] [CrossRef]
- Horváth, O.; Huszánk, R. Degradation of surfactants by hydroxyl radicals photogenerated from hydroxoiron(III) complexes. Photochem. Photobiol Sci. 2003, 2, 960–966. [Google Scholar] [CrossRef]
- Adamson, A.W. (Ed.) The surface tension of solutions. In Physical Chemistry of Surfaces; John Wiley & Sons: New York, NY, USA, 1990; pp. 51–105. [Google Scholar]
- Kosmulski, M. Zeta potentials in nonaqueous media: How to measure and control them. Colloids Surf. A Physicochem. Eng. 1999, 159, 277–281. [Google Scholar] [CrossRef]
- Walters, K.; Jones, W.M. Measurement of viscosity. In Instrumentation Reference Book, 3rd ed.; Boyes, W., Ed.; Butterworth-Heinemann: Oxford, UK, 2003; pp. 45–52. [Google Scholar]
- Sarmadi, M.; Allah Zareia, A.; Ghahrchi, M.; Sepehrnia, B.; Meshkinian, A.; Moein, H.; Nakhaei, S.; Bazrafshan, E. Carwash wastewater characteristics—A systematic review study. Desalin. Water Treat. 2021, 225, 112–148. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies. Tables and Charts, 3rd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2011. [Google Scholar]
- Alvaro, M.; Corma, A.; Das, D.; Fornés, V.; García, H. “Nafion”-functionalized mesoporous MCM-41 silica shows high activity and selectivity for carboxylic acid esterification and Friedel–Crafts acylation reactions. J. Catal. 2005, 231, 48–55. [Google Scholar] [CrossRef]
- Gutiérrez-Becerra, A.; Barcena-Soto, M.; Soto, V.; Arellano-Ceja, J.; Casillas, N.; Prévost, S.; Noirez, L.; Gradzielski, M.; Escalante, J.I. Structure of reverse microemulsion-templated metal hexacyanoferrate nanoparticles. Nanoscale Res. Lett. 2012, 7, 83. [Google Scholar] [CrossRef]
- Bellamy, T.L.J. The Infrared Spectra of Complex Molecules, Advances in Infrared Group Frequencies; Chapman and Hall: London, UK, 1980; Volume 2. [Google Scholar]
- Montusiewicz, A.; Pasieczna-Patkowska, S.; Lebiocka, M.; Szaja, A.; Szymańska-Chargot, M. Hydrodynamic cavitation of brewery spent grain diluted by wastewater. Chem. Eng. J. 2017, 313, 946–956. [Google Scholar] [CrossRef]
- Lebiocka, M.; Montusiewicz, A.; Pasieczna–Patkowska, S.; Szaja, A. Pretreatment of herbal waste using sonication. Bioresour. Technol. 2023, 377, 128932. [Google Scholar] [CrossRef]
- Lebiocka, M. Application of Hydrodynamic Cavitation to Improve the Biodegradability of Municipal Wastewater. J. Ecol. Eng. 2020, 21, 155–160. [Google Scholar] [CrossRef]
- Paszeczko, M.I.; Montusiewicz, J. Evaluation of the wear resistance of eutectic coatings of the Fe–Mn–C–B system alloyed by Si, Ni, and Cr using multi-criteria analysis. Mater. Sci. 2012, 47, 813–821. [Google Scholar]

| Time min | Inlet Pressure | |||||||
|---|---|---|---|---|---|---|---|---|
| 2 bar | 3 bar | 4 bar | 5 bar | |||||
| Variant Number | Energy Consumption kWh | Variant Number | Energy Consumption kWh | Variant Number | Energy Consumption kWh | Variant Number | Energy Consumption kWh | |
| 0 | v1 | - | v6 | - | v11 | - | v16 | - |
| 30 | v2 | 0.182 | v7 | 0.224 | v12 | 0.279 | v17 | 0.332 |
| 60 | v3 | 0.361 | v8 | 0.448 | v13 | 0.555 | v18 | 0.664 |
| 90 | v4 | 0.546 | v9 | 0.672 | v14 | 0.833 | v19 | 0.996 |
| 120 | v5 | 0.723 | v10 | 0.896 | v15 | 1.11 | v20 | 1.328 |
| Stage of analysis | AS-1 | AS-2/1 | AS-2/2 | AS-2/3 | AS-2/4 |
| Weights | - | w1 = w2 = w3 = w4 = w5 = 0.2 | w1 = 0.2, w2 = 0.3, w3 = 0.1, w4 = 0.15, w5 = 0.25 | w1 = 0.1, w2 = 0.1, w3 = 0.1, w4 = 0.1, w5 = 0.6 | w1 = 0.5, w2 = 0.1, w3 = 0.1, w4 = 0.1, w5 = 0.2 |
| Number of variants recommended | v1–v12, v14–v20 | v10 | v10 | v10 | v10 |
| Comments | Non-dominated variants | First-order compromise variant min-max | First-order compromise variant min-max with weights | ||
| Stage of analysis | AS-3/1 | AS-3/2 | AS-3/3 | AS-3/4 | |
| Weights | w1 = w2 = w3 = w4 = w5 = 0.2 | w1 = 0.2, w2 = 0.3, w3 = 0.1, w4 = 0.15, w5 = 0.25 | w1 = 0.1, w2 = 0.1, w3 = 0.1, w4 = 0.1, w5 = 0.6 | w1 = 0.5, w2 = 0.1, w3 = 0.1, w4 = 0.1, w5 = 0.2 | |
| Number of variants recommended | v17 | v17 | v17 | v17, v9 | |
| Comments | Second-order compromise variant min-max | Second-order compromise variant min-max with weights | |||
| pH | Viscosity [Pa·s] | Surface Tension [mN/m] | Specific Conductivity [mS·cm−1] | Zeta Potential [mV] | Particle Size [nm] | ||
|---|---|---|---|---|---|---|---|
| Time | 3 bar | ||||||
| 0 | 7.56 ± 0.10 | 9.8 × 10−4 ± 2.4 × 10−5 | 59.58 ± 2.4 | 1.14 ± 0.18 | −11.70 ± 0.74 | 1560.0 ± 556 | |
| 30 | 8.11 ± 0.05 | 9.3 × 10−4 ± 3.0 × 10−5 | 66.48 ± 1.5 | 1.12 ± 0.18 | −12.15 ± 0.58 | 1306 ± 464 | |
| 60 | 8.34 ± 0.08 | 9.5 × 10−4 ± 2.7 × 10−5 | 69.13 ± 1.7 | 1.11 ± 0.17 | −12.56 ± 0.85 | 1164 ± 408 | |
| 90 | 8.41 ± 0.13 | 9.8 × 10−4 ± 5.8 × 10−5 | 71.49 ± 0.9 | 1.08 ± 0.16 | −12.89 ± 0.98 | 1008 ± 328 | |
| 120 | 8.45 ± 0.16 | 9.9 × 10−4 ± 2.6 × 10−5 | 72.45 ± 0.5 | 1.06 ± 0.016 | −13.11 ± 1.08 | 937 ± 128 | |
| Time | 5 bar | ||||||
| 0 | 7.54 ± 0.09 | 9.4 × 10−4 ± 2.3 × 10−5 | 65.93 ± 2.2 | 1.06 ± 0.3 | −12.03 ± 0.13 | 1065 ± 205 | |
| 30 | 8.26 ± 0.07 | 9.9 × 10−4 ± 6.4 × 10−5 | 70.44 ± 0.2 | 1.05 ± 0.2 | −12.13 ± 0.52 | 917 ± 136 | |
| 60 | 8.46 ± 0.07 | 9.3 × 10−4 ± 5.4 × 10−5 | 71.6 ± 0.2 | 1.03 ± 0.1 | −12.28 ± 0.74 | 876 ± 125 | |
| 90 | 8.65 ± 0.08 | 9.3 × 10−4 ± 3.9 × 10−5 | 71.81 ± 0.5 | 1.00 ± 0.5 | −12.63 ± 1.08 | 813 ± 78 | |
| 120 | 8.68 ± 0.05 | 1.0 × 10−3 ± 3.4 × 10−5 | 72.2 ± 0.1 | 0.97 ± 0.7 | −12.8 ± 0.45 | 819 ± 59 | |
| Parameter | Inlet Pressure [bar] | |||
|---|---|---|---|---|
| 2 | 3 | 4 | 5 | |
| NS concentration [mg L−1] | 164 ± 3.3 | 153 ± 3.1 | 140 ± 2.8 | 204 ± 4.1 |
| AS concentration [mg L−1] | 39.5 ± 2.0 | 50.5 ± 2.5 | 36.9 ± 1.8 | 40.6 ± 2.0 |
| CS concentration [mg L−1] | 0.87 ± 0.043 | 0.58 ± 0.058 | 1.18 ± 0.094 | 0.5 ± 0.035 |
| COD [mg L−1] | 251.1 ± 5.0 | 159 ± 3.2 | 165 ± 6.3 | 249 ± 7.5 |
| pH - | 7.51 ± 0.11 | 7.77 ± 0.13 | 7.76 ± 0.12 | 7.49 ± 0.11 |
| Turbidity [NTUs] | 56.3 ± 0.1 | 43.3 ± 0.2 | 35.7 ± 0.2 | 53.7 ± 0.1 |
| Phosphates [mg L−1] | 0.203 ± 0.011 | 0.189 ± 0.01 | 0.178 ± 0.013 | 0.216 ± 0.01 |
| Parameter | Inlet Pressure [bar] | |||
|---|---|---|---|---|
| 2 | 3 | 4 | 5 | |
| pv [kPa] | 2.337 | 2.642 | 2.642 | 2.485 |
| p2 [kPa] | 96.67 | 96.85 | 96.89 | 96.94 |
| v0 [ms−1] | 32.49 | 36.77 | 46.05 | 51.21 |
| cv - | 0.18 | 0.12 | 0.09 | 0.07 |
| Time [min] | Number of passes through cavitation zone | |||
| 30 | 13.8 | 16.9 | 19.5 | 21.7 |
| 60 | 27.6 | 33.7 | 39.1 | 43.4 |
| 90 | 41.3 | 50.6 | 58.6 | 65.2 |
| 120 | 55.1 | 67.5 | 78.1 | 86.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lebiocka, M.; Montusiewicz, A.; Grządka, E.; Pasieczna-Patkowska, S.; Montusiewicz, J.; Szaja, A. Hydrodynamic Cavitation as a Method of Removing Surfactants from Real Carwash Wastewater. Molecules 2024, 29, 4791. https://doi.org/10.3390/molecules29204791
Lebiocka M, Montusiewicz A, Grządka E, Pasieczna-Patkowska S, Montusiewicz J, Szaja A. Hydrodynamic Cavitation as a Method of Removing Surfactants from Real Carwash Wastewater. Molecules. 2024; 29(20):4791. https://doi.org/10.3390/molecules29204791
Chicago/Turabian StyleLebiocka, Magdalena, Agnieszka Montusiewicz, Elżbieta Grządka, Sylwia Pasieczna-Patkowska, Jerzy Montusiewicz, and Aleksandra Szaja. 2024. "Hydrodynamic Cavitation as a Method of Removing Surfactants from Real Carwash Wastewater" Molecules 29, no. 20: 4791. https://doi.org/10.3390/molecules29204791
APA StyleLebiocka, M., Montusiewicz, A., Grządka, E., Pasieczna-Patkowska, S., Montusiewicz, J., & Szaja, A. (2024). Hydrodynamic Cavitation as a Method of Removing Surfactants from Real Carwash Wastewater. Molecules, 29(20), 4791. https://doi.org/10.3390/molecules29204791









