Abstract
A novel and controllable synthesis of thioacetals/thioketals and β-sulfanyl ketones mediated by the reaction of aldehyde/acetone with thiols has been developed. In this protocol, β-sulfanyl ketones can be generated without the prior preparation of α, β-unsaturated carbonyl compounds. A variety of thiols reacted with aldehyde/acetone and provided the corresponding thioacetals/thioketals and β-sulfanyl ketones in good to excellent yields, respectively. This protocol is operationally simple, mild, and atom-economical, providing controllable access to thioacetals/thioketals and thia-Michael addition products under mild conditions.
1. Introduction
Sulfides have garnered significant attention from scientists due to their diverse chemical valence states and the resulting rich chemical structure and stereo conformation. Thioether, sulfoxide, sulfone, and sulfonamide are widely utilized pharmacophores in commercially available pharmaceuticals [1]. Furthermore, owing to the high reactivity of sulfides, they are frequently employed as a key intermediate in the total synthesis of natural products [2] and various synthetic reactions [3]. Thioacetals and thioketals, among a wide variety of sulfur-containing compounds, are commonly utilized in organic synthesis as precursors for fluorination and alkylation and for olefin formation [4,5,6]. Furthermore, thioketals exhibit diverse biological activities and are employed as drugs with numerous pharmacological effects (Figure 1) [7,8,9]. The construction of C-S bonds through the Michael addition reaction between mercaptan with α, β-unsaturated carbonyl compounds holds significant value in both chemistry and biology [10].
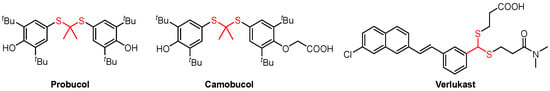
Figure 1.
Examples of applications of thioacetals/thioketals.
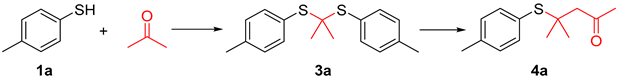
Thioacetals and thioketals are typically synthesized through the condensation of carbonyl compounds with thiols or dithiols, employing protic acids, Lewis acids, or photocatalysis (Figure 2a) [11,12,13,14,15,16,17,18]. The addition of thiol to aryl and alkyl substituted α, β-unsaturated carbonyl compounds represents the primary method for synthesizing β-sulfanyl ketones (Figure 2b) [19,20,21]. Herein, we investigated a novel and controllable synthesis of thioacetals/thioketals and β-sulfanyl ketones from acetone and thiols. With this synthetic method, β-sulfanyl ketones can be formed without preparation of α, β-unsaturated carbonyl compounds in advance (Figure 2c).
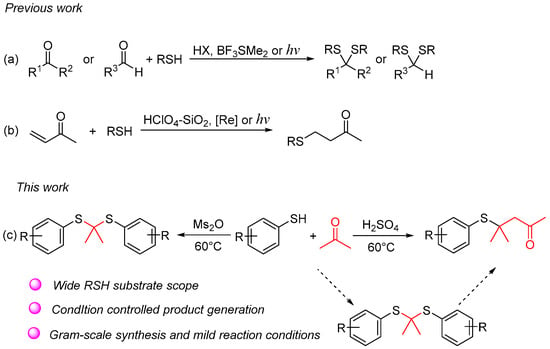
Figure 2.
Overview of synthetic strategies to access thioacetals/thioketals and β-sulfanyl ketones. (a) The synthesis strategies of thioacetals and thioketals; (b) The synthesis strategies of β-sulfanyl ketones; (c) Our work.
2. Results and Discussion
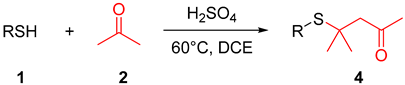
The Michael addition reaction has been extensively studied due to its high application value. In the previous investigations, we discovered that mercaptan can be transformed into a thia-Michael addition product when mediated by p-toluene sulfonic anhydride in acetone as the solvent. Thioketals and α, β-unsaturated ketone were also monitored during the reaction (Table 1). According to preliminary results, a multitude of experiments were conducted to optimize the reaction conditions.

Table 1.
Optimization of reaction conditions a.
We initiated our investigation using 4-methylbenzenethiol (1a) and acetone (2) as the model substrates and then allowed them to react at 60 °C for 24 h (Table 1, entry 1). Initially, various additives (Ts2O, Ms2O, TsOH, HCl, and H2SO4) were added to the model reaction to identify suitable accelerators. Both Ts2O and TsOH exhibited comparable facilitation abilities in this reaction (Table 1, entries 1 and 3). Compared to Ts2O, the addition of TsOH makes the reaction proceed more completely (Table 1, entry 3). There are no intermediates remaining after 24 h. Notably, it was observed that the addition of Ms2O mainly generates thioketal for an extended period, with only a limited number of products being generated over time.
Inorganic acids can also promote the reaction, and sulfuric acid displayed superior efficacy among them (Table 1, entry 5). This enhancement may be attributed to the high concentration of hydrogen ions dissociated from sulfuric acid relative to other acids used in equivalent amounts. Decreasing the amount of acetone provides better results (Table 1, entry 6), whereas reducing the amount of H2SO4 is not conducive to the reaction (Table 1, entries 7–8). Enhanced yields were achieved using DCE, DCM, and toluene as solvents (Table 1, entries 6, 9–10), while polar solvents, such as water and acetonitrile, hindered progress (Table 1, entries 11–14). Neither lower nor higher temperatures provided optimal outcomes for this process (Table 1, entries 15–18). According to the above experiments, we established optimal reaction conditions, which yielded product 4a with an isolated yield of 86% (Table 1, entry 6).
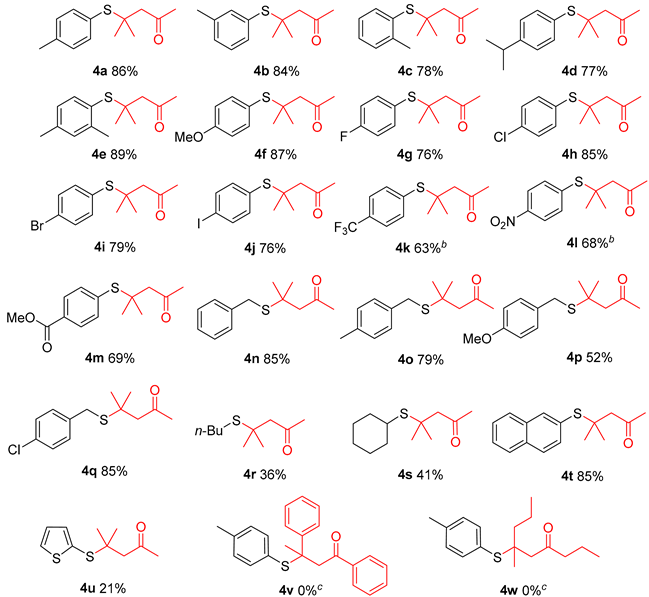
With the optimized reaction conditions established, we investigated the scope and generality of thiol substrates in conjunction with ketones (Table 2). Overall, both electron-donating and electron-withdrawing substituted substrates provided the desired products in good yields. A diverse array of functional groups, such as Me, i-Pr, OMe, F, Cl, Br, I, CF3, NO2, and COOMe, were compatible with this reaction and provided satisfactory product yields (Table 2, 4a–4m). Substituted benzyl mercaptan and naphthalene-2-thiol were well-tolerated under the optimal reaction conditions (Table 2, 4n–4q, 4t). However, alkyl mercaptans and heteroaryl mercaptans afforded the corresponding products with lower yields (Table 2, 4r–4s, 4u). Motivated by the above results, we further explored the substrate scope of ketones, such as acetophenone and 2-pentanone. Unfortunately, no desired products were obtained due to the steric hindrance.

Table 2.
H2SO4 facilitated thia-Michael addition between thiols and ketones a,d.
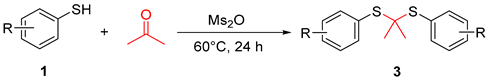
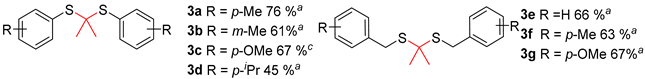
In the process of condition optimization, it was observed that when methanesulfonic anhydride was introduced into the reaction mixture, thioketal emerged as the main product, rather than β-sulfanyl ketone (Table 1, entry 2). The results prompted us to carry out an extended study of the reaction with methanesulfonic anhydride as the accelerator (Table 3). It was found that the thiophenol and benzyl mercaptan substituted with Me or OMe can obtain the target substances in medium to good yields (Table 3, 3a–3g), while thiophenol substituted with other substituents (NO2, Cl, Br, etc.) has a complex reaction system, and very little thioketal is generated, which is difficult to be separated and purified.

Table 3.
Ms2O facilitated thioacetalization of acetone to thioacetals a,b.
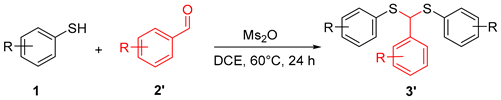
In addition to acetone, the applicability of aromatic aldehydes as substrates was also discussed. The results indicated that the target product could be obtained with medium to good yield from different substituted aromatic aldehydes (Table 4). Notably, even thiophenol substituted with NO2 achieved a yield of approximately 70% for the target material (Table 4, 3′o).

Table 4.
Ms2O facilitated thioacetalization of aldehyde to thioacetals a,b.
During the reaction process, α, β-unsaturated ketone and thioketal were identified alongside substrates and desired products at various stages of the reaction. In order to clarify the reaction mechanism, several control experiments were performed. In the absence of 4-methylbenzenethiol, acetone was converted into α, β-unsaturated ketone A under current reaction conditions (Figure 3a). When A was introduced into the reaction instead of acetone, a yield of 42% for product 4a was obtained (Figure 3b). Thioketal reacted with acetone under the optimal conditions to produce 4a in a yield of 71% (Figure 3c). Additionally, with thioketals 3a and A as substrates, only 35% of 4a was obtained (Figure 3d). The reaction process was monitored via GC-MS, and it was surprising that at the initial stage of the reaction, only thioketal was detected in the reaction mixture without a product or α, β-unsaturated ketone A. The presence of compound A became evident in later stages. Thus, we assume that the reaction (b), (c), and (d) exist at the same time, and reaction (c) is the main process (Figure 3).
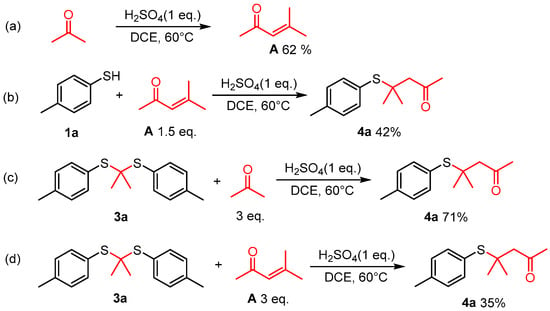
Figure 3.
Control experiments. (a) Acetone converted into α, β-unsaturated ketone in the absence of 4-methylbenzenethiol under optimal reaction conditions; (b) 4-Methylbenzenethiol reacted with A to form 4a; (c) Thioketal 3a reacted with 3 eq acetone under optimal reaction conditions to produce 4a in a yield of 71%; (d) Thioketal 3a reacted with A to produce the target compound 4a with a yield of 35%.
To demonstrate the practicality of the present method, a gram-scale experiment was performed under the standard reaction conditions, yielding the product 4a in a 78% yield (Figure 4). The gram-scale experiment shows the promising application of this method.

Figure 4.
Gram-scale experiment.
Based on the above results and the relevant literature [22,23,24,25], a possible mechanism was proposed, as shown in Figure 5. The reaction products (4) are generated more rapidly via Path A. Thioketals are formed uniformly and quickly, which is reversible under acidic conditions, leading to positively charged sulfur species that subsequently react with acetone to generate the final products. In contrast, only a minimal quantity of products is produced through Path B. The α, β-unsaturated ketone A was detected later using GC-MS. The conversion of acetone into diketone alcohol occurs at a slow rate. Diacetone alcohol eliminates one molecule of H2O and directly reacts with thiols to produce the final products. By comparing these two reaction mechanisms, we propose that β-sulfanyl ketones are preferentially formed through reactions with highly reactive charged sulfur species (Path A).
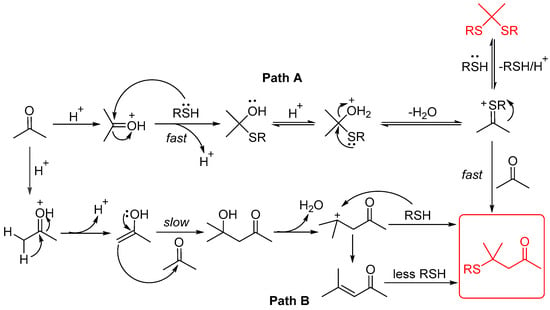
Figure 5.
Plausible mechanism.
3. Materials and Methods
General Information.
All reactions were performed in a sealed tube with magnetic stirring. Unless otherwise stated, all commercially available reagents (innochem, Beijing, China) were used without further purification. Reactions were monitored using thin-layer chromatography (TLC), GC/MS, or LC/MS. NMR spectra were recorded on Bruker DRX-300 instruments (Bruker, Rheinstetten, Germany) and were calibrated using residual undeuterated solvent (CHCl3 at 7.26 ppm for 1H NMR and 77.16 ppm for 13C NMR). Data were reported as follows: chemical shift, multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, dd = doublet of doublets, td = triplet of doublets, qd = quartet of doublets, m = multiplet), coupling constants (Hz) and integration. High-resolution mass spectra (HRMS) were recorded on an Agilent LC/Xevo G2-XS QTOF mass spectrometer (Agilent, Palo Alto, CA, USA) using electrospray ionization time of flight reflectron experiments.
Experimental Procedure.
A 10 mL sealed tube was charged with substituted various thiols (0.6 mmol), methanesulfonic anhydride (Ms2O, 0.6 mmol), acetone (1 mL), and DCE (5 mL). The resulting solution was stirred at 60 °C and monitored using TLC until the reaction was complete. Saturated aqueous Na2CO3 solution (10 mL) and EtOAc (10 mL) was added to the mixture. The layers were separated, and the aqueous layer was washed with EtOAc (2 × 10 mL). Combined organic layers were washed with brine (10 mL), dried with anhydrous Na2SO4, filtered, and concentrated. The residue was purified using column chromatography on silica gel, with hexane/ethyl acetate = 20:1 as the eluent to provide the desired products 3.
A 10 mL sealed tube was charged with thiols (0.6 mmol), methanesulfonic anhydride (Ms2O, 0.6 mmol), 2′ (0.9 mmol), and DCE (5 mL). The resulting solution was stirred at 60 °C and monitored by TLC until the reaction was complete. Saturated aqueous Na2CO3 solution (10 mL) and EtOAc (10 mL) were added to the resulting mixture. The layers were separated, and the aqueous layer was washed with EtOAc (2 × 10 mL). The combined organic layers were washed with brine (10 mL), dried with anhydrous Na2SO4, filtered, and concentrated. The residue was purified using column chromatography on silica gel, with hexane/ethyl acetate = 50:1 as the eluent to provide the desired products 3′.
A 10 mL sealed tube was charged with thiols (0.6 mmol), H2SO4 (0.6 mmol), acetone (1 mL), and DCE (5 mL). The resulting solution was stirred at 60 °C and monitored using TLC until the reaction was complete. Saturated aqueous Na2CO3 solution (10 mL) and EtOAc (10 mL) were added to the resulting mixture. The layers were separated, and the aqueous layer was washed with EtOAc (2 × 10 mL). The combined organic layers were washed with brine (10 mL), dried with anhydrous Na2SO4, filtered, and concentrated. The residue was purified using column chromatography on silica gel, with hexane/ethyl acetate = 10:1 as the eluent to provide the desired products 4.
The gram-scale experiment procedure. A 250 mL round-bottomed flask was charged with 4-methylbenzenethiol (1a) (10 mmol,1.2432 g), H2SO4 (10 mmol), acetone (16 mL), and DCE (84 mL). The bottle was sealed with a rubber stopper, and the resulting solution was stirred at 60 °C for 24 h. Saturated aqueous Na2CO3 solution (100 mL) and EtOAc (100 mL) were added to the resulting mixture. The layers were separated, and the aqueous layer was washed with EtOAc (2 × 100 mL). The combined organic layers were washed with brine (100 mL), dried with anhydrous Na2SO4, filtered, and concentrated. The residue was purified using column chromatography on silica gel, with hexane/ethyl acetate = 10:1 as the eluent to provide the desired product 4a. Characterization data for the products can be found in the Supplementary Materials, along with references of previous reports which provide support for the identities of the products [20,26,27,28,29,30,31].
Propane-2,2-diylbis(p-tolylsulfane) (3a) [26]. White solid, 76% yield. 1H NMR (300 MHz, CDCl3) δ 7.53 (d, J = 8.0 Hz, 4H), 7.16 (d, J = 7.9 Hz, 4H), 2.37 (s, 6H), 1.49 (s, 6H). 13C NMR (75 MHz, CDCl3) δ 139.37, 137.16, 129.48, 128.92, 59.24, 30.77, 21.43.
Propane-2,2-diylbis(m-tolylsulfane) (3b). White solid, 61% yield. 1H NMR (300 MHz, Chloroform-d) δ 7.45 (d, J = 6.5 Hz, 4H), 7.20 (dd, J = 12.1, 7.7 Hz, 4H), 2.35 (s, 6H), 1.51 (s, 6H). 13C NMR (75 MHz, CDCl3) δ 138.35, 137.66, 134.08, 132.14, 129.98, 128.44, 59.34, 30.99, 21.42.
Propane-2,2-diylbis((4-methoxyphenyl)sulfane) (3c). White solid, 67% yield. 1H NMR (300 MHz, CDCl3) δ 7.54 (d, J = 8.6 Hz, 4H), 6.88 (d, J = 8.7 Hz, 4H), 3.82 (s, 6H), 1.45 (s, 6H). 13C NMR (75 MHz, CDCl3) δ = 160.62, 138.82, 123.28, 114.14, 59.33, 55.41, 30.52.
Propane-2,2-diylbis((4-isopropylphenyl)sulfane) (3d). White solid, 45% yield. 1H NMR (300 MHz, Chloroform-d) δ 7.56 (d, J = 8.1 Hz, 4H), 7.21 (d, J = 8.0 Hz, 4H), 2.93 (hept, J = 6.8 Hz, 2H), 1.50 (s, 6H), 1.26 (d, J = 6.9 Hz, 12H). 13C NMR (75 MHz, CDCl3) δ 150.14, 137.19, 129.31, 126.83, 59.51, 34.02, 30.88, 24.01.
Propane-2,2-diylbis(benzylsulfane) (3e). White solid, 66% yield. 1H NMR (300 MHz, CDCl3) δ 7.34 (dt, J = 14.4, 7.5 Hz, 10H), 3.93 (s, 4H), 1.67 (s, 6H). 13C NMR (75 MHz, CDCl3) δ 137.87, 129.23, 128.62, 127.03, 57.30, 35.19, 30.85.
Propane-2,2-diylbis((4-methylbenzyl)sulfane) (3f). White solid, 63% yield. 1H NMR (300 MHz, CDCl3) δ 7.45 (d, J = 7.8 Hz, 4H), 7.33 (d, J = 7.8 Hz, 4H), 4.07 (s, 4H), 2.54 (s, 6H), 1.83 (s, 6H). 13C NMR (75 MHz, CDCl3) δ 136.62, 134.72, 129.31, 129.12, 57.18, 34.86, 30.84, 21.22.
Propane-2,2-diylbis((4-methoxybenzyl)sulfane) (3g). White solid, 67% yield. 1H NMR (300 MHz, CDCl3) δ 7.37 (d, J = 8.5 Hz, 4H), 6.95 (d, J = 8.5 Hz, 4H), 3.95 (s, 4H), 3.89 (s, 6H), 1.72 (s, 6H). 13C NMR (75 MHz, CDCl3) δ 158.66, 130.28, 129.71, 114.03, 57.04, 55.36, 34.52, 30.85.
(Phenylmethylene)bis(p-tolylsulfane) (3′a) [27]. White solid, 79% yield. 1H NMR (300 MHz, CDCl3) δ 7.30 (dd, J = 7.6, 1.7 Hz, 2H), 7.25–7.20 (m, 7H), 7.02 (d, J = 7.9 Hz, 4H), 5.29 (s, 1H), 2.27 (s, 6H). 13C NMR (75 MHz, CDCl3) δ 140.08, 138.13, 133.25, 130.95, 129.69, 128.48, 128.00, 61.39, 21.31.
(p-Tolylmethylene)bis(p-tolylsulfane) (3′b). White solid, 75% yield. 1H NMR (300 MHz, CDCl3) δ 7.35 (d, J = 8.0 Hz, 6H), 7.16 (t, J = 7.9 Hz, 6H), 5.43 (s, 1H), 2.42 (s, 3H), 2.40 (s, 6H). 13C NMR (75 MHz, CDCl3) δ 137.91, 137.69, 137.14, 133.03, 131.29, 129.64, 129.17, 127.85, 61.18, 21.25.
(m-Tolylmethylene)bis(p-tolylsulfane) (3′c). White solid, 74% yield. 1H NMR (300 MHz, CDCl3) δ 7.29 (d, J = 8.1 Hz, 4H), 7.22–7.15 (m, 3H), 7.09 (d, J = 7.9 Hz, 5H), 5.34 (s, 1H), 2.34 (s,9H). 13C NMR (75 MHz, CDCl3) δ 139.91, 138.13, 138.00, 133.12, 131.10, 129.63, 128.78, 128.56, 128.31, 125.00, 61.44, 21.47, 21.26.
((3, 5-Dimethylphenyl)methylene)bis(p-tolylsulfane) (3′d). White solid, 77% yield. 1H NMR (300 MHz, CDCl3) δ 7.29 (d, J = 8.1 Hz, 4H), 7.09 (d, J = 7.9 Hz, 4H), 7.00 (s, 2H), 6.91 (s, 1H), 5.31 (s, 1H), 2.34 (s, 6H), 2.30 (s, 6H). 13C NMR (75 MHz, CDCl3) δ 139.82, 137.98, 137.92, 133.05, 131.27, 129.73, 129.61, 125.64, 61.54, 21.35, 21.26.
((4-Ethylphenyl)methylene)bis(p-tolylsulfane) (3′e). White solid, 76% yield. 1H NMR (300 MHz, CDCl3) δ 7.31 (t, J = 8.5 Hz, 6H), 7.12 (dd, J = 16.9, 8.0 Hz, 6H), 5.38 (s, 1H), 2.66 (q, J = 7.6 Hz, 2H), 2.34 (s, 6H), 1.26 (t, J = 7.6 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 144.03, 137.89, 137.24, 132.99, 131.21, 129.62, 127.96, 127.84, 61.17, 28.61, 21.25, 15.52.
((4-Methoxyphenyl)methylene)bis(p-tolylsulfane) (3′f). White solid, 74% yield. 1H NMR (300 MHz, CDCl3) δ 7.24–7.20 (m, 6H), 7.03 (d, J = 7.9 Hz, 4H), 6.77 (d, J = 8.7 Hz, 2H), 5.29 (s, 1H), 3.77 (s, 3H), 2.29 (s, 6H). 13C NMR (75 MHz, CDCl3) δ 159.27, 138.02, 133.12, 132.14, 131.15, 129.68, 129.22, 113.84, 60.70, 55.40, 21.31.
((4-Fluorophenyl)methylene)bis(p-tolylsulfane) (3′g). White solid, 87% yield. 1H NMR (300 MHz, CDCl3) δ 7.39–7.27 (m, 6H), 7.10 (d, J = 8.1 Hz, 4H), 6.97 (t, J = 8.6 Hz, 2H), 5.38 (s, 1H), 2.35 (s, 6H). 13C NMR (75 MHz, CDCl3) δ 162.20 (d, J = 246.9 Hz), 138.27, 135.85 (d, J = 3.2 Hz), 133.35, 130.53, 129.72, 129.61, 115.28 (d, J = 21.7 Hz), 60.42, 21.25. 19F NMR (282 MHz, CDCl3) δ -113.83 (tt, J = 8.6, 5.3 Hz).
((4-Chlorophenyl)methylene)bis(p-tolylsulfane) (3′h). White solid, 88% yield. 1H NMR (300 MHz, CDCl3) δ 7.36–7.27 (m, 8H), 7.12 (d, J = 7.9 Hz, 4H), 5.37 (s, 1H), 2.37 (s, 6H). 13C NMR (75 MHz, CDCl3) δ 138.62, 138.32, 133.51, 133.36, 130.38, 129.74, 129.31, 128.53, 60.51, 21.25.
((4-Bromophenyl)methylene)bis(p-tolylsulfane) (3′i). White solid, 87% yield. 1H NMR (300 MHz, CDCl3) δ 7.41 (d, J = 8.5 Hz, 2H), 7.26 (m, 6H), 7.10 (d, J = 8.0 Hz, 4H), 5.33 (s, 1H), 2.35 (s, 6H). 13C NMR (75 MHz, CDCl3) δ 139.14, 138.34, 133.36, 131.49, 130.34, 129.75, 129.63, 121.72, 60.58, 21.28.
4-(Bis(p-tolylthio)methyl)benzonitrile (3′j). White solid, 86% yield. 1H NMR (300 MHz, CDCl3) δ 7.44 (d, J = 8.2 Hz, 2H), 7.31 (d, J = 8.2 Hz, 2H), 7.18 (d, J = 8.0 Hz, 4H), 7.01 (d, J = 8.0 Hz, 4H), 5.27 (s, 1H), 2.25 (s, 6H). 13C NMR (75 MHz, CDCl3) δ 145.29, 138.64, 133.54, 132.02, 129.74, 129.50, 128.56, 118.57, 111.30, 60.55, 21.15.
4-(Bis(p-tolylthio)methyl)benzaldehyde (3′k). White solid, 86% yield. 1H NMR (300 MHz, CDCl3) δ 9.70 (s, 1H), 7.51 (d, J = 8.1 Hz, 2H), 7.21 (d, J = 8.1 Hz, 2H), 7.01 (d, J = 8.0 Hz, 4H), 6.81 (d, J = 7.9 Hz, 4H), 5.12 (s, 1H), 2.06 (s, 6H). 13C NMR (75 MHz, CDCl3) δ 191.66, 146.82, 138.53, 135.66, 133.51, 129.87, 129.74, 128.54, 60.90, 21.19.
((4-Chlorophenyl)methylene)bis((4-methoxyphenyl)sulfane) (3′l). White solid, 72% yield. 1H NMR (300 MHz, CDCl3) δ 7.73–7.66 (m, 4H), 7.65–7.53 (m, 4H), 7.23–7.17 (m, 4H), 5.54 (s, 1H), 4.19 (s, 6H). 13C NMR (75 MHz, CDCl3) δ 160.16, 138.83, 136.15, 133.45, 129.34, 128.50, 124.33, 114.50, 62.11, 55.39.
Dimethyl 4,4’-((phenylmethylene)bis(sulfanediyl))dibenzoate (3′m). White solid, 75% yield. 1H NMR (300 MHz, CDCl3) δ 7.89 (d, J = 8.5 Hz, 4H), 7.46 (dd, J = 7.7, 1.5 Hz, 2H), 7.35 (d, J = 8.5 Hz, 4H), 7.29 (d, J = 7.4 Hz, 2H), 5.69 (s, 1H), 3.87 (s, 6H). 13C NMR (75 MHz, CDCl3) δ 166.49, 140.94, 138.13, 130.01, 129.97, 128.85, 128.71, 128.66, 127.93, 57.59, 52.18.
4-(Bis((4-bromophenyl)thio)methyl)benzonitrile (3′n). White solid, 75% yield. 1H NMR (300 MHz, CDCl3) δ 7.55 (d, J = 8.3 Hz, 2H), 7.38 (d, J = 8.4 Hz, 6H), 7.17 (dd, J = 8.8, 2.1 Hz, 4H), 5.33 (s, 1H). 13C NMR (75 MHz, CDCl3) δ 144.25, 134.79, 132.43, 132.30, 131.97, 128.59, 123.20, 118.39, 112.07, 60.03.
(p-tolylmethylene)bis((4-nitrophenyl)sulfane) (3′o). White solid, 70% yield. 1H NMR (300 MHz, CDCl3) δ 8.08 (d, J = 8.8 Hz, 4H), 7.42 (dd, J = 8.3, 6.5 Hz, 6H), 7.17 (d, J = 7.9 Hz, 2H), 5.80 (s, 1H), 2.34 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 146.34, 144.20, 139.42, 133.67, 129.97, 129.36, 127.88, 124.05, 56.18, 21.29.
(p-tolylmethylene)bis(naphthalen-2-ylsulfane) (3′p). White solid, 71% yield. 1H NMR (300 MHz, CDCl3) δ 7.86 (s, 2H), 7.78 (dd, J = 5.8, 3.5 Hz, 2H), 7.74–7.65 (m, 4H), 7.52–7.34 (m, 8H), 7.12 (d, J = 7.9 Hz, 2H), 5.69 (s, 1H), 2.34 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 138.11, 136.69, 133.61, 132.62, 132.25, 131.51, 129.57, 129.42, 128.44, 127.90, 127.77, 127.69, 126.54, 126.43, 60.34, 21.31, 1.16.
(Phenylmethylene)bis(benzylsulfane) (3′q) [28]. White solid, 73% yield. 1H NMR (300 MHz, CDCl3) δ 7.32–7.10 (m, 11H), 7.09–7.00 (m, 4H), 4.38 (s, 1H), 3.68 (d, J = 13.4 Hz, 2H), 3.45 (d, J = 13.4 Hz, 2H). 13C NMR (75 MHz, CDCl3) δ 139.64, 137.81, 129.05, 128.68, 128.57, 128.09, 128.03, 127.07, 50.96, 36.61.
4-Methyl-4-(p-tolylthio)pentan-2-one (4a) [20]. Oil liquid, 86% yield. 1H NMR (300 MHz, CDCl3) δ 7.39 (d, J = 8.0 Hz, 2H), 7.14 (d, J = 7.9 Hz, 2H), 2.64 (s, 2H), 2.34 (s, 3H), 2.13 (s, 3H), 1.36 (s, 6H). 13C NMR (75 MHz, CDCl3) δ 206.75, 139.31, 137.62, 129.54, 127.92, 54.41, 46.91, 32.26, 28.13, 21.30. HRMS (ESI-MS) [M + Na]+: found 245.0985; calculated for C13H18NaOS+: 245.0971.
4-Methyl-4-(m-tolylthio)pentan-2-one (4b) [29]. Oil liquid, 84% yield. 1H NMR (300 MHz, CDCl3) δ 7.38–7.26 (m, 2H), 7.20 (m, J = 17.9, 7.3 Hz, 2H), 2.65 (s, 2H), 2.34 (s, 3H), 2.13 (s, 3H), 1.38 (s, 6H). 13C NMR (75 MHz, CDCl3) δ 206.66, 138.39, 138.22, 134.61, 131.12, 129.91, 128.47, 54.43, 46.94, 32.21, 28.23, 21.27. HRMS (ESI-MS) [M + Na]+: found 245.0982; calculated for C13H18NaOS+: 245.0971.
4-Methyl-4-(o-tolylthio)pentan-2-one (4c) [29]. Oil liquid, 78% yield. 1H NMR (300 MHz, CDCl3) δ 7.54 (d, J = 7.6 Hz, 1H), 7.34–7.25 (m, 2H), 7.23–7.13 (m, 1H), 2.75 (s, 2H), 2.54 (s, 3H), 2.17 (s, 3H), 1.42 (s, 6H). 13C NMR (75 MHz, CDCl3) δ 206.66, 144.03, 138.99, 130.99, 130.66, 129.32, 125.97, 54.63, 48.56, 32.27, 28.22, 21.87. HRMS (ESI-MS) [M + Na]+: found 245.0984; calculated for C13H18NaOS+: 245.0971.
4-((4-Isopropylphenyl)thio)-4-methylpentan-2-one (4d). Oil liquid, 77% yield. 1H NMR (300 MHz, CDCl3) δ 7.42 (d, J = 8.1 Hz, 2H), 7.18 (d, J = 8.1 Hz, 2H), 2.89 (dt, J = 13.8, 6.9 Hz, 1H), 2.65 (s, 2H), 2.13 (s, 3H), 1.36 (s, 6H), 1.23 (d, J = 6.9 Hz, 6H). 13C NMR (75 MHz, CDCl3) δ 206.81, 150.07, 137.69, 128.23, 126.87, 54.45, 46.93, 33.86, 32.26, 28.15, 23.90. HRMS (ESI-MS) [M + Na]+: found 273.1290; calculated for C15H22NaOS+: 273.1284.
4-((2,4-Dimethylphenyl)thio)-4-methylpentan-2-one (4e). Oil liquid, 89% yield. 1H NMR (300 MHz, CDCl3) δ 7.39 (d, J = 7.8 Hz, 1H), 7.11 (s, 1H), 6.97 (d, J = 7.7 Hz, 1H), 2.71 (s, 2H), 2.47 (s, 3H), 2.32 (s, 3H), 2.15 (s, 3H), 1.37 (s, 6H). 13C NMR (75 MHz, CDCl3) δ 206.94, 143.90, 139.50, 139.06, 131.58, 127.59, 126.92, 54.73, 48.46, 32.37, 28.21, 21.84, 21.25. HRMS (ESI-MS) [M + Na]+: found 259.1136; calculated for C14H20NaOS+: 259.1127.
4-((4-Methoxyphenyl)thio)-4-methylpentan-2-one (4f) [20]. Oil liquid, 87% yield. 1H NMR (300 MHz, CDCl3) δ 7.38 (d, J = 8.6 Hz, 2H), 6.82 (d, J = 8.6 Hz, 2H), 3.75 (s, 3H), 2.59 (s, 2H), 2.09 (s,3H), 1.31 (s, 6H). 13C NMR (75 MHz, CDCl3) δ 206.71, 160.47, 139.01, 122.19, 114.15, 55.24, 54.22, 46.74, 32.16, 27.94. HRMS (ESI-MS) [M + Na]+: found 261.0920; calculated for C13H18NaO2S+: 261.0920.
4-((4-Fluorophenyl)thio)-4-methylpentan-2-one (4g) [30]. Oil liquid, 76% yield. 1H NMR (300 MHz, CDCl3) δ 7.67–7.33 (m, 2H), 7.01 (t, J = 8.5 Hz, 2H), 2.62 (s, 2H), 2.12 (s, 3H), 1.34 (s, 6H). 13C NMR (75 MHz, CDCl3) δ 206.53 (s), 163.68 (d, J = 249.7 Hz), 139.63 (d, J = 8.4 Hz), 126.94 (d, J = 3.5 Hz), 115.93 (d, J = 21.6 Hz), 54.39, 47.20 (d, J = 1.3 Hz), 32.28, 28.18. 19F NMR (282 MHz, Chloroform-d) δ -111.91 (tt, J = 8.6, 5.5 Hz). HRMS (ESI-MS) [M + Na]+: found 249.0726;calculated for C12H15FNaOS+: 249.0720.
4-((4-Chlorophenyl)thio)-4-methylpentan-2-one (4h) [20]. Oil liquid, 85% yield. 1H NMR (300 MHz, CDCl3) δ 7.43 (d, J = 8.4 Hz, 2H), 7.30 (d, J = 8.4 Hz, 2H), 2.63 (s, 2H), 2.12 (s, 3H), 1.36 (s, 6H). 13C NMR (75 MHz, CDCl3) δ 206.35, 138.89, 135.74, 130.09, 128.99, 54.39, 47.45, 32.23, 28.24. HRMS (ESI-MS) [M + Na]+: found 265.0429; calculated for C12H15ClNaOS+: 265.0424.
4-((4-Bromophenyl)thio)-4-methylpentan-2-one (4i) [20]. Oil liquid, 79% yield. 1H NMR (300 MHz, CDCl3) δ 7.45 (d, J = 8.4 Hz, 2H), 7.35 (d, J = 8.4 Hz, 2H), 2.62 (s, 2H), 2.11 (s, 3H), 1.35 (s, 6H). 13C NMR (75 MHz, CDCl3) δ 206.32, 139.13, 131.93, 130.63, 124.02, 54.32, 47.40, 32.21, 28.21. HRMS (ESI-MS) [M + Na]+: found 308.9923; calculated for C12H15BrNaOS+: 308.9919.
4-((4-Iodophenyl)thio)-4-methylpentan-2-one (4j). Oil liquid, 76% yield. 1H NMR (300 MHz, CDCl3) δ 7.64 (d, J = 8.2 Hz, 2H), 7.21 (d, J = 8.2 Hz, 2H), 2.62 (s, 2H), 2.11 (s, 3H), 1.34 (s, 6H). 13C NMR (75 MHz, CDCl3) δ 206.26, 139.25, 137.85, 131.32, 95.94, 54.27, 47.38, 32.20, 28.18. HRMS (ESI-MS) [M + Na]+: found 356.9786; calculated for C12H15INaOS+: 356.9780.
4-Methyl-4-((4-(trifluoromethyl)phenyl)thio)pentan-2-one (4k). Oil liquid, 63% yield. 1H NMR (300 MHz, CDCl3) δ 7.62 (q, J = 8.4 Hz, 4H), 2.68 (s, 2H), 2.15 (s, 3H), 1.40 (s, 6H). 13C NMR (75 MHz, CDCl3) δ 206.28, 137.77, 136.40, 131.19 (q, J = 32.7 Hz), 125.59 (q, J = 3.6 Hz). 124.05 (q, J = 270.75 Hz), 54.46, 48.00, 32.25, 28.42. 19F NMR (282 MHz, CDCl3) δ = −62.74. HRMS (ESI-MS) [M + Na]+: found 299.0678; calculated for C13H15F3NaOS+: 299.0688.
4-Methyl-4-((4-nitrophenyl)thio)pentan-2-one (4l) [20]. Oil liquid, 68% yield. 1H NMR (300 MHz, CDCl3) δ 8.18 (d, J = 8.7 Hz, 2H), 7.68 (d, J = 8.7 Hz, 2H), 2.71 (s,2H), 2.15 (s, 3H), 1.44 (s, 6H). 13C NMR (75 MHz, CDCl3) δ 205.88, 148.19, 140.87, 137.59, 123.61, 54.47, 48.80, 32.21, 28.61. HRMS (ESI-MS) [M + Na]+: found 276.0668; calculated for C12H15NNaO3S+: 276.0665.
Methyl 4-((2-methyl-4-oxopentan-2-yl)thio)benzoate (4m). Oil liquid, 69% yield. 1H NMR (300 MHz, CDCl3) δ 7.92 (d, J = 8.1 Hz, 2H), 7.52 (d, J = 8.1 Hz, 2H), 3.84 (s, 3H), 2.61 (s, 2H), 2.06 (s, 3H), 1.33 (s, 6H). 13C NMR (75 MHz, CDCl3) δ 206.10, 166.44, 137.51, 137.08, 130.45, 129.56, 54.27, 52.22, 47.84, 32.06, 28.29. HRMS (ESI-MS) [M + Na]+: found 289.0872; calculated for C14H18NaO3S+: 289.0869.
4-(Benzylthio)-4-methylpentan-2-one (4n). Oil liquid, 85% yield. 1H NMR (300 MHz, CDCl3) δ 7.46–7.27 (m, 5H), 3.84 (s, 2H), 2.74 (s, 2H), 2.19 (s, 3H), 1.52 (s, 6H). 13C NMR (75 MHz, CDCl3) δ 206.76, 137.99, 129.03, 128.57, 127.00, 54.56, 44.32, 33.32, 32.28, 28.49. HRMS (ESI-MS) [M + Na]+: found 245.0978; calculated for C13H18NaOS+: 245.0971.
4-Methyl-4-((4-methylbenzyl)thio)pentan-2-one (4o). Oil liquid, 79% yield. 1H NMR (300 MHz, CDCl3) δ 7.22 (d, J = 7.9 Hz, 2H), 7.10 (d, J = 7.8 Hz, 2H), 3.75 (s, 2H), 2.69 (s, 2H), 2.31 (s, 3H), 2.14 (s, 3H), 1.46 (s, 6H). 13C NMR (75 MHz, CDCl3) δ 206.67, 136.47, 134.72, 129.17, 128.83, 54.47, 44.16, 32.89, 32.20, 28.42, 21.04. HRMS (ESI-MS) [M + Na]+: found 259.1130; calculated for C14H20NaOS+: 259.1127.
4-((4-Methoxybenzyl)thio)-4-methylpentan-2-one (4p) [31]. Oil liquid, 52% yield. 1H NMR (300 MHz, CDCl3) δ 7.33 (d, J = 8.6 Hz, 2H), 6.91 (d, J = 8.6 Hz, 2H), 3.86 (s, 3H), 3.82 (s, 2H), 2.77 (s, 2H), 2.24 (s, 3H), 1.53 (s, 6H). 13C NMR (75 MHz, CDCl3) δ 206.95, 158.66, 130.14, 129.79, 114.04, 55.35, 54.68, 44.30, 32.70, 32.40, 28.56. HRMS (ESI-MS) [M + Na]+: found 275.0924; calculated for C14H20NaO2S+: 275.1076.
4-((4-Chlorobenzyl)thio)-4-methylpentan-2-one (4q) [31]. Oil liquid, 85% yield. 1H NMR (300 MHz, CDCl3) δ 7.29 (s,4H), 3.77 (s, 2H), 2.72 (s, 2H), 2.18 (s, 3H), 1.47 (s, 6H). 13C NMR (75 MHz, CDCl3) δ 206.52, 136.56, 132.65, 130.35, 128.63, 54.54, 44.43, 32.58, 32.24, 28.48. HRMS (ESI-MS) [M + Na]+: found 279.0586; calculated for C13H17ClNaOS+: 279.0581.
4-(Butylthio)-4-methylpentan-2-one (4r). Oil liquid, 36% yield. 1H NMR (300 MHz, CDCl3) δ 2.66 (s, 2H), 2.51 (t, J = 7.3 Hz, 2H), 2.16 (s, 3H), 1.59–1.45 (m, 2H), 1.45–1.29 (m, 8H), 0.89 (t, J = 7.2 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 207.10, 54.73, 43.35, 32.46, 31.64, 28.57, 27.86, 22.43, 13.81. HRMS (ESI-MS) [M + Na]+: found 211.1126; calculated for C10H20NaOS+: 211.1127.
4-(Cyclohexylthio)-4-methylpentan-2-one (4s). Oil liquid, 41% yield. 1H NMR (300 MHz, CDCl3) δ 2.67 (s, 2H), 2.66–2.57 (m, 1H), 2.15 (s, 3H), 1.91 (d, J = 9.0 Hz, 2H), 1.73–1.64 (m, 2H), 1.56–1.49 (m, 1H), 1.39 (s, 6H), 1.35–1.09 (m, 5H).13C NMR (75 MHz, CDCl3) δ 207.05, 55.50, 44.70, 41.22, 36.24, 32.54, 29.07, 26.44, 25.52. HRMS (ESI-MS) [M + Na]+: found 237.1288; calculated for C12H22NaOS+: 237.1284.
4-Methyl-4-(naphthalen-2-ylthio)pentan-2-one (4t) [29]. Oil liquid, 85% yield. 1H NMR (300 MHz, CDCl3) δ 8.08 (s, 1H), 7.89–7.73 (m, 3H), 7.59 (dd, J = 8.4, 1.4 Hz, 1H), 7.51 (dd, J = 6.2, 3.3 Hz, 2H), 2.72 (s, 2H), 2.14 (s, 3H), 1.46 (s, 6H). 13C NMR (75 MHz, CDCl3) δ 206.52, 137.45, 134.17, 133.32, 133.16, 128.85, 128.05, 127.91, 127.61, 126.94, 126.43, 54.38, 47.51, 32.16, 28.30. HRMS (ESI-MS) [M + Na]+: found 281.0980; calculated for C16H18NaOS+: 281.0971.
4-Methyl-4-(thiophen-2-ylthio)pentan-2-one (4u). Oil liquid, 21% yield. 1H NMR (300 MHz, CDCl3) δ 7.45 (dd, J = 5.4, 0.9 Hz, 1H), 7.17 (dd, J = 3.4, 0.9 Hz, 1H), 7.06 (dd, J = 5.3, 3.6 Hz, 1H), 2.70 (s, 2H), 2.16 (s, 3H), 1.41 (s, 6H). 13C NMR (75 MHz, CDCl3) δ 206.68, 137.63, 131.52, 130.63, 127.87, 54.04, 48.05, 32.30, 27.8 HRMS (ESI-MS) [M + Na]+: found 237.0383; calculated for C10H14NaOS2+: 237.0378.
4. Conclusions
In summary, the synthesis methods of thioacetal, thioacetone, and 4-methyl-4-(arylsulfide)pentane-2-ketone promoted by methanesulfonic anhydride/sulfuric acid were discussed under mild conditions. In this paper, the mechanism of reaction is explored, and it is suggested that two reaction processes may have occurred simultaneously. Notably, using this synthetic approach eliminates the need for the prior preparation of α, β-unsaturated ketones. This strategy is characterized as simple and efficient while demonstrating good substrate compatibility. It is an effective method to prepare thioacetals/thioketals and thio-Michael addition products.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules29204785/s1: NMR spectra of products.
Author Contributions
Conceptualization, X.B.; methodology, H.Y. and J.L.; investigation, H.Y., X.Z. and Y.F.; data curation, H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study did not involve humans or animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article; further inquiries can be directed to the corresponding author/s.
Conflicts of Interest
There are no conflicts of interest to declare.
References
- Xiao, X.; Feng, M.; Jiang, X. Transition-metal-free persulfuration to construct unsymmetrical disulfides and mechanistic study of the sulfur redox process. Chem. Commun. 2015, 51, 4208–4211. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.B.; Adams, C.M. Evolution of dithiane-based strategies for the construction of architecturally complex natural products. Acc. Chem. Res. 2004, 37, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Althoff, F.; Benzing, K.; Comba, P.; McRoberts, C.; Boyd, D.R.; Greiner, S.; Keppler, F. Abiotic methanogenesis from organosulphur compounds under ambient conditions. Nat. Commun. 2014, 5, 4205. [Google Scholar] [CrossRef]
- Sasson, R.; Hagooly, A.; Rozen, S. Novel method for incorporating the CHF2 group into organic molecules using BrF3. Org. Lett. 2003, 5, 769–771. [Google Scholar] [CrossRef] [PubMed]
- Chiba, K.; Uchiyama, R.; Kim, S.; Kitano, Y.; Tada, M. Benzylic intermolecular carbon-carbon bond formation by selective anodic oxidation of dithioacetals. Org. Lett. 2001, 3, 1245–1248. [Google Scholar]
- Luh, T.Y. New synthetic applications of the dithioacetal functionality. Acc. Chem. Res. 1991, 24, 257–263. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, S. Involvement of Caveolin-1 in probucol-reduced hERG membrane expression. Biophys. J. 2011, 100, 102a. [Google Scholar] [CrossRef]
- Bursell, S.E.; Della Vecchia, K.M.; Clermont, A.C.; Takahashi, J.; Sundell, C.L.; Luchoomun, J.; Aiello, L.P. Early diabetes–induced retinal vascular abnormalities are ameliorated by the antioxidant Agix-4207 in diabetic rats. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3207. [Google Scholar]
- Nicoll-Griffith, D.A.; Gupta, N.; Twa, S.P.; Williams, H.; Trimble, L.A.; Yergey, J.A. Verlukast (MK-0679) conjugation with glutathione by rat liver and kidney cytosols and excretion in the bile. Drug Metab. Dispos. 1995, 23, 1085–1093. [Google Scholar]
- Sharma, G.; Kumar, R.; Chakraborti, A.K. Fluoroboric acid adsorbed on silica-gel (HBF4-SiO2) as a new, highly efficient and reusable heterogeneous catalyst for thia-Michael addition to α, β-unsaturated carbonyl compounds. Tetrahedron Lett. 2008, 49, 4272–4275. [Google Scholar] [CrossRef]
- Perni, R.B. Amberlyst-15 as a convenient catalyst for chemoselective thioacetalization. Synth. Commun. 1989, 19, 2383–2387. [Google Scholar] [CrossRef]
- Shinde, P.D.; Borate, H.B.; Wakharkar, R.D. Thioacetalization of the carbonyl function, transthioacetalization of acetals, ketals, oximes and hydrazones catalysed by aqueous hydrobromic acid. Arkivoc 2004, 14, 110–117. [Google Scholar] [CrossRef]
- Miyake, H.; Nakao, Y.; Sasaki, M. Oxalic acid-promoted preparation of dithioacetals from carbonyl compounds or acetals. Chem. Lett. 2007, 36, 104–105. [Google Scholar] [CrossRef]
- Soderstrom, M.; Matt, C.; Odell, L.R. Thioacetalation and multi-component thiomethylative Friedel-Crafts arylation using BF3SMe2. ACS Omega 2023, 8, 4320–4330. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.C.; Zhu, J. Hafnium trifluoromethanesulfonate (hafnium triflate) as a highly efficient catalyst for chemoselective thioacetalization and transthioacetalization of carbonyl compounds. J. Org. Chem. 2008, 73, 9522–9524. [Google Scholar] [CrossRef]
- Madabhushi, S.; Mallu, K.K.R.; Chinthala, N.; Beeram, C.R.; Vangipuram, V.S. Efficient and chemoselective acetalization and thioacetalization of carbonyls and subsequent deprotection using InF3 as a reusable catalyst. Tetrahedron Lett. 2012, 53, 697–701. [Google Scholar] [CrossRef]
- Xing, Z.; Yang, M.; Sun, H.; Wang, Z.; Chen, P.; Liu, L.; She, X. Visible-light promoted dithioacetalization of aldehydes with thiols under aerobic and photocatalyst-free conditions. Green Chem. 2018, 20, 5117–5122. [Google Scholar] [CrossRef]
- Du, K.; Wang, S.C.; Basha, R.S.; Lee, C.F. Visible-light Photoredox-catalyzed thioacetalization of aldehydes under metal-free and solvent-free conditions. Adv. Synth. Catal. 2019, 361, 1597–1605. [Google Scholar] [CrossRef]
- Khatik, G.L.; Sharma, G.; Kumar, R.; Chakraborti, A.K. Scope and limitations of HClO4-SiO2 as an extremely efficient, inexpensive, and reusable catalyst for chemoselective carbon-sulfur bond formation. Tetrahedron Lett. 2007, 63, 1200–1210. [Google Scholar] [CrossRef]
- Peng, A.; Rosenblatt, R.; Nolin, K. Conjugate addition of unactivated thiols to α, β-unsaturated ketones catalyzed by a bifunctional rhenium (V)-oxo complex. Tetrahedron Lett. 2012, 53, 2712–2714. [Google Scholar] [CrossRef]
- Lauder, K.; Toscani, A.; Qi, Y.; Lim, J.; Charnock, S.J.; Korah, K.; Castagnolo, D. Photo-biocatalytic one-pot cascades for the enantioselective synthesis of 1, 3-mercaptoalkanol volatile sulfur compounds. Angew. Chem. Int. Ed. 2018, 130, 5905–5909. [Google Scholar] [CrossRef]
- Cong, Z.S.; Li, Y.G.; Du, G.F.; Gu, C.Z.; Dai, B.; He, L. N-Heterocyclic carbene-catalyzed sulfa-Michael addition of enals. Chem. Commun. 2017, 53, 13129–13132. [Google Scholar] [CrossRef] [PubMed]
- Lorette, N.B.; Howard, W.L.; Brown, J.H., Jr. Preparation of ketone acetals from linear ketones and alcohols. J. Org. Chem. 1959, 24, 1731–1733. [Google Scholar] [CrossRef]
- Leitemberger, A.; Böhs, L.M.; Peixoto, M.L.; Rosa, C.H.; Rosa, G.R.; Godoi, M. Sulfamic acid-catalyzed thioacetalization of aldehydes under solvent and metal-free conditions. Chem. Sel. 2020, 5, 8253–8257. [Google Scholar] [CrossRef]
- Hirschbeck, V.; Boldl, M.; Gehrtz, P.H.; Fleischer, I. Tandem acyl substitution/Michael addition of thioesters with vinylmagnesium bromide. Org. Lett. 2019, 21, 2578–2582. [Google Scholar] [CrossRef]
- Silva-Cuevas, C.; Paleo, E.; León-Rayo, D.F.; Lujan-Montelongo, J.A. An expeditious and efficient bromomethylation of thiols: Enabling bromomethyl sulfides as useful building blocks. RSC Adv. 2018, 8, 24654–24659. [Google Scholar] [CrossRef]
- Arunprasath, D.; Sekar, G. A Transition-metal-free and base-mediated carbene insertion into sulfur-sulfur and selenium-selenium bonds: An easy access to thio-and selenoacetals. Adv. Synth. Catal. 2017, 359, 698–708. [Google Scholar] [CrossRef]
- Bognar, S.; van Gemmeren, M. Direct synthesis of unsymmetrical dithioacetals. Chem. Eur. J. 2021, 27, 4859–4863. [Google Scholar] [CrossRef]
- MacNicol, D.D.; McKendrick, J.J. Formation of benzo [b] thiophens and related compounds by a rearrangement involving ring contraction. J. Chem. Soc. Perkin Trans. 1 1974, 6, 2493–2496. [Google Scholar] [CrossRef]
- Xu, B.; Ye, Y.; Lin, Y.; Bai, R.; Ye, X.Y.; Xie, T. Cu-catalyzed coupling of unactivated tertiary alkyl alcohols with thiols via C-O bond cleavage. Tetrahedron Lett. 2022, 89, 153604. [Google Scholar] [CrossRef]
- Tian, X.; Cassani, C.; Liu, Y.; Moran, A.; Urakawa, A.; Galzerano, P.; Melchiorre, P. Diastereodivergent asymmetric sulfa-Michael additions of α-branched enones using a single chiral organic catalyst. J. Am. Chem. Soc. 2011, 133, 17934–17941. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).