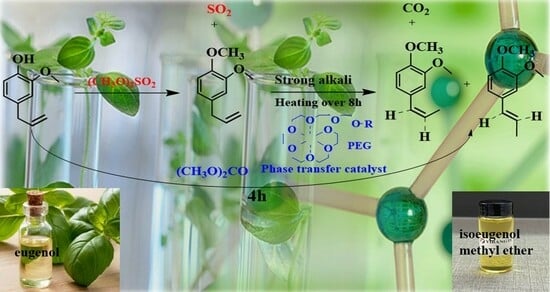
One-Step Green Synthesis of Isoeugenol Methyl Ether from Eugenol by Dimethyl Carbonate and Phase-Transfer Catalysts
Abstract
1. Introduction
1.1. O-Methylation
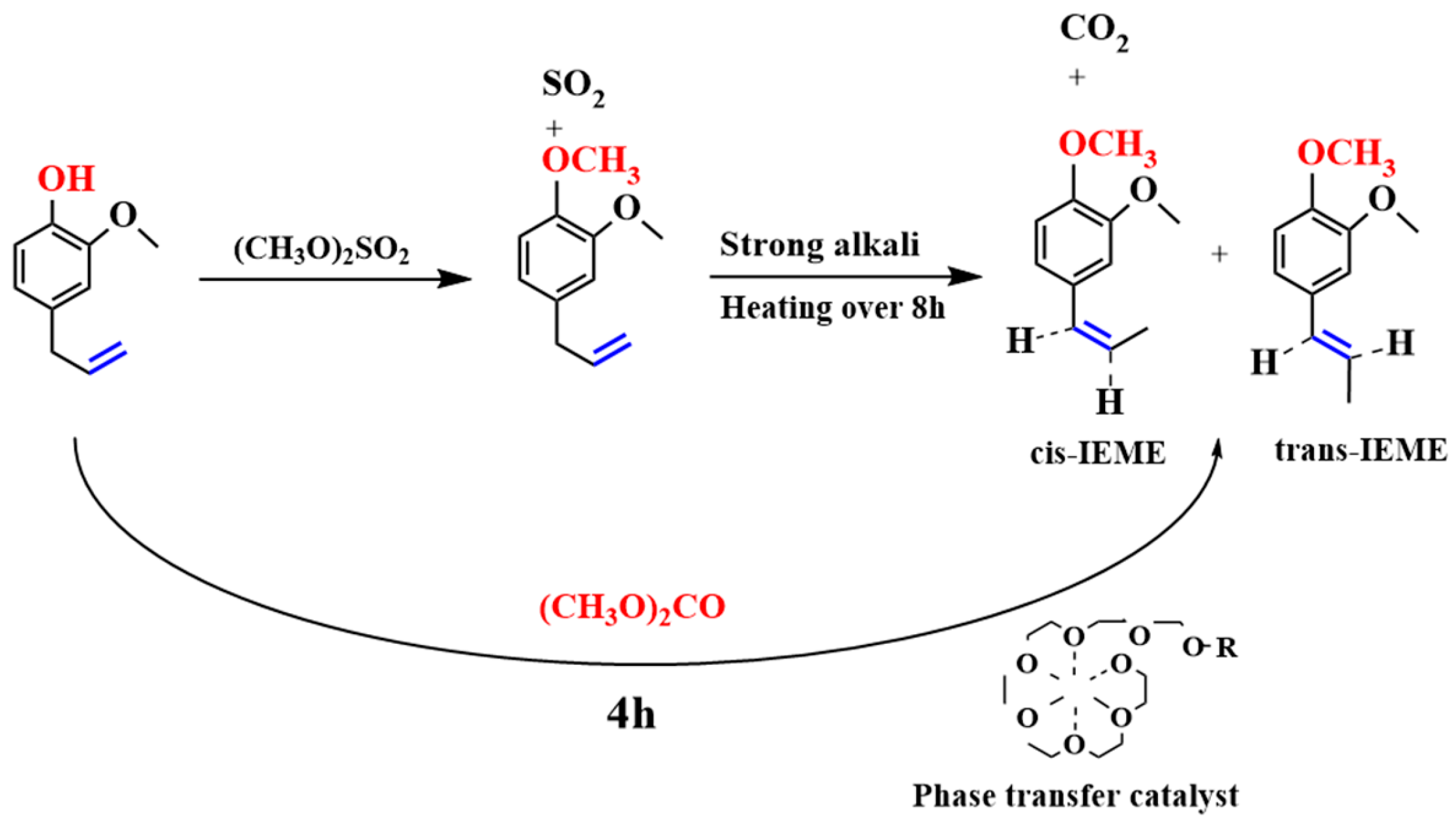
1.2. Allylbenzene Isomerization
2. Results and Discussion
2.1. Catalytic System Categories
2.2. Single-Factor Influence
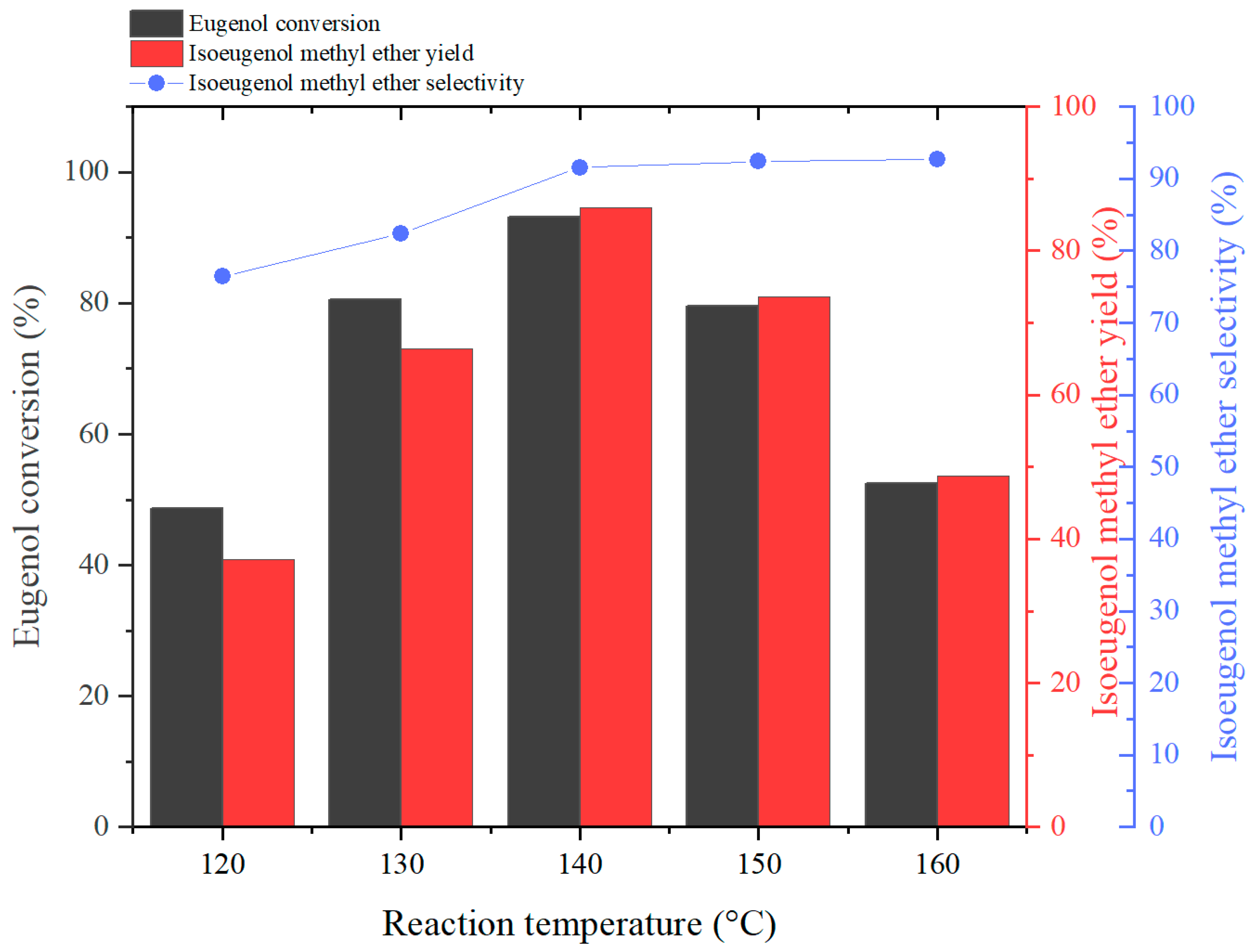
2.2.1. Effect of Reaction Temperature on Reaction
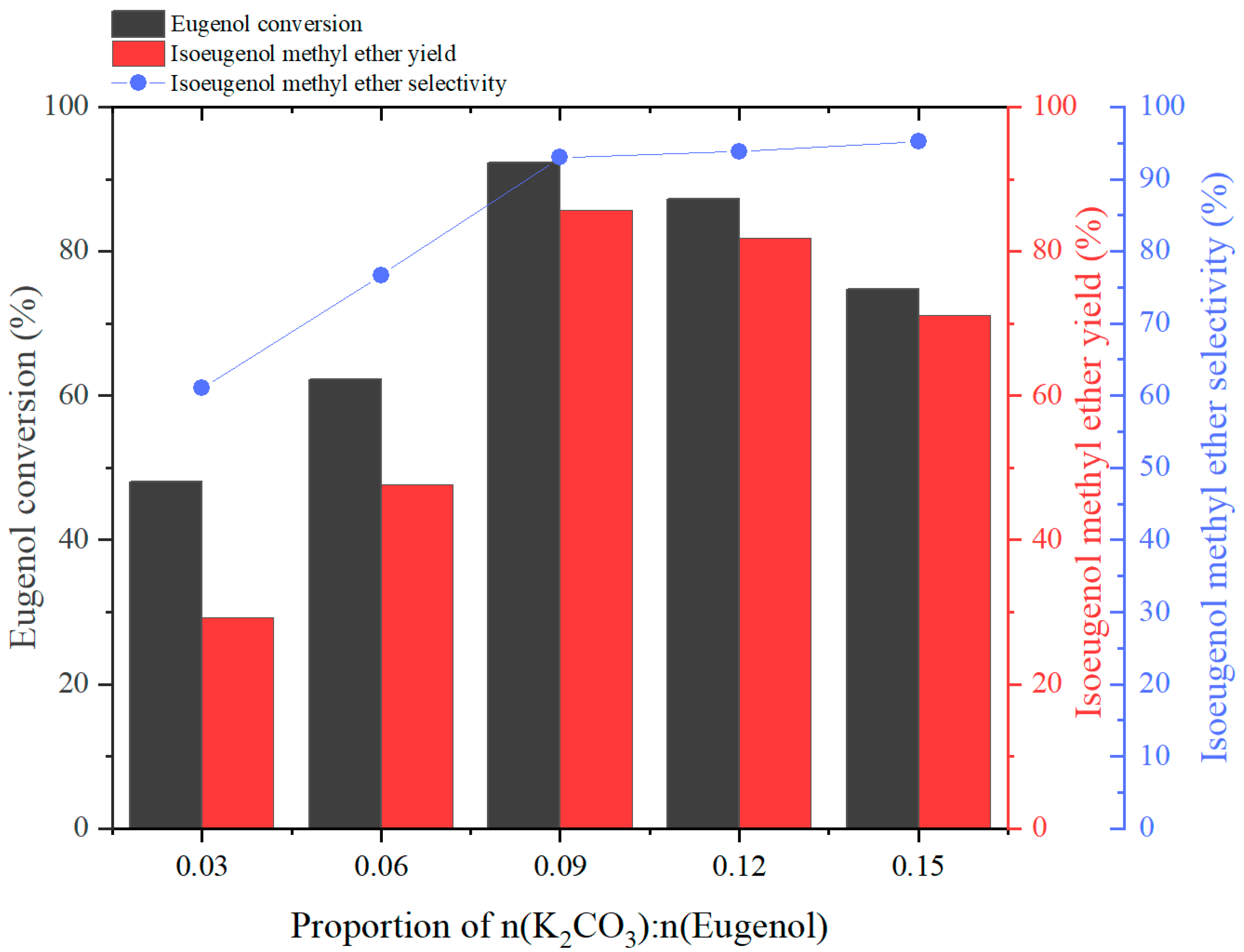
2.2.2. Effect of n(Eugenol):n(K2CO3) Ratio on the Reaction
2.2.3. Effect of n(Eugenol):n(PEG-800) Ratio on the Reaction
2.2.4. Effect of n(Eugenol):n(DMC) Ratio on the Reaction
2.2.5. Effect of DMC Drip Rates on the Reaction
3. Experimental Section
3.1. Materials
3.2. Methods
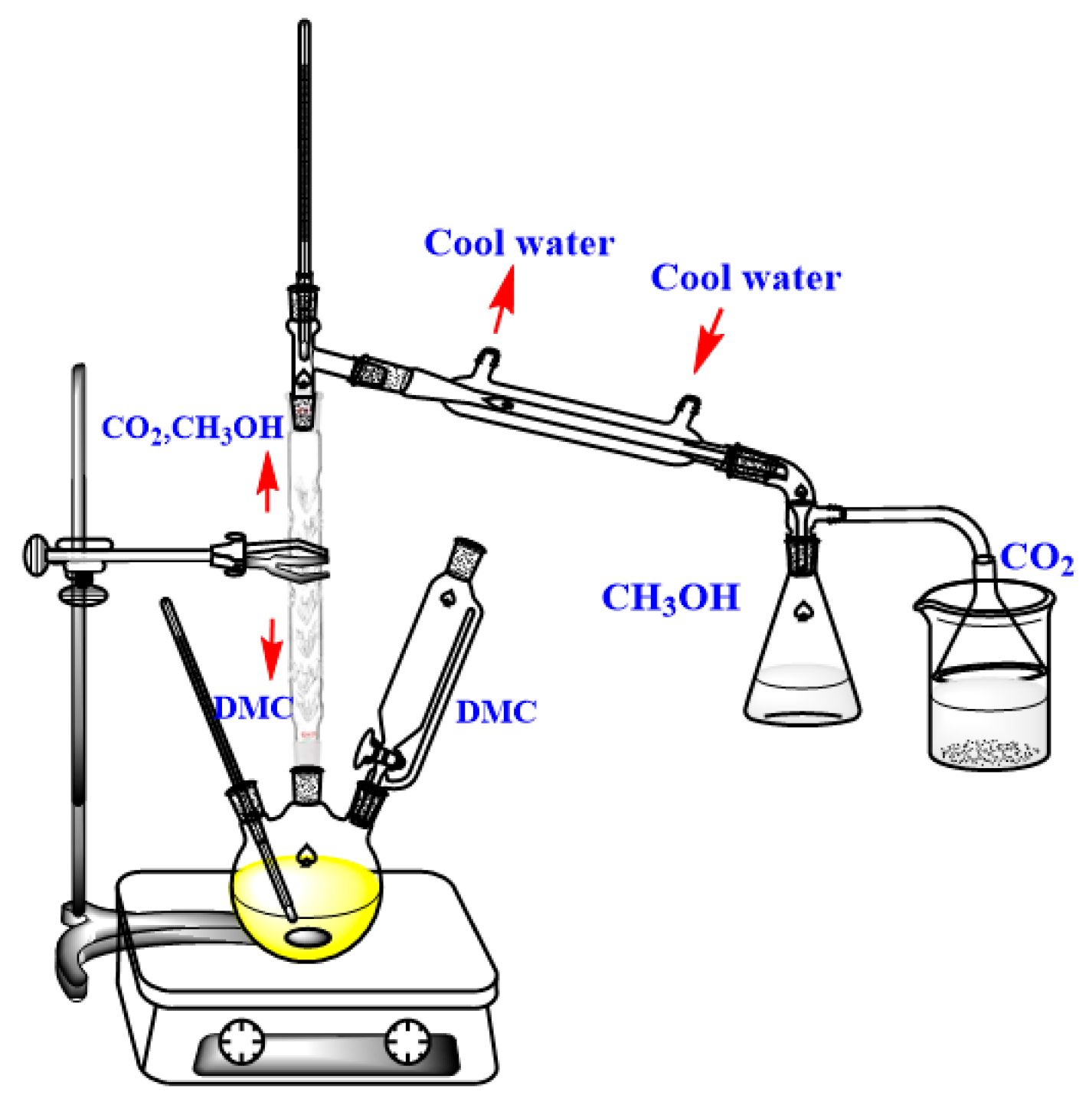
3.2.1. Synthesis Process
3.2.2. GC Analysis
3.2.3. IEME Yield Analysis
- w: content of IEME in the product, %, detected by GC;
- M: relative molecular mass of IEME, g/mol;
- n: theoretical molar value of IEME, mol.
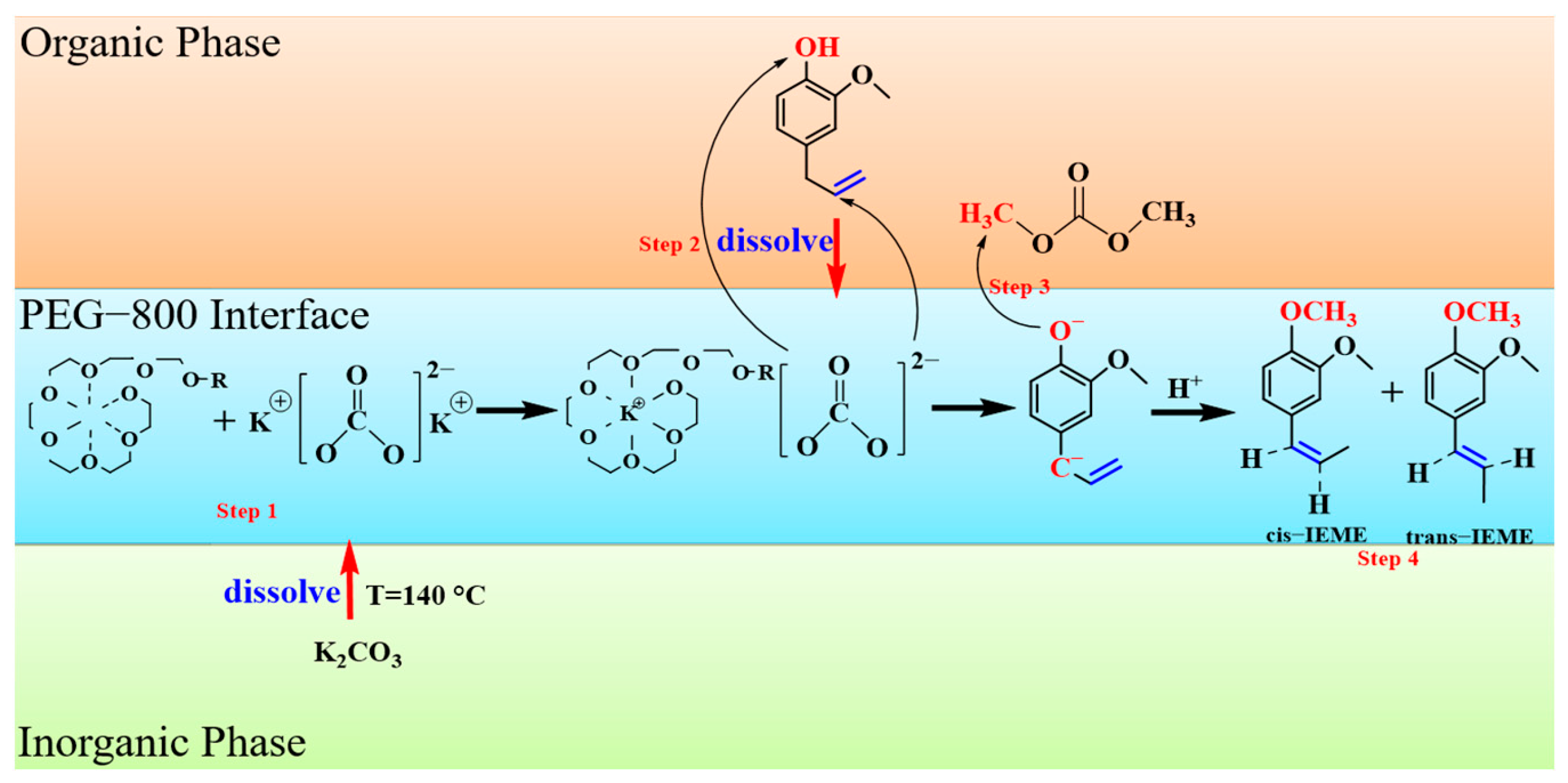
3.2.4. Reaction Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, C.; Prakash, D.; Gupta, S. A biotechnological approach to microbial based perfumes and flavours. J. Microbiol. Exp. 2015, 2. [Google Scholar] [CrossRef]
- Andraos, J.; Matlack, A.S. Introduction to Green Chemistry; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Mazzucotelli, M.; Bicchi, C.; Marengo, A.; Rubiolo, P.; Galli, S.; Anderson, J.L.; Sgorbini, B.; Cagliero, C. Ionic liquids as stationary phases for gas chromatography—Unusual selectivity of ionic liquids with a phosphonium cation and different anions in the flavor, fragrance and essential oil analyses. J. Chromatogr. A 2019, 1583, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Akagi, J.-I.; Cho, Y.-M.; Mizuta, Y.; Tatebe, C.; Sato, K.; Ogawa, K. Subchronic toxicity evaluation of isoeugenyl methyl ether in F344/DuCrj rats by 13-week oral administration. Regul. Toxicol. Pharmacol. 2019, 102, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Api, A.; Belsito, D.; Botelho, D.; Bruze, M.; Burton, G., Jr.; Buschmann, J.; Cancellieri, M.; Dagli, M.; Date, M.; Dekant, W. RIFM fragrance ingredient safety assessment, isoeugenyl ethyl ether, CAS Registry Number 7784-67-0. Food Chem. Toxicol. 2022, 161, 112873. [Google Scholar] [CrossRef] [PubMed]
- Velikorodov, A.V.; Kovalev, V.B.; Nosachev, S.B.; Tyrkov, A.G.; Pitelina, M.V.; Shchepetova, E.V. The Chemical Composition of Essential Oils from Wildgrowing and Introduced Plants of the Astrakhan Region. In Chemistry and Technology of Plant Substances; Apple Academic Press: Palm Bay, NJ, USA, 2017; pp. 309–335. [Google Scholar]
- Da Silva, C.E.; Minguzzi, S.; da Silva, R.C.; Matos, M.F.; Tofoli, D.; de Carvalho, J.E.; Ruiz, A.L.; de Costa, W.F.; Simionatto, E. Chemical composition and cytotoxic activity of the root essential oil from Jatropha ribifolia (Pohl) Baill (Euphorbiaceae). J. Braz. Chem. Soc. 2015, 26, 233–238. [Google Scholar]
- Gholipour, F.; Rahmani, M.; Panahi, F. Efficient and selective microwave-assisted O-methylation of phenolic compounds using tetramethylammonium hydroxide (TMAH). Green Process. Synth. 2019, 8, 584–589. [Google Scholar] [CrossRef]
- Moulay, S. O-Methylation of hydroxyl-containing organic substrates: A comprehensive overview. Curr. Org. Chem. 2018, 22, 1986–2016. [Google Scholar] [CrossRef]
- Chen, Y. Recent advances in methylation: A guide for selecting methylation reagents. Chem. Eur. J. 2019, 25, 3405–3439. [Google Scholar] [CrossRef]
- Prakoso, N.; Pangestu, P.; Wahyuningsih, T. O-methylation of natural phenolic compounds based on green chemistry using dimethyl carbonate. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2016. [Google Scholar]
- Selva, M.; Perosa, A. Green chemistry metrics: A comparative evaluation of dimethyl carbonate, methyl iodide, dimethyl sulfate and methanol as methylating agents. Green Chem. 2008, 10, 457–464. [Google Scholar] [CrossRef]
- Wu, M.; Long, Z.; Xiao, H.; Dong, C. Preparation of N,N,N-trimethyl chitosan via a novel approach using dimethyl carbonate. Carbohydr. Polym. 2017, 169, 83–91. [Google Scholar] [CrossRef]
- Sánchez, A.; Gil, L.M.; Martín, M. Sustainable DMC production from CO2 and renewable ammonia and methanol. J. CO2 Util. 2019, 33, 521–531. [Google Scholar] [CrossRef]
- Pyo, S.-H.; Park, J.H.; Chang, T.-S.; Hatti-Kaul, R. Dimethyl carbonate as a green chemical. Curr. Opin. Green Sustain. Chem. 2017, 5, 61–66. [Google Scholar] [CrossRef]
- Tang, Y.; Slaný, M.; Yang, Y.; Li, S.; Qin, F.; Zhao, Y.; Zhang, Z.; Zhang, L. Highly active Mg–Al hydrotalcite for efficient O-methylation of phenol with DMC based on soft colloidal templates. J. Chem. Technol. Biotechnol. 2022, 97, 79–86. [Google Scholar] [CrossRef]
- Tundo, P. New developments in dimethyl carbonate chemistry. Pure Appl. Chem. 2001, 73, 1117–1124. [Google Scholar] [CrossRef][Green Version]
- Ouk, S.; Thiébaud, S.; Borredon, E.; Le Gars, P. High performance method for O-methylation of phenol with dimethyl carbonate. Appl. Catal. A Gen. 2003, 241, 227–233. [Google Scholar] [CrossRef]
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach; John Wiley & Sons: Hoboken, NJ, USA, 2002. [Google Scholar]
- Liu, X.; Li, B.; Liu, Q. Base-metal-catalyzed olefin isomerization reactions. Synthesis 2019, 51, 1293–1310. [Google Scholar] [CrossRef]
- Hassam, M.; Taher, A.; Arnott, G.E.; Green, I.R.; van Otterlo, W.A. Isomerization of allylbenzenes. Chem. Rev. 2015, 115, 5462–5569. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Q. Catalytic asymmetric olefin isomerization: Facile access to chiral carbon-stereogenic olefinic compounds. Chem. Catal. 2022, 2, 2852–2864. [Google Scholar] [CrossRef]
- Goodajdar, B.M.; Akbari, F.; Davarpanah, J. PEG-DIL-based MnCl42−: A novel phase transfer catalyst for nucleophilic substitution reactions of benzyl halides. Appl. Organomet. Chem. 2019, 33, e4647. [Google Scholar] [CrossRef]
- Jose, D.E.; Kanchana, U.; Mathew, T.V.; Anilkumar, G. Recent studies in Suzuki-Miyaura cross-coupling reactions with the aid of phase transfer catalysts. J. Organomet. Chem. 2020, 927, 121538. [Google Scholar] [CrossRef]
- Rao, N.M.; Choudary, B.M.; Chary, M.T. Polyethylene Glycol (PEG-400): An Efficient Green Reaction Medium for the Alkylation of 3, 4-Dihydroxy Thiophene-2, 5-Dicarboxylic Esters or Their Alkali Metal or Alkaline Earth Metal Salts. 2017. Available online: https://www.semanticscholar.org/paper/polyethylene-glycol-(peg-400)%3A-an-efficient-green-rao/6ae51ef7fc27628502839c2538e42d2a3b260284 (accessed on 16 January 2024).
- Jagtapa, J.R.; Jadhava, P.A.; Deodhara, M.N.; Chavana, R.S. Phase Transfer Catalysis: A Green Approach in Organic Synthesis. J. Basic Sci. 2022, 22, 292–310. [Google Scholar]
- Kasséhin, U.C.; Gbaguidi, F.A.; Carato, P.; McCurdy, C.R.; Accrombessi, G.C.; Poupaert, J.H. Solid-Liquid Phase Transfer Catalysis and Microwave-Assisted Green Synthesis of Tetracyclone. Am. J. Org. Chem. 2015, 5, 149–152. [Google Scholar]
- Steinbauer, J.; Werner, T. Poly(ethylene glycol)s as Ligands in Calcium-Catalyzed Cyclic Carbonate Synthesis. ChemSusChem 2017, 10, 3025–3029. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Do, L.H. Thermally robust heterobimetallic palladium–alkali catalysts for ethylene and alkyl acrylate copolymerization. Organometallics 2018, 37, 3874–3882. [Google Scholar] [CrossRef]
- D’souza, A.A.; Shegokar, R. Polyethylene glycol (PEG): A versatile polymer for pharmaceutical applications. Expert Opin. Drug Deliv. 2016, 13, 1257–1275. [Google Scholar] [CrossRef] [PubMed]
- Ouk, S.; Thiebaud, S.; Borredon, E.; Legars, P.; Lecomte, L.C. O-Methylation of phenolic compounds with dimethyl carbonate under solid/liquid phase transfer system. Tetrahedron Lett. 2002, 43, 2661–2663. [Google Scholar] [CrossRef]
- Selva, M.; Perosa, A.; Rodríguez-Padrón, D.; Luque, R. Applications of dimethyl carbonate for the chemical upgrading of biosourced platform chemicals. ACS Sustain. Chem. Eng. 2019, 7, 6471–6479. [Google Scholar] [CrossRef]
- Li, K.-M.; Zhang, Q.; Xu, Z.-M.; Chen, R.; Liu, T.-T.; Luo, J.-Y.; Wu, Y.-W.; Huang, Y.-B.; Lu, Q. Tunable mono- and di-methylation of amines with methanol over bimetallic CuCo nanoparticle catalysts. Green Chem. 2022, 24, 5965–5977. [Google Scholar] [CrossRef]
- Saputra, L.; Gustini, N.; Sinambela, N.; Indriyani, N.P.; Sakti, A.W.; Arrozi, U.S.F.; Martoprawiro, M.A.; Patah, A.; Permana, Y. Nitrile modulated-Ni(0) phosphines in trans-selective phenylpropenoids isomerization: An allylic route by a regular η1-N (end-on) or an alkyl route via a flipped-nitrile? Mol. Catal. 2022, 533, 112768. [Google Scholar] [CrossRef]
- Tundo, P.; Musolino, M.; Aricò, F. The reactions of dimethyl carbonate and its derivatives. Green Chem. 2018, 20, 28–85. [Google Scholar] [CrossRef]
- Aricò, F.; Evaristo, S.; Tundo, P. The neighbouring effect of isosorbide and its epimers in their reactions with dimethyl carbonate. Sci. Res. 2014. [Google Scholar]
- Liu, Z.; Tang, B.; Zhang, S. Novel network structural PEG/PAA/SiO2 composite phase change materials with strong shape stability for storing thermal energy. Sol. Energy Mater. Sol. Cells 2020, 216, 110678. [Google Scholar] [CrossRef]
- Memboeuf, A.; Vékey, K.; Lendvay, G. Structure and energetics of poly(ethylene glycol) cationized by Li+, Na+, K+ and Cs+: A first-principles study. Eur. J. Mass Spectrom. 2011, 17, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Kabra, S.K.; Huuhtanen, M.; Keiski, R.L.; Yadav, G.D. Selectivity engineering of O-methylation of hydroxybenzenes with dimethyl carbonate using ionic liquid as catalyst. React. Chem. Eng. 2016, 1, 330–339. [Google Scholar] [CrossRef]
- Zhang, S.; Findlater, M. Cobalt-catalyzed isomerization of alkenes. Synthesis 2021, 53, 2787–2797. [Google Scholar]
- Coyanis, E.M.; Panayides, J.-L.; Fernandes, M.A.; de Koning, C.B.; van Otterlo, W.A. Ring-closing metathesis for the synthesis of substituted indenols, indenones, indanones and indenes: Tandem RCM-dehydrogenative oxidation and RCM-formal redox isomerization. J. Organomet. Chem. 2006, 691, 5222–5239. [Google Scholar] [CrossRef]
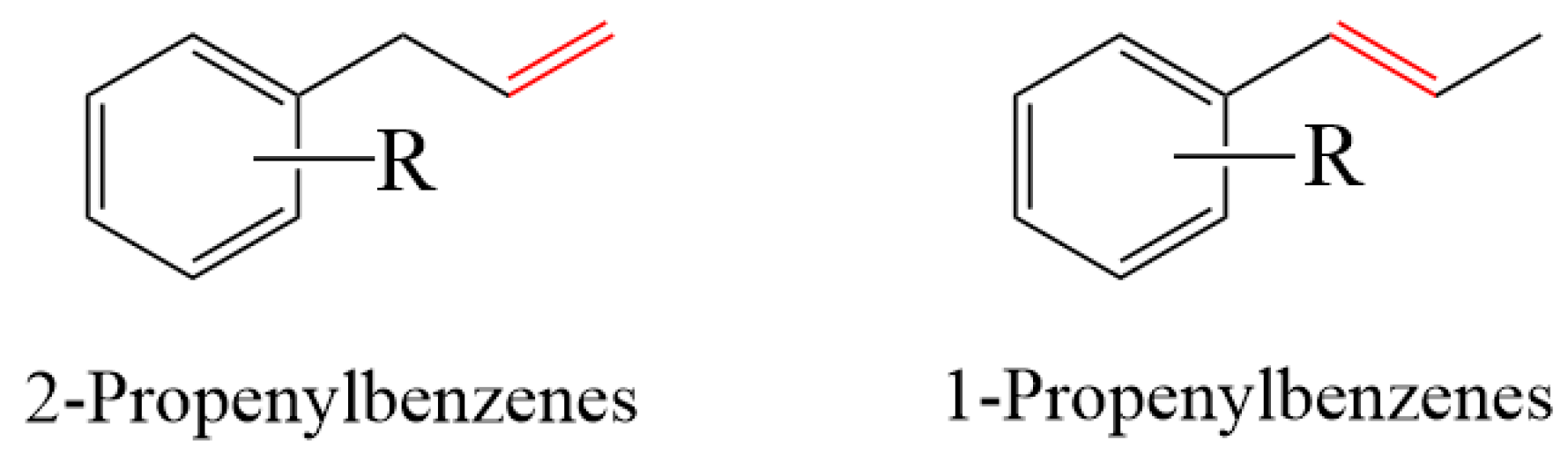



| Catalytic System | Catalyst | Eugenol Conversion (%) | IEME Yield (%) | IEME Selectivity (%) |
|---|---|---|---|---|
| Inorganic catalyst | KOH | 42.1 | 35.2 | 83.6 |
| KF | 11.5 | 7.4 | 64.3 | |
| K2CO3 | 89.7 | 10.9 | 12.2 | |
| CH3COOK | 7.4 | 0.6 | 8.1 | |
| Na2CO3 | 65.2 | 3.3 | 5.0 | |
| NaOH | 34.7 | 24.6 | 70.8 | |
| Inorganic catalyst and PTC | K2CO3 + 18-Crown-6 | 88.2 | 78.6 | 89.1 |
| KOH + 18-Crown-6 | 45.6 | 40.1 | 87.9 | |
| K2CO3 + TBAB | 80.7 | 65.6 | 81.3 | |
| K2CO3 + PEG-400 | 84.2 | 71.3 | 84.7 | |
| K2CO3 + PEG-600 | 88.9 | 77.6 | 87.3 | |
| K2CO3 + PEG-800 | 92.6 | 86.1 | 93.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Gong, Y.; Xue, X.; Hu, M.; Zhou, M.; Zhao, Y.; Hu, Z. One-Step Green Synthesis of Isoeugenol Methyl Ether from Eugenol by Dimethyl Carbonate and Phase-Transfer Catalysts. Molecules 2024, 29, 551. https://doi.org/10.3390/molecules29020551
Zhang Z, Gong Y, Xue X, Hu M, Zhou M, Zhao Y, Hu Z. One-Step Green Synthesis of Isoeugenol Methyl Ether from Eugenol by Dimethyl Carbonate and Phase-Transfer Catalysts. Molecules. 2024; 29(2):551. https://doi.org/10.3390/molecules29020551
Chicago/Turabian StyleZhang, Zhihai, Yin Gong, Xinru Xue, Mengshuang Hu, Min Zhou, Yao Zhao, and Zhiqiang Hu. 2024. "One-Step Green Synthesis of Isoeugenol Methyl Ether from Eugenol by Dimethyl Carbonate and Phase-Transfer Catalysts" Molecules 29, no. 2: 551. https://doi.org/10.3390/molecules29020551
APA StyleZhang, Z., Gong, Y., Xue, X., Hu, M., Zhou, M., Zhao, Y., & Hu, Z. (2024). One-Step Green Synthesis of Isoeugenol Methyl Ether from Eugenol by Dimethyl Carbonate and Phase-Transfer Catalysts. Molecules, 29(2), 551. https://doi.org/10.3390/molecules29020551