A Statistical Physics Approach to Understanding the Adsorption of Methylene Blue onto Cobalt Oxide Nanoparticles
Abstract
1. Introduction
2. Results
2.1. X-ray Diffraction Pattern Results
2.2. FT-IR Results for the Materials
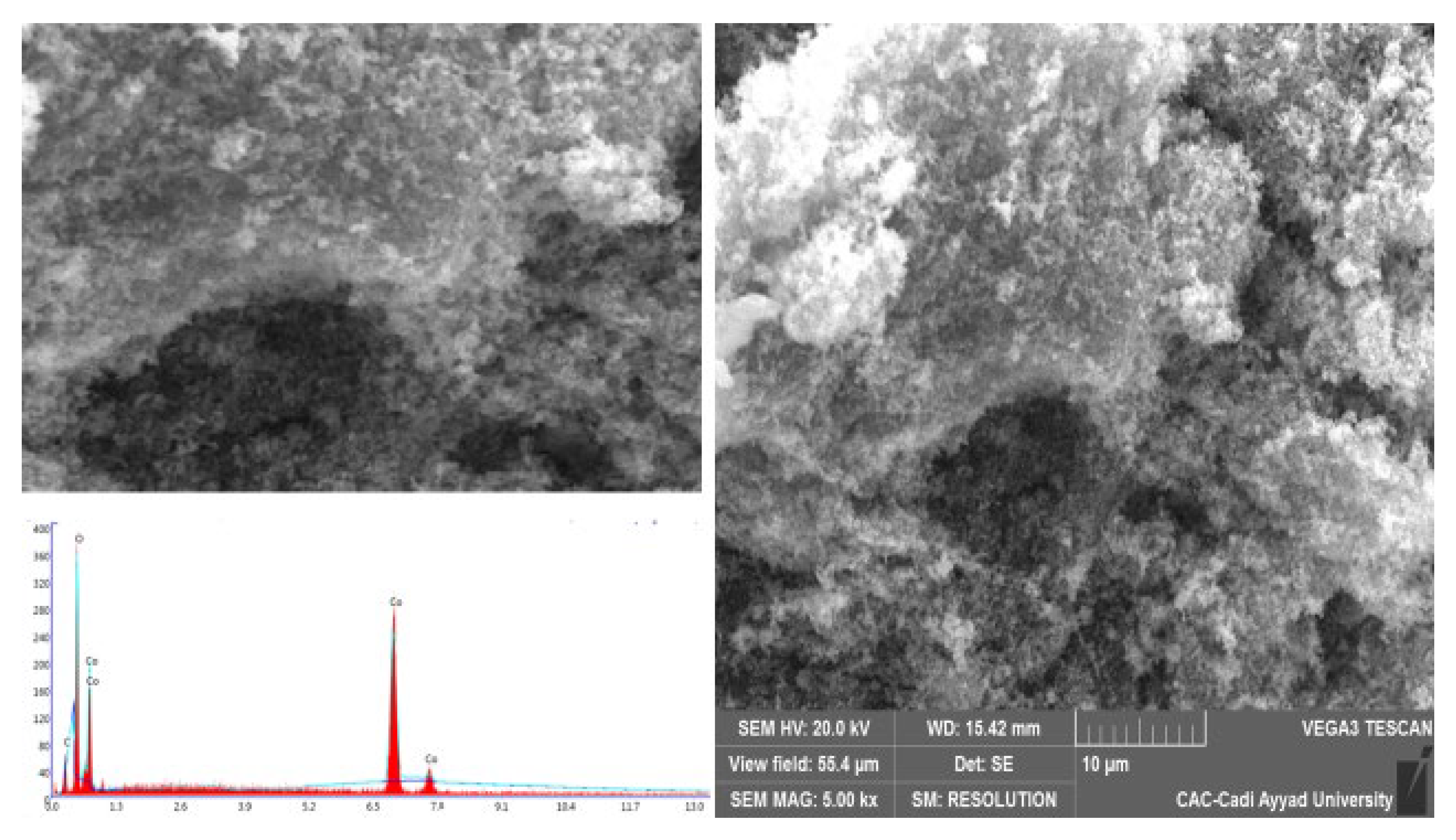
2.3. SEM Images
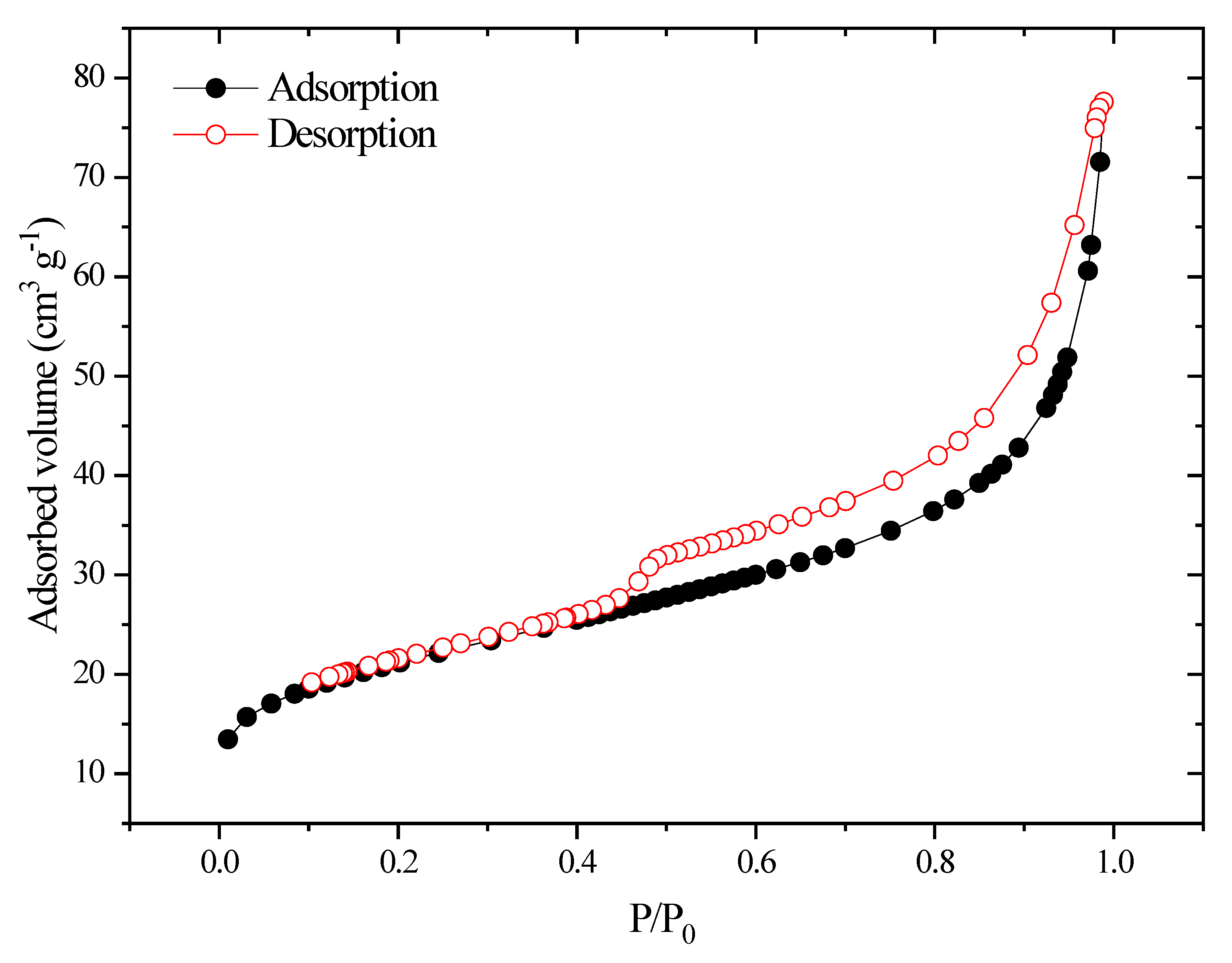
2.4. Textural Characterization Using the N2 Adsorption/Desorption Isotherm
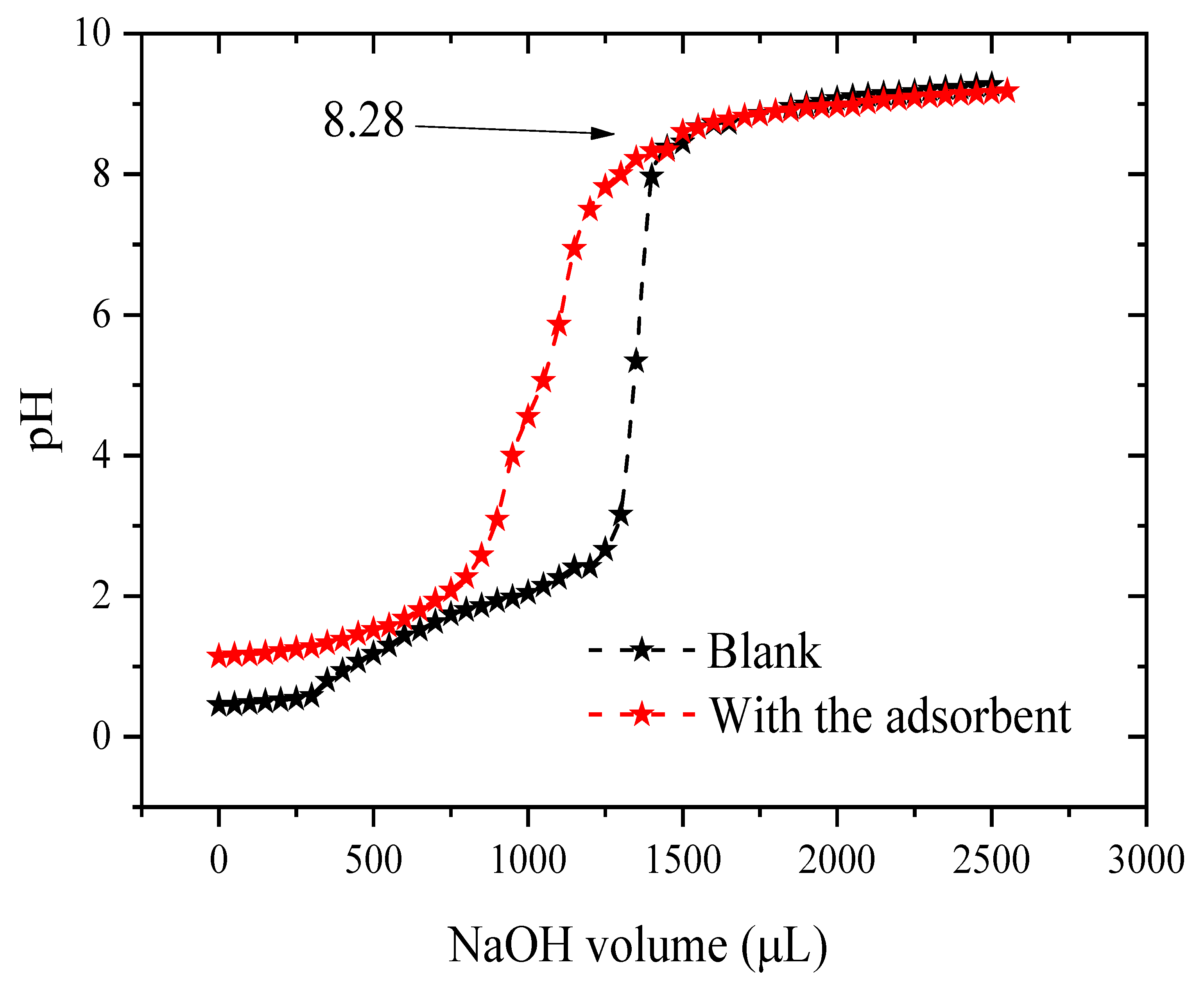
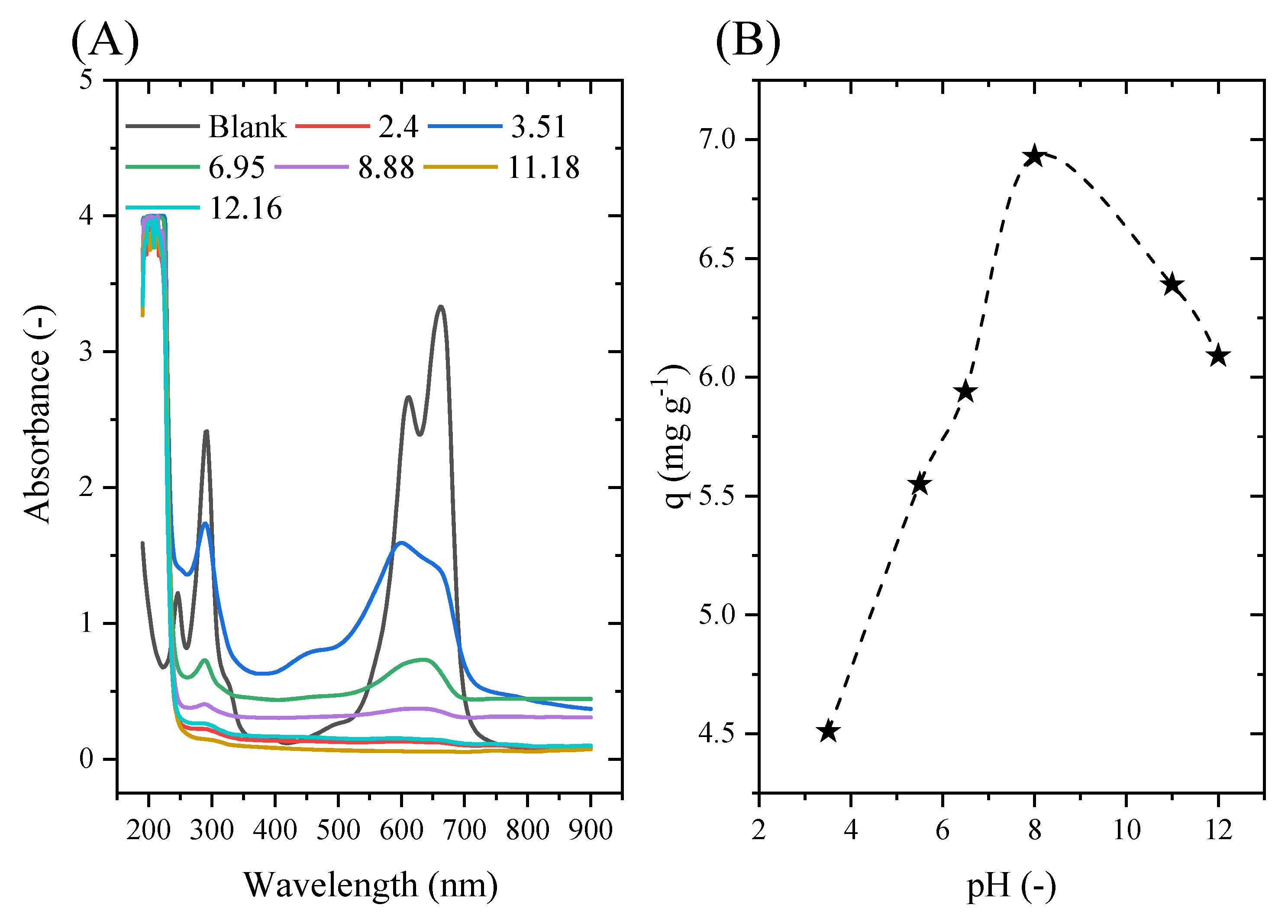
2.5. Point of Zero Charge
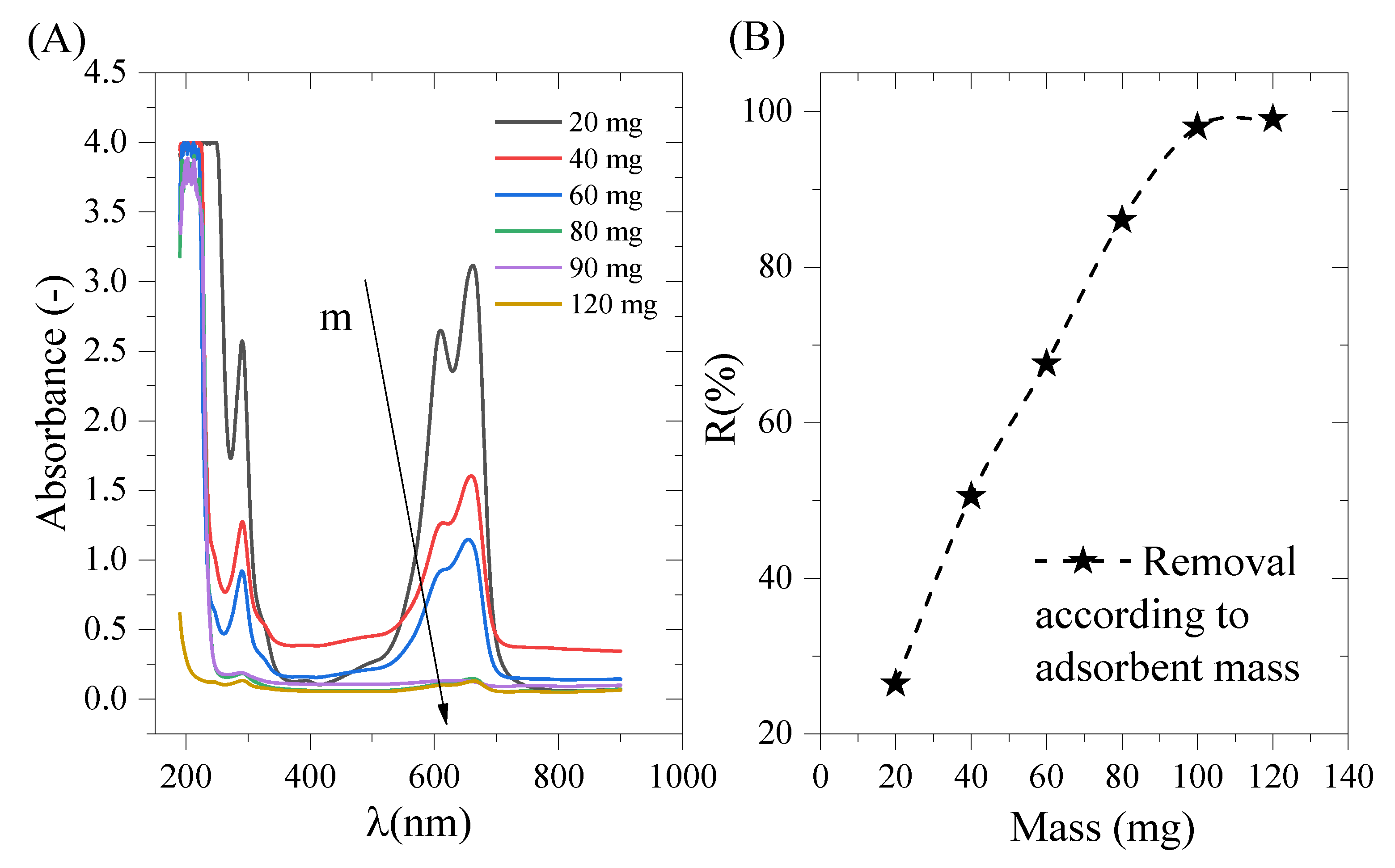
2.6. Adsorbent Mass and the Initial pH Effect
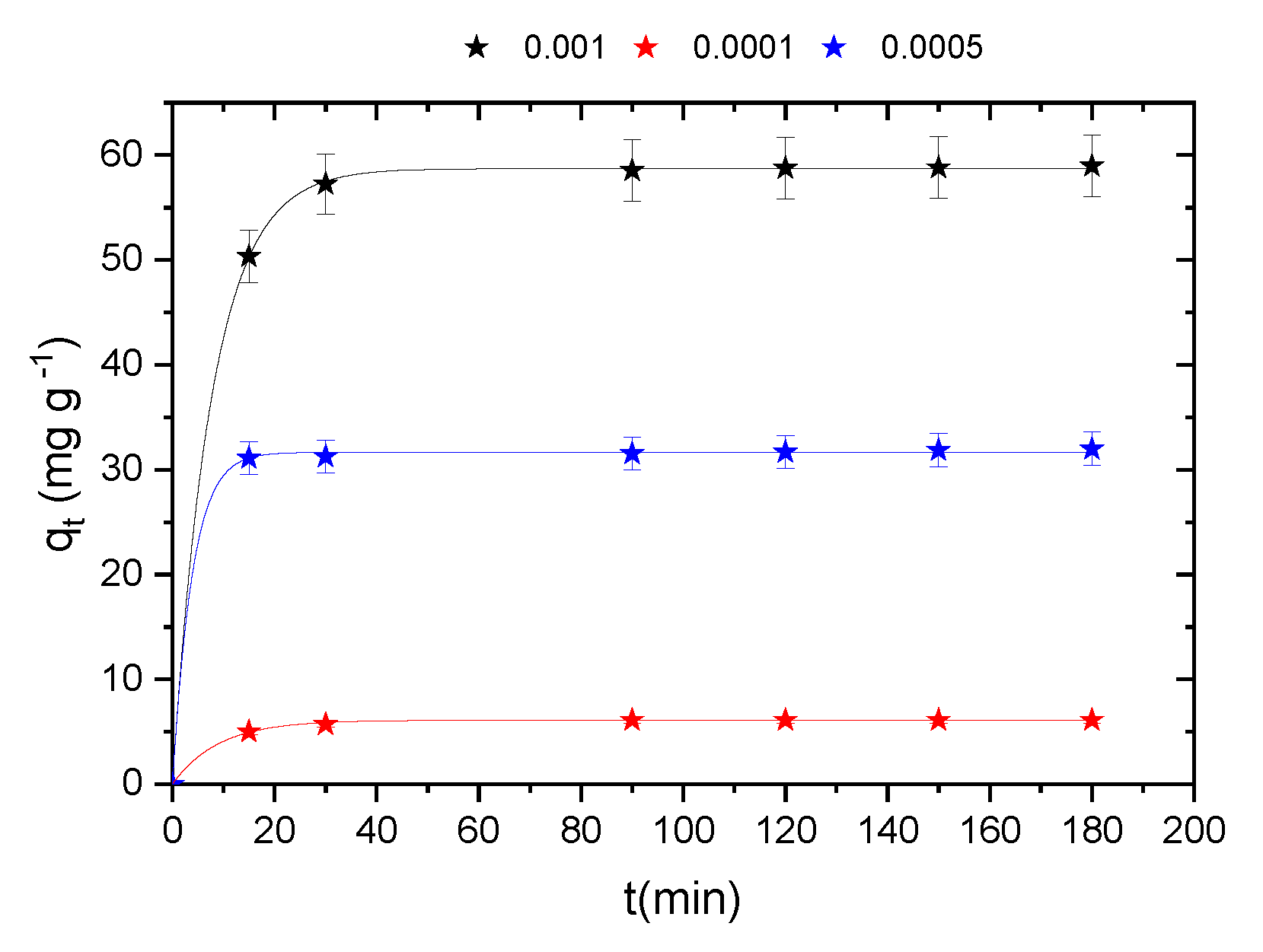
2.7. Initial Concentration and Adsorption Kinetics
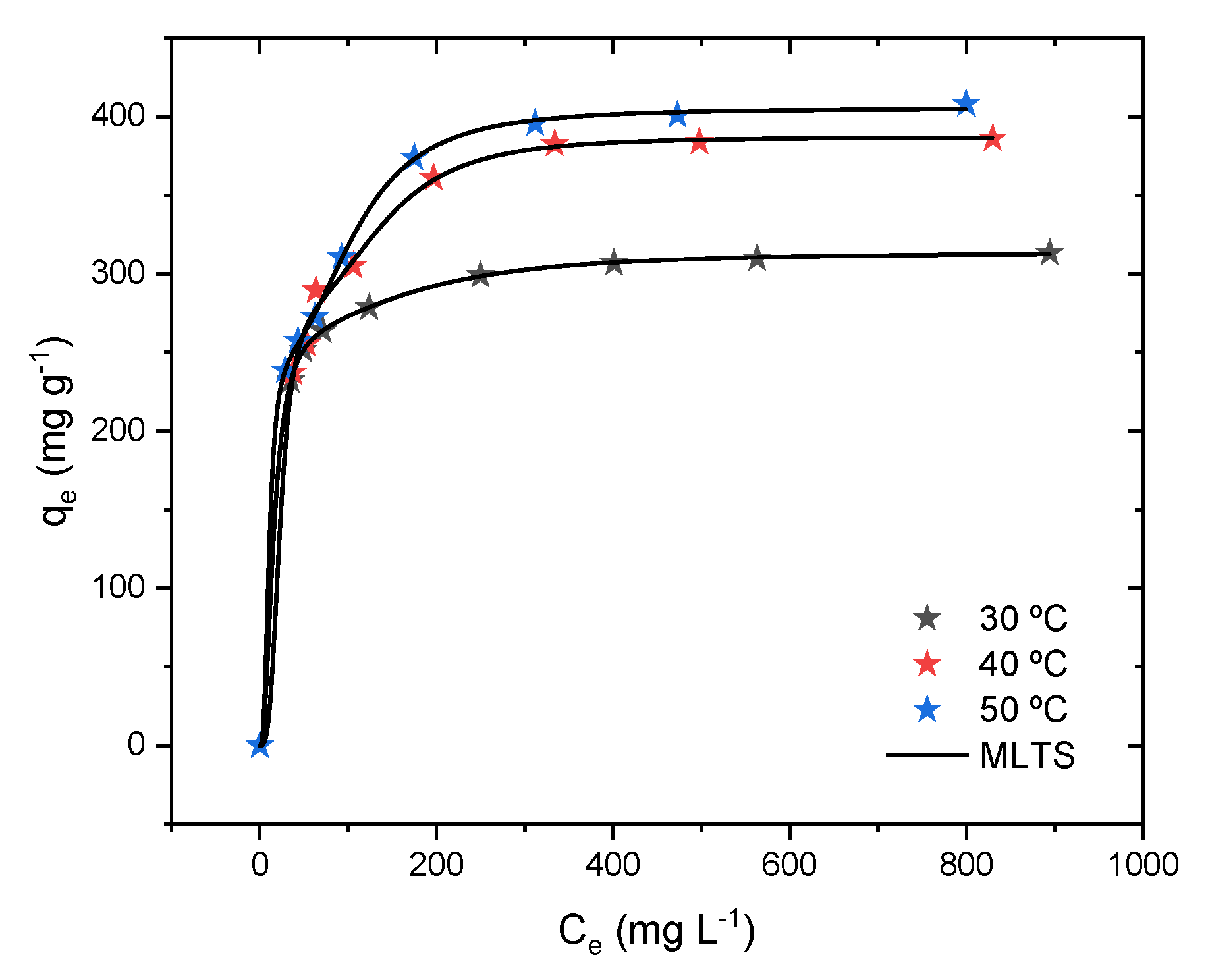
2.8. Isotherm Results and Physical–Statistical Modeling
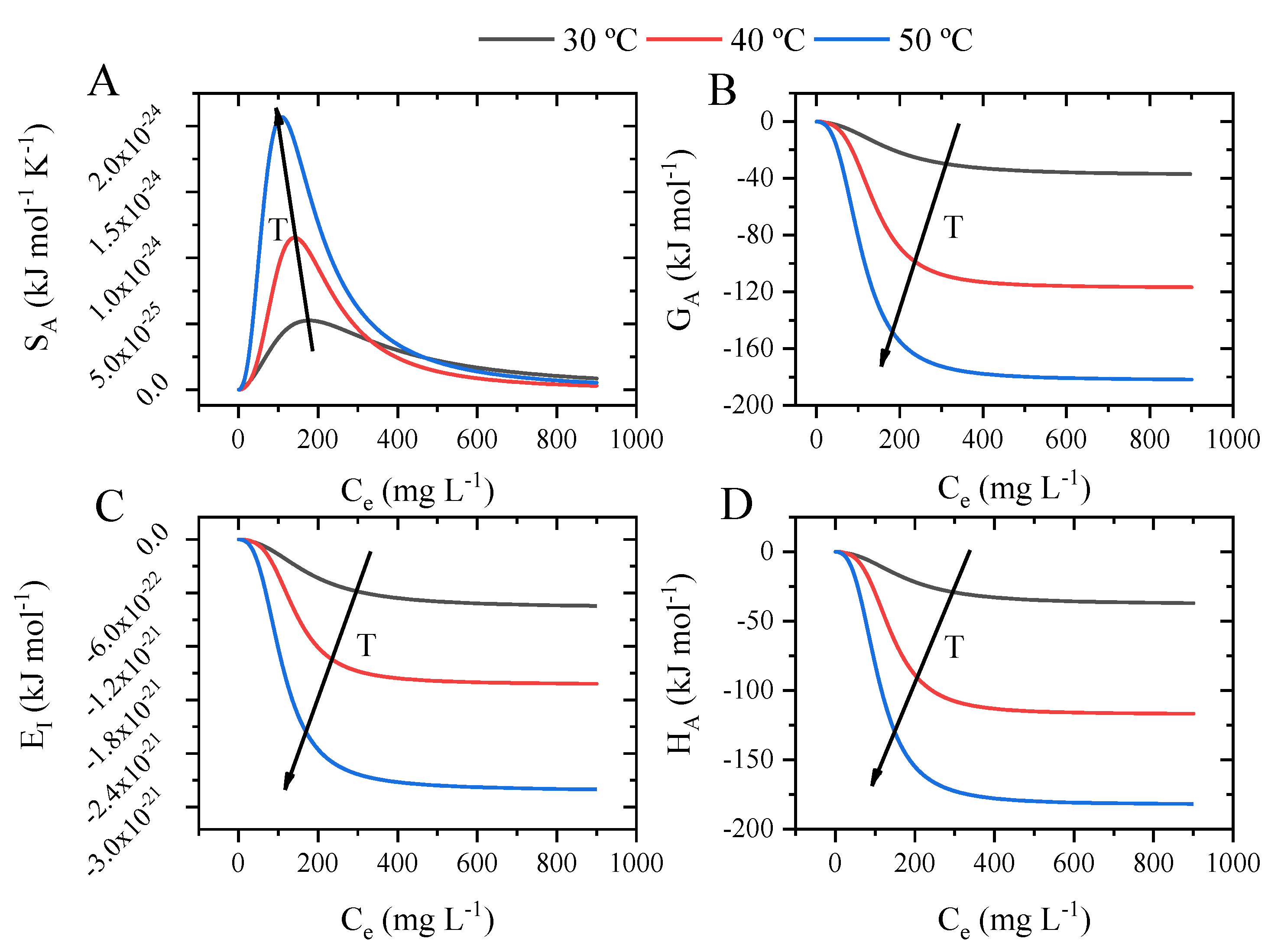
2.9. Potential Thermodynamic Functions and Thermodynamic Simulations
2.10. Adsorption Mechanism Proposal
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of Co3O4 and Its Characterization
3.3. Adsorption Kinetics and Isotherms for the Methylene Blue
3.4. Kinetics Modeling
3.5. Isothermal Modeling
3.6. Parameter Estimation and Model Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dehmani, Y.; Dridi, D.; Lamhasni, T.; Abouarnadasse, S.; Chtourou, R.; Lima, E.C. Review of phenol adsorption on transition metal oxides and other adsorbents. J. Water Process Eng. 2022, 49, 102965. [Google Scholar] [CrossRef]
- Ali, I.; Alothman, Z.A.; Sanagi, M.M. Green Synthesis of Iron Nano-Impregnated Adsorbent for Fast Removal of Fluoride from Water. J. Mol. Liq. 2015, 211, 457–465. [Google Scholar] [CrossRef]
- Catherine, H.N.; Ou, M.H.; Manu, B.; Shih, Y.H. Adsorption mechanism of emerging and conventional phenolic compounds on graphene oxide nanoflakes in water. Sci. Total Environ. 2018, 635, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.K.; Shahriar, A.; Jim, K.U. Water pollution in Bangladesh and its impact on public health. Heliyon 2019, 5, e02145. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Atar, N.; Yola, M.L.; Üstündaǧ, Z.; Uzun, L. A novel magnetic Fe@Au core-shell nanoparticles anchored graphene oxide recyclable nanocatalyst for the reduction of nitrophenol compounds. Water Res. 2014, 48, 210–217. [Google Scholar] [CrossRef]
- Kamal, M.S.; Razzak, S.A.; Hossain, M.M. Catalytic oxidation of volatile organic compounds (VOCs)—A review. Atmos. Environ. 2016, 140, 117–134. [Google Scholar] [CrossRef]
- Yousef, R.I.; El-Eswed, B.; Al-Muhtaseb, A.H. Adsorption characteristics of natural zeolites as solid adsorbents for phenol removal from aqueous solutions: Kinetics, mechanism, and thermodynamics studies. Chem. Eng. J. 2011, 171, 1143–1149. [Google Scholar] [CrossRef]
- Elwakeel, K.Z.; El-Bindary, A.A.; Kouta, E.Y. Retention of copper, cadmium and lead from water by Na-Y-Zeolite confined in methyl methacrylate shell. J. Environ. Chem. Eng. 2017, 5, 3698–3710. [Google Scholar] [CrossRef]
- Piri, F.; Mollahosseini, A.; Khadir, A.; Milani Hosseini, M. Enhanced adsorption of dyes on microwave-assisted synthesized magnetic zeolite-hydroxyapatite nanocomposite. J. Environ. Chem. Eng. 2019, 7, 103338. [Google Scholar] [CrossRef]
- Ho, Y.S.; Mckay, G. Sorption of dyes from aqueous solution by peat. Chem. Eng. J. 1998, 70, 115–124. [Google Scholar] [CrossRef]
- Al Hamadi, A.; Güven, U.R.A.Z.; Katircioğlu, H.; Osmanağaoğlu, Ö. Adsorption of azo dyes from textile wastewater by Spirulina Platensis. Eurasian J. Environ. Res. 2017, 1, 19–27. [Google Scholar]
- Javaid, R.; Qazi, U.Y. Catalytic oxidation process for the degradation of synthetic dyes: An overview. Int. J. Environ. Res. Public Health 2019, 16, 2066. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, R.; Sacari, E.; Gracia, F.; Khan, M.M.; Mosquera, E.; Gupta, V.K. Conducting PANI stimulated ZnO system for visible light photocatalytic degradation of coloured dyes. J. Mol. Liq. 2016, 221, 1029–1033. [Google Scholar] [CrossRef]
- Hussain, S.; Kamran, M.; Khan, S.A.; Shaheen, K.; Shah, Z.; Suo, H.; Khan, Q.; Shah, A.B.; Rehman, W.U.; Al-Ghamdi, Y.O.; et al. Adsorption, kinetics and thermodynamics studies of methyl orange dye sequestration through chitosan composites films. Int. J. Biol. Macromol. 2021, 168, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Makrigianni, V.; Giannakas, A.; Deligiannakis, Y.; Konstantinou, I. Adsorption of phenol and methylene blue from aqueous solutions by pyrolytic tire char: Equilibrium and kinetic studies. J. Environ. Chem. Eng. 2015, 3, 574–582. [Google Scholar] [CrossRef]
- Meili, L.; Lins, P.V.S.; Costa, M.T.; Almeida, R.L.; Abud, A.K.S.; Soletti, J.I.; Dotto, G.L.; Tanabe, E.H.; Sellaoui, L.; Carvalho, S.H.V.; et al. Adsorption of methylene blue on agroindustrial wastes: Experimental investigation and phenomenological modelling. Prog. Biophys. Mol. Biol. 2019, 141, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Sellaoui, L.; Franco, D.S.P.; Dotto, G.L.; Lima, É.C.; Ben Lamine, A. Single and binary adsorption of cobalt and methylene blue on modified chitin: Application of the Hill and exclusive extended Hill models. J. Mol. Liq. 2017, 233, 543–550. [Google Scholar] [CrossRef]
- Aarfane, A.; Tahiri, S.; Salhi, A.; El Kadiri Boutchich, G.; Siniti, M.; Bensitel, M.; Sabour, B.; El Krati, M. Adsorption of methylene blue and Red195 dyes in aqueous medium by palm bark and sugarcane bagasse: Kinetic and thermodynamic study. J. Mater. Environ. Sci. 2015, 6, 2944–2957. [Google Scholar]
- Fayoud, N.; Younssi, S.A.; Tahiri, S.; Albizane, A. Etude cinétique et thermodynamique de l’adsorption de bleu de méthylène sur les cendres de bois. J. Mater. Environ. Sci. 2015, 6, 3295–3306. [Google Scholar]
- Mittal, H.; Al Alili, A.; Alhassan, S.M. High efficiency removal of methylene blue dye using κ-carrageenan-poly(acrylamide-co-methacrylic acid)/AQSOA-Z05 zeolite hydrogel composites. Cellulose 2020, 27, 8269–8285. [Google Scholar] [CrossRef]
- Bulut, Y.; Aydın, H. A kinetics and thermodynamics study of methylene blue adsorption on wheat shells. Desalination 2006, 194, 259–267. [Google Scholar] [CrossRef]
- Sharma, P.; Laddha, H.; Agarwal, M.; Gupta, R. Selective and effective adsorption of malachite green and methylene blue on a non-toxic, biodegradable, and reusable fenugreek galactomannan gum coupled MnO2 mesoporous hydrogel. Microporous Mesoporous Mater. 2022, 338, 111982. [Google Scholar] [CrossRef]
- Chahkandi, M. Mechanism of Congo red adsorption on new sol-gel-derived hydroxyapatite nano-particle. Mater. Chem. Phys. 2017, 202, 340–351. [Google Scholar] [CrossRef]
- Kifuani, K.M.; Kifuani, A.; Mayeko, K.; Vesituluta, P.N.; Lopaka, B.I.; Bakambo, G.E. Adsorption of basic dye, Methylene Blue, in aqueous solution on bioadsorbent from agricultural waste of Cucumeropsis Naudin mannii. Int. J. Biol. Chem. Sci. 2018, 12, 558–575. [Google Scholar] [CrossRef]
- Bentahar, S.; Dbik, A.; El Khomri, M.; El Messaoudi, N.; Lacherai, A. Adsorption of methylene blue, crystal violet and congo red from binary and ternary systems with natural clay: Kinetic, isotherm, and thermodynamic. J. Environ. Chem. Eng. 2017, 5, 5921–5932. [Google Scholar] [CrossRef]
- Ifguis, O.; Ziat, Y.; Belkhanchi, H.; Ammou, F.; Moutcine, A.; Laghlimi, C. Adsorption mechanism of Methylene Blue from polluted water by Opuntia ficus indica of Beni Mellal and Sidi Bou Othmane areas: A comparative study. Chem. Phys. Impact 2023, 6, 100235. [Google Scholar] [CrossRef]
- Wang, D.C.; Li, Y.H.; Li, D.; Xia, Y.Z.; Zhang, J.P. A review on adsorption refrigeration technology and adsorption deterioration in physical adsorption systems. Renew. Sustain. Energy Rev. 2010, 14, 344–353. [Google Scholar] [CrossRef]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents: A review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef]
- Liu, T.; Li, Y.; Du, Q.; Sun, J.; Jiao, Y.; Yang, G.; Wang, Z.; Xia, Y.; Zhang, W.; Wang, K.; et al. Adsorption of methylene blue from aqueous solution by graphene. Colloids Surf. B Biointerfaces 2012, 90, 197–203. [Google Scholar] [CrossRef]
- Feitoza, U.D.S.; Thue, P.S.; Lima, E.C.; Reis, G.S.D.; Rabiee, N.; de Alencar, W.S.; Mello, B.L.; Dehmani, Y.; Rinklebe, J.; Dias, S.L.P. Use of Biochar Prepared from the Açaí Seed as Adsorbent for the Uptake of Catechol from Synthetic Effluents. Molecules 2022, 27, 7570. [Google Scholar] [CrossRef]
- Hameed, B.H.; Din, A.M.; Ahmad, A.L. Adsorption of methylene blue onto bamboo-based activated carbon: Kinetics and equilibrium studies. J. Hazard. Mater. 2007, 141, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Dehmani, Y.; Lainé, J.; Daouli, A.; Sellaoui, L.; Bonilla-Petriciolet, A.; Lamhasni, T.; Abouarnadasse, S.; Badawi, M. Unravelling the adsorption mechanism of phenol on zinc oxide at various coverages via statistical physics, artificial neural network modeling and ab initio molecular dynamics. Chem. Eng. J. 2023, 452, 139171. [Google Scholar] [CrossRef]
- Kipling, J.J.; Wilson, R.B. Adsorption of methylene blue in the determination of surface areas. J. Appl. Chem. 1960, 10, 109–113. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Kang, W.; Han, H.; Song, H.; Zhang, C.; Wang, H.; Yang, X.; Gong, X.; Zhai, C.; et al. Excellent adsorption of Zn(II) using NaP zeolite adsorbent synthesized from coal fly ash via stage treatment. J. Clean. Prod. 2020, 258, 120736. [Google Scholar] [CrossRef]
- Zhao, F.; Zou, Y.; Lv, X.; Liang, H.; Jia, Q.; Ning, W. Synthesis of CoFe2O4-zeolite materials and application to the adsorption of gallium and indium. J. Chem. Eng. Data 2015, 60, 1338–1344. [Google Scholar] [CrossRef]
- Na Chat, N.; Sangsuradet, S.; Tobarameekul, P.; Worathanakul, P. Modified hierarchical zeolite X derived from riceberry rice husk for propionic acid adsorption. Mater. Chem. Phys. 2022, 282, 125933. [Google Scholar] [CrossRef]
- Mandal, A.; Das, S.K. Phenol adsorption from wastewater using clarified sludge from basic oxygen furnace. J. Environ. Chem. Eng. 2019, 7, 103259. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, X.; Wei, Q.; Lan, Y.; Guo, J. Co3O4 anchored on biochar derived from chitosan (Co3O4 @BCC) as a catalyst to efficiently activate peroxymonosulfate (PMS) for degradation of phenacetin. J. Environ. Manag. 2023, 327, 116895. [Google Scholar] [CrossRef]
- Alhaddad, M.; Ismail, A.A.; Alghamdi, Y.G.; Al-khathami, N.D. Co3O4 Nanoparticles Accommodated Mesoporous TiO2 framework as an Excellent Photocatalyst with Enhanced Photocatalytic Properties. Opt. Mater. 2022, 131, 112643. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.-F.; Wang, X.; Deng, C.-B.; Cao, M.-M. Effect of highly dispersed Co3O4 on the catalytic performance of LaCoO3 perovskite in the combustion of lean methane. J. Fuel Chem. Technol. 2023, 51, 367–375. [Google Scholar] [CrossRef]
- Gu, Y.; Ding, J.; Tong, X.; Yao, H.; Yang, R.; Zhong, Q. Photothermal catalyzed hydrogenation of carbon dioxide over porous nanosheet Co3O4. J. CO2 Util. 2022, 61, 102003. [Google Scholar] [CrossRef]
- Humelnicu, D.; Dinu, M.V.; Drăgan, E.S. Adsorption characteristics of UO22+ and Th4+ ions from simulated radioactive solutions onto chitosan/clinoptilolite sorbents. J. Hazard. Mater. 2011, 185, 447–455. [Google Scholar] [CrossRef]
- Dhaouadi, F.; Sellaoui, L.; Reynel-Ávila, H.E.; Landín-Sandoval, V.; Mendoza-Castillo, D.I.; Jaime-Leal, J.E.; Lima, E.C.; Bonilla-Petriciolet, A.; Lamine, A.B. Adsorption mechanism of Zn2+, Ni2+, Cd2+, and Cu2+ ions by carbon-based adsorbents: Interpretation of the adsorption isotherms via physical modelling. Environ. Sci. Pollut. Res. 2021, 28, 30943–30954. [Google Scholar] [CrossRef]
- Belaid, K.D. Étude cinétique et thermodynamique de l’adsorption d’un colorant basique sur la sciure de bois. Rev. Sci. L’eau 2015, 24, 131–144. [Google Scholar] [CrossRef]
- Kamacı, U.D.; Kamacı, M. Hydrogel beads based on sodium alginate and quince seed nanoparticles for the adsorption of methylene blue. Inorg. Chem. Commun. 2023, 160, 111919. [Google Scholar] [CrossRef]
- Xue, H.; Wang, X.; Xu, Q.; Dhaouadi, F.; Sellaoui, L.; Seliem, M.K.; Ben Lamine, A.; Belmabrouk, H.; Bajahzar, A.; Bonilla-Petriciolet, A.; et al. Adsorption of methylene blue from aqueous solution on activated carbons and composite prepared from an agricultural waste biomass: A comparative study by experimental and advanced modeling analysis. Chem. Eng. J. 2022, 430, 132801. [Google Scholar] [CrossRef]
- Cai, Y.; Tang, B.; Bin, L.; Huang, S.; Li, P.; Fu, F. Constructing a multi-layer adsorbent for controllably selective adsorption of various ionic dyes from aqueous solution by simply adjusting pH. Chem. Eng. J. 2020, 382, 122829. [Google Scholar] [CrossRef]
- Radoor, S.; Karayil, J.; Jayakumar, A.; Parameswaranpillai, J.; Siengchin, S. An efficient removal of malachite green dye from aqueous environment using ZSM-5 zeolite/polyvinyl alcohol/carboxymethyl cellulose/sodium alginate bio composite. J. Polym. Environ. 2021, 29, 2126–2139. [Google Scholar] [CrossRef]
- Kausar, A.; Iqbal, M.; Javed, A.; Aftab, K.; Nazli, Z.; Nawaz, H.; Nouren, S. Dyes adsorption using clay and modified clay: A review. J. Mol. Liq. 2018, 256, 395–407. [Google Scholar] [CrossRef]
- Asuha, S.; Fei, F.; Wurendaodi, W.; Zhao, S.; Wu, H.; Zhuang, X. Activation of kaolinite by a low-temperature chemical method and its effect on methylene blue adsorption. Powder Technol. 2019, 361, 624–632. [Google Scholar] [CrossRef]
- Sadki, H.; Saidi, K.Z.M. Adsorption d ’ un colorant cationique d’un milieu aqueux sur une argile locale activée (adsorption of dyes on activated local clay in aqueous solution). Mater. Environ. Sci. 2014, 5, 2060–2065. [Google Scholar]
- El Hajam, M.; Kandri, N.I.; Harrach, A.; El Khomsi, A.; Zerouale, A. Adsorption of Methylene Blue on industrial softwood waste “Cedar” and hardwood waste “Mahogany”: Comparative study. Mater. Today Proc. 2019, 13, 812–821. [Google Scholar] [CrossRef]
- Knani, S.; Mathlouthi, M.; Lamine, A.B. Modeling of the psychophysical response curves using the grand canonical ensemble in statistical physics. Food Biophys. 2007, 2, 183–192. [Google Scholar] [CrossRef]
- Sellaoui, L.; Soetaredjo, F.E.; Ismadji, S.; Lima, É.C.; Dotto, G.L.; Lamine, A.B.; Erto, A. New insights into single-compound and binary adsorption of copper and lead ions on a treated sea mango shell: Experimental and theoretical studies. Phys. Chem. Chem. Phys. 2017, 19, 25927–25937. [Google Scholar] [CrossRef] [PubMed]
- Özer, A.; Dursun, G. Removal of methylene blue from aqueous solution by dehydrated wheat bran carbon. J. Hazard. Mater. 2007, 146, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Youcef, L.D.; Belaroui, L.S.; López-Galindo, A. Adsorption of a cationic methylene blue dye on an Algerian palygorskite. Appl. Clay Sci. 2019, 179, 105145. [Google Scholar] [CrossRef]
- Neris, A.M.; Chantelle, L.; Souza, J.J.N.; Ferreira, J.M.; Fonseca, M.G.; Santos, I.M.G. Environmental remediation and synthesis of a new pigment by irradiation-induced adsorption of methylene blue onto undoped tetragonal zirconia. Mater. Lett. 2019, 255, 126588. [Google Scholar] [CrossRef]
- Wang, H.; Li, Z.; Yahyaoui, S.; Hanafy, H.; Seliem, M.K.; Bonilla-Petriciolet, A.; Dotto, G.L.; Sellaoui, L.; Li, Q. Effective adsorption of dyes on an activated carbon prepared from carboxymethyl cellulose: Experiments, characterization and advanced modelling. Chem. Eng. J. 2021, 417, 128116. [Google Scholar] [CrossRef]
- Novais, R.M.; Caetano, A.P.F.; Seabra, M.P.; Labrincha, J.A.; Pullar, R.C. Extremely fast and efficient methylene blue adsorption using eco-friendly cork and paper waste-based activated carbon adsorbents. J. Clean. Prod. 2018, 197, 1137–1147. [Google Scholar] [CrossRef]
- Belhachemi, M.; Addoun, F. Comparative adsorption isotherms and modeling of methylene blue onto activated carbons. Appl. Water Sci. 2011, 1, 111–117. [Google Scholar] [CrossRef]
- Yang, J.; Liu, H.; Martens, W.N.; Frost, R.L. Synthesis and characterization of Cobalt hydroxide, cobalt oxyhydroxide, and cobalt oxide nanodiscs. J. Phys. Chem. C 2010, 114, 111–119. [Google Scholar] [CrossRef]
- Georgin, J.; da Boit Martinello, K.; Franco, D.S.P.; Netto, M.S.; Piccilli, D.G.A.; Foletto, E.L.; Silva, L.F.O.; Dotto, G.L. Efficient removal of naproxen from aqueous solution by highly porous activated carbon produced from Grapetree (Plinia cauliflora) fruit peels. J. Environ. Chem. Eng. 2021, 9, 106820. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, Z.H.; Coomes, A.; Haghseresht, F.; Lu, G.Q. The physical and surface chemical characteristics of activated carbons and the adsorption of methylene blue from wastewater. J. Colloid Interface Sci. 2005, 284, 440–446. [Google Scholar] [CrossRef]
- Vieira, Y.; Schnorr, C.; Piazzi, A.C.; Netto, M.S.; Piccini, W.M.; Franco, D.S.P.; Mallmann, E.S.; Georgin, J.; Silva, L.F.O.; Dotto, G.L. An advanced combination of density functional theory simulations and statistical physics modeling in the unveiling and prediction of adsorption mechanisms of 2,4-D pesticide to activated carbon. J. Mol. Liq. 2022, 361, 119639. [Google Scholar] [CrossRef]
- Atkins, P.; Paula, J.D.; Keeler, J. Physical Chemistry, 11th ed.; Oxford University Press: Oxford, UK, 2018; pp. 1–940. [Google Scholar]
- Sharma, Y.C.; Uma. Optimization of parameters for adsorption of methylene blue on a low-cost activated carbon. J. Chem. Eng. Data 2010, 55, 435–439. [Google Scholar] [CrossRef]
- Khalfaoui, M.; Baouab, M.H.V.; Gauthier, R.; Ben Lamine, A. Dye adsorption by modified cotton. Steric and energetic interpretations of model parameter behaviours. Adsorpt. Sci. Technol. 2002, 20, 33–48. [Google Scholar] [CrossRef]

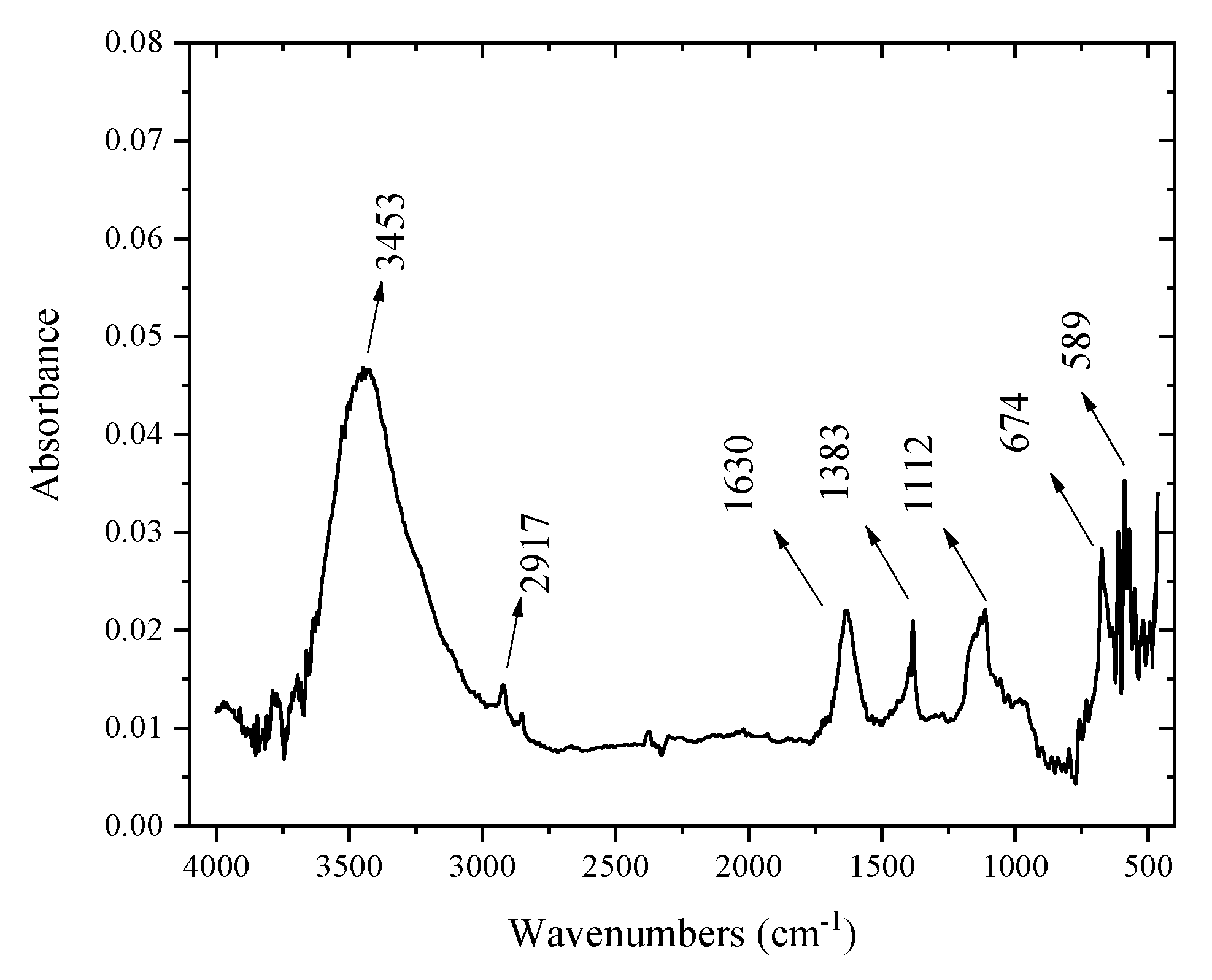


| Bands (cm−1) | Types of Vibration |
|---|---|
| 3453 | H–O–H |
| 1630 | H–O–H |
| 1383 | O=C=O |
| 1112 | H–O–H |
| 674 | Co–O |
| 609 | Co–O |
| 589 | Co–O |
| Kinetic Model | Parameters | Concentration (M) | ||
|---|---|---|---|---|
| 10−3 | 5 × 10−4 | 10−4 | ||
| Qexp (mg g−1) | 58.99 | 31.98 | 6.12 | |
| Pseudo-first order | k1 (min−1) | 11.69 | 6.73 | 1.69 |
| qe (mg g−1) | 57.11 | 31.57 | 5.86 | |
| R2 | 0.97 | 0.99 | 0.96 | |
| Pseudo-second order | k2 (g mg−1 min−1) | 0.0064 | 0.079 | 0.043 |
| qe (mg g−1) | 60 | 31.84 | 6.30 | |
| R2 | 0.99 | 0.99 | 0.99 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dehbi, A.; Dehmani, Y.; Franco, D.S.P.; Omari, H.; Georgin, J.; Brahmi, Y.; Elazhari, K.; Messaoudi, M.; Aadnan, I.; Lamhasni, T.; et al. A Statistical Physics Approach to Understanding the Adsorption of Methylene Blue onto Cobalt Oxide Nanoparticles. Molecules 2024, 29, 412. https://doi.org/10.3390/molecules29020412
Dehbi A, Dehmani Y, Franco DSP, Omari H, Georgin J, Brahmi Y, Elazhari K, Messaoudi M, Aadnan I, Lamhasni T, et al. A Statistical Physics Approach to Understanding the Adsorption of Methylene Blue onto Cobalt Oxide Nanoparticles. Molecules. 2024; 29(2):412. https://doi.org/10.3390/molecules29020412
Chicago/Turabian StyleDehbi, Ali, Younes Dehmani, Dison S. P. Franco, Hind Omari, Jordana Georgin, Younes Brahmi, Kaoutar Elazhari, Mohammed Messaoudi, Imane Aadnan, Taibi Lamhasni, and et al. 2024. "A Statistical Physics Approach to Understanding the Adsorption of Methylene Blue onto Cobalt Oxide Nanoparticles" Molecules 29, no. 2: 412. https://doi.org/10.3390/molecules29020412
APA StyleDehbi, A., Dehmani, Y., Franco, D. S. P., Omari, H., Georgin, J., Brahmi, Y., Elazhari, K., Messaoudi, M., Aadnan, I., Lamhasni, T., Alrashdi, A. A., Abdallaoui, A., Abouarnadasse, S., & Lamini, A. (2024). A Statistical Physics Approach to Understanding the Adsorption of Methylene Blue onto Cobalt Oxide Nanoparticles. Molecules, 29(2), 412. https://doi.org/10.3390/molecules29020412








