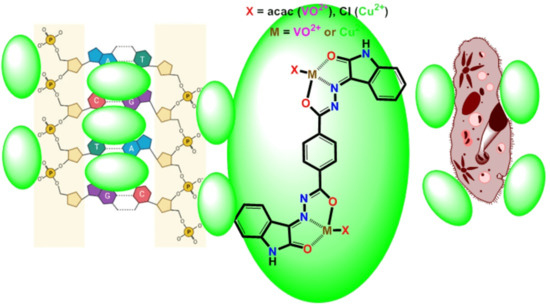
Bimetallic bis-Aroyldihydrazone-Isatin Complexes of High O=V(IV) and Low Cu(II) Valent Ions as Effective Biological Reagents for Antimicrobial and Anticancer Assays
Abstract
1. Introduction
2. Results and Discussion
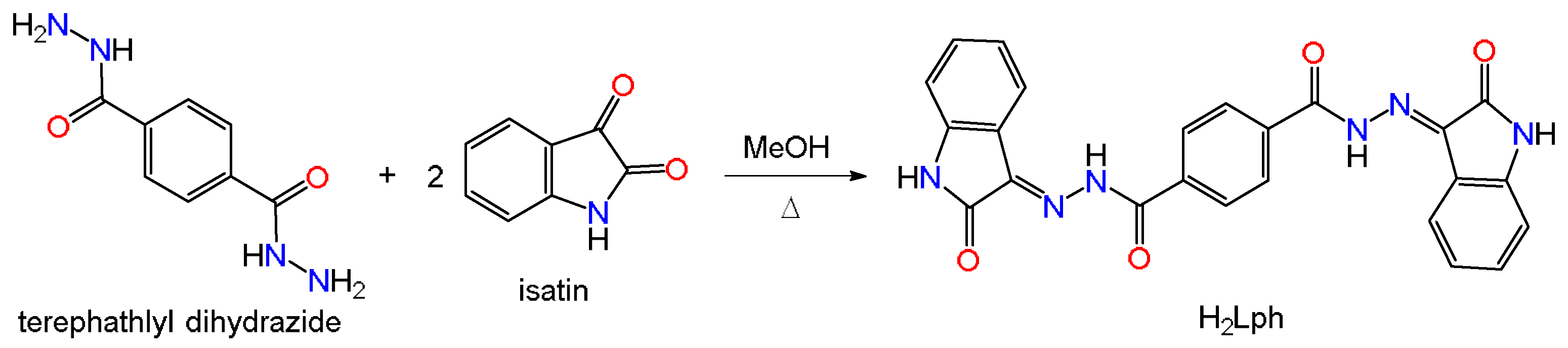
2.1. Preparation and Structural Elucidation
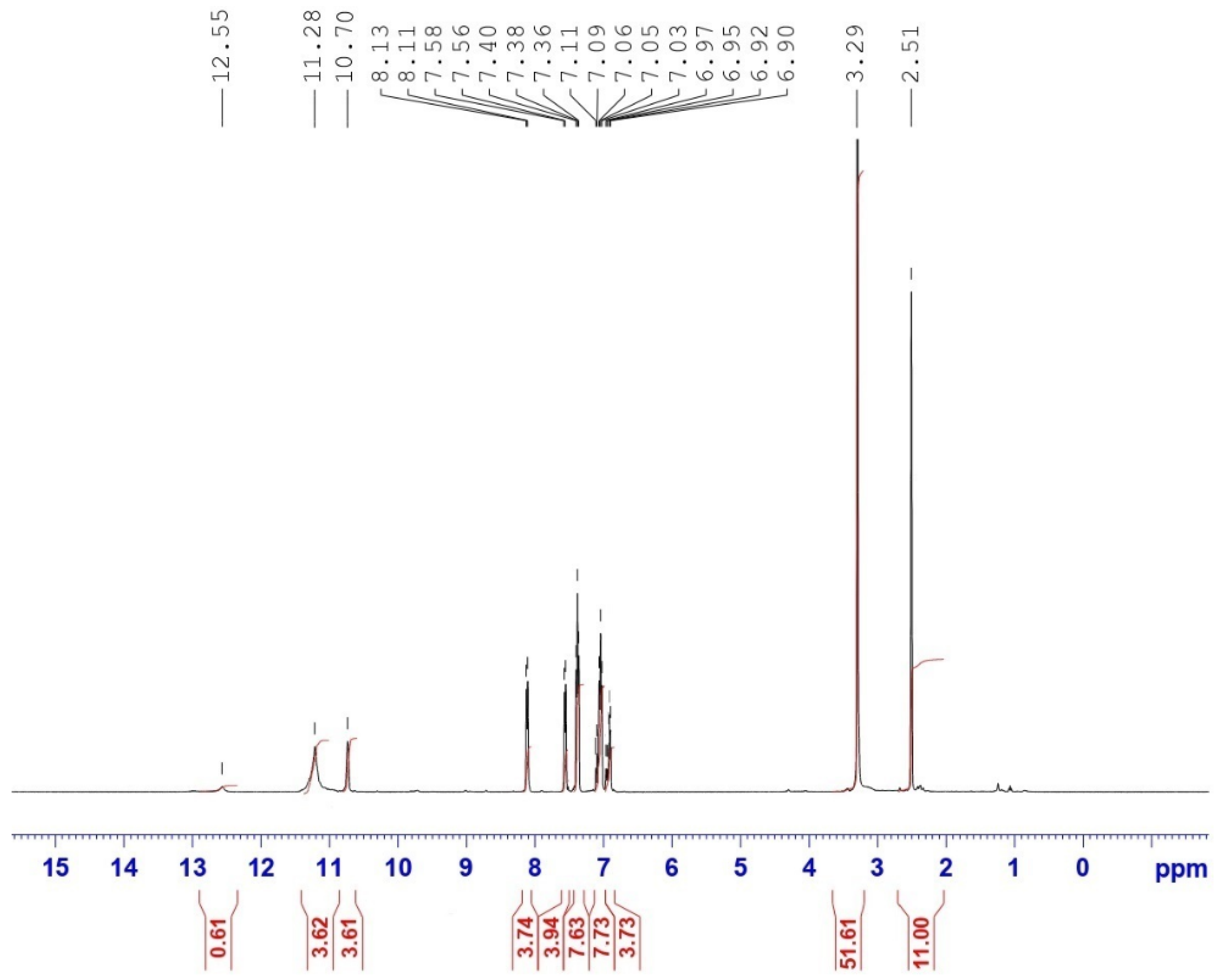
2.1.1. NMR Spectra of H2Lph Organo-Ligand
2.1.2. UV and Vis. Spectroscopy
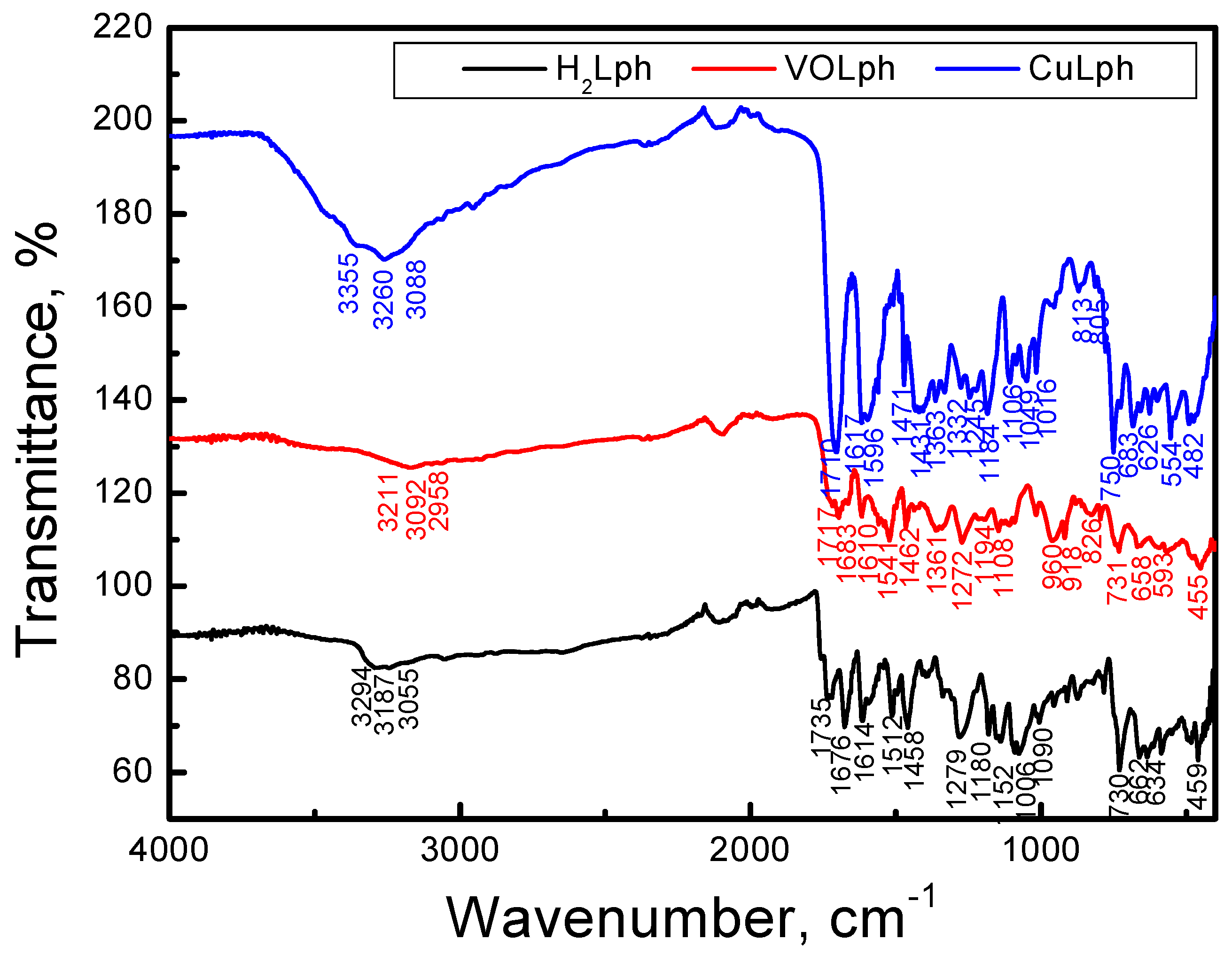
2.1.3. FT-IR Spectroscopy
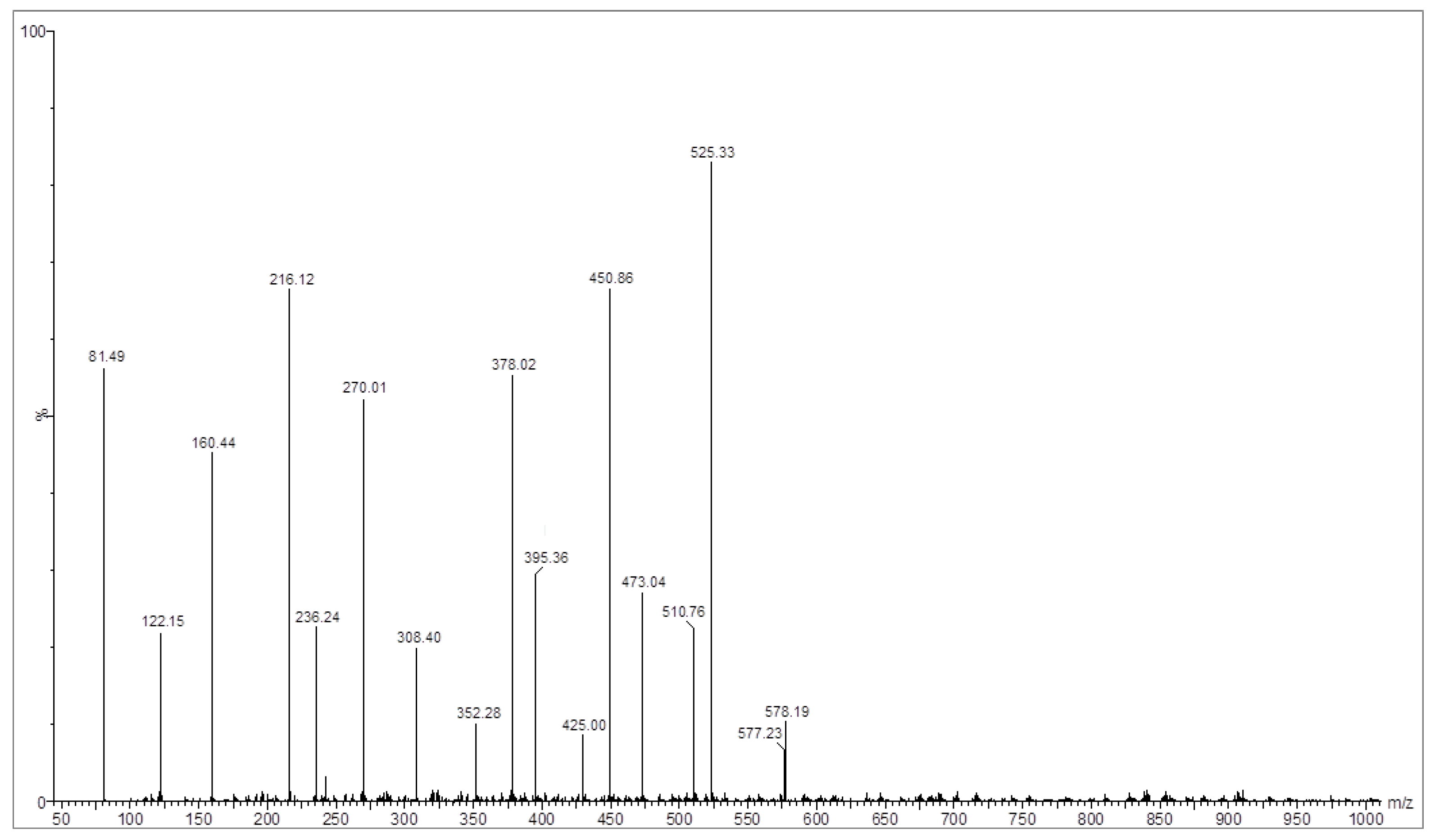
2.1.4. EI-Mass Spectroscopy
2.1.5. Stability of the Studied Compounds
2.2. Biological Studies
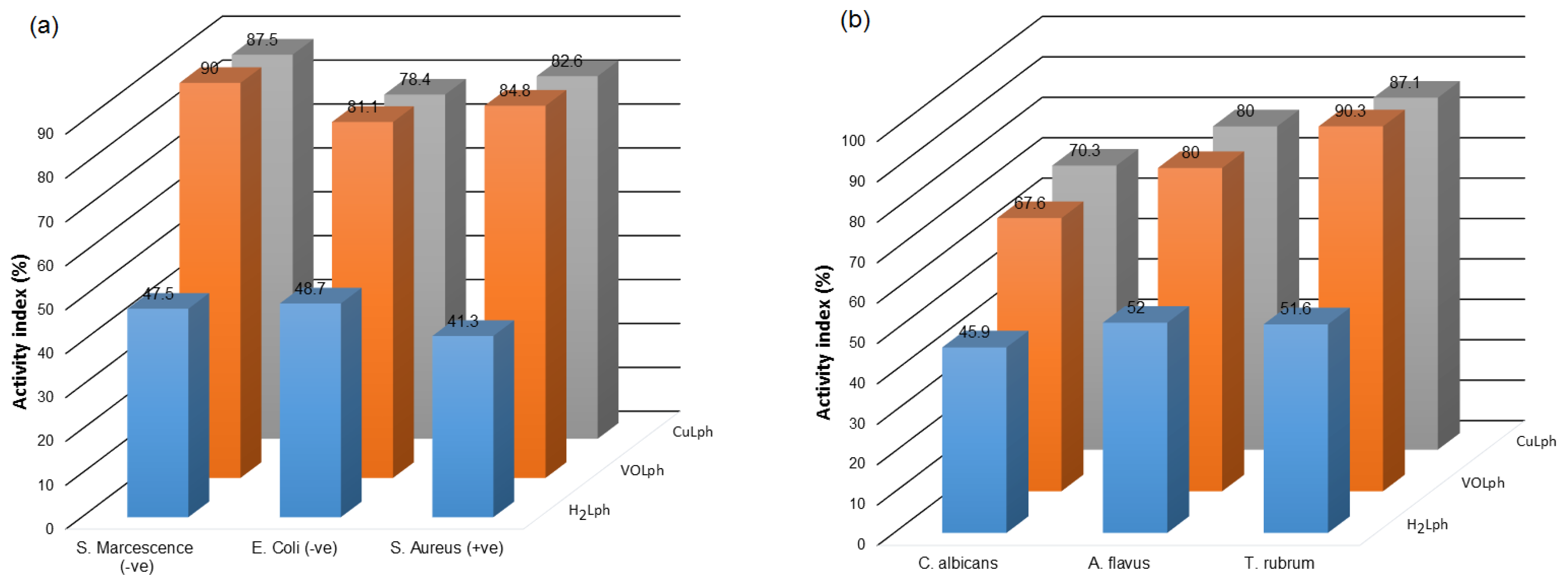
2.2.1. Antimicrobial Assays
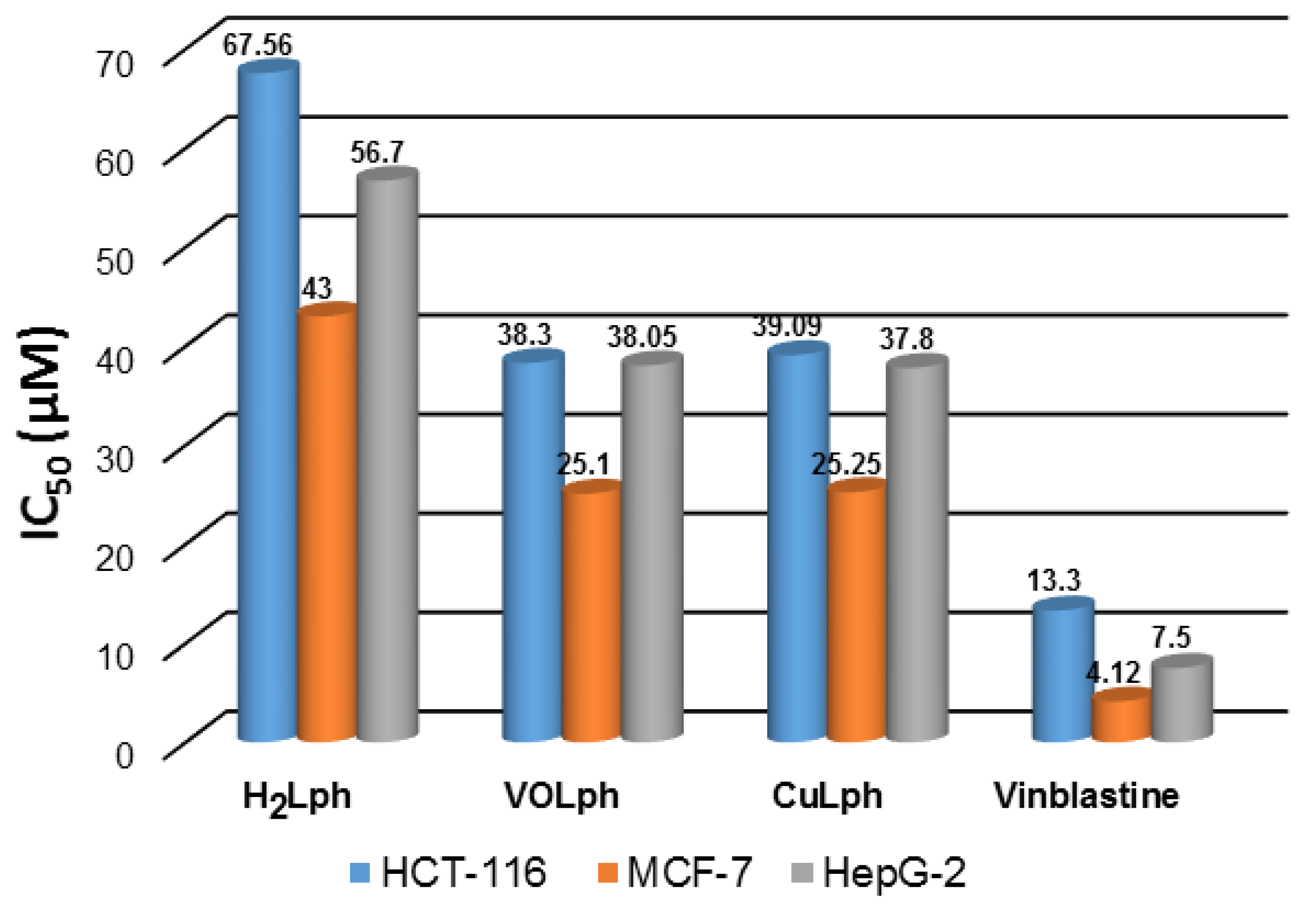
2.2.2. Antiproliferative Action
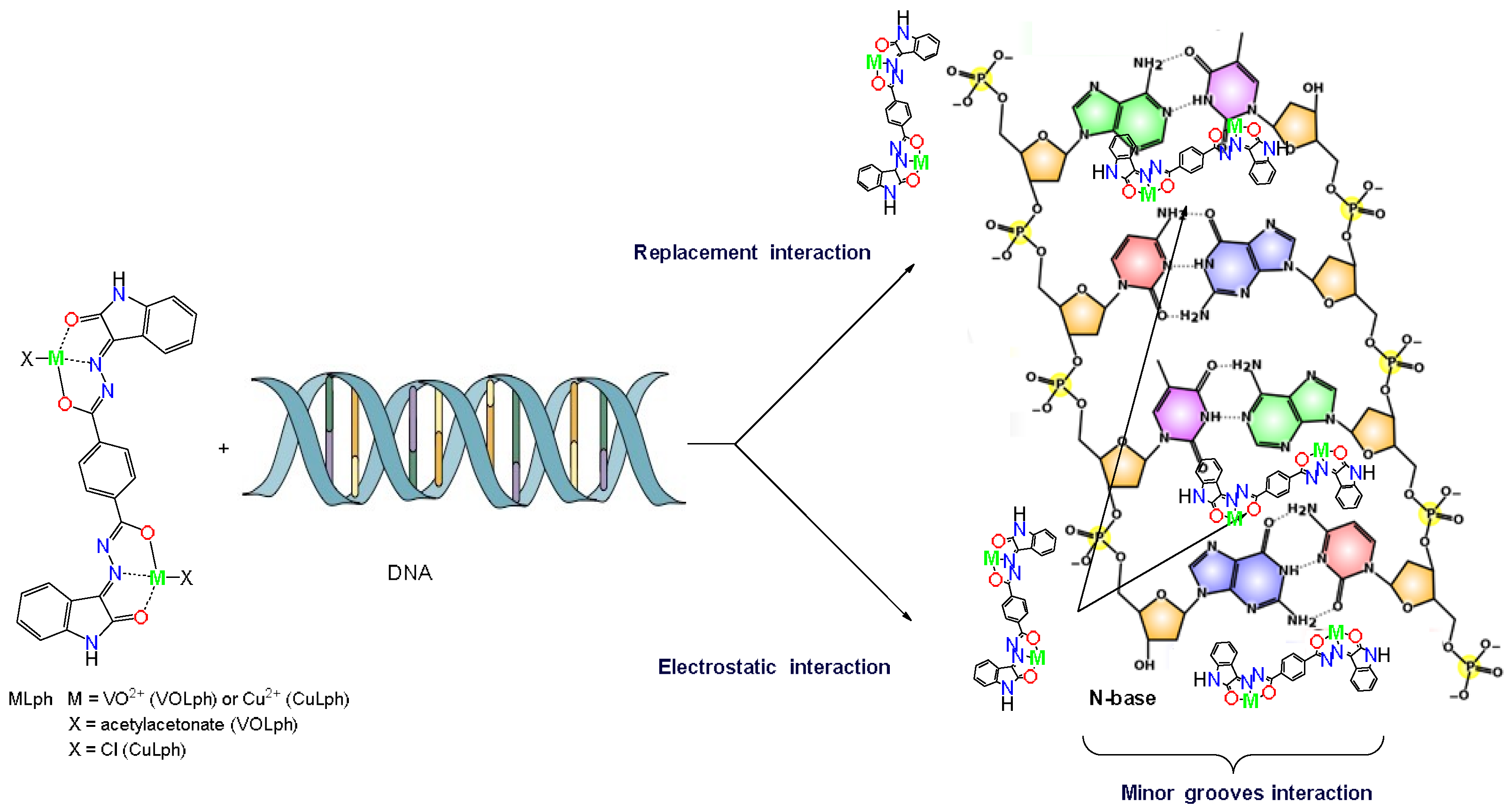
2.2.3. ctDNA Action
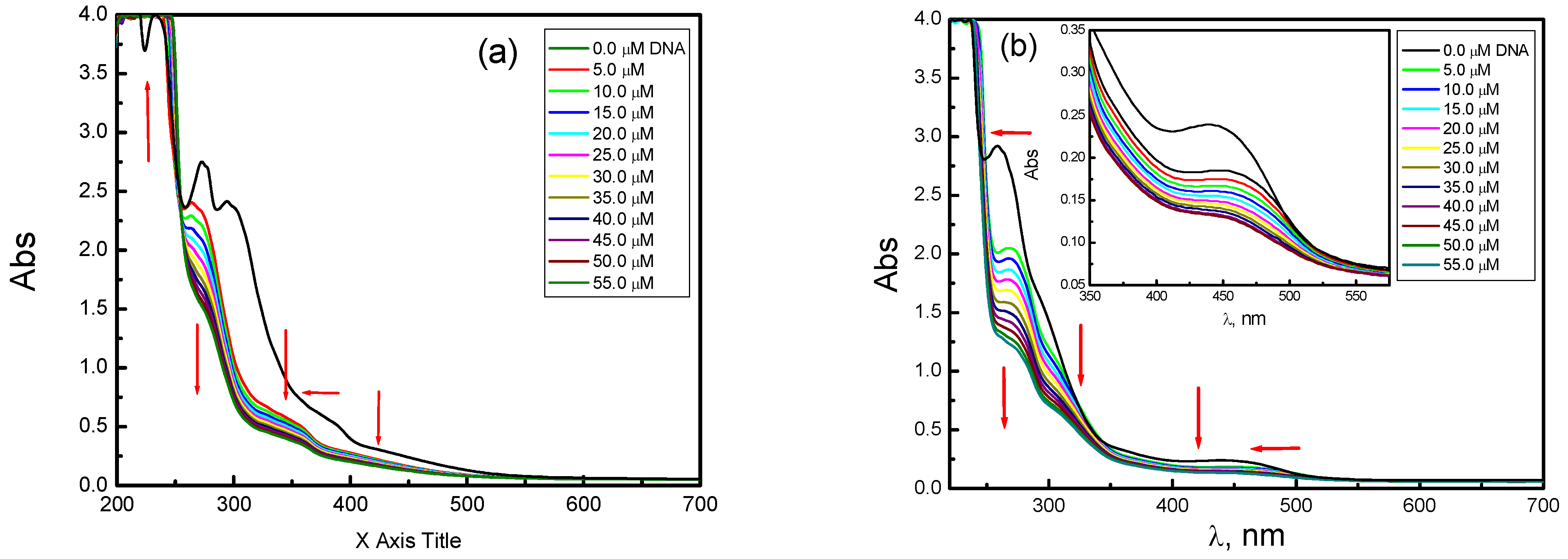
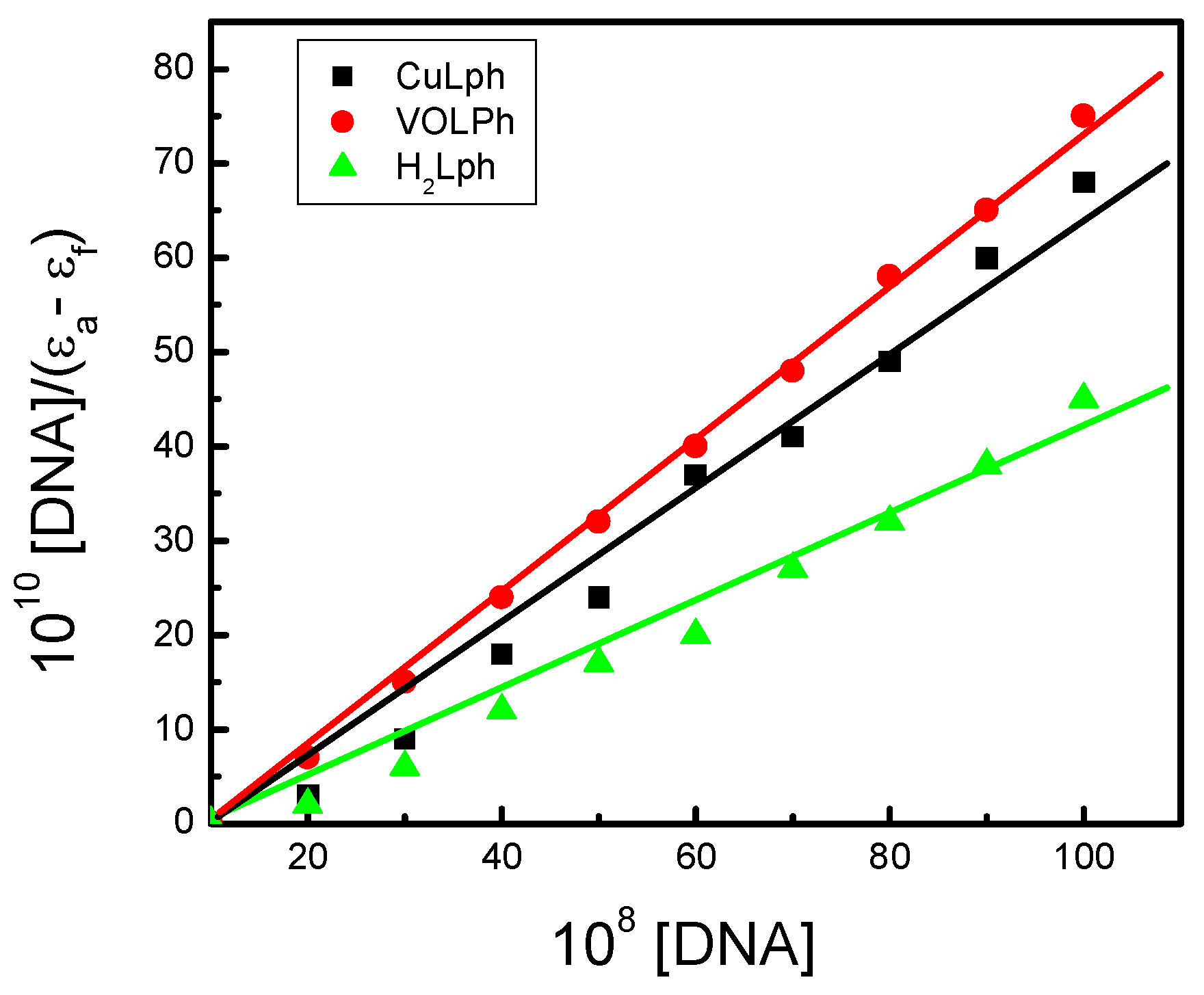
Electronic Spectroscopic Studies
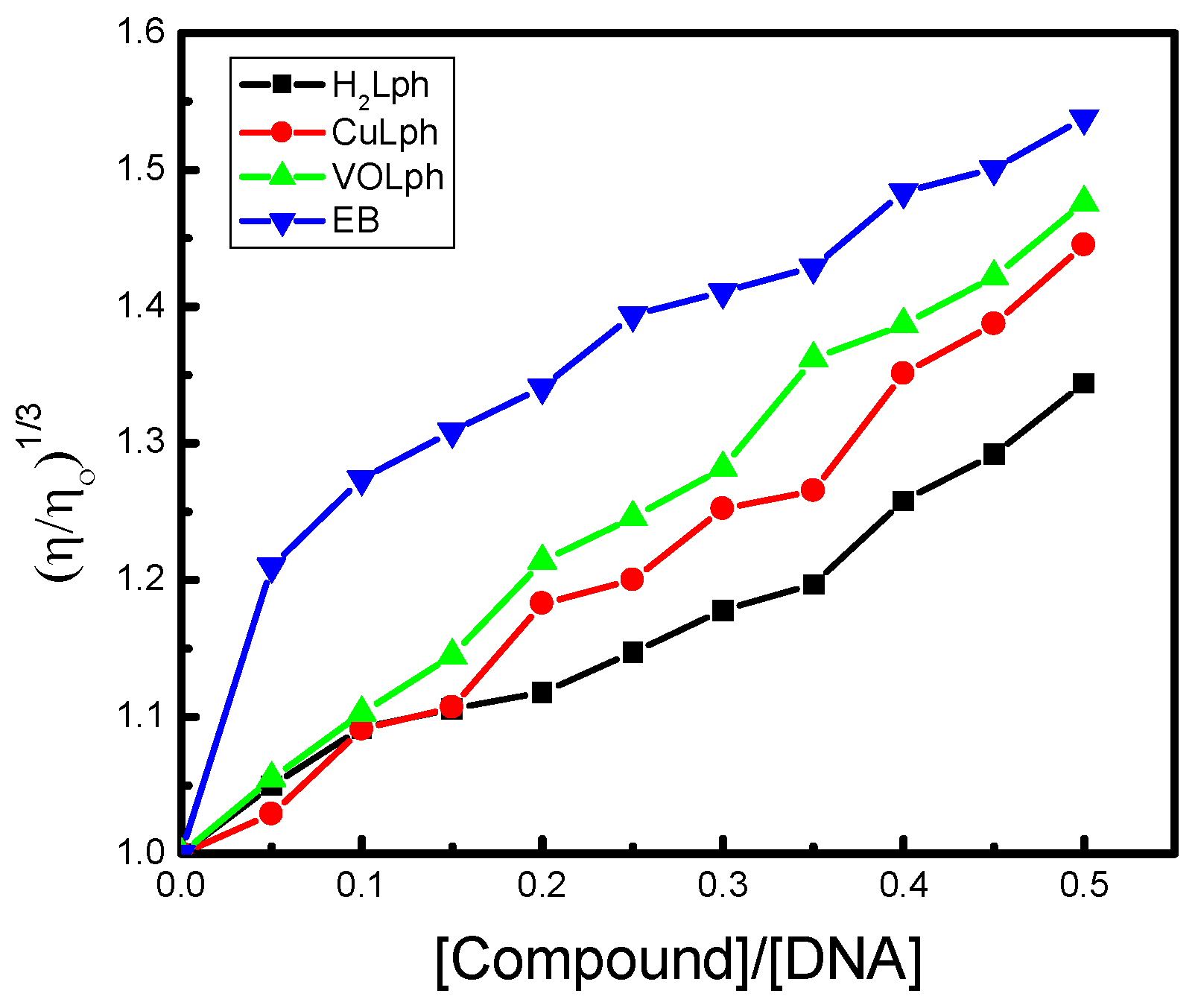
Viscosity Assay
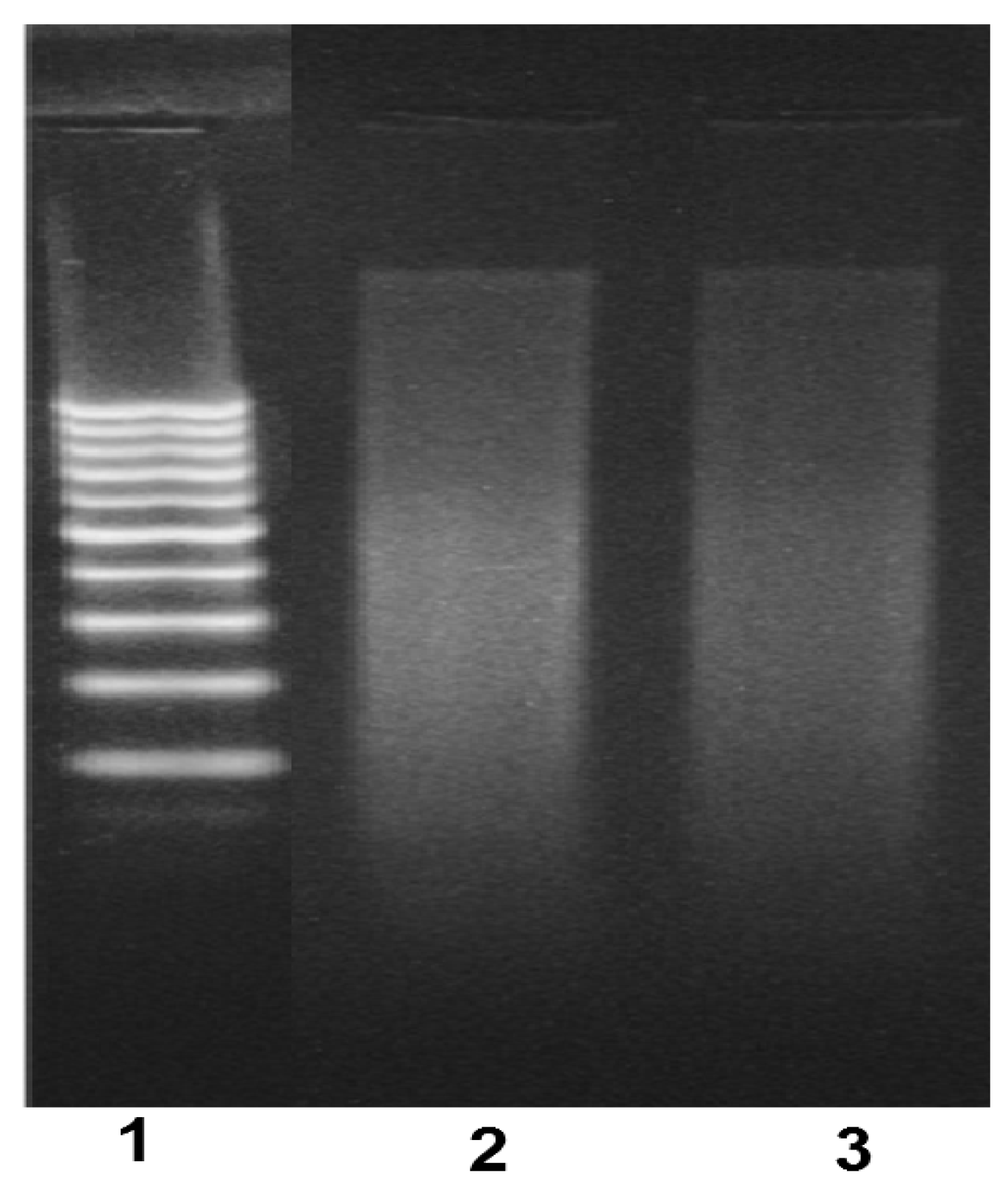
Gel Electrophoresis
3. Experimental
3.1. Materials and Methods
3.2. Synthesis
3.2.1. Synthesis of H2Lph Organo-Ligand
3.2.2. Preparation of MLph Chelates
3.3. Biological Studies
3.3.1. Antimicrobial Studies
3.3.2. Activity Index (A) and Minimal Inhibited Concentration (MIC)
3.3.3. Antitumor Assays
3.3.4. Nature of Interaction with ctDNA
Electronic Spectroscopic Assay
Viscosimetric Study
Gel Electrophoresis Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frei, A.; Zuegg, J.; Elliott, A.G.; Baker, M.; Braese, S.; Brown, C.; Chen, F.; Dowson, C.G.; Dujardin, G.; Jung, N.; et al. Metal complexes as a promising source for new antibiotics. Chem. Sci. 2020, 11, 2627–2639. [Google Scholar] [CrossRef]
- Liu, X.; Manzur, C.; Novoa, N.; Celedón, S.; Carrillo, D.; Hamon, J.-R. Multidentate unsymmetrically-substituted Schiff bases and their metal complexes: Synthesis, functional materials properties, and applications to catalysis. Coord. Chem. Rev. 2018, 357, 144–172. [Google Scholar] [CrossRef]
- Jeevadason, A.W.; Murugavel, K.K.; Neelakantan, M.A. Review on Schiff bases and their metal complexes as organic photovoltaic materials. Renew. Sustain. Energy Rev. 2014, 36, 220–227. [Google Scholar] [CrossRef]
- Zhou, L.; Kwok, C.C.; Cheng, G.; Zhang, H.; Che, C.M. Efficient red organic electroluminescent devices by doping platinum(II) Schiff base emitter into two host materials with stepwise energy levels. Opt. Lett. 2013, 38, 2373–2375. [Google Scholar] [CrossRef] [PubMed]
- Sezgin, C.; Erden, I. Photovoltaic Properties of Thiophene-Containing Derivatives of 4,5-Diazafluorene and Their Ruthenium(II) Complexes. Theor. Exp. Chem. 2023, 59, 186–192. [Google Scholar] [CrossRef]
- Pessoa, J.C.; Garribba, E.; Santos, M.F.A.; Santos-Silva, T. Vanadium and proteins: Uptake, transport, structure, activity and function. Coord. Chem. Rev. 2015, 301–302, 49–86. [Google Scholar] [CrossRef]
- Mu, M.; Zhan, J.; Dai, X.; Gao, H. Research progress of azido-containing Pt(IV) antitumor compounds. Eur. J. Med. Chem. 2022, 227, 113927. [Google Scholar] [CrossRef]
- Schons, A.B.; Appelt, P.; Correa, J.S.; Cunha, M.A.A.; Rodrigues, M.G.; Anaissi, F.J. Green Synthesis of Na abietate Obtained from the Salification of Pinus elliottii Resin with Promising Antimicrobial Action. Antibiotics 2023, 12, 514. [Google Scholar] [CrossRef]
- Pessoa, J.C.; Etcheverry, S.; Gambino, D. Vanadium compounds in medicine. Coord. Chem. Rev. 2015, 301–302, 24–48. [Google Scholar] [CrossRef]
- Rehder, D. The role of vanadium in biology. Metallomics 2015, 7, 730–742. [Google Scholar] [CrossRef]
- Meenatchi, V.; Cheng, L.; Han, S.S. Twisted intramolecular charge transfer, nonlinear optical, antibacterial activity, and DFT analysis of ultrasound processed (E)-N′-(4-isopropylbenzylidene)nicotinohydrazide. J. Mol. Liq. 2023, 376, 121489. [Google Scholar] [CrossRef]
- Gurusamy, S.; Krishnaveni, K.; Sankarganesh, M.; Asha, R.N.; Mathavan, A. Synthesis, characterization, DNA interaction, BSA/HSA binding activities of VO(IV), Cu(II) and Zn(II) Schiff base complexes and its molecular docking with biomolecules. J. Mol. Liq. 2022, 345, 117045. [Google Scholar] [CrossRef]
- Fioravanço, L.P.; Pôrto, J.B.; Martins, F.M.; Siqueira, J.D.; Iglesias, B.A.; Rodrigues, B.M.; Chaves, O.A.; Back, D.F. Vanadium(V) complexes derived from pyridoxal/salicylaldehyde. Interaction with CT-DNA/HSA, and molecular docking assessments. J. Inorg. Biochem. 2023, 239, 112070. [Google Scholar] [CrossRef] [PubMed]
- Damena, T.; Alem, M.B.; Zeleke, D.; Demissie, T.B.; Desalegn, T. Synthesis and computational studies of novel cobalt(II) and oxovanadium(IV) complexes of quinoline carbaldehyde derivative ligand for antibacterial and antioxidant applications. J. Mol. Struct. 2023, 1280, 134994. [Google Scholar] [CrossRef]
- Biswas, B.K.; Biswas, N.; Saha, S.; Rahaman, A.; Mandal, D.P.; Bhattacharjee, S.; Sepay, N.; Zangrando, E.; Garribba, E.; Choudhury, C.R. Interaction with bioligands and in vitro cytotoxicity of a new dinuclear dioxide vanadium(V) complex. J. Inorg. Biochem. 2022, 237, 111980. [Google Scholar] [CrossRef]
- Mato-López, L.; Sar-Rañó, A.; Fernández, M.R.; Díaz-Prado, M.L.; Gil, A.; Sánchez-González, Á.; Fernández-Bertólez, N.; Méndez, J.; Valdiglesias, V.; Avecilla, F. Relationship between structure and cytotoxicity of vanadium and molybdenum complexes with pyridoxal derived ligands. J. Inorg. Biochem. 2022, 235, 111937. [Google Scholar] [CrossRef]
- Ge, E.J.; Bush, A.I.; Casini, A.; Cobine, P.A.; Cross, J.R.; Nicola, G.M.; Dou, Q.P.; Franz, K.J.; Gohil, V.M.; Gupta, S.; et al. Connecting copper and cancer: From transition metal signalling to metalloplasia. Nat. Rev. Cancer 2022, 22, 102–113. [Google Scholar] [CrossRef]
- Kumar, S.S.; Sadasivan, V.; Meena, S.S.; Sreepriya, R.S.; Biju, S. Synthesis, structural characterization and biological studies of Ni(II), Cu(II) and Fe(III) complexes of hydrazone derived from 2-(2-(2,2-dimethyl-4,6-dioxo-1,3-dioxan-5-ylidene)hydrazinyl)benzoic acid. Inorg. Chim. Acta 2022, 536, 120919. [Google Scholar] [CrossRef]
- Samy, F.; Shebl, M. Ligational behavior of a new bis (bidentate NO) donor hydrazone towards Co (II), Ni (II), and Cu (II) ions: Preparation, spectral, thermal, biological, docking, and theoretical studies. App. Organomet. Chem. 2022, 36, e6898. [Google Scholar] [CrossRef]
- Alaysuy, O.; Abumelha, H.M.; Alsoliemy, A.; Alharbi, A.; Alatawi, N.M.; Osman, H.E.M.; Zay, R.; El-Metwaly, N.M. Elucidating of new hydrazide-based complexes derived from Pd(II), Cu(II) and Cd(II) ions: Studies concerning spectral, DFT, Hirshfeld-crystal, biological screening beside Swiss-ADME verification. J. Mol. Struct. 2022, 1259, 132748. [Google Scholar] [CrossRef]
- Almalki, S.A.; Bawazeer, T.M.; Asghar, B.; Alharbi, A.; Aljohani, M.M.; Khalifa, M.E.; El-Metwaly, N. Synthesis and characterization of new thiazole-based Co(II) and Cu(II) complexes; therapeutic function of thiazole towards COVID-19 in comparing to current antivirals in treatment protocol. J. Mol. Struct. 2021, 1244, 130961. [Google Scholar] [CrossRef]
- Al-Qahtani, S.D.; Alsoliemy, A.; Almehmadi, S.J.; Alkhamis, K.; Alrefaei, A.F.; Zaky, R.; El-Metwaly, N. Green synthesis for new Co(II), Ni(II), Cu(II) and Cd(II) hydrazone-based complexes; characterization, biological activity and electrical conductance of nano-sized copper sulphate. J. Mol. Struct. 2021, 1244, 131238. [Google Scholar] [CrossRef]
- Munshi, A.M.; Bayazeed, A.A.; Abualnaja, M.; Morad, M.; Alzahrani, S.; Alkhatib, F.; Shah, R.; Zaky, R.; El-Metwaly, N.M. Ball-milling synthesis technique for Cu(II)-Schiff base complexes with variable anions; characterization, potentiometric study and in-vitro assay confirmed by in-silico method. Inorg. Chem. Commun. 2021, 127, 108542. [Google Scholar] [CrossRef]
- Elsayed, S.A.; Elnabky, I.M.; di Biase, A.; El-Hendawy, A.M. New mixed ligand copper(II) hydrazone-based complexes: Synthesis, characterization, crystal structure, DNA/RNA/BSA binding, in vitro anticancer, apoptotic activity, and cell cycle analysis. App. Organomet. Chem. 2022, 36, e6481. [Google Scholar] [CrossRef]
- Zengin, A.; Serbest, K.; Emirik, M.; Özil, M.; Menteşe, E.; Faiz, Ö. Binuclear Cu(II), Ni(II) and Zn(II) complexes of hydrazone Schiff bases: Synthesis, spectroscopy, DFT calculations, and SOD mimetic activity. J. Mol. Struct. 2023, 1278, 134926. [Google Scholar] [CrossRef]
- Patil, P.R.; Kumar, A.C.; Naik, N. Synthesis of Co(II), Ni(II) and Zn(II) Metal Complexes Derived from a Hydrazone Schiff Base: Electrochemical Behavior and Comprehensive Biological Studies. ChemSelect 2022, 35, e202200752. [Google Scholar] [CrossRef]
- Elokil, A.A.; Imbabi, T.A.; Mohamed, H.I.; Abouelezz, K.F.M.; Ahmed-Farid, O.; Shishay, G.; Sabike, I.I.; Liu, H. Zinc and Copper with New Triazine Hydrazone Ligand: Two Novel Organic Complexes Enhanced Expression of Peptide Growth Factors and Cytokine Genes in Weaned V-Line Rabbit. Animals 2019, 9, 1134. [Google Scholar] [CrossRef]
- Adam, M.S.S. Zn(II)-complexes of diisatin dihydrazones as effective catalysts in the oxidation protocol of benzyl alcohol and effective reagents for biological studies. J. Mol. Liq. 2023, 381, 121841. [Google Scholar] [CrossRef]
- Adam, M.S.S.; Elsawy, H.; Sedky, A.; Makhlouf, M.M.; Taha, A. Catalytic potential of sustainable dinuclear (Cu2+ and ZrO2+) metal organic incorporated frameworks with comprehensive biological studies. J. Taiwan Inst. Chem. Eng. 2023, 144, 104747. [Google Scholar] [CrossRef]
- Adam, M.S.S.; Mohamed, M.A. Effect of Cu+/2+ ions on the biological activity of their bis-oxalyldihydrazone complexes: ctDNA interaction, antimicrobial and anticancer action. Appl. Organomet. Chem. 2023, 37, e7190. [Google Scholar] [CrossRef]
- Yousif, E.; Majeed, A.; Al-Sammarrae, K.; Salih, N.; Salimon, J.; Abdullah, B. Metal complexes of Schiff base: Preparation, characterization and antibacterial activity. Arab. J. Chem. 2017, 10, S1639–S1644. [Google Scholar] [CrossRef]
- Roy, M.; Biswal, V.; Sarkar, O.; Pramanik, N.R.; Paul, S.; Manna, C.K.; Mondal, T.K.; Chakrabarti, S. Synthesis, characterization, DFT calculations, protein binding and molecular docking studies of mononuclear dioxomolybdenum(VI) complexes with ONS donor ligand. J. Mol. Struct. 2021, 1234, 130192. [Google Scholar] [CrossRef]
- Bonaccorso, C.; Marzo, T.; La Mendola, D. Biological Applications of Thiocarbohydrazones and Their Metal Complexes: A Perspective Review. Pharmaceuticals 2020, 13, 4. [Google Scholar] [CrossRef] [PubMed]
- Climova, A.; Pivovarova, E.; Szczesio, E.; Gobis, K.; Ziembicka, D.; Korga-Plewko, A.; Kubik, J.; Iwan, M.; Antos-Bielska, M.; Krzyżowska, M.; et al. Anticancer and antimicrobial activity of new copper (II) complexes. J. Inorg. Biochem. 2023, 240, 112108. [Google Scholar] [CrossRef] [PubMed]
- Bertini, S.; Coletti, A.; Floris, B.; Conte, V.; Galloni, P. Investigation of VO-salophen complexes electronic structure. J. Inorg. Biochem. 2015, 147, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, H.; Kojima, Y.; Yoshikawa, Y.; Kawabe, K.; Yasui, H. Antidiabetic vanadium(IV) and zinc(II) complexes. Coord. Chem. Rev. 2002, 226, 187–198. [Google Scholar] [CrossRef]
- Malik, M.A.; Dar, O.A.; Gull, P.; Wani, M.Y.; Hashmi, A.A. Heterocyclic Schiff base transition metal complexes in antimicrobial and anticancer chemotherapy. MedChemComm 2018, 9, 409–436. [Google Scholar] [CrossRef]
- Li, Y.; Xu, L.; Duan, M.; Wu, J.; Wang, Y.; Dong, K.; Han, M.; You, Z. An acetohydroxamate-coordinated oxidovanadium(V) complex derived from pyridinohydrazone ligand with urease inhibitory activity. Inorg. Chem. Commun. 2019, 105, 212–216. [Google Scholar] [CrossRef]
- Bala, I.; Singh, K.; Kataria, R.; Sindhu, M. Exploration of structural, electrostatic and photophysical behaviour of novel Ni(II), Cu(II) and Zn(II) complexes and their utility as potent antimicrobial agents. Appl. Organomet. Chem. 2022, 36, e6698. [Google Scholar] [CrossRef]
- Jaafar, A.; Mansour, N.; Fix-Tailler, A.; Allain, M.; Faour, W.H.; Shebaby, W.N.; Tokajian, S.; El-Ghayoury, A.; Naoufal, D.; Larcher, G.; et al. Synthesis, Characterization, Antibacterial and Antifungal Activities Evaluation of Metal Complexes With Benzaldehyde-4-methylthiosemicarbazone Derivatives. ChemistrySelect 2022, 7, e202104497. [Google Scholar] [CrossRef]
- Yao, Y.; Xu, H.-L.; Qiu, Y.-Q.; Su, Z.-M. The second-order nonlinear optical property of hydrazones-based photochromic complexes: A DFT study. J. Mol. Liq. 2021, 327, 114882. [Google Scholar] [CrossRef]
- Adam, M.S.S.; Khalil, A.K. Nickel (II), copper (II), and vanadyl (II) complexes with tridentate nicotinoyl hydrazone derivative functionalized as effective catalysts for epoxidation processes and as biological reagents. J. Taiwan Inst. Chem. Eng. 2022, 132, 104192. [Google Scholar] [CrossRef]
- Hassan, E.A.; Ebrahium, M.M.; Ebrahium, A.M. Metal complexes of hydrazone–oxime derivative as promising in vitro antimicrobial agents against some fungal and bacterial strains. Appl. Organomet. Chem. 2022, 36, e6663. [Google Scholar] [CrossRef]
- Tweedy, B.G. Plant extracts with metal ions as potential antimicrobial agents. Phytopathology 1964, 55, 910–917. [Google Scholar]
- Mahmoud, W.H.; Deghadi, R.G.; El Desssouky, M.M.I.; Mohamed, G.G. Transition metal complexes of nano bidentate organometallic Schiff base: Preparation, structure characterization, biological activity, DFT and molecular docking studies. Appl. Organometal. Chem. 2018, 32, e4556. [Google Scholar] [CrossRef]
- Fahim, A.M.; Magar, H.S.; Mahmoud, N.H. Synthesis, antimicrobial, antitumor activity, docking simulation, theoretical studies, and electrochemical analysis of novel Cd(II), Co(II), Cu(II), and Fe(III) complexes containing barbituric moiety. Appl. Organomet. Chem. 2023, 37, e7023. [Google Scholar] [CrossRef]
- Asha, T.M.; Kurup, M.R.P. DMSO coordinated dioxidomolybdenum(VI) complexes chelated with 3-methoxybenzhydrazone related ligands: Synthesis, structural studies and in vitro cytotoxicity. Polyhedron 2019, 169, 151–161. [Google Scholar] [CrossRef]
- Dharmaraj, N.; Viswanathamurthi, P.; Natarajan, K. Ruthenium(II) complexes containing bidentate Schiff bases and their antifungal activity. Transit. Met. Chem. 2001, 26, 105–109. [Google Scholar] [CrossRef]
- Alorini, T.; Daoud, I.; Al-Hakimi, A.N.; Alminderej, F.; Albadri, A.E.A.E. An experimental and theoretical investigation of antimicrobial and anticancer properties of some new Schiff base complexes. Res. Chem. Intermed. 2023, 49, 1701–1730. [Google Scholar] [CrossRef]
- Adam, M.S.S.; Abdel-Rahman, O.S.; Makhlouf, M.M. Metal ion induced changes in the structure of Schiff base hydrazone chelates and their reactivity effect on catalytic benzyl alcohol oxidation and biological assays. J. Mol. Struct. 2023, 1272, 134164. [Google Scholar] [CrossRef]
- Abualreish, M.J.A.; Mohamad, A.D.M.; Adam, M.S.S. Comprehensive catalytic and biological studies on new designed oxo- and dioxo-metal (IV/VI) organic arylhydrazone frameworks. J. Mol. Liq. 2023, 375, 121309. [Google Scholar] [CrossRef]
- Ji, Y.; Dai, F.; Zhou, B. Designing salicylaldehyde isonicotinoyl hydrazones as Cu(II) ionophores with tunable chelation and release of copper for hitting redox Achilles heel of cancer cells. Free Radic. Biol. Med. 2018, 129, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Kovacevic, Z.; Sahni, S.; Richardson, D.R. Copper That Cancer with Lysosomal Love! Aging 2016, 8, 210. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, S.A.; Badr, H.E.; di Biase, A.; El-Hendawy, A.M. Synthesis, characterization of ruthenium(II), nickel(II), palladium(II), and platinum(II) triphenylphosphine-based complexes bearing an ONS-donor chelating agent: Interaction with biomolecules, antioxidant, in vitro cytotoxic, apoptotic activity and cell cycle analysis. J. Inorg. Biochem. 2021, 223, 111549. [Google Scholar]
- Anjum, R.; Palanimuthu, D.; Kalinowski, D.S.; Lewis, W.; Park, K.C.; Kovacevic, Z.; Khan, I.U.; Richardson, D.R. Synthesis, Characterization, and in Vitro Anticancer Activity of Copper and Zinc Bis(Thiosemicarbazone) Complexes. Inorg. Chem. 2019, 58, 13709–13723. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Xu, X.; Qiu, H.; Li, N. Analytical characterization of DNA and RNA oligonucleotides by hydrophobic interaction liquid chromatography-tandem mass spectrometry. J. Chromatogh. A 2021, 1648, 462184. [Google Scholar] [CrossRef] [PubMed]
- Rehder, D. Vanadium in biological systems and medicinal applications. Inorg. Chim. Acta 2023, 549, 121387. [Google Scholar] [CrossRef]
- Mostafavinia, S.E.; Hoshyar, R. Spectroscopic studies on the interaction of anti-cancer rosemary with ctDNA, Gene. Cell Tissue 2016, 3, 35368–35372. [Google Scholar]
- Husain, M.A.; Rehman, S.U.; Ishqi, H.M.; Sarwar, T.; Tabish, M. Spectroscopic and molecular docking evidence of aspirin and diflunisal binding to DNA: A comparative study. RSC Adv. 2015, 5, 64335–64345. [Google Scholar] [CrossRef]
- Richards, A.D.; Rodger, A. Synthetic metallomolecules as agents for the control of DNA structure. Chem. Soc. Rev. 2007, 36, 471–483. [Google Scholar] [CrossRef]
- Adam, M.S.S.; Khalil, A. Bioreactivity of divalent bimetallic vanadyl and zinc complexes bis-oxalyldihydrazone ligand against microbial and human cancer series. ctDNA interaction mode. Int. J. Biol. Macromol. 2023, 249, 125917. [Google Scholar] [CrossRef] [PubMed]
- Philip, J.E.; Shahid, M.; Kurup, M.R.P.; Velayudhan, M.P. Metal based biologically active compounds: Design, synthesis, DNA binding and antidiabetic activity of 6-methyl-3-formyl chromone derived hydrazones and their metal (II) complexes. J. Photochem. Photobiol. B 2017, 175, 178–191. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.N.; Ahmed, S.S.; Alam, S.M.R. Biomedical applications of Schiff base metal complexes. J. Coord. Chem. 2020, 73, 3109–3149. [Google Scholar] [CrossRef]

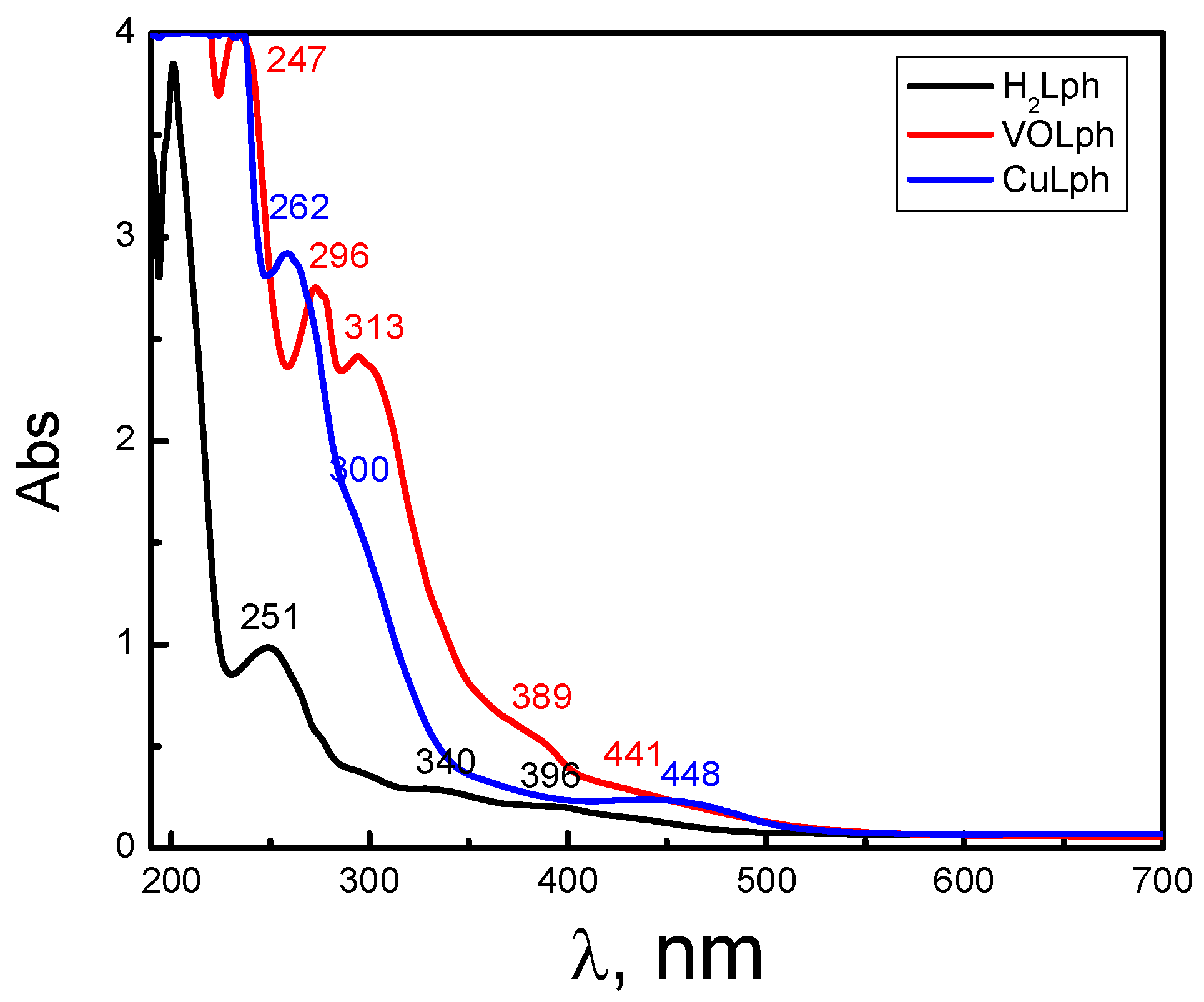
| Comp. (M.W.) | Color | CHN Analyses, % | Electronic Spectra | Λm, Ω−1 cm2 mol−1 | |||||
|---|---|---|---|---|---|---|---|---|---|
| C | H | N | λmax, nm | ε, mol−1·cm−1 | Assign. | DMSO | DMF | ||
| H2Lph (452.43 g·mol−1) | Dark yellow | 63.99 (63.71) | 3.12 (3.56) | 18.31 (18.58) | 251 | 9021 | π→π* | 25 | 31 |
| 340 | 4005 | n→π* | |||||||
| 396 | 3119 | LCT | |||||||
| VOLph (782.51 g·mol−1) | Greenish brown | 51.88 (52.19) | 3.90 (3.61) | 10.29 (10.74) | 247 | 10,185 | π→π* | 37 | 41 |
| 296 | 7922 | π→π* | |||||||
| 313 | 7031 | n→π* | |||||||
| 389 | 5204 | L-MCT | |||||||
| 441 | 4692 | d→d | |||||||
| CuLph (648.41 g·mol−1) | Green | 44.82 (44.46) | 1.92 (2.18) | 12.51 (12.96) | 262 | 8161 | π→π* | 32 | 44 |
| 300 | 6562 | n→π* | |||||||
| 448 | 4603 | d→d | |||||||
| Comp. | Zone of Effective Inhibition of the Growth of Three Bacteria (mm) | Zone of Effective Inhibition for the Growth of Three Fungi (mm) | ||||
|---|---|---|---|---|---|---|
| S. marcescens (−ve) | E. coli (−ve) | S. aureus (+ve) | C. albicans | A. flavus | T. rubrum | |
| H2Lph | 19 ± 0.05 | 18 ± 0.45 | 19 ± 0.10 | 17 ± 0.20 | 13 ± 0.26 | 16 ± 0.75 |
| VOLph | 36 ± 0.55 | 30 ± 0.45 | 39 ± 0.65 | 25 ± 0.80 | 20 ± 0.85 | 28 ± 0.55 |
| CuLph | 35 ± 0.75 | 29 ± 0.80 | 38 ± 0.35 | 26 ± 0.00 | 20 ± 0.75 | 27 ± 0.40 |
| VO(acac)2 | 27 ± 0.35 | 23 ± 0.75 | 25 ± 0.25 | 20 ± 0.65 | 17 ± 0.45 | 22 ± 0.50 |
| CuCl2·2H2O | 22 ± 0.20 | 18 ± 0.10 | 20 ± 0.05 | 17 ± 0.70 | 14 ± 0.15 | 17 ± 0.35 |
| Gentamycin | 40 ± 0.33 | 37 ± 0.72 | 46 ± 0.11 | -- | -- | -- |
| Fluconazole | -- | -- | -- | 37 ± 0.62 | 25 ± 0.90 | 31 ± 0.88 |
| Comp. | MIC (Minimum Inhibition Concentration), μM | |||||
|---|---|---|---|---|---|---|
| S. marcescens (−ve) | E. coli (−ve) | S. aureus (+ve) | C. albicans | A. flavus | T. rubrum | |
| H2Lph | 6.25 | 6.00 | 5.80 | 7.05 | 6.25 | 6.75 |
| VOLph | 1.50 | 1.25 | 1.00 | 1.25 | 1.50 | 1.25 |
| CuLph | 1.75 | 1.50 | 1.00 | 1.25 | 1.75 | 1.25 |
| DMSO | -- | -- | -- | -- | -- | -- |
| Comp. | Inhibited Zone (mm) of Bacterial Growth | Inhibited Zone (mm) of Fungal Growth | ||||
|---|---|---|---|---|---|---|
| S. marcescens (−ve) | E. coli (−ve) | S. aureus (+ve) | C. albicans | A. flavus | T. rubrum | |
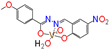 [49] | 31 ± 0.99 | 33 ± 0.05 | 37 ± 0.12 | 30 ± 0.52 | 17 ± 0.07 | 22 ± 0.83 |
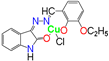 [50] | 32 ± 0.19 | 30 ± 0.29 | 35 ± 0.05 | 30 ± 0.77 | 17 ± 0.82 | 22 ± 0.35 |
 [29] | 33 ± 0.75 | 33 ± 0.42 | 37 ± 0.85 | 30 ± 0.90 | 19 ± 0.75 | 26 ± 0.05 |
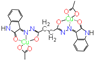 [29] | 32 ± 0.10 | 34 ± 0.00 | 38 ± 0.05 | 31 ± 0.52 | 18 ± 0.35 | 24 ± 0.60 |
| Comp. | Selectivity Index | ||
|---|---|---|---|
| HCT-116 | MCF-7 | HepG-2 | |
| H2Lph | 1.91 | 2.98 | 2.26 |
| VOLph | 2.42 | 3.68 | 2.42 |
| CuLph | 3.01 | 4.68 | 3.16 |
| Vinblastine | -- | -- | -- |
| Comp. | λfree (nm) | λbound (nm) | ∆n | Chromism | Kb 107 mol−1 dm3 | kJ mol−1 | |
|---|---|---|---|---|---|---|---|
| % | Type | ||||||
| H2Lph | 251 | 273 | 22 | 17.0 | Hypo | 12.41 | −44.05 |
| 340 | 347 | 7 | 6.6 | ||||
| 399 | 440 | 41 | 36.9 | ||||
| VOLph | 296 | 270 | 26 | 29.1 | Hypo | 16.31 | −47.11 |
| 389 | 347 | 42 | 15.8 | ||||
| 441 | 428 | 13 | 20.9 | ||||
| CuLph | 262 | 275 | 14 | 22.6 | Hypo | 16.04 | −46.89 |
| 300 | 311 | 11 | 16.3 | ||||
| 448 | 453 | 5 | 8.1 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalil, A.; Adam, M.S.S. Bimetallic bis-Aroyldihydrazone-Isatin Complexes of High O=V(IV) and Low Cu(II) Valent Ions as Effective Biological Reagents for Antimicrobial and Anticancer Assays. Molecules 2024, 29, 414. https://doi.org/10.3390/molecules29020414
Khalil A, Adam MSS. Bimetallic bis-Aroyldihydrazone-Isatin Complexes of High O=V(IV) and Low Cu(II) Valent Ions as Effective Biological Reagents for Antimicrobial and Anticancer Assays. Molecules. 2024; 29(2):414. https://doi.org/10.3390/molecules29020414
Chicago/Turabian StyleKhalil, Ahmed, and Mohamed Shaker S. Adam. 2024. "Bimetallic bis-Aroyldihydrazone-Isatin Complexes of High O=V(IV) and Low Cu(II) Valent Ions as Effective Biological Reagents for Antimicrobial and Anticancer Assays" Molecules 29, no. 2: 414. https://doi.org/10.3390/molecules29020414
APA StyleKhalil, A., & Adam, M. S. S. (2024). Bimetallic bis-Aroyldihydrazone-Isatin Complexes of High O=V(IV) and Low Cu(II) Valent Ions as Effective Biological Reagents for Antimicrobial and Anticancer Assays. Molecules, 29(2), 414. https://doi.org/10.3390/molecules29020414