Abstract
Several types of 1,4-diphenylanthracene derivatives 1–4 were prepared, and their photophysical properties were observed in the solid and solution states. Interestingly, the CN-group-substituted 1,4-diphenylanthracene derivative 2 was found to exhibit a higher fluorescence quantum yield (ϕf = 0.71) in the solid state than in the solution state, probably due to the formation of an intermolecular Ar–CN⋯H–Ar hydrogen bond and antiparallel type locked packing structure in the solid state. Furthermore, for some derivatives, an increase in the fluorescence quantum yield was observed in the PMMA film (1 wt%) over both the solid state and the solution state. More interestingly, some of the 1,4-diphenylanthracene derivatives exhibited unusual mechanofluorochromic properties with a “hypsochromic shift” in luminous color depending on the substituents of the phenyl group, and with the derivatives having CF3, OMe, CN, and two F substituents (1d–1f, 2–4) showing a significant luminous color change with a “hypsochromic shift” after grinding. However, no change in the luminous color was observed for the derivatives having H, Me, and one F substituent (1a–1c), and especially for some of the CN-substituted derivatives, a reversible luminous color change with a “hypsochromic shift” was observed, probably due to the formation of an antiparallel type packing structure. These “hypsochromic” anthracene derivatives could probably be utilized as new mechanofluorochromic materials.
1. Introduction
Organic solid-state light-emitting materials have received significant attention for their applications in light-emitting diodes (LEDs) [1,2,3,4,5,6,7,8], lasers [9,10,11,12,13,14], and optical waveguides [15,16,17,18,19]. Mechanofluorochromic materials, which are solid organic light-emitting materials that change their luminous color upon mechanical stimuli by crushing or grinding, have attracted attention for their applications in security inks, memory chips, and pressure sensors [20,21,22,23,24,25,26,27,28,29,30]. Numerous organic mechanofluorochromic materials have been reported, including metal complexes [31,32,33,34,35,36,37,38,39,40,41,42,43,44], anthracene derivatives [45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65], pyrene derivatives [66,67,68,69,70,71,72,73,74,75,76], tetraphenylethylene derivatives [77,78,79,80,81,82,83], and organoboron compounds [84,85,86,87,88,89,90,91,92,93,94]. However, most of these mechanofluorochromic materials have complex molecular structures and involve a multi-step synthesis, so the practical application of mechanofluorochromic materials with simple structures and easy synthesis is an important issue. Additionally, most of these mechanofluorochromic materials show a “bathochromic shift” (red shift) of their luminous color upon grinding, and mechanofluorochromic materials that show a “hypsochromic shift” (blue shift) in their luminous color are relatively rare [67,87,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110].
Recently, our research group reported that structurally simple 1,8-diphenylanthracene derivatives exhibit characteristic mechanofluorochromic properties with the normal “bathochromic shift” [62]. Interestingly, the core anthracene itself does not exhibit any mechanofluorochromic properties at room temperature, but the introduction of phenyl substituents causes it to exhibit mechanofluorochromic properties [65]. In order to develop this structurally simple mechanofluorochromic material and to clarify the reason for the role of the substituted phenyl groups and their substitution positions (i.e., whether or not the substitution at the 1,8-positions of the phenyl group is essential), we synthesized 1,4-diphenylanthracene derivatives 1–4 and studied several mechanofluorochromic properties. For the mechanofluorochromic properties of the 1,4-diphenylanthracene derivatives 1a (R = H), 1b (R = Me), and 1c (R = F), the blue luminous color of the pristine sample did not change after grinding. Interestingly, however, “hypsochromic shift” of the luminous color changes (e.g., yellow-green to blue-green) was observed after grinding for derivatives 1e, 1f, and 2–4 having OMe, CF3, CN, and two F groups. Especially the 1,4-diphenylanthracene derivatives 1f (R = CN), 2 (3-CN), and 3 (3,5-CN2, R′ = CN) substituted with CN groups underwent repeated reversible luminous color changes with “hypsochromic shift” upon grinding, heating, and fuming with solvent vapors, although some of the derivatives could not be fully recovered. This unique “hypsochromic shift” with repeated reversible emission color changes is thought to be controlled by the hydrogen bonding between the Ar–CN and Ar–H groups (Ar–CN⋯H–Ar) and the formation of an antiparallel type packing structure. These unique “hypsochromic” anthracene derivatives could probably be applied as new mechanofluorochromic materials.
2. Results and Discussion
2.1. Synthesis and Solid-State Photophysical Properties of 1,4-Diphenylanthracene Derivatives
The 1,4-diphenylanthracene derivatives 1–4 were prepared from 1,4-dibromoanthracene and the corresponding phenylboronic acids using the Suzuki–Miyaura cross-coupling reaction catalyzed by Pd(PPh3)4 (Scheme 1), and all the derivatives were obtained in 45–99% yields (see experimental part).
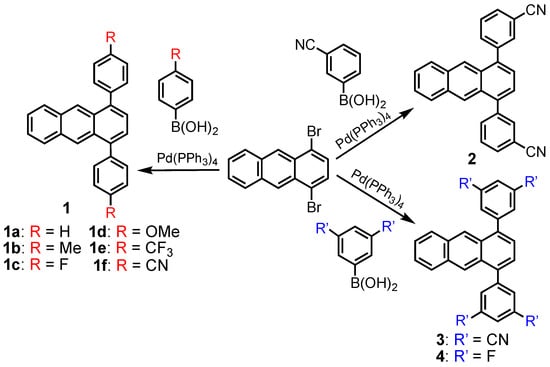
Scheme 1.
Synthetic route of 1,4-diphenylanthracene derivatives 1–4 (Red is substituent R and blue is substituent R’).
The photophysical properties of the obtained 1,4-diphenylanthracene derivatives (1–4) in the solid and solution state are shown in Table 1, Figures S1, S2, and S15, and the absorption spectra are shown in Figures S13 and S14. All the synthesized 1,4-diphenylanthracene derivatives 1–4 showed a light-blue (LB), green (G), or yellow-green (YG) luminous color in the pristine solid state (λmax = 452–536 nm) under UV irradiation at 365 nm (Table 1, Figure S2). For the solid-state fluorescence spectra of the pristine samples of the 1,4-diphenylanthracene derivatives, the fluorescence bands around 455–480 nm (λmax) were observed in the light-blue luminous solids (1a–1c, LB, Table 1, Figure 1a, Figures S1 and S2). On the other hand, in the yellow-green luminous solids (1d, 1f, 2, 3, YG), the fluorescence bands were observed around 514–522 nm (λmax). Additionally, for the green luminous solids (1e, 4, G), two fluorescence bands were observed around 480 and 530 nm (λmax, Table 1, Figure 1b, Figures S1 and S2).

Table 1.
Fluorescence properties of 1,4-diphenylanthracene derivatives.
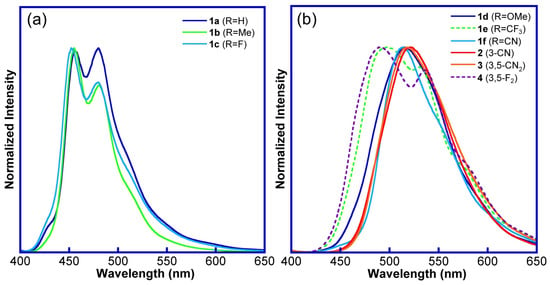
Figure 1.
Fluorescence spectra of 1,4-diphenylanthracene derivatives (pristine solids, λex = 365 nm). (a) 1,4-diphenylanthracene derivatives 1a–1c (LB), (b) 1,4-diphenylanthracene derivatives 1d–1f, 2–4 (YG: solid line, G: dotted line).
The fluorescence quantum yields (Φf) of the 1,4-diphenylanthracene derivatives were 0.15–0.71 in the pristine solids, and a relatively high fluorescence quantum yield was observed for 2 (3-CN, Φf = 0.71). The fluorescence lifetimes of the light-blue luminous crystals of 1a–1c are 2.27–3.96 ns, indicating that the short lifetimes are probably due to the monomer emission of the diphenylanthracene part (Table 1, Figure S20). On the other hand, slightly longer fluorescence lifetimes (4.69–5.22 ns) were observed for the green luminous crystals (1e, 4). Additionally, much longer fluorescence lifetimes (7.39–109 ns) were observed for the yellow-green luminous crystals (1f, 2, 3), and these greenish emission bands are probably due to the excimer emission of the diphenylanthracene part [47,48,51,61,62,63,64,65].
To understand the relationship between the crystal packing and solid state photophysical properties (fluorescence quantum yield, fluorescence lifetime, and luminous color) of the 1,4-diphenylanthrcene derivatives, the single crystal structures of 1a (R = H, light-blue luminous color, LB), 1b (R = Me, light-blue luminous color, LB), 1c (R = F, light-blue luminous color, LB), 1d (R = OMe, yellow-green luminous color, YG), 1f (R = CN, yellow-green luminous color, YG), and 2 (3-CN, yellow-green luminous color, YG) were analyzed. Single crystals were obtained from dimethoxyethane (DME) solutions for 1d and 1f, from hexane solutions for 1a and 1c, from a CHCl3 solution for 2, and from a CH2Cl2 solution for 1b. For the bimolecular stacked crystal packing of 1a (R = H), 1b (R=Me), 1c (R = F), and 1d (R = OMe), the 1,4-diphenylanthracene rings are arranged in the same direction, called the “parallel orientation” (Figure 2). On the other hand, in the bimolecular stacked crystal packing of the CN-group substituted derivatives 1f (R = CN) and 2 (3-CN), the 1,4-diphenylanthracene rings are arranged in the “antiparallel orientation”, and a stronger π-stacking is observed than in the “parallel orientation” (Figure 2). Additionally, Ar–CN⋯H–Ar or Ar–F⋯H–Ar hydrogen bonds were observed in the crystal packing of 1c (R = F), 1f (R = CN), and 2 (3-CN) with CN or F substituents (Figures S28, S32 and S34). To understand the reason for the formation of the “parallel” and “antiparallel” orientation packings of the 1,4-diphenylanthracene rings in the crystal structure, the electrostatic potential surfaces were calculated using DFT calculations according to the obtained crystal data (Figures S41 and S42). For the CN-group substituted derivatives 1f (R = CN) and 2 (3-CN), the positive charges are located at the edge of the substituted phenyl ring, as shown in Figures S41 and S42, and these positively charged edges are stacked with the center part of the phenyl ring of the other molecules to form a stable stacking bimolecular “antiparallel” packing. Additionally, in the crystal structure of the bimolecular packing of 1f (R = CN) and 2 (3-CN), many interactions, such as CH–π interactions between two 1,4-diphenylanthracen rings, were observed compared to the other non-CN substituted derivatives (1a–1d), as shown in Figure S36, and it may be showing that the “antiparallel” orientation is more stable than the “parallel” orientation in the case of electron withdrawing CN-group substituted derivatives.
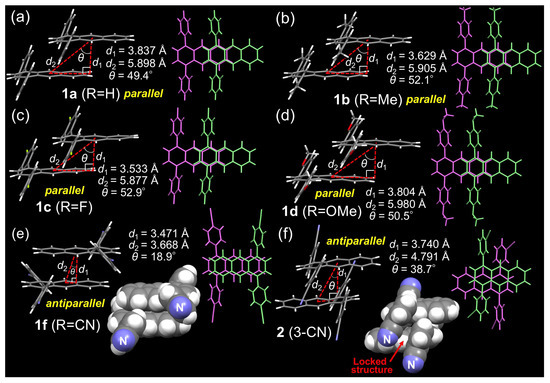
Figure 2.
Crystal structures of the 1,4-diphenylanthracene derivatives: (a) 1a (R = H), (b) 1b (R = Me), (c) 1c (R = F), (d) 1d (R = OMe), (e) 1f (R = CN), (f) 2 (3-CN).
According to the crystal structure analysis and photophysical data of the 1,4-diphenylanthracene derivatives, we can discuss the following:
- (1)
- Relation between solid-state luminous color, crystal packing, Hirshfeld surface analysis, and fluorescence lifetimes: As already mentioned, the luminous colors of the pristine samples of the 1,4-diphenylanthracene derivatives show light-blue (1a–1c, LB), green (1e, 4, G), and yellow-green (1d, 1f, 2, 3, YG) colors. In the case of the light-blue luminous derivatives 1a–1c, the emission is probably derived from the monomer of the 1,4-diphenylanthracene rings since the crystal packing of 1a–1c showed only partial overlapping of the anthracene ring, as indicated by the purple and green colors in Figure 2a,c. On the other hand, in the case of the yellow-green luminous derivatives 1f (R = CN) and 2 (3-CN), the emission is probably derived from the excimer state of the 1,4-diphenylanthracene rings, and a greater overlapping of the anthracene rings compared to 1a–1c is observed, as shown in Figure 2e,f. In the case of the yellow-green luminous MeO-group substituted derivative 1d, the crystal packing is very similar (overlapping only one benzene ring) to the light-blue luminous derivatives 1a–1c, as shown in Figure 2a–d, suggesting that an intermolecular charge transfer may be operating (see Figures S39 and S40, DFT calculation) and showing a yellow-green luminous color. To further understand the contribution of the π–π stacking for the crystal packing, the Hirshfeld surface analysis according to the crystal data was performed (Figure 3). The contribution of π–π stacking for 1f (R = CN, contribution of the C–C interactions: 13.8%) and 2 (3-CN, contribution of the C–C interactions: 13.5%) is greater than that for 1a–1c (contribution of the C–C interactions: 6.4–9.8%), indicating that the yellow-green emission of 1f and 2 is due to the formation of the π–π stacking (excimer emission). The fluorescence lifetimes of the 1,4-diphenylanthracene derivatives for the pristine state also show the difference in the contribution of the π–π stacking interactions as follows. Short fluorescence lifetimes between 2.27–3.96 ns were observed for the light-blue luminous samples 1a–1c; however, slightly longer fluorescence lifetimes were observed for the green luminous samples 1e (4. 69 ns, R = CF3) and 4 (5.22 ns, R′ = F, 3,5-F2) and for the yellow-green luminous samples 1f (R = CN), 2 (3-CN) and 3 (R ′= CN, 3,5-CN2), longer fluorescence lifetimes were observed between 7.39 and 109 ns (Table 1, Figure S20). These observed fluorescence lifetimes are increasing in the order of light-blue < green < yellow-green luminous color (the fluorescence wavelengths also increased in the same order). These results indicated that the contribution of the π–π stacking interactions is also increasing in the same order, which is in good agreement with the crystal data only except for 1d.
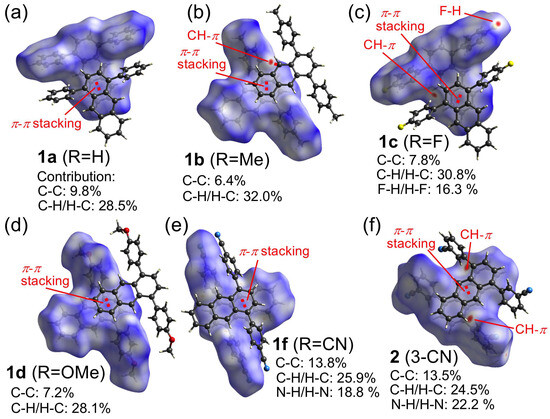 Figure 3. Hirshfeld surface showing the intermolecular interactions over dnorm and the percentages of the contact’s contribution to the total Hirshfeld surface area of the molecules: (a) 1a (R = H), (b) 1b (R = Me), (c) 1c (R = F), (d) 1d (R = OMe), (e) 1f (R = CN), (f) 2 (R′ = CN, 3-CN).
Figure 3. Hirshfeld surface showing the intermolecular interactions over dnorm and the percentages of the contact’s contribution to the total Hirshfeld surface area of the molecules: (a) 1a (R = H), (b) 1b (R = Me), (c) 1c (R = F), (d) 1d (R = OMe), (e) 1f (R = CN), (f) 2 (R′ = CN, 3-CN). - (2)
- Relation between fluorescence quantum yield, fluorescence lifetime, and crystal packing of 1f (R=CN) and 2 (3-CN): The pristine sample of 2 (3-CN) exhibited a relatively high fluorescence quantum yield (ϕf = 0.71) compared to the other 1,4-diphenylanthracene derivatives (ϕf = 0.15–0.44, Table 1), as already described. To consider the reason for the higher fluorescence quantum yield of 2 (3-CN), we calculated the rate constant kf for the fluorescence and the rate constant knr for non-radiative decay (Table S1) and compared kf and knr to that of 1f (R = CN) and 2 (3-CN). The rate constants kf for the fluorescence of 1f (kf = 0.008 ns−1) and 2 (kf = 0.006 ns−1) were almost the same, but the rate constant knr for the non-radiative decay of 2 (knr = 0.003 ns−1) was about seven times lower than that of 1f (knr = 0.022 ns−1), and the difference in knr caused the fluorescence quantum yield of 2 (ϕf = 0.71) to be higher than that of 1f (ϕf = 0.26) in the pristine solid state. The difference in knr between 1f and 2 may be due to the difference in the crystal packing structure. Due to the substitution of a CN group at the 3-position (meta-position) of the phenyl group in the derivative 2 and the intermolecular hydrogen bond (Ar–CN⋯H–Ar) formed by the CN group, the crystal packing structure of 2 forms a tight, lock-and-key type packing compared to the loose packing structure of 1f (Figure 2e,f and Figure S37). The tight packing structure of 2 is considered to suppress the molecular vibration in the solid state compared to the packing structure of 1f and exhibits a very low knr (0.003 ns−1 for 2) and a high fluorescence quantum yield (ϕf = 0.71).
2.2. Photophysical Properties of 1,4-Diphenylanthracene Derivatives in Solution and in PMMA Films
To compare the photophysical properties of the 1,4-diphenylanthracene derivatives in the solid and solution states, we measured their UV spectra, fluorescence spectra, fluorescence quantum yields, and fluorescence lifetimes of the 1,4-diphenylanthracene derivatives in chloroform solutions (Table 1, Figures S14–S16 and S21). In the case of 1,4-diphenylanthracene derivatives 1a–1c, 1e, 2, and 4, a blue luminous color (421–443 nm) was observed in the CHCl3 solution. However, for derivatives 1d (R = OMe), 1f (R = CN), and 3 (3,5-CN2), which have electron-donating and electron-withdrawing groups, a light-blue luminous color (454–458 nm) was observed in the CHCl3 solution (Figures S15 and S16). These derivatives may be causing a charge-transfer interaction in the solution state, but only a small solvatochromic effect on the fluorescence spectra was observed in different solvents (Figure S17). For all the 1,4-diphenylanthracene derivatives, the fluorescence quantum yields were observed with a moderate efficiency around ϕf = 0.41–0.56, and for almost all derivatives, the CHCl3 solution state showed higher fluorescence quantum yields than the pristine solid state. Additionally, the fluorescence lifetimes were observed around τ = 2.57–3.36 ns for all the derivatives, probably indicating a monomer emission in the solution state. Interestingly, only derivative 2 (3-CN) showed a higher fluorescence quantum yield (ϕf = 0.71) in the pristine solid state than in the solution state (ϕf = 0.46 in CHCl3).
In order to develop new fluorescent materials, it is important that the newly synthesized fluorescent materials exhibit high fluorescence properties even in the polymer-supported solid state. Therefore, we measured the photophysical properties of the 1,4-diphenylanthracene derivatives in PMMA (polymethyl methacrylate). The desired 1,4-diphenylanthracene derivative-containing PMMA film (1 wt%) was prepared by casting on a glass plate using a CHCl3 solution. For most of the PMMA films obtained, a blue or light-blue luminous color was observed, and the fluorescence spectra was similar to that of the CHCl3 solution but was significantly different from that of the pristine solid (Figures S18 and S19). These observations indicated that the blue emission of the 1,4-diphenylanthracene derivatives in the PMMA films is derived from the monomer emission, which is a significant difference from the solid-state emission (Figure S18). The fluorescence quantum yields of the PMMA films (1 wt%) of the 1,4-diphenylanthracene derivatives show relatively high efficiencies around ϕf = 0.62–0.71. Interestingly, the fluorescence quantum yields in the PMMA films are increased compared to the solid and solution states, except for 2 (3-CN). Additionally, the fluorescent lifetime of the PMMA film (1 wt%) of the 1,4-diphenylanthracene derivatives exhibited a slightly longer lifetime (τ = 3.45–4.16 ns) than the solution state (Tables S1 and S2, Figure S22). Based on these photophysical measurements, the rate constant kf for fluorescence of the PMMA films (kf = 0.1425–0.2058) was found to be similar to that of the corresponding solution state (Tables S1 and S2). However, the rate constants knr for non-radiative decay of the PMMA film (knr = 0.0837–0.0936) was found to be much lower than for the solution and solid states (Tables S1 and S2). The lower knr value showed the inhibition of the molecular motion in the PMMA film and showed an increasing fluorescence quantum yield in the PMMA films than in the solution state. In the pristine solid state of the 1,4-diphenylanthracene derivatives, the aggregation-caused quenching (ACQ) effect that may have occurred and the fluorescence quantum yields were reduced, except for derivative 2 (3-CN).
2.3. Mechanofluorochromic Properties of 1,4-Diphenylanthracene Derivatives
To further investigate the fluorescence properties of these 1,4-diphenylanthracene derivatives, we studied their mechanofluorochromic properties in the solid state. The light-blue luminous color (λmax = 452–455, 480 nm) of the pristine samples of the 1,4-diphenylanthracene derivatives 1a (R = H), 1b (R = Me), and 1c (R = F) did not change their luminous color after grinding in a mortar at room temperature under 365 nm UV light (Table 1, Figure 4, Figures S1 and S2). On the other hand, the yellow-green or green luminous color of the 1,4-diphenylanthracene derivatives 1d (R = OMe, from 515 nm YG to 486 nm BG), 1e (R = CF3, from 497, 530 nm G to 488 nm BG), 1f (R = CN, from 514 nm YG to 497 nm G), 2 (3-CN, from 522 nm YG to 493 nm BG), 3 (3,5-CN2, R′ = CN, from 518 nm YG to 497 nm G), and 4 (3,5-F2, R′ = F, from 489, 536 nm G to 489 nm BG) changed to shorter wavelength luminous colors (i.e., hypsochromic shift) when ground in a mortar (Table 1, Figure 4 and Figure 5a, Figures S1 and S2), and the fluorescence spectra also significantly changed, as shown in Figure S1. The compounds that show a “hypsochromic shift” in fluorescent color after grinding are very rare and interesting, but compared to mechanofluorochromic materials that show a “bathochromic shift”, the mechanistic studies are unclear at the present moment [67,87,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110].
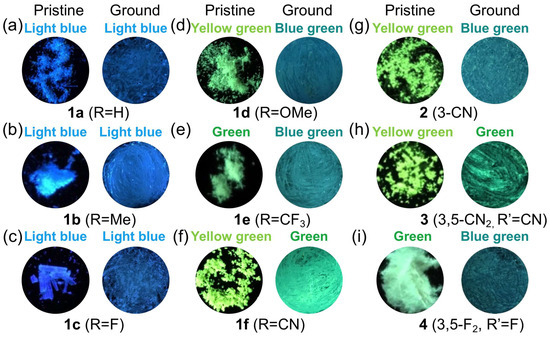
Figure 4.
Fluorescent images of the pristine and ground samples of 1,4-diphenylanthracene derivatives 1–4 during a 365 nm UV irradiation: (a) 1a (R = H), (b) 1b (R = Me), (c) 1c (R = F), (d) 1d (R = OMe), (e) 1e (R = CF3), (f) 1f (R = CN), (g) 2 (3-CN), (h) 3 (3,5-CN2, R′ = CN), (i) 4 (3,5-F2, R′ = F).
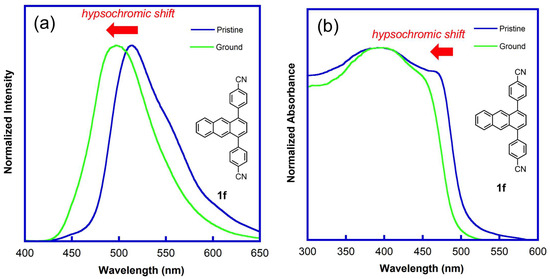
Figure 5.
Fluorescence and UV-vis spectra of 1,4-diphenylanthracene derivative 1f (R=CN) in the solid state: (a) fluorescence spectra of the pristine and ground samples (λex = 365 nm), (b) UV-vis absorption spectra of the pristine and ground samples.
For the mechanofluorochromic properties of the 1,8-diphenylanthracene derivatives previously reported, some of the derivatives recovered to their original fluorescent color after grinding within 5 min. at room temperature, but the CN-group substituted derivatives did not recover to their original emission color at room temperature [62]. However, this mechanofluorochromism of the 1,4-diphenylanthracene derivatives was not observed as a self-recovering phenomenon within 5 min. at room temperature in the luminous color changed derivatives 1d–1f and 2–4 (Figures S1 and S2).
For the yellow-green (YG) emitting CN-substituted derivatives 1f, 2, and 3, the fluorescence lifetimes (τ) of the ground samples became shorter than those of the pristine samples (Table 1), along with a “hypsochromic shift” of the luminous color. These results indicated that the π–π stacking structure dissociates after grinding, exhibiting a “hypsochromic shift” in the luminous color and a shortening of the fluorescence lifetimes.
To further understand the mechanofluorochromism of the 1,4-diphenylanthracene derivatives, the UV and IR spectra were observed in the solid state for the pristine and ground samples. In the UV spectra, a blue shift (hypsochromic shift) and decreasing of the longer wavelength peaks were observed for the derivatives 1d–1f, 2–4 (Figure 5b and Figure S13), which showed a “hypsochromic shift” in the fluorescence spectra after grinding, indicating a decrease in the contribution of the π-stacking structure after grinding. Furthermore, infrared (IR) spectra of the CN-substituted 1,4-diphenylanthracene derivatives 1f (R = CN) and 2 (3-CN) before and after grinding were observed, and the CN stretching band of the 1f and 2 shifted to the higher wavenumber side from the pristine to ground one, indicating that the hydrogen bond (Ar–CN⋯H–Ar) strength decreased after grinding (Figure 6, Figures S10 and S11).
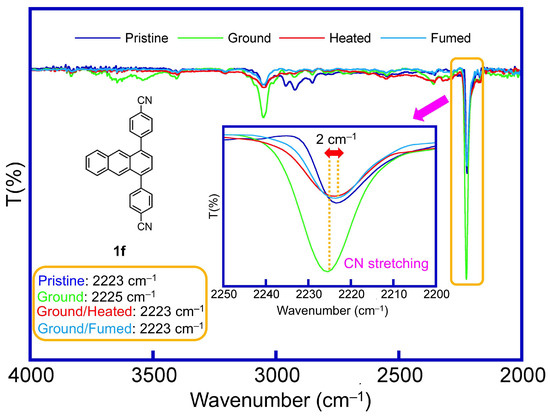
Figure 6.
IR spectra of the 1,4-diphenylanthracene derivative 1f (pristine, ground, ground-heated, ground-fumed samples) in the CN stretching region.
2.4. Vapochromic and Thermochromic Properties of 1,4-Diphenylanthracene Derivatives
To understand the reversibility of the luminous color of the 1,4-diphenylanthracene derivatives, fluorescence images and fluorescence spectra of the ground samples were observed after fuming with the CH2Cl2 vapor. The blue-green luminescent ground samples of derivatives 1d (R = OMe), 1e (R = CF3), and 4 (3,5-F2, R′ = F) showed no recovery of the luminous color (recovery to green or yellow-green, bathochromic shift) after fuming with the CH2Cl2 vapor for 20 h (Figures S3 and S4). Interestingly, for the CN-group-substituted 1,4-diphenylanthracene derivatives 1f (R = CN), 2 (3-CN), and 3 (3,5-CN2, R′ = CN), the yellow-green luminous color of the pristine sample turned to the green or blue-green luminous color (“hypsochromic shift”) with grinding, and the original yellow-green luminous color was recovered by fuming with solvent vapor (Figure 7, Figures S3 and S4). Additionally, repeated grinding–fuming cycles were also observed, but in some cases, a decrease in the fluorescence intensity and decreasing of the color-switching ability were observed (Figure 8, Figures S5 and S6). To understand the reason for the decrease in the fluorescence intensity and decreasing of the color-switching ability, we measured fluorescence spectra and 1H NMR spectra of the derivatives 1a (R = H) and 2 (3-CN) after UV irradiation in the solid state (365 nm, 5 min.). In the fluorescence spectra of 1a, no change in fluorescence intensity was observed after 365 nm UV irradiation. On the other hand, a slight decrease in fluorescence intensity was observed for derivative 2, but no spectral change was observed in the 1H NMR spectrum after dissolving the irradiated samples (Figures S43 and S44).
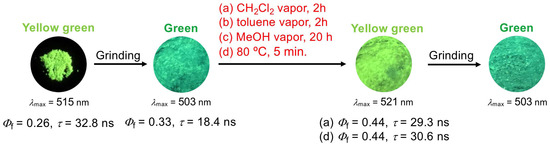
Figure 7.
Typical fluorescence images of ground, fumed (CH2Cl2, toluene, and MeOH vapors), and heated (80 °C for 5 min.) samples of 1,4-diphenylanthracene derivative 1f (R = CN) upon irradiation by 365 nm UV lamp.
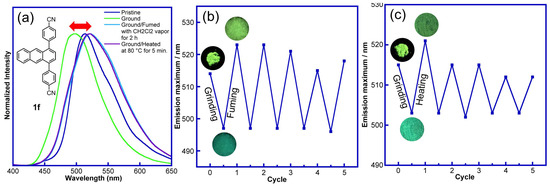
Figure 8.
Vapochromic and thermochromic properties of 1,4-diphenylanthracene derivative 1f (R = CN): (a) fluorescence spectra of pristine, ground, ground-fumed (CH2Cl2 vapor, 2 h), and ground-heated (80 °C, 5 min.) samples, (b) repeated grinding–fuming cycles, (c) repeated grinding–heating cycles.
Again, for derivatives 1d (R = OMe), 1e (R = CF3), and 4 (3,5-F2, R′ = F), no recovery to the original luminous color (pristine state) by heating the ground sample was observed (Figures S3 and S4). However, for the CN-group-substituted 1,4-diphenylanthracene derivatives 1f (R = CN), 2 (3-CN), and 3 (3,5-CN2, R′ = CN), a similar recovery of the luminous color of the ground sample to the luminous color of the pristine sample by heating was also observed (Figure 7, Figures S3, S4, and S7–S9). The green luminous ground sample of 1f (R = CN) turned yellow-green when heated at 80 °C for 5 min., a recovery of the green luminous color was observed upon regrinding (Figure 7 and Figure 8a), and the repeated cycles of grinding–heating are shown in Figure 8c. Although repeated grinding–heating cycles were observed, in some cases, a decrease in the fluorescence intensity and decreasing of the color-switching ability were observed.
To further understand the grinding–heating process of the CN-substituted 1,4-diphenylanthracene derivative 1f (R = CN), differential scanning calorimetry (DSC) was conducted to observe the phase transition between the ground and ground-heated samples. For the yellow-green luminous pristine sample of 1f, only the endothermic peak of melting (281.6 °C, 285.9 °C) was observed (Figure 9a). On the other hand, a broad exothermic peak was observed around 89.1 °C in the green luminous ground sample, which may have caused the transition from the green to yellow-green luminous state. Similar exothermic peaks were also observed in the ground samples for derivative 2 with a substituted CN group (Figure 9b). The DSC analysis of the other 1,4-diphenylanthracene derivatives 1a–1e was examined, but only endothermic melting peaks were observed (Figure S12).
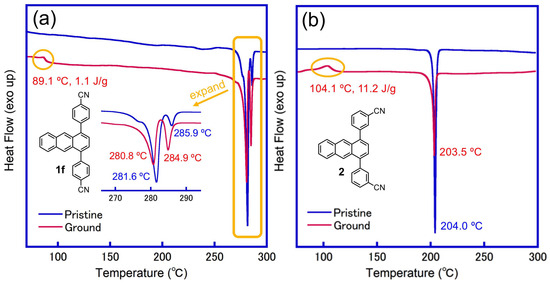
Figure 9.
DSC profiles of 1,4-diphenylanthracene derivatives before grinding (pristine) and after grinding (ground): (a) 1f (R = CN), (b) 2 (3-CN).
To understand in detail the mechanofluorochromic properties of the 1,4-diphenylanthracene derivatives, the powder X-ray diffraction patterns (PXRD) of the pristine, ground, ground-fumed, and ground-heated samples of derivative 1f (R = CN) were observed. The “yellow-green” luminous pristine sample of 1f showed a sharp and strong peak in the PXRD measurement (Figure 10a), but no diffraction peak was observed in the ground “green luminous” sample (Figure 10b). After heating at 80 °C for 5 min., the ground sample recovered its “yellow-green luminous color” and showed small crystallization peaks (Figure 10c). Similar crystallization of the ground sample was also observed when fuming with CH2Cl2 vapor for 2 h (Figure 10d). These observations, like those in the previously reported paper, show that this mechanofluorochromism may be operating under a phase transition from the crystalline state to the amorphous state [32,33,34,35,36,37,38,39,40,41,42,43,44,46,47,48,49,52,53,55,57,58,59,71,72,77,80,83,84,86,87,89,90,91,92,93,94]. Furthermore, during the vapochromic and thermochromic recovery of the ground sample of 1f, a reversible change in the fluorescence lifetimes (pristine: τ = 32.8 ns, ground: τ = 18.4 ns, ground-fumed with CH2Cl2 vapor: τ = 29.3 ns, ground-heated: τ = 30.6 ns) was observed (Figure 7). These changes in the fluorescence lifetimes of 1f are indicated as follows: (i) the π–π stacked pristine sample showed a yellow-green luminous color (λmax = 514 nm) and showed a longer fluorescence lifetime (τ = 32.8 ns) due to π–π stacking structure; (ii) after grinding, the sample showed a green luminous color (λmax = 497 nm, “hypsochromic shift”) and showed a shorter fluorescence lifetime (τ = 18.4 ns) due to the decreasing of the π–π stacked structure; (iii) after fuming and heating of the ground sample, the longer fluorescence lifetimes (τ = 29.3 and 30.6 ns) and yellow-green luminous color were recovered due to the recovering of the π–π stacked structure (crystallization was observed in the PXRD analysis).
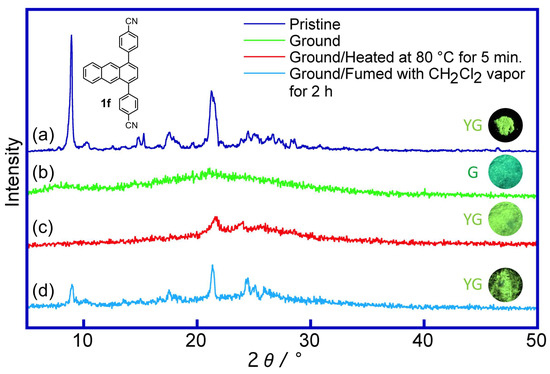
Figure 10.
Powder X-ray diffraction (PXRD) patterns of 1,4-diphenylanthracene derivative 1f (R = CN): (a) pristine, (b) ground, (c) ground-heated sample (80 °C, 5 min.), and (d) ground-fumed sample (with CH2Cl2 vapor, 2 h).
Furthermore, the IR spectra of the ground-heated and ground-fumed samples showed a recovery of the wavenumber of the CN stretching bands from the ground state in derivatives 1f and 2 (a shorter wavenumber shift from the ground sample was observed; Figure 6, Figures S10 and S11), and only the CN-substituted 1,4-diphenylanthracene derivatives showed a reversible emission color change, indicating that the hydrogen bond between the Ar–CN group and the Ar–H hydrogen atom (Ar–CN⋯H–Ar) is important for the controllable reversible mechanofluorochromism in the CN-substituted 1,4-diphenylanthracene derivatives.
Although it is difficult to further understand the critical mechanism of this mechanofluorochromism, it can be classified as follows: (Type-A) the 1,4-diphenylanthracene derivatives 1a–1c show a blue luminous color in the pristine state, and the crystal packing showed a parallel-type orientation. Only a weaker π–π stacking was observed, and its blue luminous color probably comes from monomer emission. After grinding the pristine sample, it does not change the original luminous color (Scheme 2a); (Type-B) the derivatives 1d, 1e, and 4 show green and yellow-green luminous colors in the pristine state, and the crystal packing of 1d showed a parallel-type orientation. Only weak π–π stacking was observed, and its green and yellow-green luminous color probably comes from the partial excimer emission or charge-transfer interactions. After grinding the pristine sample, it changes its original luminous color to a bluish color, but its original luminous color does not recover by heating and fuming of the ground samples (Scheme 2b); (Type-C) the derivatives 1f, 2, and 3, which having CN-groups, show a yellow-green luminous color in the pristine state, and the crystal packing showed an antiparallel-type orientation. A stronger π–π stacking was observed, and its yellow-green luminous color probably comes from the excimer emission. After grinding the pristine sample, its original luminous color changes to the shorter wavelength side (yellow-green to green or yellow-green to blue-green), and its original luminous color was repeatedly recovered by heating and fuming of the ground samples. Thus, only some of the CN-substituted 1,4-diphenylanthracene derivatives exhibited a repetitive reversible mechanofluorochromism with a “hypsochromic” luminous color change upon grinding, heating, and fuming with solvent vapors, which may be due to the stronger π–π stacking attributed to the antiparallel crystal packing structure (Scheme 2c). The mechanofluorochromic properties of other phenylanthracence derivatives, such as 1,5-diphenylanthracence and fused phenylanthracene derivatives [111] are currently under investigation.
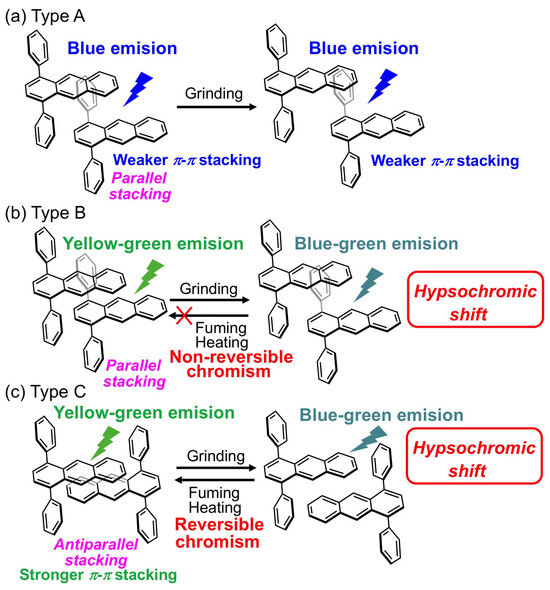
Scheme 2.
Schematic diagram of different types of mechanofluorochromic properties of 1,4-diphenylanthracene derivatives: (a) Type-A, non-mechanofluorochromic phenomenon for derivatives 1a–1c; (b) Type-B shows mechanofluorochromic phenomenon but non-reversible change by fuming and heating for derivatives 1d, 1e, and 4; (c) Type-C shows mechanofluorochromic phenomenon repeatedly changing by fuming and heating for the CN-substituted derivatives 1f, 2, and 3.
3. Materials, Equipment and Methods
3.1. General Methods
The 1H and 13C NMR spectra were recorded using Bruker (Billerica, MA, USA) Avance III (1H: 500 and 13C: 125 MHz) spectrometers. The FAB-mass spectra were recorded by a JEOL (Tokyo, Japan) JMS-700 mass spectrometer. The absorption spectra (solid and solution samples) were recorded by a Jasco (Tokyo, Japan) V-750 UV-Visible spectrometer. The fluorescence spectra for the solution samples were recorded by a Jasco (Tokyo, Japan) FP-8300 spectrometer, and the fluorescence spectra were calibrated using rhodamine B as the reference. The fluorescence spectra for the solid samples were recorded by a Flame-S, Ocean Optics (Orlando, FL, USA) fiber-optic spectrometer (λex = 365 nm). The absolute PL quantum yields (Φ) were determined by a Quantaurus-QY (Hamamatsu Photonics, Hamamatsu, Japan) instrument. The emission lifetimes were measured by a Quantaurus-Tau (Hamamatsu Photonics) instrument. The DSC thermograms of the solid samples were recorded by a TA Instruments (New Castle, Delaware, USA) DSC 2920 modulated DSC and were recorded at the constant heating rate of 10 °C/min. The infrared spectra were recorded using a Jasco (Tokyo, Japan) FTIR 4600 spectrometer equipped with an attenuated total reflection (ATR) unit. All the solvents and reagents were purified according to standard procedures. 3,5-dicyanophenylboronic acid [65] and 1,4-dibromoanthracene [112,113,114] were prepared according to published procedure.
3.2. Synthesis of 1,4-Diphenylanthracene Derivatives 1–4
General Procedure: 1,4-dibromoanthracene (103 mg, 0.307 mmol), phenylboronic acid (90.6 mg, 0.743 mmol), Pd(PPh3)4 (35.6 mg, 30.8 μmol) were mixed in 20 mL of DME. Then a 1 M K2CO3 aqueous solution (3.0 mL, 3.00 mmol) was added. The obtained mixture was degassed 3 times by a freeze-pump-through method, and then the mixture was heated at 85 °C for 18 h under an Ar atmosphere. After removing the solvent in vacuo and adding water (80 mL), the mixture was extracted with CH2Cl2 (80 mL), dried over anhydrous Na2SO4, and the solvent evaporated. The 1,4-diphenylanthracene 1a was isolated (98.2 mg, 97%) by column chromatography (SiO2, CH2Cl2, or CH2Cl2/hexane) followed by recrystallization from MeOH or CH2Cl2/MeOH.
- 1a (R = H): yield 87%, pale-yellow needles, mp: 157.8–158.8 °C; 1H NMR (500 MHz, CDCl3): δ (ppm) = 8.52 (s, 2H), 7.87 (dd, J = 6.4, 3.3 Hz, 2H), 7.63 (d, J = 8.2 Hz, 4H), 7.56 (t, J = 7.5 Hz, 4H), 7.49 (t, J = 7.3 Hz, 2H), 7.44 (s, 2H), 7.40 (dd, J = 6.6, 3.2 Hz, 2H), 13C NMR (125 MHz, CDCl3): δ (ppm) = 141.2 (Cq), 140.0 (Cq), 131.5 (Cq), 130.8 (Cq), 130.4 (CH), 128.6 (CH), 128.5 (CH), 127.6 (CH), 125.9 (CH), 125.7 (CH), 125.5 (CH), HRMS (FAB, NBA) m/z = 330.1417 (calculated for M+·: 330.1409), Anal. Calcd. for C26H18: C: 94.51, H: 5.49, Found: C: 94.41, H: 5.58.
- 1b (R = Me): yield 76%, pale-yellow prisms, mp: 192.3–193.3 °C; 1H NMR (500 MHz, CDCl3): δ (ppm) = 8.54 (s, 2H), 7.87 (dd, J = 6.4, 3.3 Hz, 2H), 7.52 (d, J = 8.0 Hz, 4H), 7.42 (s, 2H), 7.39 (dd, J = 6.6, 3.2 Hz, 2H), 7.37 (d, J = 7.7 Hz, 4H), 2.50 (s, 6H), 13C NMR (125 MHz, CDCl3): δ (ppm) = 139.6 (Cq), 138.1 (Cq), 137.1 (Cq), 131.3 (Cq), 130.7 (Cq), 130.1 (CH), 129.1 (CH), 128.3 (CH), 125.8 (CH), 125.4 (CH), 125.3 (CH), 21.3 (CH3), HRMS (FAB, NBA) m/z = 358.1723 (calculated for M+·: 358.1722), Anal. Calcd. for C28H22·0.4H2O: C: 91.97, H: 6.28, Found: C: 92.02, H: 6.17.
- 1c (R = F): yield 94%, pale-yellow prisms, mp: 160.0–161.0 °C; 1H NMR (500 MHz, CDCl3): δ (ppm) = 8.43 (s, 2H), 7.87 (dd, J = 6.4, 3.3 Hz, 2H), 7.55 (m, 4H), 7.42 (dd, J = 6.6, 3.2 Hz, 2H), 7.38 (s, 2H), 7.24 (m, 4H), 13C NMR (125 MHz, CDCl3): δ (ppm) = 162.6 (Cq, d, 1JC-F = 246.7 Hz), 139.1 (Cq), 136.9 (Cq, d, 4JC-F = 3.0 Hz), 131.9 (CH), 131.8 (CH), 131.6 (Cq), 130.8 (Cq), 128.4 (CH), 126.0 (CH), 125.3 (CH), 115.6 (CH, d, 2JC-F = 21.4 Hz), 19F NMR (470 MHz, CDCl3): δ (ppm) = −116.1, HRMS (FAB, NBA) m/z = 366.1230 (calculated for M+·: 366.1220), Anal. Calcd. for C26H16F2: C: 85.23, H: 4.40, Found: C: 85.15, H: 4.46.
- 1d (R = OMe): yield 80%, yellow solids, mp: 246.9–247.9 °C; 1H NMR (500 MHz, CDCl3): δ (ppm) = 8.53 (s, 2H), 7.88 (dd, J = 6.4, 3.3 Hz, 2H), 7.54 (d, J = 8.7 Hz, 4H), 7.404 (s, 2H), 7.401 (dd, J = 6.4, 3.3 Hz, 2H), 7.10 (d, J = 8.7 Hz, 4H), 3.93 (s, 6H), 13C NMR (125 MHz, CDCl3): δ (ppm) = 159.2 (Cq), 139.4 (Cq), 133.6 (Cq), 131.5 (Cq), 131.4 (CH), 131.1 (Cq), 128.5 (CH), 125.9 (CH), 125.6 (CH), 125.5 (CH), 114.0 (CH), 55.5 (CH3), HRMS (FAB, NBA) m/z = 390.1619 (calculated for M+·: 390.1620), Anal. Calcd. for C28H24O3·H2O: C: 82.33, H: 5.92, Found: C: 82.30, H: 5.57.
- 1e (R = CF3): yield 88%, yellow solids, mp: 207.5–208.5 °C; 1H NMR (500 MHz, CDCl3): δ (ppm) = 8.42 (s, 2H), 7.89 (dd, J = 6.4, 3.2 Hz, 2H), 7.83 (d, J = 8.0 Hz, 4H), 7.73 (d, J = 8.0 Hz, 4H), 7.45 (dd, J = 6.6, 3.1 Hz, 2H), 7.43 (s, 2H), 13C NMR (125 MHz, CDCl3): δ (ppm) = 144.5 (Cq), 139.1 (Cq), 131.6 (Cq), 130.5 (CH), 130.1 (Cq), 129.83 (q, 2JC-F = 32.5 Hz), 128.3 (CH), 126.1 (CH), 125.8 (CH), 125.5 (CH, q, 3JC-F = 3.6 Hz), 125.1 (CH), 124.3 (CF3, q, 1JC-F = 272.1 Hz), 19F NMR (470 MHz, CDCl3): δ (ppm) = −63.5, HRMS (FAB, NBA) m/z = 466.1154 (calculated for M+·: 466.1156), Anal. Calcd. for C28H16F6·0.2H2O: C: 71.55, H: 3.52, Found: C: 71.41, H: 3.50.
- 1f (R = CN): yield 88%, yellow solids, mp: 278.5–279.5 °C; 1H NMR (500 MHz, CDCl3): δ (ppm) = 8.39 (s, 2H), 7.90 (dd, 2H), 7.89 (d, J = 8.2 Hz, 4H), 7.75 (d, J = 8.2 Hz, 4H), 7.49 (dd, J = 6.6, 3.2 Hz, 2H), 7.45 (s, 2H), 13C NMR (125 MHz, CDCl3): δ (ppm) = 145.5 (Cq), 139.0 (Cq), 132.4 (CH), 131.7 (Cq), 130.8 (CH), 129.7 (Cq), 128.2 (CH), 126.4 (CH), 125.8 (CH), 125.0 (CH), 118.8 (Cq), 111.6 (Cq), HRMS (FAB, NBA) m/z = 380.1324 (calculated for M+·: 380.1313), Anal. Calcd. for C28H16N2·0.2H2O: C: 87.57, H: 4.30, N: 7.29, Found: C: 87.51, H: 4.37, N: 7.17.
- 2 (3-CN): yield 88%, yellow solids, mp: 208.1–209.1 °C; 1H NMR (500 MHz, CDCl3): δ (ppm) = 8.35 (s, 2H), 7.92 (t, J = 1.6 Hz, 2H), 7.92 (dd, 2H), 7.87 (dt, J = 7.7, 1.5 Hz, 2H), 7.83 (dt, J = 7.8, 1.4 Hz, 2H), 7.50 (dd, J = 6.6, 3.2 Hz, 2H), 7.43 (s, 2H), 13C NMR (125 MHz, CDCl3): δ (ppm) = 141.9 (Cq), 138.4 (Cq), 134.5 (CH), 133.5 (CH), 131.7 (Cq), 131.3 (CH), 129.9 (Cq), 129.4 (CH), 128.2 (CH), 126.4 (CH), 125.9 (CH), 124.9 (CH), 118.7 (Cq), 112.9 (Cq), HRMS (FAB, NBA) m/z = 380.1306 (calculated for M+·: 380.1313), Anal. Calcd. for C28H16N2·0.4H2O: C: 86.75, H: 4.37, N: 7.23, Found: C: 86.65, H: 4.29, N: 7.12.
- 3 (3,5-CN2, R′=CN): yield 45%, yellow solids, mp: >300 °C; 1H NMR (500 MHz, CDCl3): δ (ppm) = 8.24 (s, 2H), 8.13 (d, J = 1.5 Hz, 4H), 8.11 (t, J = 1.5 Hz, 2H), 7.95 (dd, J = 6.5, 3.3 Hz, 2H), 7.58 (dd, J = 6.6, 3.1 Hz, 2H), 7.44 (s, 2H), 13C NMR (125 MHz, CDCl3): δ (ppm) = 143.3 (Cq), 137.2 (CH), 137.0 (Cq), 134.5 (CH), 132.1 (Cq), 129.2 (Cq), 128.2 (CH), 127.2 (CH), 126.2 (CH), 124.6 (CH), 116.5 (Cq), 114.7 (Cq), HRMS (FAB, NBA) m/z = 430.1214 (calculated for M+·: 430.1218).
- 4 (3,5-F2, R′ = F): yield 99%, yellow solids, mp: 196.8–197.8 °C; 1H NMR (500 MHz, CDCl3): δ (ppm) = 8.45 (s, 2H), 7.92 (dd, J = 6.4, 3.3 Hz, 2H), 7.47 (dd, J = 6.6, 3.1 Hz, 2H), 7.40 (s, 2H), 7.13 (dd, 3JF-H = 7.8 Hz, 4JH-H = 2.0 Hz, 4H), 6.96 (tt, 3JF-H = 9.0 Hz, 4JH-H = 2.3 Hz, 2H), 13C NMR (125 MHz, CDCl3): δ (ppm) = 163.2 (Cq, dd, 1JC-F = 249.1, 3JC-F = 12.9 Hz), 144.1 (Cq, t, 3JC-F = 9.5 Hz), 138.6 (Cq), 131.9 (Cq), 130.0 (Cq), 128.5 (CH), 126.4 (CH), 125.7 (CH), 125.1 (CH), 113.3 (CH, dd, 2JC-F = 19.3, 4JC-F = 6.1 Hz), 103.3 (CH, t, 2JC-F = 25.3 Hz), 19F NMR (470 MHz, CDCl3): δ (ppm) = −110.8, HRMS (FAB, NBA) m/z = 402.1045 (calculated for M+·: 402.1032), Anal. Calcd. for C26H14F4·0.5H2O: C: 75.91, H: 3.68, Found: C:76.01, H: 3.75.
3.3. Computational Methods
Density functional theory (DFT) with the B3LYP functional at the 6–31G* basis set level was used to calculate the electro cloud distribution of the HOMO and LUMO in a vacuum. For the calculation from the obtained single crystal structures, two molecules, which were stacked on each other, were located, and the non-hydrogen atom coordinates were fixed. Only the hydrogen atoms were optimized to obtain the conformation for the DFT calculation. All of the calculations were performed using Gaussian 16W C.01 [115].
3.4. Crystallographic Analysis
Crystals suitable for X-ray crystallography were grown from the solutions of 1a (R = H), 1c (R = F) in hexane solution that were stored in a test tube and left to stand for several days. For compounds 1d (R = OMe) and 1f (R = CN), the crystals were obtained from DME solution. For compounds 1b (R = Me) and 2 (3-CN), the crystals were obtained from CH2Cl2 and CHCl3 solutions, respectively. For the crystallographic data collection of 1a (R = H) and 2 (3-CN), Rigaku XtaLAB mini with graphite-monochromated Mo-Kα radiation was used. Calculations were performed using the Olex2, version 1.3.0 crystallographic software packages [116]. The crystal structures were solved by a direct method using Shelxt Version 2014/2015 [117,118]. The structure refinements were performed by a full-matrix least squares method using Shelxl Version 2014/2015 [119,120]. For the crystallographic data collection of 1b (R = Me), 1c (R = F), 1d (R = OMe), and 1f (R = CN), a Bruker D8 VENTURE equipped with a rotating anode of Mo-Kα or Cu-Kα radiation and a PHOTON III detector was used. Calculations were performed using the Bruker APEX3, 2019 [121] software package. Details of the data are summarized in Tables S3 and S4. All non-hydrogen atoms were anisotropically refined. All the hydrogen atoms were placed in idealized positions and were included in the structure factor calculations but were not refined. CCDC-2314555-2314560 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
3.5. PXRD Analysis
Powder X-ray diffraction (PXRD) patterns of the samples were recorded using a Rigaku Smartlab X-ray diffractometer with CuKα radiation (1.5406 Å) at a voltage of 40 kV and a current of 30 mA, and the data were collected with a step size of 0.01° over a 2θ range of 5–50°.
4. Conclusions
In this study, several types of 1,4-diphenylanthracene derivatives 1–4 were prepared, and their photophysical properties were observed in the solid and solution states. The CN-group substituted 1,4-diphenylanthracene derivative 2 exhibited a relatively high fluorescence quantum yield (Φ = 0.71) in the solid state than that in the solution state. Interestingly, for some derivatives, an increase in the fluorescence quantum yield was observed in the PMMA film (1 wt%) over both the solid state and the solution state. More interestingly, some of the 1,4-diphenylanthracene derivatives exhibited mechanofluorochromic properties with “hypsochromic shift” of the luminous color depending on the substituents of the phenyl group. Especially for some CN-substituted derivatives, a reversible luminous color change with a “hypsochromic shift” was observed, probably due to the formation of the antiparallel-type packing structure. These “hypsochromic” anthracene derivatives could probably be applied as new mechanofluorochromic materials.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29020407/s1. Fluorescence spectra of the pristine, ground, and stored samples of 1,4-diphenylanthracene derivatives (Figure S1), fluorescence images of the pristine, ground, stored, heated and fumed sample of 1,4-diphenylanthracene derivatives (Figures S2 and S3), normalized fluorescence spectra of the pristine, ground, ground-fumed and ground-heated samples (Figure S4), vapochromic properties of 1,4-diphenylanthracene derivatives (Figures S5 and S6),thermochromic properties of 1,4-diphenylanthracene derivatives (Figures S7–S9), IR spectra of the CN-substituted 1,4-diphenylanthracene derivatives (Figures S10 and S11), DSC profiles of 1,4-diphenylanthracene derivatives (Figure S12), UV-vis absorption spectra of the pristine and ground samples (Figure S13), UV-vis absorption spectra of 1,4-diphenylanthracene derivatives in solution (Figure S14), fluorescence spectra of 1,4-diphenylanthracene derivatives in solution (Figures S15–S17), fluorescence spectra of 1,4-diphenylanthracene derivatives in PMMA film (Figures S18 and S19), fluorescence lifetime decay profiles of pristine, ground, ground-heated and ground-fumed samples (Figure S20), fluorescence lifetime decay profiles of 1,4-diphenylanthracene derivatives in solution (Figure S21), fluorescence lifetime decay profiles of 1,4-diphenylanthracene derivatives in PMMA film (Figure S22), photophysical properties of 1,4-diphenylanthracene derivatives (Tables S1 and S2), single crystal X-ray analysis of 1,4-diphenylanthracene derivatives (Tables S3 and S4, Figures S23–S37), Powder X-ray diffraction (PXRD) patterns (Figure S38), DFT calculation of the obtained crystal structures (Figures S39–S42), UV irradiation experiments for 1,4-diphenylanthracene derivatives (Figures S43 and S44), andNMR spectra of new compounds.
Author Contributions
F.K. experiment, formal analysis, data curating, computation, and writing. T.A. experiment, formal analysis, data curating, M.N. experiment, formal analysis, K.Y. crystallographic analysis, T.K. design, supervision, editing, project administration, and writing-review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and supplementary materials.
Acknowledgments
We thank K. Naka and H. Imoto (Kyoto Institute of Technology) for the single crystal X-ray analysis. This study was the result of using research equipment shared in the MEXT Project for promoting public utilization of advanced research infrastructure (program for supporting the introduction of the new sharing system) Grant Number JPMXS0421800120.
Conflicts of Interest
Author Kenji Yoza was employed by the company Bruker Japan K.K. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Aziz, H.; Popovic, Z.D.; Hu, N.-X.; Hor, A.-M.; Xu, G. Degradation Mechanism of Small Molecule–Based Organic Light-Emitting Devices. Science 1999, 283, 1900. [Google Scholar] [CrossRef] [PubMed]
- Friend, R.H.; Gymer, R.W.; Holmes, A.B.; Burroughes, J.H.; Marks, R.N.; Taliani, C.; Bradley, D.D.C.; Santos, D.A.D.; Brédas, J.L.; Lögdlung, M.; et al. Electroluminescence in Conjugated Polymers. Nature 1999, 397, 121. [Google Scholar] [CrossRef]
- Yanagi, H.; Morikawa, T.; Hotta, S. Electroluminescence from Low-Dimensionally Confined Crystals of Thiophene/p-Phenylene Co-Oligomers. Appl. Phys. Lett. 2002, 81, 1512. [Google Scholar] [CrossRef]
- Lee, J.; Yuan, Y.-Y.; Kang, Y.; Jia, W.-L.; Lu, Z.-H.; Wang, S. 2,5-Functionalized Spiro-Bisiloles as Highly Efficient Yellow-Light Emitters in Electroluminescent Devices. Adv. Funct. Mater. 2006, 16, 681. [Google Scholar] [CrossRef]
- Yuan, W.Z.; Lu, P.; Chen, S.; Lam, J.W.Y.; Wang, Z.; Liu, Y.; Kwok, H.S.; Ma, Y.; Tang, B.Z. Changing the Behavior of Chromophores from Aggregation-Caused Quenching to Aggregation-Induced Emission: Development of Highly Efficient Light Emitters in the Solid State. Adv. Mater. 2010, 22, 2159. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.L.; Bao, Z. Halogenated Materials as Organic Semiconductors. Chem. Mater. 2011, 23, 446. [Google Scholar] [CrossRef]
- Yee, K.W.; Yokoyama, M.; Hiramoto, M. Very-Thin-Perylene-Crystal-Based Electroluminescent Devices. Appl. Phys. Lett. 2006, 88, 083511. [Google Scholar] [CrossRef]
- Figueira-Duarte, T.M.; Müllen, K. Pyrene-Based Materials for Organic Electronics. Chem. Rev. 2011, 111, 7260. [Google Scholar] [CrossRef]
- Gao, F.; Liano, Q.; Xu, Z.-Z.; Yue, Y.-H.; Wang, Q.; Zhang, H.-L.; Fu, H.-B. Strong Two-Photon Excited Fluorescence and Stimulated Emission from an Organic Single Crystal of an Oligo(Phenylene Vinylene). Angew. Chem. Int. Ed. 2010, 49, 732. [Google Scholar] [CrossRef]
- Zhao, Y.S.; Peng, A.; Fu, H.; Ma, Y.; Yao, J. Nanowire Waveguides and Ultraviolet Lasers Based on Small Organic Molecules. Adv. Mater. 2008, 20, 1661. [Google Scholar] [CrossRef]
- Bulovic, V.; Kozlov, V.G.; Khalfin, V.B.; Forrest, S.R. Transform-Limited, Narrow-Linewidth Lasing Action in Organic Semiconductor Microcavities. Science 1998, 279, 553. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Huang, Y.; Agarwal, R.; Lieber, C.M. Single-Nanowire Electrically Driven Lasers. Nature 2003, 421, 241. [Google Scholar] [CrossRef] [PubMed]
- Schmidtke, J.; Stille, W.; Finkelmann, H.; Kim, S.T. Laser Emission in a Dye Doped Cholesteric Polymer Network. Adv. Mater. 2002, 14, 746–749. [Google Scholar] [CrossRef]
- Samuel, I.D.W.; Turnbull, G.A. Organic Semiconductor Lasers. Chem. Rev. 2007, 107, 1272. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Fu, H.; Yao, J. Waveguide Modulator by Energy Remote Relay from Binary Organic Crystalline Microtubes. Adv. Mater. 2009, 21, 4153. [Google Scholar] [CrossRef]
- Tong, L.; Gattass, R.R.; Ashcom, J.B.; He, S.; Lou, J.; Shen, M.; Maxwell, I.; Mazur, E. Subwavelength-Diameter Silica Wires for Low-Loss Optical Wave Guiding. Nature 2003, 426, 816. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, Y.; Lei, T.; Hu, N.; Chen, E.-Q.; Pei, J. Structural-Property Relationship in Pyrazino[2,3-g]quinoxaline Derivatives: Morphology, Photophysical, and Waveguide Properties. Chem. Mater. 2010, 22, 3735. [Google Scholar] [CrossRef]
- Takazawa, K.; Kitahama, Y.; Kimura, Y.; Kido, G. Optical Waveguide Self-Assembled from Organic Dye Molecules in Solution. Nano. Lett. 2005, 5, 1293. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, Y.S.; Yao, J. Optical Waveguides at Micro/Nanoscale Based on Functional Small Organic Molecules. Phys. Chem. Chem. Phys. 2011, 13, 9060. [Google Scholar] [CrossRef]
- Goyal, H.; Kumar, P.; Gupta, R. Polycyclic Aromatic Hydrocarbon-based Soft Materials: Applications in Fluorescent Detection, Gelation, AIEE and Mechanochromism. Chem. Asian J. 2023, e202300355. [Google Scholar] [CrossRef]
- Ito, S. Mechanochromic Luminescence of Soft Crystals: Recent Systematic Studies in Controlling the Molecular Packing and Mechanoresponsive Properties. J. Photochem. Photobiol. C 2022, 51, 100481. [Google Scholar] [CrossRef]
- Sun, Y.; Lei, Z.; Ma, H. Twisted Aggregation-Induced Emission Luminogens (AIEgens) Contribute to Mechanochromism Materials: A Review. J. Mater. Chem. C 2022, 10, 14834. [Google Scholar] [CrossRef]
- Mutai, T.; Takamizawa, S. Organic Soft Crystals Exhibiting Spontaneously Reversible Mechano-Responsive Luminescence. J. Photochem. Photobiol. C 2022, 51, 100479. [Google Scholar] [CrossRef]
- Sagara, Y.; Kato, T. Mechanically Induced Luminescence Changes in Molecular Assemblies. Nature Chem. 2009, 1, 605. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Chi, Z.; Mao, Z.; Zhang, Y.; Liu, S.; Zhao, J.; Aldred, M.P.; Chi, Z. Recent Advances in Mechano-Responsive Luminescence of Tetraphenylethylene Derivatives with Aggregation-Induced Emission Properties. Mater. Chem. Front. 2018, 2, 861. [Google Scholar] [CrossRef]
- Luo, X.; Li, J.; Li, C.; Heng, L.; Dong, Y.Q.; Liu, Z.; Bo, Z.; Tang, B.Z. Reversible Switching of the Emission of Diphenyldibenzofulvenes by Thermal and Mechanical Stimuli. Adv. Mater. 2011, 23, 3261. [Google Scholar] [CrossRef] [PubMed]
- Sagara, Y.; Yamane, S.; Mitani, M.; Weder, C.; Kato, T. Mechanoresponsive Luminescent Molecular Assemblies: An Emerging Class of Materials. Adv. Mater. 2016, 28, 1073. [Google Scholar] [CrossRef]
- Huang, X.; Qian, L.; Zhou, Y.; Liu, M.; Cheng, Y.; Wu, H. Effective Structural Modification of Traditional Fluorophores to Obtain Organic Mechanofluorochromic Molecules. J. Mater. Chem. C 2018, 6, 5075. [Google Scholar] [CrossRef]
- Xue, S.; Qiu, X.; Sun, Q.; Yang, W. Alkyl Length Effects on Solid-State Fluorescence and Mechanochromic Behavior of Small Organic Luminophores. J. Mater. Chem. C 2016, 4, 1568. [Google Scholar] [CrossRef]
- Ito, H.; Saito, T.; Oshima, N.; Kitamura, N.; Ishizaka, S.; Hinatsu, Y.; Wakeshima, M.; Kato, M.; Tsuge, K.; Sawamura, M. Reversible Mechanochromic Luminescence of [(C6F5Au)2(μ-1,4-Diisocyanobenzene)]. J. Am. Chem. Soc. 2008, 130, 10044. [Google Scholar] [CrossRef]
- Seki, T.; Kobayashi, K.; Ito, H. Low-Temperature-Selective Luminescent Mechanochromism of a Thienyl Gold Isocyanide Complex. Chem. Commun. 2017, 53, 6700. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Xu, Z. Reversible Mechanochromic Studies on AIE-Inspired Smart Materials and Their Applications in HCHO Sensing. Dalton Trans. 2022, 51, 6332. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; He, L.-H.; Ju, P.; Chen, J.-L.; Ye, H.-Y.; Wang, J.-Y.; Liu, S.-J.; Wen, H.-R. Reversible Mechanochromic Luminescence of Tetranuclear Cuprous Complexes. Inorg. Chem. 2020, 59, 17213. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wen, T.; Fu, W.-Q.; Liu, M.; Chen, S.; Zhang, J. Structure-Dependent Mechanochromism of Two Ag(I) Imidazolate Chains. CrystEngComm 2016, 18, 218. [Google Scholar] [CrossRef]
- Geng, H.; Luo, K.; Zou, G.; Wang, H.; Ni, H.; Yu, W.; Li, Q.; Wang, Y. New Phosphorescent Platinum(II) Complexes: Lamellar Mesophase and Mechanochromism. New J. Chem. 2016, 40, 10371. [Google Scholar] [CrossRef]
- Seki, T.; Takamatsu, Y.; Ito, H. A Screening Approach for the Discovery of Mechanochromic Gold(I) Isocyanide Complexes with Crystal-To-Crystal Phase Transitions. J. Am. Chem. Soc. 2016, 138, 6252. [Google Scholar] [CrossRef]
- Ai, Y.; Li, Y.; Chan, M.H.-Y.; Xiao, G.; Zou, B.; Yam, V.W.-W. Realization of Distinct Mechano- and Piezochromic Behaviors Via Alkoxy Chain Length-Modulated Phosphorescent Properties and Multidimensional Self-Assembly Structures of Dinuclear Platinum(II) Complexes. J. Am. Chem. Soc. 2021, 143, 10659. [Google Scholar] [CrossRef]
- Seki, T.; Ida, K.; Ito, H. A Meta-Diisocyanide Benzene-Based Aryl Gold Isocyanide Complex Exhibiting Multiple Solid-State Molecular Arrangements and Luminescent Mechanochromism. Mater. Chem. Front. 2018, 2, 1195. [Google Scholar] [CrossRef]
- Zhao, K.-Y.; Shan, G.-G.; Fu, Q.; Su, Z.-M. Tuning Emission of AIE-Active Organometallic Ir(III) Complexes by Simple Modulation of Strength of Donor/Acceptor on Ancillary Ligands. Organometallics 2016, 35, 3996. [Google Scholar] [CrossRef]
- Yagai, S.; Seki, T.; Aonuma, H.; Kawaguchi, K.; Karatsu, T.; Okura, T.; Sakon, A.; Uekusa, H.; Ito, H. Mechanochromic Luminescence Based on Crystal-To-Crystal Transformation Mediated by a Transient Amorphous State. Chem. Mater. 2016, 28, 234. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, G.; Wang, R.; Pu, S. Highly Emissive Carbazole-Based Gold(I) Complex with a Long Room-Temperature Phosphorescence Lifetime and Self-Reversible Mechanochromism Characteristics. RSC Adv. 2017, 7, 15112. [Google Scholar] [CrossRef]
- Huitorel, B.; Benito, Q.; Fargues, A.; Garcia, A.; Gacoin, T.; Boilot, J.-P.; Perruchas, S.; Camerel, F. Mechanochromic Luminescence and Liquid Crystallinity of Molecular Copper Clusters. Chem. Mater. 2016, 28, 8190. [Google Scholar] [CrossRef]
- Jin, M.; Seki, T.; Ito, H. Luminescent Mechanochromism of a Chiral Complex: Distinct Crystal Structures and Color Changes of Racemic and Homochiral Gold(I) Isocyanide Complexes with a Binaphthyl Moiety. Chem. Commun. 2016, 52, 8083. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Tokodai, N.; Omagari, S.; Nakanishi, T.; Hasegawa, Y.; Iwasa, T.; Taketsugu, T.; Ito, H. Luminescent Mechanochromic 9-Anthryl Gold(I) Isocyanide Complex with an Emission Maximum at 900 nm After Mechanical Stimulation. J. Am. Chem. Soc. 2017, 139, 6514. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Xu, B.; Zhang, J.; Tan, X.; Wang, L.; Chen, J.; Lv, H.; Wen, S.; Li, B.; Ye, L.; et al. Piezochromic Luminescence Based on the Molecular Aggregation of 9,10-Bis((E)-2-(pyrid-2-yl)vinyl)anthracene. Angew. Chem. Int. Ed. 2012, 51, 10782. [Google Scholar] [CrossRef] [PubMed]
- Tu, D.; Leong, P.; Li, Z.; Hu, R.; Shi, C.; Zhang, K.Y.; Yan, H.; Zhao, Q. A Carborane-Triggered Metastable Charge Transfer State Leading to Spontaneous Recovery of Mechanochromic Luminescence. Chem. Commun. 2016, 52, 12494. [Google Scholar] [CrossRef]
- Li, H.; Zhang, X.; Chi, Z.; Xu, B.; Zhou, W.; Liu, S.; Zhang, Y.; Xu, J. New Thermally Stable Piezofluorochromic Aggregation-Induced Emission Compounds. Org. Lett. 2011, 13, 556. [Google Scholar] [CrossRef]
- Wu, X.; Guo, J.; Cao, Y.; Zhao, J.; Jia, W.; Chen, Y.; Jia, D. Mechanically Triggered Reversible Stepwise Tricolor Switching and Thermochromism of Anthracene-o-Carborane Dyad. Chem. Sci. 2018, 9, 5270. [Google Scholar] [CrossRef]
- Li, R.; Xiao, S.; Li, Y.; Lin, Q.; Zhang, R.; Zhao, J.; Yang, C.; Zou, K.; Li, D.; Yi, T. Polymorphism-Dependent and Piezochromic Luminescence Based on Molecular Packing of a Conjugated Molecule. Chem. Sci. 2014, 5, 3922. [Google Scholar] [CrossRef]
- Fedorenko, E.; Tretyakova, G.; Mirochnik, A.; Gerasimenko, A.; Beloliptsev, A. Anthracene-Containing Complexes of Boron Difluoride. Dual Luminescence, Formation of Excimers, and Mechanochromism. Spectrochim. Acta A 2021, 262, 120114. [Google Scholar] [CrossRef]
- Suresh, S.K.; Raju, P.D.; Krishnan, A.; Ramachandran, L.M.; Suneesh, C.V. Supramolecular Interactions-Assisted Polymorphism and Unique Mechanofluorochromism in 9,10-Bis((E)-4-(trifluoromethyl)styryl)anthracene. Chem. Eur. J. 2023, 29, e202204030. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Zhang, J.; Tan, R.; Wang, S.; Li, Y.; Li, Q.; Xiao, S. High-Contrast Reversible Mechanochromic Luminescence of a Conjugated Anthracene-Based Molecule. Mater. Lett. 2016, 164, 239. [Google Scholar] [CrossRef]
- Xiong, Y.; Yan, X.; Ma, Y.; Li, Y.; Yin, G.; Chen, L. Regulating the Piezofluorochromism of 9,10-Bis(butoxystyryl)anthracenes by Isomerization of Butyl Groups. Chem. Commun. 2015, 51, 3403. [Google Scholar] [CrossRef]
- Tanikubo, H.; Matsuo, T.; Hayashi, S. Alkyl-Chain Bridged Acrylonitrile-Appended Anthracene Chromophore Toward Mechanically-Induced Bathochromic Shift. Bull. Chem. Soc. Jpn. 2023, 96, 178. [Google Scholar] [CrossRef]
- Naeem, K.C.; Subhakumari, A.; Varughese, S.; Nair, V.C. Heteroatom Induced Contrasting Effects on the Stimuli Responsive Properties of Anthracene Based Donor–π–Acceptor Fluorophores. J. Mater. Chem. C 2015, 3, 10225. [Google Scholar] [CrossRef]
- Naito, H.; Morisaki, Y.; Chujo, Y. o-Carborane-Based Anthracene: A Variety of Emission Behaviors. Angew. Chem. Int. Ed. 2015, 54, 5084. [Google Scholar] [CrossRef]
- Zhang, Z.; Yao, D.; Zhou, T.; Zhang, H.; Wang, Y. Reversible Piezo- and Photochromic Behaviors Accompanied by Emission Color Switching of Two Anthracene-Containing Organic Molecules. Chem. Commun. 2011, 47, 7782. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Zhang, D.T.; Sun, M.X.; Li, Y.P.; Liu, T.L.; Xue, S.F.; Yang, W.J. Cruciform 9,10-Distyryl-2,6-Bis(p-dialkylamino-styryl)anthracene Homologues Exhibiting Alkyl Length-Tunable Piezochromic Luminescence and Heat-Recovery Temperature of Ground States. J. Mater. Chem. C 2014, 2, 1913. [Google Scholar] [CrossRef]
- Bu, L.; Sun, M.; Zhang, D.; Liu, W.; Wang, Y.; Zheng, M.; Xue, S.; Yang, W. Solid-State Fluorescence Properties and Reversible Piezochromic Luminescence of Aggregation-Induced Emission-Active 9,10-Bis[(9,9-dialkylfluorene-2-yl)vinyl]anthracenes. J. Mater. Chem. C 2013, 1, 2028. [Google Scholar] [CrossRef]
- Kohmoto, S.; Chuko, T.; Hisamatsu, S.; Okuda, Y.; Masu, H.; Takahashi, M.; Kishikawa, K. Piezoluminescence and Liquid Crystallinity of 4,4′-(9,10-Anthracenediyl)bispyridinium Salts. Cryst. Growth Des. 2015, 15, 2723. [Google Scholar] [CrossRef]
- Gogoi, G.; Kashyap, D.; Sarma, R.J. Mechano-Luminescent Behavior of a Pyridine-Containing Anthracene Derivative: Role of Aromatic Stacking Interactions. Cryst. Growth Des. 2018, 18, 4963. [Google Scholar] [CrossRef]
- Kusukawa, T.; Kojima, Y.; Kannen, F. Mechanofluorochromic Properties of 1,8-Diphenylanthracene Derivatives. Chem. Lett. 2019, 48, 1213. [Google Scholar] [CrossRef]
- Kusukawa, T.; Kannen, F.; Kojima, Y.; Yoza, K. Crystal Polymorphism-Dependent Fluorescence of Fluoroarene-Substituted Anthracene Derivatives. Chem. Lett. 2021, 50, 31. [Google Scholar] [CrossRef]
- Kusukawa, T.; Shibata, S.; Kannen, F.; Yoza, K. Mechnofluorochromic Properties of N-Alkyl Amide Anthracene Derivatives. Tetrahedron 2022, 111, 132735. [Google Scholar] [CrossRef]
- Kannen, F.; Nishimura, M.; Yoza, K.; Kusukawa, T. Mechanofluorochromic Properties of 1-Phenylanthracene Derivatives with Extremely Simple Structures. Tetrahedron 2023, 149, 133710. [Google Scholar] [CrossRef]
- Zhu, G.; Yu, T.; Chen, J.; Hu, R.; Yang, G.; Zeng, Y.; Li, Y. Dipyrene-Terminated Oligosilanes Enable Ratiometric Fluorescence Response in Polymers Toward Mechano- and Thermo-Stimuli. ACS Appl. Mater. Interfaces. 2023, 15, 11033. [Google Scholar] [CrossRef]
- Sagara, Y.; Kato, T. Stimuli-Responsive Luminescent Liquid Crystals: Change of Photoluminescent Colors Triggered by a Shear-Induced Phase Transition. Angew. Chem. Int. Ed. 2008, 47, 5175. [Google Scholar] [CrossRef]
- Ma, Z.; Teng, M.; Wang, Z.; Yang, S.; Jia, X. Mechanically Induced Multicolor Switching Based on a Single Organic Molecule. Angew. Chem. Int. Ed. 2013, 52, 12268. [Google Scholar] [CrossRef]
- Zhang, H.; Chang, X.; Ma, C.; Huang, G.; Li, B.S.; Tang, B.Z. Two Cholesterol-Containing Pyrene Derivatives: Subtle Spacer Difference, Diverse Stimuli-Responsive Luminescence, Chirality, and Self-Assembly Behaviors. ACS Appl. Mater. Interfaces 2022, 14, 43926. [Google Scholar] [CrossRef]
- Hirai, Y.; Laize-Générat, L.; Wrona-Piotrowicz, A.; Zakrzewski, J.; Makal, A.; Brosseau, A.; Michely, L.; Versace, D.-L.; Allain, C.; Métivier, R. Multi-Directional Mechanofluorochromism of Acetyl Pyrenes and Pyrenyl Ynones. ChemPhysChem 2021, 22, 1638. [Google Scholar] [CrossRef]
- Li, W.; Wang, L.; Zhang, J.-P.; Wang, H. Bis-Pyrene-Based Supramolecular Aggregates with Reversibly Mechanochromic and Vapochromic Responsiveness. J. Mater. Chem. C 2014, 2, 1887. [Google Scholar] [CrossRef]
- Teng, M.-J.; Jia, X.-R.; Yang, S.; Chen, X.-F.; Wei, Y. Reversible Tuning Luminescent Color and Emission Intensity: A Dipeptide-Based Light-Emitting Material. Adv. Mater. 2012, 24, 1255. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Xu, Y.; Zhuang, W.; Wang, Y. Preparation of Organic Mechanochromic Fluorophores with Simple Structures and Promising Mechanochromic Luminescence Properties. RSC Adv. 2016, 6, 84787. [Google Scholar] [CrossRef]
- Sagara, Y.; Mutai, T.; Yoshikawa, I.; Araki, K. Material Design for Piezochromic Luminescence: Hydrogen-Bond-Directed Assemblies of a Pyrene Derivative. J. Am. Chem. Soc. 2007, 129, 1520. [Google Scholar] [CrossRef]
- Sagara, Y.; Komatsu, T.; Ueno, T.; Hanaoka, K.; Kato, T.; Nagano, T. Covalent Attachment of Mechanoresponsive Luminescent Micelles to Glasses and Polymers in Aqueous Conditions. J. Am. Chem. Soc. 2014, 136, 4273. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.-D.; Dong, X.-X.; Cao, J.-Y.; Zhao, W.-X.; Bi, G.-H.; Wang, C.-Z.; Zhang, T.; Rahman, S.; Georghiou, P.E.; Lin, J.-B.; et al. Substituent Effects on the Intermolecular Interactions and Emission Behaviors in Pyrene-Based Mechanochromic Luminogens. J. Mater. Chem. C 2022, 10, 9310. [Google Scholar] [CrossRef]
- Tong, J.; Wang, Y.; Mei, J.; Wang, J.; Qin, A.; Sun, J.Z.; Tang, B.Z. A 1,3-Indandione-Functionalized Tetraphenylethene: Aggregation-Induced Emission, Solvatochromism, Mechanochromism, and Potential Application as a Multiresponsive Fluorescent Probe. Chem. Eur. J. 2014, 20, 4661. [Google Scholar] [CrossRef]
- Khan, F.; Ekbote, A.; Misra, R. Reversible Mechanochromism and Aggregation Induced Enhanced Emission in Phenothiazine Substituted Tetraphenylethylene. New J. Chem. 2019, 43, 16156. [Google Scholar] [CrossRef]
- Zhang, G.-F.; Wang, H.; Aldred, M.P.; Chen, T.; Chen, Z.-Q.; Meng, X.; Zhu, M.-Q. General Synthetic Approach Toward Geminal-Substituted Tetraarylethene Fluorophores with Tunable Emission Properties: X-Ray Crystallography, Aggregation-Induced Emission and Piezofluorochromism. Chem. Mater. 2014, 26, 4433. [Google Scholar] [CrossRef]
- Jia, J.; Zhao, H. Remarkable Isomeric Effects on the Mechanofluorochromism of Tetraphenylethylene-Based D–π–A Derivatives. New J. Chem. 2019, 43, 2231. [Google Scholar] [CrossRef]
- Wang, X.; Qian, M.; Jiang, J.; Gao, Q.; Zhang, C.; Qi, H. Mechano-Chromic and Mechano-Enhanced Electrogenerated Chemiluminescence of Tetra[4-(4-cyanophenyl)phenyl]ethene. Chem. Commun. 2022, 58, 12847. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Cai, Z.; Zou, H.; Li, J.; Zhang, S.; Ying, L.; Deng, W. Achieving Halogen Bonding Enhanced Ultra-Highly Efficient AIE and Reversible Mechanochromism Properties of TPE-Based Luminogens: Position of Bromine Substituents. J. Mater. Chem. C 2022, 10, 8390. [Google Scholar] [CrossRef]
- Wang, J.; Mei, J.; Hu, R.; Sun, J.Z.; Qin, A.; Tang, B.Z. Click Synthesis, Aggregation-Induced Emission, E/Z Isomerization, Self-Organization, and Multiple Chromisms of Pure Stereoisomers of a Tetraphenylethene-Cored Luminogen. J. Am. Chem. Soc. 2012, 134, 9956. [Google Scholar] [CrossRef] [PubMed]
- Suenaga, K.; Tanaka, K.; Chujo, Y. Design and Luminescence Chromism of Fused Boron Complexes Having Constant Emission Efficiencies in Solution and in the Amorphous and Crystalline States. Eur. J. Org. Chem. 2017, 5191. [Google Scholar] [CrossRef]
- Taguchi, J.; Matsuura, S.; Seki, T.; Ito, H. Synthesis and Tunable Optical Properties of C,N-Chelated Borate Luminophores Derived from Potassium Acyltrifluoroborates. Chem. Eur. J. 2020, 26, 2450. [Google Scholar] [CrossRef] [PubMed]
- Butler, T.; Morris, W.A.; Samonina-Kosicka, J.; Fraser, C.L. Mechanochromic Luminescence and Aggregation Induced Emission of Dinaphthoylmethane β-Diketones and Their Boronated Counterparts. ACS Appl. Mater. Interfaces 2016, 8, 1242. [Google Scholar] [CrossRef]
- Qi, Y.; Wang, Y.; Ge, G.; Liu, Z.; Yu, Y.; Xue, M. Multi-State Emission Properties and the Inherent Mechanism of D–A–D Type Asymmetric Organic Boron Complexes. J. Mater. Chem. C 2017, 5, 11030. [Google Scholar] [CrossRef]
- Potopnyk, M.A.; Kravets, M.; Luboradzki, R.; Volyniuk, D.; Sashuk, V.; Grazulevicius, J.V. Carbazole-Modified Thiazolo[3,2-c][1,3,5,2]oxadiazaborinines Exhibiting Aggregation-Induced Emission and Mechanofluorochromism. Org. Biomol. Chem. 2021, 19, 406. [Google Scholar] [CrossRef]
- Morris, W.A.; Liu, T.; Fraser, C.L. Mechanochromic Luminescence of Halide-Substituted Difluoroboron β-Diketonate Dyes. J. Mater. Chem. C 2015, 3, 352. [Google Scholar] [CrossRef]
- Liao, C.-W.; Rao, M.R.; Sun, S.-S. Structural Diversity of New Solid-State Luminophores Based on Quinoxaline-β-Ketoiminate Boron Difluoride Complexes with Remarkable Fluorescence Switching Properties. Chem. Commun. 2015, 51, 2656. [Google Scholar] [CrossRef]
- Guo, C.; Li, M.; Yuan, W.; Wang, K.; Zou, B.; Chen, Y. Tuning the Mechanochromic Luminescence of BOPIM Complexes by Rational Introduction of Aromatic Substituents. J. Phys. Chem. C 2017, 121, 27009. [Google Scholar] [CrossRef]
- Zhang, G.; Lu, J.; Sabat, M.; Fraser, C.L. Polymorphism and Reversible Mechanochromic Luminescence for Solid-State Difluoroboron Avobenzone. J. Am. Chem. Soc. 2010, 132, 2160. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Liu, W.; Wang, Y.; Ma, L.; Yu, Y.; Zhang, Y.; Ren, L. The Inherent Mechanism of Mechanochromism Under Different Stress: Electron Cloud Density Distribution, J-Type Stacking, Pore Structure and Collapse of J-Type Stacking. New J. Chem. 2018, 42, 11373. [Google Scholar] [CrossRef]
- Nguyen, N.D.; Zhang, G.; Lu, J.; Sherman, A.E.; Fraser, C.L. Alkyl Chain Length Effects on Solid-State Difluoroboron β-Diketonate Mechanochromic Luminescence. J. Mater. Chem. 2011, 21, 8409. [Google Scholar] [CrossRef]
- Yoshii, R.; Suenaga, K.; Tanaka, K.; Chujo, Y. Mechanofluorochromic Materials Based on Aggregation-Induced Emission-Active Boron Ketoiminates: Regulation of the Direction of the Emission Color Changes. Chem. Eur. J. 2015, 21, 7231. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Cheng, Y.; Lan, J.; Wu, D.; Qian, S.; Yan, L.; He, Z.; Li, X.; Wang, K.; Zou, B.; et al. Molecular Engineering of Mechanochromic Materials by Programmed C−H Arylation: Making a Counterpoint in the Chromism Trend. J. Am. Chem. Soc. 2016, 138, 12803. [Google Scholar] [CrossRef]
- Adak, A.; Panda, T.; Raveendran, A.; Bejoymohandas, K.S.; Asha, K.S.; Prakasham, A.P.; Mukhopadhyay, B.; Panda, M.K. Distinct Mechanoresponsive Luminescence, Thermochromism, Vapochromism, and Chlorine Gas Sensing by a Solid-State Organic Emitter. ACS Omega 2018, 3, 5291. [Google Scholar] [CrossRef]
- Li, B.; Seth, K.; Niu, B.; Pan, L.; Yang, H.; Ge, H. Transient-Ligand-Enabled ortho-Arylation of Five-Membered Heterocycles: Facile Access to Mechanochromic Materials. Angew. Chem. Int. Ed. 2018, 57, 3401. [Google Scholar] [CrossRef]
- Sagara, Y.; Kubo, K.; Nakamura, T.; Tamaoki, N.; Weder, C. Temperature-Dependent Mechanochromic Behavior of Mechanoresponsive Luminescent Compounds. Chem. Mater. 2017, 29, 1273. [Google Scholar] [CrossRef]
- Panda, M.K.; Ravi, N.; Asha, P.; Prakasham, A.P. High Contrast Mechanochromic and Thermochromic Luminescence Switching by a Deep Red Emitting Organic Crystal. CrystEngComm 2018, 20, 6046. [Google Scholar] [CrossRef]
- Yoshida, R.; Tachikawa, T.; Ito, S. Mechano- and Thermo-Responsive Luminescence of Crystalline Thienylbenzothiadiazole Derivatives: Stepwise Hypsochromic Switching of Near-Infrared Emission. Cryst. Growth Des. 2022, 22, 547. [Google Scholar] [CrossRef]
- Chen, S.; Liu, W.; Zhang, W.; Ge, Z.; Wang, K.-P.; Gan, L.-H.; Hu, Z.-Q. Dimethylamine Substituted Bisbenzocoumarin Amides with Solvatochromic and Mechanochromic Properties. Tetrahedron 2019, 75, 3504. [Google Scholar] [CrossRef]
- Nagura, K.; Saito, S.; Yusa, H.; Yamawaki, H.; Fujihisa, H.; Sato, H.; Shimoikeda, Y.; Yamaguchi, S. Distinct Responses to Mechanical Grinding and Hydrostatic Pressure in Luminescent Chromism of Tetrathiazolylthiophene. J. Am. Chem. Soc. 2013, 135, 10322. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Kobayashi, K.; Mashimo, T.; Ito, H. A Gold Isocyanide Complex with a Pendant Carboxy Group: Orthogonal Molecular Arrangements and Hypsochromically Shifted Luminescent Mechanochromism. Chem. Commun. 2018, 54, 11136. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-Z.; Wang, J.-X.; Niu, L.-Y.; Chen, Y.-Z.; Yang, Q.-Z. Carbazole-Containing Difluoroboron β-Diketonate Dyes: Two-Photon Excited Fluorescence in Solution and Grinding-Induced Blue-Shifted Emission in the Solid State. J. Mater. Chem. C 2017, 5, 12538. [Google Scholar] [CrossRef]
- Ying, S.; Chen, M.; Liu, Z.; Zheng, M.; Zhang, H.; Xue, S.; Yang, W. Unusual Mechanohypsochromic Luminescence and Unique Bidirectional Thermofluorochromism of Long-Alkylated Simple DPP Dyes. J. Mater. Chem. C 2017, 5, 5994. [Google Scholar] [CrossRef]
- Khan, F.; Ekbote, A.; Singh, G.; Misra, R. Mechanochromic Luminogens with Hypsochromically Shifted Emission Switching Property: Recent Advances and Perspectives. J. Mater. Chem. C 2022, 10, 5024. [Google Scholar] [CrossRef]
- Khan, F.; Urbonas, E.; Volyniuk, D.; Grazulevicius, J.V.; Mobin, S.M.; Misra, R. White Hyperelectrofluorescence from Solution-Processable OLEDs Based on Phenothiazine Substituted Tetraphenylethylene Derivatives. J. Mater. Chem. C 2020, 8, 13375. [Google Scholar] [CrossRef]
- Nagai, S.; Yamashita, M.; Tachikawa, T.; Ubukata, T.; Asami, M.; Ito, S. Efficient and Versatile Mechanochromic Luminescence of Phenanthroimidazolylbenzothiadiazoles: Tricolor Switching and Directional Control over the Chromism. J. Mater. Chem. C 2019, 7, 4988. [Google Scholar] [CrossRef]
- Wang, B.; Wu, Z.; Fang, B.; Yin, M. Blue-Shifted Mechanochromism of a Dimethoxynaphthalene-Based Crystal with Aggregation-Induced Emission. Dyes Pigm. 2020, 182, 108618. [Google Scholar] [CrossRef]
- Kumar, G.J.; Bogoslavsky, B.; Debnath, S.; Bedi, A. Effect of Chalcogenophenes on Chiroptical Activity of Twisted Tetracenes: Computational Analysis, Synthesis and Crystal Structure Thereof. Molecules 2023, 28, 5074. [Google Scholar] [CrossRef]
- Feofanov, M.; Akhmetov, V.; Sharapa, D.I.; Amsharov, K. Modular Approach to the Synthesis of Two-Dimensional Angular Fused Acenes. Org. Lett. 2020, 22, 1698. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, S.E.; McNeil, A.J.; Müller, P.; Swager, T.M.; Houk, K.N. Probing Substituent Effects in Aryl-Aryl Interactions Using Stereoselective Diels-Alder Cycloadditions. J. Am. Chem. Soc. 2010, 132, 3304. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkov, N.P.; Gonzalez-Rodriguez, E.; Hughes, A.; Gomes, G.D.P.; White, F.D.; Kuriakose, F.; Alabugin, I.V. Radical Alkyne Peri-Annulation Reactions for the Synthesis of Functionalized Phenalenes, Benzanthrenes, and Olympicene. Angew. Chem. Int. Ed. 2018, 57, 3651. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2019. [Google Scholar]
- Olex2, Crystallographic Software Package, version 1.3.0; OlexSys Ltd.: Durham, UK, 2024.
- Sheldrick, G.M. SHELXT: Integrating Space Group Determination and Structure Solution. Acta Crystallogr. Sect. A 2014, 70, C1437. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT - Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- APEX3, Bruker AXS Inc.: Madison, WI, USA, 2019.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).