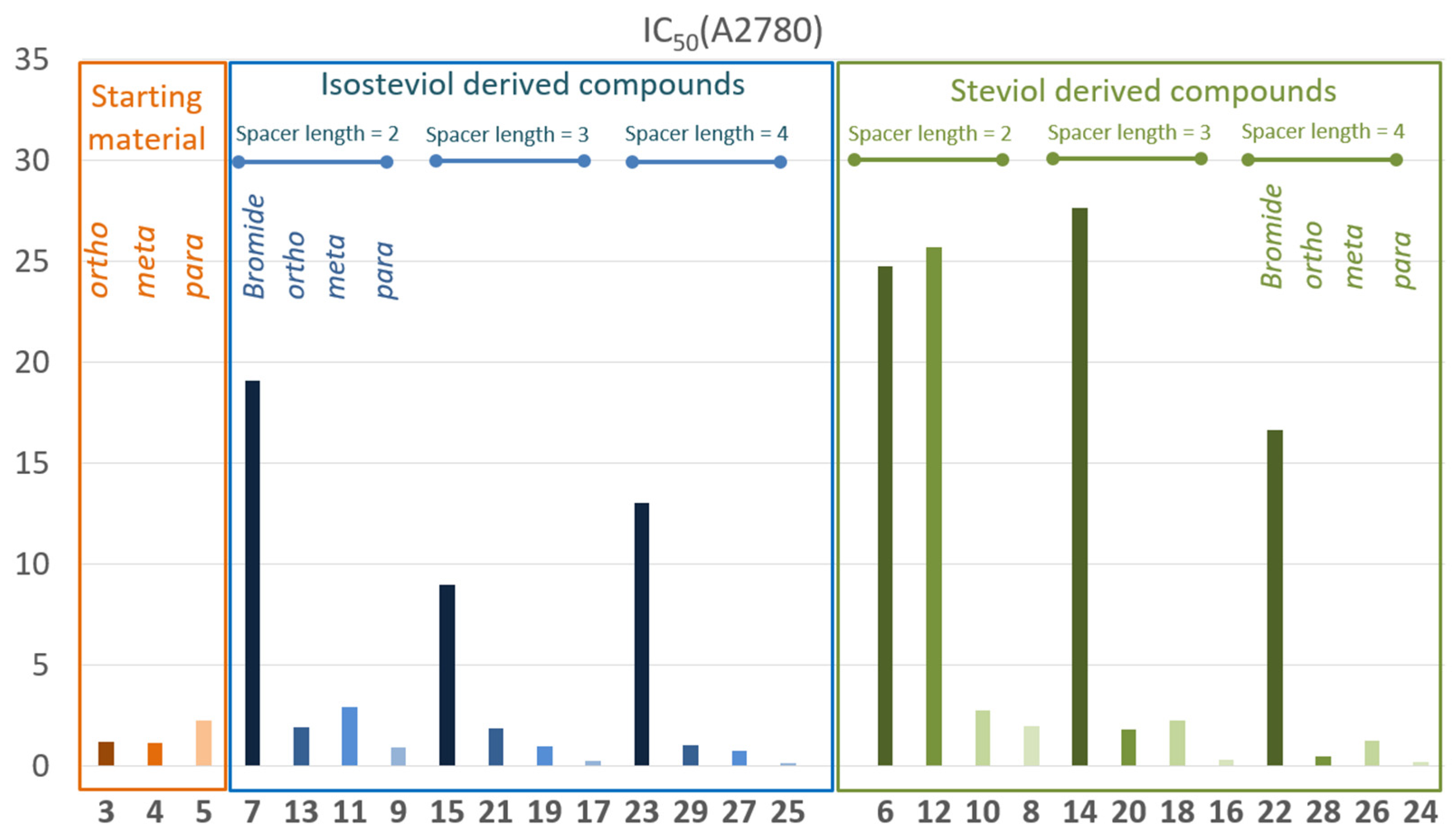
4.2. Syntheses
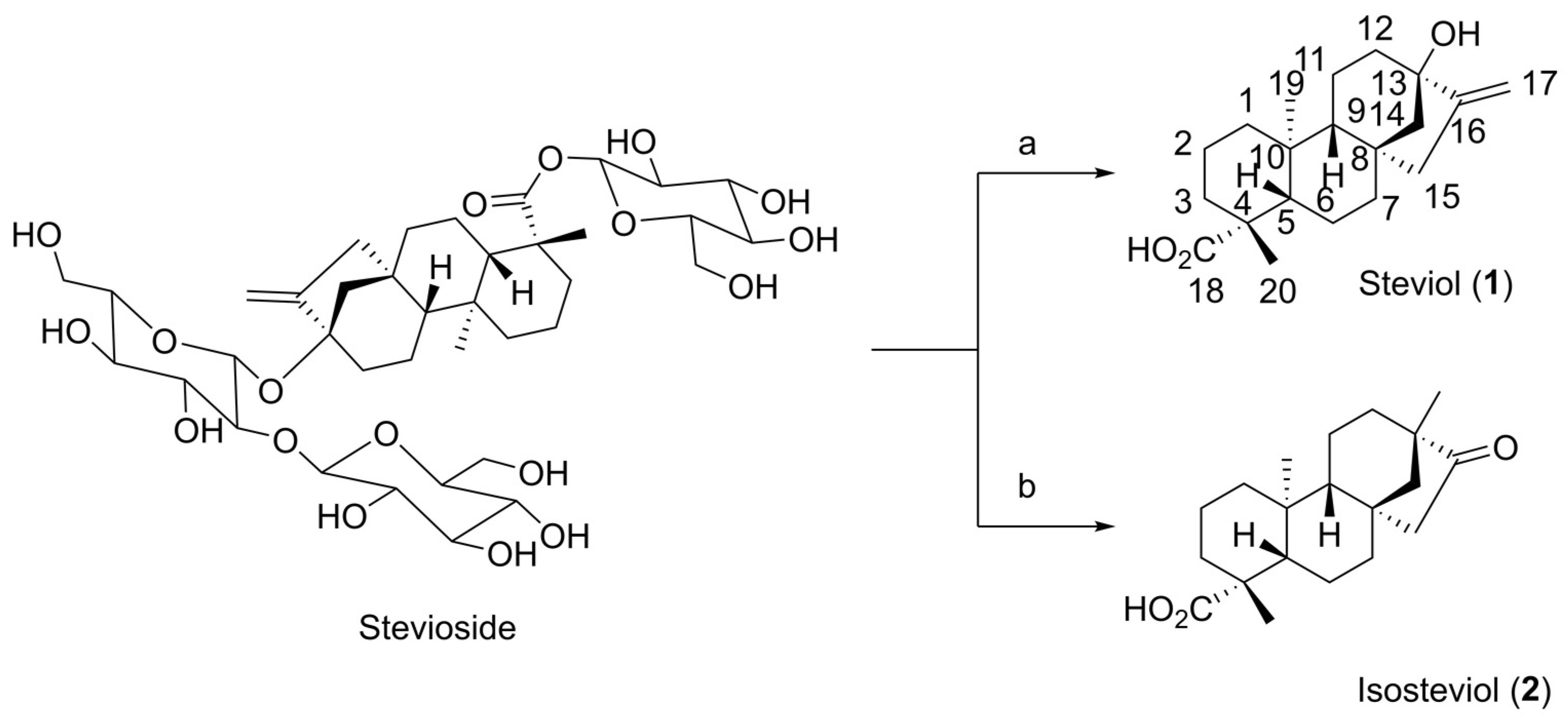
4.2.1. (4α) 13-Hydroxy-kaur-16-en-18-oic Acid (1)
To a solution of stevioside (100.0 g, 0.12 mol) in dist. water (8.0 L), NaIO
4 (160.0 g, 0.75 mol) was added and the mixture was stirred for 1 day at 21 °C. Finely ground KOH (650.0 g, 11.6 mol) was added, and the mixture was stirred under reflux for 3 h. The mixture was cooled to 0 °C and HOAc (650 mL) was slowly added; the mixture was extracted with ether (1.8 L). The combined organic layers were washed with water, dried (MgSO
4), and the solvents were removed under reduced pressure. The residue was subjected to re-crystallization from MeOH to yield
1 (10.5 g, 26%) as a colorless solid:
Rf = 0.47 (SiO
2, CHCl
3/MeOH, 9:1); m.p. = 204–206 °C [lit.: [
39] 212–213 °C];
= −62.88° (
c = 0.09, CHCl
3) [lit.: [
40]
= −55.2° (CHCl
3)]; IR (ATR): ν = 2945
br, 1694
w, 1456
w, 1184
w, 757
w cm
−1;
1H NMR (500 MHz, CDCl
3): δ = 4.98 (
s, 1H, 17-H), 4.81 (
s, 1H, 17-H), 2.20 (
dt,
J = 17.0, 2.9 Hz, 1H, 15-H), 2.14 (
td,
J = 13.4, 3.3 Hz, 1H, 3-H), 2.12–2.04 (
m, 2H, 14-H, 15-H), 1.94 (
td,
J = 13.8, 4.1 Hz, 1H, 2-H), 1.92–1.70 (
m, 5H, 1-H, 6-H, 11-H, 12-H), 1.65–1.51 (
m, 3H, 7-H, 11-H, 12-H), 1.48–1.39 (
m, 2H, 2-H, 7-H), 1.30 (
dd,
J = 10.5, 2.7 Hz, 1H, 14-H), 1.23 (
s, 3H, 19-H), 1.08 (
dd,
J = 12.1, 2.3 Hz, 1H, 5-H), 1.04–0.99 (
m, 1H, 3-H), 0.99–0.96 (
m, 1H, 9-H), 0.95 (
s, 3H, 20-H), 0.84–0.79 (
m, 1H, 1-H) ppm;
13C NMR (126 MHz, CDCl
3): δ = 183.77 (C-18), 155.80 (C-16), 103.20 (C-17), 80.51 (C-13), 57.03 (C-5), 53.96 (C-9), 47.54 (C-15), 47.05 (C-14), 43.74 (C-4), 41.87 (C-8), 41.36 (C-7), 40.64 (C-1), 39.65 (C-10), 39.50 (C-12), 37.84 (C-3), 28.92 (C-19), 21.93 (C-6), 20.59 (C-11), 19.14 (C-2), 15.55 (C-20) ppm; MS (ESI, MeOH):
m/z (%) 317 (100%, [M − H]
−).
4.2.2. (4α, 8β, 13β) 13-Methyl-16-oxo-17-norkauran-18-oic Acid (2)
A solution of stevioside (86.0 g, 0.10 mol) in MeOH (500 mL) and aq. HCl (33%, 90 mL) was heated under reflux for 2 h. Stirring at 21 °C was continued overnight, water (1200 mL) was added slowly, and the precipitate was filtered off, dried and re-crystallized from EtOH (300 mL) to yield
2 (21.6 g, 63%) as a colorless solid:
Rf = 0.71 (SiO
2, CHCl
3/MeOH 9:1); m.p. = 229–231 °C [lit.: [
41] 228–230 °C];
= −84.02° (
c = 0.15, CHCl
3) [lit.: [
39]
= −79.3° (EtOH)]; IR (ATR),
1H NMR and
13C NMR as previously reported; MS (ESI, MeOH/CHCl
3, 4:1):
m/
z (%) 317 (100%, [M − H]
−).
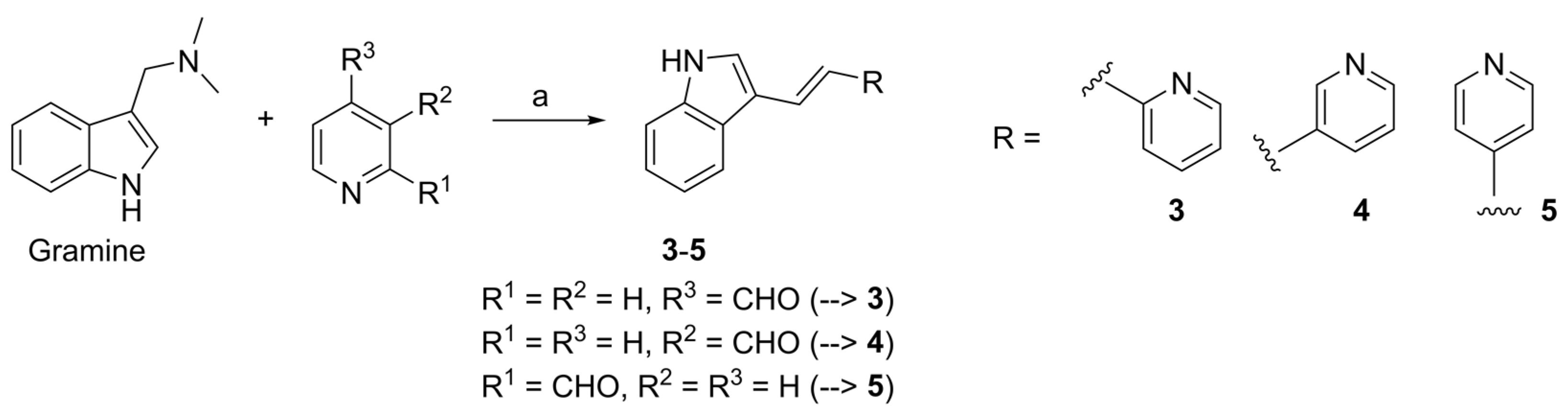
4.2.3. General Procedure for the Synthesis of Pyridyl-ethenylindoles 3–5 (GPA)
A mixture of 2-, 3- or 4-pyridinecarboxaldehyde (1.5 equiv), tri-
n-butyl-phosphine (1.5 equiv) and gramine (3-dimethylaminomethyl-indole, 3 equiv) in dry acetonitrile (75 mL) was stirred at 85 °C for 1 day; [
35] the volatiles were removed under reduced pressure, and the residue was purified by chromatography.
4.2.4. 3-[(E)-2-Pyridin-2-yl-ethenyl]-1H-indole (3)
Following GPA from gramine (5.25 g, 30 mmol), acetonitrile (75 mL),
n-PBu
3 (12 mL, 46 mmol) and 24-pyridinecarbaldehyde (4.2 mL, 45 mmol) followed by chromatography (SiO
2; hexanes/ethyl acetate, 1:1),
5 (3.05 g, 46%) was obtained as a yellowish solid:
Rf = 0.52 (SiO
2, hexanes/ethyl acetate, 1:1); m.p. = 185–187 °C (lit: [
41] 190–191 °C); IR (ATR): ν = 3128
w, 3086
w, 3041
w, 2972
w, 2919
w, 2879
w, 1632
m, 1565
m, 1523
w, 1500
m, 1451
m, 1417
m, 1346
br, 1283
m, 1252
m, 1222
w, 1185
w, 1151
w, 1138
w, 1117
m, 1084
w, 1042
w, 1025
w, 977
w, 952
s, 913
w, 881
w, 853
w, 817
w, 771
br, 739
s, 701
s, 627
m, 618
m, 564
w, 547
w, 426
m cm
−1; UV-Vis (MeOH): λ
max (log ε) = 303.67 nm (2.38);
1H NMR (500 MHz, DMSO-d
6): δ = 11.42 (
s, 1H, NH), 8.50 (
ddd,
J = 4.8, 1.8, 0.7 Hz, 1H, 15-H), 7.99 (
d,
J = 7.8 Hz, 1H, 3-H), 7.88 (
d,
J = 16.1 Hz, 1H, 9-H), 7.73 (
d,
J = 2.7 Hz, 1H, 8-H), 7.69 (
td,
J = 7.6, 1.8 Hz, 1H, 13-H), 7.47 (
d,
J = 7.9 Hz, 1H, 12-H), 7.45–7.41 (
m, 1H, 2-H), 7.18 (
dd,
J = 7.0, 1.2 Hz,1H, 5-H), 7.15(
d,
J = 16.3 Hz, 1H, 10-H), 7.20–7.09 (m, 1H, 4-H), 7.12–7.10 (m, 1H, 14-H) ppm;
13C NMR (126 MHz, DMSO-d
6): δ = 156.42 (C-15), 149.20 (C-11), 137.19 (C-1), 136.51 (C-13), 127.85 (C-9), 127.45 (C-6), 126.38 (C-8), 125.12 (C-3), 124.15 (C-10), 122.63 (C-12), 121.93 (C-4), 121.22 (C-14), 119.99 (C-5), 112.03 (C-7), 111.68 (C-2) ppm; MS (ESI, DMSO/CHCl
3, 4:1):
m/
z (%) 221 (100%, [M + H]
+); analysis calcd. for C
15H
12N
2 (220.28): C 81.79, H 5.49, N 12.72; found: C 81.63, H 5.70, N 12.45.
4.2.5. 3-[(E)-2-Pyridin-3-yl-ethenyl]-1H-indole (4)
Following GPA from gramine (5.25 g, 30 mmol), acetonitrile (75 mL),
n-PBu
3 (12 mL, 46 mmol) and 3-pyridinecarbaldehyde (4.2 mL, 45 mmol), followed by chromatography (SiO
2; hexanes/ethyl acetate, 1:1),
4 (3.32 g, 50%) [
42,
43,
44,
45] was obtained as a yellowish solid:
Rf = 0.25 (SiO
2, hexanes/ethyl acetate, 1:1); m.p. = 194–195 °C (lit.: [
35] 194–195 °C); IR (ATR): ν = 3129
w, 3089
w, 3039
w, 2970
w, 2921
w, 2881
w, 1630
m, 1568
m, 1524
w, 1499
m, 1452
m, 1416
m, 1348
br, 1281
m, 1250
m, 1224
w, 1186
w, 1153
w, 1136
w, 1118
m, 1083
w, 1042
w, 1026
w, 976
w, 953
s, 911
w, 882
w, 851
w, 816
w, 772
br, 740
s, 699
s, 629
m, 617
m, 563
w, 548
w, 424
s cm
−1; UV-Vis (MeOH): λ
max (log ε) = 335.01 nm (2.51);
1H NMR (500 MHz, DMSO-d
6): δ = 11.40 (
s, 1H, NH), 8.77 (
d,
J = 2.2 Hz, 1H, 15-H), 8.37 (
dd,
J = 4.6, 1.6 Hz, 1H, 14-H), 8.05 (
d,
J = 7.8 Hz, 1H, 2-H), 7.99 (
dt,
J = 7.9, 1.8 Hz, 1H, 12-H), 7.68 (
dt,
J = 2.6 Hz, 1H, 8-H), 7.56 (
d,
J = 16.6 Hz, 1H, 9-H), 7.45 (
d,
J = 7.8 Hz, 1H, 3-H), 7.34 (
dd,
J = 7.9, 4.7 Hz, 1H, 13-H), 7.21–7.16 (m1H, 5-H), 7.14 (
td,
J = 7.4, 6.8, 1.2 Hz, 1H, 4-H), 7.12 (
d,
J = 16.6 Hz, 1H, 10-H) ppm;
13C NMR (126 MHz, DMSO-d
6): δ = 147.95 (C-15), 147.47 (C-14), 137.54 (C-6), 134.76 (C-11), 131.91 (C-12), 127.17 (C-8), 125.57 (C-1), 125.14 (C-9), 124.10 (C-13), 122.37 (C-5), 120.38 (C-2), 120.31 (C-4), 119.93 (C-10), 114.02 (C-7), 112.45 (C-3) ppm; MS (ESI, MeOH/DMSO, 4:1):
m/
z (%) 219 (77%, [M − H]
−); analysis calcd. for C
15H
12N
2 (220.28): C 81.79, H 5.49, N 12.72; found: C 81.58, H 5.79, N 12.40.
4.2.6. 3-[(E)-2-Pyridin-4-yl-ethenyl]-1H-indole (5)
Following GPA from gramine (5.25 g, 30 mmol), acetonitrile (75 mL),
n-PBu
3 (12 mL, 46 mmol) and 4-pyridinecarbaldehyde (4.2 mL, 45 mmol), followed by chromatography (SiO
2; hexanes/ethyl acetate, 1:1),
3 (4.31 g, 65%) was obtained as a reddish solid [
37,
46,
47,
48,
49,
50,
51]:
Rf = 0.26 (SiO
2, hexanes/ethyl acetate, 1:1); m.p. = 254–255 °C (lit.: [
37] 255–256 °C); C; IR (ATR): ν = 3127
w, 3086
w, 3064
w, 3026
w, 2958
w, 2915
w, 2869
w, 1625
m, 1592
s, 1547
m, 1519
m, 1493
m, 1444
s, 1420
s, 1352
w, 1332
w, 1303
w, 1279
m, 1247
s, 1215
m, 1200
m, 1153
w, 1135
w, 1121
m, 1093
m, 1065
w, 1018
w, 999
m, 962
s, 902
w, 869
m, 830
m, 802
m, 772
w, 732
s, 666
w, 618
m, 559
w, 546
w, 526
m, 496
w, 422
m cm
−1; UV-Vis (MeOH): λ
max (log ε) = 352.44 nm (1.26);
1H NMR (400 MHz, DMSO-d
6): δ = 11.63–11.45 (
s, 1H, NH), 8.47 (
d,
J = 6.1 Hz, 2H, 13-H, 14-H), 8.03 (
dd,
J = 7.5, 1.5 Hz, 1H, 5-H), 7.73 (
d,
J = 13.8 Hz, 1H, 9-H,), 7.75 (
s, 1H, 8-H), 7.52 (
d, J = 6.2 Hz, 2H, 12-H, 15-H), 7.43 (
d,
J = 7.9 Hz, 1H, 2-H), 7.20–7.11 (
m, 2H, 3-H, 4-H), 7.04 (
d,
J = 16.5 Hz, 1H, 10-H) ppm;
13C NMR (101 MHz, DMSO-d
6): δ = 150.18 (C-13, C-14), 146.38 (C-11), 137.61 (C-1), 128.44 (C-8), 127.83 (C-9), 125.50 (C-6), 122.54 (C-4), 120.67 (C-10), 120.55 (C-3), 120.43 (C-5), 120.37 (C-12, C-15), 113.65 (C-7), 112.54 (C-2) ppm; MS (ESI, MeOH/DMSO, 4:1):
m/
z (%) 219 (83%, [M − H]
−); analysis calcd. for C
15H
12N
2 (220.28): C 81.79, H 5.49, N 12.72; found: C 81.50, H 5.71, N 12.46.
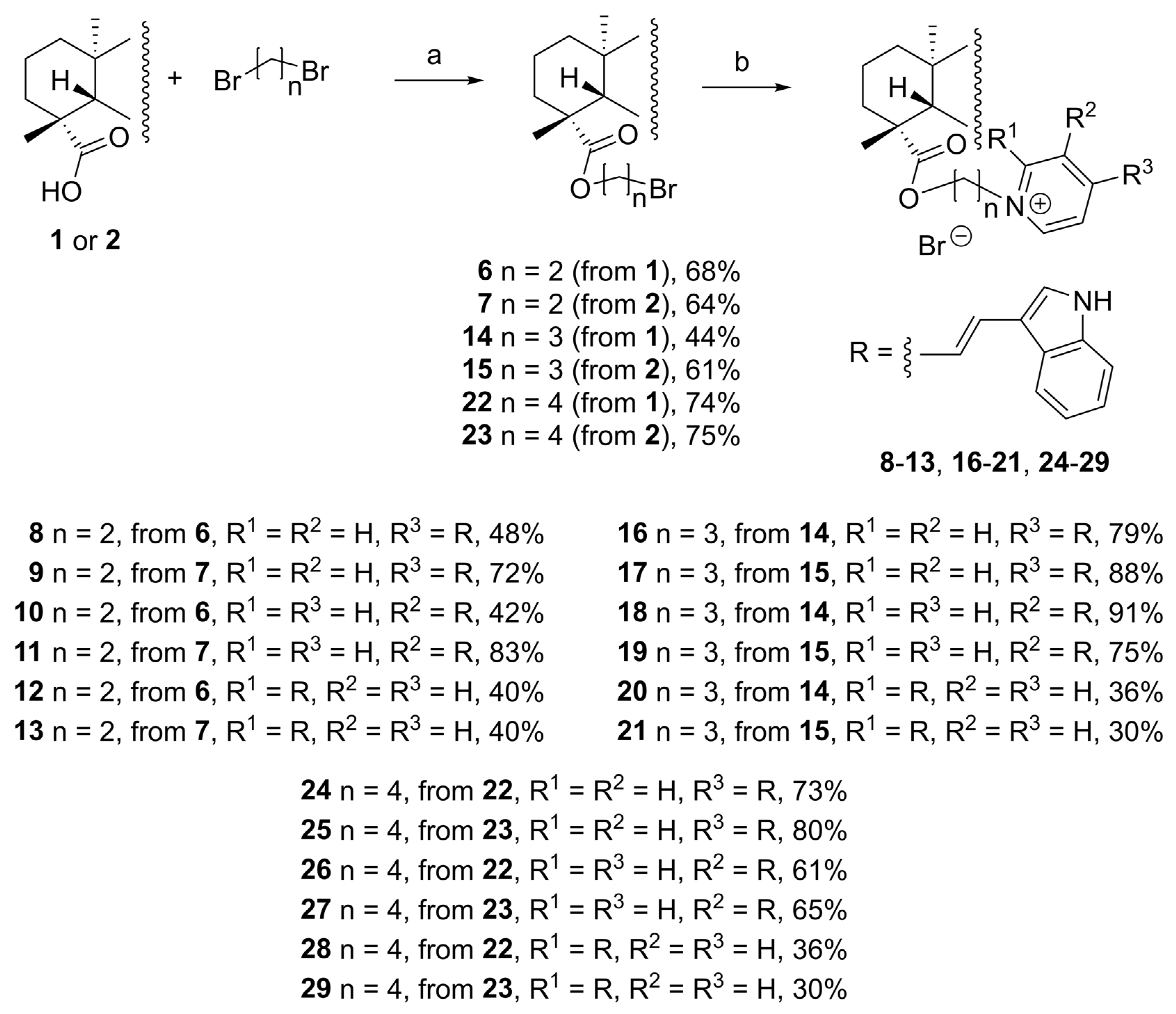
4.2.7. General Procedure for the Synthesis of Alkyl Bromides 6, 7, 14, 15, 22 and 23 (GPB)
To a solution of
1 or
2 in dry DMF/ACN (3:1), finely ground K
2CO
3 (2 equiv.) and 1,2-dibromoethane, 1,3-dibromopropane or 1,4-dibromobutane (4 equiv.) were added, and the mixture was stirred for 3 h at 50 °C [
8]. The volatiles were removed under reduced pressure, and the residue was subjected to chromatography to yield compounds
6 and
7 (from dibromoethane),
14 and
15 (from dibromopropane) and
22 and
23 (from dibromobutane), respectively.
4.2.8. General Procedure for the Synthesis of the F16 Conjugates 8–13, 16–21 and 24–29 (GPC)
A mixture of bromide 6, 7, 14, 15, 22 or 23 (1.0 mmol) and pyridyl-ethenylindoles 3–5 (1 equiv.) in dry DMF (10 mL) was stirred in a microwave for 14 h at 120 °C (microwave assisted; Anton Parr Monowave apparatus; Anton Paar GmbH, Graz, Austria). The volatiles were removed under reduced pressure and the residue was subjected to chromatography to yield products 8–13, 16–21 and 24–29.
4.2.9. 2-Bromoethyl (4α)-13-Hydroxykaur-16-en-18-oate (6)
Following GPB from 1 (0.5 g, 1.57 mmol), K2CO3 (0.434 g, 3.1 mmol), 1,2-dibromoethane (0.55 mL, 6.3 mmol) followed by chromatography (SiO2, hexanes/ethyl acetate, 6:1), 6 (0.45 g, 68%) was obtained as a colorless solid: Rf = 0.33 (SiO2, hexanes/ethyl acetate, 8:2); m.p. = 115–117 °C; = −57.06° (c = 0.145, CHCl3); IR (ATR): ν = 3554m, 2934m, 2853m, 1709s, 1460m, 1443m, 1388w, 1366w, 1316w, 1282w, 1208m, 1138s, 1113m, 1092m, 1057w, 1008w, 967m, 877w, 819w, 774w, 678w, 578w, 531w cm−1; 1H NMR (500 MHz, CDCl3): δ = 4.97 (s, 1H, 17-H), 4.81 (s, 1H, 17-H), 4.42–4.30 (m, 2H, 21-H), 3.53 (t, J = 5.8 Hz, 2H, 22-H), 2.17 (dd, J = 9.0, 5.6 Hz, 2H, 12-Ha, 15-Ha), 1.93–1.71 (m, 4H, 2-Ha, 6-H, 1-Ha), 1.71–1.57 (m, 5H, 3-H, 7-H, 11-Ha), 1.57–1.35 (m, 5H, 2-Hb, 11-H, 14-H), 1.20 (s, 3H, 19-H), 1.10–0.94 (m, 2H, 12-Hb, 5-H), 0.88 (s, 3H, 20-H), 0.87–0.76 (m, 2H, 1-H, 9-H) ppm; 13C NMR (126 MHz, CDCl3): δ = 177.27 (C-18), 143.61 (C-16), 103.04 (C-17), 82.73 (C-13), 63.99, 56.85 (C-5), 53.88 (C-9), 51.00 (C-15), 48.08 (C-14), 44.13 (C-4), 41.45 (C-8), 40.98 (C-1), 39.75 (C-7), 39.50 (C-10), 38.16 (C-12), 31.89 (C-3), 29.05 (C-22), 28.96 (C-19), 21.15 (C-6), 20.93 (C-11), 19.18 (C-2), 15.47 (C-20) ppm; MS (ESI, MeOH/CHCl3, 4:1): m/z (%) 345 (100%, [M − Br]+); analysis calcd. for C22H33O3Br (425.41): C 62.12, H 7.82; found: C 61.97, H 8.03.
4.2.10. 2-Bromoethyl (4α, 8β, 13β) 13-Methyl-16-oxo-17-norkauran-18-oate (7)
Following GPB from 2 (0.5 g, 1.57 mmol), K2CO3 (0.434 g, 3.1 mmol), 1,2-dibromoethane (0.55 mL, 6.3 mmol) followed by chromatography (SiO2, hexanes/ethyl acetate, 9:1), 7 (0.43 g, 64%) was obtained as a colorless solid: Rf = 0.7 (SiO2; hexanes/ethyl acetate, 4:1); m.p. = 141–144 °C; = −63.27° (c = 0.156, CHCl3); IR (ATR): ν = 2935m, 2883w, 2839m, 1723s, 1470m, 1451m, 1431w, 1386w, 1322w, 1291w, 1229m, 1209m, 1176s, 1146s, 1132s, 1093m, 1060w, 1030w, 1017w, 996w, 974m, 952w, 928w, 880w, 856w, 803w, 759m, 696w, 587w, 574w, 561w, 535w, 505w, 462w cm−1; 1H NMR (500 MHz, CDCl3): δ = 4.42–4.32 (m, 2H, 21-H), 3.53 (t, J = 5.7 Hz, 2H, 22-H), 2.64 (dd, J = 18.6, 3.8 Hz, 1H, 15-H), 2.21 (d, J = 13.5 Hz, 1H, 3-H), 1.91 (dd, J = 14.1, 2.6 Hz, 2H, 2-H, 15-H), 1.86–1.76 (m, 3H, 1-H, 6-H, 11-H), 1.75–1.52 (m, 6H, 6-H, 7-H, 11-H, 12-H, 14-H), 1.52–1.34 (m, 3H, 2-H, 12-H, 14-H), 1.23 (s, 3H, 19-H), 1.22–1.13 (m, 2H, 5-H, 9-H), 1.04 (td, J = 13.5, 4.2 Hz, 1H, 3-H), 0.98 (s, 3H, 17-H), 0.92 (td, J = 13.3, 4.2 Hz, 1H, 1-H), 0.75 (s, 3H, 20-H) ppm; 13C NMR (126 MHz, CDCl3): δ = 222.39 (C-16), 177.03 (C-18), 63.94 (C-21), 57.09 (C-5), 54.69 (C-9), 54.28 (C-14), 48.70 (C-13), 48.42 (C-15), 43.97 (C-4), 41.51 (C-7), 39.79 (C-1), 39.48 (C-8), 38.09 (C-10), 37.93 (C-3), 37.31 (C-12), 28.95 (C-22), 28.93 (C-19), 21.73 (C-6), 20.35 (C-11), 19.85 (C-17), 18.89 (C-2), 13.45 (C-20) ppm; MS (ESI, MeOH:CHCl3 4:1): m/z (%) 345 (100%, [M − Br]+); analysis calcd. for C22H33O3Br (425.41): C 62.12, H 7.82; found: C 61.87, H 7.97.
4.2.11. 2-{4-[(E)-2-(1H-Indol-3-yl)ethenyl]-pyridinium-1-yl}-ethyl (4α)-13-Hydroxykaur-16-en-18-oate Bromide (8)
Following GPC from 6 (0.23 g, 0.54 mmol) and 3 (0.12 g, 0.54 mmol) followed by chromatography (SiO2, CHCl3/MeOH, 9:1), 8 (0.15 g, 48%) was obtained as a reddish solid: Rf = 0.2 (SiO2, CHCl3/MeOH, 9:1); m.p. = 188–190 °C; = −27.39° (c = 0.076, MeOH); IR (ATR): ν = 2924w, 2850w, 1721w, 1594m, 1574m, 1500m, 1430m, 1363w, 1317br, 1275w, 1245m, 1203w, 1178m, 1131m, 1043w, 958w, 870w, 744m, 611w, 565br, 509w, 424w cm−1; UV-Vis (MeOH): λmax (log ε) = 436.98 nm (4.46); 1H NMR (500 MHz, DMSO-d6): δ = 11.99 (s, 1H, NH), 8.83 (d, J = 6.9 Hz, 2H, 23-H, 27-H), 8.32 (s, 1H, 37-H), 8.29 (d, J = 16.2 Hz, 1H, 29-H), 8.19–8.13 (m, 2H, 24-H, 26-H), 7.98 (d, J = 2.9 Hz, 1H, 35-H), 7.51 (d, J = 7.5 Hz, 1H, 32-H), 7.30 (d, J = 16.2 Hz, 1H, 28-H), 7.27–7.19 (m, 2H, 33-H, 34-H), 4.83–4.71 (m, 2H, 22-H), 4.75–4.64 (m, 2H, 17-H), 4.58 (m, 2H, 21-H), 2.54–2.46 (m, 1H, 15-H), 1.95 (s, 1H, 12-Ha), 1.84–1.65 (m, 4H, 3-H, 6-Ha, 11-Ha, 15-Ha), 1.65–1.43 (m, 4H, 1-Ha, 2-Ha, 11-Hb, 14-Ha), 1.43–1.17 (m, 3H, 2-Hb, 6-Hb, 7-Ha), 1.14 (d, J = 10.0 Hz, 2H, 12-Hb, 14-Hb), 1.03 (s, 3H, 19-H), 0.96 (q, J = 13.6, 12.6 Hz, 2H, 3-H, 7-Hb), 0.84 (d, J = 8.0 Hz, 2H, 5-H, 9-H), 0.72 (t, 1H, 1-Hb), 0.61 (s, 3H, 20-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 176.64 (C-18), 156.59 (C-16), 155.67 (C-25), 144.13 (C-23, C-27), 138.00 (C-36), 137.66 (C-29), 133.12 (C-35), 125.39 (C-31), 123.42 (C-31), 122.23 (C-33), 121.67 (C-24, C-26), 120.94 (C-34), 117.23 (C-28), 114.16 (C-30), 113.09 (C-32), 102.96 (C-17), 79.03 (C-13), 62.73 (C-21), 57.94 (C-22), 56.21 (C-5), 53.50 (C-9), 47.63 (C-15), 46.50 (C-14), 43.74 (C-4), 43.70 (C-8), 41.43 (C-7), 41.19 (C-1), 39.39 (C-10), 39.12 (C-12), 37.72 (C-3), 28.63 (C-19), 21.93 (C-6), 20.32 (C-11), 18.99 (C-2), 15.42 (C-20) ppm; MS (ESI, MeOH/DMSO, 4:1): m/z (%) 566 (66%, [M − Br]+); analysis calcd. for C37H45N2O3Br (645.68): C 68.83, H 7.03, N 4.34; found: C 68.59, H 7.26, N 4.11.
4.2.12. 2-{4-[(E)-2-(1H-Indol-3-yl)-ethenyl]pyridinium-1-yl}-ethyl (4α, 8β, 13β) 13-Methyl-16-oxo-17-norkauran-18-oate Bromide (9)
Following GPC from 7 (0.215 g, 0.51 mmol) and 3 (0.115 g, 0.51 mmol) followed by chromatography (SiO2, CHCl3/MeOH, 9:1), 9 (0.2 g, 72%) was obtained as a reddish solid: Rf = 0.32 (SiO2, CHCl3/MeOH, 9:1); m.p. = 194–196 °C; = −43.56° (c = 0.101, MeOH); IR (ATR): ν = 2925br, 2848w, 1726s, 1645w, 1594s, 1574s, 1500s, 1431s, 1356w, 1317w, 1275w, 1246m, 1204m, 1176s, 1130s, 1043w, 960w, 870w, 745s, 663w, 612w, 563w, 509w, 425w cm−1; UV-Vis (MeOH): λmax (log ε) = 438.15 nm (4.79); 1H NMR (500 MHz, DMSO-d6): δ = 11.98 (s, 1H, NH), 8.83 (d, J = 6.8 Hz, 2H, 23-H, 27-H), 8.31 (s, 1H, 37-H), 8.29 (d, J = 16.1 Hz, 1H, 29-H), 8.19 (d, J = 7.0 Hz, 2H, 24-H, 26-H), 8.12 (d, J = 7.6 Hz, 1H, 32-H), 7.51 (d, J = 7.6 Hz, 1H, 35-H), 7.29 (d, J = 16.1 Hz, 1H, 28-H), 7.27–7.19 (m, 2H, 33-H, 34-H), 4.77 (s, 2H, 22-H), 4.57–4.50 (m, 1H, 21-Ha), 4.44–4.37 (m, 1H, 21-Hb), 2.29–2.20 (m, 1H, 15-Ha), 1.95 (d, J = 12.8 Hz, 1H, 3-Ha), 1.75 (d, J = 18.5 Hz, 1H, 15-Hb), 1.65–1.46 (m, 5H, 1-Ha, 6-Ha, 2-Ha, 7-Ha, 11-Ha), 1.40–1.20 (m, 6H, 2-Hb, 7-Hb, 12-H, 14-H), 1.13–1.02 (m, 3H, 5-H, 6-Hb, 9-H), 1.06 (s, 3H, 19-H), 1.02–0.91 (m, 2H, 3-Hb, 11-Hb), 0.88–0.78 (m, 1H, 1-Hb), 0.75 (s, 3H, 17-H), 0.44 (s, 3H, 20-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 220.8 (C-16), 176.6 (C-18), 155.7 (C-25), 144.2 (C-23, C-27), 138.0 (C-36), 137.8 (C-29), 133.2 (C-37), 125.4 (C-31), 123.4 (C-24, C-26), 122.2 (C-28), 121.7 (C-34), 120.9 (C-33), 117.2 (C-32), 114.2 (C-30), 113.1 (C-35), 63.0, 57.9, 56.1 (C-5), 54.0 (C-9), 53.6 (C-14), 48.2 (C-13), 48.1 (C-15), 43.7 (C-4), 40.8 (C-7), 39.4 (C-1), 39.3 (C-8), 37.8 (C-10), 37.7 (C-3), 36.9 (C-12), 28.7 (C-19), 21.8 (C-6), 20.2 (C-11), 20.1 (C-17), 18.8 (C-2), 13.2 (C-20) ppm; MS (ESI, MeOH/DMSO, 4:1): m/z (%) 566 (85%, [M − Br]+); C37H45N2O3Br (645.68): C 68.83, H 7.03, N 4.34; found: C 68.71, H 7.19, N 4.05.
4.2.13. 2-{3-[(E)-2-(1H-Indol-3-yl)ethenyl]-pyridinium-1-yl}-ethyl (4α)-13-Hydroxykaur-16-en-18-oate Bromide (10)
Following GPC from 6 (0.23 g, 0.54 mmol) and 4 (0.12 g, 0.54 mmol) followed by chromatography (SiO2, CHCl3/MeOH, 9:1), 10 (0.13 g, 42%) was obtained as a yellowish solid: Rf = 0.12 (SiO2, CHCl3/MeOH, 9:1); m.p. = 184–187 °C; = −6.74° (c = 0.095, MeOH); IR (ATR): ν = 3370br, 2928m, 2851m, 1725m, 1662w, 1632m, 1615m, 1576m, 1526w, 1503w, 1459m, 1434m, 1387w, 1364w, 1330w, 1276w, 1229m, 1167w, 1148m, 1118m, 1083m, 1048w, 967w, 920w, 874w, 819w, 747s, 681m, 611w, 571w, 528w, 500w, 475w, 425w cm−1; UV-Vis (MeOH): λmax (log ε) = 360.00 nm (4.17); 1H NMR (400 MHz, DMSO-d6): δ = 11.6 (s, 1H, NH), 9.4 (s, 1H, 23-H), 8.9 (d, J = 5.9 Hz, 1H, 27-H), 8.7 (d, J = 8.3 Hz, 1H, 25-H), 8.3 (s, 1H), 8.1 (dd, J = 8.1, 6.0 Hz, 1H, 26-H), 8.0 (d, J = 7.6 Hz, 1H, 32-H), 7.9 (s, 1H, 37-H), 7.9 (d, J = 16.5 Hz, 1H, 29-H), 7.5 (d, J = 7.4 Hz, 1H, 35-H), 7.2 (d, J = 16.4 Hz, 1H, 28-H), 7.3–7.1 (m, 2H, 33-H, 34-H), 5.0–4.8 (m, 3H, 17-Ha, 22-H), 4.7–4.4 (m, 3H, 17-Hb, 21-H), 2.0–1.9 (m, 3H, 15-H, 3-Ha), 1.7–1.5 (m, 6H, 1-Hb, 2-H, 6-Hb, 11-Ha, 14-Hb), 1.5–1.4 (m, 1H, 6-Ha), 1.3–1.1 (m, 6H, 7-H, 11-Hb, 12-H, 14-Ha), 1.0 (s, 3H, 19-H), 1.0–0.9 (m, 2H, 3-Hb, 5-H), 0.9–0.8 (m, 1H, 9-H), 0.7 (s, 1H, 1-Ha), 0.5 (s, 3H, 20-H) ppm; 13C NMR (101 MHz, DMSO-d6): δ = 176.7 (C-18), 156.7 (C-16), 142.4 (C-23), 141.1 (C-27), 140.6 (C-25), 140.0 (C-36), 137.7 (C-24), 133.5 (C-37), 130.8 (C-29), 128.2 (C-26), 125.3 (C-31), 122.9 (C-34), 120.9 (C-33), 120.4 (C-32), 115.7 (C-28), 113.4 (C-30), 112.8 (C-35), 103.0 (C-17), 81.1 (C-13), 63.0 (C-21), 59.8 (C-22), 55.9 (C-5), 53.4 (C-9), 47.5 (C-15), 47.0 (C-14), 43.7 (C-4), 43.7 (C-8), 41.3 (C-7), 40.6 (C-1), 39.4 (C-10), 39.2 (C-12), 37.8 (C-3), 28.5 (C-19), 20.9 (C-6), 18.9 (C-11), 16.8 (C-2), 15.1 (C-20) ppm; MS (ESI, MeOH/DMSO, 4:1): m/z (%) 566 (70%, [M − Br]+); analysis calcd. for C37H45N2O3Br (645.67): C 68.83, H 7.02, N 4.34; found: C 68.61, H 7.22, N 4.17.
4.2.14. 2-{3-[(E)-2-(1H-Indol-3-yl)ethenyl]-pyridinium-1-yl}-ethyl (4α)-13-Hydroxykaur-16-en-18-oate bromide Bromide (11)
Following GPC from 7 (0.215 g, 0.51 mmol) and 4 (0.115 g, 0.51 mmol) followed by chromatography (SiO2, CHCl3/MeOH, 9:1), 11 (0.24 g, 83%) was obtained as a yellowish solid: Rf = 0.22 (SiO2, CHCl3/MeOH, 9:1); m.p. = 193–194 °C; = +0.25° (c = 0.081, MeOH); IR (ATR): ν = 2926w, 2848w, 1725m, 1663w, 1630m, 1615w, 1576w, 1525w, 1501w, 1456m, 1433m, 1340w, 1319w, 1276w, 1250m, 1229m, 1176w, 1146w, 1130w, 1111w, 1095w, 1029w, 964w, 928w, 882w, 825w, 744m, 681m, 616w, 590w, 569w, 528w, 505w, 473w, 425w cm−1; UV-Vis (MeOH): λmax (log ε) = 360.42 nm (4.22); 1H NMR (400 MHz, DMSO-d6): δ = 11.66 (s, 1H, NH), 9.43 (s, 1H, 23-H), 8.88 (d, J = 6.0 Hz, 1H, 27-H), 8.77 (d, J = 8.4 Hz, 1H, 25-H), 8.30 (s, 1H, 37-H), 8.12 (dd, J = 8.2, 6.0 Hz, 1H, 26-H), 8.04 (d, J = 7.7 Hz, 1H, 32-H), 7.89 (d, J = 16.5 Hz, 1H, 29-H), 7.47 (d, J = 7.5 Hz, 1H, 35-H), 7.20 (d, J = 16.5 Hz, 1H, 28-H), 7.27–7.10 (m, 2H, 33-H, 34-H), 5.01–4.79 (m, 2H, 22-H), 4.63 (dt, J = 12.3, 4.4 Hz, 1H, 21-Ha), 4.47 (dd, J = 11.9, 3.5 Hz, 1H, 21-Hb), 2.20 (dd, J = 18.4, 3.4 Hz, 1H, 15-Ha), 1.94 (d, J = 12.8 Hz, 1H, 3-Ha), 1.74 (d, J = 18.4 Hz, 1H, 15-Hb), 1.60–1.40 (m, 5H, 1-Ha, 2-Ha, 6-Ha, 7-Ha, 11-Ha), 1.38–1.16 (m, 6H, 2-Hb, 7-Hb, 12-H, 14-H), 1.10–1.00 (m, 2H, 5-H, 9-H), 1.04 (s, 3H, 19-H), 1.02–0.86 (m, 3H, 3-Hb, 6-Hb, 11-Hb), 0.81 (s, 3H, 17-H), 0.85–0.73 (m, 1H, 1-Hb), 0.41 (s, 3H, 20-H) ppm; 13C NMR (101 MHz, DMSO-d6): δ = 221.0 (C-16), 176.6 (C-18), 142.3 (C-23), 141.2 (C-27), 140.5 (C-25), 139.9 (C-36), 137.7 (C-24), 130.8 (C-29), 129.7 (C-37), 128.2 (C-26), 125.3 (C-31), 122.9 (C-28), 120.9 (C-34), 120.4 (C-32), 115.6 (C-33), 113.4 (C-30), 112.8 (C-35), 63.1 (C-21), 59.8 (C-22), 56.1 (C-5), 54.0 (C-9), 53.5 (C-14), 48.2 (C-13), 48.2 (C-15), 43.7 (C-4), 40.6 (C-7), 39.4 (C-1), 39.3 (C-8), 37.8 (C-10), 37.6 (C-3), 36.9 (C-12), 28.6 (C-19), 21.8 (C-6), 20.2 (C-11), 20.1 (C-17), 18.7 (C-2), 13.1 (C-20) ppm; MS (ESI, MeOH/DMSO, 4:1): m/z (%) 566 (60%, [M − Br]+); analysis calcd. for C37H45N2O3Br (645.67): C 68.83, H 7.02, N 4.34; found: C 68.64, H 7.19, N 4.21.
4.2.15. 2-{2-[(E)-2-(1H-Indol-3-yl)ethenyl]-pyridinium-1-yl}-ethyl (4α)-13-Hydroxykaur-16-en-18-oate Bromide (12)
Following GPC from 6 (0.23 g, 0.54 mmol) and 5 (0.12 g, 0.54 mmol) followed by chromatography (SiO2, CHCl3/MeOH, 8:2), 12 (0.12 g, 40%) was obtained as a yellowish solid: Rf = 0.11 (SiO2, CHCl3/MeOH, 8.5:1.5); m.p. = 90–93 °C; = −11.07° (c = 0.042, MeOH); IR (ATR): ν = 3386br, 2926w, 2854w, 1654s, 1495w, 1436m, 1420m, 1389m, 1351m, 1252w, 1166w, 1110m, 1061w, 823w, 666w, 481br cm−1; UV-Vis (MeOH): λmax (log ε) = 433.17 nm (3.48); 1H NMR (500 MHz, DMSO-d6): δ = 12.17 (s, 1H, NH), 8.87 (d, J = 6.2 Hz, 1H, 23-H), 8.62 (d, J = 8.7 Hz, 1H, 26-H), 8.40 (t, J = 8.1 Hz, 2H, 25-H), 8.35 (d, J = 15.8 Hz, 1H, 29-H), 8.28 (s, 1H, 37-H), 8.13 (d, J = 7.2 Hz, 1H, 32-H), 7.76 (t, J = 6.4 Hz, 1H, 24-H), 7.52 (d, J = 7.2 Hz, 1H, 35-H), 7.40 (d, J = 15.6 Hz, 1H, 28-H), 7.28–7.17 (m, 2H, 33-H, 34-H), 5.26–5.07 (m, 2H, 22-H), 4.94 (s, 1H, 17-Ha), 4.73 (s, 1H, 17-Hb), 4.69–4.57 (m, 1H, 21-Ha), 4.41–4.31 (m, 1H, 21-Hb), 2.03–1.80 (m, 2H, 3-Ha, 15-H), 1.74–1.36 (m, 6H, 1-Ha,2-Ha, 6-Ha, 11-Ha, 12-Ha, 14-Ha), 1.35–1.03 (m, 7H, 2-Hb, 6-Hb, 7-H, 11-Hb, 12-Hb, 14-Hb), 0.98 (s, 3H, 19-H), 0.96–0.73 (m, 3H, 3-Hb, 5-H, 9-H), 0.72–0.61 (m, 1H, 1-Ha), 0.51 (s, 3H, 20-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 176.9 (C-18), 154.5 (C-16), 145.6 (C-23), 145.1 (C-27), 144.1 (C-25), 139.3 (C-29), 137.8 (C-36), 132.9 (C-37), 125.8 (C-31), 124.6 (C-26), 123.5, 123.4 (C-24, 34), 121.8 (C-33), 121.4 (C-30), 120.6 (C-32), 114.0 (C-17), 113.1 (C-35), 110.3 (C-28), 81.2 (C-13), 62.2 (C-21), 56.0 (C-5), 55.5 (C-22), 53.5 (C-9), 47.7 (C-14, 15), 43.7 (C-4), 41.4 (C-8), 41.1 (C-7), 40.7 (C-1), 39.4 (C-10), 39.2 (C-12), 37.8 (C-3), 28.4 (C-19), 21.0 (C-6), 20.8 (C-11), 19.0 (C-2), 15.1 (C-20) ppm; MS (ESI, MeOH/DMSO, 4:1): m/z (%) 566 (55%, [M − Br]+); analysis calcd. For C37H45N2O3Br (645.67): C 68.83, H 7.02, N 4.34; found: C 68.63, H 7.27, N 4.19.
4.2.16. 2-{2-[(E)-2-(1H-Indol-3-yl)ethenyl]-pyridinium-1-yl}-ethyl (4α, 8β, 13β) 13-Methyl-16-oxo-17-norkauran-18-oate Bromide (13)
Following GPC from 7 (0.215 g, 0.51 mmol) and 5 (0.12 g, 0.54 mmol) followed by chromatography (SiO2, CHCl3/MeOH, 8:2), 13 (0.10 g, 40%) was obtained as a yellowish solid: Rf = 0.35 (SiO2, CHCl3/MeOH, 8.5:1.5); m.p. = 94–97 °C; = −12.83° (c = 0.073, MeOH); IR (ATR): ν = 3368br, 2923m, 2850m, 1725s, 1629w, 1604s, 1562m, 1496m, 1446br, 1433m, 1375w, 1318w, 1280w, 1244m, 1209w, 1166w, 1146w, 1131m, 1112w, 1095w, 1056m, 1040w, 966w, 928w, 818w, 745m, 665w, 570w, 508w, 423w cm−1; UV-Vis (MeOH): λmax (log ε) = 434.17 nm (4.02); 1H NMR (500 MHz, DMSO-d6): δ =12.14 (s, 1H, NH), 8.83 (d, J = 5.5 Hz, 1H, 23-H), 8.61 (d, J = 7.9 Hz, 1H, 26-H), 8.41 (t, J = 7.6 Hz, 1H, 25-H), 8.35 (d, J = 15.6 Hz, 1H, 29-H), 8.32 (s, 1H, 37-H), 8.12 (d, J = 7.2 Hz, 1H, 32-H), 7.76 (t, J = 6.8 Hz, 1H, 24-H), 7.52 (d, J = 7.0 Hz, 1H, 35-H), 7.38 (d, J = 15.7 Hz, 1H, 28-H), 7.30–7.20 (m, 2H, 33-H, 34-H), 5.25–4.95 (m, 2H, 22-H), 4.65–4.53 (m, 1H, 21-Ha), 4.43–4.30 (m, 1H, 21-Hb), 2.20 (dd, J = 18.4, 3.6 Hz, 1H, 15-Ha), 1.91 (d, J = 12.8 Hz, 1H, 3-Ha), 1.73 (d, J = 18.5 Hz, 1H, 15-Hb), 1.60–1.48 (m, 6H, 1-Hb, 2-H, 6-Hb, 7-Hb, 11-Hb), 1.46–1.36 (m, 4H, 7-Ha, 12-H, 14-Hb), 1.32 (d, J = 7.7 Hz, 2H, 11-Ha, 14-Ha), 1.17–1.04 (m, 2H, 5-H, 9-H), 1.03 (s, 2H, 3-Hb, 6-Ha), 0.99 (s, 3H, 19-H), 0.87 (s, 1H, 1-Ha), 0.85 (s, 3H, 17-H), 0.39 (s, 3H, 20-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 221.1 (C-16), 176.8 (C-18), 154.4 (C-27), 145.5 (C-23), 145.4 (C-37), 144.0 (C-25), 139.2 (C-29), 137.7 (C-36), 125.7 (C-31), 124.4 (C-26), 123.4 (C-34), 123.2 (C-24), 121.8 (C-33), 120.4 (C-32), 119.6, 113.9 (C-30), 113.1 (C-35), 110.1 (C-28), 62.2 (C-21), 56.4 (C-22), 56.1 (C-5), 54.1 (C-9), 53.6 (C-14), 48.3 (C-13), 48.2 (C-15), 43.6 (C-4), 40.7 (C-7), 39.4 (C-1), 39.3 (C-8), 37.8 (C-10), 37.7 (C-3), 37.0 (C-12), 28.4 (C-19), 21.7 (C-6), 20.2 (C-17), 18.9 (C-11), 18.8 (C-2), 13.0 (C-20) ppm; MS (ESI, MeOH/DMSO, 4:1): m/z (%) 566 (65%, [M − Br]+; analysis calcd. for C37H45N2O3Br (645.67): C 68.83, H 7.02, N 4.34; found: C 68.60, H 7.19, N 4.08.
4.2.17. 3-Bromopropyl (4α) -13-hydroxykaur-16-en-18-oate (14)
Following GPB from 1 (0.5 g, 1.57 mmol), K2CO3 (0.434 g, 3.1 mmol) and 1,3-dibromopropane (0.64 mL, 6.3 mmol) followed by chromatography (SiO2, hexanes/ethyl acetate, 6:1), 14 (0.3 g, 44%) was obtained as a colorless solid: Rf = 0.3 (SiO2, hexanes/ethyl acetate, 8:2); m.p. = 97–99 °C; = −56.12° (c = 0.104, CHCl3); IR (ATR): ν = 3493m, 2990w, 2974w, 2958w, 2939m, 2851m, 1703s, 1469w, 1458w, 1444w, 1392w, 1369w, 1330m, 1275w, 1237m, 1220w, 1201w, 1178w, 1155w, 1119m, 1097w, 1018w, 1001m, 967w, 952w, 938w, 891w, 875w, 849w, 813w, 770w, 694w, 620w, 571w, 518w, 420w cm−1; 1H NMR (400 MHz, CDCl3): δ = 4.99–4.95 (m, 1H, 17-Ha), 4.83–4.79 (m, 1H, 17-Hb), 4.26–4.18 (m, 1H, 21-Ha), 4.17–4.08 (m, 1H, 21-Hb), 3.48 (t, J = 6.5 Hz, 2H, 23-H), 2.23–2.00 (m, 6H, 3-Ha, 14-Ha, 15-H, 22-H), 1.89–1.79 (m, 3H, 1-Ha, 2-Ha 6-Ha), 1.79–1.71 (m, 3H, 6-Hb, 11-Ha, 12-Ha), 1.62–1.55 (m, 1H, 11-Hb), 1.55–1.48 (m, 2H, 7-Ha, 12-Hb), 1.48–1.35 (m, 2H, 2-Hb, 7-Hb), 1.29–1.23 (m, 1H, 14-Hb), 1.17 (d, J = 1.7 Hz, 3H, 19-H), 1.08–0.93 (m, 3H, 3-Hb, 5-H, 9-H), 0.85 (d, J = 2.3 Hz, 3H, 20-H), 0.80 (dd, J = 13.4, 4.8 Hz, 1H, 1-Hb) ppm; 13C NMR (101 MHz, CDCl3): δ = 177.3 (C-18), 156.0 (C-16), 102.9 (C-17), 82.6, 80.2 (C-13), 61.8, 56.9 (C-5), 53.7 (C-9), 50.9, 47.4 (C-15), 47.0 (C-14), 43.9 (C-4), 41.6 (C-8), 41.3 (C-7), 40.6 (C-1), 39.3 (C-10), 39.2 (C-12), 38.0 (C-3), 31.5 (C-22), 29.6 (C-23), 28.8 (C-19), 21.9 (C-6), 20.4 (C-11), 19.1 (C-2), 15.5 (C-20) ppm; MS (ESI, MeOH:CHCl3 4:1): m/z (%) 359 (90%, [M − Br]+); analysis calcd. for C23H35O3Br (439.43): C 62.87, H 8.03; found: C 62.77, H 8.20.
4.2.18. 3-Bromopropyl (4α, 8β, 13β) 13-Methyl-16-oxo-17-norkauran-18-oate (15)
Following GPB from 2 (0.5 g, 1.57 mmol), K2CO3 (0.434 g, 3.1 mmol) and 1,3-dibromopropane (0.64 mL, 6.3 mmol) followed by chromatography (SiO2, hexanes/ethyl acetate, 6:1), 15 (0.42 g, 61%) was obtained as a colorless solid: Rf = 0.69 (SiO2, hexanes/ethyl acetate,8:2); m.p. = 103–106 °C; = −58.31° (c = 0.15, CHCl3); IR (ATR): ν = 2932m, 2899w, 2884w, 2854w, 2839w, 1720s, 1470m, 1457m, 1423w, 1389w, 1369w, 1332m, 1321w, 1288m, 1257m, 1233m, 1219w, 1209w, 1179s, 1149s, 1135m, 1110m, 1097m, 1062w, 1018m, 978m, 942w, 929w, 895w, 876w, 852w, 827w, 767m, 738w, 696w, 660w, 632w, 612w, 587m, 532w, 507w, 462w, 436w, 418w cm−1; 1H NMR (400 MHz, CDCl3): δ = 4.25–4.17 (m, 1H, 21-Ha), 4.15–4.06 (m, 1H, 21-Hb), 3.46 (t, J = 6.5 Hz, 2H, 23-H), 2.62 (dd, J = 18.6, 3.8 Hz, 1H, 15-Ha), 2.16 (p, J = 6.2 Hz, 3H, 3-Ha, 22-H), 1.92–1.84 (m, 1H, 6-Ha), 1.85–1.78 (m, 1H, 2-Ha), 1.79 (d, J = 18.7 Hz, 1H, 15-Hb), 1.74–1.60 (m, 5H, 1-Ha, 6-Hb, 7-Ha, 11-H), 1.61–1.49 (m, 2H, 12-Ha, 14-Ha), 1.48–1.31 (m, 4H, 2-Hb, 7-Hb, 12-Hb, 14-Hb), 1.29–1.21 (m, 1H, 9-H), 1.19 (s, 3H, 19-H), 1.13 (dd, J = 12.0, 2.1 Hz, 1H, 5-H), 1.02 (td, J = 13.5, 4.2 Hz, 1H, 3-Hb), 0.96 (s, 3H, 17-H), 0.90 (td, J = 13.1, 4.2 Hz, 1H, 1-Hb), 0.70 (s, 3H, 20-H) ppm; 13C NMR (101 MHz, CDCl3): δ = 222.3 (C-16), 177.1 (C-18), 61.8 (C-21), 57.0 (C-5), 54.7, 54.3 (C-14), 48.7 (C-13), 48.4 (C-15), 43.9 (C-4), 41.5 (C-7), 39.8 (C-1), 39.4 (C-8), 38.0 (C-10), 37.9 (C-3), 37.3 (C-12), 31.4 (C-22), 29.6 (C-23), 28.9 (C-19), 21.7 (C-6), 20.3 (C-11), 19.8 (C-17), 18.9 (C-2), 13.4 (C-20) ppm; MS (ESI, MeOH/CHCl3, 4:1): m/z (%) 359 (80%, [M − Br]+); analysis calcd. for C23H35O3Br (439.43): C 62.87, H 8.03; found: C 62.66, H 8.24.
4.2.19. 3-{4-[(E)-2-(1H-Indol-3-yl)ethenyl]-pyridinium-1-yl}-propyl (4α)-13-Hydroxykaur-16-en-18-oate Bromide (16)
Following GPC from 14 (0.155 g, 0.35 mmol) and 3 (0.08 g, 0.36 mmol) followed by chromatography (SiO2, CHCl3/MeOH, 6:1), 16 (0.161 g, 79%) was obtained as a reddish solid: Rf = 0.84 (SiO2, CHCl3/MeOH, 9:1); m.p. = 209–211 °C; = −20.78° (c = 0.074, MeOH); IR (ATR): ν = 3316w, 3163w, 2935w, 1720m, 1607s, 1575m, 1503m, 1462m, 1433m, 1362w, 1330w, 1251m, 1204w, 1180m, 1165w, 1142m, 1104m, 1086w, 1049w, 1038w, 960m, 873m, 832w, 745s, 608w, 570w, 513w, 429w cm−1; UV-Vis (MeOH): λmax (log ε) = 445.33 nm (4.59); 1H NMR (500 MHz, DMSO-d6): δ = 11.98 (s, 1H, NH), 8.78 (d, J = 6.8 Hz, 2H, 25-H, 27-H), 8.28 (d, J = 16.3 Hz, 1H, 30-H), 8.19–8.12 (m, 3H, 24-H, 28-H, 33-H), 7.98 (d, J = 2.8 Hz, 1H, 38-H), 7.51 (d, J = 7.2 Hz, 1H, 36-H), 7.31 (d, J = 16.1 Hz, 1H, 29-H), 7.29–7.19 (m, 2H, 34-H, 35-H), 4.92–4.86 (m, 1H, 17-Ha), 4.70–4.65 (m, 1H, 17-Hb), 4.51 (t, J = 7.0 Hz, 2H, 23-H), 4.17–3.99 (m, 2H, 21-H), 2.32–2.23 (m, 2H, 22-H), 1.99 (dd, J = 26.6, 9.8 Hz, 3H, 3-Ha, 15-H), 1.86 (dd, J = 10.8, 1.1 Hz, 1H, 14-Hb), 1.80–1.55 (m, 6H, 1-Ha, 2-Ha, 6-H, 11-Ha, 12-Ha), 1.55–1.39 (m, 2H, 7-Ha, 11-Hb), 1.34 (s, 3H, 2-Hb, 7-Hb, 12-Hb), 1.20 (dd, J = 11.1, 1.7 Hz, 1H, 14-Ha), 1.08 (s, 3H, 19-H), 1.03 (dd, J = 11.9, 1.5 Hz, 1H, 5-H), 0.95 (td, J = 13.5, 4.1 Hz, 1H, 3-Hb), 0.90 (d, J = 7.9 Hz, 1H, 9-H), 0.80–0.76 (m, 1H, 1-Hb), 0.74 (s, 3H, 20-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 176.9 (C-18), 156.7 (C-16), 145.0 (C-26), 143.9 (C-25, C-27), 138.0 (C-37), 137.2 (C-30), 133.0 (C-38), 125.4 (C-32), 123.4 (C-34), 122.4 (C-24, C-28), 121.6 (C-35), 120.9 (C-33), 117.2 (C-29), 114.1 (C-31), 113.1 (C-36), 103.0 (C-17), 81.2 (C-13), 61.4 (C-21), 57.2 (C-23), 56.4 (C-5), 53.6 (C-9), 47.7 (C-15), 46.6 (C-14), 43.7 (C-4), 41.5 (C-8), 41.3 (C-7), 40.4 (C-1), 39.5 (C-10), 39.2 (C-12), 37.8 (C-3), 29.9 (C-22), 28.7 (C-19), 22.0 (C-6), 20.4 (C-11), 19.2 (C-2), 15.7 (C-20) ppm; MS (ESI, MeOH/DMSO, 4:1): m/z (%) 580 (80%, [M − Br]+); analysis calcd. for C38H47N2O3Br (659.70): C 69.18, H 7.18, N 4.25; found: C 68.88, H 7.30, N 4.03.
4.2.20. 3-{4-[(E)-2-(1H-Indol-3-yl)ethenyl]-pyridinium-1-yl}-propyl (4α, 8β, 13β) 13-Methyl-16-oxo-17-norkauran-18-oate Bromide (17)
Following GPC from 15 (0.212 g, 0.48 mmol), 3 (0.11 g, 0.05 mmol) followed by chromatography (SiO2, CHCl3/MeOH, 6:1), 17 (0.246 g, 88%) was obtained as a reddish solid: Rf = 0.85 (SiO2, CHCl3/MeOH, 9:1); m.p. = 184–186 °C; = −15.67° (c = 0.052, MeOH); IR (ATR): ν = 2924w, 2847w, 1722m, 1644w, 1598s, 1574m, 1500m, 1457w, 1432m, 1371w, 1355w, 1317w, 1275w, 1246m, 1205m, 1174s, 1131s, 1096w, 1043w, 965w, 871w,745s, 663w, 611w, 568w, 512w, 425w cm−1; UV-Vis (MeOH): λmax (log ε) = 444.49 nm (4.70); 1H NMR (400 MHz, DMSO-d6): δ = 11.94 (s, 1H, NH), 8.75 (d, J = 6.9 Hz, 2H, 24-H, 28-H), 8.25 (d, J = 16.2 Hz, 1H, 30-H), 8.14 (d, J = 6.9 Hz, 3H, 25-H, 27-H, 33-H), 7.96 (d, J = 2.7 Hz, 1H, 38-H), 7.50 (d, J = 7.0 Hz, 1H, 36-H), 7.29 (d, J = 16.1 Hz, 1H, 29-H), 7.26–7.18 (m, 2H, 34-H, 35-H), 4.48 (t, J = 7.0 Hz, 2H, 23-H), 4.17–3.96 (m, 2H, 21-H), 2.41 (d, J = 18.4 Hz, 1H, 15-Ha), 2.31–2.21 (m, 2H, 22-H), 1.96 (td, J = 12.5, 2.8 Hz, 1H, 3-Ha), 1.87 (d, J = 18.5 Hz, 1H, 6-Ha), 1.72 (td, J = 13.3, 2.4 Hz, 1H, 15-Hb), 1.68–1.54 (m, 5H, 1-Ha, 2-Ha, 6-Hb, 7-Ha, 11-Ha), 1.54–1.44 (m, 2H, 12-Ha, 14-Ha), 1.44–1.29 (m, 4H, 2-Hb, 7-Hb, 12-Hb, 14-Hb), 1.23–1.11 (m, 3H, 5-H, 9-H, 11-Hb), 1.09 (s, 3H, 19-H), 0.97 (td, J = 13.5, 4.2 Hz, 1H, 3-Hb), 0.92–0.87 (m, 1H, 1-Hb), 0.85 (s, 3H, 17-H), 0.60 (s, 3H, 20-H) ppm; 13C NMR (101 MHz, DMSO-d6): δ = 220.8 (C-16), 176.4 (C-18), 154.8 (C-26), 143.4 (C-24, C-28), 137.5 (C-37), 136.7 (C-30), 132.5 (C-38), 124.9 (C-32), 123.0 (C-29), 121.9 (C-25, C-27), 121.2 (C-35), 120.4 (C-34), 116.8 (C-33), 113.6 (C-31), 112.6 (C-36), 61.0 (C-21), 56.8 (C-23), 55.9 (C-5), 53.7 (C-9), 53.1 (C-14), 47.9 (C-15), 47.7 (C-13), 43.2 (C-4), 40.1 (C-7), 39.0 (C-1), 38.9 (C-8), 37.4 (C-3), 37.2 (C-10), 36.6 (C-12), 29.4 (C-22), 28.3 (C-19), 21.3 (C-6), 19.8 (C-11), 19.7 (C-17), 18.5 (C-2), 13.0 (C-20) ppm; MS (ESI, MeOH/DMSO, 4:1): m/z (%) 580 (80%, [M − Br]+); analysis calcd. for C38H47N2O3Br (659.70): C 69.18, H 7.18, N 4.25; found: C 68.89, H 7.33, N 4.02.
4.2.21. 3-{3-[(E)]-2-(1H-Indol-3-yl)-ethenyl]-pyridinium-1-yl}-propyl (4α)-13-Hydroxykaur-16-en-18-oate Bromide (18)
Following GPC from 14 (0.305 g, 0.7 mmol) and 4 (0.154 g, 0.7 mmol) followed by chromatography (SiO2, CHCl3/MeOH, 6:1), 18 (0.366 g, 91%) was obtained as a yellowish solid: Rf = 0.13 (SiO2, CHCl3/MeOH, 9:1); m.p. = 175–177 °C; = −43.33° (c = 0.111, MeOH); IR (ATR): ν = 2932br, 2850w, 1713m 1631m, 1577m, 1525w, 1501w, 1459w, 1432m, 1365w, 1331w, 1277w, 1235m, 1148m, 1096w, 1055w, 1020w, 958w, 880w, 818w, 745m, 679w, 568w, 527w, 501w, 426w cm−1; UV-Vis (MeOH): λmax (log ε) = 358.18 nm (4.66); 1H NMR (500 MHz, DMSO-d6): δ = 11.66 (s, 1H, NH), 9.35 (s, 1H, 28-H), 8.83 (d, J = 6.0 Hz, 1H, 24-H), 8.75 (d, J = 8.4 Hz, 1H, 26-H), 8.12–8.04 (m, 2H, 25-H, 33-H), 7.89 (d, J = 16.6 Hz, 1H, 30-H), 7.78 (d, J = 2.7 Hz, 1H, 38-H), 7.48 (d, J = 7.5 Hz, 1H, 36-H), 7.22 (d, J = 16.5 Hz, 1H, 29-H), 7.24–7.14 (m, 2H, 34-H, 35-H), 4.87 (s, 1H, 17-Ha), 4.69 (d, J = 7.2 Hz, 3H, 17-Hb, 23-H), 4.21–4.03 (m, 2H, 21-H), 2.42–2.33 (m, 2H, 22-H), 2.05–1.90 (m, 3H, 3-Ha, 15-H), 1.85 (d, J = 11.0 Hz, 1H, 14-Hb), 1.79–1.65 (m, 3H, 1-Ha, 2-Ha, 6-Ha), 1.65–1.49 (m, 3H, 6-Hb, 11-Ha, 12-Ha), 1.50–1.39 (m, 2H, 7-Ha, 11-Hb), 1.33 (s, 3H, 2-Hb, 7-Hb, 12-Hb), 1.19 (dd, J = 10.8, 1.6 Hz, 1H, 14-Ha), 1.06 (s, 3H, 19-H), 1.03 (dd, J = 12.0, 1.7 Hz, 1H, 5-H), 0.95 (td, J = 13.5, 4.2 Hz, 1H, 3-Hb), 0.89 (d, J = 8.1 Hz, 1H, 9-H), 0.79–0.76 (m, 1H, 1-Hb), 0.75 (s, 3H, 20-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ =176.9 (C-18), 156.7 (C-16), 142.0 (C-28), 141.1 (C-24), 140.1 (C-37), 140.0 (C-26), 137.8 (C-27), 130.7 (C-30), 129.7 (C-38), 128.2 (C-25), 125.3 (C-32), 122.9 (C-29), 120.9 (C-35), 120.5 (C-33), 115.8 (C-34), 113.4 (C-31), 112.8 (C-36), 103.0 (C-17), 79.1 (C-13), 61.5 (C-21), 59.1 (C-23), 56.4 (C-5), 53.6 (C-9), 47.7 (C-15), 46.6 (C-14), 43.6 (C-4), 41.4 (C-8), 41.3 (C-7), 40.5 (C-1), 39.5 (C-10), 39.2 (C-12), 37.8 (C-3), 30.0 (C-22), 28.7 (C-19), 22.0 (C-6), 20.4 (C-11), 19.2 (C-2), 15.7 (C-20) ppm; MS (ESI, MeOH/DMSO, 4:1): m/z (%) 580 (63%, [M − Br]+); analysis calcd. for C38H47N2O3Br (659.70): C 69.18, H 7.18, N 4.25; found: C 68.88, H 7.37, N 3.99.
4.2.22. 3-{3[(E)-2-(1H-Indol-3-yl)-ethenyl]-pyridinium-1-yl}-propyl (4α, 8β, 13β) 13-Methyl-16-oxo-17-norkauran-18-oate Bromide (19)
Following GPC from 15 (0.423 g, 0.96 mmol) and 4 (0.212 g, 0.96 mmol) followed by chromatography (SiO2, CHCl3/MeOH, 6:1), 19 (0.418 g, 75%) was obtained as a yellowish solid: Rf = 0.27 (SiO2, CHCl3/MeOH 9:1); m.p. = 171–173 °C; = −29.97° (c = 0.109, MeOH); IR (ATR): ν = 3164br, 3058w, 2937m, 2847w, 2454w, 1732m, 1718s, 1632m, 1584m, 1526w, 1500m, 1456m, 1427m, 1374w, 1336w, 1321w, 1273w, 1246m, 1225m, 1159s, 1130m, 1108m, 1056w, 1016w, 976w, 953m, 926w, 905w, 873w, 822w, 807w, 746s, 678m, 662m, 610w, 590w, 567m, 508w, 441w, 423m cm−1; UV-Vis (MeOH): λmax (log ε) = 358.18 nm (4.02); 1H NMR (500 MHz, DMSO-d6): δ = 11.65 (s, 1H, NH), 9.32 (s, 1H, 28-H), 8.81 (d, J = 6.0 Hz, 1H, 26-H), 8.75 (d, J = 8.3 Hz, 1H, 24-H), 8.12–8.04 (m, 2H, 25-H, 33-H), 7.88 (d, J = 16.5 Hz, 1H, 30-H), 7.77 (d, J = 2.6 Hz, 2H, 38-H), 7.48 (d, J = 7.9 Hz, 1H, 36-H), 7.22 (d, J = 16.3 Hz, 1H, 29-H), 7.23–7.13 (m, 1H, 34-H, 35-H), 4.66 (t, J = 6.9 Hz, 2H, 23-H), 4.12 (m, 2H, 21-H), 2.46–2.34 (m, 3H, 15-Ha, 22-H), 1.95 (d, J = 13.0 Hz, 1H, 3-Ha), 1.84 (d, J = 18.3 Hz, 1H, 15-Hb), 1.73–1.49 (m, 6H, 1-Ha, 2-Ha, 6-H, 7-Ha, 11-Hb), 1.49–1.26 (m, 6H, 7-Hb, 11-Ha, 12-H, 14-H), 1.19–1.04 (m, 3H, 2-Hb, 5-H, 9-H), 1.08 (s, 3H, 19-H), 0.98 (td, J = 13.5, 3.9 Hz, 1H, 3-Hb), 0.88 (td, J = 13.4, 3.7 Hz, 1H, 1-Hb), 0.83 (s, 3H, 17-H), 0.61 (s, 3H, 20-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 221.2 (C-16), 176.9 (C-18), 142.0 (C-28), 141.2 (C-26), 140.1 (C-37), 140.0 (C-24), 137.8 (C-27), 130.7 (C-30), 129.7 (C-38), 128.2 (C-25), 125.3 (C-32), 122.9 (C-29), 120.9 (C-35), 120.4 (C-33), 115.8 (C-34), 113.4 (C-31), 112.8 (C-36), 61.6 (C-21), 59.3 (C-23), 56.4 (C-5), 54.1 (C-9), 53.6 (C-14), 48.3 (C-13), 48.1 (C-15), 43.6 (C-4), 40.9 (C-7), 40.5 (C-1), 39.5 (C-8), 39.4 (C-10), 37.9 (C-3), 37.7 (C-12), 29.9 (C-22), 28.7 (C-19), 21.8 (C-6), 20.3 (C-2), 20.2 (C-17), 19.0 (C-11), 13.5 (C-20) ppm; MS (ESI, MeOH/DMSO, 4:1): m/z (%) 580 (64%, [M − Br]+); analysis calcd. for C38H47N2O3Br (659.70): C 69.18, H 7.18, N 4.25; found: C 68.96, H 4.45, N 4.02.
4.2.23. 3-{2-[(E)-2-(1H-Indol-3-yl)-ethenyl]-pyridinium-1-yl}-propyl (4α)-13-Hydroxykaur-16-en-18-oate bromide (20)
Following GPC from 14 (0.305 g, 0.7 mmol) and 5 (0.154 g, 0.7 mmol) followed by chromatography (SiO2, CHCl3/MeOH, 4:1), 21 (0.12 g, 30%) was obtained as a yellowish solid: Rf = 0.2 (SiO2, CHCl3/MeOH, 8.5:1.5); m.p. = 91–94 °C; = −31.96° (c = 0.097, MeOH); IR (ATR): ν = 3370br, 2925w, 2851w, 1716w, 1628w, 1605m, 1564m, 1499m, 1461w, 1431m, 1364w, 1325w, 1274w, 1243m, 1164w, 1152w, 1135w, 1116w, 1051w, 955w, 816w, 745m, 520br, 423w cm−1; UV-Vis (MeOH): λmax (log ε) = 427.47 nm (4.24); 1H NMR (400 MHz, DMSO-d6): δ =12.14 (s, 1H, NH), 8.81 (d, J = 6.1 Hz, 1H, 24-H), 8.57 (d, J = 8.4 Hz, 1H, 27-H), 8.37 (t, J = 7.7 Hz, 1H, 26-H), 8.32 (d, J = 16.1 Hz, 1H, 30-H), 8.21 (s, 1H, 38-H), 8.06 (d, J = 7.7 Hz, 1H, 33-H), 7.74 (t, J = 6.6 Hz, 1H, 25-H), 7.50 (d, J = 7.8 Hz, 1H, 36-H), 7.31–7.14 (m, 2H, 34-H, 35-H), 7.23 (d, J = 15.2 Hz, 1H, 29-H), 4.94 (s, 1H, 17-Ha), 4.84 (t, 2H, 23-H), 4.56 (s, 1H, 17-Hb), 4.23–4.03 (m, 2H, 21-H), 2.33–2.12 (m, 2H, 22-H), 1.99–1.70 (m, 4H, 3-Ha, 14-H, 15-Ha), 1.69–1.31 (m, 10H, 1-Ha, 2-Ha, 6-H, 7-H, 11-H, 12-H), 1.32–1.04 (m, 2H, 2-Hb, 15-Hb), 0.99 (s, 3H, 19-H), 0.97–0.69 (m, 4H, 1-Hb, 3-Hb, 5-H, 9-H), 0.67 (s, 3H, 20-H) ppm; 13C NMR (101 MHz, DMSO-d6): δ = 176.8 (C-18), 153.8 (C-16), 145.1 (C-24), 144.9 (C-28), 143.8 (C-26), 139.1 (C-30), 137.7 (C-37), 133.6, 132.8 (C-38), 125.6 (C-32), 124.7 (C-27), 123.5 (C-25), 123.3 (C-35), 121.7 (C-34), 120.3 (C-33), 113.8 (C-31), 113.1 (C-36), 110.1 (C-29), 102.9 (C-17), 81.2 (C-13), 61.6 (C-21), 56.7 (C-5), 56.1 (C-9), 55.4 (C-23), 50.9 (C-15), 49.0, 47.6 (C-14), 46.7, 43.6 (C-8), 41.6 (C-7), 39.4 (C-1), 38.4 (C-10), 37.8 (C-3), 37.8, 32.2, 29.4 (C-22), 28.7 (C-19), 22.5 (C-6), 20.8 (C-11), 19.1 (C-2), 15.4 (C-20) ppm; MS (ESI, MeOH/DMSO, 4:1): m/z (%) 580 (90%, [M − Br]+); analysis calcd. For C38H47N2O3Br (659.70): C 69.18, H 7.18, N 4.25; found: C 68.96, H 7.34, N 4.13.
4.2.24. 3-{2-[(E)-2-(1-H-Indol-3-yl)-ethenyl]-pyridinium-1-yl}-propyl (4α)-13-Hydroxykaur-16-en-18-oate Bromide (21)
Following GPC from 15 (0.371 g, 0.72 mmol) and 5 (0.158 g, 0.72 mmol) followed by chromatography (SiO2, CHCl3/MeOH, 6:1), 20 (0.174 g, 36%) was obtained as a yellowish solid: Rf = 0.1 (SiO2, CHCl3/MeOH, 9:1); m.p. = 89–92 °C; = −57.04° (c = 0.301, MeOH); IR (ATR): ν = 3360br, 2925w, 2848w, 1721m, 1628w, 1605m, 1564m, 1524w, 1499m, 1449w, 1432m, 1374w, 1318w, 1274w, 1237m, 1156m, 1131m, 1112w, 1096w, 1057w, 1039w, 1016w, 966w, 877w, 853w, 816w, 745s, 663w, 567w, 508w, 423w cm−1; UV-Vis (MeOH): λmax (log ε) = 430.32 nm (4.24); 1H NMR (500 MHz, DMSO-d6): δ = 12.08 (s, 1H, NH), 8.77 (d, J = 6.2 Hz, 1H, 24-H), 8.57 (d, J = 8.1 Hz, 1H, 27-H), 8.38 (dd, J = 7.8 Hz, 1H, 26-H), 8.31 (d, J = 15.6 Hz, 1H, 30-H), 8.19 (d, J = 2.9 Hz, 1H, 38-H), 8.07 (d, J = 7.6 Hz, 1H, 33-H), 7.75 (t, J = 6.6 Hz, 1H, 25-H), 7.51 (d, J = 7.6 Hz, 1H, 36-H), 7.27 (d, J = 28.6 Hz, 1H, 29-H), 7.28–7.17 (m, 2H, 34-H, 35-H), 4.89–4.76 (m, 2H, 23-H), 4.23–4.09 (m, 2H, 21-H), 2.33–2.22 (m, 3H, 15-Ha, 22-H), 1.93 (d, J = 13.2 Hz, 1H, 3-Ha), 1.70–1.42 (m, 7H, 1-Ha, 6-H, 7-Ha, 11-Ha, 15-Hb), 1.43–1.20 (m, 6H, 2-H, 7-Hb, 12-H, 14-H), 1.20–1.03 (m, 2H, 5-H, 9-H), 1.01 (s, 3H, 19-H), 1.00–0.86 (m, 3H, 1-Hb, 3-Hb, 11-Hb), 0.83 (s, 3H, 17-H), 0.53 (s, 3H, 20-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 221.0 (C-16), 176.9 (C-18), 153.8 (C-28), 145.1 (C-24), 143.8 (C-26), 139.1 (C-30), 137.8 (C-37), 132.6 (C-38), 125.7 (C-32), 124.6 (C-27), 123.6 (C-25), 123.4 (C-35), 121.7 (C-34), 120.3 (C-33), 113.9 (C-31), 113.1 (C-36), 110.2 (C-29), 61.8 (C-21), 56.4 (C-5), 55.6 (C-23), 54.1 (C-9), 53.6 (C-14), 48.3 (C-13), 47.9 (C-15), 43.6 (C-4), 40.8 (C-7), 39.3 (C-1, 8), 37.8 (C-10), 37.7 (C-3), 37.0 (C-12), 28.7 (C-19), 28.6 (C-22), 21.7 (C-6), 20.2 (C-11), 20.2 (C-17), 19.0 (C-2), 13.4 (C-20) ppm; MS (ESI, MeOH/DMSO, 4:1): m/z (%) 580 (70%, [M + H]+); analysis calcd. for C38H47N2O3Br (579.8): C 78.72, H 8.17, N 4.83; found: C 78.56, H 8.37, N 4.65.
4.2.25. 4-Bromobutyl (4α) -13-Hydroxykaur-16-en-18-oate Bromide (22)
Following GPB from 1 (0.5 g, 1.57 mmol), K2CO3 0.434 g, 3.1 mmol), 1,4-dibromobutane (0.74 mL, 6.3 mmol) followed by chromatography (SiO2, hexanes/ethyl acetate, 6:1), 22 (0.53 g, 74%) was obtained as a colorless solid: Rf = 0.37 (SiO2, hexanes/ethyl acetate, 8:2); m.p. = 92–96 °C; = −50.12° (c = 0.353, CHCl3); IR (ATR): ν = 3419br, 2934m, 2848m, 1719s, 1465w, 1444m, 1387w, 1365w, 1329m, 1270w, 1229m, 1202w, 1151s, 1118m, 1081m, 1047w, 1020w, 973w, 953w, 920w, 882w, 868w, 818w, 754m, 694w, 647w, 562w, 531w, 501w, 432w cm−1; 1H NMR (500 MHz, CDCl3): δ = 5.09 (s, 1H, OH), 4.96 (s, 1H, 17-Ha), 4.80 (s, 1H, 17-Hb), 4.13–3.98 (m, 2H, 21-H), 3.43 (t, J = 6.6 Hz, 2H, 24-H), 2.19–2.11 (m, 3H, 3-Ha, 14-Ha, 15-Ha), 2.00–1.91 (m, 2H, 22-H), 1.87–1.72 (m, 5H, 1-Ha, 2-Ha, 6-Ha, 23-H), 1.71–1.56 (m, 3H, 11-H, 12-Ha), 1.56–1.34 (m, 4H, 2-Hb, 6-Hb, 12-Hb, 15-Hb), 1.30–1.22 (m, 1H, 14-Hb), 1.16 (s, 3H, 19-H), 1.07–0.93 (m, 3H, 3-Hb, 5-H, 9-H), 0.84 (s, 3H, 20-H), 0.82–0.78 (m, 1H, 1-Hb) ppm; 13C NMR (126 MHz, CDCl3): δ = 177.4 (C-18), 143.5 (C-16), 102.9 (C-17), 82.6 (C-13), 63.1 (C-21), 56.7 (C-5), 53.8 (C-9), 50.9 (C-15), 47.9 (C-14), 43.9 (C-4), 41.3 (C-8), 40.9 (C-7), 40.7 (C-1), 39.6 (C-10), 39.4 (C-12), 38.1 (C-3), 31.8 (C-24), 29.6 (C-22), 28.8 (C-19), 27.2 (C-23), 21.0 (C-6), 20.8 (C-11), 19.1 (C-2), 15.3 (C-20) ppm; MS (ESI, MeOH:CHCl3 4:1): m/z (%) 373 (70%, [M − Br]+); analysis calcd. for C24H37O3Br (453.46): C 63.57, H 8.22; found: C 63.41, H 8.39.
4.2.26. 4-Bromobutyl (4α, 8β, 13β) 13-Methyl-16-oxo-17-norkauran-18-oate (23)
Following GPB from 2 (0.5 g, 1.57 mmol), K2CO3 (0.434 g, 3.1 mmol) and 1,4-dibromobutane (0.74 mL, 6.3 mmol) followed by chromatography (SiO2, hexanes/ethyl acetate, 6:1), 23 (0.53 g, 75%) was obtained as a colorless solid: Rf = 0.68 (SiO2, hexanes/ethyl acetate, 4:1); m.p. = 104–106 °C; = −54.38° (c = 0.158, CHCl3); IR (ATR): ν = 2937m, 2891w, 2845w, 1734s, 1718s, 1448m, 1387w, 1355w, 1320w, 1299w, 1245w, 1231m, 1208w, 1180s, 1153s, 1133w, 1109w, 1095w, 1060w, 1031w, 1017w, 1002w, 978w, 928w, 868w, 850w, 827w, 809w, 776w, 750w, 735w, 694w, 652w, 594w, 557w, 513w, 462w cm−1; 1H NMR (400 MHz, CDCl3): δ = 4.13–3.96 (m, 2H, 21-H), 3.43 (t, J = 6.6 Hz, 2H, 24-H), 2.61 (dd, J = 18.6, 3.8 Hz, 1H, 15-Ha), 2.17 (dt, J = 13.4, 3.8 Hz, 1H, 3-Ha), 1.99–1.85 (m, 3H, 6-Ha, 22-H), 1.85–1.74 (m, 4H, 2-Ha, 15-Hb, 23-H), 1.74–1.57 (m, 5H, 1-Ha, 6-Hb, 7-Ha, 11-Ha), 1.53 (td, J = 11.4, 3.6 Hz, 1H, 14-Ha), 1.48–1.31 (m, 4H, 2-Hb, 7-Hb, 12-H, 14-Hb), 1.18 (s, 3H, 19-H), 1.15 (s, 2H, 9-H, 11-Hb), 1.12 (dd, J = 11.9, 2.2 Hz, 1H, 5-H), 1.03 (td, J = 13.4, 4.1 Hz, 1H, 3-Hb), 0.97 (s, 3H, 17-H), 0.90 (td, J = 13.1, 4.2 Hz, 1H, 1-Hb), 0.70 (s, 3H, 20-H) ppm; 13C NMR (101 MHz, CDCl3): δ = 222.4 (C-16), 177.3 (C-18), 63.2 (C-21), 57.0 (C-5), 54.7 (C-9), 54.3 (C-14), 48.4 (C-15), 41.5 (C-7), 39.8 (C-8), 39.4 (C-1), 38.0 (C-10), 37.9 (C-3), 37.3 (C-12), 32.9 (C-24), 29.5 (C-22), 29.0 (C-19), 27.2 (C-23), 21.8 (C-6), 20.3 (C-11), 19.8 (C-17), 19.0 (C-2), 13.4 (C-20) ppm; MS (ESI, MeOH/CHCl3, 4:1): m/z (%) 373 (90%, [M − Br]+); analysis calcd. for C24H37O3Br (453.46): C 63.57, H 8.22; found: C 63.41, H 8.39.
4.2.27. 4-{4-[(E)-2-(1H-Indol-3-yl)ethenyl-pyridinium-1-yl-butyl (4α)-13-Hydroxykaur-16-en-18-oate Bromide (24)
Following GPC from 22 (0.3 g, 0.66 mmol) and 3 (0.146 g, 0.66 mmol) followed by chromatography (SiO2, CHCl3/MeOH, 6:1), 24 (0.287 g, 73%) was obtained as a reddish solid: Rf = 0.11 (SiO2, CHCl3/MeOH, 9:1); m.p. = 179–182 °C; = −44.96° (c = 0.045, MeOH); IR (ATR): ν = 3308br, 2953w, 2934m, 2857w, 2843w, 1716m, 1647w, 1591m, 1573m, 1556w, 1492m, 1460w, 1439m, 1385w, 1371w, 1356w, 1316w, 1278w, 1245m, 1207w, 1179m, 1150w, 1136w, 1116m, 1083w, 1062w, 1047w, 1021w, 984w, 967w, 940w, 899w, 872w, 841w, 820w, 802w, 766w, 751m, 696w, 617w, 574w, 551w, 527w, 513w, 499w, 429w cm−1; UV-Vis (MeOH): λmax (log ε) = 438.12 nm (4.14); 1H NMR (500 MHz, DMSO-d6): δ = 11.97 (s, 1H, NH), 8.78 (d, J = 7.0 Hz, 2H, 25-H, 29-H), 8.27 (d, J = 16.1 Hz, 1H, 31-H), 8.15 (m, 3H, 26-H, 28-H, 34-H), 7.98 (d, J = 2.7 Hz, 1H, 39-H), 7.51 (d, J = 7.3 Hz, 1H, 37-H), 7.30 (d, J = 16.2 Hz, 3H, 30-H), 7.27–7.19 (m, 2H, 35-H, 36-H), 5.00 (s, 1H, 17-Ha), 4.64 (s, 1H, 17-Hb), 4.48 (t, J = 7.1 Hz, 2H, 24-H), 4.12–3.94 (m, 2H, 21-H), 2.07–1.90 (m, 6H, 3-Ha, 14-H, 15-Ha, 22-H), 1.78–1.66 (m, 3H, 1-Ha, 2-Ha, 6-Ha), 1.65–1.48 (m, 4H, 11-H, 23-H), 1.48–1.40 (m, 2H, 12-H), 1.39–1.18 (m, 3H, 2-Hb, 6-Hb, 15-Hb), 1.10 (s, 3H, 19-H), 1.06–0.87 (m, 3H, 3-Hb, 5-H, 9-H), 0.82–0.76 (m, 1H, 1-Hb), 0.74 (s, 3H, 20-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 176.4 (C-18), 154.7 (C-16), 144.5 (C-27), 143.3 (C-25, 29), 137.5 (C-38), 136.7 (C-31), 133.2 (C-39), 132.4, 124.9 (C-33), 122.9 (C-35), 121.9 (C-26, 28), 121.1 (C-36), 120.4 (C-34), 116.8 (C-30), 113.6 (C-32), 112.6 (C-37), 102.5 (C-17), 80.7 (C-13), 62.9 (C-21), 58.5 (C-24), 55.7 (C-5), 53.1 (C-9), 50.5 (C-15), 47.2 (C-14), 43.3 (C-4), 43.2 (C-8), 41.0 (C-7), 40.2 (C-1), 39.0 (C-12), 38.8 (C-10), 37.4 (C-3), 28.3 (C-19), 27.4 (C-22), 24.8 (C-23), 20.5 (C-6), 20.4 (C-11), 18.7 (C-2), 15.0 (C-20) ppm; MS (ESI, MeOH/DMSO, 4:1): m/z (%) 594 (75%, [M − Br]+); analysis calcd. for C39H49N2O3Br (673.72): C 69.53, H 7.33, N 4.16; found: C 69.39, H 7.54, N 3.87.
4.2.28. 4-{4[(E)-2-(1H-Indol-3-yl)ethenyl]-pyridinium-1-yl}butyl (4α, 8β, 13β) 13-Methyl-16-oxo-17-norkauran-18-oate Bromide (25)
Following GPC from 23 (0.3 g, 0.66 mmol) and 3 (0.146 g, 0.66 mmol) followed by chromatography (SiO2, CHCl3/MeOH, 6:1), 25 (0.314 g, 80%) was obtained as a reddish solid: Rf = 0.17 (SiO2, CHCl3/MeOH, 9:1); m.p. = 174–176 °C; = −7.30° (c = 0.178, MeOH); IR (ATR): ν = 3406br, 3128br, 2925m, 2848w, 1721m, 1645w, 1597s, 1575m, 1557w, 1531w, 1501m, 1457m, 1432m, 1371w, 1356w, 1318w, 1274w, 1246m, 1206m, 1173s, 1132s, 1112m, 1097w, 1058w, 1044w, 1016w, 965m, 928w, 871w, 744s, 663w, 612w, 590w, 569w, 510w, 425w cm−1; UV-Vis (MeOH): λmax (log ε) = 443.61 nm (4.58); 1H NMR (500 MHz, DMSO-d6): δ = 11.97 (s, 1H, NH), 8.79 (d, J = 6.9 Hz, 2H, 25-H, 29-H), 8.26 (d, J = 16.3 Hz, 1H, 31-H), 8.17–8.12 (m, 3H, 26-H, 28-H, 34-H), 7.96 (d, J = 2.4 Hz, 1H, 39-H), 7.51 (d, J = 7.3 Hz, 1H, 37-H), 7.29 (d, J = 16.2 Hz, 1H, 30-H), 7.27–7.20 (m, 2H, 35-H, 36-H), 4.47 (t, J = 6.1 Hz, 2H, 21-H), 4.06–3.93 (m, 2H, 24-H), 2.35 (dd, J = 18.3, 3.5 Hz, 1H, 15-Ha), 2.02 (d, J = 13.0 Hz, 1H, 3-Ha), 1.99–1.88 (m, 2H, 23-H), 1.80 (d, J = 18.3 Hz, 1H, 15-Hb), 1.79–1.70 (m, 1H, 6-Hb), 1.71–1.51 (m, 6H, 1-Ha,2-Ha, 7-Ha, 11-Ha, 22-H), 1.49 (d, J = 14.0 Hz, 1H, 6-Ha), 1.44 (dd, J = 11.3, 1.9 Hz, 1H, 14-Ha), 1.39 (td, J = 13.1, 3.1 Hz, 1H, 7-Hb), 1.39–1.18 (m, 4H, 2-H, 12-H, 14-Hb), 1.19–1.13 (m, 2H, 5-H, 9-H), 1.12 (s, 3H, 19-H), 1.08–0.94 (m, 2H, 3-Hb, 11-Hb), 0.87 (td, J = 13.4, 13.0, 4.2 Hz, 1H, 1-Hb), 0.78 (s, 3H, 17-H), 0.57 (s, 3H, 20-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 221.1 (C-16), 176.9 (C-18), 155.2 (C-27), 143.8 (C-25, 29), 138.0 (C-38), 137.2 (C-31), 133.0 (C-39), 125.4 (C-33), 123.4 (C-36), 122.4 (C-26, 28), 121.6 (C-35), 120.9 (C-34), 117.2 (C-30), 114.1 (C-32), 113.1 (C-37), 63.5 (C-24), 59.0 (C-21), 56.4 (C-5), 54.1 (C-9), 53.6 (C-14), 48.3 (C-13), 48.0 (C-15), 43.7 (C-4), 41.0 (C-7), 39.5 (C-1), 39.4 (C-8), 37.9 (C-10), 37.8 (C-3), 37.0 (C-12), 28.9 (C-19), 28.0 (C-23), 25.2 (C-22), 21.8 (C-6), 20.3 (C-11), 20.1 (C-17), 19.0 (C-2), 13.5 (C-20) ppm; MS (ESI, MeOH/DMSO, 4:1): m/z (%) 594 (80%, [M − Br]+); analysis calcd. for C39H49N2O3Br (673.72): C 69.53, H 7.33, N 4.16; found: C 69.40, H 7.53, N 3.89.
4.2.29. 4-{3-[(E)-2-(1H-Indol-3-yl)-ethenyl]-pyridinium-1-yl}butyl (4α)-13-Hydroxykaur-16-en-18-oate Bromide (26)
Following GPC from 22 (0.526 g, 1.15 mmol) and 4 (0.256 g, 1.16 mmol) followed by chromatography (SiO2, CHCl3/MeOH, 6:1), 26 (0.420 g, 61%) was obtained as a yellowish solid: Rf = 0.06 (SiO2, CHCl3/MeOH, 9:1); m.p. = 182–184 °C; = −33.43° (c = 0.157, MeOH); IR (ATR): ν = 3376br, 3210br, 2933w, 2849w, 1707m, 1632m, 1577m, 1525w, 1501w, 1459w, 1434m, 1386w, 1364w, 1329w, 1276w, 1236m, 1202w, 1152m, 1116m, 1082w, 1055w, 1018w, 954w, 819w, 745s, 680w, 618w, 570w, 500w, 424w cm−1; UV-Vis (MeOH): λmax (log ε) = 359.12 nm (4.17); 1H NMR (500 MHz, DMSO-d6): δ = 11.65 (s, 1H, NH), 9.36 (s, 1H, 25-H), 8.82 (d, J = 5.9 Hz, 1H, 29-H), 8.74 (d, J = 8.4 Hz, 1H, 27-H), 8.32 (s, 1H), 8.11–8.05 (m, 2H, 28-H, 34-H), 7.90 (d, J = 16.5 Hz, 1H, 31-H), 7.78 (d, J = 2.7 Hz, 1H, 39-H), 7.49 (d, J = 7.7 Hz, 2H, 37-H), 7.22 (d, J = 16.4 Hz, 1H, 30-H), 7.25–7.14 (m, 2H, 35-H, 36-H), 4.99 (s, 1H, 17-Ha), 4.73–4.55 (m, 3H, 17-Hb, 24-H), 4.10–3.95 (m, 2H, 21-H), 2.11–1.97 (m, 5H, 3-Ha, 14-H, 23-H), 1.90 (d, J = 9.3 Hz, 1H, 15-Ha), 1.82–1.57 (m, 6H, 1-Ha, 2-Ha, 6-Ha, 11-Ha, 22-H), 1.56–1.40 (m, 3H, 11-Hb, 12-H), 1.39–1.15 (m, 3H, 2-Hb, 6-Hb, 15-Hb), 1.10 (s, 3H, 19-H), 1.02 (dd, J = 12.1, 1.6 Hz, 1H, 5-H), 0.95 (td, J = 13.5, 9.3, 3.7 Hz, 1H, 3-Hb), 0.89 (d, J = 8.0 Hz, 1H, 9-H), 0.77 (d, J = 8.5 Hz, 1H, 1-Hb), 0.74 (s, 3H, 20-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 176.4 (C-18), 156.2 (C-16), 144.5 (C-38), 141.3 (C-25), 140.5 (C-29), 139.5 (C-27), 137.3 (C-26), 133.2, 130.2 (C-31), 129.1 (C-39), 127.7 (C-28), 124.8 (C-33), 122.4 (C-35), 120.4 (C-36), 120.0 (C-34), 115.3 (C-30), 112.9 (C-32), 112.3 (C-37), 102.5 (C-17), 80.7 (C-13), 62.9 (C-24), 60.3 (C-21), 55.7 (C-5), 53.0 (C-9), 50.5 (C-15), 47.2 (C-14), 43.2 (C-4), 41.5 (C-8), 41.0 (C-7), 40.8 (C-1), 40.2 (C-10), 38.7 (C-12), 37.4 (C-3), 31.8, 28.3 (C-19), 27.5 (C-23), 24.8 (C-22), 20.5 (C-6), 20.4 (C-11), 18.7 (C-2), 15.0 (C-20) ppm; MS (ESI, MeOH/DMSO, 4:1): m/z (%) 594 (70%, [M − Br]+); analysis calcd. For C39H49N2O3Br (673.72): C 69.53, H 7.33, N 4.16; found: C 69.40, H 7.52, N 3.97.
4.2.30. 4-{3-[(E)-2-(1H-Indol-3-yl)ethenyl]-pyridinium-1-yl}butyl (4α, 8β, 13β) 13-Methyl-16-oxo-17-norkauran-18-oate Bromide (27)
Following GPC from 23 (0.533 g, 1.17 mmol) and 4 (0.26 g, 1.18 mmol) followed by chromatography (SiO2, CHCl3/MeOH 6:1), 27 (0.447 g, 65%) was obtained as a yellowish solid: Rf = 0.09 (SiO2, CHCl3/MeOH 9:1); m.p. = 167–169 °C; = −28.15° (c = 0.115, MeOH); IR (ATR): ν = 3417br, 4198br, 2928w, 2848w, 1722m, 1633m, 1578m, 1525w, 1501m, 1456w, 1434m, 1372w, 1337w, 1321w, 1276w, 1250w, 1233m, 1179w, 1151w, 1132w, 1111w, 1059w, 1029w, 1016w, 959w, 929w, 824w, 744m, 681w, 618w, 598w, 569w, 504w, 424w cm−1; UV-Vis (MeOH): λmax (log ε) = 357.9 nm (4.25); 1H NMR (500 MHz, DMSO-d6): δ = 11.65 (s, 1H, NH), 9.37–9.31 (m, 1H, 25-H), 8.83 (d, J = 5.9 Hz, 1H, 29-H), 8.76 (d, J = 8.4 Hz, 1H, 27-H), 8.15–8.06 (m, 2H, 28-H, 34-H), 7.89 (d, J = 16.5 Hz, 1H, 31-H), 7.78 (d, J = 2.7 Hz, 1H, 39-H), 7.50 (d, J = 7.7 Hz, 1H, 37-H), 7.22 (d, J = 15.7 Hz, 1H, 30-H), 7.27–7.16 (m, 2H, 35-H, 36-H), 4.65 (t, J = 7.1 Hz, 2H, 24-H), 4.11–3.97 (m, 2H, 21-H), 2.35 (d, J = 21.9 Hz, 1H, 15-Ha), 2.14–1.98 (m, 3H, 3-Ha, 23-H), 1.82 (d, J = 18.3 Hz, 1H, 15-Hb), 1.82–1.75 (m, 1H, 6-Ha), 1.73–1.47 (m, 7H, 1-Ha, 2-Ha, 6-Hb, 7-Ha, 11-Ha, 22-H), 1.43 (dd, J = 11.4, 2.1 Hz, 1H, 14-Ha), 1.40–1.21 (m, 5H, 2-Hb, 7-Hb, 12-H, 14-Hb), 1.19–1.15 (m, 2H, 5-H, 9-H), 1.14 (s, 3H, 19-H), 1.02 (m, 2H, 3-Hb, 11-Hb), 0.89 (td, J = 13.3, 3.9 Hz, 1H, 1-Hb), 0.84 (s, 3H, 17-H), 0.59 (s, 3H, 20-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 221.1 (C-16), 176.9 (C-18), 141.7 (C-25), 141.0 (C-29), 140.0 (C-38), 140.0 (C-27), 137.8 (C-26), 130.7 (C-31), 129.7 (C-39), 128.2 (C-28), 125.3 (C-33), 122.9 (C-30), 120.9 (C-36), 120.4 (C-34), 115.8 (C-35), 113.4 (C-32), 112.8 (C-37), 63.5 (C-24), 60.9 (C-21), 56.4 (C-5), 54.1 (C-9), 53.5 (C-14), 48.3 (C-13), 48.0 (C-15), 43.7 (C-4), 41.0 (C-7), 39.4 (C-1, 8), 37.9 (C-10), 37.8 (C-3), 37.0 (C-12), 28.9 (C-19), 28.0 (C-23), 25.2 (C-22), 21.8 (C-6), 20.2 (C-11), 20.1 (C-17), 19.0 (C-2), 13.5 (C-20) ppm; MS (ESI, MeOH/DMSO, 4:1): m/z (%) 594 (80%, [M + H]+); analysis calcd. for C39H49N2O3Br (673.72): C 69.53, H 7.33, N 4.16; found: C 69.46, H 7.58, N 3.86.
4.2.31. 4-{2-[(E{-2-(1H-Indol-3-yl)ethenyl]pyridinium-1-yl}-butyl (4α)-13-Hydroxykaur-16-en-18-oate Bromide (28)
Following GPC from 22 (0.263 g, 0.58 mmol) and 5 (0.128 g, 0.58 mmol) followed by chromatography (SiO2, CHCl3/MeOH, 4:1), 28 (0.123 g, 36%) was obtained as a yellowish solid: Rf = 0.19 (SiO2, CHCl3/MeOH 8.5:1.5); m.p. = 84–87 °C; = −21.46° (c = 0.135, MeOH); IR (ATR): ν = 3375br, 2925w, 2851w, 1715w, 1627w, 1605m, 1563m, 1499w, 1472w, 1432m, 1364w, 1329w, 1319w, 1278w, 1242m, 1153m, 1135w, 1117w, 1082w, 1055w, 1019w, 955w, 864w, 815w, 743w, 694w, 610w, 567w, 515w, 422w cm−1; UV-Vis (MeOH): λmax (log ε) = 426.84 nm (4.14); 1H NMR (400 MHz, DMSO-d6): δ = 12.11 (s, 1H, NH), 8.81 (d, J = 5.8 Hz, 1H, 25-H), 8.57 (d, J = 8.2 Hz, 1H, 28-H), 8.37 (d, J = 8.1 Hz, 1H, 26-H), 8.32 (d, J = 15.4 Hz, 1H, 31-H), 8.19 (s, 1H, 39-H), 8.06 (d, J = 7.2 Hz, 1H, 34-H), 7.72 (t, 1H, 27-H), 7.50 (d, J = 7.1 Hz, 1H, 37-H), 7.28 (d, J = 16.1 Hz, 1H, 30-H), 7.22 (s, 2H, 35-H, 36-H), 4.94 (s, 1H, 17-Ha), 4.90–4.74 (m, 2H, 24-H), 4.66 (s, 1H, 17-Hb), 4.12–3.87 (m, 2H, 21-H), 2.03–1.83 (m, 5H, 3-Ha, 14-H, 23-H), 1.75 (d, 1H, 15-Ha), 1.73–1.36 (m, 10H, 1-Ha, 2-Ha, 6-Ha, 7-Ha, 11-H, 12-H, 22-H), 1.26 (m, 4H, 2-Hb, 6-Hb, 7-Hb, 15-Hb), 0.92 (s, 3H, 19-H), 0.90–0.74 (m, 3H, 3-Hb, 5-H, 9-H), 0.72–0.64 (m, 1H, 1-Hb), 0.62 (s, 3H, 20-H) ppm; 13C NMR (101 MHz, DMSO-d6): δ = 176.8 (C-18), 156.7 (C-16), 153.5 (C-29), 145.1 (C-25), 144.9 (C-38), 143.7 (C-26), 139.0 (C-31), 137.8 (C-33), 132.8 (C-39), 125.6 (C-32), 124.4 (C-28), 123.4 (C-27), 123.3 (C-36), 121.7 (C-35), 120.3 (C-34), 113.9 (C-17), 113.1 (C-37), 110.0 (C-30), 81.2 (C-13), 63.4 (C-21), 61.5 (C-24), 56.1 (C-5), 53.5 (C-9), 51.0 (C-15), 47.6 (C-14), 43.6 (C-4), 42.3 (C-8), 41.7 (C-7), 40.5 (C-1), 40.1 (C-10), 39.1 (C-12), 37.8 (C-3), 28.6 (C-19), 26.6 (C-23), 25.4 (C-22), 21.0 (C-6), 20.8 (C-11), 19.1 (C-2), 15.4 (C-20) ppm; MS (ESI, MeOH/DMSO, 4:1): m/z (%) 594 (80%, [M − Br]+); analysis calcd. for C39H49N2O3Br (673.72): C 69.53, H 7.33, N 4.16; found: C 69.42, H 7.57, N 3.91.
4.2.32. 4-{2-[(E)-2-(1H-Indol-3-yl)ethenyl]pyridinium-1-yl}-butyl (4α, 8β, 13β) 13-Methyl-16-oxo-17-norkauran-18-oate Bromide (29)
Following GPC from 23 (0.267 g, 0.59 mmol) and 5 (0.13 g, 0.59 mmol) followed by chromatography (SiO2, CHCl3/MeOH, 4:1), 29 (1.04 g, 30%) was obtained as a yellowish solid: Rf = 0.35 (SiO2, CHCl3/MeOH, 8.5:1.5); m.p. = 86–89 °C; = −5.25° (c = 0.294, MeOH); IR (ATR): ν = 3381br, 2924w, 2850w, 1719m, 1660w, 1627w, 1605m, 1562m, 1523w, 1499w, 1469w, 1432m, 1380w, 1335w, 1319w, 1278w, 1242m, 1156w, 1131w, 1112w, 1059w, 1016w, 966w, 855w, 816w, 744m, 661w, 564w, 506w, 459w, 423w cm−1; UV-Vis (MeOH): λmax (log ε) = 427.68 nm (4.67); 1H NMR (500 MHz, DMSO-d6): δ = 12.10 (s, 1H, NH), 8.81 (d, J = 6.2 Hz, 1H, 25-H), 8.58 (d, J = 8.4 Hz, 1H, 28-H), 8.40–8.32 (m, 1H, 26-H), 8.33 (d, J = 15.9 Hz, 1H, 31-H), 8.20 (d, J = 2.8 Hz, 1H, 39-H), 8.07 (d, J = 7.0 Hz, 1H, 34-H), 7.73 (t, J = 6.9 Hz, 1H, 27-H), 7.50 (d, J = 7.7 Hz, 1H, 37-H), 7.30 (d, J = 15.6 Hz, 1H, 30-H), 7.27–7.17 (m, 2H, 35-H, 36-H), 4.83 (t, J = 7.3 Hz, 2H, 24-H), 4.14–3.86 (m, 2H, 21-H), 2.24 (dd, J = 18.3, 3.3 Hz, 1H, 15-Ha), 2.05–1.87 (m, 3H, 3-Ha, 23-H), 1.78–1.51 (m, 5H, 2-Ha, 6-Ha, 15-Hb, 22-H), 1.51–1.18 (m, 10H, 1-Ha, 2-Hb, 6-Hb, 7-H, 11-Ha, 12-H, 14-H), 1.08–0.98 (m, 2H, 5-H, 9-H), 0.96 (s, 3H, 19-H), 0.94–0.86 (m, 2H, 3-Hb, 11-Hb), 0.86–0.80 (m, 3H, 17-H), 0.75 (td, J = 12.9, 12.4, 4.9 Hz, 1H, 1-Hb), 0.48 (s, 3H, 20-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 221.0 (C-16), 176.8, 153.5 (C-29), 145.1 (C-25), 143.7 (C-26), 139.0 (C-31), 137.8 (C-38), 132.7 (C-39), 129.8, 125.7 (C-33), 124.4 (C-28), 123.4 (C-27), 122.7 (C-36), 121.7 (C-35), 120.3 (C-34), 113.9 (C-32), 113.1 (C-37), 110.0 (C-30), 63.6 (C-21), 57.2 (C-24), 56.3 (C-5), 54.0 (C-9), 53.5 (C-14), 48.3 (C-13), 47.9 (C-15), 43.6 (C-4), 40.8 (C-7), 39.4 (C-1), 39.4 (C-8), 39.3 (C-10), 37.8 (C-3), 37.0 (C-12), 28.7 (C-19), 26.8 (C-23), 25.4 (C-22), 21.7 (C-6), 20.2 (C-17), 20.2 (C-11), 18.9 (C-2), 13.4 (C-20) ppm; MS (ESI, MeOH/DMSO, 4:1): m/z (%) 594 (75%, [M + H]+); analysis calcd. For C39H49N2O3Br (673.72): C 69.53, H 7.33, N 4.16; found: C 69.36, H 7.59, N 3.94.