Preparation of Surface Dispersed WO3/BiVO4 Heterojunction Arrays and Their Photoelectrochemical Performance for Water Splitting
Abstract
1. Introduction
2. Results and Discussion
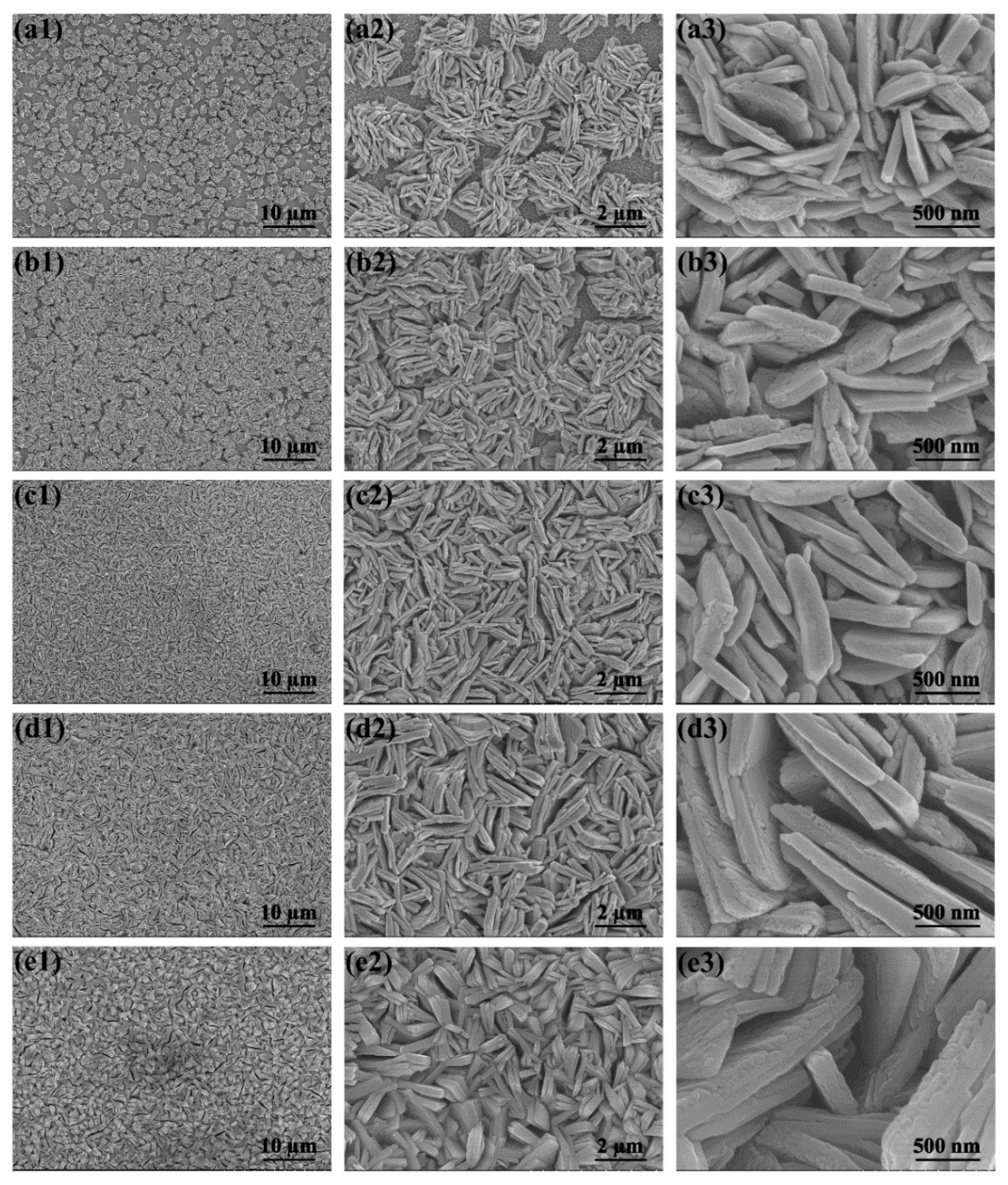
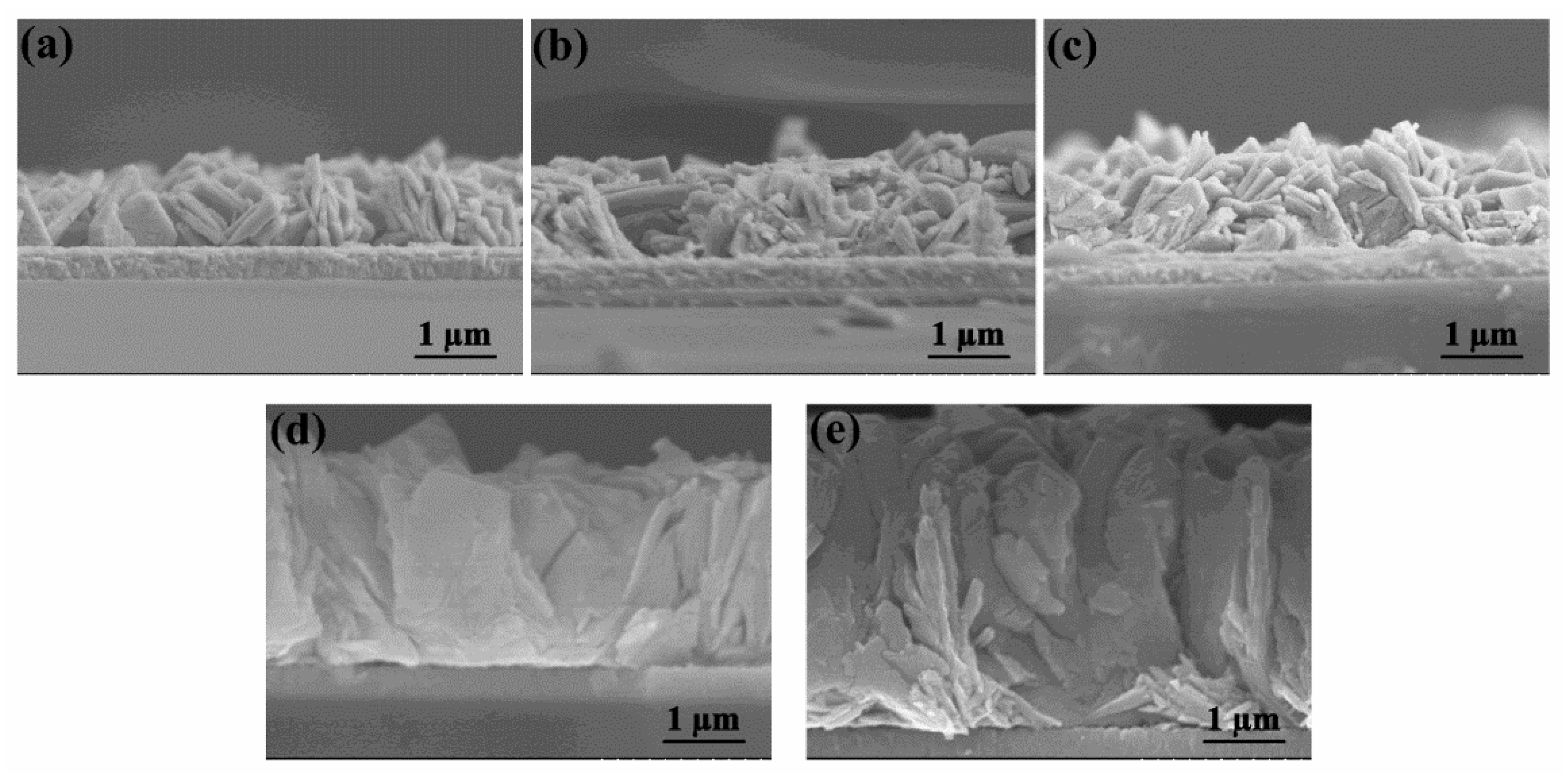
2.1. Structure and Properties of WO3 Nanoflake Arrays
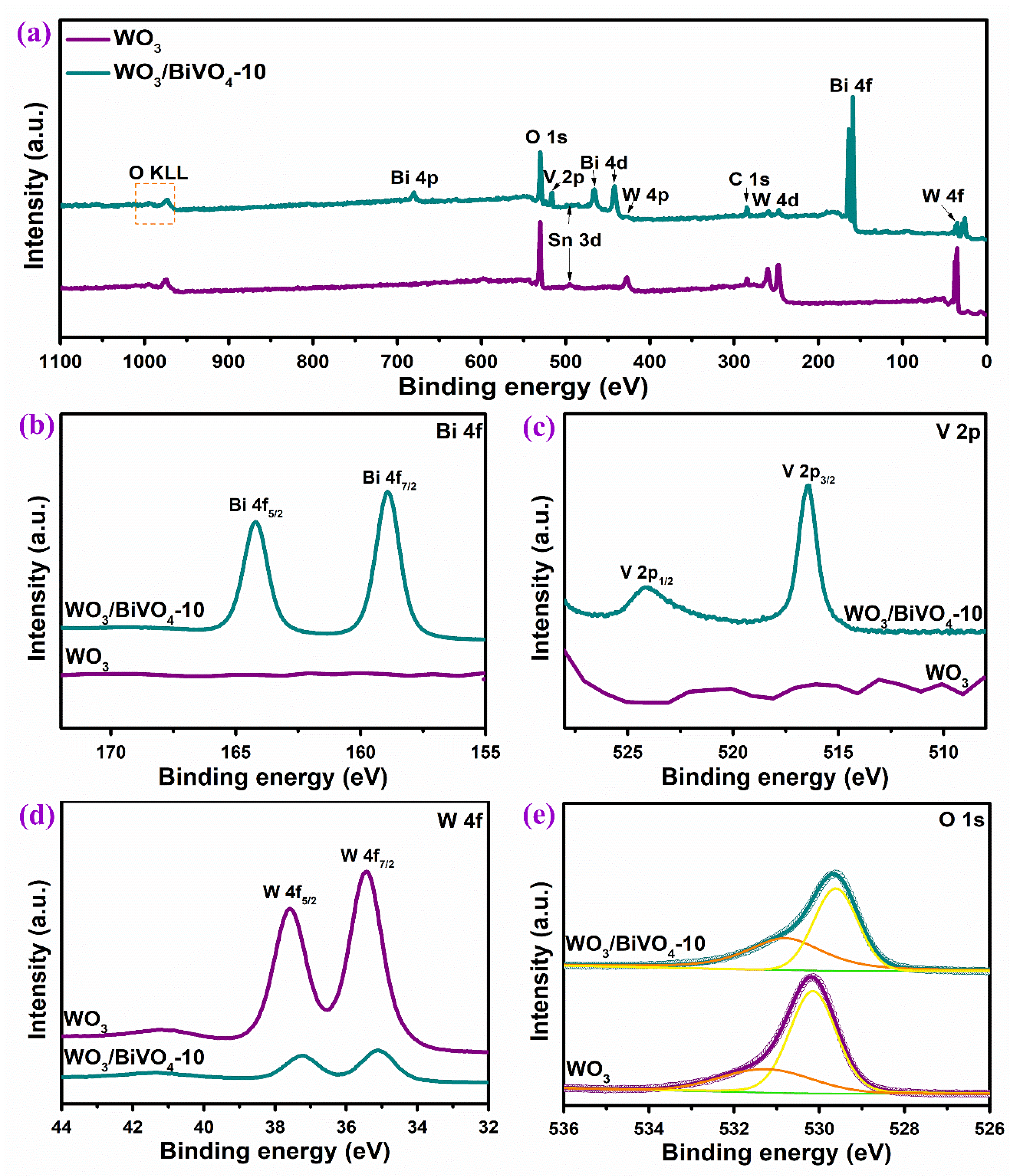
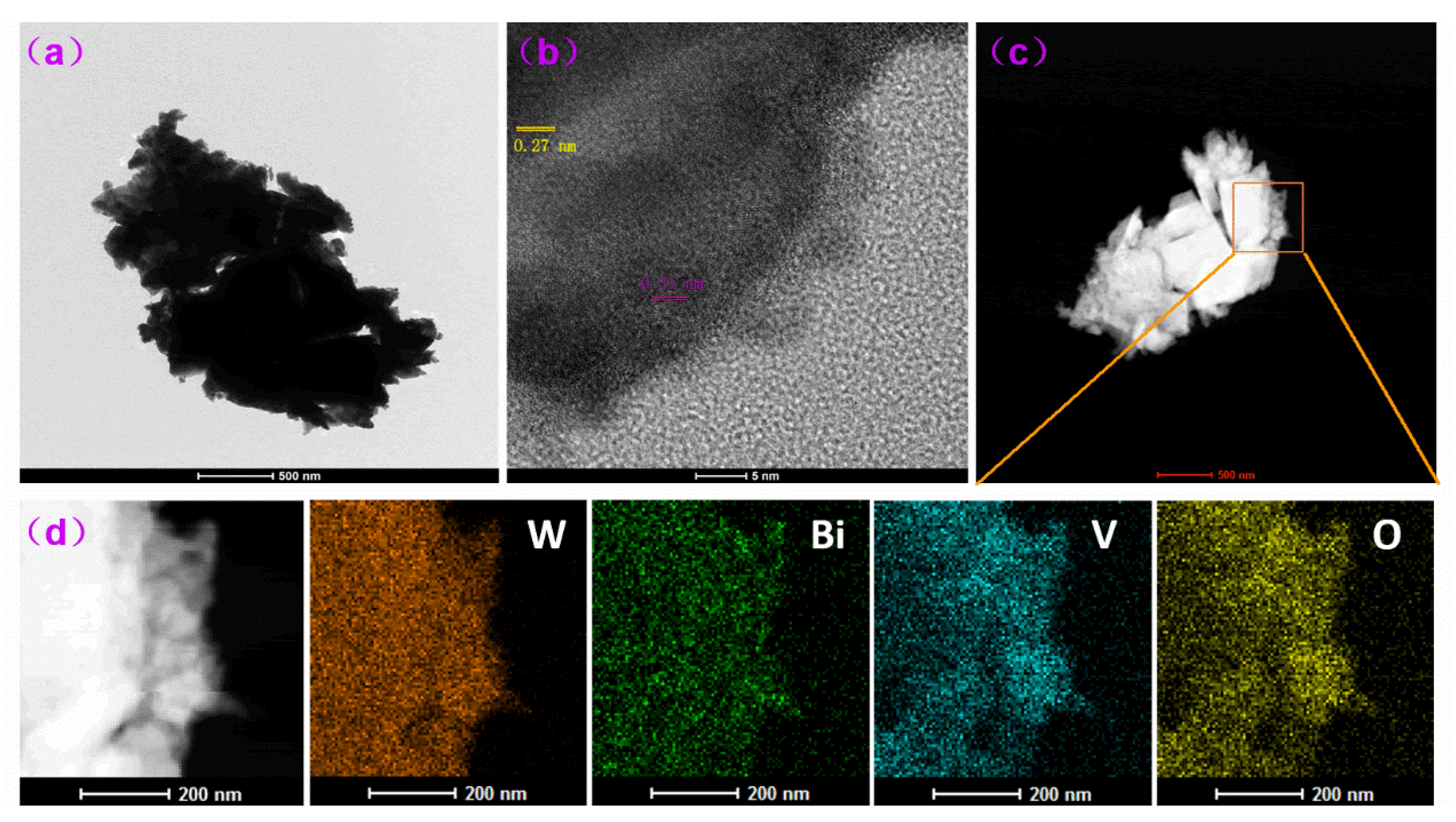
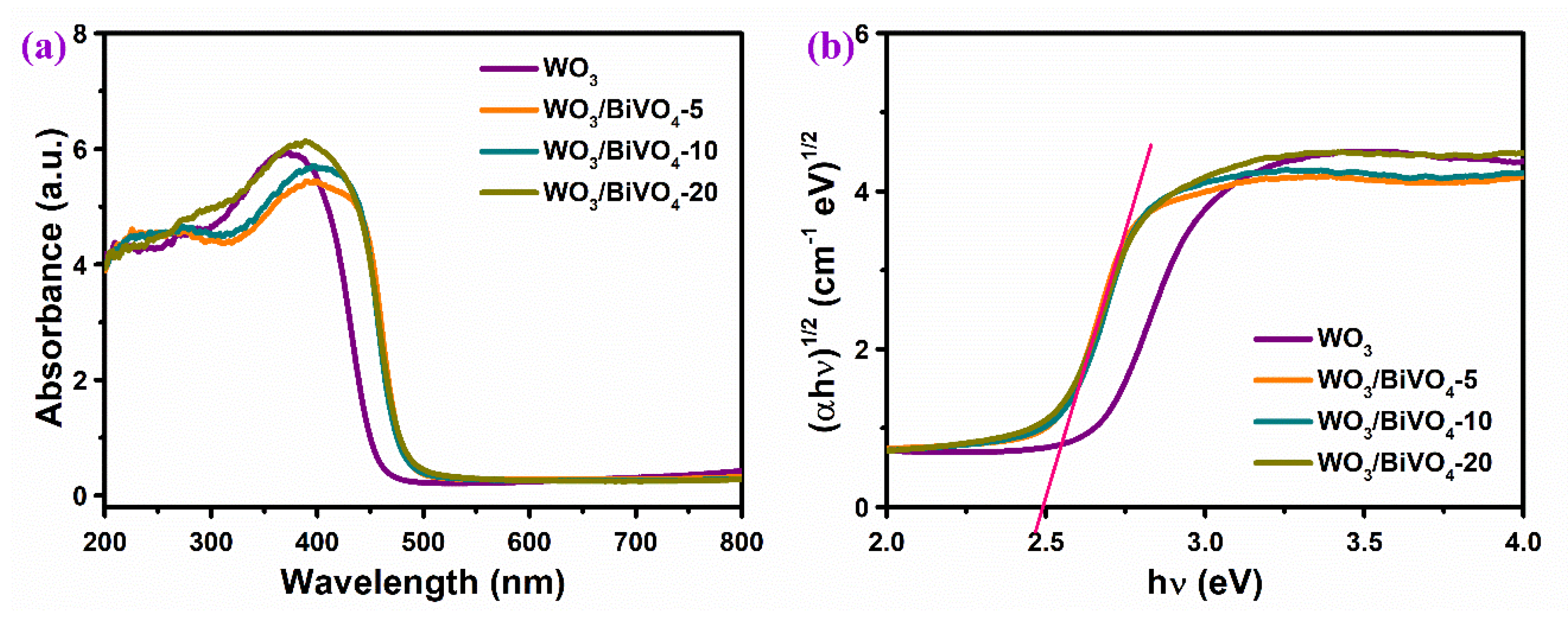
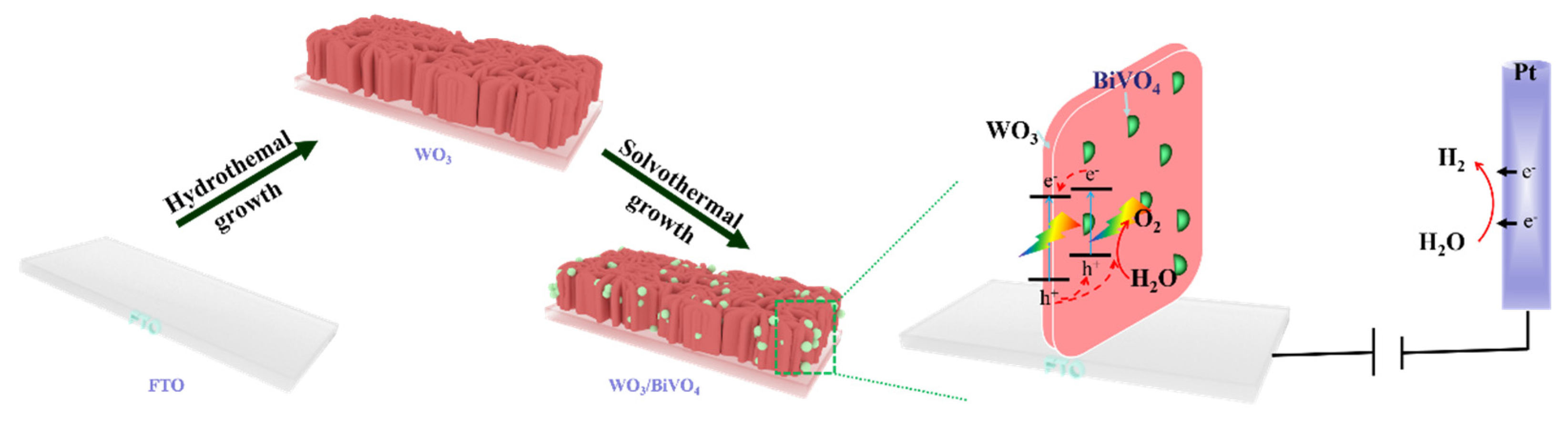
2.2. Structure and Properties of WO3/BiVO4 Nanoarrays
3. Materials and Methods
3.1. Preparation of the WO3 Nanoflake Arrays
3.2. Preparation of the WO3/BiVO4 Nanoflake Arrays
3.3. Characterization
3.4. Photoelectrochemical Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Samuel, E.; Joshi, B.; Kim, M.W.; Swihart, M.T.; Yoon, S.S. Morphology engineering of photoelectrodes for efficient photoelectrochemical water splitting. Nano Energy 2020, 72, 104648. [Google Scholar] [CrossRef]
- Mushtaq, M.A.; Kumar, A.; Yasin, G.; Arif, M.; Tabish, M.; Ibraheem, S.; Cai, X.; Ye, W.; Fang, X.; Saad, A.; et al. 3D interconnected porous Mo-doped WO3@CdS hierarchical hollow heterostructures for efficient photoelectrochemical nitrogen reduction to ammonia. Appl. Catal. B Environ. 2022, 317, 121711. [Google Scholar] [CrossRef]
- Daulbayev, C.; Sultanov, F.; Bakbolat, B.; Daulbayev, O. 0D, 1D and 2D nanomaterials for visible photoelectrochemical water splitting. A Review. Int. J. Hydrogen Energy 2020, 45, 33325–33342. [Google Scholar] [CrossRef]
- Marwat, M.A.; Humayun, M.; Afridi, M.W.; Zhang, H.; Karim, M.R.A.; Ashtar, M.; Usman, M.; Waqar, S.; Ullah, H.; Wang, C.; et al. Advanced catalysts for photoelectrochemical water splitting. ACS Appl. Energy Mater. 2021, 4, 12007–12031. [Google Scholar] [CrossRef]
- Tang, J.; Durrant, J.R.; Klug, D.R. Mechanism of photocatalytic water splitting in TiO2. Reaction of water with photoholes, importance of charge carrier dynamics, and evidence for four-hole chemistry. J. Am. Chem. Soc. 2008, 130, 13885–13891. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Zhang, J.; Fan, X.; He, J.; Li, Y. Surface engineering of nanomaterials for photo-electrochemical water splitting. Small 2019, 15, 1803746. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Wang, D.; Ye, J. Conformal BiVO4-layer/WO3-nanoplate-array heterojunction photoanode modified with cobalt phosphate cocatalyst for significantly enhanced photoelectrochemical performances. ACS Appl. Mater. Interfaces 2019, 11, 5623–5631. [Google Scholar] [CrossRef]
- Li, H.; Lin, C.; Yang, Y.; Dong, C.; Min, Y.; Shi, X.; Wang, L.; Lu, S.; Zhang, K. Boosting Reactive Oxygen Species Generation Using Inter-Facet Edge Rich WO3 Arrays for Photoelectrochemical Conversion. Angew. Chem. Int. Ed. 2023, 62, e202210804. [Google Scholar] [CrossRef]
- Han, H.; Riboni, F.; Karlický, F.; Kment, S.; Goswami, A.; Sudhagar, P.; Yoo, J.; Wang, L.; Tomanec, O.; Petr, M.; et al. α-Fe2O3/TiO2 3D hierarchical nanostructures for enhanced photoelectrochemical water splitting. Nanoscale 2017, 9, 134–142. [Google Scholar] [CrossRef]
- Wang, L.; Si, W.; Ye, Y.; Wang, S.; Hou, F.; Hou, X.; Cai, H.; Dou, S.X.; Liang, J. Cu-ion-implanted and polymeric carbon nitride-decorated TiO2 nanotube array for unassisted photoelectrochemical water splitting. ACS Appl. Mater. Interfaces 2021, 13, 44184–44194. [Google Scholar] [CrossRef]
- Chaulagain, N.; Alam, K.M.; Kadian, S.; Kumar, N.; Garcia, J.; Manik, G.; Shankar, K. Synergistic enhancement of the photoelectrochemical performance of TiO2 nanorod arrays through embedded plasmon and surface carbon nitride co-sensitization. ACS Appl. Mater. Interfaces 2022, 14, 24309–24320. [Google Scholar] [CrossRef]
- Han, J.; Liu, Z. Optimization and modulation strategies of zinc oxide-based photoanodes for highly efficient photoelectrochemical water splitting. ACS Appl. Energy Mater. 2021, 4, 1004–1013. [Google Scholar] [CrossRef]
- Xie, X.; Wang, R.; Ma, Y.; Chen, J.; Cui, Q.; Shi, Z.; Li, Z.; Xu, C. Photothermal-effect-enhanced photoelectrochemical water splitting in MXene-nanosheet-modified ZnO nanorod arrays. ACS Appl. Nano Mater. 2022, 5, 11150–11159. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Ji, H.; Ma, W.; Chen, C.; Zhao, J. Pivotal role and regulation of proton transfer in water oxidation on hematite photoanodes. J. Am. Chem. Soc. 2016, 138, 2705–2711. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, S.; Gao, L.; Long, X.; Chai, H.; Li, F.; Wang, Q.; Jin, J. Achieving surface-sealing of hematite nanoarray photoanode with controllable metal–organic frameworks shell for enhanced photoelectrochemical water oxidation. J. Catal. 2022, 413, 398–406. [Google Scholar] [CrossRef]
- Yuan, S.Y.; Jiang, L.W.; Hu, J.S.; Liu, H.; Wang, J.J. Fully dispersed irox atomic clusters enable record photoelectrochemical water oxidation of hematite in acidic media. Nano Lett. 2023, 23, 2354–2361. [Google Scholar] [CrossRef]
- Kong, W.; Zhang, X.; Liu, S.; Zhou, Y.; Chang, B.; Zhang, S.; Fan, H.; Yang, B. N doped carbon dot modified WO3 nanoflakes for efficient photoelectrochemical water oxidation. Adv. Mater. Interfaces 2019, 6, 1801653. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Q.; Zhang, L.; Tang, Y.W.; Wang, G.; Mao, G.B. Novel nanostructured WO3@prussian blue heterojunction photoanodes for efficient photoelectrochemical water splitting. ACS Appl. Energy Mater. 2021, 4, 12508–12514. [Google Scholar] [CrossRef]
- Bu, Y.; Ren, J.; Zhang, H.; Yang, D.; Chen, Z.; Ao, J.P. Photogenerated-carrier separation along edge dislocation of WO3 single-crystal nanoflower photoanode. J. Mater. Chem. A 2018, 6, 8604–8611. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.S. Elaborately modified BiVO4 photoanodes for solar water splitting. Adv. Mater. 2019, 31, 1806938. [Google Scholar] [CrossRef]
- Lin, J.; Han, X.; Liu, S.; Lv, Y.; Li, X.; Zhao, Y.; Li, Y.; Wang, L.; Zhu, S. Nitrogen-doped cobalt-iron oxide cocatalyst boosting photoelectrochemical water splitting of BiVO4 photoanodes. Appl. Catal. B Environ. 2023, 320, 121947. [Google Scholar] [CrossRef]
- Jiang, W.; An, Y.; Wang, Z.; Wang, M.; Bao, X.; Zheng, L.; Cheng, H.; Wang, P.; Liu, Y.; Zheng, Z.; et al. Stress-induced BiVO4 photoanode for enhanced photoelectrochemical performance. Appl. Catal. B Environ. 2022, 304, 121012. [Google Scholar] [CrossRef]
- Grigioni, I.; Stamplecoskie, K.G.; Selli, E.; Kamat, P.V. Dynamics of photogenerated charge carriers in WO3/BiVO4 heterojunction photoanodes. J. Phys. Chem. C 2015, 119, 20792–20800. [Google Scholar] [CrossRef]
- Afroz, K.; Moniruddin, M.; Bakranov, N.; Kudaibergenov, S.; Nuraje, N. A heterojunction strategy to improve the visible light sensitive water splitting performance of photocatalytic materials. J. Mater. Chem. A 2018, 6, 21696–21718. [Google Scholar] [CrossRef]
- Tateno, H.; Chen, S.Y.; Miseki, Y.; Nakajima, T.; Mochizuki, T.; Sayama, K. Photoelectrochemical oxidation of glycerol to dihydroxyacetone over an acid-resistant Ta: BiVO4 photoanode. ACS Sustain. Chem. Eng. 2022, 10, 7586–7594. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, D.H.; Yoon, J.W.; Dai, Z.; Lee, J.-H. Rational design of branched WO3 nanorods decorated with BiVO4 nanoparticles by all-solution processing for efficient photoelectrochemical water splitting. ACS Appl. Energy Mater. 2019, 2, 4535–4543. [Google Scholar] [CrossRef]
- Hong, S.J.; Lee, S.; Jang, J.S.; Lee, J.S. Heterojunction BiVO4/WO3 electrodes for enhanced photoactivity of water oxidation. Energy Environ. Sci. 2011, 4, 1781–1787. [Google Scholar] [CrossRef]
- Rao, P.M.; Cai, L.; Liu, C.; Cho, I.S.; Lee, C.H.; Weisse, J.M.; Yang, P.; Zheng, X. Simultaneously efficient light absorption and charge separation in WO3/BiVO4 core/shell nanowire photoanode for photoelectrochemical water oxidation. Nano Lett. 2014, 14, 1099–1105. [Google Scholar] [CrossRef]
- Shi, X.; Choi, Y.; Zhang, K.; Kwon, J.; Kim, D.Y.; Lee, J.K.; Oh, S.H.; Kim, J.K.; Park, J.H. Efficient photoelectrochemical hydrogen production from bismuth vanadate-decorated tungsten trioxide helix nanostructures. Nat. Commun. 2014, 5, 4775. [Google Scholar] [CrossRef]
- Ma, Z.; Song, K.; Wang, L.; Gao, F.; Tang, B.; Hou, H.; Yang, W. WO3/BiVO4 type-II heterojunction arrays decorated with oxygen-deficient ZnO passivation layer: A highly efficient and stable photoanode. ACS Appl. Mater. Interfaces 2019, 11, 889–897. [Google Scholar] [CrossRef]
- Ma, M.; Kim, J.K.; Zhang, K.; Shi, X.; Kim, S.J.; Moon, J.H.; Park, J.H. Double-deck inverse opal photoanodes: Efficient light Absorption and charge separation in heterojunction. Chem. Mater. 2014, 26, 5592–5597. [Google Scholar] [CrossRef]
- Ma, M.; Shi, X.; Zhang, K.; Kwon, S.; Li, P.; Kim, J.K.; Phu, T.T.; Yi, G.-R.; Park, J.H. A 3D triple-deck photoanode with a strengthened structure integrality: Enhanced photoelectrochemical water oxidation. Nanoscale 2016, 8, 3474–3481. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Li, J.; Li, L.; Bai, J.; Xia, L.; Zhou, B. Synthesis of WO3/BiVO4 photoanode using a reaction of bismuth nitrate with peroxovanadate on WO3 film for efficient photoelectrocatalytic water splitting and organic pollutant degradation. Appl. Catal. B Environ. 2017, 217, 21–29. [Google Scholar] [CrossRef]
- Park, E.; Patil, S.S.; Lee, H.; Kumbhar, V.S.; Lee, K. Photoelectrochemical H2 evolution on WO3/BiVO4 enabled by single-crystalline TiO2 overlayer modulations. Nanoscale 2021, 13, 16932–16941. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.S.; Lee, J.; Park, E.; Nagappagari, L.R.; Lee, K. Interstitial M+ (M+ = Li+ or Sn4+) doping at interfacial BiVO4/WO3 to promote photoelectrochemical hydrogen production. ACS Appl. Energy Mater. 2021, 4, 13636–13645. [Google Scholar] [CrossRef]
- Grigioni, I.; Liberto, G.D.; Dozzi, M.V.; Tosoni, S.; Pacchioni, G.; Selli, E. WO3/BiVO4 photoanodes: Facets matching at the heterojunction and BiVO4 layer thickness effects. ACS Appl. Energy Mater. 2021, 4, 8421–8431. [Google Scholar] [CrossRef]
- Quang, N.D.; Van, P.C.; Majumder, S.; Jeong, J.-R.; Kim, D.; Kim, C. Optimization of photogenerated charge transport using type-II heterojunction structure of CoP/BiVO4:WO3 for high efficient solar-driver water splitting. J. Alloys Compd. 2022, 899, 163292. [Google Scholar] [CrossRef]
- Li, Y.; Mei, Q.; Liu, Z.; Hu, X.; Zhou, Z.; Huang, J.; Bai, B.; Liu, H.; Ding, F.; Wang, Q. Fluorine-doped iron oxyhydroxide cocatalyst: Promotion on the WO3 photoanode conducted photoelectrochemical water splitting. Appl. Catal. B Environ. 2022, 304, 120995. [Google Scholar] [CrossRef]
- Kalanur, S.S.; Yoo, I.-H.; Park, J.; Seo, H. Insights into the electronic bands of WO3/BiVO4/TiO2, revealing high solar water splitting efficiency. J. Mater. Chem. A 2017, 5, 1455–1461. [Google Scholar] [CrossRef]
- Lee, M.G.; Kim, D.H.; Sohn, W.; Moon, C.W.; Park, H.; Lee, S.; Jang, H.W. Conformally coated BiVO4 nanodots on porosity-controlled WO3 nanorods as highly efficient type II heterojunction photoanodes for water oxidation. Nano Energy 2016, 28, 250–260. [Google Scholar] [CrossRef]
- Kumbhar, V.S.; Lee, H.; Lee, J.; Lee, K. Interfacial growth of the optimal BiVO4 nanoparticles onto self-assembled WO3 nanoplates for efficient photoelectrochemical water splitting. J. Colloid Interface Sci. 2019, 557, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Chi, Z.; Zhao, J.; Zhang, Y.; Yu, H.; Yu, H. Coral-like WO3/BiVO4 photoanode constructed via morphology and facet engineering for antibiotic wastewater detoxifcation and hydrogen recovery. Chem. Eng. J. 2022, 428, 131817. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, Y.; Wang, S.; Li, L.; Wang, W.; Sun, Q. In-situ generation of Bi2S3 to construct WO3/BiVO4/Bi2S3 heterojunction for photocathodic protection of 304SS. J. Electroanal. Chem. 2022, 907, 116033. [Google Scholar] [CrossRef]
- Li, L.; Xiao, S.; Li, R.; Cao, Y.; Chen, Y.; Li, Z.; Li, G.; Li, H. Nanotube array-like WO3 photoanode with dual-layer oxygen evolution cocatalysts for photoelectrocatalytic overall water splitting. ACS Appl. Energy Mater. 2018, 1, 6871–6880. [Google Scholar] [CrossRef]
- Baek, J.H.; Kim, B.J.; Han, G.S.; Hwang, S.W.; Kim, D.R.; Cho, I.S.; Jung, H.S. BiVO4/WO3/SnO2 double-heterojunction photoanode with enhanced charge separation and visible-transparency for bias-free solar water-splitting with a perovskite solar cell. ACS Appl. Mater. Interfaces 2017, 9, 1479–1487. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Y.; Yang, G.; Li, S.; Xiao, N.; Xu, B.; Liu, S.; Qiu, P.; Hao, S.; Ge, L. Fe2TiO5 as an efficient co-catalyst to improve the photoelectrochemical water splitting performance of BiVO4. ACS Appl. Mater. Interfaces 2018, 10, 39713–39722. [Google Scholar] [CrossRef]
- Chen, H.-Q.; Lin, L.-Y.; Chen, S.-L. Direct growth of BiVO4/Bi2S3 nanorod array on conductive glass as photocatalyst for enhancing the photoelectrochemical performance. ACS Appl. Energy Mater. 2018, 1, 6089–6100. [Google Scholar] [CrossRef]





| Structure of Photoelectrodes | Method | Electrolyte | Photocurrent Density at 1.23 V vs. RHE (mA cm−2) | Ref. |
|---|---|---|---|---|
| BiVO4 layer/nanoplate-like WO3 | hydrothermal method + spin coating (20 cycles) | 0.5 M Na2SO4 | 0.43 | [34] |
| BiVO4 layer/WO3 nanoplates | hydrothermal method + spin coating | 0.5 M Na2SO4 | 0.62 | [35] |
| BiVO4 layers/a WO3 layer | spin-coating + repeated spin-coating | 0.5 M Na2SO4 | 1.25 | [36] |
| BiVO4 layer/WO3 nanoplates | hydrothermal method + spin-coating deposition (4 cycles) | 0.5 M Na2SO4 | 1.93 | [37] |
| WO3 nanoplates coated by BiVO4 nanoparticles | hydrothermal + spinning calcination | 0.1 M KPi buffer solution | 1.2 | [38] |
| Island-like BiVO4/Plate-like WO3 | hydrothermal + spin coating | 0.1 M Na2SO4 | 4.2 | [39] |
| BiVO4 nanodot/WO3 nanorods | glancing angle deposition + Pulsed electrodeposition | 0.5 M KPi + 1 M Na2SO3 | 4.55 | [40] |
| BiVO4 nanoparticles/WO3 nanoplates | hydrothermal method + chemical bath deposition (20 times) | 0.5 M Na2SO4 | 1.7 | [41] |
| coral-like BiVO4/WO3 | hydrothermal method + electrodeposition process | 0.5 M phosphate buffer solution | 2.9 | [42] |
| BiVO4 nanoparticles/WO3 nanoplates | hydrothermal method + hydrothermal method | 0.5 M (Na2SO4 + Na2SO3) | 1.35 | [43] |
| surface dispersed BiVO4/WO3 nanoplates | hydrothermal method + solvothermal method | 0.2 M KPi buffer solution | 3.53 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, X.; Chen, Q.; Zhu, F.; Wang, T.; Gao, B.; Song, L.; He, J. Preparation of Surface Dispersed WO3/BiVO4 Heterojunction Arrays and Their Photoelectrochemical Performance for Water Splitting. Molecules 2024, 29, 372. https://doi.org/10.3390/molecules29020372
Fan X, Chen Q, Zhu F, Wang T, Gao B, Song L, He J. Preparation of Surface Dispersed WO3/BiVO4 Heterojunction Arrays and Their Photoelectrochemical Performance for Water Splitting. Molecules. 2024; 29(2):372. https://doi.org/10.3390/molecules29020372
Chicago/Turabian StyleFan, Xiaoli, Qinying Chen, Fei Zhu, Tao Wang, Bin Gao, Li Song, and Jianping He. 2024. "Preparation of Surface Dispersed WO3/BiVO4 Heterojunction Arrays and Their Photoelectrochemical Performance for Water Splitting" Molecules 29, no. 2: 372. https://doi.org/10.3390/molecules29020372
APA StyleFan, X., Chen, Q., Zhu, F., Wang, T., Gao, B., Song, L., & He, J. (2024). Preparation of Surface Dispersed WO3/BiVO4 Heterojunction Arrays and Their Photoelectrochemical Performance for Water Splitting. Molecules, 29(2), 372. https://doi.org/10.3390/molecules29020372







